Chapter 206 Nerve-Grafting Procedures for Birth-Related Peripheral Nerve Injuries
A birth-related peripheral nerve injury (BRPNI) is caused by traction to the brachial plexus during labor.1,2 In the majority of cases, delivery of the upper shoulder is blocked by the mother’s symphysis (shoulder dystocia). If additional traction is applied to the child’s head, the angle between the neck and shoulder is forcefully widened, overstretching the ipsilateral brachial plexus.
The incidence of BRPNI varies from 0.42 to 2.9 per 1000 births in prospective studies.3–5 Risk factors that have been identified for the occurrence of BRPNI reflect the disproportion between the child and the birth canal. The main fetal risk factor is macrosomia,6,7 and maternal factors include gestational diabetes and multiparity.6 Shoulder dystocia and assisted delivery by forceps or vacuum cup are well-known risk factors for the development of BRPNI.6 A relationship has been described between the risk and severity of a BRPNI and the amount of downward traction.8 A less-common delivery pattern concerns infants, usually with low birth weight, born in a breech position. Infants born in breech carry a high risk for the presence of root avulsions.9
There is an ongoing debate whether the BRPNI can be prevented and whether the obstetrician can be held responsible. This debate is fed by numerous malpractice suits in which large sums of money are compensated.10 Case reports describing spontaneous deliveries without traction applied to the child during labor with the occurrence of brachial plexus injury have been published to acquit the obstetrician.11
The upper brachial plexus is most commonly affected, resulting in paresis of the supraspinatus, infraspinatus, deltoid, and biceps muscles, as first described by Erb and Duchenne.12 Typically, in the C5, C6 lesion type, the affected arm rests on the surface in adduction, internal rotation, and extension. The wrist and fingers are continuously flexed when C7 is damaged as well. Hand function is additionally impaired in approximately 15% of patients.3,13,14 An isolated injury to the lower plexus (Déjèrine–Klumpke’s type) is rare.15
The traction injury can vary from neurapraxia or axonotmesis to neurotmesis and avulsion of rootlets from the spinal cord.16 The severity of neural damage will become clear by evaluation of recovery in the course of time, because nerve lesions of different severity initially manifest with the same clinical features. Neurapraxia and axonotmesis eventually result in complete recovery. Neurotmesis and root avulsion, on the other hand, result in permanent loss of arm function, and in time development of skeletal malformations, cosmetic deformities, behavioral problems and socioeconomic limitations.17–21
Neuropathophysiology
The number of axons that do not pass the lesion site depends on the severity of the lesion, which is determined by the magnitude and angle of the exerted traction forces. There is a minimum number of axons that should reconnect with the end organs in order to regain function. In addition, for the regain of useful function there is a minimum of axons that should be properly routed to their original end organ. We presume that those axons in the BRPNI neuroma in continuity are particularly prone to abnormal branching and misrouting. Because the direction of outgrowth after severe lesions is essentially random,22 outgrowing axons growing through a neuroma in continuity are likely to end up in the wrong tube.
Each BRPNI case is unique on an axonal level in the sense that the number of ruptured axons and basal laminal tubes differ for each intraplexal involved nerve element. This subsequently leads to the wide variety in level of functional recovery that can be found in individual cases. Branching and misrouting can also explain co-contraction,23 a typical feature of BRPNI at a later age, in which shoulder abduction and elbow flexion or elbow flexion and extension become irreversibly linked. Misrouting can even result in the phenomenon of the breathing arm: Clinically, when the proximal arm is at rest, involuntary movements simultaneous with the breathing rhythm can be observed. This can be explained by misrouting of ruptured C4 or C5 axons that were originally connected to the diaphragm through the phrenic nerve and erroneously grow into a superior trunk neuroma-in-continuity to shoulder muscles or the biceps muscle.24
An additional factor to inadequate number of outgrowing axons and misrouting that can reduce functional regeneration is that improper central motor programming can occur.25 There are various reasons that the formation of motor programs fail in BRPNI. Firstly, BRPNI causes deafferentation as well as weakness; many functions in the central nervous system depend on afferent input in a specific time window or else they are not formed correctly. Secondly, aberrant outgrowth of motor axons can present the central nervous system with conflicting information. A motor command for shoulder abduction can, for instance, cause elbow flexion in addition to abduction through misrouted motor axons. The resulting feedback may well hamper the formation of a selective abduction program, because there is probably no way for the central nervous system to identify the “misbehaving” motor units.26,27 Thirdly, sensory axons might also be prone to misrouting, compounding the problem. A final hurdle for the central nervous system may be the severity of paresis. In such cases the only way to effect certain movements may be through trick movements (such as scapular rotation instead of glenohumeral rotation), which then represent a functional adaptation.
Natural History
The prognosis of BRPNI is generally considered to be very good, with complete or almost complete spontaneous recovery in more than 90% of patients.28–33 However, this opinion is based on a limited number of series,34,35 which are cited indiscriminately, and without considering methodological aspects of these studies. In a systematic literature review, we discussed the methodologic flaws in the available natural history studies.36 We found that no study presented a prospective population-based cohort that was scored with a proper scoring system with adequate follow-up of 3 years. In other words, there is no scientifically sound evidence to support the common perception of complete spontaneous recovery from BRPNI. The often-cited excellent prognosis may be too optimistic.
Analysis of the most methodologically sound studies led us to estimate the percentage of children with residual deficits at 20% to 30%. This analysis was subsequently confirmed by two other studies, which prospectively investigated a population-based cohort. In the first, the British Paediatric Surveillance Unit notification system performed a nationwide registration of BRPNI injuries. Although follow-up was restricted to 6 months only, in this period only half of the infants showed full recovery.5 A prospective population-based study from a Swedish region revealed that 18% of the children had residual deficits at 18 months of age.37
Electromyography and Prognosis
Ancillary testing, in particular electromyography (EMG), is not considered reliable enough for prognostication of BRPNI.26,38 A needle EMG might seem a useful tool in this respect, but at present its role is debated. A main reason for this is that EMG findings may be discordant with clinical findings at 3 months of age, at which the biceps test is performed.39 In a paralytic biceps brachii muscle, the expected findings are an absence of motor unit potentials (MUPs) and the presence of positive sharp waves and/or fibrillation potentials (denervation activity). But in a typical BRPNI case, MUPs are present and denervation is absent in a paralytic biceps muscle at 3 months of age. This confusing finding has been noted by others40,41 and might have contributed to the opinion that the EMG is not useful in BRPNI.32,42,43 We previously outlined several possible explanations for inactive MUPs, that is, MUPs in a paralytic muscle.26 These explanations suggest that the presence of inactive MUPs might depend on time after injury, because they reflect incomplete outgrowth of damaged axons and the hampered formation of motor programs in the central nervous system.
Spontaneous recovery of useful extremity function has been observed in subsets of patients without elbow flexion at 3 months of age.44 In one study, even 20 of 28 infants who had no biceps function at 3 months had developed biceps contraction at 6 months.45 Together with our findings46 that MUPs can almost always be found in the biceps muscle at 3 months, this strongly suggests that the age of 3 months does not represent a stable state in BRPNI. In fact, the outgrowing axons might well have only just arrived in the various muscles, and the central nervous system might not yet have learned to cope with the situation. In nerve lesions in adults, one may expect all motor programs to be ready and waiting for the restoration of peripheral connections. In BRPNI, axonal outgrowth may only be the starting point for restoration of function, as formation of central nervous system motor programs might only commence after enough axons have arrived to start exerting force. At the same time, forming such central motor programs may be more difficult and thus take longer than in healthy children, because the central nervous system must somehow take aberrant outgrowth and the confusing feedback it causes into account. Faced with a degree of inescapable co-contraction, it might not be easy to program effective elbow flexion, abduction, or rotation. In this hypothetical view, the age of 3 months may well be the very worst period imaginable to correlate the EMG with clinical findings: it is late enough to show evidence of axonal outgrowth but too early for the brain to control contraction efficiently. This leaves the role of the EMG for prognosis at 3 months undetermined at present; we showed that severe cases of BRPNI can be identified reliably at 1 month of age based on clinical findings and needle EMG of the biceps.46 These findings will be reported in full after validation, which is currently in progress.
For less-extreme cases, that is, the majority of BRPNI cases, the challenge lies in predicting whether function will be best after spontaneous outgrowth through a neuroma in continuity, resulting in reinnervation through tangled paths, or after nerve grafting, in which the grafts serve as a straight path that can be targeted. Results achieved by surgery are claimed to be superior to the outcome in conservatively treated subjects with equally severe lesions.39,47,48 However, this comparison relies on historical controls49; no randomized study has been performed.50,51 The best way to answer this question may be a controlled trial comparing nerve surgery to spontaneous recovery. In view of the current standard of treatment practice, it seems extremely difficult to perform such a prospective randomized trial.
Conservative Treatment the First Few Months of Life
In the past there has been a tendency to immobilize the arm directly after birth to prevent secondary damage to the injured nerve elements of the brachial plexus. It is, however, highly unlikely that secondary damage to the brachial plexus can occur during the passive movements of the arm in a physiologic range of motion during exercises or caregiving. We recommend frequent mobilization of the joints from the beginning to prevent formation of joint contractures. Additionally, there is no scientific proof that immobilization might be of any benefit to accelerate or improve the nerve-regeneration process. Joint contracture formation, however, might be detrimental to final functional outcome when contractures limit the effective contraction of reinnervated muscles. It can also lead to improper modeling of the joints, of which the glenohumeral joint is most commonly affected.20 Contractures can start to form as early as 2 to 3 weeks after birth. The type of joint contractures that we most often see are those resulting in a fixed internal rotation, flexion, and pronation position of the upper limb.
Surgical Treatment
Selecting Patients for Surgery
Surgery should be restricted to severe cases in which spontaneous restoration of function will not occur, namely, in neurotmesis or root avulsions.16 Herein lies the root of the problem, because most infants with BRPNI initially present with paralysis, regardless of the severity of the underlying nerve lesion. At present, the earliest accepted indication of the severity of the lesion can be obtained at 3 months of age. Paralysis of the biceps muscle at 3 months is associated with a poor prognosis52 and is considered an indication for nerve surgery by some authors.39,53–56 However, biceps paralysis at age 3 months does not preclude satisfactory spontaneous recovery.44,45,47,57 Additionally, biceps muscle testing might not be reliable in infants.44,58,59 Alternative tests56,58,60 are complex or are done at an even later age. These difficulties in the diagnostic process can also lead to parental distress.61
In the current surgical selection process at the Leiden University Medical Center, we seek to identify all patients with neurotmetic lesions or nerve root avulsions as surgical candidates. In our patient-selection process, we try to assess the severity of the brachial plexus lesion(s) as early as possible for surgical and psychosocial reasons: Parents and caregivers need time to consider the recommended treatment options. We proposed a paradigm to identify severe nerve lesions at 1 month of age as a result of our prospective study.46 Elbow extension and elbow flexion are clinically assessed, and needle EMG of the biceps muscle is performed. Severe lesions of C5 and C6 and the upper trunk can be predicted in the vast majority of infants at 1 month of age in whom elbow extension is absent or in whom both elbow flexion and motor unit action potentials (MUAPs) are absent in the biceps muscle on EMG. Infants who meet these criteria at 1 month of age should be promptly referred to a specialized center. Early diagnosis of severe BRPNI lesions and admission to a specialized center open opportunities to start appropriate and rigorous child physiotherapy and/or (appropriately) early surgery, if necessary.
As the infant reaches the age of 3 months, we consider impaired hand function to be an absolute indication for nerve surgery as soon as possible.62 Similarly, we recommend operative intervention to BRPNI patients who demonstrate no spontaneous recovery of shoulder external rotation and elbow flexion and forearm supination by 3 to 4 months of age.63 Radiographic assessment via ultrasound of the diaphragm (to detect phrenic nerve palsy) and CT-myelography (to detect nerve root avulsions) can provide additional evidence for severe BRPN injuries that are amenable to surgical repair and reconstruction.64–66 If the presence of true shoulder and elbow movements is doubtful, we proceed with surgical exploration for further intraoperative assessment of the severity of the lesion. In our opinion, the potential benefits from repairing neurotmetic lesions at an early age generally outweigh the risks of a diagnostic exploration. In case a mainly axonotmetic lesion is found during exploration and only neurolysis is performed, at least clarity can be given to the parents about the diagnosis and prognosis. Surgery for BRPNI is rarely performed before 3 months of age and is almost always performed before 7 months of age.
Surgical Procedure
Supraclavicular Exposure
• The phrenic nerve cannot always be macroscopically seen directly because it is covered by the deep transverse cervical fascia; the transparency of this fascia varies depending on its thickness and any scar present. Nerve stimulation to identify the course of the phrenic nerve from medial to lateral over the surface of the anterior scalene muscle is extremely helpful and is, in our opinion, indispensable.
• The phrenic nerve usually originates from C3 and C4 and occasionally has a thin C5 contribution. Because C4 is already identified at this stage, the phrenic nerve origin can be located at the caudal aspect of C4.
• The artery and vein that are adjacent to the phrenic nerve should not be identified erroneously as the nerve.
• We have occasionally encountered a separate auxiliary phrenic nerve at higher cervical levels.
The phrenic nerve courses lateral to medial toward the diaphragm, while the contents of the plexus and the surrounding nerves course from medial to lateral. As the phrenic nerve approaches the lateral edge of the anterior scalene, the C5 spinal nerve root emerges; this is a reliable site for the identification of the C5 nerve root. The phrenic nerve is completely neurolysed in its trajectory ventral to the anterior scalene muscle to allow gentle medial retraction without significant traction. In some patients, the phrenic nerve is adherent to the neuroma of C5; to preserve diaphragmatic function, leaving some neuroma scar tissue on the phrenic nerve is preferable to dissecting flush on the phrenic nerve and removing all the C5 neuroma. Resection or partial resection of the anterior scalene muscle is always performed to allow optimal exposure of the proximal intraforaminal part of the spinal nerve roots. During such proximal exposure, a pseudomeningocele that extends extraforaminally may be encountered; every attempt should be made to identify extraforaminal expansion such structures on CT-myelography or MRI.
Following the C5 root distally leads to the upper trunk, and following the upper trunk proximally leads to the C6 spinal nerve root. The C6 spinal nerve root is located caudal and dorsal to the C5 spinal nerve root. The anterior tubercle of C6 can be very prominent (Chassignac’s tubercle). The C7, C8, and T1 spinal nerve roots are sequentially more caudal and dorsal. A transverse cervical artery and vein cross the C7 spinal nerve root and can be ligated. Following the C7 spinal nerve distally will reveal the middle trunk. The C8 and T1 spinal nerves combine quickly to form the lower trunk, which is adjacent to the subclavian vessels. The roots of the lower trunk surround the first rib; therefore, care should be taken to avoid injury to the pleura. Special attention should be given to the vertebral artery, because in proximal dissection it runs unprotected at the level of the roots C8–T1 before it enters the vertebral canal in the lateral mass of C7.67
Exposure of Extraplexal Nerves for Nerve-Transfer Procedures
Another commonly used donor nerve is the medial pectoral nerve (MPN); it is used for nerve transfer to the MCN to restore elbow flexion. The MCN can be identified in its course dorsal to the pectoralis major and minor muscles. Generally the MPNs can be reached by retracting the pectoralis major muscle cranially through an incision in the lower part of the deltoideopectoral groove, which further extends distally over the proximal medial bicipital groove. The MPN originates from the medial cord, and its function remains intact in C5–C6 or C5–C6–C7 lesions.68 Intraoperative nerve stimulation is an indispensable step for identifying the MPNs because small vessels simulate their appearance and course. There are usually two individual medial pectoral nerve branches, and they should be cut as distally as possible then coapted to the MCN. The total cross-sectional area of the medial pectoral nerve branches is usually less than that of the MCN. If so, the epineurium of the MCN is opened approximately 270° and subsequently the perineurium is opened and the cross-sectional diameter of the individual fascicles is assessed. Subsequently, the MCN fascicle with a diameter comparable to the MPNs is cut and a direct coaptation is made between the MCN fascicle and the MPNs. Usually, more than half of the MCN can be covered with the MPN donor.
Another nerve-transfer technique to restore elbow flexion uses intercostal nerves (ICNs) as donors and the MCN as recipient. We previously described the technique for intercostal nerve transfer in adults.69 We apply the same surgical technique in infants with BRPNI. Either ICNs 3 to 5 or 4 to 6 are exposed by means of an undulating skin incision over the ipsilateral chest. The incision starts at the anterior axillary line at the inferior border of the pectoralis major muscle and continues beneath the nipple, extending medially to the costosternal junction. The inferior part of the pectoralis major muscle is shifted upward, with partial detachment of its sternal insertion if necessary. The rib attachments of the serratus anterior muscle usually remain intact. The main branch of the ICN is identified halfway in its ventral course between the external and internal interosseus intercostal muscle by means of blunt dissection in the muscle fiber direction and dissected free over its entire anterior course. Care should be taken to keep the periosteum of the ribs intact to avoid rib cage deformities during growth.
Other nerve-transfer techniques have been described in the literature using the phrenic nerve as donor, or using an isolated fascicle of the ulnar or median nerve or an intact nerve in a end-to-side fashion. The use of the phrenic nerve at an early age might carry the risk of pulmonary problems in the immediate postoperative period lasting to adulthood, so it is not employed in our center. We do not use the ulnar nerve fascicle–to–MCN (biceps muscle branch) transfer or the median nerve fascicle–to–MCN (brachialis muscle branch) transfer,70–72 because these techniques carry potential risks for the growth of the hand. The end-to-side option is not reliable enough for routine use.73 The use of the hypoglossal nerve as donor was abandoned after it became apparent that volitional control after reinnervation is not restored. The reinnervated muscle contracts only when the tongue is pushed against the hard palate. Consequently, when patients talk or eat they cannot move the limb.74 Much less is known about the quality of central control following transfer of the contralateral C7 spinal nerve in BRPNI infants. Therefore, we have not used the contralateral C7 spinal nerve as a donor so far.
Intraoperative Assessment of the Severity of the Brachial Plexus Lesion
The most common finding is that a functionally intact long thoracic nerve branch exits from the neurotmetic part of the spinal nerve. Careful proximal dissection of the long thoracic nerve branch within the neurotmetic part of the spinal nerve usually reveals an essentially normal fascicle that can be dissected away from the neurotmetic tissue and should be left intact. Transection of the neurotmetic spinal nerve tissue or neuroma (in preparation for repair) should be performed just distal to the exit of the long thoracic nerve branch.
Proximal stumps are evaluated by frozen section. The total quantity of myelin in the entire cross-sectional area of the donor stump that corresponds to the viability of the proximal stump is expressed semi-quantitatively in quartiles: less than 25%, 25% to 50%, 50% to 75%, or more than 75%.75 The neuropathologist can also assess for the presence of dorsal root ganglion cells (indicating nerve root avulsion) and for the presence of fibrosis or neuroma in the proximal and distal stumps. In our institution, proximal stumps are considered viable to be used as a donor for nerve grafting when the total myelin quantity is at least 50% (more than 75% is preferred) and preoperative CT-myelography demonstrated intact rootlets.
Intraoperative Neurophysiologic Recordings
Direct electrical stimulation during surgery is important to obtain a nonquantitative evaluation of the presence of axons in a regenerating nerve segment. A more sophisticated quantitative evaluation consists of the recording of intraoperative nerve action potentials (NAPs), and evoked compound motor action potential (CMAP) is advocated in adults to objectively distinguish between axonotmetic and neurotmetic lesions.76,77 It has been shown that the presence of a NAP across the lesion site requires at least 3000 to 4000 nerve fibers with a diameter greater than 5 μm. The presence of these fibers in a recovering nerve indicates that spontaneous functional recovery will take place, and that, therefore, resection and grafting is not indicated.78
We analyzed the results of intraoperative NAP and CMAP recordings in 95 patients with BRPNI to assess the predictive values for the diagnosis of axonotmesis, neurotmesis, avulsion, or normal spinal nerves.79 We found statistically significant differences between diagnosis groups. For the individual patient, however, a clinically useful cutoff point for NAP and CMAP recordings to differentiate between avulsion, neurotmesis, axonotmesis, and normal could not be found. The sensitivity for an absent NAP or CMAP was too low for clinical use. This finding is in accordance with the findings of others that the presence of conduction across the lesion in continuity does not guarantee good spontaneous recovery.80 Intraoperative NAP and CMAP recordings, therefore, do not add to the decision making during surgery.
Principles Underlying Strategies for Surgical Reconstruction
For the purposes of the discussion of surgical strategies, the most common lesions are divided as shown in Table 206-1.
TABLE 206-1 Different Types of Lesions in Birth-Related Peripheral Nerve Injury
| Group 1 |
| N C5, C6 |
| N C5, Av C6 |
| Av C5, C6 |
| Group 2 |
| N C5, C6, C7 |
| N C 5, C6, Av C7 |
| N C5, Av C6, C7 |
| N C5, Av C6, C7, C8 |
| N C5, C6; Av C7, C8 |
| Group 3 |
| N C5, C6, C7, Av C8, T1 |
| N C5, C6, Av C7, C8, T1 |
| N C5, Av C6, C7, C8, T1 |
| Av C5–T1 |
Av, avulsion; N, neurotmesis.
Group 1: C5, C6, and Upper Trunk Lesions
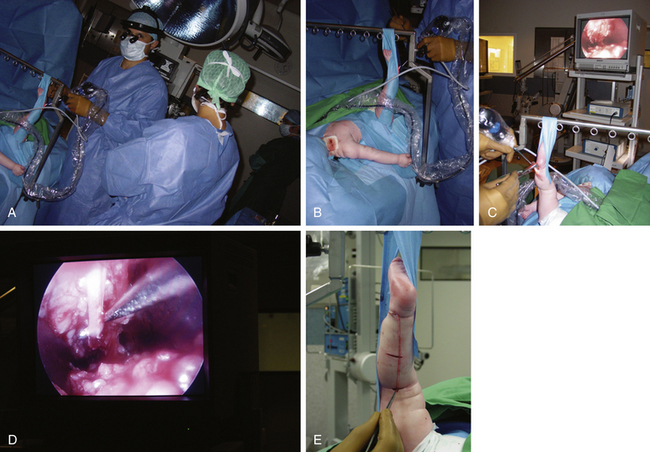
Lesions of C5, C6, and/or the upper trunk account for the lesions in the majority of BRPNI patients. Three different types of C5 and C6 lesions are observed clinically. In type 1, neurotmetic lesions of C5, C6, or the upper trunk are present. Because hand-grasp function is essentially normal, the first priority of surgical intervention is restoration of elbow flexion followed by reanimation of shoulder movements. The most common lesion observed intraoperatively is a C5, C6, or upper trunk neuroma-in-continuity. The neuroma should be resected, and the nerve is repaired using sural nerve grafts. When the child is 4 to 5 months of age, the gap between the proximal and distal stumps is usually 2.5 to 3.5 cm, necessitating the harvest of the sural nerves from both legs. We harvest sural nerves endoscopically using three small horizontal incisions, allowing improved cosmesis. The length of each harvested nerve is usually 11 to 13 cm (Fig. 206-1).
The usual strategy for nerve repair includes the use of one graft from C5 to the SSN (placed at the rostroventral quadrant of the proximal stump of C5),81 two grafts from C5 to the posterior division of the upper trunk, and four grafts from C6 to the anterior division of the upper trunk. Depending on the size of the proximal stumps and the availability of nerve graft, an alternative strategy involves an SAN-to-SSN transfer. Our results indicate that no difference in external rotation exists between nerve repair and nerve transfer to the SSN, which has been confirmed by other surgeons.63,82
In type 3, both C5 and C6 roots are avulsed; this injury usually results from a breech presentation at birth.9,83 Neither root is useful for nerve repair or reconstruction. In these instances, the C5 and C6 roots are left untouched in their foramina, and nerve transfers are the most viable option. Our patients undergo an SAN-to-SSN transfer coupled with MPN-to-MCN transfer.
Group 2: C5, C6, C7, (C8) Lesions
Patients in group 2 retain useful preoperative finger grasp function, so restoration of hand function is not a goal of surgical intervention. However, in this group, elbow, wrist, and finger extension are impaired, and elbow flexion and shoulder movements are minimal or absent. Group 2 lesions can be divided into four types (see Table 206-1).
In type 1, a neurotmetic lesion of C5, C6, and C7 is present. Intraoperative findings include C5, C6, and upper trunk neuroma-in-continuity coupled with a C7 and middle trunk neuroma. The repair is performed in a stepwise fashion. The C5, C6, and upper trunk neuroma is resected. We then harvest the sural nerves from both legs to determine the quantity and quality of nerve graft. Based on the availability of graft material, the C7 neuroma either is resected or undergoes extensive neurolysis unless graft material can be harvested from other sites (superficial branch of the radial nerve and/or medial or lateral supraclavicular nerves) (Fig. 206-2). We opt for neurolysis only if some fascicular continuity is seen within the neuroma. The nerve repair strategy is similar to that for patients in group 1, with the addition of C7 and middle trunk repair.
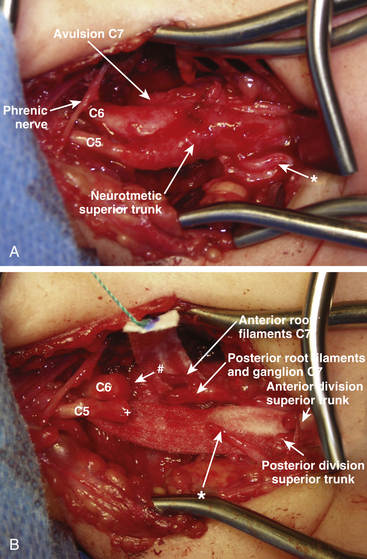
In type 2, a C5, C6, and upper trunk neuroma-in-continuity along with a C7 nerve root avulsion is present. The upper trunk neuroma is resected. The avulsed C7 root is transected as proximally as possible. If the dorsal root ganglion can be morphologically identified (and confirmed by the microscopic presence of dorsal root ganglion cells), it is dissected from the ventral root and resected. We then perform an intraplexal nerve transfer with direct coaptation from the caudal aspect of the C6 proximal stump to the C7 ventral root filaments (Fig. 206-3). When direct coaptation to C6 is technically impossible, the C7 ventral root can be the target for nerve grafting from the caudal aspect of the proximal C6 nerve stump. To restore sensory function, a direct coaptation of the medial supraclavicular nerve (arising from C4) to the postganglionic sensory part of the C7 root can be performed to innervate the C7 dermatome in the hand. The C5, C6, and upper trunk repair is undertaken as described earlier.
Group 3: C5, C6, C7, C8, T1 Lesions (Panplexopathy)
The infants suffering group 3 lesions present with a flail arm. Our first priority is the restoration of hand function, followed by elbow flexion and shoulder movements. Selection of the distal targets for reinnervation are key to the strategy of nerve repair and reconstruction. Fortunately, complete nerve root avulsion in group 3 patients is extremely rare. Usually, C5 and C6 suffer postganglionic neurotmetic lesions and C7, C8, and T1 suffer avulsions. Therefore, C5 and C6 proximal stumps are available as donors for graft repair to restore C8, T1, and lower or middle trunk functions. If possible, we divide the C8 and/or T1 roots into their respective motor and sensory parts at the neural foramen without violating the vertebral artery. Direct coaptation of the C6 proximal stump to the motor portion of C8 (and T1, if possible) is then performed to restore hand function.62 For restoration of sensory function, the supraclavicular nerves can be directly coapted to the postganglionic sensory portions of C8 and/or T1. Graft repair from the remaining part of C6 to the anterior division of the upper trunk and from the C5 proximal stump to the posterior division of the upper trunk is performed, followed by SAN-to-SSN transfer. Either the C7 root can remain in place, or the supraclavicular nerves can be coapted to C7 to augment sensory function to the hand. When only one proximal stump is available as a donor for nerve repair and reconstruction, it is used entirely for restoration of hand function. Restoration of elbow flexion is achieved with intercostal nerve transfer to MCN, and restoration of shoulder movement is achieved via an SAN-to-SSN transfer.
Postoperative Care
After nerve repair or reconstruction, the infant’s upper body is placed in a prefabricated cast to limit movement of the head and affected arm for 2 weeks. Patients undergo clinical examinations at our outpatient clinic at 6-month intervals. Active and passive ranges of motion are recorded in degrees, and motor function is assessed by the Medical Research Council grading system. In addition, the Mallet score84 is recorded for assessment of shoulder function, and the Raimondi hand score is recorded for assessment hand function.85
Results of Nerve Surgery
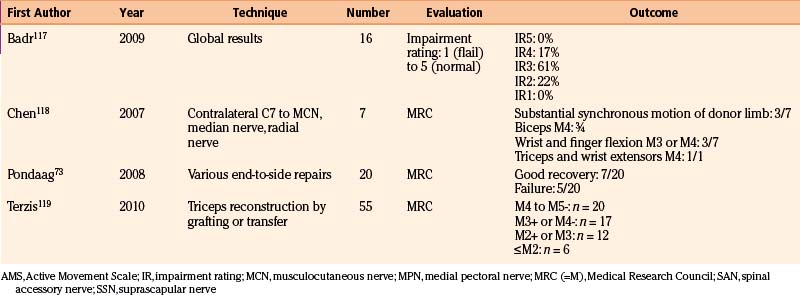
To provide as much of the available data as possible, we summarize and comment on our own surgical results in Table 206-2. In addition, we present the literature results in Table 206-3 based on the following PubMed searches using Medical Subject Heading (MeSH) terms: “brachial plexus/surgery” AND “birth injuries”; “brachial plexus/surgery” AND “infant”; “brachial plexus neuropathies/surgery” AND “birth injuries”; and “brachial plexus neuropathies/surgery” AND “child”. Occasionally, similar patient series were published in different papers. When this could be identified, only one of these papers is mentioned in Table 206-3.
TABLE 206-2 Results of Nerve Reconstructive Surgery of Patients with Birth-Related Peripheral Nerve Injury at the Leiden University Medical Center
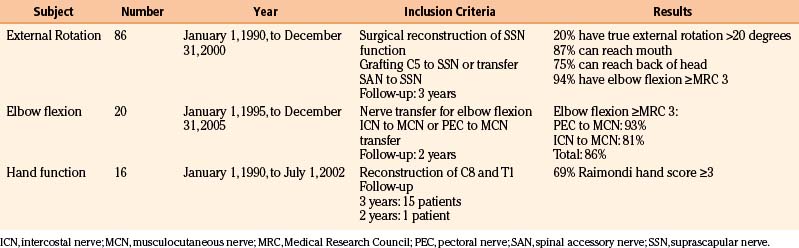
TABLE 206-3 Literature Results of Nerve Reconstructive Surgery of Patients with Birth-Related Peripheral Nerve Injury
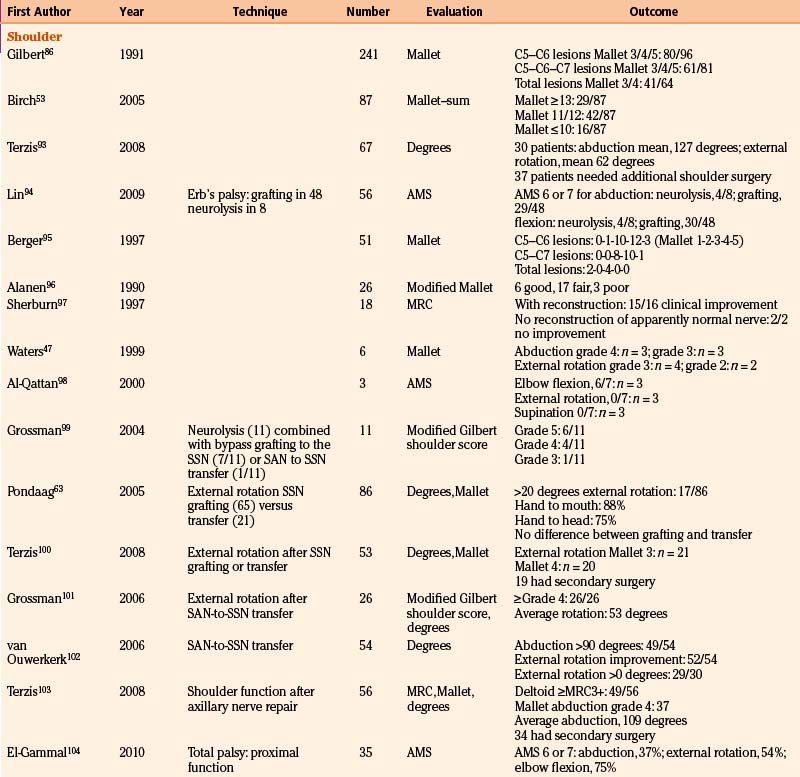
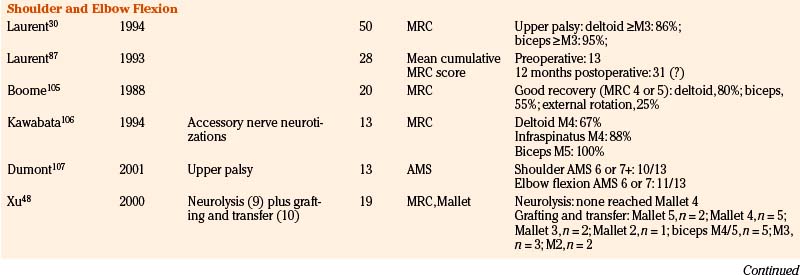
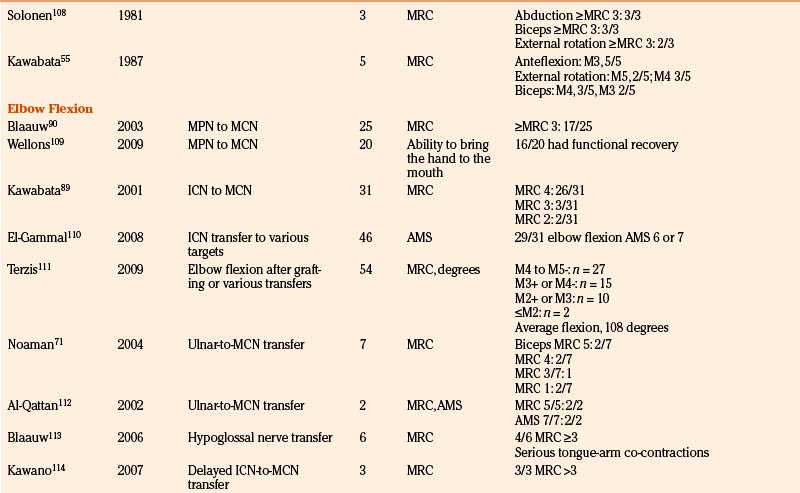
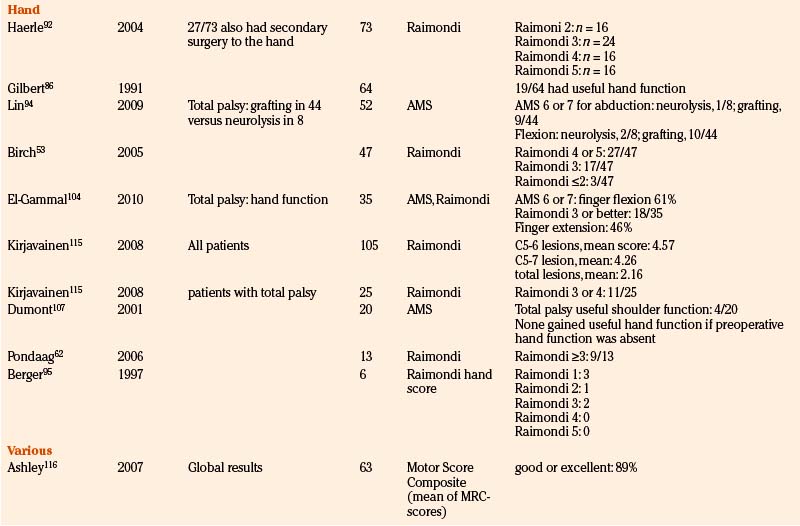
Shoulder Function
The results of nerve repairs to improve shoulder function have been published in a number of series, from which at first glance it can be concluded that global shoulder function recovery is good.47,53,55,86,87 We performed a study (N = 86) that focused on the recovery of true glenohumeral external rotation as a solitary movement in order to determine specific factors affecting recovery after neurotization of the SSN.63 During the neurologic evaluation of these children, trick movements were eliminated for a clean comparison of two surgical techniques and of other prognostic factors. We found that only 20% of the patients gained more than 20 degrees of range of true external rotation and that restoration of true glenohumeral external rotation failed in as many as 41% of all patients. In contrast to this disappointing result of true external rotation, functional evaluation showed that 87% of patients could reach their mouth and 75% of children could reach the back of their head. This illustrates the great ability of the infants to compensate their limited true external rotation by thoracoscapular movements. We found no difference between nerve grafting C5 to SSN compared to nerve transfer of SAN to SSN.
Elbow Flexion
In the previously mentioned cohort, biceps muscle force against gravity or more was gained in 92% of patients.63 In most of these patients nerve grafting had been performed.
We analyzed 30 consecutive patients (1995 to 2005) in whom nerve transfers for biceps reanimation had been applied.88 From 1995 to 2000, only intercostal-to-MCN transfers were performed; from 2001 to 2005 the pectoral-to-MCN transfer was preferentially applied when the C8–T1 trajectory to the inferior trunk was intact. In 15 of 16 intercostal-to-MCN transfers, three intercostal nerves were coapted directly to the MCN; in one patient a 1cm graft proved necessary. In all patients with pectoral-to-MCN transfers we were able to perform a direct coaptation. Elbow flexion of at least MRC 3 was achieved in 87% of patients after a mean follow-up of 40 months. The results in the pectoral-to-MCN group were somewhat better than in the intercostal-to-MCN group (93% vs. 81%, respectively), which may be explained by the more-severe brachial plexus lesions that were included in the intercostal-to-MCN group (8 of 16 patients in the intercostal-to-MCN group had a flail arm). In the intercostal-to-MCN group we performed a Steindler flexorplasty as secondary surgery in one patient.
These results correspond well to the limited number of reports in the literature. Kawabata reported the results of 31 intercostal-to-MCN transfers in BRPNI patients: 94% reached MRC 3 or better.89 For the pectoral-to-MCN transfer, a success rate of 88% MRC3 or better was reported by Blaauw and Slooff.90
Recovery of Hand Function
Without reanimation of the hand, the maximal function that can be obtained is the use of the affected limb as a hook. In the past, reanimation of hand function in adults with a total brachial plexus lesion has been tried, but it did not result in useful function.91 Because of better nerve regeneration and neural plasticity in infants compared to adult patients, restoration of hand function in BRPNI infants is feasible. The primary aim of surgery in patients with a flail arm due to BRPNI is, therefore, restoration of hand function. We analyzed 16 patients with a flail arm who had discontinuity of the outflow of the spinal nerves C7, C8, and T1 as a result of avulsion injury or neurotmetic parts of the outflow were resected, followed by neurotization of C8, T1, and the inferior trunk or median nerve. The postoperative recovery of hand function could, therefore, only be attributed to the nerve reconstruction.62 The analysis of our surgical results showed that useful reanimation of the hand was obtained in 69% of patients (Raimondi score ≥3).85
Only a few other reports concerning recovery of hand function have been published. Gilbert reported 76% good recovery of hand function, but in this series secondary surgery (i.e., tendon transfers) had also been performed on a number of patients.92 In the series of Birch et al. of 47 patients, 57% regained Raimondi 4 or better and even 93% Raimondi 3 or better.53
Birch R., Ahad N., Kono H., Smith S. Repair of obstetric brachial plexus palsy: results in 100 children. J Bone Joint Surg Br. 2005;87(8):1089-1095.
Blaauw G., Slooff A.C. Transfer of pectoral nerves to the musculocutaneous nerve in obstetric upper brachial plexus palsy. Neurosurgery. 2003;53(2):338-341.
Brown T., Cupido C., Scarfone H., et al. Developmental apraxia arising from neonatal brachial plexus palsy. Neurology. 2000;55(1):24-30.
Clarke H.M., Al Qattan M.M., Curtis C.G., Zuker R.M. Obstetrical brachial plexus palsy: results following neurolysis of conducting neuromas-in-continuity. Plast Reconstr Surg. 1996;97(5):974-982.
Evans-Jones G., Kay S.P., Weindling A.M., et al. Congenital brachial palsy: incidence, causes, and outcome in the United Kingdom and Republic of Ireland. Arch Dis Child Fetal Neonatal Ed. 2003;88(3):F185-F189.
Fisher D.M., Borschel G.H., Curtis C.G., Clarke H.M. Evaluation of elbow flexion as a predictor of outcome in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2007;120(6):1585-1590.
Gilbert A., Tassin J.L. Surgical repair of the brachial plexus in obstetric paralysis. [in French]. Chirurgie. 1984;110(1):70-75.
Haerle M., Gilbert A. Management of complete obstetric brachial plexus lesions. J Pediatr Orthop. 2004;24(2):194-200.
Kawabata H., Shibata T., Matsui Y., Yasui N. Use of intercostal nerves for neurotization of the musculocutaneous nerve in infants with birth-related brachial plexus palsy. J Neurosurg. 2001;94(3):386-391.
Lagerkvist A.L., Johansson U., Johansson A., et al. Obstetric brachial plexus palsy: a prospective, population-based study of incidence, recovery, and residual impairment at 18 months of age. Dev Med Child Neurol. 2010;52(6):529-534.
Lin J.C., Schwentker-Colizza A., Curtis C.G., Clarke H.M. Final results of grafting versus neurolysis in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2009;123(3):939-948.
Malessy M.J., Pondaag W., Yang L.J. Severe obstetric brachial plexus palsies can be identified at one month of age. PLoS One. 2011;6(10):e26193. Epub 2011 Oct 17
Mollberg M., Wennergren M., Bager B., et al. Obstetric brachial plexus palsy: a prospective study on risk factors related to manual assistance during the second stage of labor. Acta Obstet Gynecol Scand. 2007;86(2):198-204.
Pondaag W., de Boer R., Van Wijlen-Hempel M.S., et al. External rotation as a result of suprascapular nerve neurotization in obstetric brachial plexus lesions. Neurosurgery. 2005;57(3):530-537.
Pondaag W., Gilbert A. Results of end-to-side nerve coaptation in severe obstetric brachial plexus lesions. Neurosurgery. 2008;62(3):656-663.
Pondaag W., Malessy M.J., van Dijk J.G., Thomeer R.T. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46(2):138-144.
Pondaag W., Malessy M.J. Recovery of hand function following nerve grafting and transfer in obstetric brachial plexus lesions. J Neurosurg (1 Suppl Pediatrics). 2006;105(suppl 1):33-40.
Pondaag W., van der Veken L.P., van Someren P.J., et al. Intraoperative nerve action and compound motor action potential recordings in patients with obstetric brachial plexus lesions. J Neurosurg. 2008;109(5):946-954.
Smith N.C., Rowan P., Benson L.J., et al. Neonatal brachial plexus palsy. Outcome of absent biceps function at three months of age. J Bone Joint Surg Am. 2004;86-A(10):2163-2170.
Sunderland S. Nerve Injuries and Their Repair: A Critical Appraisal. Edinburgh: Churchill Livingstone; 1991.
Tassin J.L. Obstetric paralysis of the brachial plexus. Spontaneous recovery: results of interventions. [in French] (Thesis). Université Paris. 1983.
van Dijk J.G., Malessy M.J., Stegeman D.F. Why is the electromyogram in obstetric brachial plexus lesions overly optimistic? (letter). Muscle Nerve. 1998;21(2):260-261.
van Dijk J.G., Pondaag W., Malessy M.J. Obstetric lesions of the brachial plexus. Muscle Nerve. 2001;24(11):1451-1461.
Waters P.M. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am. 1999;81(5):649-659.
Waters P.M. Update on management of pediatric brachial plexus palsy. J Pediatr Orthop B. 2005;14(4):233-244.
1. Clark L.P., Taylor A.S., Prout T.P. A study on brachial birth palsy. Am J Med Sci. 1905;130(4):670-705.
2. Metaizeau J.P., Gayet C., Plenat F. Brachial plexus birth injuries. An experimental study [in French]. Chir Pediatr. 1979;20(3):159-163.
3. Bager B. Perinatally acquired brachial plexus palsy—a persisting challenge. Acta Paediatr. 1997;86(11):1214-1219.
4. Dawodu A., Sankaran-Kutty M., Rajan T.V. Risk factors and prognosis for brachial plexus injury and clavicular fracture in neonates: a prospective analysis from the United Arab Emirates. Ann Trop Paediatr. 1997;17(3):195-200.
5. Evans-Jones G., Kay S.P., Weindling A.M., et al. Congenital brachial palsy: incidence, causes, and outcome in the United Kingdom and Republic of Ireland. Arch Dis Child Fetal Neonatal Ed. 2003;88(3):F185-F189.
6. Gilbert W.M., Nesbitt T.S., Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93(4):536-540.
7. Levine M.G., Holroyde J., Woods J.R.J., et al. Birth trauma: incidence and predisposing factors. Obstet Gynecol. 1984;63(6):792-795.
8. Mollberg M., Wennergren M., Bager B., et al. Obstetric brachial plexus palsy: a prospective study on risk factors related to manual assistance during the second stage of labor. Acta Obstet Gynecol Scand. 2007;86(2):198-204.
9. Geutjens G., Gilbert A., Helsen K. Obstetric brachial plexus palsy associated with breech delivery. A different pattern of injury. J Bone Joint Surg Br. 1996;78(2):303-306.
10. Gurewitsch E.D., Allen R.H. Shoulder dystocia. Clin Perinatol. 2007;34(3):365-385.
11. Lerner H.M., Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e7-e8.
12. Brody I.A., Wilkins R.H. On a characteristic sit of injury in the brachial plexus. W Erb Arch Neurol. 1969;21(4):442-444.
13. Jacobsen S. Occurrence of obstetrical injuries to the brachial plexus on the islands of Lolland and Falster 1960-1970 [in Danish]. Nord Med. 1979;86(42):1200-1201.
14. Sjoberg I., Erichs K., Bjerre I. Cause and effect of obstetric (neonatal) brachial plexus palsy. Acta Paediatr Scand. 1988;77(3):357-364.
15. Al Qattan M.M., Clarke H.M., Curtis C.G. Klumpke’s birth palsy. Does it really exist? J Hand Surg Br. 1995;20(1):19-23.
16. Sunderland S. Nerve Injuries and Their Repair: A Critical Appraisal. Edinburgh: Churchill Livingstone; 1991.
17. Adler J.B., Patterson R.L.J. Erb’s palsy. Long-term results of treatment in eighty-eight cases. J Bone Joint Surg Am. 1967;49(6):1052-1064.
18. Bellew M., Kay S.P., Webb F., Ward A. Developmental and behavioural outcome in obstetric brachial plexus palsy. J Hand Surg Br. 2000;25(1):49-51.
19. Gjorup L. Obstetrical lesion of the brachial plexus. Acta Neurol Scand. 1966;42(suppl 18):1-80.
20. Pearl M.L., Edgerton B.W. Glenoid deformity secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998;80(5):659-667.
21. Pollock A.N., Reed M.H. Shoulder deformities from obstetrical brachial plexus paralysis. Skeletal Radiol. 1989;18(4):295-297.
22. Gramsbergen A., IJkema-Paassen J., Meek M.F. Sciatic nerve transection in the adult rat: abnormal EMG patterns during locomotion by aberrant innervation of hindleg muscles. Exp Neurol. 2000;161(1):183-193.
23. Roth G. Reinnervation in obstetrical brachial plexus paralysis. [in French]. J Neurol Sci. 1983;58(1):103-115.
24. Swift T.R. The breathing arm. Muscle Nerve. 1994;17(1):125-129.
25. Brown T., Cupido C., Scarfone H., et al. Developmental apraxia arising from neonatal brachial plexus palsy. Neurology. 2000;55(1):24-30.
26. van Dijk J.G., Pondaag W., Malessy M.J. Obstetric lesions of the brachial plexus. Muscle Nerve. 2001;24(11):1451-1461.
27. van Dijk J.G., Pondaag W., Malessy M.J. Botulinum toxin and the pathophysiology of obstetric brachial plexus lesions (letter). Dev Med Child Neurol. 2007;49(4):318-319.
28. Bradley W.G., Daroff R.B., Fenichel G.M., Marsden C.D. Neurology in Clinical Practice, 2nd ed., Boston: Butterworth-Heinemann, 1996.
29. Greenberg M.S. Handbook of Neurosurgery, 5th ed., New York: Thieme, 2001.
30. Laurent J.P., Lee R.T. Birth-related upper brachial plexus injuries in infants: operative and nonoperative approaches. J Child Neurol. 1994;9(2):111-117.
31. Painter M.J., Bergman I. Obstetrical trauma to the neonatal central and peripheral nervous system. Semin Perinatol. 1982;6(1):89-104.
32. Shenaq S.M., Berzin E., Lee R., et al. Brachial plexus birth injuries and current management. Clin Plast Surg. 1998;25(4):527-536.
33. Terzis J.K., Papakonstantinou K.C. Management of obstetric brachial plexus palsy. Hand Clin. 1999;15(4):717-736.
34. Gordon M., Rich H., Deutschberger J., Green M. The immediate and long-term outcome of obstetric birth trauma. I. Brachial plexus paralysis. Am J Obstet Gynecol. 1973;117(1):51-56.
35. Walle T., Hartikainen-Sorri A.L. Obstetric shoulder injury. Associated risk factors, prediction and prognosis. Acta Obstet Gynecol Scand. 1993;72(6):450-454.
36. Pondaag W., Malessy M.J., van Dijk J.G., Thomeer R.T. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46(2):138-144.
37. Lagerkvist A.L., Johansson U., Johansson A., et al. Obstetric brachial plexus palsy: a prospective, population-based study of incidence, recovery, and residual impairment at 18 months of age. Dev Med Child Neurol. 2010;52(6):529-534.
38. van Dijk J.G., Malessy M.J., Stegeman D.F. Why is the electromyogram in obstetric brachial plexus lesions overly optimistic? (letter). Muscle Nerve. 1998;21(2):260-261.
39. Gilbert A., Tassin J.L. Surgical repair of the brachial plexus in obstetric paralysis. [in French]. Chirurgie. 1984;110(1):70-75.
40. Vredeveld J.W., Blaauw G., Slooff B.A., et al. The findings in paediatric obstetric brachial palsy differ from those in older patients: a suggested explanation. Dev Med Child Neurol. 2000;42(3):158-161.
41. Zalis O.S., Zalis A.W., Barron K.D., Oester Y.T. Motor patterning following transitory sensory-motor deprivations. Arch Neurol. 1965;13(5):487-494.
42. Heise C.O., Siqueira M.G., Martins R.S., Gherpelli J.L. Clinical-electromyography correlation in infants with obstetric brachial plexopathy. J Hand Surg Am. 2007;32(7):999-1004.
43. Grossman J.A. Early operative intervention for birth injuries to the brachial plexus. Semin Pediatr Neurol. 2000;7(1):36-43.
44. Fisher D.M., Borschel G.H., Curtis C.G., Clarke H.M. Evaluation of elbow flexion as a predictor of outcome in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2007;120(6):1585-1590.
45. Smith N.C., Rowan P., Benson L.J., et al. Neonatal brachial plexus palsy. Outcome of absent biceps function at three months of age. J Bone Joint Surg Am. 2004;86(10):2163-2170.
46. Malessy M.J. Severe obstetric brachial plexus injuries can be identified easily and reliably at one month of age. American Society for Peripheral Nerve. Annual Scientific Meeting. Jan 13-16, 2007.
47. Waters P.M. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am. 1999;81(5):649-659.
48. Xu J., Cheng X., Gu Y. Different methods and results in the treatment of obstetrical brachial plexus palsy. J Reconstr Microsurg. 2000;16(6):417-420.
49. Kline D.G. Different methods and results in the treatment of obstetrical brachial plexus palsy (Letter). J Reconstr Microsurg. 2000;16(6):420-422.
50. Bodensteiner J.B., Rich K.M., Landau W.M. Early infantile surgery for birth-related brachial plexus injuries: justification requires a prospective controlled study. J Child Neurol. 1994;9(2):109-110.
51. Kay S.P. Obstetrical brachial palsy. Br J Plast Surg. 1998;51(1):43-50.
52. Tassin J.L. Obstetric paralysis of the brachial plexus. Spontaneous recovery: results of interventions. [in French] (Thesis). Université Paris. 1983.
53. Birch R., Ahad N., Kono H., Smith S. Repair of obstetric brachial plexus palsy: results in 100 children. J Bone Joint Surg Br. 2005;87(8):1089-1095.
54. Clarke H.M., Al Qattan M.M., Curtis C.G., Zuker R.M. Obstetrical brachial plexus palsy: results following neurolysis of conducting neuromas-in-continuity. Plast Reconstr Surg. 1996;97(5):974-982.
55. Kawabata H., Masada K., Tsuyuguchi Y., et al. Early microsurgical reconstruction in birth palsy. Clin Orthop Relat Res, 1987;215:233-242
56. Waters P.M. Update on management of pediatric brachial plexus palsy. J Pediatr Orthop B. 2005;14(4):233-244.
57. Michelow B.J., Clarke H.M., Curtis C.G., et al. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93(4):675-680.
58. Borrero J.L., de Pawlikowski W. Obstetrical Brachial Plexus Palsy. Lima: MAD Corp S.A; 2005.
59. Clarke H.M., Curtis C.G. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11(4):563-580.
60. Bisinella G.L., Birch R., Smith S.J. Neurophysiological prediction of outcome in obstetric lesions of the brachial plexus. J Hand Surg Br. 2003;28(2):148-152.
61. Bellew M., Kay S.P. Early parental experiences of obstetric brachial plexus palsy. J Hand Surg Br. 2003;28(4):339-346.
62. Pondaag W., Malessy M.J. Recovery of hand function following nerve grafting and transfer in obstetric brachial plexus lesions. J Neurosurg (1 Suppl Pediatrics). 2006;105(suppl 1):33-40.
63. Pondaag W., de Boer R., Van Wijlen-Hempel M.S., et al. External rotation as a result of suprascapular nerve neurotization in obstetric brachial plexus lesions. Neurosurgery. 2005;57(3):530-537.
64. Walker A.T., Chaloupka J.C., de Lotbiniere A.C., et al. Detection of nerve rootlet avulsion on CT myelography in patients with birth palsy and brachial plexus injury after trauma. AJR Am J Roentgenol. 1996;167(5):1283-1287.
65. Chow B.C., Blaser S., Clarke H.M. Predictive value of computed tomographic myelography in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2000;106(5):971-977.
66. Steens S, Verbist B. CT myelography in obstetric brachial plexopathy. Poster presentation at European Congress of Radiology, Vienna, 2010.
67. Tubbs R.S., Salter E.G., Wellons J.C.III, et al. The triangle of the vertebral artery. Neurosurgery. 2005;56(suppl 2):252-255.
68. Aszmann O.C., Rab M., Kamolz L., Frey M. The anatomy of the pectoral nerves and their significance in brachial plexus reconstruction. J Hand Surg [Am]. 2000;25(5):942-947.
69. Malessy M.J., Thomeer R.T. Evaluation of intercostal to musculocutaneous nerve transfer in reconstructive brachial plexus surgery. J Neurosurg. 1998;88(2):266-271.
70. Liverneaux P.A., Diaz L.C., Beaulieu J.Y., et al. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast Reconstr Surg. 2006;117(3):915-919.
71. Noaman H.H., Shiha A.E., Bahm J. Oberlin’s ulnar nerve transfer to the biceps motor nerve in obstetric brachial plexus palsy: indications, and good and bad results. Microsurgery. 2004;24(3):182-187.
72. Oberlin C., Beal D., Leechavengvongs S., et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5–C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19(2):232-237.
73. Pondaag W., Gilbert A. Results of end-to-side nerve coaptation in severe obstetric brachial plexus lesions. Neurosurgery. 2008;62(3):656-663.
74. Malessy M.J., Hoffmann C.F., Thomeer R.T. Initial report on the limited value of hypoglossal nerve transfer to treat brachial plexus root avulsions. J Neurosurg. 1999;91(4):601-604.
75. Malessy M.J., van Duinen S.G., Feirabend H.K., Thomeer R.T. Correlation between histopathological findings in C-5 and C-6 nerve stumps and motor recovery following nerve grafting for repair of brachial plexus injury. J Neurosurg. 1999;91(4):636-644.
76. Kline D.G. Nerve surgery as it is now and as it may be. Neurosurgery. 2000;46(6):1285-1293.
77. Tiel R.L., Happel L.T.Jr., Kline D.G. Nerve action potential recording method and equipment. Neurosurgery. 1996;39(1):103-108.
78. Kline D.G., Hackett E.R., May P.R. Evaluation of nerve injuries by evoked potentials and electromyography. J Neurosurg. 1969;31(2):128-136.
79. Pondaag W., van der Veken L.P., van Someren P.J., et al. Intraoperative nerve action and compound motor action potential recordings in patients with obstetric brachial plexus lesions. J Neurosurg. 2008;109(5):946-954.
80. König R.W., Antoniadis G., Börm W., et al. Role of intraoperative neurophysiology in primary surgery for obstetrical brachial plexus palsy (OBPP). Childs Nerv Syst. 2006;22(7):710-714.
81. Siqueira M.G., Foroni L., Martins R.S., et al. The fascicular topography of the suprascapular nerve in the C5 root and upper trunk of the brachial plexus: a microanatomic study from a nerve surgeon’s perspective. Neurosurgery. 2010;67(suppl Operative 2):402-406.
82. Clarke HM. Suprascapular nerve reconstruction in obstetrical brachial plexus palsy revisited: spinal accessory nerve transfer vs C5 nerve grafting. Presented at the American Society of Peripheral Nerve, Boca Raton, FL, 2010.
83. Ubachs J.M., Slooff A.C., Peeters L.L. Obstetric antecedents of surgically treated obstetric brachial plexus injuries. Br J Obstet Gynaecol. 1995;102(10):813-817.
84. Mallet J. Obstetrical paralysis of the brachial plexus. II. Therapeutics. Treatment of sequelae. Priority for the treatment of the shoulder. Method for the expression of results [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1972;58(suppl 1):166-168.
85. Raimondi P. Evaluation of results in obstetric brachial plexus palsy. The hand. Presented at the International Meeting on Obstetric Brachial Plexus Palsy, Heerlen, the Netherlands, 1993.
86. Gilbert A., Brockman R., Carlioz H. Surgical treatment of brachial plexus birth palsy. Clin Orthop Relat Res. 1991;264:39-47.
87. Laurent J.P., Lee R., Shenaq S., et al. Neurosurgical correction of upper brachial plexus birth injuries. J Neurosurg. 1993;79(2):197-203.
88. Pondaag W. Nerve transfers for elbow flexion in obstetric brachial plexus lesions. American Society for Peripheral Nerve. Annual Scientific Meeting. 2009.
89. Kawabata H., Shibata T., Matsui Y., Yasui N. Use of intercostal nerves for neurotization of the musculocutaneous nerve in infants with birth-related brachial plexus palsy. J Neurosurg. 2001;94(3):386-391.
90. Blaauw G., Slooff A.C. Transfer of pectoral nerves to the musculocutaneous nerve in obstetric upper brachial plexus palsy. Neurosurgery. 2003;53(2):338-341.
91. Narakas A.O., Allieu Y., Alnot J.Y., et al. Complete supraclavicular paralysis. Surgical possibilities and Results. [in French]. In: Alnot J.Y., Narakas A.O. Paralysis of the Brachial Plexus. Paris: Expansion Scientifique Francaise; 1989:130-162.
92. Haerle M., Gilbert A. Management of complete obstetric brachial plexus lesions. J Pediatr Orthop. 2004;24(2):194-200.
93. Terzis J.K., Kokkalis Z.T. Primary and secondary shoulder reconstruction in obstetric brachial plexus palsy. Injury. 2008;39(suppl 3):S5-S14.
94. Lin J.C., Schwentker-Colizza A., Curtis C.G., Clarke H.M. Final results of grafting versus neurolysis in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2009;123(3):939-948.
95. Berger A.C., Hierner R., Becker M.H. Early microsurgical revision of the brachial plexus in traumatic birth injuries. Patient selection and outcome. Orthopade. 1997;26(8):710-718.
96. Alanen M., Ryoppy S., Varho T. Twenty-six early operations in brachial birth palsy. Z Kinderchir. 1990;45(3):136-139.
97. Sherburn E.W., Kaplan S.S., Kaufman B.A., et al. Outcome of surgically treated birth-related brachial plexus injuries in twenty cases. Pediatr Neurosurg. 1997;27(1):19-27.
98. Al-Qattan M.M. The outcome of Erb’s palsy when the decision to operate is made at 4 months of age. Plast Reconstr Surg. 2000;106(7):1461-1465.
99. Grossman J.A., DiTaranto P., Yaylali I., et al. Shoulder function following late neurolysis and bypass grafting for upper brachial plexus birth injuries. J Hand Surg Br. 2004;29(4):356-358.
100. Terzis J.K., Kostas I. Outcomes with suprascapular nerve reconstruction in obstetrical brachial plexus patients. Plast Reconstr Surg. 2008;121(4):1267-1278.
101. Grossman J.A., Di T.P., Alfonso D., et al. Shoulder function following partial spinal accessory nerve transfer for brachial plexus birth injury. J Plast Reconstr Aesthet Surg. 2006;59(4):373-375.
102. van Ouwerkerk W.J., Uitdehaag B.M., Strijers R.L., et al. Accessory nerve to suprascapular nerve transfer to restore shoulder exorotation in otherwise spontaneously recovered obstetric brachial plexus lesions. Neurosurgery. 2006;59(4):858-867.
103. Terzis J.K., Kokkalis Z.T. Shoulder function following primary axillary nerve reconstruction in obstetrical brachial plexus patients. Plast Reconstr Surg. 2008;122(5):1457-1469.
104. El-Gammal T.A., El-Sayed A., Kotb M.M., et al. Total obstetric brachial plexus palsy: results and strategy of microsurgical reconstruction. Microsurgery. 2010;30(3):169-178.
105. Boome R.S., Kaye J.C. Obstetric traction injuries of the brachial plexus. Natural history, indications for surgical repair and results. J Bone Joint Surg Br. 1988;70(4):571-576.
106. Kawabata H., Kawai H., Masatomi T., Yasui N. Accessory nerve neurotization in infants with brachial plexus birth palsy. Microsurgery. 1994;15(11):768-772.
107. Dumont C.E., Forin V., Asfazadourian H., Romana C. Function of the upper limb after surgery for obstetric brachial plexus palsy. J Bone Joint Surg Br. 2001;83(6):894-900.
108. Solonen K.A., Telaranta T., Ryoppy S. Early reconstruction of birth injuries of the brachial plexus. J Pediatr Orthop. 1981;1(4):367-370.
109. Wellons J.C., Tubbs R.S., Pugh J.A., et al. Medial pectoral nerve to musculocutaneous nerve neurotization for the treatment of persistent birth-related brachial plexus palsy: an 11-year institutional experience. J Neurosurg Pediatr. 2009;3(5):348-353.
110. El-Gammal T.A., bdel-Latif M.M., Kotb M.M., et al. Intercostal nerve transfer in infants with obstetric brachial plexus palsy. Microsurgery. 2008;28(7):499-504.
111. Terzis J.K., Kokkalis Z.T. Elbow flexion after primary reconstruction in obstetric brachial plexus palsy. J Hand Surg Eur. 2009;34(4):449-458.
112. Al Qattan M.M. Oberlin’s ulnar nerve transfer to the biceps nerve in Erb’s birth palsy (letter). Plast Reconstr Surg. 2002;109(1):405-407.
113. Blaauw G., Sauter Y., Lacroix C.L., Slooff A.C. Hypoglossal nerve transfer in obstetric brachial plexus palsy. J Plast Reconstr Aesthet Surg. 2006;59(5):474-478.
114. Kawano K., Nagano A., Ochiai N., et al. Restoration of elbow function by intercostal nerve transfer for obstetrical paralysis with co-contraction of the biceps and the triceps. J Hand Surg Eur. 2007;32(4):421-426.
115. Kirjavainen M., Remes V., Peltonen J., et al. The function of the hand after operations for obstetric injuries to the brachial plexus. J Bone Joint Surg Br. 2008;90(3):349-355.
116. Ashley W.W.Jr., Baty J.D., Hollander T., et al. Long-term motor outcome analysis using a motor score composite following surgical brachial plexus repair. J Neurosurg. 2007;106(suppl 4):276-281.
117. Badr Y., O’Leary S., Kline D.G. Management of one hundred seventy-one operative and nonoperative obstetrical birth palsies at the Louisiana State University Health Sciences Center. Neurosurgery. 2009;65(suppl 4):A67-A73.
118. Chen L., Gu Y.D., Hu S.N., et al. Contralateral C7 transfer for the treatment of brachial plexus root avulsions in children—a report of 12 cases. J Hand Surg Am. 2007;32(1):96-103.
119. Terzis J.K., Kokkalis Z.T. Restoration of elbow extension after primary reconstruction in obstetric brachial plexus palsy. J Pediatr Orthop. 2010;30(2):161-168.