Chapter 198 Nerve Transfers: Indications and Techniques
Traumatic brachial plexus injuries (BPIs) produce psychologically and functionally devastating handicaps afflicting, generally, a subset of young, healthy males in the prime of life. Seventy percent are caused by motor vehicle accidents, of which 70% are due to the use of two-wheelers.1 The disease burden is estimated to be about 2162 cases per annum in the United States; costs of treatment amount to about $34,733 per capita.2 It is prudent to mention at the outset that the management of traumatic BPI requires a complex multidisciplinary approach involving neurosurgeons, orthopedists, plastic/hand surgeons, neurologists, neuroradiologists, neurophysiologists, physiotherapists, and pain specialists.
Nerve Transfers (Neurotizations)
The use of and enthusiasm for nerve transfers have increased dramatically in the past two decades largely due to the creative contributions of innovators such as Drs. Christophe Oberlin and Susan Mackinnon. The introduction of a fascicular transfer by Oberlin et al. (1994)3 for upper trunk injuries catalyzed the transformation. This procedure introduced the concept of fascicular transfer by using functioning ulnar nerve fascicles supplying the flexor carpi ulnaris to be selectively transferred onto the nerve to biceps with excellent results.3–5
The new era of nerve transfer has created a major controversy in brachial plexus surgery now—the role of nerve grafts versus nerve transfers in postganglionic injuries. This issue remains largely unresolved. The advantages and disadvantages of performing nerve transfers over nerve grafts are listed in Tables 198-1 and 198-2. As can be seen, the advantages of performing nerve transfers may outweigh those of brachial plexus nerve grafting. In fact, in the event of patients sustaining panplexal nerve injuries secondary to multiple root avulsions, nerve transfers may be the only viable form of repair.
TABLE 198-1 Advantages of Performing Nerve Transfer versus Nerve Graft
| Nerve Transfer | Nerve Graft |
|---|---|
| A distal procedure performed close to the motor point of muscles. This decreases the time to reinnervation. The direct repair is at a single suture line. This improves reinnervation as well. | Performed at the site of injury, typically proximally, and thus more anatomical. These are also more physiologic and make relearning when muscle power returns easier. |
| Surgical dissection occurs in uninvolved pristine tissue. | These repairs make available more donor motor axons as nerve stumps are typically largest proximally. |
| Directed (selective) neurotization makes targeting motor recipient axons easier as it is closer to the motor end plate. | As the entire cut surface is coapted with the injured stump, sensory reinnervation is also a possibility. |
| Is the only procedure possible after nerve root avulsion. | Grafts enable entire motor groups to recover as opposed to single targeted muscles. |
| Can be performed with minimal technology. |
TABLE 198-2 Disadvantages of Performing Nerve Transfer versus Nerve Graft
| Nerve Transfer | Nerve Graft |
|---|---|
| Involves some loss of existing function, by definition. | Typically requires use of nerve grafts, e.g., non-degenerated sensory nerves like the sural. The harvest has cosmetic and functional sequelae. |
| Requires muscle re-education that can hamper autonomy because of co-contractions. | Has to be performed at the site of injury. Dissection is more difficult, especially in the presence of a concomitant vascular repair. |
| Number of available donor nerves is limited especially in patients with panplexal injuries. | Cannot be performed in patients with root avulsive injuries. |
| Number of available motor axons is necessarily limited. | Directed (motor donor to motor recipient) repair is seldom possible, especially when the loss of length is high. All proximal components of the brachial plexus are mixed sensorimotor. |
| Longer grafts should be vascularized as free grafts may fibrose secondary to ischemic injury. | |
| Grafts require axonal sprouts to cross two suture lines. This further delays repair. If the distal suture line scars densely, the entire repair may fail here. | |
| Grafts ideally require the use of intraoperative sensory and motor-evoked potentials to determine the viability of the donor nerve stump (especially proximal cervical nerves) even if it appears structurally intact. These have several technical and observer-dependent confounding factors. |
Indications
1. “Irreparable” nerves that are avulsed from the spinal cord.
Preganglionic injuries are not amenable to any other form of repair as the motor axon has been physically disconnected from the neuronal cell body across the intervertebral foramen. Reconnection via axonal regeneration is not physically possible. In such patients, motor end plate degeneration starts at the time of injury and may be irreversible after 12 to 18 months.6,7 To beat the biological clock, it is imperative to operate as soon as possible and transfer viable axons to as close to the motor end point as possible. This is the procedure of choice in this form of injury.
2. More rapid or reliable recovery of motor function.
Most experts have now begun to recognize that nerve transfers allow a faster and perhaps more reliable way of regaining function even in postganglionic injuries bucking the previous trend of using proximal nerve grafts (Table 198-3).
3. To power free-functioning muscle transfers (FFMT).
Free-functioning muscle transfer is a reliable way to reconstruct the damaged upper extremity by moving a functioning muscle with its nerve and blood supply to another location where it can subserve a new function. This can occur after a successful nerve repair and anastomosis of the artery and vein have been accomplished. This microsurgical technique therefore does not have a time window like typical nerve reconstruction. Most commonly, FFMTs have thereby been performed for delayed cases to provide elbow flexion (such as in neglected injuries or those with poor or incomplete recovery). FFMTs in this setting can augment motor function. The native anatomy of the transferred muscle/tendon unit (gracilis, for example) has important features which allow FFMTs to achieve more distal function, ordinarily unachievable with standard techniques of nerve reconstruction (e.g., recovery of prehension of the hand in patients with flail limbs). The nerve supply is relatively close to the muscle and the tendon is long (and can be prolonged). This technique then can also be incorporated into an armamentarium in combination with other nerve techniques in the early setting.
TABLE 198-3 Commonly and Uncommonly Performed Nerve Donors/Transfers
| Recipient Nerve | Common Donor Nerves | Uncommon Donor Nerves |
|---|---|---|
| Suprascapular | Spinal accessory C5 nerve C6 nerve |
C4 nerve C7 nerve/middle trunk Phrenic nerve Contralateral C7 Dorsal scapular |
| Axillary | Triceps branch of radial Medial pectoral Motor intercostals |
Thoracodorsal Contralateral C7 |
| MCN, biceps, or brachialis branch | Ulnar nerve fascicle Median nerve fascicle Motor intercostal Medial pectoral |
Thoracodorsal Spinal accessory Phrenic Contralateral C7 Hemi-hypoglossal |
| Median | Sensory intercostals Contralateral C7 Ulnar nerve fascicle Brachialis branch of MCN |
Supinator/ECRB branch of radial |
| Radial | Motor intercostals Proximal branches of radial |
Contralateral C7 Dorsal scapular nerve Median nerve branches |
| Ulnar nerve | Brachialis branch of MCN Anterior interosseous |
Contralateral C7 |
ECRB, extensor carpi radialis brevis; MCN, musculocutaneous.
Contraindications
The only absolute contraindications for a nerve transfer are the absence of a donor nerve and the presence of a fibrosed atrophic recipient nerve with no viable fascicles seen under magnification on sequential cut sections at the motor point. Relative contraindications would include the availability of a poor quality donor as seen with direct electrical stimulation at surgery, the presence of a stimulatable motor nerve recipient at surgery, or a short segment rupture/neuroma that can easily be repaired end-to-end without the use of a graft.
Principles
1. Accurate documentation of pre-existing muscle power, vascular injury, and joint contractures
2. Clear, coherent, and exhaustive discussion of options and priorities with the patient
3. Fall-back planning and prior consent in case favored donors are poorly functioning
4. Elaboration of realistic goals prior to surgery, including risks related to possible donor morbidity (transient and permanent) and slow nature of recovery
5. Selection of the ideal donor nerve (Table 198-4)
6. Transection of recipient nerve as proximal as possible to determine adequacy before donor dissection
7. Donor nerve dissected distal to recipient to gain length
8. Selective neurotization based on knowledge of fascicular anatomy (Table 198-5)
9. Awareness of proximal and distal orientation of transected nerve segments
| Pure | Motor or sensory. Example: Medial pectoral nerve (motor); sensory intercostal nerves. |
| Adjacent | Minimal donor dissection required to gain length. Example: Double fascicular transfer for elbow flexion. |
| Expendable | Unlikely to affect donor function. Example: Intercostal nerve transfer. |
| Uninjured | Donor fascicle muscle groups must have at least grade 4/5 MRC power. Example: Spinal accessory transfer is contraindicated when shoulder function is poor. |
| Remote | From zone of injury (dissection is easier). Example: Oberlin’s transfer in upper trunk injury. |
| Proximate | To motor point so reinnervation is faster and likely better. Example: Triceps to axillary nerve transfer. |
| Adequate | Diameter mismatch is prevented. Example: Three motor intercostals are used to neurotize the nerve to biceps. |
| Educatable | Same muscle compartment is ideal, as relearning and subsequent autonomy are better and faster as antagonistic co-contractions do not occur. |
MRC, Medical Research Council.
TABLE 198-5 Fascicular Anatomy of Major Nerves
| Nerve | Donor/Recipient | Fascicular Anatomy |
|---|---|---|
| CC7 | Donor | Posterior division has 2x motor axons. |
| Median (axilla) | Recipient | Lateral root is mainly sensory. |
| Median (arm) | Donor | FCR/FDS fascicle is medial. |
| Median (arm) | Recipient | Posterior fascicle (AIN). Anterior fascicle (PT, FCR). Middle fascicle (FDS, thenar, sensory). |
| Median (forearm) | Donor | Terminal AIN branch to PQ. |
| Nerve to biceps | Recipient | Lateral in musculocutaneous nerve. |
| Nerve to brachialis | Donor/recipient | Lateral to LABC. |
| Suprascapular | Recipient | Lateral within upper trunk. |
| Ulnar (arm) | Donor | FCU fascicle is posteromedial. |
AIN, anterior interosseous nerve; CC7, contralateral C7; FCR, flexor carpi radialis; FCU, flexor carpi ulnaris; FDS, flexor digitorum superficialis; LABC, lateral antebrachial cutaneous nerve; PQ, pronator quadratus; PT, pronator teres.
Common Targets
The common recipient nerves for transfers are the suprascapular, axillary, and musculocutaneous nerves. A list of donors is provided in Table 198-3.
Surgical Anatomy
The brachial plexus is formed by the ventral rami of the fifth to eighth cervical and the first thoracic nerve roots. It is essential to remember that the nerves emerge between the scalenus anterior and medius (interscalene triangle) to form trunks in the supraclavicular fossa. The divisions of the brachial plexus are retroclavicular while the cords are infraclavicular and named as such in relation to the axillary artery.
Supraclavicular Exploration
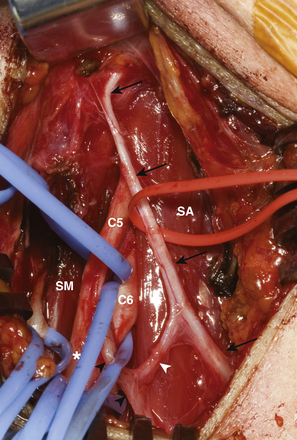
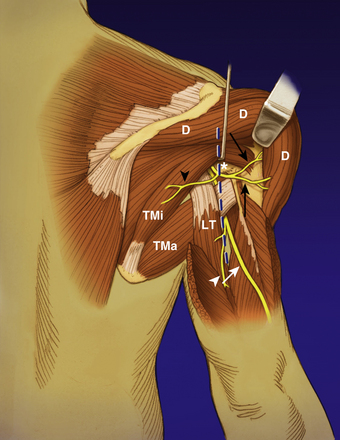
The key muscles for the supraclavicular exploration of the brachial plexus are the omohyoid (deep to which lies the fat pad covering the plexus) and the scalenus anterior (deep to which lie the nerves). The latter is often difficult to identify especially when there is extensive scarring. They are then identified indirectly as the structures that lie beneath the phrenic nerve. The phrenic nerve may be identified visually as the only neural structure (other than the nerve to subclavius) that passes from lateral to medial in the root of the neck (Fig. 198-1). If scarring is extensive, blind neural stimulation using 0.1–2 mA of current may provoke capnographic changes if not frank diaphragmatic contraction that may guide the surgeon to the approximate location of this key structure before sharp dissection is commenced.8 The phrenic nerve can then be traced back to the C5 nerve via its contribution to the former. From here, the C5 nerve can then be traced to the upper trunk. Proximal and distal dissection allows identification of C6 and the divisions of the upper trunk. The suprascapular nerve is the key target in this exploration and can be found superior, lateral, and posterior to the upper trunk. Its direction confirms its identity. It parallels the omohyoid and runs posteriorly obliquely to the scapular notch. In the event of a complete rupture of the C5 and C6 nerves, the distal components of the plexus can be found near, behind, or even below the clavicle.
Care has to be taken, during dissection, not to injure the lymphatic duct on the right and the chyle carrying thoracic duct on the left side. These elements are at risk during medial dissection such as with exposure of the lower trunk elements.
Infraclavicular Exploration
It is important to realize that the musculocutaneous nerve provides its first branch to the coracobrachialis muscle and then penetrates this muscle. This must not be mistaken for the nerve to the biceps which is given off its lateral aspect in the mid-arm (typically 12 cm distal to the acromion). The nerve to the brachialis is given off further distally (approximately 17 cm distal to the acromion) and typically lies lateral to the terminal branch, the lateral antebrachial cutaneous nerve.9 The former is a pure motor branch, while the latter is a sensory nerve. The authors verify the nature of both these terminal divisions by tracing the brachialis branch until it arborizes on the muscle surface. A variation that has to be considered is the low take-off of the musculocutaneous nerve from the median nerve. This is important to consider especially when the median nerve is being considered as a donor for a simultaneous nerve transfer.
General Principles: Surgical Technique
Positioning and Anesthesia
Patients are operated on under general anesthesia. Strict instructions are issued to ensure that no neuromuscular blocking agents are used. The patient is typically placed in the supine position with the head slightly extended and turned to the opposite side. An interscapular towel roll can also be used to make the supraclavicular fossa more prominent. Occasionally, the patient is bumped up to provide access if a posterior approach is anticipated. The arm is placed, initially, in an adducted and depressed position to enhance the exposure. However, it should be draped in such a manner as to ensure that it can be freely abducted or crossed over the chest, so as to facilitate further surgery. The hands and fingers should be kept exposed for visual inspection of evoked movements. As a substantial part of the total body surface area has to be kept exposed, it is vital to ensure that patients do not slip into hypothermia. The appropriate use of blankets and warming units is encouraged. As nerve transfers may well be done in combination with nerve grafting, wide prepping of the neck, chest, arm, and both legs is always done for various exposures and harvest of nerve graft(s).
Technique of Nerve Coaption
First we prepare all of the donor and recipient nerves or fascicles and clip them with redundancy to green backgrounds, once transected, to ensure high visibility. Then we perform all of the microsurgery of the various nerves. We trim back on the recipient so that its length is as short as possible so as to minimize reinnervation time to the end organ. A repair with tension is avoided. The repair is tested in the maximum anticipated range of joint movements in all directions. Diameter mismatches are avoided wherever possible and selective neurotization is practiced when it is not. Donor and recipient nerve fascicles are further skeletonized and soft tissue is denuded at the coapting ends to prevent scar formation. The fascicular ends are finally sectioned using an ultrafine diamond knife. Coaption is then carried out using interrupted, nylon, monofilament 9-0 sutures passed through the epineurium of adjacent fascicles. An operating microscope with twin mobile opposable eyepieces is used so that two surgeons can operate simultaneously. Frequent heparinized saline flushes ensure adequate visibility at the time of coaption. Typically, two diametrically opposite sutures are used with three throws each. The initial knot is tensioned gradually so as to just appose the cut sections of the participating fascicles without buckling them. Knots are initially cut long so as to rotate the line of coaption to look for any unevenness. If the suture line is uneven or buckled, more sutures or else release of existing sutures and redo may be preferred as this is the most vital part of the operation. Once the suture line is observed to be satisfactory, we place a bivalved nerve tube of an appropriate diameter to protect the repair. This is then cemented in with the use of tissue adhesive.9
Common Presentations: Strategies and Nerve Transfers
Panplexal Injury
Management Rationale
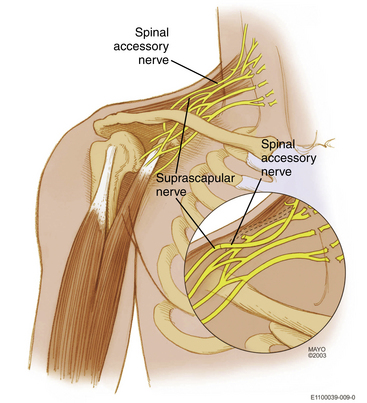
In the event of panroot avulsion, nerve transfers alone can provide the only solution. The authors’ preferred standard solution is the spinal accessory-suprascapular nerve (Fig. 198-2), motor intercostal-musculocutaneous nerve (Fig. 198-3) and sensory intercostal-lateral root of median nerve transfer. This algorithm provides stability of the shoulder, elbow flexion, and protective sensation in the hand. In many situations, a C5 stump is available for nerve grafting, even in the panplexal situation. This is why we recommend exploration of the brachial plexus and electrophysiologic monitoring of these patients. One or more donors may be available for use.
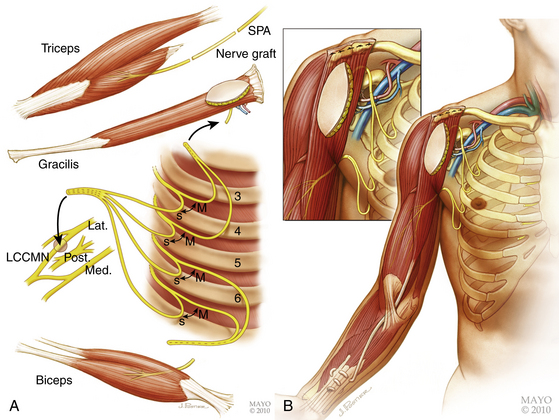
We have also employed a technique to offer patients a chance of hand prehension. We have modified Doi’s original procedure detailing the double functioning free muscle transfer (FFMT) into a single-stage procedure to provide some chance of hand recovery in a patient with a flail anesthetic limb.10 If one cervical nerve is viable, it is used as a donor for nerve grafting to the suprascapular and axillary nerves. If it is not available, the shoulder is not reconstructed, or at a delayed stage, shoulder fusion or tendon transfer can be considered. Four intercostals (T3–T6) are harvested for nerve transfers for elbow flexion: two of these (T5 and T6) are transferred to the native nerve to biceps and the remaining two (T3 and T4) are used to power a FFMT destined for combined elbow flexion/finger flexion. The contralateral gracilis muscle is transferred with its neurovascular pedicle onto the clavicle and its tendon sutured into the long flexors of all five digits. Vascular anastomoses are then carried out and the nerve to gracilis is coapted. Sensory innervation is provided for by coapting all available sensory intercostal nerves with the lateral root of the median nerve (Fig. 198-4).9 The spinal accessory nerve is lengthened with a nerve graft and coapted to a triceps branch.
Approaches
The spinal accessory nerve is then identified on the medial border of the trapezius, stimulated to confirm its identity and viability after detecting trapezius contraction, encircled with vessel loops, and then traced distally as far as is feasible. The proximal branch of the spinal accessory nerve to the trapezius is preserved to retain some of the muscle’s function.
The suprascapular nerve is then prepared. It is dissected off the neuroma/posterior division and its fascicle traced into the upper trunk to gain additional length. It is transected at that level. Good fascicular structure is obtained by trimming back as necessary. The donor and recipient fascicles are then secured on a green background. These nerves may be difficult to find later in a bloody field and the suprascapular nerve may retract inferiorly after being cut. This repair is then carried out using a standardized technique described in an earlier section (Fig. 198-2).
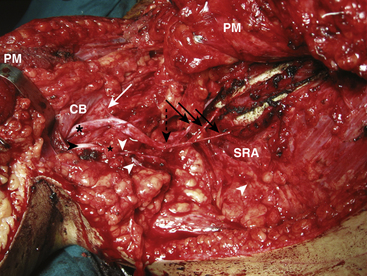
Intercostal–Musculocutaneous/Median Nerve Transfer
With the axillary sheath exposed, the lateral cord, its contribution to the median nerve and the take-off of the musculocutaneous nerve are identified. The musculocutaneous nerve is then followed to its biceps motor branch. This branch is then lengthened with retrograde dissection, separating it from the parent nerve until sufficient length is obtained to allow direct transfer of the prepared intercostal motor nerves. In a similar fashion, the lateral root of the median nerve is also skeletonized from the musculocutaneous nerve and the lateral cord itself to gain additional length to allow for a direct transfer of the previously harvested intercostal sensory nerves (Fig. 198-3). Nerve coaption is performed using the operating microscope with the arm abducted to 90 degrees in external rotation.
Upper Trunk (C5/C6) Brachial Plexus Injury Pattern
Management Rationale
In this pattern of partial brachial plexis injury (BPI), several intraplexal donors are available for nerve transfers. Most importantly, hand function remains intact as the C8, T1 nerves are viable. However, this hand function is of limited use in the absence of useful elbow and shoulder function. Thus, while the management priorities are the same as that for panplexal injuries (namely, shoulder abduction and external rotation and elbow flexion), the major difference is that more options exist. These include newer, better motor intraplexal donors that can be transferred closer to the end organ. Classical proximal nerve grafts or nerve transfers, which were previously performed for these injuries, are now being replaced by distal directed nerve transfers for reasons that have been discussed previously.11–39 In these cases, to optimize results, double reinnervation of shoulder and elbow flexor targets is attempted.
For shoulder function the suprascapular nerve is still targeted using the spinal accessory. For restoring axillary nerve function, the authors prefer to use the adjacent triceps branch of the radial nerve via a posterior approach (Leechavengvongs’ transfer; Figs. 198-5 and 198-6).40 Elbow flexion is gained using either the Oberlin transfer alone (ulnar nerve to biceps) or a double fascicular transfer (ulnar nerve to biceps and a simultaneous median—nerve to brachialis; Fig. 198-7).41 If the C7 nerve is injured in addition, the axillary nerve may be neurotized using the medial pectoral nerve (C8, T1) or the thoracodorsal nerves (C6–C8). The more common techniques are described below.
Spinal Accessory Nerve to Suprascapular Nerve Transfer
As described previously, this is most commonly done in the supraclavicular region (Fig. 198-2). Some surgeons are performing this more distally, that is, closer to the target supraspinatus muscle. The disadvantage of this procedure is that it entails a separate exposure. Moreover, the distal myelinated axon count of the donor spinal accessory nerve is reduced due to its terminal branching pattern. Through a separate posterior incision, one can harvest both the spinal accessory and the suprascapular nerves in the vicinity of the scapula. This technique will not be further discussed here.
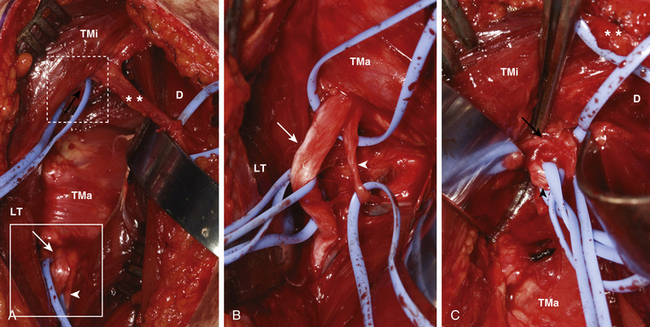
Nerve to Triceps-Axillary Nerve (Leechavengvongs) Transfer
The radial nerve and its branches to the triceps are now identified in the triangular space. The interval between the long and lateral heads of the triceps is opened, and the radial nerve is readily exposed. Several triceps branches are identified, tagged with vessel loops and tested for function using a nerve stimulator. The longest, largest functioning motor radial branch to any of the triceps heads is identified. Other branches to the triceps and the main radial nerve are protected. The selected triceps branch is then traced distally to gain as much length as feasible. It is also traced proximally and separated from the main radial nerve so as to displace the pivot point closer towards the deltoid muscle. This permits not only adequate length to perform a direct graft-free nerve transfer but also provides a little extra length. The surgeon can trim the recipient nerve back closer to the motor point of the deltoid to enable faster and perhaps better muscle reinnervation. The suture line is later rechecked with the arm in a position of abduction and external rotation before final approximation (Figs. 198-5 and 198-6).
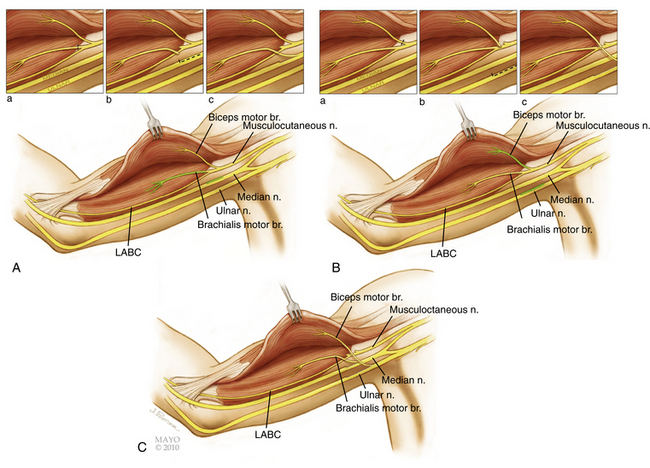
Double Fascicular Transfer for Elbow Flexion
The double fascicular transfer involves coaption of donors from the ulnar and median nerves onto the nerve to biceps and brachialis (Fig. 198-7). The theoretical advantage of performing this procedure is obvious as the brachialis is a more powerful elbow flexor than the biceps. The use of the additional donor and the inherent risk (albeit low) need to be carefully balanced. In our experience, clinical results have not demonstrated a functional advantage of the double fascicular transfer versus a single fascicular transfer.41
These donor and recipient nerves are found within the neurovascular bundle in the triceps-biceps groove of the mid-arm. A straight linear skin incision in the groove below the biceps, extending from the anterior axillary line to the distal one third–two thirds junction of the arm, is deepened through fat and fascia. The median nerve is generally the first structure to be reliably identified. The biceps muscle belly is then identified and retracted to look for the musculocutaneous nerve which lies on the deep surface of this muscle. Its separate branches to the biceps and brachialis muscles are then identified as is the lateral antebrachial cutaneous nerve. The ulnar nerve is then identified and dissected in the lower arm to a point 5 to 7 cm distal to the entry point of the musculocutaneous branch into the biceps muscle.
Free-Functioning Muscle Transfer
In early reconstruction, the authors prefer to perform either a single gracilis FFMT for elbow and finger flexion (Fig 198-4) or a two-staged double gracilis FFMT performed 6 weeks apart for combined elbow flexion and wrist extension and finger flexion. Shoulder stability, elbow extension and hand sensation is also targeted in both reconstructive algorithms. FFMT, in the chronic setting for elbow flexion, can be done using any available donor (most commonly spinal accessory nerve or intercostals; occasionally an ulnar fascicle). Details of these microsurgical procedures are beyond the scope of this chapter and have been described elsewhere by our group.9 Secondary reconstructive procedures are often performed such as arthrodesis of the glenohumeral joint, carpometacarpal joint of the thumb and the wrist joint to optimize muscle pull vectors and are considered on an individual basis.
Other Rarely Performed Nerve Transfers
Medial Pectoral-Axillary/Musculocutaneous Nerve Transfer
This procedure is performed by some for neurotizing the axillary and musculocutaneous nerves in upper and middle trunk/C5,C6,C7 nerve level injuries. A direct transfer is feasible through the same infraclavicular approach and skin incision.37,38 The problems lie in that the donor nerve here is the sole functioning nerve supplying the pectoralis major, and sectioning it decreases shoulder function. Another major problem is the diameter mismatch between donor and recipients. The transfer onto the musculocutaneous nerve is satisfactory in most hands, but this procedure is being supplanted by the use of the Oberlin’s transfer, which decreases the time to reinnervation and can be performed solely within the arm.
Phrenico-Suprascapular/Axillary/Musculocutaneous Nerve Transfer
The advantages of using the phrenic nerve are several—it is easily accessible, fires continuously and is purely motor for all realistic purposes.42–48 Sufficiently long lengths can also be isolated especially with the use of endoscopy to harvest it in the chest.48 This, theoretically, enables direct nerve transfers without the use of grafts. Practically, when this nerve is harvested in the supraclavicular region, grafts are required for axillary and musculocutaneous nerve transfers. Though several reports indicate that the clinical effects of diaphragmatic palsy are transient, the authors’ experience indicates otherwise especially when simultaneous intercostal nerves are harvested. This may perhaps be related to racial variations in build and body mass indices as well as regional variations involving low- versus high-velocity motor vehicle accidents.
Contralateral C7 Transfer
This was initially developed as a significant source of remote extraplexal expendable motor neurons by Gu et al. especially in the restoration of hand function in patients with panplexal injuries.35,36,49–54 It is classically performed in two stages. In the first stage, standard nerve transfers aimed at gaining shoulder and elbow function are performed. Simultaneously, the normal contralateral plexus is explored and the C7 nerve/middle trunk is either used in toto or after hemisection as a donor coapted with either a reversed pedicled or a vascularized free ulnar (ipsilateral injured) nerve graft passed subcutaneously across the chest. Axonal growth is monitored clinically along the ulnar nerve graft using Tinel’s sign and SSEPs. Once the Tinel’s sign reaches the axilla, the patient undergoes the second stage of surgery when the distal part of the ulnar graft is connected to the injured median nerve. This theoretically provides for both sensory innervation of the hand as well as long finger flexor function. Although initial results were promising, they have not been reproducible, especially regarding independent hand function.55–60 Moreover, the risk to the only functioning extremity, though small, is not without significance. In the authors’ experience, results have been functionally disappointing.
Newer Directions
It would not be an exaggeration to state that the results obtained after performing nerve transfers have radically improved the formerly dismal prognosis after BPI (Table 198-6).61–75 Achieving hand function in patients with panplexal injury, once unobtainable, can now be achieved with aggressive techniques. We are using FFMT. Some groups are routinely using extended phrenic or contralateral C7 nerve transfers. Others are attempting direct reimplantation of ventral avulsed roots within the spinal cord.76
| Recipient Nerve | Donor Nerves | Useful Postoperative Function (≥M3 MRC) |
|---|---|---|
| Suprascapular | Spinal accessory Phrenic Contralateral C7 |
70–100% 61–6366 75–100% 44,46,61,65 23.1*–100% 24 |
| Axillary | Triceps branch of radial Spinal accessory Medial pectoral Motor intercostals Thoracodorsal Contralateral C7 |
100% 40,63,67 75% 37 81.8% 75 63.2% 37 36v93.3% 62,74 20–23.1% *,19 |
| MCN | Ulnar nerve fascicle Motor intercostal Medial pectoral Median nerve fascicle Contralateral C7 Spinal accessory Phrenic Thoracodorsal |
64.7–100% 3–563 0–100% 37,61,62 85.7–90% 62,75 90–100% 72,73 52–100% 19,54,69–71 0–100% 61,64 16.6–100% 45,61 100% 74 |
*Mayo Clinic results (in press).
MCN, musculocutaneous nerve; MRC, Medical Research Council.
The extrapolation of nerve transfer techniques for single or distal nerves in the upper limb, to major nerves in the lower limb, or even the distal transected spinal cord can potentially change current management strategies.77–80 New pharmacologic agents, such as FK 506 may enhance neural regeneration and provide us with a new window of opportunity.81 The future for novel nerve transfers is bright.
Bertelli J.A., Ghizoni M.F. Reconstruction of C5 and C6 brachial plexus avulsion injury by multiple nerve transfers: spinal accessory to suprascapular, ulnar fascicles to biceps branch, and triceps long or lateral head branch to axillary nerve. J Hand Surg Am. 2004;29:131-139.
Carlsen B.T., Kircher M.F., Spinner R.J., et al. Comparison of single versus double nerve transfers for elbow flexion after brachial plexus injury. Plast Reconstr Surg. 2011;127:269-276.
Carlstedt T., Anand P., Hallin R., et al. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93:237-247.
Chuang D.C. Contralateral C7 transfer (CC–7T) for avulsion injury of the brachial plexus. Tech Hand Up Extrem Surg. 1999;3:185-192.
Doi K., Muramatsu K., Hottori Y., Watanabe M. Reconstruction of upper extremity function in brachial plexopathy using double free gracilis flaps. Tech Hand Up Extrem Surg. 2000;4:34-43.
Gu Y., Xu J., Chen L., et al. Long term outcome of contralateral C7 transfer: a report of 32 cases. Chin Med J [Engl]. 2002;115:866-868.
Gu Y.D., Chen D.S., Zhang G.M., et al. Long-term functional results of contralateral C7 transfer. J Reconstr Microsurg. 1998;14:57-59.
Leechavengvongs S., Witoonchart K., Uerpairojkit C., Thuvasethakul P. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: a report of 7 cases. J Hand Surg Am. 2003;28:633-638.
Loy S., Bhatia A., Asfazadourian H., Oberlin C. Ulnar nerve fascicle transfer onto to the biceps muscle nerve in C5–C6 or C5–C6–C7 avulsions of the brachial plexus. Eighteen cases. Ann Chir Main Memb Super. 1997;16:275-284. [French]
Mackinnon S.E., Novak C.B., Myckatyn T.M., Tung T.H. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J Hand Surg Am. 2005;30:978-985.
Mackinnon S.E., Novak C.B. Nerve transfers. New options for reconstruction following nerve injury. Hand Clin. 1999;15:643-666.
Merrell G.A., Barrie K.A., Katz D.L., Wolfe S.W. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg Am. 2001;26:303-314.
Oberlin C., Beal D., Leechavengvongs S., et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5–C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19:232-237.
Samardzic M., Rasuli L., Grujici D., Milici B. Results of nerve transfers to the musculocutaneous and axillary nerves. Neurosurgery. 2000;46:93-101.
Samardzic M., Grujicic D., Rasulic L., Bacetic D. Transfer of the medial pectoral nerve: myth or reality? Neurosurgery. 2002;50:1277-1282.
Samii M. Nerve transfer. J Neurosurg. 2000;92:1068-1069.
Songcharoen P., Wongtrakul S., Mahaisavariya B., Spinner R.J. Hemi-contralateral C7 transfer to median nerve in the treatment of root avulsion brachial plexus injury. J Hand Surg Am. 2001;26:1058-1064.
Spinner R.J., Shin A.Y., Herbert-Blouin M., et al. Traumatic brachial plexus injury, Wolfe S.W., Hotchkiss R.N., Pederson W.C., Kozin S.H., editors. Green’s Operative Hand Surgery, 6th ed, vol. 2. Philadelphia: Elsevier Churchill Livingstone, 2010;1235-1295.
Sulaiman O.A., Kim D.D., Burkett C., Kline D.G. Nerve transfer surgery for adult brachial plexus injury: a 10-year experience at Louisiana State University. Neurosurgery. 2009;65:A55-A62.
Sungpet A., Suphachatwong C., Kawinwonggowit V. One-fascicle median nerve transfer to biceps muscle in C5 and C6 root avulsions of brachial plexus injury. Microsurgery. 2003;23:10-13.
Teboul F., Kakkar R., Ameur N., et al. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J Bone Joint Surg (Am). 2004;86:1485-1490.
Terzis J.K., Kokkalis Z.T. Selective contralateral cC7 transfer in posttraumatic brachial plexus injuries: a report of 56 cases. Plast Reconstr Surg. 2009;123:927-938.
Terzis J.K., Konofaos P. Low-dose FK506 after contralateral C7 transfer to the musculocutaneous nerve using two different tubes: a study in rats. Ann Plast Surg. 2010;64:622-631.
Tung T.H., Mackinnon S.E. Nerve transfers: indications, techniques, and outcomes. J Hand Surg Am. 2010;35:332-341.
Witoonchart K., Leechavengvongs S., Uerpairojkit C., et al. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part I: an anatomic feasibility study. J Hand Surg Am. 2003;28:628-632.
1. Narakas A.O., Hentz V.R. Neurotization in brachial plexus injuries. Indication and results. Clin Orthop. 1988;237:43-56.
2. Lad S.P., Nathan J.K., Schubert R.D., Boakye M. Trends in median, ulnar, radial, and brachioplexus nerve injuries in the United States. Neurosurgery. 2010;66:953-960.
3. Oberlin C., Beal D., Leechavengvongs S., et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5–C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19:232-237.
4. Loy S., Bhatia A., Asfazadourian H., Oberlin C. Ulnar nerve fascicle transfer onto to the biceps muscle nerve in C5–C6 or C5–C6–C7 avulsions of the brachial plexus. Eighteen cases. Ann Chir Main Memb Super. 1997;16:275-284.
5. Teboul F., Kakkar R., Ameur N., et al. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J Bone Joint Surg (Am). 2004;86:1485-1490.
6. Mackinnon S.E., Dvali L.T.. Basic pathology of the hand, wrist and forearm: nerve, Berger R.A., Weiss A.P.C., editors, Hand Surgery, Philadelphia, Lippincott Williams & Wilkins, 2004;vol. 1:37-48.
7. Aird R.B., Naffziger H.C. The pathology of human striated muscle following denervation. J Neurosurg. 1953;10:216-227.
8. Bhagat H., Agarwal A., Sharma M.S. Capnography as an aid in localizing the phrenic nerve in brachial plexus surgery. Technical note. J Brachial Plex Peripher Nerve Inj. 2008;3:14.
9. Spinner R.J., Shin A.Y., Herbert-Blouin M., et al. Traumatic brachial plexus injury, Wolfe S.W., Hotchkiss R.N., Pederson W.C., Kozin S.H., editors. Green’s Operative Hand Surgery, 6th ed, vol. 2. Philadelphia: Elsevier Churchill Livingstone, 2010;1235-1295.
10. Doi K., Muramatsu K., Hottori Y., Watanabe M. Reconstruction of upper extremity function in brachial plexopathy using double free gracilis flaps. Tech Hand Up Extrem Surg. 2000;4:34-43.
11. Thoburn W. Obstetrical paralysis. J Obstet Gynecol Br Emp. 1903;3:454-458.
12. Taylor A.S. Brachial birth palsy and injuries of a similar type in adults. Surg Gynecol Obstet. 1920;30:494-502.
13. Nulsen F.E., Slade W.W. Recovery following injury to the brachial plexus. In: Woodhal B., Beebe G.W. Peripheral nerve regeneration: a follow-up study of 3656 World War II injuries. Washington, DC: Government Printing Office; 1956:389-408.
14. Millesi H. Surgical management of brachial plexus injury. J Hand Surg Am. 1977;2:367-378.
15. Narakas A. Brachial plexus surgery—Symposium on peripheral nerve injuries. Orth Clin North Am. 1981;2:303-323.
16. Sedel L. The results of surgical repair of brachial plexus injuries. J Bone Joint Surg (Br). 1982;64-B:54-66.
17. Kline D.G., Judice D.J. Operative management of selected brachial plexus lesions. J Neurosurg. 1983;58:631-649.
18. Terzis J.K., Kokkalis Z.T. Pediatric brachial plexus reconstruction. Plast Reconstr Surg. 2009;124:e370-e385.
19. Terzis J.K., Kokkalis Z.T. Selective contralateral c7 transfer in posttraumatic brachial plexus injuries: a report of 56 cases. Plast Reconstr Surg. 2009;123:927-938.
20. Terzis J.K., Kostopoulos V.K. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2007;119:e73-e92.
21. Chuang D.C. Adult brachial plexus reconstruction with the level of injury: review and personal experience. Plast Reconstr Surg. 2009;124:e359-e369.
22. Chuang D.C. Nerve transfer with functioning free muscle transplantation. Hand Clin. 2008;24:377-388.
23. Chuang D.C. Neurotization and free muscle transfer for brachial plexus avulsion injury. Hand Clin. 2007;23:91-104.
24. Chuang D.C. Nerve transfers in adult brachial plexus injuries: my methods. Hand Clin. 2005;21:71-82.
25. Chuang D.C. Management of traumatic brachial plexus injuries in adults. Hand Clin. 1999;15:737-755.
26. Mackinnon S.E., Novak C.B. Nerve transfers. New options for reconstruction following nerve injury. Hand Clin. 1999;15:643-666.
27. Mackinnon S.E., Novak C.B., Myckatyn T.M., Tung T.H. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J Hand Surg Am. 2005;30:978-985.
28. Tung T.H., Mackinnon S.E. Nerve transfers: indications, techniques, and outcomes. J Hand Surg Am. 2010;35:332-341.
29. Colbert S.H., Mackinnon S.E. Nerve transfers for brachial plexus reconstruction. Hand Clin. 2008;24:341-361.
30. Samii M., Carvalho G.A., Nikkhah G., Penkert G. Surgical reconstruction of the musculocutaneous nerve in traumatic brachial plexus injuries. J Neurosurg. 1997;87:881-886.
31. Penkert G., Carvalho G.A., Nikkhah G., et al. Diagnosis and surgery of brachial plexus injuries. J Reconstr Microsurg. 1999;15:3-8.
32. Samii M. Nerve transfer. J Neurosurg. 2000;92:1068-1069.
33. Gu Y.D., Wu M.M., Zheng Y.L., et al. Microsurgical treatment for root avulsion of the brachial plexus. Chin Med J (Engl). 1987;100:519-522.
34. Gu Y.D., Wu M.M., Zhen Y.L., et al. Phrenic nerve transfer for brachial plexus motor neurotization. Microsurgery. 1989;10:287-289.
35. Gu Y.D., Chen D.S., Zhang G.M., et al. Long-term functional results of contralateral C7 transfer. J Reconstr Microsurg. 1998;14:57-59.
36. Wang L., Zhao X., Gao K., et al. Reinnervation of thenar muscle after repair of total brachial plexus avulsion injury with contralateral C7 root transfer: report of five cases. Microsurgery. 2011;31:323-326.
37. Samardzi M., Rasuli L., Grujici D., Milici B. Results of nerve transfers to the musculocutaneous and axillary nerves. Neurosurgery. 2000;46:93-101.
38. Samardzi M., Grujici D., Rasuli L., Milici B. Restoration of upper arm function in traction injuries to the brachial plexus. Acta Neurochir (Wien). 2002;144:327-334.
39. Witoonchart K., Leechavengvongs S., Uerpairojkit C., et al. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part I: an anatomic feasibility study. J Hand Surg Am. 2003;28:628-632.
40. Leechavengvongs S., Witoonchart K., Uerpairojkit C., Thuvasethakul P. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: a report of 7 cases. J Hand Surg Am. 2003;28:633-638.
41. Carlsen B.T., Kircher M.F., Spinner R.J., et al. Comparison of single versus double nerve transfers for elbow flexion after brachial plexus injury. Plast Reconstr Surg. 2011;127:269-276.
42. Zheng M.X., Xu W.D., Qiu Y.Q., et al. Phrenic nerve transfer for elbow flexion and intercostal nerve transfer for elbow extension. J Hand Surg Am. 2010;35:1304-1309.
43. Siqueira M.G., Martins R.S. Phrenic nerve transfer in the restoration of elbow flexion in brachial plexus avulsion injuries: how effective and safe is it? Neurosurgery. 2009;65:A125-A131.
44. Sungpet A., Suphachatwong C., Kawinwonggowith V. Restoration of shoulder abduction in brachial plexus injury with phrenic nerve transfer. Aust N Z J Surg. 2000;70:783-785.
45. Hou Z., Xu Z. Nerve transfer for treatment of brachial plexus injury: comparison study between the transfer of partial median and ulnar nerves and that of phrenic and spinal accessary nerves. Chin J Traumatol. 2002;5:263-266.
46. Chuang D.C., Lee G.W., Hashem F., Wei F.C. Restoration of shoulder abduction by nerve transfer in avulsed brachial plexus injury: evaluation of 99 patients with various nerve transfers. Plast Reconstr Surg. 1995;96:122-128.
47. Cardenas-Mejia A., O’Boyle C.P., Chen K.T., Chuang D.C. Evaluation of single-, double-, and triple-nerve transfers for shoulder abduction in 90 patients with supraclavicular brachial plexus injury. Plast Reconstr Surg. 2008;122:1470-1478.
48. Lijie T., Zhenglang X., Xu W., et al. Mobilization of the phrenic nerve in the thoracic cavity by video-assisted thoracic surgery. Techniques and initial experience. Surg Endosc. 2001;15:1156-1158.
49. Gu Y.D., Zhang G.M., Chen D.S., et al. Cervical nerve root transfer from contralateral normal side for treatment of brachial plexus root avulsions. Chin Med J. 1991;104:208-211.
50. Chen L., Gu Y.D. [An experimental study of the treatment of root avulsion of brachial plexus using contralateral C7 nerve neurotization (nerve transfer)]. Zhonghua Wai Ke Za Zhi. 1992;30:525-527. 570-571
51. Gu Y.D., Zhang G.M., Chen D.S., et al. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg Br. 1992;17:518-521.
52. Chen L., Gu Y.D. An experimental study of contralateral C7 root transfer with vascularized nerve grafting to treat brachial plexus root avulsion. J Hand Surg Br. 1994;19:60-66.
53. Gu Y.D., Shen L.Y. Electrophysiological changes after severance of the C7 nerve root. J Hand Surg Br. 1994;19:69-71.
54. Gu Y., Xu J., Chen L., et al. Long-term outcome of contralateral C7 transfer: a report of 32 cases. Chin Med J [Engl]. 2002;115:866-868.
55. Chuang D.C., Wei F.C., Noordhoff M.S. Cross-chest C7 nerve grafting followed by free muscle transplantations for the treatment of total avulsed brachial plexus injuries: a preliminary report. Plast Reconstr Surg. 1993;92:717-725.
56. Chuang D.C., Cheng S.L., Wei F.C., et al. Clinical evaluation of C7 spinal nerve transection: 21 patients with at least 2 years’ follow-up. Br J Plast Surg. 1998;51:285-290.
57. Chuang D.C. Contralateral C7 transfer (CC-7T) for avulsion injury of the brachial plexus. Tech Hand Up Extrem Surg. 1999;3:185-192.
58. Yu Z.J., Sui S., Yu S., et al. Contralateral normal C7 nerve transfer after upper arm shortening for the treatment of total root avulsion of the brachial plexus: a preliminary report. Plast Reconstr Surg. 2003;111:1465-1469.
59. Waikakul S., Orapin S., Vanadurongwan V. Clinical results of contralateral C7 root neurotization to the median nerve in brachial plexus injuries with total root avulsions. J Hand Surg Br. 1999;24:556-560.
60. Songcharoen P., Wongtrakul S., Mahaisavariya B., Spinner R.J. Hemi-contralateral C7 transfer to median nerve in the treatment of root avulsion brachial plexus injury. J Hand Surg Am. 2001;26:1058-1064.
61. Merrell G.A., Barrie K.A., Katz D.L., Wolfe S.W. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg Am. 2001;26:303-314.
62. Sulaiman O.A., Kim D.D., Burkett C., Kline D.G. Nerve transfer surgery for adult brachial plexus injury: a 10-year experience at Louisiana State University. Neurosurgery. 2009;65:A55-A62.
63. Bertelli J.A., Ghizoni M.F. Reconstruction of C5 and C6 brachial plexus avulsion injury by multiple nerve transfers: spinal accessory to suprascapular, ulnar fascicles to biceps branch, and triceps long or lateral head branch to axillary nerve. J Hand Surg Am. 2004;29:131-139.
64. Songcharoen P., Mahaisavariya B., Chotigavanich C. Spinal accessory neurotization for restoration of elbow flexion in avulsion injuries of the brachial plexus. J Hand Surg Am. 1996;21:387-390.
65. Songcharoen P. Neurotization in the treatment of brachial plexus injuries. In: Omer G.E.Jr, Spinner M., Van Beek L.A. Management of Peripheral Nerve Problems. 2nd ed. Philadelphia: W.B. Saunders Co.; 1998:459-464.
66. Millesi H. Trauma involving the brachial plexus. In: Omer G.E.Jr, Spinner M., Van Beek L.A. Management of Peripheral Nerve Problems. 2nd ed. Philadelphia: W.B. Saunders Co.; 1998:433-444.
67. Kawai H., Akita S. Shoulder muscle reconstruction in the upper type of the brachial plexus injury by partial radial nerve transfer to the axillary nerve. Tech Hand Up Extrem Surg. 2004;8:51-55.
68. Xu L., Gu Y., Xu J., et al. Contralateral C7 transfer via the prespinal and retropharyngeal route to repair brachial plexus root avulsion: a preliminary report. Neurosurgery. 2008;63:553-558.
69. Amr S.M., Moharram A.N. Repair of brachial plexus lesions by end-to-side side-to-side grafting neurorrhaphy: experience based on 11 cases. Microsurgery. 2005;25:126-146.
70. Hierner R., Berger A.K. Did the partial contralateral C7-transfer fulfill our expectations? Results after 5 year experience. Acta Neurochir Suppl. 2007;100:33-35.
71. Terzis J.K., Kokkalis Z.T., Kostopoulos E. Contralateral C7 transfer in adult plexopathies. Hand Clin. 2008;24:389-400.
72. Nath R.K., Lyons A.B., Bietz G. Physiological and clinical advantages of median nerve fascicle transfer to the musculocutaneous nerve following brachial plexus root avulsion injury. J Neurosurg. 2006;105:830-834.
73. Sungpet A., Suphachatwong C., Kawinwonggowit V. One-fascicle median nerve transfer to biceps muscle in C5 and C6 root avulsions of brachial plexus injury. Microsurgery. 2003;23:10-13.
74. Samardzic M.M., Grujicic D.M., Rasulic L.G., Milicic B.R. The use of thoracodorsal nerve transfer in restoration of irreparable C5 and C6 spinal nerve lesions. Br J Plast Surg. 2005;58:541-546.
75. Samardzic M., Grujicic D., Rasulic L., Bacetic D. Transfer of the medial pectoral nerve: myth or reality? Neurosurgery. 2002;50:1277-1282.
76. Carlstedt T., Anand P., Hallin R., et al. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93:237-247.
77. Oppenheim J.S., Spitzer D.E., Winfree C.J. Spinal cord bypass surgery using peripheral nerve transfers: review of translational studies and a case report on its use following complete spinal cord injury in a human. Experimental article. Neurosurg Focus. 2009;26:E6.
78. Viterbo F., Ripari W.T. Nerve grafts prevent paraplegic pressure ulcers. J Reconstr Microsurg. 2008;24:251-253.
79. Livshits A., Catz A., Folman Y., et al. Reinnervation of the neurogenic bladder in the late period of the spinal cord trauma. Spinal Cord. 2004;42:211-217.
80. Zhang S., Johnston L., Zhang Z., et al. Restoration of stepping-forward and ambulatory function in patients with paraplegia: rerouting of vascularized intercostal nerves to lumbar nerve roots using selected interfascicular anastomosis. Surg Technol Int. 2003;11:244-248.
81. Terzis J.K., Konofaos P. Low-dose FK506 after contralateral C7 transfer to the musculocutaneous nerve using two different tubes: a study in rats. Ann Plast Surg. 2010;64:622-631.