Chapter 9 Nerve Destruction for the Alleviation of Visceral Pain
 Visceral pain is commonly more challenging to treat. It is often more diffuse and poorly localizable.
Visceral pain is commonly more challenging to treat. It is often more diffuse and poorly localizable. Visceral organs are rarely innervated by single localizable nerve structures; most organs, especially the perineal and pelvic structures, have dual or more innervation, resulting in only partial treatment when one innervation source is treated in isolation.
Visceral organs are rarely innervated by single localizable nerve structures; most organs, especially the perineal and pelvic structures, have dual or more innervation, resulting in only partial treatment when one innervation source is treated in isolation. With the development of modern imaging techniques, procedures to provide analgesia to the visceral organs have become low to moderate risk. The majority of catastrophic outcomes associated with neurolytic procedures predated the use of the imaging to visualize the intended structure before administration of neurolytics.
With the development of modern imaging techniques, procedures to provide analgesia to the visceral organs have become low to moderate risk. The majority of catastrophic outcomes associated with neurolytic procedures predated the use of the imaging to visualize the intended structure before administration of neurolytics. Patient selection is one of the most important factors in the success of these procedures in the short and long terms. Neurolysis of visceral nerve fibers is controversial in the nonmalignant (noncancer) population because of legitimate concerns regarding deafferentation pain.
Patient selection is one of the most important factors in the success of these procedures in the short and long terms. Neurolysis of visceral nerve fibers is controversial in the nonmalignant (noncancer) population because of legitimate concerns regarding deafferentation pain. Neurolytic procedures of the visceral nervous system should be considered early in the treatment of cancer patients whose pain is no longer well controlled on moderate doses of analgesics or who are unable to tolerate systemic analgesic medications because of adverse effects.
Neurolytic procedures of the visceral nervous system should be considered early in the treatment of cancer patients whose pain is no longer well controlled on moderate doses of analgesics or who are unable to tolerate systemic analgesic medications because of adverse effects. Neurolytic procedures for visceral cancer pain are an effective adjunctive treatment that can co-occur with other allopathic and alternative analgesic modalities.
Neurolytic procedures for visceral cancer pain are an effective adjunctive treatment that can co-occur with other allopathic and alternative analgesic modalities. Given that there is redundant innervation to many of the visceral organs, it may prove difficult to impossible to alleviate all of a patient’s visceral pain with a single intervention; thus a multimodal approach should be taken with these patients.
Given that there is redundant innervation to many of the visceral organs, it may prove difficult to impossible to alleviate all of a patient’s visceral pain with a single intervention; thus a multimodal approach should be taken with these patients. Celiac plexus blocks will help to alleviate visceral pain in a multitude of organs from the distal third of the esophagus to the descending colon, including the liver, pancreas, gallbladder, stomach, spleen, kidneys, small intestine, large intestine, and adrenal gland.
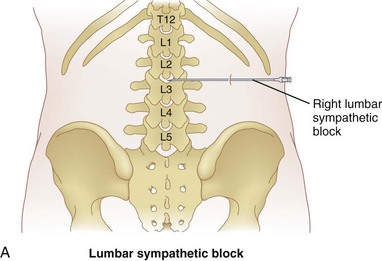
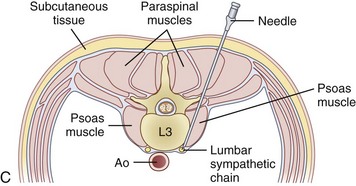
Celiac plexus blocks will help to alleviate visceral pain in a multitude of organs from the distal third of the esophagus to the descending colon, including the liver, pancreas, gallbladder, stomach, spleen, kidneys, small intestine, large intestine, and adrenal gland. The lumbar sympathetic chain is located just anterolateral to the lumbar vertebral bodies, anterior to the origin of the psoas muscle, and is best approached at L2, L3, or L4. This block is performed for visceral pain from the descending colon, upper portion of the sigmoid colon, and kidneys.
The lumbar sympathetic chain is located just anterolateral to the lumbar vertebral bodies, anterior to the origin of the psoas muscle, and is best approached at L2, L3, or L4. This block is performed for visceral pain from the descending colon, upper portion of the sigmoid colon, and kidneys. The superior hypogastric block is performed at the level of L5 to S1 for treatment of visceral pain of the lower sigmoid colon, rectum, testicles, ovaries, and uterus.
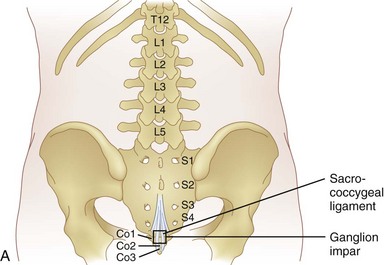
The superior hypogastric block is performed at the level of L5 to S1 for treatment of visceral pain of the lower sigmoid colon, rectum, testicles, ovaries, and uterus. A ganglion impar block is performed for pain in the perineal area. The ganglion impar is where the two sympathetic chains join together and is located retroperitoneally at the level of the sacrococcygeal ligament.
A ganglion impar block is performed for pain in the perineal area. The ganglion impar is where the two sympathetic chains join together and is located retroperitoneally at the level of the sacrococcygeal ligament. The benefits of neurolysis are time limited and patients with non-cancer originated pain may have a life expectancy well past the analgesic effect of the block. This can leave the patient with not only their original abdominal pain but also deafferentation pain, which can be more severe than the original pain.
The benefits of neurolysis are time limited and patients with non-cancer originated pain may have a life expectancy well past the analgesic effect of the block. This can leave the patient with not only their original abdominal pain but also deafferentation pain, which can be more severe than the original pain. A backache in addition to orthostatic hypotension following a celiac plexus block should be assumed to be a retroperitoneal hematoma until proven otherwise, warranting serial hematocrits.
A backache in addition to orthostatic hypotension following a celiac plexus block should be assumed to be a retroperitoneal hematoma until proven otherwise, warranting serial hematocrits. There is some controversy regarding the timing of neurolytic interventional therapy, leaving each individual practitioner to make the final decision. If you place the block early in the disease process you may decrease the likelihood of central or peripheral sensitization and you may decrease the unwanted side effects of high dose opioids. Others argue that these blocks should be performed only when undesirable side effects occur from non-invasive methods (such as opioid management).
There is some controversy regarding the timing of neurolytic interventional therapy, leaving each individual practitioner to make the final decision. If you place the block early in the disease process you may decrease the likelihood of central or peripheral sensitization and you may decrease the unwanted side effects of high dose opioids. Others argue that these blocks should be performed only when undesirable side effects occur from non-invasive methods (such as opioid management).Introduction
Visceral pain is a common complaint seen by many different types of physicians, including family medicine, gastroenterologists, oncologists, surgeons, and pain medicine. Visceral pain can be very challenging to diagnose, manage, and treat. This pain can be somatic or visceral in origin, can be related to a malignancy or be noncancer in origin, and is often very diffuse and nonlocalized. Therefore, treatment and management not only depends on the type of pain and its origin but also very often is aimed at the additional factors in the patient’s life exacerbating the pain such as stress and anxiety.1,2
The history provided by the patient involving the description of pain quality and referral pattern will help elucidate the origin of the pain symptoms. Somatic pain is generally described as well localized and constant in nature with an aching or sharp quality. Somatic pain can often be treated with a combination of nonopioid adjuvant analgesics and local pain interventions. Visceral pain is typically described as vague in origin and of a squeezing, deep, pressure-like quality. Visceral pain is a result of organ injury with resulting transmission of pain via fibers that travel with the sympathetic nervous system.3 Visceral pain may result from distention, traction, torsion, ischemia, or abnormal contraction of visceral organs and may result in referred somatic complaints.4 Interventional procedures involving the sympathetic chain may be used to ease the pain of some patients with abdominal malignancies. Finally, neuropathic pain is usually described as a shooting, numbness, tingling, burning type of pain. This type of pain is usually caused by some injury or irritation of the nervous system itself whether it is via direct postsurgical injury or from a tumor encroachment, compression, or invasion of a nerve structure. This type of pain requires a multimodal pharmacological approach, including opioids, tricyclic antidepressants, selective serotonin norepinephrine reuptake inhibitors, and N-methyl-d-aspartate blockers and early aggressive interventional management with spinal cord stimulation; intrathecal medication administration; and, in the case of malignancy-related pain, neurolysis. This chapter focuses on peripheral neurolytic treatment of visceral pain.
Visceral input arrives in the dorsal horn of the spinal cord; lamina I, II and V; and the intermediolateral cell column, sacral parasympathetic nucleus and lamina X.4 Multiple visceral inputs converge at a single site, thus making exact diagnosis of the location of pain difficult and hence the vague symptoms described by patients.1 The signal travels from the dorsal horn of the spinal cord to the spinothalamic, spinoreticular, spinomesencephalic, and spinohypothalamic tract.4 Visceral input terminates in the brain at multiple sites, including the medulla, pons, mesencephalon, hypothalamus, and thalamus,4 and results in activity of the anterior and midcingulate cortex, frontal cortex, parietal cortex, and cerebellum.5 The cortical sites associated with visceral pain have involvement in the affective perception of pain and memory and may account for the preponderance of co-morbidities such as anxiety and depression with visceral pain.
The treatment of visceral pain resulting from malignancy is integral to the overall treatment of patients with cancer, the goals being improvement in quality of life and improved functional status.6 Evidence suggests pain control plays a significant role in cancer survival.7,8 In treating patients with cancer-related pain, a multimodal and multidisciplinary approach involving medical, interventional, psychological, and social support management produces the highest level of patient satisfaction.
Chemical Neurolysis
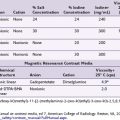
Chemical neurolysis has been used since the early 1900s when Schloesser used alcohol for the treatment of trigeminal neuralgia.9 In 1919, Kappis10 performed the first neurolytic procedure for the relief of visceral pain when he performed a percutaneous celiac plexus block in the treatment of intractable abdominal pain.9 Many pharmacological agents have been used for neurolysis, including alcohol (ethanol), phenol, glycerol, and hypertonic saline. Nerve destruction depends on the agent, amount, concentration, and rate of injection.9
The neurolytic action of alcohol is produced by the extraction of cholesterol, phospholipids, and cerebrosides and the precipitation of mucopeptides from the nerve and supporting myelin sheath.11 The basal lamina of the Schwann cell sheath remains intact, allowing for new Schwann cell growth, thereby providing the framework for subsequent nerve fiber growth. This framework encourages the regeneration of axons, but only if the cell bodies of these nerves are not completely destroyed.12 The pathway of degeneration is nonselective and can be observed in peripheral nerves and spinal nerve roots after intrathecal injection and in perineural structures (peritoneum, bowel) after peripheral injections.
Phenol is a benzene ring with one hydroxyl group substituted for a hydrogen atom. It is usually prepared by the hospital or compounding pharmacy because it is not commercially available in premixed liquid form; however, this practice has ceased in many hospital organizations because of the complexity of sterile compounding. Phenol is poorly soluble in water and, at room temperature, forms only a 6.7% aqueous solution. Consequently, phenol is frequently prepared with contrast dyes and sterile water, saline, or glycerin. When phenol is exposed to room air, it undergoes oxidation and turns a reddish color; however, it has a shelf life of approximately 1 year if refrigerated and shielded from light exposure. When phenol is prepared with glycerin, it has limited spread; hence, injections are well localized. In rats, the aqueous solution of phenol has greater ability to penetrate the perineurium and produce greater endoneurial damage than glycerin preparations, but there is no difference in results after intraneural injection.13
Putnam and Hampton14 first used phenol as a neurolytic agent in 1936, and Mandl used it for a sympathetic ganglion neurolysis in animals in 1947.15 Originally, it was surmised that phenol had a selective effect on small-diameter, unmyelinated and lightly myelinated nerve fibers, such as C-fiber afferents and A-δ afferents, respectively. Subsequent studies have shown that phenol concentrations determine the type and extent of nerve disruption that also include A-α and A-β fiber damage.16 At concentrations less than 5%, phenol causes protein denaturation of axons and surrounding blood vessels. At concentrations greater than 5%, phenol can produce protein coagulation and nonselective segmental demyelination.17
Unlike alcohol, phenol injection has an initial local anesthetic effect. It is not associated with localized burning but instead creates a sensation of warmth and numbness. Concentrations of 4% to 10% are typically used for neurolysis. Preparations of phenol in glycerin are highly viscous, which may make administration through a small gauge (25-gauge) spinal needle difficult. Careful patient positioning to allow phenol to settle into the desired location is important. When compared with alcohol, phenol seems to facilitate axonal regeneration in a shorter period of time. Electrophysiological studies comparing peripheral nerve destruction in cats showed that those injected with phenol had returned to normal by 2 months; at the end of the same time period, those injected with alcohol still demonstrated depression of compound action potentials.18 However, another study by Smith19 suggests regeneration is not completed until approximately 14 weeks after the administration of phenol.
Celiac Plexus Neurolysis
Indication
Neurolysis of the celiac plexus is one of the most commonly performed neurolytic procedures for the treatment of visceral cancer pain. The nerves of the celiac plexus are responsible for the painful sensation of an extremely large anatomical area, innervating the visceral organs from the distal third of the esophagus to the descending colon. This, therefore, includes the liver, pancreas, gallbladder, stomach, spleen, kidneys, small intestines, large intestines (to the descending colon), and adrenal glands; a partial contribution to the bladder; and a small contribution to the testes and epididymis, the ovaries, and the blood vessels surrounding them.9 Both malignant and nonmalignant pain associated with these organs can be reduced with the neurolytic destruction of the celiac plexus. Although the treatment of nonmalignant pain conditions can be achieved with this technique, it is not recommended for routine use in the treatment of noncancer pain because the procedure is associated with significant and serious complications that may not outweigh the benefits of the procedure. Celiac plexus neurolysis for the treatment of nonmalignant abdominal pain conditions such as chronic pancreatitis has been reported to be more short-lived analgesia than those treated with the same procedure for the treatment of cancer-related pain.9,20,21 Furthermore, the benefits of the neurolysis are likely to be time limited, and the noncancer patient’s life may extend well past the analgesia associated with the procedure. This can leave the patient with their original abdominal pain and partial deafferentation pain, which can be more severe than the initial pain and now with more limited treatment options.
Anatomy
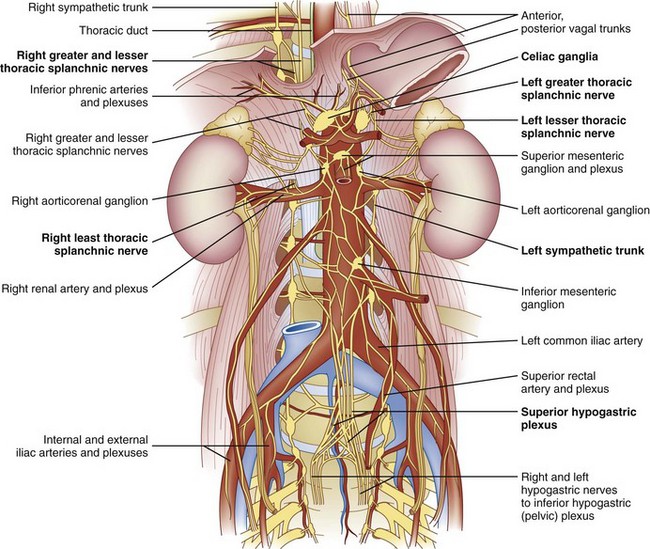
The celiac plexus is located at the level of T12 to L1. It is found in the retroperitoneal space, just anterior and caudad to the crura of the diaphragm (Fig. 9-1). It surrounds the anterior and lateral aspects of the aorta and celiac and superior mesenteric trunks as they divide from the aorta.22 Most commonly, the celiac plexus ganglia are 0.6 cm and 0.9 cm caudad to the celiac artery on the right and left, respectively.23 The location of the celiac plexus varies with respect to bony landmarks and can be located anywhere from the T12 to L1 disc space to the middle of the L2 vertebral body.24 The plexus receives sympathetic fibers from the greater (T9-T10), lesser (T10-T11), and least (T12) splanchnic nerves and parasympathetic fibers from the vagus nerve (Fig. 9-1).
Procedure
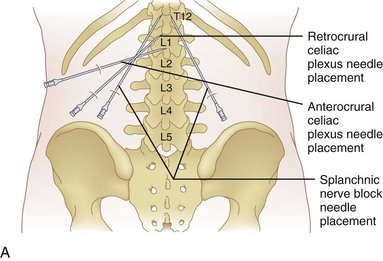
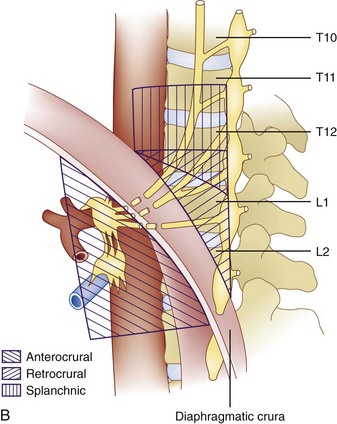
The celiac plexus may be approached in three different ways: retrocrural, anterocrural, and the splanchnic nerves (Fig. 9-2). It can be performed percutaneously in the prone and supine positions using anatomic landmarks, ultrasonography, fluoroscopy, or computed tomography (CT), or through the gastric wall with the assistance of an endoscope.
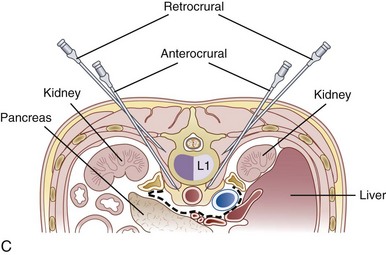
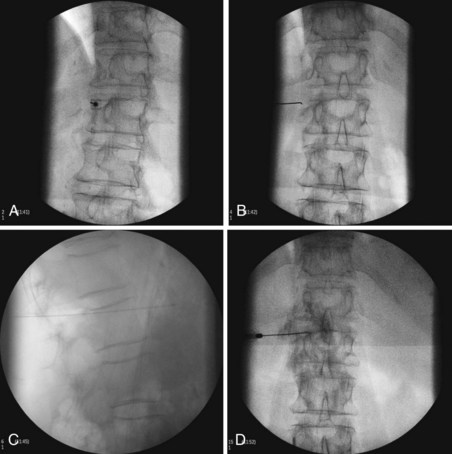
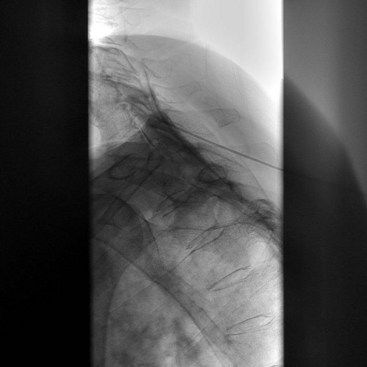
The most common approach and most thoroughly studied approach is the fluoroscopic or CT-guided posterior percutaneous approach. In this approach, the patient is placed prone, and a 5- to 7-inch small-gauge needle is inserted at the level of the L1 transverse process approximately 5 to 7 cm to the left of midline. This location should coincide with the lateral edge of the L1 vertebral body when viewed in the oblique orientation at approximately 25 to 35 degrees (Fig. 9-3, A) depending on the patient’s body mass index. After the needle is inserted, it is directed toward the upper third of L1 (Fig. 9-3, B) for the retrocrural approach and the lower third of L1 for the anterocrural approach. In performing the retrocrural approach, the needle is advanced just anterior to the anterior border of the body of L1, no more than 0.5 cm. In the anterocrural approach, from the left, the needle is advanced through the aorta (Fig. 9-3, C), and the needle is advanced until no more blood is aspirated. At this time, contrast dye should be injected to confirm location in the anteroposterior (AP) and lateral views (Fig. 9-3, D). If dye spread appears anterior to the aorta and covers the left and right sides and if the provider is intending anterocrural injection, then a second needle from the right side need not be placed. In a retrocrural injection, if the dye spread remains in the anterior third of the vertebral body when examined in the lateral view and on both sides when viewed in AP, then a second needle need not be placed. In performing the splanchnic nerve block, the needles are aimed at the body of T12 using a caudocephalad angle (camera cephalad, image intensifier caudad) of 10 to 15 degrees with skin insertion at the L1 level similar to that of the celiac plexus neurolysis to best avoid puncture of the lung pleural or parenchyma. The needle is then directed until it is just short of the anterior border of the T12 vertebral body. After the needle is placed and secure, a local anesthetic agent with epinephrine is injected to test for inadvertent intravascular access. A long-acting local anesthetic is then injected to produce analgesia before ethanol administration (this can be excluded if phenol is the neurolytic).
The volume of neurolytic injected differs for the approaches. Retrocrural celiac plexus neurolysis may require a slightly higher volume of ethanol because it has to spread to the celiac plexus across the crura or envelop the descending splanchnic nerves. Traditionally, these volumes have been in the range of 20 to 25 mL per side. These volumes are often excessive and a total of 15 to 20 mL (7.5-10 cc per side) can be sufficient. The anterocrural approach is similar, and it can require between 15 and 20 mL of the neurolytic drug (divided between the needles if using more than one). The splanchnic neurolysis requires 6 to 8 mL total.25 The ethanol concentration for these blocks is in the range of 50% to 80% obtained by diluting the neurolytic with contrast to enable visualization of neurolytic medication spread. If the provider has injected 5 to 10 mL of local anesthetic before the administration of the neurolytic, they may take that volume into account for the dilution calculation or preferably wait 5 to 10 minutes between the injections to allow for significant diffusion and absorption of the local anesthetic. If phenol is being used, the concentration most commonly used is in the range of 5% to 10% mixed with contrast, saline, or glycerin.
With advanced imaging, such as CT guidance or ultrasonography, patients who are either unable to lie flat or who have a liver that is too enlarged to allow a posterior entry, a transabdominal radiographic approach can be used but is not recommended unless all other approaches are exhausted because of the high risk of infection secondary to needle entrance into the bowel.9
Complications
Efficacy
The efficacy of the celiac plexus block has been studied in several trials.25,30–32 The prospective randomized study by Ischia et al25 looked at 61 patients with pancreatic cancer pain. Of those, 48% experienced complete pain relief from the neurolytic celiac plexus block. The remaining 52%, or 32 patients, did not experience complete pain relief secondary to either technical failure or the fact that the patient had not just visceral pain but a combination of neuropathic, visceral, and somatic complaints.25 A second study compared medical therapy with that of the celiac plexus block.33 The patients who received the neurolytic block used less opioid than those who did not receive the block and thus experienced fewer pharmacologically induced side effects.33 The presence of lymphadenopathy is a poor prognostic indicator in the success of the neurolytic block; thus, when there is advanced disease outside of the pancreas, a successful outcome is less likely. A meta-analysis looking at 21 retrospective studies showed that pain relief could be achieved in 89% of patients for the first 2 weeks after a block.34 In patients who were alive at the 3-month interval, 90% of the patients had partial to complete pain relief. Of patients who received the block within the 3-month interval preceding death, 70% to 90% experienced partial to complete pain relief.34 This meta-analysis did not take into account the approach used or choice and dosage of neurolytic agent used.
There is some controversy regarding the timing of neurolytic treatment in the course of tumor progression. Those who place the neurolytic procedure very early in the cancer progress (early after the onset of pain), such as these authors, believe that the early abolition of pain reduces the likelihood of central or peripheral sensitization and therefore the development of a more challenging pain syndrome latter in the course. Furthermore, early intervention provides an easier target to treat because the tumor growth has not yet physically obstructed access to the plexus. Advanced disease that has spread will usually result in not only visceral pain but also somatic and neuropathic pain.9 Furthermore, there is evidence showing that high doses of opioids may have a negative effect on immunity.35 A study by Lillemoe and colleagues8 showed that patients with nonresectable pancreatic cancer lived longer if they received splanchnic neurolysis. This prospective randomized trial suggested that the patients who had the neurolysis procedure used fewer narcotics and had better-preserved immune functions and also experienced less nausea and vomiting.8 Others argue that neurolytic blocks should be performed only when patients obtain undesirable side effects from significant dosages of systemic narcotics. Side effects include sedation, constipation, nausea, vomiting, and respiratory depression and may prove to be intolerable to some patients. The ideal time to provide neurolytic treatment has yet to be determined in a rigorous fashion.
Lumbar Sympathetic Neurolysis
Indications
The lumbar sympathetic chain is blocked in cases of visceral pain associated with the descending colon and upper portion of the sigmoid colon, kidney, a partial contribution to the bladder, and ovaries.36–38 Although this is not the subject of this chapter, blockade of this plexus or chain is also performed in the treatment of lower extremity sympathetically-mediated neuropathic pain syndromes and ischemic pain associated with small vessel peripheral vascular disease. The treatment of the latter pain syndromes with lumbar sympathetic chain neurolysis is very controversial. As discussed in previous sections, neurolysis is most commonly reserved for cancer-related pain secondary to the reduced life expectancy and therefore lower cumulative risk of deafferentation pain. Neurolysis of this plexus for the purpose of relieving visceral pain will result in sympatholysis to the lower extremities as well, and patients will have to provide consent for this known outcome.
Anatomy
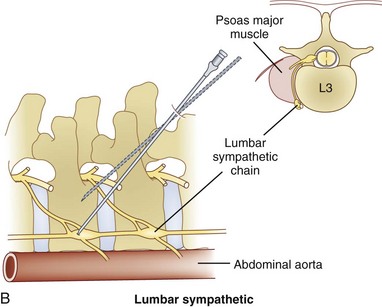
The lumbar sympathetic chain is located just anterolateral of the lumbar vertebral bodies anterior to the origin of the psoas muscle.38 The aorta is anterior and medial to the left sympathetic chain, and the right sympathetic chain approximates the inferior vena cava.22 The lumbar arteries and veins are present around the sympathetic chain as well.38
Procedure
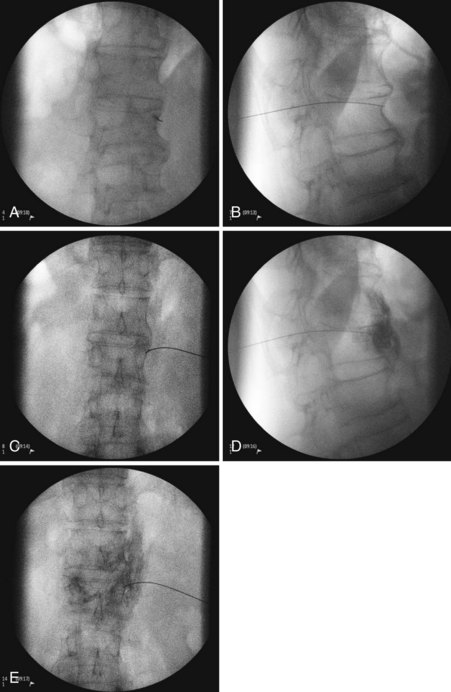
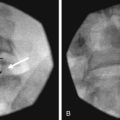
Three approaches are available for the lumbar sympathetic block. Kappis and Mandl developed the classic technique using 3 needles placed 5 to 6 cm from midline at the level of L2, L3, and L4. The needles are angled medially contacting the transverse process of their respective lumbar vertebra. Redirecting and passing inferiorly and medially, the needle is aimed at the vertebral body. The needle is advanced until it is just anterolateral to the respective vertebral body.38 Reid et al developed the lateral technique in 1970.39 They initiated their approach 10 to 12 cm lateral to midline, thus avoiding the vertebral body altogether. They designed this approach because they thought that it was difficult to navigate around the vertebral body effectively.38 A third approach developed by Bryce-Smith40 took advantage of the tendinous arches at the origin of the psoas muscle. The arches contain lumbar vessels, fat, rami communicantes, and the sympathetic chain anteriorly.38 This technique uses one insertion point at L3 5 cm lateral to the superior tip of the spinous process directed medially through the psoas muscle, missing the vertebra altogether. Although these are the described standard techniques, with the addition of fluoroscopy and CT, most pain physicians have altered their practice. The most common performed approach is using a fluoroscopic angle of approximately 30 to 35 degrees to allow for the L3 transverse process to just become buried within the vertebral body (Figs. 9-4, A and 9-5). The needle is then inserted at the level of the L3 transverse process and advanced until it contacts the most lateral portion of the vertebral body at which point in lateral view the needle is directed medially and anteriorly until the tip lies in the anterolateral portion of the vertebral body (Fig. 9-4, B and C). Contrast dye is then injected to confirm the needle tip location. Contrast dye covering the anterolateral aspect of the vertebral body without the appearance of an intramuscular injection confirms appropriate placement (Fig. 9-4, D and E). After this, a test dose with lidocaine and epinephrine can be performed, although the test is not necessary if real-time fluoroscopy or digital subtraction angiography is performed. A solution of 5 to 8 cc of bupivacaine is then injected to anesthetize the nerves before injection of the neurolytic medication. A range of 5 to 10 cc of 50% to 80% ethanol can be used for neurolysis. If contrast is included in the ethanol, one can guide the volume of injection by the cephalad–caudal and dorsal–ventral spread of the ethanol.
Complications
Complications resulting from performing a lumbar sympathetic block include the following:
Superior Hypogastric Plexus Neurolysis
Indications
Patients with pain secondary to cancer invasion or nonmalignant conditions involving the lower portion of the sigmoid colon to the rectum, testicles, ovaries, uterus, fallopian tubes, or a partial sympathetic innervation of the bladder and ureters36 may benefit from a superior hypogastric block.
Anatomy
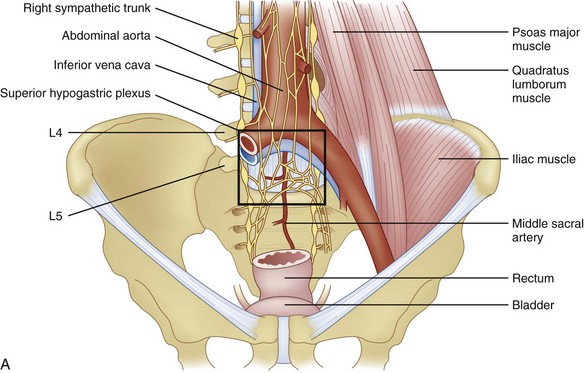
The afferent fibers innervating pelvic structures travel in the sympathetic nerves and ganglia. For pain syndromes involving cancer invasion into the pelvic organs, it has been suggested that a superior hypogastric plexus (SHP) neurolytic block should be considered even in advanced stages of disease.57 The plexus is situated retroperitoneally extending from the lower edge of L5 to the upper third of S1 (Fig. 9-6, A).
Procedure
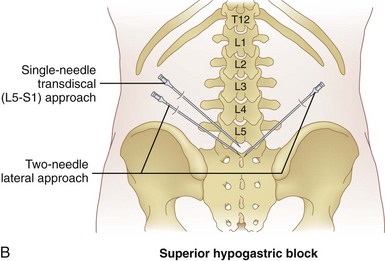
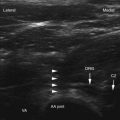
There are two main approaches to the SHP (Fig. 9-6, B). Using the two-needle technique, the patient is placed in a prone position with two 7-cm needles directed medially and caudad so that the tip of the needles lay anterolateral to the L5-S1 intervertebral disc space (Fig. 9-7, A and B).57,58 This approach avoids transgression of the intervertebral disc, but it has a higher rate of neurapraxia related to needle trauma to the L5 nerve roots as they exit the L5-S1 foramen. Avoidance of injection into the iliac vessels is accomplished with aspiration. If blood is aspirated, a transvascular approach can be used. Accurate placement is verified using fluoroscopy with the tip of the needle at the junction of L5 and S1 on an anteroposterior view.
The second approach is the transdiscal technique in which a single needle is placed in a similar manner to a discogram of the L5-S1 intervertebral disc. With the patient positioned prone, a single 7-inch spinal needle is placed through the disc using an oblique angle of approximately 25 to 30 degrees so the needle tip ends in the midline immediately anterior to the L5-S1 disc (Fig. 9-8, A and B). Contrast injection confirms proper placement (Fig. 9-8, C and D). After the needle has been confirmed to be in the correct position, a local anesthetic can be used diagnostically and then ethanol or phenol can be used as a neurolytic solution. The volume of neurolytic is guided by the spread of the agent (when mixed with contrast); common volumes range from 5 to 10 cc.
Ganglion Impar Neurolysis
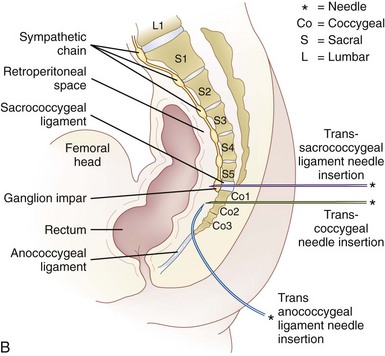
Procedure
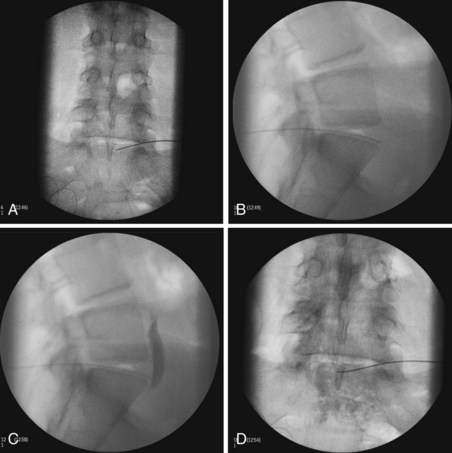
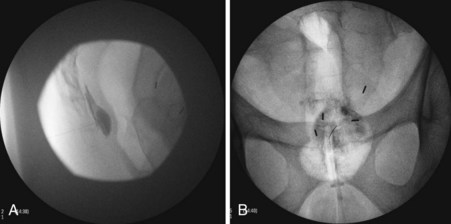
Two approaches using fluoroscopy have been described in performing a ganglion impar block (Fig. 9-9). A trans-sacrococcygeal joint approach involves placement of a 20- to 22-gauge needle through the sacrococcygeal ligament until it is just anterior to the sacrum, with the patient in a prone position (Fig. 9-10). The second approach places the patient in the lateral decubitus position with hips flexed. A 3.5-inch needle is placed through the anococcygeal ligament using fluoroscopy. A finger is placed in the rectum to avoid inadvertent puncture. The needle is guided along the anococcygeal ligament. This latter approach has fallen out of favor because of its complexity and increased risk to the patient and interventionalist of needle puncture. Phenol 6% has been the traditional neurolytic agent of choice, but ethanol provides excellent neurolysis that is comfortable for the patient provided sufficient local anesthetic is first injected. Small volumes are used for this injection; often 1 to 3 mL of 80% ethanol or 6% phenol is sufficient to produce analgesia.
Efficacy
In a prospective study by Plancarte et al,60 patients with perineal pain who had failed pharmacologic treatment were evaluated. All of the patients had pain confined to the perineal area. Using a trans-sacrococcygeal approach, eight of 16 patients experienced complete relief with the remaining eight patients experiencing a 60% to 90% reduction in pain.60 Of note, the ganglion impar block has not been shown to be efficacious in the treatment of patients with coccydynia.61
1 Ness T. Chronic abdominal, groin, and perineal pain of visceral origin. In: Breivik H, Campbell W, Nicholas M, editors. Textbook of clinical pain management: chronic pain. London: Hodder & Stroughton; 2008:567-586.
2 Bhatia V, Tandon R. Stress and the gastrointestinal tract. J Gastroenterol Hepatol. 2004;20:332-339.
3 Newman PP. Visceral afferent functions of the nervous system. Monogr Physiol Soc. 1974:1-273.
4 Ness T. Applied physiology: persistent visceral pain. In: Wilson PR, Watson PJ, Haythornthwaite JA, et al, editors. Clinical pain management: chronic pain. London: Oxford University Press; 2008:37-47.
5 Athwal BS, Berkley KJ, Hussain I, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369-377.
6 Ferrell BR, Wisdom C, Wenzl C. Quality of life as an outcome variable in the management of cancer pain. Cancer. 1989;63:2321-2327.
7 Liebeskind JC. Pain can kill. Pain. 1991;44:3-4.
8 Lillemoe KD, Cameron JL, Kaufman HS, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217:447-455. discussion 456-457
9 Williams J. Nerve blocks—chemical and physical neurolytic agents. I. In: Sykes N, Fallon MT, Patt RB, editors. Clinical pain management: cancer pain. London: Arnold; 2003:235-244.
10 Kappis M. Sensibilitat und locale Anasthesia in chirurginchen Gebiet der Bauchhohle mit besonderer Berucksichrigung der Splanchnicus. Anasthesie Beitr Z Klin Chir. 1919;115:161.
11 Rumsby MG, Finean JB. The action of organic solvents on the myelin sheath of peripheral nerve tissue. II. Short-chain aliphatic alcohols. J Neurochem. 1966;13:1509-1511.
12 Bonica J, Buckley F, Moricca G, et al. Neurolytic blockade and hypophysectomy. In: Bonica J, editor. The management of pain. Philadelphia: Lea & Febiger; 1990:1980-2039.
13 Westerlund T, Vuorinen V, Kirvela O, et al. The endoneurial response to neurolytic agents is highly dependent on the mode of application. Reg Anesth Pain Med. 1999;24:294-302.
14 Putnam TJ, Hampton AO. The technic of injection into the Gasserian ganglion under roentgenographic control. Arch Neurol Psychiatry. 1936;35:92-98.
15 Mandl F. Aqueous solution of phenol as a substitute for alcohol in sympathetic block. J Int Coll Surg. 1950;13:566-568.
16 Nathan PW, Sears TA, Smith MC. Effects of phenol solutions on the nerve roots of the cat: an electrophysiological and histological study. J Neurol Sci. 1965;2:7-29.
17 de Leon-Casasola OA. Drugs commonly used for nerve blocking: neurolytic agents. In: Raj PP, editor. Practical management of pain. St. Louis: Mosby; 2000:575-578.
18 Gregg RV, Costantini CH, Ford DJ, et al. Electrophysiologic investigation of alcohol as a neurolytic agent. Anesthesiology. 1985;63(suppl A):A250.
19 Smith MC. Histological findings following intrathecal injections of phenol solutions for relief of pain. Br J Anaesth. 1964;36:387-406.
20 Bell SN, Cole R, Roberts-Thomson IC. Coeliac plexus block for control of pain in chronic pancreatitis. Br Med J. 1980;281:1604.
21 Waldman SD. Celiac plexus block. In: Weiner RS, editor. Innovations in pain management. Orlando: PMD Press; 1990:10-15.
22 Day M. Sympathetic blocks: the evidence. Pain Pract. 2008;8:98-109.
23 Romanelli DF, Beckmann CF, Heiss FW. Celiac plexus block: efficacy and safety of the anterior approach. AJR Am J Roentgenol. 1993;160:497-500.
24 Buy JN, Moss AA, Singler RC. CT guided celiac plexus and splanchnic nerve neurolysis. J Comput Assist Tomogr. 1982;6:315-319.
25 Ischia S, Ischia A, Polati E, et al. Three posterior percutaneous celiac plexus block techniques. A prospective, randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 1992;76:534-540.
26 Matson J, Ghia J, Levy J. A case report of a potentially fatal complication associated with Ischia’s transaortic method of celiac plexus block. Reg Anesth Pain Med. 1985;10:193.
27 Sett SS, Taylor DC. Aortic pseudoaneurysm secondary to celiac plexus block. Ann Vasc Surg. 1991;5:88-91.
28 Kaplan R, Schiff-Keren B, Alt E. Aortic dissection as a complication of celiac plexus block. Anesthesiology. 1995;83:632-635.
29 Davies DD. Incidence of major complications of neurolytic coeliac plexus block. J R Soc Med. 1993;86:264-266.
30 Brown DL, Rorie DK. Altered reactivity of isolated segmental lumbar arteries of dogs following exposure to ethanol and phenol. Pain. 1994;56:139-143.
31 Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092-1099.
32 De Cicco M, Matovic M, Bortolussi R, et al. Celiac plexus block: injectate spread and pain relief in patients with regional anatomic distortions. Anesthesiology. 2001;94:561-565.
33 Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain. Pain. 1993;52:187-192.
34 Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80:290-295.
35 Yeager MP, Colacchio TA, Yu CT, et al. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology. 1995;83:500-508.
36 Mitchell G. The Innervation of the kidney, ureter, testicle and epididymis. J Anat. 1935;70:10-32.
37 Aveline C, Gautier JF, Vautier P, et al. Postoperative analgesia and early rehabilitation after total knee replacement: a comparison of continuous low-dose intravenous ketamine versus nefopam. Eur J Pain. 2009;13:613-619.
38 Middleton W, Chan V. Lumbar sympathetic block: a review of complications. Tech Reg Anesthes Pain Manage. 1998;2:137-146.
39 Reid W, Watt JK, Gray TG. Phenol injection of the sympathetic chain. Br J Surg. 1970;57:45-50.
40 Bryce-Smith R. Injection of the Lumbar Sympathetic Chain. Anaesthesia. 1951;6(3):150-153.
41 Gay GR, Evans JA. Total spinal anesthesia following lumbar paravertebral block: a potentially lethal complication. Anesthesth Analg. 1971;50:344.
42 Bradsher JTJr. Complications following paravertebral lumbar sympathetic block with Nupercaine in oil; report of a case. N Engl J Med. 1949;240:291-293.
43 Cousins MJ, Reeve TS, Glynn CJ, et al. Neurolytic lumbar sympathetic blockade: duration of denervation and relief of rest pain. Anaesth Intensive Care. 1979;7:121-135.
44 Raj P. Sympathetic nerve blocks. In: Raj P, editor. Practical management of pain. St. Louis: Mosby; 1992:792-812.
45 Boas R, Hatangdi V, Richards E. Lumbar sympathectomy: a percutaneous chemical technique. Adv Pain Res Ther. 1976;1:685-689.
46 Smith RC, Davidson NM, Ruckley CV. Hazard of chemical sympathectomy. Br Med J. 1978;1:552-553.
47 Parris WC, Kirshner HS. Motor paralysis of the lower extremities following lumbar sympathetic block. Anesthesiology. 1993;78:981-983.
48 Echenique Elizondo M, Gurutz Linazasoro C. [Reversible partial paraplegia after sympathetic lumbar block]. Neurologia. 1995;10:101-103.
49 Wood KM. The use of phenol as a neurolytic agent: a review. Pain. 1978;5:205-229.
50 Brown EM, Kunjappan V. Single-needle lateral approach for lumbar sympathetic block. Anesth Analg. 1975;54:725-729.
51 Wheatley JK, Motamedi F, Hammonds WD. Page kidney resulting from massive subcapsular hematoma. Complication of lumbar sympathetic nerve block. Urology. 1984;24:361-363.
52 Weyland A, Weyland W, Carduck H, et al. Optimization of the image intensifier-assisted technique of lumbar sympathetic block. Computed tomographic simulation of a paravertebral puncture access. Der Anaesthesist. 1993;42:710.
53 Benzon HT. Convulsions secondary to intravascular phenol: a hazard of celiac plexus block. Anesth Analg. 1979;58:150-151.
54 Egbert LD. Horner’s syndrome; complication of lumbar sympathetic block. Anesthesiology. 1955;16:811-812.
55 Stanton-Hicks M. Lumbar sympathetic nerve block and neurolysis. In Interventional pain management, ed 2, Philadelphia: WB Saunders; 2001:485-492.
56 Gee W, Ansell J, Bonica J. Pelvic and perineal pain of urologic origin. In: Bonica JJ, editor. The management of pain. Philadelphia: Lea & Febiger; 1990:1368-1394.
57 de Leon-Casasola OA, Kent E, Lema MJ. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Pain. 1993;54:145-151.
58 Plancarte R, Amescua C, Patt RB, et al. Superior hypogastric plexus block for pelvic cancer pain. Anesthesiology. 1990;73:236-239.
59 Plancarte R, de Leon-Casasola OA, El-Helaly M, et al. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Reg Anesth. 1997;22:562-568.
60 Plancarte R, Amescua C, Patt R, et al. Presacral blockade of the ganglion of Walther (ganglion impar). Anesthesiology. 1990;73(suppl A):A751.
61 Patijn J, Janssen M, Hayek S, et al. Coccygodynia. Pain Pract. 2010;10(6):554-559. doi: 10.1111/j.1533-2500.2010.00404.x. Epub 2010