Chapter 66 Neovascular (Exudative or “Wet”) Age-Related Macular Degeneration
Epidemiology
Age-related macular degeneration (AMD) is the major cause of severe visual loss in older adults1 if left untreated.2 Most AMD patients have macular drusen or retinal pigment epithelial abnormalities or both.3 Approximately 10% of AMD patients manifest the neovascular form of the disease.4 Neovascular AMD includes choroidal neovascularization (CNV) and associated manifestations such as retinal pigment epithelial detachment (PED), retinal pigment epithelial tears, fibrovascular disciform scarring, and vitreous hemorrhage.3 In the absence of antivascular endothelial growth factor (anti-VEGF) therapy, the vast majority of people with severe vision loss (20/200 or worse in either eye) from AMD have the neovascular form.4
Risk factors
The prevalence of AMD-associated vision loss in at least one eye increases with age. For example, AMD was the leading cause of blindness in white (prevalence 2.7 per 1000; 95% CI 1.2–5.4) but not black subjects randomly selected to participate in the Baltimore Eye Survey. In this study, AMD resulting in blindness affected 3% of all white subjects 80 years of age or older.5
AMD may be a multifactorial syndrome with different causative factors damaging the macula and resulting in common clinical manifestations that are recognized clinically as AMD. Risk factors implicated in clinical and laboratory studies include drusen, visible (but not ultraviolet) injury, micronutrient deficiency as measured in blood serum levels or by dietary history, cigarette smoking, family history (genetic predisposition6), and cardiovascular risk factors (including systemic hypertension).7,8 More detailed information regarding the epidemiology of AMD is reviewed in Chapter 63 (Epidemiology and risk factors for age-related macular degeneration).
Clinical (including biomicroscopic) presentation
Overview
Blurred vision and distortion, especially distorted near vision, are the symptoms most patients with CNV notice first.3,9 Patients also may complain of decreased vision, micropsia, metamorphopsia, or a scotoma; however, many times they volunteer no symptoms or report only vague visual complaints.8 Symptoms generally arise from subretinal fluid, intraretinal fluid, blood, or destruction of photoreceptors and the retinal pigment epithelium (RPE) by fibrous or fibrovascular tissue.10–13 In some cases, areas of distortion or scotoma can be mapped out on an Amsler grid. Visual acuity, although frequently decreased, may not always be affected. Functional vision generally declines in accordance with Snellen visual acuity. Thus patients with poor Snellen acuity generally report decreased ability to perform functional tasks (e.g., face recognition, telling time) with the affected eye.14
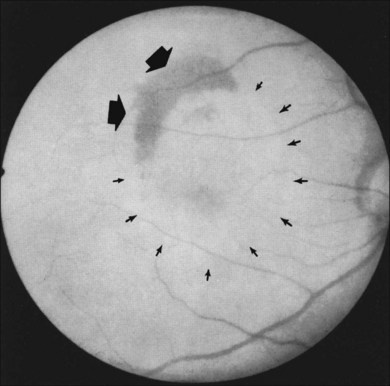
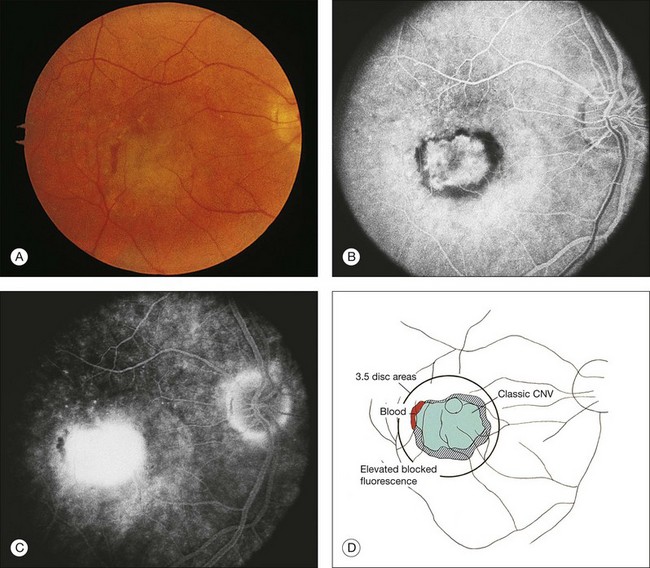
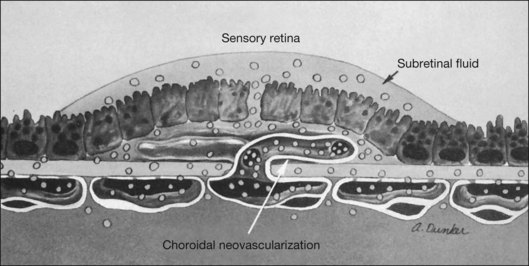
In some patients with AMD, CNV may appear as a gray–green elevation of tissue deep to the retina with overlying detachment of the neurosensory retina (Fig. 66.1). The gray–green color may arise from hyperplastic RPE in response to the CNV,15 as has typically been seen in patients, usually younger individuals, with ocular histoplasmosis syndrome (OHS), pathologic myopia, and other conditions complicated by CNV. This gray–green appearance is not always present in older individuals with AMD. Often, the presence of blood or lipid or a sensory retinal detachment in an elderly patient with vision loss indicates the presence of CNV. The CNV capillary network may become more apparent when the overlying RPE has atrophied. Occasionally, a shallow neurosensory detachment may be the only presenting sign of underlying CNV. Elevated RPE, also termed a pigment epithelial detachment (PED), even without overlying subretinal fluid, may also suggest the presence of CNV to be identified subsequently by a fluorescein angiogram. RPE folds beneath a shallow RPE elevation usually indicate the presence of CNV.16 These subtle clinical findings can easily be missed without careful stereoscopic slit-lamp biomicroscopic examination, facilitated with a contact lens.
Retinal pigment epithelial detachments
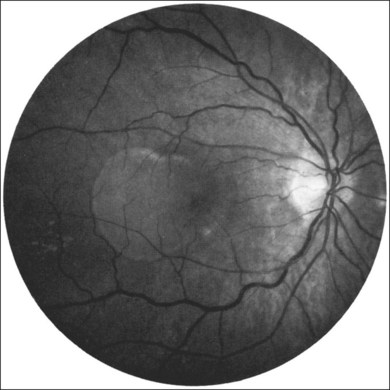
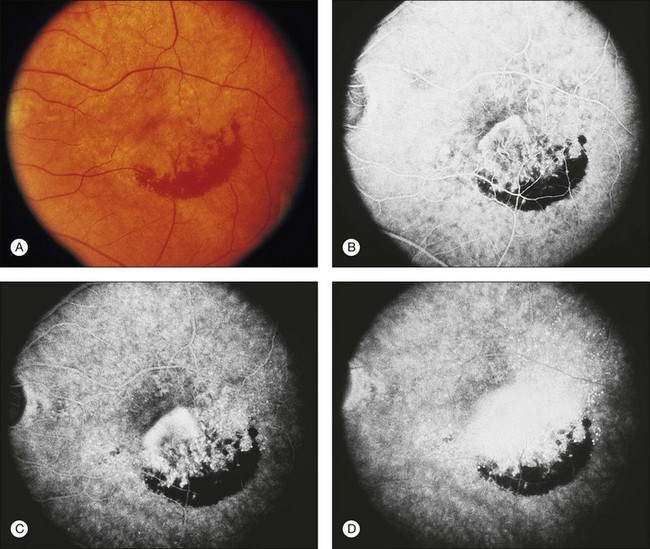
Retinal PEDs appear clinically as sharply demarcated, dome-shaped elevations of the RPE (Fig. 66.2). They usually transilluminate if they are filled with serous fluid only. Often, there is accompanying RPE atrophy and “pigment figure” formation. Pigment figures, a reticulated pattern of increased pigmentation extending radially over the PED, indicate chronicity of disease and probably have no prognostic significance. Although an overlying sensory retinal detachment may be a clue to the presence of CNV beneath a PED,17 sometimes a shallow neurosensory detachment may occur as a result of breakdown of the physiologic RPE pump or from disruption of the tight junctions between adjacent RPE cells in the absence of CNV. Unlike a PED, the borders of a neurosensory detachment are not sharply demarcated. The presence of a PED may or may not be a feature of CNV. The fluorescein angiographic pattern (see subsequent discussion) can differentiate a drusenoid PED,18 which does not have CNV, from a fibrovascular PED, which is a form of occult CNV,19 as well as from a serous PED, which may or may not overlie an area with CNV.19 Several clinical signs suggest the presence of CNV underlying an area of PED identified biomicroscopically, including overlying sensory retinal detachment and lipid, blood, and chorioretinal folds radiating from the PED.3 Blood within or surrounding a PED implies the presence of CNV (Fig. 66.3). When confined to the sub-RPE space, the blood may appear as a discretely elevated, green or dark-red mound. The hemorrhage can dissect through the RPE into the subsensory retinal space or into the retina. Rarely, blood may pass through the retina into the vitreous cavity, causing extensive vitreous hemorrhage. The Submacular Surgery Trials (SST) Research Group suggested that this event was more likely in predominantly hemorrhagic lesions that were large (>12 disc areas) or associated with very poor visual acuity (worse than 20/1280 Snellen equivalent).20
Breakthrough vitreous hemorrhage
In most cases of neovascular AMD, the peripheral visual field remains unaffected. If bleeding breaks through the retina into the vitreous cavity, however, patients may complain of severe and sudden visual loss involving the peripheral visual field, as well as the central field. This may be accompanied by pain believed to result from stretching of the nerve fibers within the choroid.21
Massive subretinal hemorrhage
Massive subretinal hemorrhage is an unusual complication of neovascular AMD. If – extremely rarely – total hemorrhagic retinal detachment occurs, secondary angle closure glaucoma may develop. These patients may report sudden visual loss followed by pain.22 Anticoagulation therapy may contribute to massive subretinal hemorrhage. In one report,23 19% of AMD patients with massive subretinal hemorrhage were taking sodium warfarin or aspirin. Although sodium warfarin therapy may have contributed to the massive subretinal hemorrhage, the antiplatelet therapy was likely a chance association because several Macular Photocoagulation Study (MPS) reports did not observe any increased risk of hemorrhage associated with the use of aspirin.24–26 Furthermore, comparing baseline characteristics in study participants with predominantly choroidal neovascular lesions in the SST Group N Trial27 with participants with predominantly hemorrhagic lesions,20 no difference in use of aspirin was detected. Recent epidemiology studies found an association of aspirin use with neovascular AMD, but this finding does not necessarily confirm or refute an association of aspirin with the development of predominantly hemorrhagic lesions or massive subretinal hemorrhages.28
Retinal pigment epithelial tears
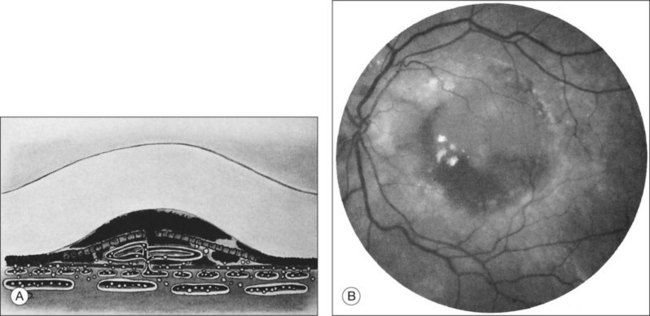
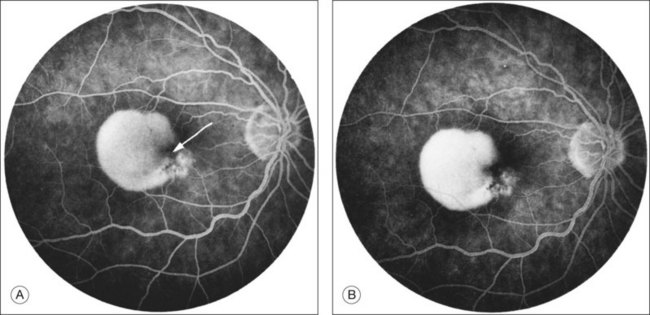
RPE dehiscence or tears have been described as a complication associated with CNV, often in an eye with a serous or fibrovascular PED, and secondary to or unassociated with laser photocoagulation.29–33 One report34 suggested that CNV underlying a detached RPE can contribute to RPE tear formation. Tears occur at the junction of attached and detached RPE, perhaps when the PED can no longer resist the stretching forces from the fluid in the sub-RPE space emanating from the underlying occult CNV (Fig. 66.4) or from the contractile forces of the underlying fibrovascular tissue that may be intimately associated or entwined with the overlying RPE. When the RPE tears, the free edge of the RPE retracts and rolls toward the mound of fibrovascular tissue. Acutely, a serous detachment of the sensory retina may be caused by the leaking of fluid from the exposed choriocapillaris.18 This is rarely seen after a few days following the tear.
Disciform scars
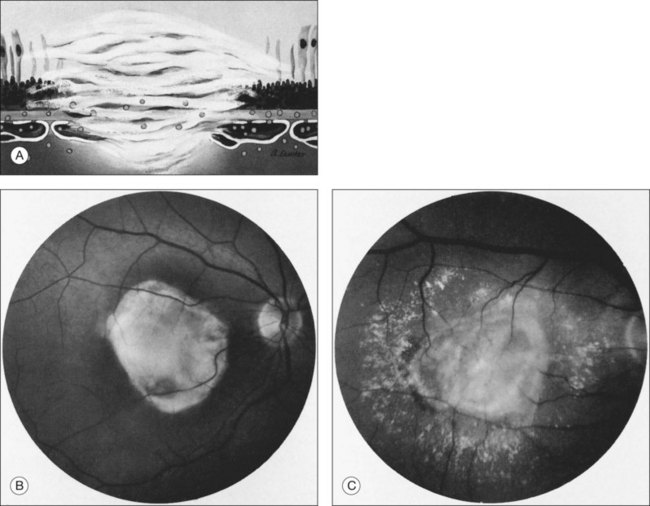
Histologically, CNV usually is accompanied by fibrous tissue, even when no fibrous tissue is readily apparent on initial presentation to an ophthalmologist.10,35,36 This fibrous tissue may be accompanied by CNV (fibrovascular tissue) or not (fibroglial tissue).10 The fibrous tissue complex may be beneath the RPE (usually proliferating within the inner aspect of an abnormally thickened Bruch’s membrane), and has been termed type I, or between the RPE and the photoreceptors, termed type II.34 While some people speculate that these types differentiate classic CNV from occult CNV,37,38 there is little evidence to support that these histologic types are always differentiated by histopathologic correlation.39 Often, over time, the plane of the RPE is destroyed by the fibrovascular or fibroglial tissue, so the location of the CNV with respect to the RPE can no longer be identified readily. When the fibrous tissue becomes apparent clinically, the CNV and fibrous tissue complex may be termed a disciform scar.
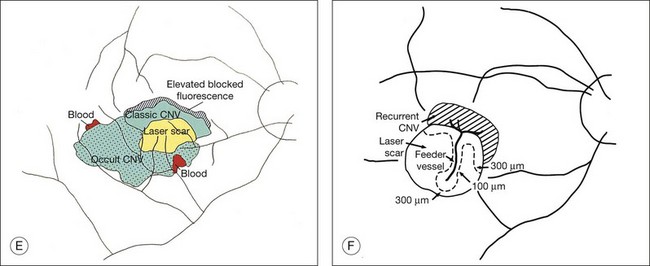
Clinically, disciform lesions may vary in color, although typically they appear white to yellow. Hyperpigmented areas may be present depending on the degree of RPE hyperplasia within the scar tissue. Disciform fibrovascular scars may continue to grow, with neovascularization recurring along the edge, invading previously unaffected areas (Fig. 66.5). Varying degrees of subretinal hemorrhage and lipid may overlie or surround the scar. Occasionally, fibrovascular scars may precipitate massive transudation of fluid, mimicking a retinal detachment. The scars may be accompanied by massive lipid, as might be seen in retinal telangiectasis from Coats disease, and hence are sometimes called a “senile Coats response” in AMD. Disciform scars occasionally masquerade as choroidal tumors when much pigment is seen.34 Not infrequently, anastomoses are observed between the retina and the fibrovascular tissue.13 As a rule, most fibrovascular scars involve the fovea and cause severe visual loss. However, surviving islands of intact photoreceptor cells noted histologically may explain the better visual performance than would be predicted from the morphologic appearance alone in some scars. Reading vision, rarely better than 20/200, becomes severely compromised in most cases with extensive scars.
Fluorescein angiographic features
Overview
Whenever one suspects CNV for which treatment might be indicated, one should consider obtaining stereoscopic fluorescein angiography promptly (Fig. 66.6), even in an era of optical coherence tomography (OCT). The treating ophthalmologist is about to embark on a recommendation for treatment involving drugs which carry risks, potentially large expenses, and requiring many years of follow-up. Although the clinical picture may be “obviously” CNV, other lesions masquerading for CNV can exist (see below) having a fluorescein angiogram at the time of diagnosis reduces the possibility that an error in diagnosis will be made. In addition, fluorescein angiography frequently allows one to determine the pattern (classic or occult), boundaries (well defined or poorly defined), composition (e.g., predominantly CNV, predominantly classic CNV, predominantly CNV with a minimally classic composition, predominantly CNV with an occult with no classic composition, predominantly hemorrhagic), and location of the neovascular lesions with respect to the geometric center of the foveal avascular zone (FAZ). Although many physicians no longer refer to CNV composition, entry criteria for many and even most of the treatment trials cited later in this chapter relied in part on lesion composition. If one chooses to treat only patients who would have been eligible for, e.g., the MARINA trial, baseline angiography is necessary. High-quality stereoscopic fluorescein angiograms, together with meticulous slit-lamp biomicroscopic examination (ideally with a contact lens examination using topical anesthesia and hard contact lens wetting solution to avoid degradation of any subsequent image acquisition that might occur if an ophthalmic demulcent is used), facilitate detecting obvious and subtle features of CNV on angiography.14,19,40 It should be noted that the descriptive terms below refer to patterns of fluorescence on fluorescein angiography that have been shown to be reliable and reproducible in multicenter clinical trials,19,41,42 and in practice, and are not related to terms based on other imaging such as OCT, indocyanine green angiography, histopathology, or immunohistochemistry.
Classic choroidal neovascularization
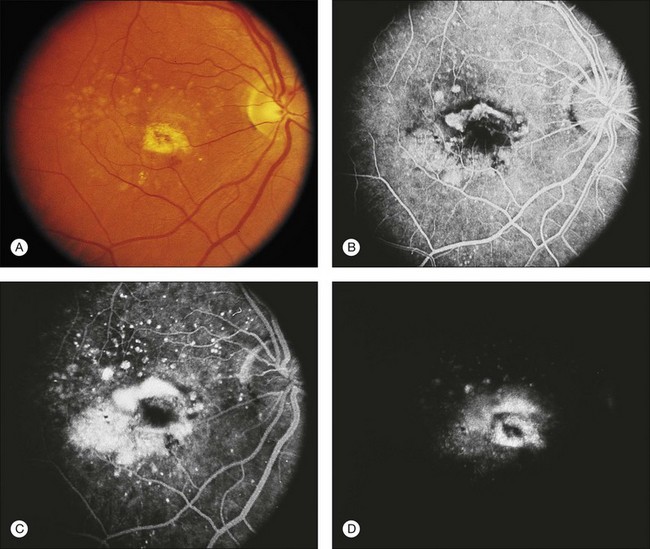
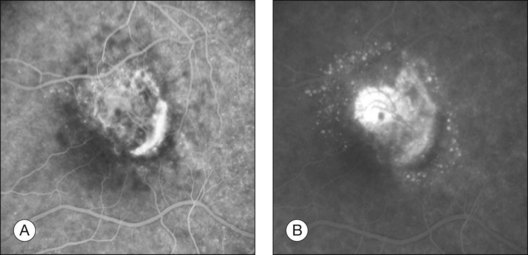
The fluorescein angiographic appearance of classic CNV consists of a discrete, well-demarcated focal area of hyperfluorescence that can be discerned in the early phases of the angiogram, sometimes before dye has completely filled the retinal vessels during choroidal filling.19,41,42 Although fluorescein can occasionally be observed within the actual capillary network of CNV in the early phase of the angiogram (Fig. 66.7A), the ability to visualize the appearance of actual new vessels is not needed to diagnose classic CNV and is not a specific feature of classic versus occult CNV.19,41–43 Since both classic and occult patterns of CNV contain new vessels histologically, early-phase angiography may be able to demonstrate these vessels in either pattern. As the angiogram is evaluated within the area of classic CNV, hyperfluorescence increases in intensity and extends beyond the boundaries of the hyperfluorescent area identified in earlier phases of the angiogram through mid- and late-phase frames. Fluorescein may also pool in subsensory retinal fluid overlying the classic CNV (Fig. 66.7B), best seen when visualizing early- and late-phase frames of classic CNV on stereoscopic images. This presentation of classic CNV is in contrast to the appearance of an area of RPE atrophy on fluorescein angiography. RPE atrophy, like classic CNV, is hyperfluorescent during the early phase of the angiogram (Fig. 66.8A). The increased fluorescence through the atrophic patch results from increased transmission of fluorescein through an overlying RPE with a reduced amount of pigment that normally obscures the choroidal blush (sometimes termed a window, or transmission, defect). Unlike the increase in extent and intensity of hyperfluorescence due to leakage from the fluorescence of classic CNV, RPE atrophy does not show leakage of fluorescein at its boundaries through the mid- and late-phase frames. The fluorescence fades after several minutes (Fig. 66.8B), without leakage of fluorescein beyond the boundaries of hyperfluorescence defined in the early stages. Two other lesions in AMD that may show an area of discrete hyperfluorescence in the early phase of the angiogram include a serous PED and a rip or tear of the RPE (angiographic features that differentiate these abnormalities from classic CNV are discussed later). Neither one of these latter abnormalities should show fluorescein leakage in later phases of the angiogram at the boundary of the hyperfluorescence noted in earlier phases.
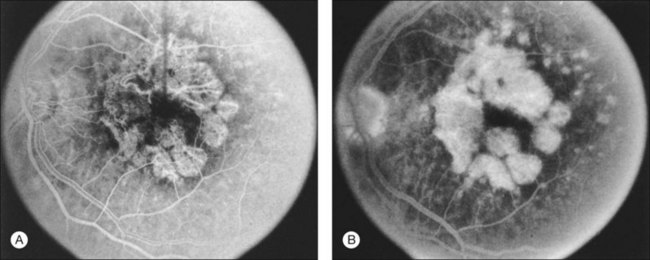
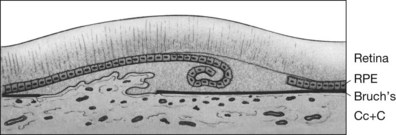
Fig. 66.8 (A) Transit phase of fluorescein angiogram, showing hyperfluorescence corresponding to atrophic zones of the retinal pigment epithelium (transmission, or window, defect) and easily visualized choroidal vessels (too large to be vessels of choroidal neovascularization). (B) Hypofluorescence does not increase in size and fades with the later phases of the angiogram. This is in contrast to the pattern seen in choroidal neovascularization (Fig. 66.5).
(Reproduced with permission from Elman MJ. Age-related macular degeneration. Int Ophthalmol Clin 1986;26:117–44.)
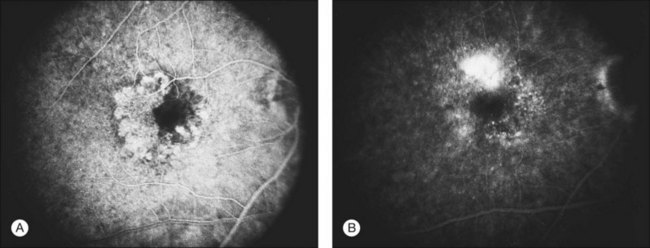
Occult choroidal neovascularization
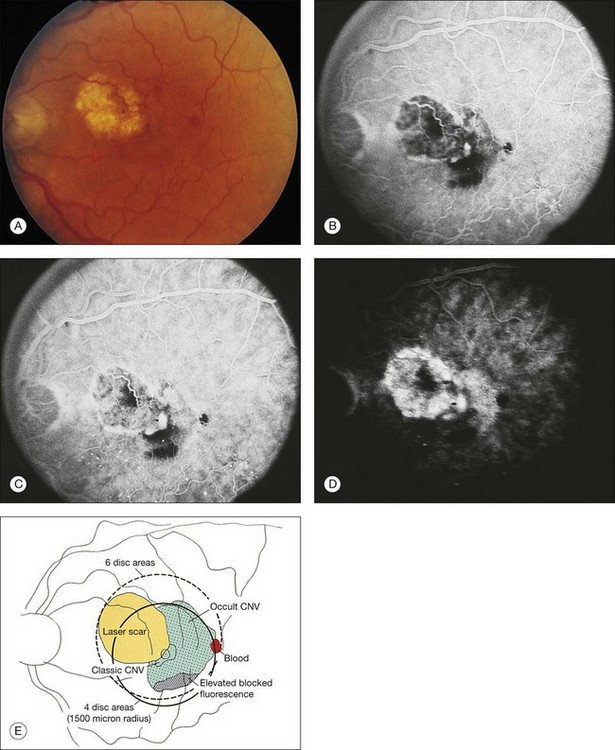
Occult CNV refers to two hyperfluorescent patterns on fluorescein angiography.19,41,42 The first pattern, termed a fibrovascular pigment epithelial detachment (FVPED), is best appreciated with stereoscopic views, usually at approximately 1–2 min after dye injection. It appears as an irregular elevation of the RPE, often stippled with hyperfluorescent dots (Fig. 66.9). The boundaries may or may not show leakage in the late-phase frames as fluorescein collects within the fibrous tissue or pools in the subretinal space overlying the FVPED. The exact boundaries of a FVPED can usually be determined most accurately only when fluorescence sharply outlines the elevated RPE. The amount of elevation depends on the quality of the stereoscopic photographs and the thickness of the fibrovascular tissue. Stereoscopic pairs of fluorescein angiogram frames can sometimes facilitate identification of the boundaries of the elevated RPE, although not always, as the elevation can slope gradually down to the normal level of the RPE. The second pattern, late leakage of an undetermined source (Fig. 66.10), refers to late choroidal-based leakage in which there is no clearly identifiable classic CNV or FVPED in the early or mid-phase of the angiogram to account for an area of leakage in the late phase. Often this pattern of occult CNV can appear as speckled hyperfluorescence with pooling of dye in the subretinal space overlying the speckles. Usually the boundaries of this type of CNV cannot be determined precisely, and a lesion with this component should not be considered for photocoagulation treatment if it contributes to poorly demarcated boundaries of the lesion.
Other terms relevant to interpreting fluorescein angiography of choroidal neovascularization
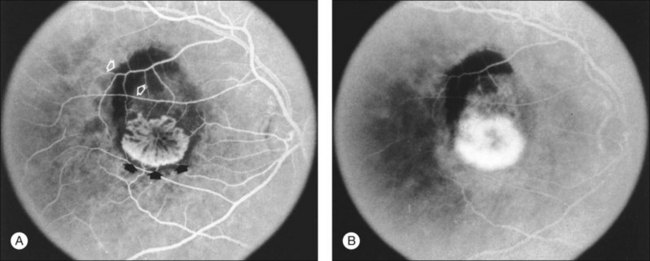
The terms lesion component versus lesion are important to differentiate in the discussion of fluorescein interpretation and treatment of CNV.19,41,42 Lesion component is classic or occult CNV or any of four angiographic features that could obscure the boundaries of classic or occult CNV. These four features include: (1) blood that is visible on color fundus photographs and thick enough to obscure the normal choroidal fluorescence; (2) hypofluorescence due to hyperplastic pigment or fibrous tissue, or blood not visible on color fundus photographs; (3) a serous detachment of the RPE (Fig. 66.11); and (4) scar from CNV which either stains or blocks fluorescence (depending on the extent of RPE within the scar). The first two of these four features block the angiographic view of the choroid, making it impossible to determine whether CNV is located in the area of this component. The bright, reasonably uniform, early hyperfluorescence associated with a serous detachment of the RPE (described later) may obscure hyperfluorescence from classic or occult CNV and therefore interfere with the ability to judge whether CNV extends under the area of the serous detachment. The term lesion, in contrast, refers to the entire complex of lesion components.
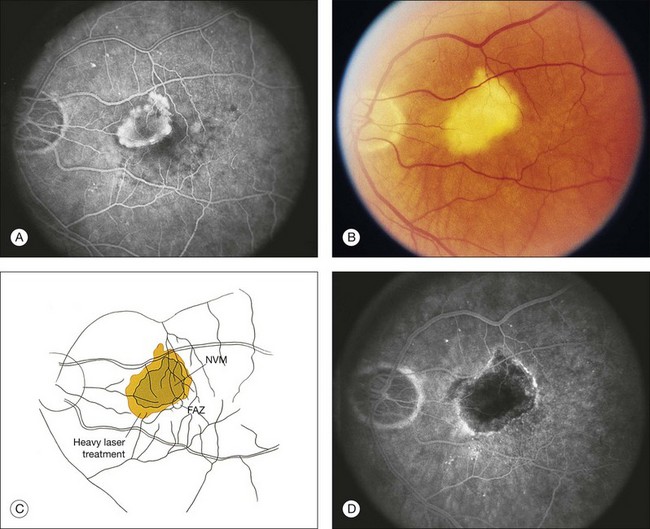
The terms well-defined (synonymous with well-demarcated) and poorly defined (synonymous with poorly demarcated or ill-defined) refer to a description of the boundaries of the entire lesion (not of individual lesion components). In a well-defined lesion, the entire boundary for 360 degrees is well demarcated (for example, Figs 66.9, 66.12, 66.13). If the entire boundary is not well demarcated for 360 degrees, then the lesion is poorly defined (for example, Fig. 61.9). Thus the terms well-defined and classic should not be used interchangeably, nor should poorly defined and occult. Well-defined and poorly defined describe lesion boundaries (for a lesion that may be composed of classic CNV, or occult CNV, or both). Classic and occult CNV refer to patterns of fluorescence. In addition, the term poorly defined should not be used to describe situations in which blood blocks the ability to see fluorescence from CNV,19,41,42 even though earlier publications had alluded to descriptions incorporating this possibility.44
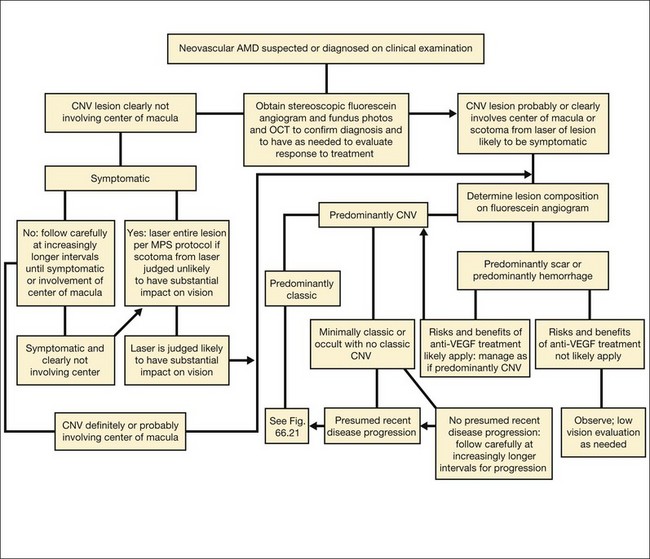
The term predominantly CNV indicates that at least 50% of the lesion is composed of either classic CNV or occult CNV, or both, while the term predominantly hemorrhagic indicates that at least 50% of the lesion is composed of hemorrhage.41,42 These terms are critical in the management of AMD (Fig. 66.14), since treatments for CNV with anti-VEGF therapy, or less frequently, with laser photocoagulation, photodynamic therapy (PDT), or surgery, have been tested only in lesions that are predominantly CNV or predominantly hemorrhagic. After determining whether a lesion’s composition is predominantly CNV, it should be determined whether the lesion is predominantly classic, rather than minimally classic or occult with no classic. If predominantly classic, then treatment could be considered with or without evidence of presumed recent disease progression (defined as evidence of blood associated with CNV, or definite visual acuity loss within 3 months, or definite growth of the lesion within 3 months). If minimally classic or occult with no classic, treatment has been shown to be beneficial compared with no treatment only with evidence of presumed recent disease progression, although a therapeutic trial of anti-VEGF therapy might be considered if visual acuity loss already had occurred and one believed that visual acuity improvement might occur with anti-VEGF therapy because of the presence of intraretinal or subretinal fluid judged to be contributing to visual acuity loss and judged likely to resolve with visual acuity improvement following initiation of anti-VEGF therapy.
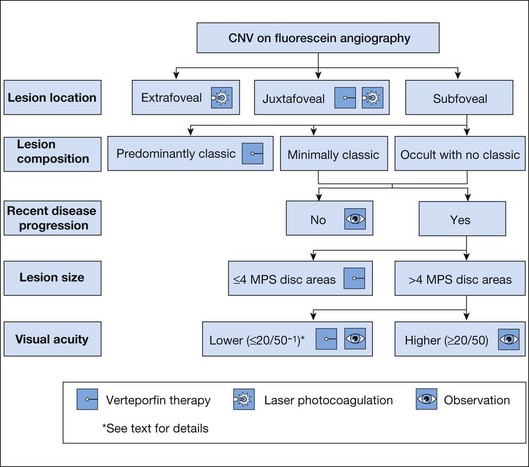
Fig. 66.14 Algorithm for verteporfin therapy or laser photocoagulation or observation for symptomatic patients with age-related macular degeneration, pathologic myopia, or other causes of choroidal neovascularization (CNV) in which the natural course is likely worse without treatment. MPS, Macular Photocoagulation Study, and now largely replaced by anti-VEGF therapy as outlined in Fig. 66.21.
Retinal pigment epithelium detachments in age-related macular degeneration
Various changes in an eye with AMD may result in elevation or detachment of the RPE, as seen on stereoscopic biomicroscopic or angiographic evaluation. The term RPE detachment or retinal pigment epithelial detachment (retinal PED) secondary to AMD in the ophthalmic literature remains confusing because various RPE detachments may have quite different compositions, fluorescein angiographic appearances, prognoses, and management. Fortunately, these various RPE detachments can usually be differentiated on the basis of fluorescein angiographic patterns of fluorescence. The patterns include the following: (1) fibrovascular PEDs,19 which are a subset of occult CNV (see Figs 66.9 and 66.10); (2) serous detachments of the RPE45 (see Fig. 66.11); (3) hemorrhagic detachments of the RPE, in which blood from a choroidal neovascular lesion is noted beneath or exterior to the RPE (see Fig. 66.3); and (4) drusenoid RPE detachments,9 in which large areas of confluent, soft drusen are noted. It is potentially difficult to differentiate between fibrovascular PEDs and serous PEDs. Using descriptions from the MPS Group, fibrovascular PEDs (as a subset of occult CNV) have been distinguished from a typical serous detachment of the RPE, in that the former do not have uniform, bright hyperfluorescence in the early phase. Instead, they show a stippled fluorescence along the surface of the RPE by the middle phase of the angiogram and may show pooling of dye in the overlying subsensory retinal space in the late phase (see Fig. 66.9). Serous PEDs show uniform, bright hyperfluorescence in the early phase, with a smooth contour to the RPE by the middle phase, and little, if any, leakage at the borders of the PED by the late phase (see Fig. 66.11). The fluorescent pattern of a serous PED obscures the ability to determine whether classic or occult CNV exists within or beneath the area of the serous PED. In contrast, a fibrovascular PED is an area of occult CNV.
A hemorrhagic detachment of the RPE will block choroidal fluorescence because of the mound-like collection of blood beneath the RPE (see Fig. 66.3). Occasionally a hemorrhagic detachment of the RPE may be mistaken for a choroidal melanoma, but usually hemorrhagic detachments of the RPE do not demonstrate low internal reflectivity, as is seen characteristically in choroidal melanomas.
One other feature of AMD that appears as an elevated or detached RPE is a drusenoid RPE detachment,18 which represents extensive areas of large, confluent drusen. Drusenoid RPE detachments can be distinguished from serous detachments of the RPE in that drusenoid RPE detachments fluoresce faintly during the transit and do not progress to bright hyperfluorescence in the late phase of the angiogram. In contrast, serous detachments of the RPE fluoresce brightly in the early-transit phase and remain brightly hyperfluorescent in the late phase. In addition, serous detachments will usually have a smoother, sharper boundary compared with drusenoid RPE detachments. Drusenoid RPE detachments can be distinguished from fibrovascular PEDs in occult CNV by noting that fibrovascular PEDs show areas of stippled hyperfluorescence with persistence of staining or leakage within a sensory retinal detachment overlying the area in the late phase of the angiogram. RPE detachments due to large, soft, confluent drusen are usually smaller, shallower, and more irregular in outline than are fibrovascular PEDs. In addition, the drusenoid RPE detachments often have reticulated pigment clumping overlying the large, soft, confluent drusen, a scalloped border, and have less fluorescence in late-phase frames as compared with earlier-phase frames.
Other angiographic features
Speckled hyperfluorescence
Speckled fluorescence (Fig. 66.15A) in the absence of fluorescein leakage consists of several punctuate spots of hyperfluorescence, usually within 500 µm of each other that are apparent between 2 and 5 minutes after fluorescein injection and cannot be detected in early phase frames in contrast to drusen.46 The fluorescence of these spots persisted or increased in intensity by the late phase of the angiogram (Fig. 66.15B), in contrast to drusen or atrophy of the retinal pigment epithelium which do not remain brightly hyperfluorescent by the late phase of the angiogram. Typically, clusters of speckles would appear at the edge of the CNV lesion, rather than the typical distribution of drusen throughout the macular area. This angiographic feature was previously reported to be associated with recurrent CNV.46 If speckled hyperfluorescence is noted in the presence of fluorescein leakage in the late phase frames in the absence of elevation of the RPE, then the pattern would be considered to meet the definition of late leakage of an undetermined source, a type of occult CNV.
Fading choroidal neovascularization
CNV may occasionally be recognized in the early- or middle-transit phase of the angiogram with fading in the late phase, so little fluorescein staining or leakage can be discerned in the late phase within an area that was presumed to harbor CNV on the basis of its early-phase features19 (Fig. 66.16). A vascular pattern of the fibrovascular tissue can occasionally be discerned in early-phase frames.43 Most ophthalmologists are reluctant to treat areas of CNV that fade. These areas are usually not associated with overlying subretinal fluid (in conjunction with the lack of fluorescein leakage on angiography), so one can only presume that this region may proceed to disciform scarring. Perhaps these areas represent CNV histologically, but without evidence of subretinal fluid or late leakage, one cannot be certain that this pattern definitively represents CNV, and laser treatment of this area may damage the retina unnecessarily.
Feeder vessels
These vessels may be identified as choroidal vessels apparent during the transit phase of the angiogram and connected unequivocally to leaking choroidal capillaries19 (see Fig. 66.9). Although feeder vessels have been described as extending from a laser-treated area to recurrent CNV across the perimeter of the laser-treated areas, feeder vessels may also be seen in untreated eyes (see Fig. 66.10). In the latter situation, peripheral, untreated areas of CNV may be connected by feeder vessels to more central areas of CNV that are evolving toward natural scar formation.
Retinal lesion anastomosis (“retinal angiomatous proliferans” or “chorioretinal anastomosis”)
Retinal vessels can anastomose with CNV from AMD.47 The vessels can be seen dividing at right angles from the surface of the retina to the neovascular lesion (as may be seen with idiopathic parafoveal telangiectasis).
Loculated fluid
This fluid consists of a well-demarcated area of hyperfluorescence that appears to represent pooling of fluorescein in a compartmentalized space anterior to the choroidal neovascular leakage, usually seen in the late phase of the angiogram.48 Although the loculated fluid may conform to a pattern of typical cystoid macular edema, it can also pool within an area deep to the sensory retina in a shape that does not bear any resemblance to cystoid macular edema.
Retinal pigment epithelial tears
RPE tears have a characteristic fluorescein angiographic appearance.49 The denuded RPE displays marked early hyperfluorescence. Later, staining of the choroid and sclera may be observed, but fluorescein generally does not leak from the denuded area. The folded pigment epithelial mound blocks fluorescence; However, this area may leak later during the angiogram, presumably from underlying CNV. Tears may occur following development of a serous PED in the absence of CNV. In addition, tears may occur following development of CNV, sometimes accompanied by large areas of hemorrhage. Tears in any of these situations may occur without any antecedent treatment or may occur soon after laser photocoagulation or PDT.
Disciform scars
Fibrovascular scars frequently hyperfluoresce from both fluorescein leakage and staining. One may also notice chorioretinal anastomoses or, more precisely, retinal anastomoses into fibrovascular tissue (also called retinal angiomatous proliferans47 or chorioretinal anastomoses or retinal lesion anastomoses). Some descriptions of these vessels have suggested that they can develop prior to the development of CNV (as is seen in the subretinal neovascularization that can develop in an individual with idiopathic parafoveal telangiectasis). Theoretically, these descriptions seem plausible if sufficient VEGF production, which typically would be involved in the development of CNV, first led to the development of proliferation of retinal capillaries. However, there is no evidence that these vessels develop in the absence of CNV from AMD on histopathology. Furthermore, most cases show evidence of CNV in the presence of these anastomoses of retinal vessels with the neovascular lesion, and those cases that do not show obvious CNV often have difficult angiograms to interpret to state with certainty that CNV is not present. The area of anastomosis, when noted before development of extensive visible scar tissue, often shows a bright area of fluorescence in the early phase, occasionally accompanied by a small area of intraretinal hemorrhage. While some reports have suggested that the natural history of lesions with these anastomoses is worse than the natural history without these anastomoses, there is no strong evidence to support this impression at this time.
Pathogenesis
Choroidal neovascularization
Histopathology
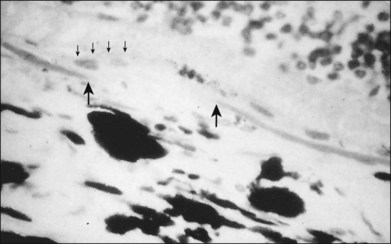
CNV appears as a neovascular sprout growing under or through the RPE through breaks in Bruch’s membrane13 (Figs 66.17, 66.18). Usually this occurs in association with evidence of fibroblasts, myofibroblasts, lymphocytes, and macrophages.50 Various growth factors are suspected to be involved in the development of this CNV, such as vascular endothelial growth factor (VEGF).51 However, while drugs designed to interfere with VEGF have been shown to reduce the risk of vision loss,52–57 vision loss still occurred in many treated cases, and it is not yet clear how much these drugs produce an antiangiogenic effect or an antipermeability effect. Following penetration of the inner aspect of Bruch’s membrane, the new vessels proliferate laterally between the RPE and Bruch’s membrane.13 As these neovascular twigs mature, they develop a more organized vascular system stemming from a trunk of feeder vessels off the choroid, as well as proliferation of fibrous tissue. The endothelial cells in the arborizing neovascular tufts lack the barrier function of more mature endothelial cells. Hence these new vessels can leak fluid (and fluorescein) in the neurosensory, subsensory, and RPE layers of the retina. Proteins and lipids may accompany this process and precipitate in any layer of the retina. In addition, the fragile vessels are prone to hemorrhage. Occasionally, blood may extend through all the layers of the retina, breaking through into the vitreous cavity. Ultimately, a fibrovascular scar results, usually causing disruption and death of the overlying sensory retinal tissue accompanied by severe visual loss.
Associated factors
The stimulus for vascular ingrowth of choroidal vessels remains unknown, but several theories have been advanced. Soft drusen have been associated histopathologically with CNV. The soft drusen represent focal accumulation of membranous debris (ultrastructurally termed basal linear deposits) accumulated as a diffuse, shallow layer between the RPE basement membrane and the inner aspect of Bruch’s membrane.11–13,58–63 This material should not be confused with basal laminar deposit, which is material that collects between the RPE plasma membrane and the basement membrane of the RPE and accumulates with age but may not lead to vision loss from CNV or geographic atrophy and therefore may not be part of AMD.58,59 The term should also not be confused with basal laminar drusen, also called cuticular drusen. (Basal laminar, or cuticular, drusen usually present in midlife with a myriad of small, translucent drusen that appear like a starry sky on a fluorescein angiogram and may be associated with vitelliform macular detachments: see below.3,64) Some investigators believe that soft drusen represent extracellular matrix material produced by the RPE.58,65 Deposition of this material may suggest a widespread RPE abnormality.20 The diffusely thickened area is weakly attached, allowing the development of localized detachments seen clinically as soft drusen. These localized detachments can coalesce into larger drusenoid or serous RPE detachments.12 Alternatively, drusen may act as an indirect angiogenic factor by attracting macrophages from the choroid.50
Breaks in Bruch’s membrane permit ingrowth of new vessels from the choriocapillaris. However, these breaks can also be seen without ingrowth of choroidal new vessels. Some investigators have suggested that endothelial cells of growing CNV may actually produce the break in Bruch’s membrane rather than grow through pre-existing breaks in Bruch’s membrane.66 An inflammatory component seen in association with AMD may play a role in the development of CNV.67 Eyes with AMD show an increased prevalence of lymphocytes, fibroblasts, and macrophages within Bruch’s membrane as compared with control eyes without AMD. However, these findings are not specific to eyes with neovascular AMD.67,68 The presence of macrophages and lymphocytes near breaks in Bruch’s membrane suggests that leukocytes may be involved in the induction of CNV growth and the release of collagenases from endothelial cells. It is postulated that leukocytes may initially stimulate neovascular proliferation, promote the release of factors leading to breakdown of Bruch’s membrane, and even affect (with pericytes) the dilation of new vessels.69 Whether these inflammatory cells act as mediators of the degenerative changes seen in Bruch’s membrane or directly stimulate new vessel growth remains unknown. Finally, as mentioned above, other angiogenic factors, such as VEGF or a platelet-derived growth factor (PDGF),51,53,70 may contribute to the ingrowth of new vessels from the choroid through Bruch’s membrane into the sub-RPE space. Growth factors leading to neovascular formation may arise from an imbalance between stimulating and inhibiting chemical modulators. The RPE has been implicated as the source of these factors, but RPE cells may also act indirectly through the attraction of macrophages.71
Differential diagnosis
Vitreous hemorrhage
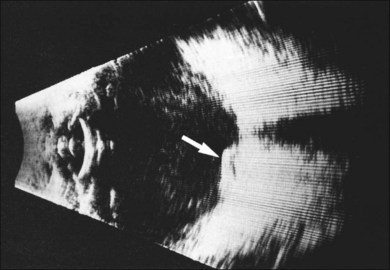
If a new patient has vitreous hemorrhage in one eye and signs of AMD in the other eye, other causes of vitreous hemorrhage must first be ruled out. Common causes of vitreous hemorrhage include retinal tear formation and retinal vascular diseases such as diabetic retinopathy or branch vein occlusion. Ultrasonography usually differentiates breakthrough vitreous hemorrhage secondary to neovascular AMD or retinal vascular causes from that caused by a tumor (Fig. 66.19).
Natural history
Well-defined extrafoveal and juxtafoveal choroidal neovascularization
Patients assigned to observation in the MPS trials provide natural-history information on cases that met criteria for these studies. When lesions have well-defined boundaries, the CNV lesion can be classified according to the location of the most posterior boundary of the lesion with respect to the center of the FAZ on the fluorescein angiogram. CNV lesions located more than 200 µm from the FAZ center are termed extrafoveal; those between 1 and 199 µm from the center are juxtafoveal; CNV lesions extending under the center of the FAZ are termed subfoveal. In contrast to other pathologic conditions predisposing to CNV (e.g., OHS and pathologic myopia) in eyes with AMD, CNV presents more frequently under the FAZ center. In addition, subfoveal CNV from AMD tends to be larger on initial presentation than when seen in other conditions.72 In a retrospective review of fluorescein angiograms of AMD ordered at a community hospital over a 9.5-year period, only 8% (19/244) of the CNV lesions were extrafoveal.73 Other retrospective reports not derived solely from a retinal referral practice have suggested that most patients with CNV from AMD present to an ophthalmologist with subfoveal CNV with poorly demarcated boundaries.72,74 However, there is no information from population-based studies to describe precisely how many choroidal neovascular lesions are subfoveal versus not subfoveal, and for those that are not subfoveal, how many are well demarcated on laser photocoagulation. Furthermore, there are no studies to determine how many cases that are subfoveal are predominantly classic, and if not predominantly classic, how many have presumed recent disease progression after developing.
In the MPS, 62% of untreated eyes with well-defined, extrafoveal CNV (posterior boundary of lesion located at least 200 µm from the FAZ center) lost 6 or more lines of visual acuity by 3 years of follow-up.75 By 5 years, this percentage was similar at 64%.76 Furthermore, in nearly 75% of the untreated eyes, the CNV extended under the FAZ center.77 For juxtafoveal lesions, 49% of untreated eyes had lost 6 or more lines of visual acuity by 3 years of follow-up.26
Subfoveal choroidal neovascularization
For subfoveal lesions, the MPS reported that 48% of untreated eyes with well-defined subfoveal CNV that was predominantly CNV and included a component of classic CNV in which the entire lesion was no greater than 3.5 MPS disc areas lost 6 or more lines of vision from baseline by 48 months.24,78 Similar outcomes were noted for untreated eyes with recurrent subfoveal CNV with similar features as for new subfoveal lesions except the total size of the lesion plus any area of prior treatment was to be no greater than 6 MPS disc areas.25,78 A broader group of subfoveal lesions in the Treatment of Age-related macular degeneration with Photodynamic therapy (TAP) investigation described the natural history in subfoveal lesions that were predominantly CNV with evidence of classic CNV and a lesion size no greater than 9 disc areas in which 62% lost 3 or more lines at 2 years after entry, including 30% losing 6 or more lines of visual acuity.79 With increasing size of predominantly classic lesions on presentation, the average visual acuity is more likely to be worse by 2 years.80 Similar natural history outcomes were noted for untreated eyes with occult with no classic CNV with presumed recent disease progression,54 in which almost half of the eyes lost 3 or more lines at 2 years after entry, including 23% losing 6 or more lines of visual acuity.
A variety of studies have shown that cases of predominantly CNV with a composition that is occult with no classic CNV, or minimally classic CNV have a more heterogeneous outcome.44,80–85 Most of this information is from series in clinical trials with presumed recent disease progression. Some cases may remain stable for years without visual loss, whereas other cases may develop severe visual loss at a rate similar to the deterioration noted for cases of classic CNV only. Furthermore, increasing size of minimally classic or occult with no classic lesions is not associated with a worse natural history outcome.80
Up to 50% of the cases with no classic CNV may develop classic CNV within a year of presentation.44,81,82,86 Cases that develop some classic CNV may be more likely to have severe visual acuity loss.81,82,86 Results from several clinical trials suggest that the natural history of lesions with classic CNV but no occult CNV is worse than the natural history of lesions with classic and occult CNV or occult with no classic CNV.82,84,87 It is likely that the natural history of lesions with classic and occult CNV may lie somewhere between the natural course of lesions with classic CNV only and occult CNV only.82
Natural course of large subfoveal subretinal hemorrhage in age-related macular degeneration
Some eyes with subfoveal subretinal hemorrhage associated with AMD have poor outcomes.88–90 However, the visual acuity of other eyes does not deteriorate or may improve spontaneously.88,90 Such findings underscore the importance of evaluating the role of therapeutic interventions for these cases, such as surgery91,92 to remove subretinal hemorrhage and associated CNV, in randomized clinical trials.93 The SST Group B Trial of relatively large, predominantly hemorrhagic subfoveal lesions demonstrated that 41% of untreated eyes remained stable or improved, although 36% had severe visual acuity loss after enrollment into the trial. Furthermore, this trial suggested that 18% of large subfoveal subretinal hemorrhages will progress to a vitreous hemorrhage as blood dissects from the subretinal space through the retina and into the vitreous.27
Retinal pigment epithelial tears
Patients with RPE tears involving the foveal center may initially maintain good vision but usually develop severe visual loss. However, cases with RPE tears through the fovea and preservation of good visual acuity have been reported.94 Unfortunately, there is a substantial risk of AMD-related visual loss in the fellow eye. Schoeppner and associates95 reported a cumulative risk of visual loss in the fellow eye of patients who had an RPE tear in the eye as 37% at 1 year, 59% at 2 years, and 80% at 3 years of follow-up. Visual loss usually arose from development of a PED, RPE tear, or CNV.
Laser photocoagulation treatment
Laser treatment of well-defined choroidal neovascular lesions
While laser photocoagulation has been shown to be beneficial for well-defined lesions not involving the foveal center or very small lesions involving the foveal center with a component of classic CNV, failure to cover the entire lesion increases the likelihood of recurrent CNV96–98 and, for extrafoveal and juxtafoveal lesions, of additional visual acuity loss,96,98,99 and treatment will destroy retinal tissue (and corresponding function).98 Therefore, such treatment generally is reserved for cases that are extrafoveal and in which it is judged that a scotoma from the laser is preferred over proceeding with anti-VEGF therapy.
Preparation for laser photocoagulation treatment
Macular Photocoagulation Study photocoagulation techniques
Using a 200 µm spot size and duration of at least 0.2 s, a test burn is placed along the lesion’s perimeter to determine the necessary power setting. Then, the lesion is outlined with laser burns. Ideally, one should start at the inferior edge of a lesion and work superiorly, as if case intraoperative bleeding occurs and tracks inferiorly, obscuring retinal landmarks, this area will have been treated. To obliterate the CNV, the burns should be sufficiently intense to produce retinal whitening. Finally, the center of the CNV is obliterated (Fig. 66.20).
To ensure adequate treatment, the MPS protocol required that the entire CNV lesion be covered by a uniformly intense area of whitening extending 100 µm beyond the angiographically visible lesion. Excessive treatment can contribute to visual loss. However, failure to cover the CNV in its entirety could lead to increased persistence within 6 weeks of treatment.96–98
When treating CNV that is contiguous with the optic nerve, it should be kept in mind that laser treatment directly over the optic nerve can cause thermal necrosis of disc tissue and nerve fiber bundle defects. Therefore one should consider refraining from treatment within 100–200 µm of the optic nerve. Similarly, when treating a peripapillary area of CNV, one may want to consider treatment only when at least 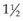 clock-hours of papillomacular bundle on the temporal side of the disc is uninvolved with CNV so that at least
clock-hours of papillomacular bundle on the temporal side of the disc is uninvolved with CNV so that at least 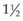 clock-hours of papillomacular bundle can be spared of treatment, as was done in several of the MPS trials. Treatment to nasal or peripapillary lesions that met criteria for MPS trials will likely not lead to severe visual loss from damage to the nerve fiber layer that serves the central macula if the treatment guidelines outlined previously are followed.100 In these situations, the MPS group reported that severe visual acuity loss was noted after treatment only when recurrent CNV extended through the center of the fovea.100 This finding suggests that, in the absence of subfoveal recurrence, severe visual loss only from nerve fiber layer damage after this treatment approach must be a rare complication.
clock-hours of papillomacular bundle can be spared of treatment, as was done in several of the MPS trials. Treatment to nasal or peripapillary lesions that met criteria for MPS trials will likely not lead to severe visual loss from damage to the nerve fiber layer that serves the central macula if the treatment guidelines outlined previously are followed.100 In these situations, the MPS group reported that severe visual acuity loss was noted after treatment only when recurrent CNV extended through the center of the fovea.100 This finding suggests that, in the absence of subfoveal recurrence, severe visual loss only from nerve fiber layer damage after this treatment approach must be a rare complication.
On rare occasions, an area of extrafoveal CNV is contiguous to a serous detachment of the RPE in which the serous detachment extends under the center of the FAZ. There have been case reports in which only the extrafoveal CNV in these lesions is treated, resulting in prompt flattening of the RPE detachment with improvement of vision in selected cases.101 Nevertheless, with follow-up, many of these eyes have acquired recurrent CNV with extensive scarring and visual loss. In these situations, one should consider the possibility that the extrafoveal CNV is associated with a subfoveal fibrovascular PED – a pattern of occult CNV in which treatment of the extrafoveal CNV alone has not been shown to be of any benefit. In such cases, anti-VEGF therapy typically would be indicated.
Evaluations following laser photocoagulation
Clinical examination probably cannot replace fluorescein angiography in detecting all recurrent CNV after laser treatment or PDT (as described below) or anti-VEGF therapy (as described below). In one prospective evaluation in which recurrent CNV was not suspected on biomicroscopy within approximately 1 year after laser photocoagulation to CNV, definite or questionable recurrent CNV was identified on the fluorescein angiogram 12% of the time.40 Most of these recurrent cases that were not identified on biomicroscopy alone were treated promptly after review of the fluorescein angiogram. This study also showed that questionable recurrences that showed focal staining along the edge of the laser lesion and speckled hyperfluorescence were the patterns that were most likely to progress to definite recurrence.14
Complications of laser photocoagulation
Immediate complications from treatment of CNV are uncommon. Rates of choroidal hemorrhage, which can occur when attempting to achieve a white burn, may be minimized by avoiding a spot size less than 200 µm and an exposure time shorter than 0.2 s. Macular pucker, observed with some frequency after argon blue–green laser treatment, is rarely clinically significant and is far less common when employing argon green or krypton red wavelengths.73 Rates of inadvertent treatment of the foveola in extrafoveal or juxtafoveal lesions, the most serious treatment complication, are rare now that anti-VEGF therapy or PDT is used for these lesions.
Delayed perfusion of choroidal vessels, thought to result from vascular spasm, has been reported following the use of the krypton red laser.102 In most cases, normal choroidal perfusion returns rapidly without any permanent visual sequelae. The krypton red laser has also been implicated in RPE tears arising from treatment of CNV.33 However, RPE tears may be observed spontaneously and after treatment of CNV using any wavelength, particularly in patients with associated RPE detachments, either serous or fibrovascular.
Visual acuity might decline several years after treatment with late remodeling and enlargement of the area of RPE disturbance. Such occurrences may be less frequent now that anti-VEGF therapy, or less commonly, PDT, is used to treat most CNV, while laser is reserved for lesions that are relatively far away from the center of the macula. These areas of enlargement of RPE abnormalities following thermal laser have been reported by Morgan and Schatz103 to increase in size by 50–100 µm over serial follow-up. In their series, 4 (3%) of 174 eyes lost vision when the area of disturbance extended into the fovea. The peripheral zone of atrophy in a laser-treated area may correspond with the runoff or spread of the laser burn during the treatment. “Runoff” refers to the border of less-intense whitening surrounding the area of heavy treatment; it probably represents less severe damage to the RPE, retina, and choroid.
Photodynamic therapy
Until 1999, no treatment other than laser photocoagulation had been shown to reduce the risk of vision loss in patients with CNV from AMD in large-scale, randomized clinical trials. The TAP Study Group,79,104 reported that PDT with verteporfin (Visudyne) can reduce the risk of moderate and severe visual acuity loss for at least 2 years in patients who present with subfoveal lesions in AMD with a predominantly classic lesion composition (in which the area of classic CNV is at least 50% of the area of the lesion). The results were even better in the absence of occult CNV.87 The therapy also reduced the risk of contrast sensitivity loss at a level likely to be beneficial for a patient’s visual function.105 For occult with no classic lesions, the Verteporfin In Photodynamic therapy (VIP) Trial in AMD showed that PDT could reduce the risk of moderate and severe visual acuity loss by 2 years after randomization compared with a sham treatment.85 While the primary outcome for the VIP trial, avoiding at least 15 letters (or 3 lines) of visual acuity loss at 1 year after randomization, was not statistically significantly different between the PDT and control group, the totality of the vision results (including avoiding 3 lines of loss at 2 years, avoiding 6 lines of loss at 1 and 2 years, mean visual acuity loss at 1 and 2 years, percentage 20/200 or worse) support considering this therapy for selected occult with no classic lesions. Specifically, occult with no classic lesions for which therapy was shown to reduce the risk of vision loss included lesions with recent disease progression (having blood associated with CNV, or definite loss of visual acuity within the past 3 months, or growth of the lesion on fluorescein angiography). Furthermore, therapy appeared to be beneficial for relatively small occult with no classic lesions or larger lesions with relatively poorer levels of visual acuity. While minimally classic lesions evaluated within a subgroup of classic-containing lesions in the TAP investigation showed no benefit of therapy, subsequent retrospective analyses of relatively small minimally classic lesions85 and a small randomized clinical trial of relatively smaller minimally classic lesions,106 showed a treatment benefit.
PDT involves the use of an intravenously injected photosensitizing drug combined with a low-intensity laser light to cause damage of choroidal neovascular tissue through a photochemical reaction by the light-activated drug that appears to result in direct cellular injury, including damage to vascular endothelial cells and vessel thrombosis.107,108 An important advantage of this therapy is the potentially selective destruction of the CNV tissue. Retinal and choroidal tissue surrounding the CNV may be minimally disturbed, thus maintaining function of surrounding and overlying sensory retina, RPE, and choroid. Three multicenter, randomized clinical trials using benzoporphyrin-derivative monoacid verteporfin as the photosensitizing agent are currently underway to evaluate the effectiveness of this therapy, with additional trials evaluating other drugs, which have yet to show benefit in randomized clinical trials. When complexed with low-density lipoprotein (LDL) and injected intravenously, verteporfin may be taken up selectively by rapidly proliferating endothelial cells that have an increased number of LDL receptors active in their plasma membranes. Infrared laser light of low power (to avoid thermal damage) is used to irradiate the neovascular complex after verteporfin injection. Although the treatment can result in cessation of fluorescein dye leakage from CNV without significant visual loss at 1 and 4 weeks after treatment, fluorescein leakage from CNV is often apparent by 12 weeks after treatment,107 with fewer and fewer treated cases showing fluorescein leakage with each subsequent follow-up after treatment is given.
Results of photodynamic therapy treatment
Preparation for photodynamic therapy
Before PDT, patients with a potentially treatable CNV lesion should be informed that PDT will not likely improve vision (in contrast to anti-VEGF therapy, described below, in which approximately one-third of patients experience substantial visual acuity gain). Nevertheless, although PDT does not reduce the chance of improving vision compared with no treatment, it does reduce the risk of substantial visual acuity loss. Patients should understand that most individuals lose some vision after PDT, usually within the first 12 months, although this may not be true for patients with a polypoidal choroidal vasculopathy pattern of CNV in AMD, as suggested in small studies within an Asian population.109 Patients also should understand that there is a small risk of acute severe visual acuity decrease (loss of at least 4 lines of visual acuity compared with pretreatment levels within 7 days of treatment). Such events can occur from sudden hemorrhages (sometimes associated with tears or rips of the RPE), or choroidal perfusion abnormalities (sometimes associated with extensive subretinal fluid accumulation).87
Pharmacologic therapy with anti-VEGF products and overall management approach to CNV in AMD110
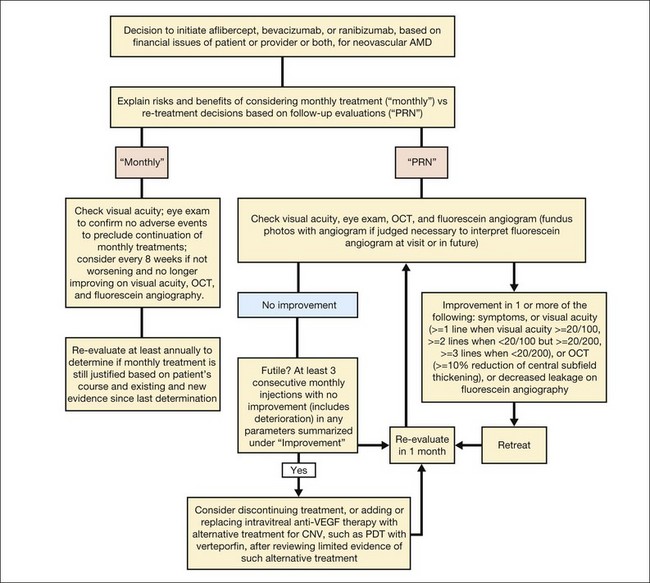
Based on the results from several clinical trials evaluating anti-VEGF therapies for CNV in AMD,54–57 a current approach to management of subfoveal CNV in AMD in which the CNV is the predominant component (that is, the area of any classic CNV plus any occult CNV is at least 50% of the area of the lesion) is summarized in Fig. 66.21. For lesions that are predominantly scar or predominantly a serous PED, it is unknown if any treatment is beneficial. For lesions that are predominantly hemorrhagic (area of blood at least 50% of the area of the lesion), anti-VEGF therapy might be considered to reduce the risk of additional severe visual acuity loss although it is not known if such therapy might increase the chance of visual acuity improvement compared with no treatment.111
Recombinant interferon-alpha-2a administered systemically is a weak inhibitor of angiogenesis that is commercially available. When tested in a randomized clinical trial, however, the treatment was found to be of no benefit, and possibly harmful, compared to placebo treatment.112 This outcome, which confirmed the variable course of untreated subfoveal CNV in AMD, especially within the first year after presentation, underscores the need for randomized clinical trials in any evaluation of pharmacologic or other therapies for CNV in AMD.
Pegaptanib sodium, a modified oligonucleotide that binds to an isoform of vascular endothelial growth factor (VEGF), has been studied in two recent phase II/III multicenter trials involving 1186 patients randomized 1 : 1 : 1 : 1 to sham, or 0.3 mg, 1 mg, or 3 mg of pegaptanib intraocular injections. Injections were given every 6 weeks. The trials enrolled patients with subfoveal CNV due to AMD with lesions smaller than 12 disc areas, of which at least 50% of the lesion had to be CNV (i.e., the lesion was predominantly CNV). In addition, there could not be atrophy or fibrosis in the center of the macula. Individuals with minimally classic or occult with no classic lesion compositions required evidence of recent disease progression.113 For the primary endpoint of the proportion of patients losing less than 3 lines of visual acuity by week 54, a combined analysis found an absolute difference of 15% in favor of treatment (70% vs 55%; P <0.001) for the 294 patients receiving pegaptanib 0.3 mg. No dose response was seen in the pegaptanib studies; pegaptanib at 1.0 mg or 3.0 mg was less effective than at 0.3 mg for all outcomes. Although the incidence of endophthalmitis was low when expressed on a per-injection basis, the need to administer treatment at 6-week intervals increases this risk. During the 12 months of the studies, 1.3% of pegaptanib recipients had endophthalmitis; of these, few (8%) lost 6 or more lines of visual acuity. This anti-VEGF therapy largely has been replaced based on the more favorable results utilizing ranibizumab,54,55 bevacizumab,56 and aflibercept.57
Efficacy of ranibizumab vs PDT with verteporfin for predominantly classic subfoveal CNV lesions
Trial participants in the ANCHOR study had CNV lesions that were to be predominantly classic CNV (at least 50% of the total area of the lesion was classic CNV).55 Unlike eligibility requirements for the MARINA study (see following section), participants were not required to have presumed recent disease progression (usually defined as presence of blood, documented recent loss of visual acuity, or documented recent growth of the choroidal neovascular lesion). Participants received ranibizumab monthly for 24 months.
Mean visual acuity letter score at baseline was 47.1 (approximate Snellen equivalent of 20/125+2) in the 0.5 mg ranibizumab group. For the primary outcome (visual acuity decline of fewer than 15 letters from baseline at 12 months), 96.4% of the 0.5 mg group (n = 140) and 64.3% of the verteporfin group (n = 143) avoided this loss (P <0.001).55 These outcomes were maintained at 24 months for most subjects, with 89.9% of the 0.5 mg group and 65.7% of the verteporfin group avoiding a loss of 3 lines or greater. Investigators also reported that visual acuity improved by 15 letters or more in 40.3% of the 0.5 mg group, as compared with 5.6% of the verteporfin group (P <0.001) at 12 months55 and 41.0% of the 0.5 mg group gained 3 or more lines of visual acuity versus only 6.3% of the verteporfin group at 24 months. Differences in the mean visual acuity from baseline visual acuity were noted as early as 1 month, when 8.4 letters of improvement occurred in the 0.5 mg ranibizumab group compared with 0.5 letters in the PDT group (P <0.001).55 At 12 months, mean visual acuity improved by 11.3 letters in the 0.5 mg ranibizumab group but decreased by a mean of 9.5 letters in the verteporfin group (P <0.001).55 At 24 months, mean visual acuity improved by 10.7 letters in the 0.5 mg ranibizumab group but decreased by 9.8 letters in the verteporfin group.
Efficacy of ranibizumab vs sham treatment for minimally classic or occult with no classic subfoveal choroidal neovascular lesions and presumed recent disease progression
In a phase 3, double-blind, sham-controlled, randomized clinical trial, eligibility criteria included subfoveal CNV with lesion composition on FA that was minimally classic or occult with no classic component accompanied by presumed recent disease progression.54 Mean visual acuity letter score at baseline was 53.7 (20/80−1) in the 0.5 mg ranibizumab group.54 Using the primary outcome of visual acuity decline of fewer than 15 letters from baseline as a measure of efficacy, 94.6% of those in the 0.5 mg ranibizumab group and 62.2% of those in the sham-injection group met the primary goal (P <0.001) after 12 months of treatment.54 At 24 months, 90.0% of those in the 0.5 mg ranibizumab group and 52.9% of those in the sham-injection group met the primary goal (P <0.001).54
At 12 months, visual acuity improved by 15 or more letters in 33.8% of the 0.5 mg ranibizumab group, as compared with 5% of the sham-injection group (P <0.001).54 Participants in the 0.5 mg ranibizumab group achieved mean increases in visual acuity of 7.2 letters, whereas the sham-injection group lost an average of 10.4 letters (P <0.001).54 At 24 months, participants in the 0.5 mg ranibizumab group gained a mean of 6.6 letters of visual acuity, compared with a mean loss of 14.9 letters in the sham-injection group (P <0.001).54
In addition to experiencing improvements in objectively measurable visual outcomes, at 12 and 24 months, patients treated with ranibizumab were more likely to improve with respect to self-reported vision-related quality of life (QOL)114–116 regardless of whether the better- or worse-seeing eye was treated. This information is complementary to the visual acuity outcomes described above because although most physicians and patients make decisions based on functional parameters such as a patient’s ability to read, watch television, and avoid dependence on others because of vision, the primary outcome measure in most recent trials evaluating treatments for neovascular AMD has been visual acuity in the treated eye.54,55Although changes in visual acuity on an eye chart are important in understanding treatment effects, measures of visual acuity do not always completely capture the degree of visual function. Clinicians may assume that visual function or patient’s perception of visual function improves along with visual acuity; however, this is not always the case.117 Among MARINA participants, scores on the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) relating to activities that require both near vision (such as sewing) and distance vision (such as watching television) were more likely to improve by 10 or more points, a clinically relevant amount, in the ranibizumab group than in the sham-injection group (P <0.001).118 In addition, ranibizumab-treated patients were less likely to perceive themselves as being dependent on others because of their vision.118 Similar results were confirmed in the ANCHOR trial.
Safety of ranibizumab
Safety issues with ranibizumab intravitreal injections include local ocular adverse events (AEs) from the drug or the injection, as well as potential systemic AEs of the drug. Ocular AEs may be categorized as common but not serious and rare but potentially serious. AEs that are common but not serious include subconjunctival hemorrhage, vitreous floaters from medication or vitreous hemorrhage, and discomfort from antiseptic used to prepare the cornea before the injection. Endophthalmitis is a rare but potentially serious ocular AE that may develop after any intravitreal injection, regardless of what is being injected. Just how much preparation is necessary to minimize the development of postinjection infections is controversial. Based on data from the Diabetic Retinopathy Clinical Research Network, there is little evidence to support the need for pretreatment or post-treatment topical antibiotics.119 Some clinicians use sterile gloves and drapes. All clinicians seem to agree that the use of a lid speculum and povidone-iodine treatment of lids, lashes, and the area to receive the injection are generally recommended to minimize postinjection colonization with normal flora. Other rare but serious ocular AEs include inflammation (as opposed to infection), vitreous hemorrhage, retinal tear or detachment, and increased intraocular pressure, which in the trials was listed as a serious AE (SAE) but might not always be considered an SAE by a treating ophthalmologist.
There was no evidence that ranibizumab 0.5 mg was associated with increases in either diastolic or systolic blood pressure. In fact, treatment-related hypertension emerged in a larger proportion of PDT patients (12 of 143, or 8.4%) than among ranibizumab 0.5 mg patients (9 of 140, or 6.4%).55 Among participants in the MARINA trial, approximately 16% in both the ranibizumab 0.5 mg and sham-injection groups developed hypertension.54
Impact of noninferiority results on frequency of treatment and the role of aflibercept or bevacizumab in place of ranibizumab
The major outcomes during the first year which are relevant to these important questions included the following: (1) visual acuity outcomes when using bevacizumab every 4 weeks were equivalent (not inferior) to those when using ranibizumab every 4 weeks; (2) visual acuity outcomes when using ranibizumab as needed, based on examinations every 4 weeks, were equivalent to those when ranibizumab was given every 4 weeks; (3) visual acuity outcomes when bevacizumab as needed was compared with ranibizumab every 4 weeks were inconclusive, i.e., possibly equivalent but also possibly inferior; (4) CATT participants assigned to ranibizumab as needed received an average of approximately seven treatments in the first year and those assigned to bevacizumab as needed received an average of approximately eight treatments; (5) although no difference between ranibizumab and bevacizumab participants was identified with respect to proportions of participants who had myocardial infarction, cerebrovascular accidents, or endophthalmitis, the combined bevacizumab groups had a higher rate of systemic serious AEs than the combined ranibizumab groups (24% for bevacizumab, 19% for ranibizumab) – of note, the point estimates for these systemic serious adverse events was higher in the bevacizumab as-needed group than the bevacizumab every-4-weeks group; and (6) the average cost of drug per patient for the first year was $23 400 in the ranibizumab every-4-weeks group, $13 800 in the ranibizumab as-needed group, and $595 in the bevacizumab every-4-weeks group. Two-year data confirmed that every-4-week bevacizumab was equivalent to ranibizumab, the increased risk of serious systemic adverse events with bevacizumab was sustained and still requires further study, and the average visual acuity in both the bevacizumab-as-needed and ranibizumab-as-needed groups continued to decline from year 1 to year 2.120
The 1-year results from another trial comparing bevacizumab and ranibizumab, and continuous and discontinuous treatment121 confirmed that the bevacizumab group appeared to have a slightly smaller mean gain in visual acuity at 1 year compared with the ranibizumab group (when combining both continuous and discontinuous treatment groups), and the discontinuous group (which required three consecutive monthly injections whenever treatment was resumed) appeared to have only a slightly smaller gain in mean visual acuity when compared with the continuous group (combining the ranibizumab and bevacizumab groups). Two-year data are anticipated in 2013.
Yet another set of trials confirmed that aflibercept (Eyelea) given every 4 weeks for three doses followed by every 8 weeks through 48 weeks gave equivalent outcomes to ranibizuamb-every-4 weeks.57 In the second year of these trials, when either aflibercept or ranibizumab was given at least every 12 weeks as well as when retreatment was judged to be indicated based on clinical parameters, including OCT, evaluated every 4 weeks, showed a small decline in the average visual acuity for each of the groups.
Potential implications of anti-VEGF noninferiority trials results on clinical practice
The results should be interpreted with the finding that as-needed ranibizumab or aflibercept costs about $13 000 more at least for the first year than every-4-week bevacizumab, and every-4-week ranibizumab costs about $10 000 more than ranibizumab as needed. Whether the greater costs of ranibizumab or aflibercept are worth the possibility that a small percentage of patients might have substantially better vision or that a larger number of patients might have slightly better vision or a slightly higher risk of systemic AEs, or clusters of endophthalmitis when not compounded properly, are beyond the scope of this chapter. However, these trials provide important data for both private (commercial) and government-sponsored insurances to make decisions on utilization of funds from the perspective of the public health and societal costs for patients who cannot pay for the costs of these very beneficial treatments which, when indicated and available, could reduce at least 75%2,122 of the prevalence of blindness from what was the leading cause of blindness just a decade ago.
Follow-up after deciding to initiate anti-VEGF therapy for neovascular AMD
Figure 66.21 provides one approach123 to consider if monthly anti-VEGF therapy with bevacizumab or ranibizumab, or 3 monthly doses of aflibercept followed by every-2-months aflibercept, is not employed. The approach considers repeating therapy as long as improvement is noted on subjective symptoms, visual acuity, biomicroscopic examination, OCT, or fluorescein angiography. The approach errs on the side of treatment since failure to treat when VEGF is present could result in irreversible visual acuity loss from fibrosis involving the retina or RPE.
Early identification of choroidal neovascularization
It has become critically important to identify, while potentially still at a treatable stage, those eyes at high risk for visual loss. Unfortunately, the likelihood of finding a case that will benefit from treatment appears to be time-dependent. Fluorescein angiographic studies have suggested growth of CNV lesions at an average rate of 10–18 µm per day.124,125 Grey and colleagues126 reported that patients with acute visual loss from AMD are more likely to have extrafoveal CNV if they are examined within the first month after the onset of symptoms.
Prevention of choroidal neovascularization
The Age-Related Eye Disease Study (AREDS) suggested that dietary supplements of vitamins C, E, beta-carotene, and zinc can reduce the chance of CNV developing in eyes with large drusen, especially when retinal pigment epithelial abnormalities were present and when these features were in both eyes. The Complications of Age-related Macular Degeneration Prevention Trial (CAPT) was not able to demonstrate that light laser photocoagulation, which has been associated with a decreased visualization of drusen,127 can prevent the development of complications of AMD associated with visual loss, including CNV and geographic atrophy.
Risk of fellow-eye involvement
When one eye has developed CNV, it is important to monitor the other eye for CNV, especially since substantial loss of vision in the first eye is highly likely.128 Development of CNV in the second eye can be devastating, since patients may have functioned quite well with good vision in their remaining eye but would be abruptly confronted for the first time with severe lifestyle impairment with development of CNV in both eyes. Often, they may not have planned for this eventuality despite proper counseling at the time of the first eye involvement. It is therefore paramount to discover and treat CNV as soon as possible to maximize the possibility of detecting treatable lesions. Because patients may be aware of symptoms only when the fovea has become involved, it would be useful to stratify risk based on fundus appearance and perhaps employ more aggressive monitoring strategies in those deemed at high risk.
The investigators of the MPS Group have studied the eyes of 670 patients enrolled in the trials of laser therapy for extrafoveal, juxtafoveal, and subfoveal CNV to identify fundus characteristics that might be predictive of CNV development in the uninvolved fellow eyes.129–131 Follow-up ranged from 3 to 5 years and revealed an overall incidence of 35%, which is consistent with previously reported figures. Application of life table estimation methods to this data yielded cumulative incidence rates of 10%, 28%, and 42% at 1, 3, and 5 years respectively. Three characteristics of the central macula and one systemic factor were associated independently with increased risk of developing CNV: the presence of five or more drusen, one or more large drusen, focal hyperpigmentation, and systemic hypertension. A substantial difference in prognosis was seen depending on the number of risk factors present. Estimated 5-year incidence rates ranged from 7% for the subgroup with no risk factors to 87% for the subgroup with all four risk factors. Similarly, in AREDS, subjects enrolled with advanced AMD (either neovascular AMD or central atrophy of the RPE) in one eye only at study entry had a 43% chance of developing advanced AMD in the fellow eye by 5 years.132 The type of CNV present in the affected eye, classic versus occult, appeared to have no effect on the rate of CNV development in the fellow eye. It must be stressed that these figures do not apply to patients with non-neovascular abnormalities only in both eyes. They are, however, very important in counseling the AMD patient who has just experienced the initial development of CNV in one eye with respect to prognosis and frequency of follow-up to try to protect the vision in the other eye.
Additional therapies
Submacular surgery
Studies have shown that visual acuity outcomes with submacular surgery are no different compared with observation for subfoveal CNV in patients with AMD in which a majority of the lesion is CNV and there is evidence of classic CNV.27 However, for lesions that are predominantly hemorrhagic (at least 50% of the lesion is blood), submacular surgery can reduce the risk of additional severe visual acuity loss compared with observation, although many patients undergoing surgery lost at least two lines of visual acuity.20 Specifically, 21% of patients undergoing removal of hemorrhage lost 6 or more lines of visual acuity compared with 36% assigned to observation. Almost half of the patients who were phakic at the time of undergoing submacular surgery had cataract surgery by 2 years. Furthermore, there was a relatively high risk of rhegmatogenous retinal detachment, especially when the lesion was greater than 16 disc areas or the visual acuity was very poor after enrollment. For this reason, submacular surgery for predominantly hemorrhagic lesions could be considered for lesions that were no greater than 16 disc areas if identified before visual acuity was very poor. More details on the findings of the trials investigating surgery for CNV in patients with AMD are included in the surgery volume of this book.
Although there is no strong evidence to support benefits of submacular surgery,27,93,104,133 except for reducing the risk of additional severe visual acuity loss in selected cases of predominantly hemorrhagic lesions, alternative surgical approaches under investigation for managing CNV and its neovascular complications include macular translocation and mechanical displacement of relatively large submacular hemorrhages using intraocular gas injection. Macular translocation can be accomplished by vitrectomy combined with retinotomy or by vitrectomy combined with external sclerochoroidal foreshortening.134 After translocation has been accomplished and the CNV lesion is no longer subfoveal, laser photocoagulation is applied with sparing of the foveal center from photocoagulation. The American Academy of Ophthalmology published an Ophthalmic Procedure Preliminary Assessment of this technique,135 highlighting the need for large-scale case series and randomized trials before widespread acceptance of this approach should be considered.
When AMD-related CNV is associated with a large submacular hemorrhage, intraocular injection of gas and face-down positioning may allow for mechanical displacement of the blood and at least temporary recovery of visual acuity.136 The improvement may not have long-term benefit if the underlying neovascular lesion and its accompanying destruction of the retina, not the blood, determines the ultimate visual outcome. More experience is needed to evaluate the safety and benefit of these surgical approaches.
Indocyanine green angiography
Indocyanine green is a dye that is more highly protein-bound than sodium fluorescein and that fluoresces in the near-infrared wavelength. These properties were suggested to be useful in the evaluation and management of CNV (see Chapter 2, Indocyanine green angiography). Three basic patterns of fluorescence have been reported in indocyanine green angiography of CNV judged to be occult on fluorescein angiography: a small, focal “hot spot” (a bright area of fluorescence more than one disc area that usually shows by the mid-phase of the angiogram), a plaque (a well-demarcated area of fluorescence more than one disc area in size that emerges relatively late in the angiogram), and ill-defined fluorescence.137,138 Because the hot spot is small and may be extrafoveal in location, attention is drawn to it as a possible treatment site. Pilot studies utilizing photocoagulation of this site have reported improvement or stabilization of visual acuity, as well as resolution of “exudative features.”137–140 However, similar outcomes have been observed in eyes with occult CNV that have not received any treatment.82,139 For example, a reanalysis of data used in the MPS juxtafoveal trial suggested that eyes with occult only CNV may have a more favorable natural course than eyes with classic CNV only. It is possible that the outcome of treating hot spots is no better than that of the natural course of these lesions. As described previously, eyes with both classic and occult CNV did not benefit from photocoagulation of the classic component alone. Treatment of the entire neovascular complex appears to be necessary. Treatment of a “hot spot” alone, identified on indocyanine green and associated with a larger area judged to be occult CNV on fluorescein angiography, may be similar to the unsuccessful strategy of treating just the classic CNV in an eye with both classic and occult CNV on fluorescein angiography. Until carefully designed, randomized clinical trials are conducted that show that indocyanine green-guided laser therapy of AMD-related CNV results in a better visual outcome than no treatment, one cannot know for sure if this particular intervention is beneficial.141 The role of ICG angiography in the setting of polypoidal choroidal vasculopathy patterns of CNV also is discussed further in Chapter 71 (Polypoidal choroidal vasculopathy).
Radiation therapy
The use of radiation therapy for CNV in AMD is based on the possibility that radiation can damage rapidly proliferating neovascular tissue. Studies have been inconsistent in documenting a benefit to this approach, either using external beam, plaques, or use of a probe with local irradiation.142–144
Other pharmacologic therapies and combination therapies
A variety of other pharmacologic therapies, delivery devices for pharmacologic therapies, and combinations of therapies are under investigation,145,146 but given the public health importance and biologic variability of visual acuity outcomes with CNV in AMD, changes in the management of CNV in AMD should be influenced strongly by large-scale, appropriately designed randomized clinical trials with adequate follow-up that show benefit before new therapies are incorporated into standard practice.
Patient education and rehabilitation
Patient education is an extremely important part of the management of patients with AMD. All patients over the age of 50 who have drusen should be made aware of the importance of regular central visual acuity testing in each eye to facilitate early detection of CNV. Although the Amsler grid is often recommended, it is not particularly sensitive or specific; frequently, changes in near or distance vision herald underlying neovascularization not detected by the patient on an Amsler grid.9 Therefore patients experiencing any change in vision in eyes at risk for neovascularization should be encouraged to contact their ophthalmologist promptly. Although patients should be counseled about the risk of severe central visual loss, they should also be reassured that AMD almost never leads to total blindness. Patients at risk should be informed that, in its more severe neovascular form, central visual tasks can be severely and permanently impaired. In light of the therapeutic implications of prompt anti-VEGF therapy when indicated, the importance of quickly reporting new visual symptoms must be stressed to all patients. At the same time, they should be reassured that no harm will come by continuing to read or perform routine visual tasks. On the contrary, patients should be encouraged to continue reading and to pursue vigorously any visual activity that they enjoy.
1 Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572.
2 Bressler NM, Doan QV, Varma R, et al. Estimated cases of legal blindness and visual impairment avoided using ranibizumab for choroidal neovascularization. Arch Ophthalmol. 2011;129:709–717.
3 Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413.
4 Ferris FL, III., Fine SL, Hyman LA. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642.
5 Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–1417.
6 Schultz DW, Klein M, Humpert AJ, et al. Analysis of the ARMDI locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum Mol Genet. 2003;12:3315–3323.
7 Hyman L, Schachat AP, He Q, et al. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351–358.
8 Loewenstein A, Bressler NM, Bressler SB. Epidemiology of RPE disease. In: Marmor MF, Wolfensberger TJ. Retinal pigment epithelium: current aspects of function and disease. New York: Oxford University Press, 1999.
9 Fine AM, Elman MJ, Ebert JE, et al. Earliest symptoms caused by neovascular membranes in the macula. Arch Ophthalmol. 1986;104:513–514.
10 Bressler SB, Silva JC, Bressler NM, et al. Clinicopathologic correlation of occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol. 1992;110:827–832.
11 Green WR, Key SN. Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180–254.
12 Green WR, McDonnell PH, Yeo JH. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92:615–627.
13 Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman lecture. Ophthalmology. 1993;100:1519–1535.
14 Dyer DS, Brant AM, Schachat AP, et al. Questionable recurrent choroidal neovascularization: angiographic features and outcome. Am J Ophthalmol. 1995;120:497–505.
15 Doyle WJ, Davidorf FH, Makley TA, et al. Histopathology of an active lesion of ocular histoplasmosis. Ophthalm Forum. 1984;2:105–111.
16 Schatz H, McDonald HR, Johnson RN. Retinal pigment epithelial folds associated with retinal pigment epithelial detachment in macular degeneration. Ophthalmology. 1990;97:658–665.
17 Elman MJ, Fine SL, Murphy RP, et al. The natural history of serous retinal pigment epithelium detachments in patients with age-related macular degeneration. Ophthalmology. 1986;93:224–230.
18 Bird AC, Marshal J. Retinal pigment epithelial detachment in the elderly. Trans Ophthalmol Soc UK. 1986;105:674–682.
19 Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration: guidelines for evaluation and treatment in the Macular Photocoagulation Study. Arch Ophthalmol. 1991;109:1242–1257.
20 Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal lesions of age-related macular degeneration: ophthalmic findings. SST report no. 13. Submacular Surgery Trials (SST) Research Group. Ophthalmology. 2004;111:1993–2006.
21 Taylor HR, West S, Munoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110:99–104.
22 Wood WJ, Smith TR. Senile disciform macular degeneration complicated by massive hemorrhagic retinal detachment and angle closure glaucoma. Retina. 1983;3:296–303.
23 el Baba F, Jarrett WH, II., Harbin IS, et al. Massive hemorrhage complicating age-related macular degeneration. Ophthalmology. 1986;93:1281–1592.
24 Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–1231.
25 Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1232–1241.
26 Macular Photocoagulation Study Group. Laser photocoagulation of juxtafoveal choroidal neovascularization: 5-year results from randomized clinical trials. Arch Ophthalmol. 1994;111:500–509.
27 Hawkins BS, Bressler NM, Miskala PH, et al. Surgery for subfoveal choroidal neovascularization of age-related macular degeneration: ophthalmic findings. SST report no. 11. Submacular Surgery Trials (SST) Research Group. Ophthalmology. 2004;111:1967–1980.
28 De Jong PTVM, Chakravarthy U, Rahu M, et al. Associations between aspirin use and aging macula disorder. The European Eye Study. Ophthalmology. 2012. in press
29 Cantrill HL, Ramsay RC, Knoblock WH. Rips in the pigment epithelium. Arch Ophthalmol. 1983;101:1074–1079.
30 Decker WL, Sanborn GF, Ridley M, et al. Retinal pigment epithelial tears. Ophthalmology. 1983;90:507–512.
31 Green SN, Yarian D. Acute tears of the retinal pigment epithelium. Retina. 1983;3:16–20.
32 Hoskin A, Bird AC, Sehow K. Tears of detached retinal pigment epithelium. Br J Ophthalmol. 1981;65:417–422.
33 Yeo JH, Marcus S, Murphy RP. Retinal pigment epithelial tears: patterns and prognosis. Ophthalmology. 1988;95:813.
34 Gass JDM. Stereoscopic atlas of macular disease and treatment, 4th ed. St Louis: Mosby; 1997.
35 Grossniklaus HE, Green WR, for the Submacular Surgery Trials Research Group. Histopathologic and ultrastructural findings of surgically-excised choroidal neovascularization. Arch Ophthalmol. 1998;116:745–749.
36 Grossniklaus HE, Miskala PH, Green WR, et al. Histopathological and ultrastructural features of surgically-excised subfoveal choroidal neovascularization and associated tissue. SST report no. 7. Submacular Surgery Trials (SST) Research Group. Arch Ophthalmol. 2005;123:914–921.
37 Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503.
38 Lafaut BA, Bartz-Schmidt KU, Vanden Broecke C, et al. Clinicopathologic correlation in exudative age related macular degeneration: histological differentiation between classic and occult choroidal neovascularization. Br J Ophthalmol. 2000;84:239–243.
39 Submacular Surgery Trials Research Group. Comparison of two-dimensional reconstructions of surgically-excised subfoveal choroidal neovascularization with fluorescein angiographic features: SST report no. 15. Ophthalmology. 2006;113:279.
40 Sykes SO, Bressler NM, Maguire MG, et al. Detecting recurrent choroidal neovascularization: comparison of clinical examination with and without fluorescein angiography. Arch Ophthalmol. 1994;111:1561–1566.
41 Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) and Verteporfin in Photodynamic Therapy Study Groups. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment – TAP and VIP report no. 2. Arch Ophthalmol. 2003;121:1253–1268.
42 Solomon SD, Bressler SB, Hawkins BS, et al. Guidelines for interpreting retinal photographs in the Submacular Surgery Trials (SST). SST report no. 8. Submacular Surgery Trials (SST) Research Group. Retina. 2005;25:253–268.
43 Koenig F, Soubrane G, Coscas G. Angiographic aspects of senile macular degeneration: spontaneous course. J Fr Ophtalmol. 1984;7:93–98.
44 Frost L, Bressler NM, Bressler SB, et al. Natural course of poorly defined choroidal neovascularization associated with age-related macular degeneration. Invest Ophthalmol. 1988;29(Suppl):120.
45 Hyman L, Lilienfeld AM, Ferris FL, III., et al. Senile macular degeneration: a case-control study. Am J Epidemiol. 1983;118:213–227.
46 Dyer DS, Brant AM, Schachat AP, et al. Angiographic features and outcome of questionable recurrent choroidal neovascularization. Am J Ophthalmol. 1995;120:497–505.
47 Yannuzzi LA, Negrao S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–434.
48 Bressler NM, Bressler SB, Alexander J, et al. Loculated fluid: a previously undescribed angiographic finding in macular degeneration. Arch Ophthalmol. 1991;109:211–215.
49 Chuang EL, Bird AC. The pathogenesis of tears of the retinal pigment epithelium. Am J Ophthalmol. 1988;105:285–290.
50 Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye. 1990;4:613–621.
51 Ambati J, Ambati BK, Yoo SH, et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293.
52 Gragoudas ES, Adamis AP, Cunningham ET, Jr., et al. Pegaptanib for neovascular age-related macular degeneration. for the VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. N Engl J Med. 2004;351:2805–2816.
53 Genentech [Internet]. Preliminary phase III data show Lucentis maintained or improved vision in nearly 95 percent of patients with wet age-related macular degeneration. Press release May 23, 2005 [cited June 2005] http://www.gene.com/gene/news/press-releases/
54 Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431.
55 Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444.
56 The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908.
57 Aflibercept Product Insert. 11/2011. http://www.regeneron.com/Eylea/eylea-fpi.pdf [accessed 2012 July 4]
58 Bressler NM, Silva JC, Bressler SB, et al. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–142.
59 Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related macular degeneration. Arch Ophthalmol. 1999;117:329–339.
60 Sarks SH. Aging and degeneration in the macular region: a clinicopathological study. Br J Ophthalmol. 1976;60:324–341.
61 Sarks SH. Drusen and their relationship to senile macular degeneration. Aust J Ophthalmol. 1980;8:117–130.
62 Sarks SH, Penfold PL, Killingsworth MC, et al. Patterns in macular degeneration. In: Ryan SJ, Dawson AK, Little, HL. Retinal diseases. Orlando, FL: Grune & Stratton, 1985.
63 Sarks SH, Van Driel D, Maxwell L, et al. Softening of drusen and subretinal neovascularization. Trans Ophthalmol Soc UK. 1980;100:414–422.
64 Kenyon KR, Maumenee AE, Ryan SJ, et al. Diffuse drusen and associated complications. Am J Ophthalmol. 1985;100:119–128.
65 Eagle RC. Mechanisms of maculopathy. Ophthalmology. 1984;91:613–625.
66 Heriot WJ, Henkind P, Bellhorn RW, et al. Choroidal neovascularization can digest Bruch’s membrane: a prior break is not essential. Ophthalmology. 1984;91:1603–1608.
67 Loffer KU, Lee WR. Basal linear deposit in the human macula. Graefes Arch Clin Exp Ophthalmol. 1986;224:493–501.
68 Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76.
69 Penfold PL, Provis JM, Billson FA. Age-related macular degeneration: ultrastructural studies of the relationship of leukocytes to angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1987;225:70–76.
70 Campochiaro PA, Hackett SF, Vinores SA, et al. Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci. 1994;107:2459–2469.
71 Glaser BM. Extracellular modulating factors and the control of intraocular neovascularization. Arch Ophthalmol. 1988;106:603–607.
72 Bressler NM, Bressler SB, Gragoudas ES. Clinical characteristics of choroidal neovascular membranes. Arch Ophthalmol. 1987;105:209–213.
73 Berkow JW. Subretinal neovascularization in senile macular degeneration. Am J Ophthalmol. 1984;97:143–147.
74 Moisseiev J, Alhalel A, Masuri R, et al. The impact of the Macular Photocoagulation Study results on the treatment of exudative age-related macular degeneration. Arch Ophthalmol. 1995;113:185–189.
75 Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy: 3-year results from randomized clinical trials. Arch Ophthalmol. 1986;104:694–701.
76 Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy after 5 years: results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–1114.
77 Macular Photocoagulation Study Group. Argon laser photocoagulation for senile macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1982;100:912–918.
78 Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration: updated findings from two clinical trials. Arch Ophthalmol. 1993;111:1200–1209.
79 Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two year results of 2 randomized clinical trials – TAP report no. 2. Arch Ophthalmol. 2001;119:198–207.
80 Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Effect of lesion size, visual acuity, and lesion composition on visual acuity change from baseline with and without verteporfin therapy in choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP report no. 1. Am J Ophthalmol. 2003;136:407–418.
81 Bressler NM, Maguire MG, Murphy PL, et al. Macular scatter (“grid”) laser treatment of poorly demarcated subfoveal choroidal neovascularization in age-related macular degeneration: results of a randomized pilot trial. Arch Ophthalmol. 1996;114:1456–1464.
82 Macular Photocoagulation Study Group. Occult choroidal neovascularization: influence on visual outcome in patients with age-related macular degeneration. Arch Ophthalmol. 1996;114:400–412.
83 Soubrane G, Coscas G, Francais C, et al. Occult subretinal new vessels in age-related macular degeneration: natural history and early laser treatment. Ophthalmology. 1990;97:649–657.
84 Tani PM, Buettner H, Robertson DM. Massive vitreous hemorrhage and senile macular choroidal degeneration. Am J Ophthalmol. 1980;90:525–533.
85 Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two year results of a randomized clinical trial including lesions with occult but no classic neovascularization – VIP report no. 2. Am J Ophthalmol. 2001;131:541–560.
86 Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Natural history of minimally classic subfoveal choroidal neovascular lesions in the Treatment of Age-related macular degeneration with Photodynamic therapy (TAP) Investigation: Outcomes potentially relevant to management – TAP report no. 6. Arch Ophthalmol. 2004;122:325–329.
87 Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in patients with age-related macular degeneration: additional information regarding baseline lesion composition’s impact on vision outcomes – TAP Report No. 3. Arch Ophthalmol. 2002;120:1443–1454.
88 Avery RL, Fekrat S, Hawkins BS, et al. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16:183–189.
89 Bennett SR, Folk JC, Blodi CF, et al. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990;109:33–37.
90 Berrocal MH, Lewis ML, Flynn HW, Jr. Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol. 1996;122:486–493.
91 Hanscom TA, Diddie KP. Early surgical drainage of macular subretinal hemorrhage. Arch Ophthalmol. 1987;105:1722–1723.
92 Lewis H, Jaffe GJ, Blumenkranz MS. Management of submacular hemorrhage with vitreoretinal surgery and subretinal injection of tissue plasminogen activator. Invest Ophthalmol Vis Sci. 1992;33:898.
93 Bressler NM. Submacular surgery: are randomized trials necessary? Arch Ophthalmol. 1995;113:1557–1560.
94 Bressler NM, Finkelstein D, Sunness JS, et al. Retinal pigment epithelial tears through the fovea with preservation of good visual acuity. Arch Ophthalmol. 1990;108:1694–1697.
95 Schoeppner G, Chuang EL, Bird AC. The risk of fellow eye visual loss with unilateral retinal pigment epithelial tears. Am J Ophthalmol. 1989;108:683–685.
96 Macular Photocoagulation Study Group. Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Arch Ophthalmol. 1990;108:825–833.
97 Macular Photocoagulation Study Group. Persistent and recurrent choroidal neovascularization after laser photocoagulation for subfoveal choroidal neovascularization of age-related macular degeneration. Arch Ophthalmol. 1994;112:489–499.
98 Macular Photocoagulation Study Group. The influence of treatment coverage on the visual acuity of eyes treated with krypton laser for juxtafoveal choroidal neovascularization. Arch Ophthalmol. 1995;113:190–194.
99 Macular Photocoagulation Study Group. Recurrent choroidal neovascularization after argon laser photocoagulation for neovascular maculopathy. Arch Ophthalmol. 1986;104:503–512.
100 Macular Photocoagulation Study Group. Laser photocoagulation for neovascular lesions nasal to the fovea associated with ocular histoplasmosis or idiopathic causes. Arch Ophthalmol. 1995;113:56–61.
101 Maguire JI, Benson WE, Brown GC. Treatment of foveal pigment epithelial detachments with contiguous extrafoveal choroidal neovascular membranes. Am J Ophthalmol. 1990;109:523–529.
102 Cohen SMZ, Fine SL, Murphy RP, et al. Transient delay in choroidal filling after krypton red laser photocoagulation for choroidal neovascular membranes. Retina. 1983;3:284–290.
103 Morgan CM, Schatz H. Atrophic creep of the retinal pigment epithelium after focal macular photocoagulation. Ophthalmology. 1989;96:96–103.
104 Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Verteporfin (Visudyne) therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 1-year results of two randomized clinical trials, TAP report no 1. Arch Ophthalmol. 1999;117:1329–1345.
105 Rubin GS, Bressler NM, Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Effects of verteporfin therapy on contrast sensitivity; results from the Treatment of Age-related Macular Degeneration with Photodynamic therapy (TAP) Investigation: TAP report no. 4. Retina. 2002;22:536–544.
106 Visudyne® In Minimally Classic Choroidal Neovascularization Study Group. Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial. Arch Ophthalmol. 2005;123:448–457.
107 Miller JW, Schmidt-Erfurth U, Sickenberg M, et al. Photodynamic therapy for choroidal neovascularization due to age-related macular degeneration with verteporfin: results of a single treatment in a phase I and II study. Arch Ophthalmol. 1999;117:1161–1173.
108 Schmidt-Erfurth U, Miller JW, Sickenberg M, et al. Photodynamic therapy of choroidal neovascularization due to age-related macular degeneration with verteporfin: results of retreatments in a phase I and II study. Arch Ophthalmol. 1999;117:1177–1187.
109 Phase IV EVEREST PCV trial. Clinical trials identifier NCT00674323.
110 Bressler NM. Update on treatment of neovascular age-related macular degeneration: focus on antiangiogenesis in clinical practice. Ophthalmology. 2009;116(Suppl):S15–S23.
111 Chang MA, Do DV, Bressler SB, et al. Prospective one-year study of ranibizumab for predominantly hemorrhagic choroidal neovascular lesions in age-related macular degeneration. Retina. 2010;30:1171–1176.
112 Pharmacological Therapy for Macular Degeneration Study Group. Interferon alfa-2a is ineffective for patients with choroidal neovascularization secondary to age-related macular degeneration: results of a prospective, randomized, placebo-controlled clinical trial. Arch Ophthalmol. 1997;115:865–872.
113 Gragoudas ES, Adamis AP, Cunningham ET, Jr., et al. Pegaptanib for neovascular age-related macular degeneration. VEGF, Inhibition Study in Ocular Neovascularization Clinical Trial Group. N Engl J Med. 2004;351:2805–2816.
114 Chang TS, Bressler NM, Fine JT, et al. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration. Arch Ophthalmol. 2007;125:1460–1469.
115 Bressler NM, Chang TS, Fine JT, et al. Improved vision-related function after ranibizumab vs photodynamic therapy. for the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) Research Group. Arch Ophthalmol. 2009;127:13–21.
116 Bressler NM, Chang TS, Suñer IJ, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: clinical trial results from MARINA and ANCHOR. for the MARINA and ANCHOR Research Groups. Ophthalmology. 2010;117:747–756.
117 Miskala PH, Bressler NM, Meinert CL. Relative contributions of reduced vision and general health to NEI-VFQ scores in patients with neovascular age-related macular degeneration. Arch Ophthalmol. 2004;122:758–766.
118 Chang TS, Bressler NM, Fine JT, et al. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration. Arch Ophthalmol. 2007;125:1460–1469.
119 Bhavsar AR, Googe JM, Stockdale CR, et al. Risk of endophthalmitis after intravitreal drug injection when topical antibiotics are not required: The DRCR.net Diabetic Retinopathy Clinical Research Network Laser-Ranibizumab-Triamcinolone Clinical Trials. Arch Ophthalmol. 2009;127:1581–1583.
120 Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398.
121 The IVAN Study Investigators Writing CommitteeChakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN Randomized Trial. Ophthalmology. 2012. in press
122 Campbell JP, Bressler SB, Bressler NM. Impact of availability of anti-VEGF therapy on vision impairment and blindness due to neovascular AMD. Arch Ophthalmol. 2012. in press
123 Bressler NM. Retina Times 2010. (published online by the American Society of Retina Specialists)
124 Klein ML, Jorizzo PA, Watzke RC. Growth features of choroidal neovascular membranes in age-related macular degeneration. Ophthalmology. 1989;96:1416–1419.
125 Vander JF, Morgan CM, Schatz H. Growth rate of subretinal neovascularization in age-related macular degeneration. Ophthalmology. 1989;96:1422–1426.
126 Grey RHB, Bird AC, Chisholm IH. Senile disciform macular degeneration: features indicating suitability for photocoagulation. Br J Ophthalmol. 1986;104:702–705.
127 Choroidal Neovascularization Prevention Trial Research Group. Laser treatment in eyes with large drusen. Ophthalmology. 1998;105:11–23.
128 Thomas MS, Grand MG, Williams DF, et al. Surgical management of subfoveal choroidal neovascularization. Ophthalmology. 1992;99:952–966.
129 Bressler SB, Maguire MG, Bressler NM, et al. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. The Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108:1442–1447.
130 Macular Photocoagulation Study Group. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Arch Ophthalmol. 1993;111:1189–1199.
131 Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115:741–747.
132 The Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436.
133 Berger AS, Kaplan HJ. Clinical experience with the surgical removal of subfoveal neovascular membranes. Ophthalmology. 1992;99:969–976.
134 deJuan E, Jr., Loewenstein A, Bressler NM, et al. Translocation of the retina for management of subfoveal choroidal neovascularization. II. A preliminary report in humans. Am J Ophthalmol. 1998;125:635–646.
135 American Academy of Ophthalmology. Macular translocation. Ophthalmic procedure preliminary assessment. Ophthalmology. 2000;107:1015–1018.
136 Hassan AS, Johnson MW, Regillo CD, et al. Management of submacular hemorrhage with intravitreal tPA injection and pneumatic displacement. Invest Ophthalmol Vis Sci. 1998;39(Suppl):227.
137 Regillo CD, Benson WE, Maguire JI, et al. Indocyanine green angiography and occult neovascularization. Ophthalmology. 1994;101:280–288.
138 Yannuzzi LA, Slakter JS, Sorenson JA, et al. Digital indocyanine green videoangiography and choroidal neovascularization. Retina. 1992;12:191–223.
139 Guyer DR, Yannuzzi LA, Ladas I, et al. Indocyanine green-guided laser photocoagulation of focal spots at the edge of plaques of choroidal neovascularization. Arch Ophthalmol. 1996;114:693–697.
140 Regillo CD, Blade KA, Custis PH, et al. Evaluating persistent and recurrent choroidal neovascularization: the role of indocyanine green angiography. Ophthalmology. 1998;105:1821–1826.
141 Bressler NM, Bressler SB. Indocyanine green angiography: can it help preserve the vision of our patients? Arch Ophthalmol. 1996;114:747–749.
142 Bergink GJ, Hoyng CB, van der Maazen RW, et al. A randomized controlled clinical trial on the efficacy of radiation therapy in the control of subfoveal choroidal neovascularization in age-related macular degeneration: radiation versus observation. Graefes Arch Clin Exp Ophthalmol. 1998;236:321–325.
143 Holz FG, Unnebrink K, Engenhart-Cabillic R, et al. Results of a prospective, randomized, controlled, double-blind multicenter trial on external beam radiation therapy for subfoveal choroidal neovascularization secondary to ARMD (RAD-study). Invest Ophthalmol Vis Sci. 1999;40(Suppl):2115.
144 Spaide RF, Guyer DR, McCormick B, et al. External beam radiation therapy for choroidal neovascularization. Ophthalmology. 1998;105:24–30.