15 Neoplastic Meningitis
Introduction
Neoplastic meningitis (NM) is the result of seeding of the leptomeninges by malignant cells. NM is not infrequent, and is becoming more common as cancer patients live longer.1–5 NM results in significant morbidity, and median survival is short despite therapy. However, treatment can result in significant palliation of the CNS and is best approached by a multidisciplinary team.
Epidemiology
Neoplastic meningitis is diagnosed in 1% to 5% of patients with solid tumors (in which case it is termed carcinomatous meningitis), 5% to 15% of patients with leukemia (termed leukemic meningitis) and lymphoma (termed lymphomatous meningitis), and 1% to 2% of patients with primary brain tumors.5 Autopsy studies show that 19% of patients with cancer and neurologic signs and symptoms have evidence of meningeal involvement.6 Adenocarcinoma is the most frequent histology, and breast, lung, and melanoma are the most common primary sites to metastasize to the leptomeninges.3,7,8 Although small cell lung cancer and melanoma have the highest rates of spread to the leptomeninges (11% and 20%, respectively), because of the higher incidence of breast cancer (with a 5% rate of spread), the later accounts for most cases in large series of the disorder.1,7,9–11 Carcinomas of unknown primary constitute 1% to 7% of all cases of NM.7
NM usually presents in patients with widely disseminated and progressive systemic cancer (>70%) but it can present after a disease-free interval (20%) and may even be the first manifestation of cancer (5% to 10%), occasionally in the absence of other evidence of systemic disease.7,12–14
Pathogenesis
Cancer cells reach the meninges by various routes: (1) hematogenous spread, either through the venous plexus of Batson or by arterial dissemination; (2) direct extension from contiguous tumor deposits; (3) and through centripetal migration from systemic tumors along perineural or perivascular spaces.15–18
Once cancer cells have entered the subarachnoid space, cancer cells are transported by CSF flow, resulting in disseminated and multifocal neuraxis seeding of the leptomeninges. Tumor infiltration is most prominent at the base of the brain, the dorsal surface of the spinal cord, and, in particular, the cauda equina.5,18 Hydrocephalus or impairment of CSF flow may occur at any level of the neuraxis and is due to ependymal nodules or tumor deposits obstructing CSF outflow.
Clinical Features
The most common manifestations of cerebral hemisphere dysfunction are headache and mental status changes. Other signs include confusion, cognitive impairment, seizures and hemiparesis. Diplopia is the most common symptom of cranial nerve dysfunction with cranial nerve VI being the most frequently affected, followed by cranial nerves III and IV. Trigeminal sensory or motor loss, cochlear dysfunction and optic neuropathy are also common findings. Spinal signs and symptoms include weakness (lower extremities more often than upper), dermatomal or segmental sensory loss, and pain in the neck, back, or following radicular patterns. Nuchal rigidity is only present in 15% of cases.3,7,8,13,19
New neurological signs and symptoms may represent progression of NM but must be distinguished from the manifestations of parenchymal disease (30% to 40% of patients with NM will have coexistent parenchymal brain metastases), from side effects of chemotherapy or radiation used for treatment, and, rarely, from paraneoplastic syndromes. At presentation, NM must also be differentiated from chronic meningitis due to tuberculosis, fungal infection or sarcoidosis, as well as from metabolic and toxic encephalopathies in the appropriate clinical setting.7,20
Diagnosis
CSF EXAMINATION
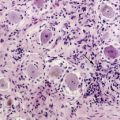
The most useful laboratory test in the diagnosis of NM is the CSF examination. Abnormalities include increased opening pressure (>200 mm H2O), increased leukocytes (>4/mm3), elevated protein (>50 mg/dl) or decreased glucose (<60 mg/dl), which though suggestive of NM are not diagnostic. The presence of malignant cells in the CSF is diagnostic of NM, but in general, as is true for most cytological analysis, assignment to a particular tumor is not possible.21
In patients with positive CSF cytology (discussed later), up to 45% will be cytologically negative on initial examination.6 The yield is increased to 80% with a second CSF examination, but little benefit is obtained from repeat lumbar punctures after the second.7 Of note, a series by Kaplan, including lymphomatous and leukemic meningitis, observed the frequent dissociation between CSF cell count and malignant cytology (29% of cytologically-positive CSF had concurrent CSF counts of less than 4/mm3).3 Murray showed that CSF levels of protein, glucose, and malignant cells vary at different levels of the neuraxis, even if there is no obstruction of the CSF flow.22,23 This finding reflects the multifocal nature of neoplastic meningitis and explains that CSF obtained from a site distant to that of the pathologically-involved meninges may yield a negative cytology.
Of the 90 patients reported by Wasserstrom, 5% had positive CSF cytology only from either the ventricles or cisterna magna.7 In a series of 60 patients with NM, positive lumbar CSF cytology at diagnosis, and no evidence of CSF flow obstruction, ventricular and lumbar cytologies obtained simultaneously were discordant in 30% of cases.24 The authors observed that in the presence of spinal signs or symptoms, the lumbar CSF was more likely to be positive and, conversely, in the presence of cranial signs or symptoms, the ventricular CSF was more likely to be positive. Not obtaining CSF from a site of symptomatic or radiographically demonstrated disease was found, in a prospective evaluation of 39 patients, to correlate with false-negative cytology results, as did withdrawing small CSF volumes (<10.5 ml), delaying processing of specimens, and obtaining less than two samples.25 Even after correcting for these factors, there remains a substantial group of patients with NM who have persistently negative CSF cytology. Glass reported on a postmortem study of the value of premortem CSF cytology,6 and demonstrated that up to 40% of patients with clinically suspected NM proven at the time of autopsy are cytologically negative. This figure increased to over 50% in patients with focal NM.
Numerous biochemical markers have been evaluated but, in general, their use has been limited by poor sensitivity and specificity. Particular tumor markers, such as CEA (carcinoembryogenic antigen) from adenocarcinomas, and AFP (α-fetoprotein) and β-HCG (β-human chorionic gonadotropin) from testicular cancers and primary extragonadal CNS tumors, can be relatively specific for NM when elevated in CSF in the absence of markedly elevated serum levels.16,26 Nonspecific tumor markers such as CK-BB (creatine-kinase BB isoenzyme), TPA (tissue polypeptide antigen, β2microglobulin, β-glucoronidase, LDH isoenzyme-5 and more recently VEGF (vascular endothelial growth factor) can be strong indirect indicators of NM, but none are sensitive enough to improve the cytological diagnosis.27–31 The use of these biochemical markers can be helpful as adjunctive diagnostic tests and, when followed serially, to assess response to treatment. Occasionally, in patients with clinically suspected NM and negative CSF cytology, they may support the diagnosis of NM.32
Use of monoclonal antibodies for immunohistochemical analysis in NM does not significantly increase the sensitivity of cytology alone.33–35 However, in the case of leukemia and lymphoma, antibodies against surface markers can be used to distinguish between reactive and neoplastic lymphocytes in the CSF.36
Cytogenetic studies have also been evaluated in an attempt to improve the diagnostic accuracy of NM. Flow cytometry and DNA single cell cytometry, techniques that measure the chromosomal content of cells, and fluorescent in situ hybridization (FISH), that detects numerical and structural genetic aberrations as a sign of malignancy, can give additional diagnostic information, but still have a low sensitivity.37–39 Polymerase-chain reaction (PCR) can establish a correct diagnosis when cytology is inconclusive, but the genetic alteration of the neoplasia must be known for it to be amplified with this technique, and this is generally not the case, particularly in solid tumors.40
In cases where there is no manifestation of systemic cancer and CSF examinations remain inconclusive, a meningeal biopsy may be diagnostic. The yield of this test increases if the biopsy is taken from an enhancing region on MRI (see below).41
NEURORADIOGRAPHIC STUDIES
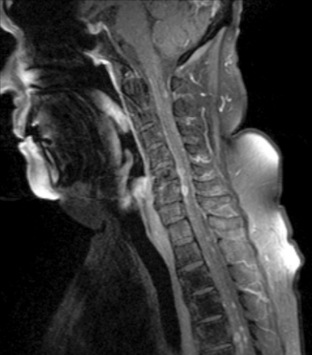
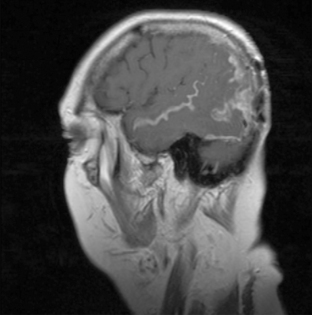
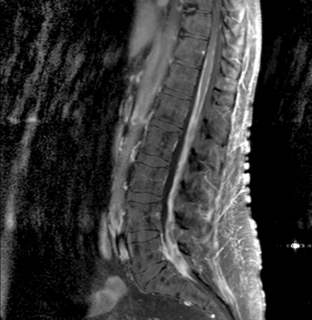
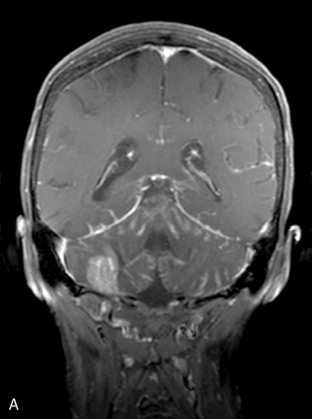
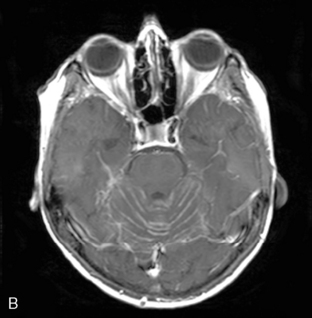
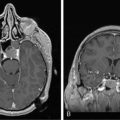
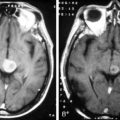
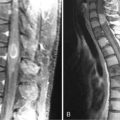
Magnetic resonance imaging with gadolinium enhancement (MR-Gd) is the technique of choice to evaluate patients with suspected NM (Figures 15-1 through 15-4).42–44 Because NM involves the entire neuraxis, imaging of the entire CNS is required in patients considered for further treatment. T1-weighted sequences, with and without contrast, combined with fat-suppression T2-weighted sequences constitute the standard examination.42–45 MRI has been shown to have a higher sensitivity than cranial contrast-enhanced computed tomography (CE-CT) in several series, and is similar to computerized tomographic myelography (CT-M) for the evaluation of the spine, but significantly better tolerated.42,44–46
Any irritation of the leptomeninges (i.e., subarachnoid blood) will result in their enhancement on MRI, which is seen as a fine signal-intense layer that follows the gyri and superficial sulci. Subependymal involvement of the ventricles often results in ventricular enhancement. Some changes, such as cranial nerve enhancement on cranial imaging and intradural extramedullary enhancing nodules on spinal MR (most frequently seen in the cauda equina), can be considered diagnostic of NM in patients with cancer.47 Lumbar puncture itself can rarely cause a meningeal reaction leading to dural-arachnoidal enhancement, so imaging should, preferably, be obtained prior to the procedure.48 MR-Gd still has a ≥30% incidence of false-negative results, so a normal study does not exclude the diagnosis of NM. On the other hand, in cases with a typical clinical presentation, abnormal MR-Gd alone is adequate to establish the diagnosis of NM.32,42,46,47
Radionuclide studies, using either 111Indium-diethylenetriamine pentaacetic acid or 99Tc macro-aggregated albumin, constitute the technique of choice to evaluate CSF flow dynamics.17,49 Abnormal CSF circulation has been demonstrated in 30% to 70% of patients with NM, with blocks commonly occurring at the skull base, the spinal canal, and over the cerebral convexities.46,49,50 Patients with interruption of CSF flow demonstrated by radionuclide ventriculography have been shown, in three clinical series, to have decreased survival when compared to those with normal CSF flow.49,51,52 Involved-field radiotherapy to the site of CSF flow obstruction restores flow in 30% of patients with spinal disease and 50% of patients with intracranial disease.53,54 Reestablishment of CSF flow with involved-field radiotherapy followed by intrathecal chemotherapy led to longer survival, lower rates of treatment-related morbidity, and a lower rate of death from progressive NM, compared to the group that had persistent CSF blocks.49,51
Prognosis
The median survival of untreated patients with NM is 4 to 6 weeks; death generally occurs due to progressive neurological dysfunction.7 Treatment is intended to improve or stabilize the neurological status, maintain neurological quality of life, and prolong survival. Fixed neurological defects are rarely improved with treatment, but progression of neurological deterioration may be halted in some patients and median survival can be increased to 4 to 6 months.16 Of the solid tumors, breast cancer responds best, with median survivals of 6 months and 11% to 25% 1-year survivals.26,55 Numerous prognostic factors for survival and response have been studied (age, gender, duration of signs of NM, increased protein or low glucose in CSF, ratio of lumbar/ventricular CEA, etc.), but many remain controversial.55 It is commonly accepted, however, that patients will do poorly with intensive treatment of NM if they have poor performance status, multiple fixed neurologic deficits, bulky CNS disease, coexistent carcinomatous encephalopathy, and CSF flow abnormalities demonstrated by radionuclide ventriculography. In general, patients with widely metastatic aggressive cancers that do not respond well to systemic chemotherapies are also less likely to benefit from intensive therapy.56,57 What appears clear is that, optimally, NM should be diagnosed in the early stages of disease to prevent progression of disabling neurological deficits, analogous to the clinical situation of epidural spinal cord compression.
Treatment
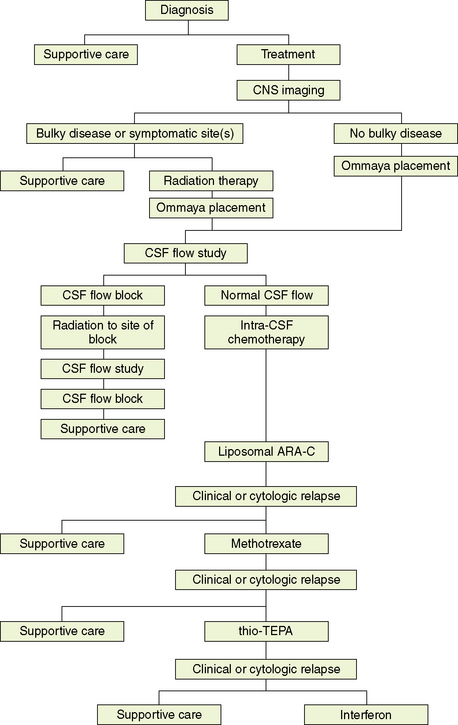
The evaluation of treatment of NM is complicated by the lack of standard treatments, the difficulty of determining response to treatment given the suboptimal sensitivity of the diagnostic procedures and that most patients will die of systemic disease, and the fact that most studies are small, nonrandomized, and retrospective. However, it is clear that treatment of NM can provide effective palliation and, in some cases, result in prolonged survival. Treatment in most cases requires the combination of surgery, radiation, and chemotherapy (Box 15-1). Figure 15-5 outlines a treatment algorithm for NM.
SURGERY
Drugs can be instilled into the subarachnoid space by lumbar puncture or via an intraventricular reservoir system. The latter is the preferred approach because it is simpler, more comfortable for the patient, and safer than repeated lumbar punctures. It also results in a more uniform distribution of the drug in the CSF space and produces the most consistent CSF levels. In up to 10% of lumbar punctures, the drug is delivered to the epidural space (even if there is CSF return after placement of the needle), and drug distribution has been shown to be better after drug delivery through a reservoir.58–60
RADIOTHERAPY
Radiotherapy is used in the treatment of NM for (1) palliation of symptoms, such as a cauda equina syndrome, (2) to decrease bulky disease such as coexistent parenchymal brain metastases, and (3) to correct CSF flow abnormalities demonstrated by radionuclide ventriculography. Patients may have significant symptoms without radiographic evidence of bulky disease and still benefit from radiation. For example, patients with low back pain and leg weakness should be considered for radiation to the cauda equina, and those with cranial neuropathies should be offered whole-brain or base-of-skull radiotherapy.26
CHEMOTHERAPY
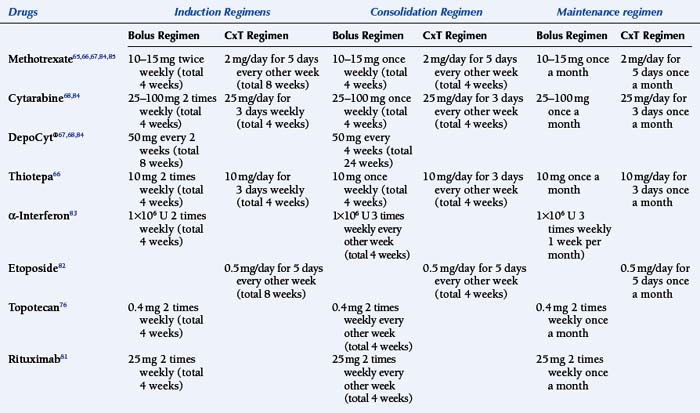
Intrathecal chemotherapy is the mainstay of treatment for NM (Table 15-1). Retrospective analysis or comparison to historical series suggest that the administration of chemotherapy to the CSF improves the outcome of patients with NM.1,20,51,61,62 However, it is noted that most series will exclude patients that are too sick to receive any treatment, which may be up to one third of patients with NM.63 Three agents are routinely used: methotrexate, cytarabine (including liposomal cytarabine or DepoCyt®), and thioTEPA. No difference in response has been seen when comparing single agent methotrexate with thioTEPA or when using multiple agent (methotrexate, thioTEPA and cytarabine, or methotrexate and cytarabine) versus single agent methotrexate treatment.64–66 Table 15-2 outlines the randomized clinical trials conducted for NM while Table 15-1 is an outline of the common treatment regimens for intra-CSF chemotherapy. A sustained-release form of cytarabine (DepoCyt®) results in cytotoxic cytarabine levels in the CSF for greater than or equal to 10 days, and when given bimonthly and compared to biweekly methotrexate, resulted in longer time to neurological progression in patients with NM .67,68 Furthermore, quality of life and cause of death favored DepoCyt® over methotrexate. These findings were confirmed in a study of lymphomatous meningitis and in an open label study, suggesting that DepoCyt® should be considered the drug of first choice in the treatment of NM.68,69
Complications of intrathecal chemotherapy include those related to the ventricular reservoir and those related to the chemotherapy administered (Box 15-2). The most frequent complications of ventricular reservoir placement are malposition (rates reported 3% to 12%), obstruction, and infection (usually skin flora). CSF infection occurs in 2% to 13% of patients receiving intrathecal chemotherapy. It commonly presents with headache, changes in neurologic status, fever, and malfunction of the reservoir. CSF pleocytosis is commonly encountered. The most frequently isolated organism is Staphylococcus epidermidis. Treatment requires intravenous antibiotics, with or without oral and intraventricular antibiotics. Some authors advocate the routine removal of the ventricular reservoir, whilst others reserve device removal for those that do not clear with antibiotic therapy.
BOX 15-2 Common Complications of Intraventricular Chemotherapy86
Myelosuppression may occur after administration of intrathecal chemotherapies, and some recommend that folinic acid rescue (10 mg every 6 hours for 24 hours) be given orally after the administration of methotrexate to mitigate this complication. Chemical aseptic meningitis occurs in nearly half of patients treated by intrathecal administration and is manifested by fever, headache, nausea, vomiting, meningismus, and photophobia. In the majority of patients, this inflammatory reaction can be treated in the outpatient setting with oral antipyretics, antiemetics, and corticosteroids. Rarely, treatment-related neurotoxicity occurs and may result in a symptomatic subacute leukoencephalopathy or myelopathy. However, in patients with NM and prolonged survival, the combination of radiotherapy and chemotherapy frequently results in a late leukoencephalopathy evident on neuroradiographic studies, which is occasionally symptomatic.1,5,70–72
The rationale to give intrathecal chemotherapy is based on the presumption that most chemotherapeutic agents, when given systemically, have poor CSF penetration and do not reach therapeutic levels. Exceptions to this would be systemic high-dose methotrexate, cytarabine, and thioTEPA, all of which result in cytotoxic CSF levels. Their systemic administration, however, is limited by systemic toxicity and the difficulty to integrate these regimens into other chemotherapeutic programs being used to manage systemic disease. Some authors argue that intrathecal chemotherapy does not add to improved outcome in the treatment of NM, since systemic therapy can obtain access to the subarachnoid deposits through their own vascular supply.63 In a retrospective comparison of patients treated with systemic chemotherapy and radiation to involved areas, plus or minus intrathecal chemotherapy, Bokstein did not find significant differences in response rates, median survival, or proportion of long-term survivors between the two groups but, of course, the group that did not receive the intrathecal treatment was spared the complications of this modality.73 Glantz treated 16 patients with high-dose intravenous methotrexate and compared their outcome with a reference group of 15 patients treated with intrathecal methotrexate.74 They found response rates and survival were significantly better in the group treated with intravenous therapy. Finally, a recent report describes two patients with breast cancer in whom NM was controlled with systemic hormonal treatment.75
Nonetheless, intrathecal chemotherapy remains the preferred treatment route for NM at this time. New drugs are being explored to try to improve the efficacy of treatment. These include mafosphamide, diaziquone, topotecan, interferon-α, and temozolomide. Immunotherapy, using IL-2 and IFN-α, 131I-radiolabelled monoclonal antibodies, gene therapy, and retuximab are other modalities that are being explored in clinical trials.76–81
SUPPORTIVE CARE
Not all patients with NM are candidates for the aggressive treatment outlined above (Box 15-3). Most authors agree that combined-modality therapy should be offered to patients with life expectancy greater than 3 months and a Karnofsky performance status of greater than 60%.
Supportive care should be offered to every patient, regardless of whether they receive NM-directed therapy. These therapies include anticonvulsants for seizure control (seen in 10% to 15% of patients with NM), adequate analgesia with opioid drugs as needed, as well as antidepressants and anxiolytics if necessary. Corticosteroids have a limited use in NM-related neurological symptoms, but can be useful to treat vasogenic edema associated with intraparenchymal or epidural metastases, or for the symptomatic treatment of nausea and vomiting together with routine antiemetics. Decreased attention and somnolence secondary to whole brain radiation can be treated with psychostimulants.5
Conclusions
A second feature of NM, which complicates therapy, is deciding whom to treat (Box 15-3). Not all patients necessarily warrant aggressive CNS-directed therapy, however, few guidelines exist directing appropriate choice of therapy. Based on the prognostic variables determined clinically and by evaluation of extent of disease, a sizable minority of patients will not be candidates for aggressive NM-directed therapy. Therefore, supportive comfort care (radiotherapy to symptomatic disease, antiemetics, and narcotics) is reasonably offered to patients with NM considered poor candidates for aggressive therapy, as seen in Figure 15-1.
1. W.R. Shapiro, J.B. Posner, Y. Ushio, et al. Treatment of meningeal neoplasms. Cancer Treat Rep. 1977;61:733-743.
2. J.L. Nugent, P.A. BunnJr, M.J. Matthews, et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44:1885-1893.
3. J.G. Kaplan, T.G. DeSouza, A. Farkash, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemia’s. J Neuro Oncol. 1990;9:225-229.
4. T. Siegal, A. Lossos, M.R. Pfeffer. Leptomeningeal metastases: analysis of 31 patients with sustained off- therapy response following combined-modality therapy. Neurology. 1994;44:1463-1469.
5. M.C. Chamberlain. Carcinomatous meningitis. Arch Neurol. 1997;54(1):16-17.
6. J.P. Glass, M. Melamed, N.L. Chernik, et al. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29(10):1369-1375.
7. W.R. Wasserstrom, J.P. Glass, J.B. Posner. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759-772.
8. J.R. Little, A.J. Dale, H. Okazaki. Meningeal carcinomatosis. Clinical manifestations. Arch Neurol. 1974;30(2):138-143.
9. S.T. Rosen, J. Aisner, R.W. Makuch, et al. Carcinomatous leptomeningitis in small cell lung cancer: a clinicopathologic review of the National Cancer Institute experience. Medicine (Baltimore). 1982;61(1):45-53.
10. M.H. Amer, M. Al Sarraf, L.H. Baker, et al. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42(2):660-668.
11. H.Y. Yap, B.S. Yap, C.K. Tashima, et al. Meningeal carcinomatosis in breast cancer. Cancer. 1978;42(1):283-286.
12. R.J. van Oostenbrugge, A. Twijnstra. Presenting features and value of diagnostic procedures in leptomeningeal metastases. Neurology. 1999;53(2):382-385.
13. M. Balm, J. Hammack. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53(7):626-632.
14. S.C. Sorensen, R.T. Eagan, M. Scott. Meningeal carcinomatosis in patients with primary breast or lung cancer. Mayo Clin Proc. 1984;59(2):91-94.
15. J.C. Gonzalez-Vitale, R. Garcia-Bunuel. Meningeal carcinomatosis. Cancer. 1976;37(6):2906-2911.
16. S.A. Grossman, M.J. Krabak. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25(2):103-119.
17. M.C. Chamberlain. Radioisotope CSF flow studies in leptomeningeal metastases. J Neuro Oncol. 1998;38(2–3):135-140.
18. R. Boyle, M. Thomas, J.H. Adams. Diffuse involvement of the leptomeninges by tumour—a clinical and pathological study of 63 cases. Postgrad Med J. 1980;56(653):149-158.
19. G. Wolfgang, D. Marcus, S. Ulrike. Leptomeningeal Carcinomatosis; Clinical syndrome in different primaries. J Neuro Oncol. 1998;38:103-110.
20. M.C. Chamberlain, P.R. Kormanik. Carcinomatous meningitis secondary to breast cancer: predictors of response to combined modality therapy. J Neuro Oncol. 1997;35(1):55-64.
21. H.W. Kolmel. Cytology of neoplastic meningosis. J Neuro Oncol. 1998;38(2–3):121-125.
22. J.J. Murray, F.A. Greco, S.N. Wolff, et al. Neoplastic meningitis. Marked variations of cerebrospinal fluid composition in the absence of extradural block. Am J Med.. 1983;75(2):289-294.
23. L.R. Rogers, P.M. Duchesneau, C. Nunez, et al. Comparison of cisternal and lumbar CSF examination in leptomeningeal metastasis. Neurology. 1992;42(6):1239-1241.
24. M.C. Chamberlain, P.A. Kormanik, M.J. Glantz. A comparison between ventricular and lumbar cerebrospinal fluid cytology in adult patients with leptomeningeal metastases. Neuro-Oncol. 2001;3(1):42-45.
25. M.J. Glantz, B.F. Cole, L.K. Glantz, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733-739.
26. L.M. DeAngelis. Current diagnosis and treatment of leptomeningeal metastasis. J Neuro Oncol. 1998;38(2–3):245-252.
27. W.R. Wasserstrom, M.K. Schwartz, M. Fleisher, et al. Cerebrospinal fluid biochemical markers in central nervous system tumors: a review. Ann Clin Lab Sci. 1981;11(3):239-251.
28. A.P. Van Zanten, A. Twijnstra, A.A. Hart, et al. Cerebrospinal fluid lactate dehydrogenase activities in patients with central nervous system metastases. Clin Chim Acta. 1986;161(3):259-268.
29. G.G. Klee, R.D. Tallman, J.R. Goellner, et al. Elevation of carcinoembryonic antigen in cerebrospinal fluid among patients with meningeal carcinomatosis. Mayo Clin Proc. 1986;61(1):9-13.
30. A. Twijnstra, A.P. van Zanten, A.A. Hart, et al. Serial lumbar and ventricle cerebrospinal fluid lactate dehydrogenase activities in patients with leptomeningeal metastases from solid and haematological tumours. J Neurol Neurosurg Psychiatry. 1987;50(3):313-320.
31. G. Stockhammer, W. Poewe, S. Burgstaller, et al. Vascular endothelial growth factor in CSF: a biological marker for carcinomatous meningitis. Neurology. 2000;54(8):1670-1676.
32. M.C. Chamberlain. Cytologically negative carcinomatous meningitis: usefulness of CSF biochemical markers. Neurology. 1998;50(4):1173-1175.
33. J.A. Garson, H.B. Coakham, J.T. Kemshead, et al. The role of monoclonal antibodies in brain tumour diagnosis and cerebrospinal fluid (CSF) cytology. J Neuro Oncol. 1985;3(2):165-171.
34. A. Hovestadt, SC. Henzen-Logmans, C.J. Vecht. Immunohistochemical analysis of the cerebrospinal fluid for carcinomatous and lymphomatous leptomeningitis. Br J Cancer. 1990;62(4):653-654.
35. W. Boogerd, T.M. Vroom, P. van Heerde, et al. CSF cytology versus immunocytochemistry in meningeal carcinomatosis. J Neurol Neurosurg Psychiatry. 1988;51(1):142-145.
36. R.J. Van Oostenbrugge, A.H. Hopman, F.C. Ramaekers, et al. In situ hybridization: a possible diagnostic aid in leptomeningeal metastasis. J Neuro Oncol. 1998;38(2–3):127-133.
37. E.S. Cibas, M.G. Malkin, J.B. Posner, et al. Detection of DNA abnormalities by flow cytometry in cells from cerebrospinal fluid. Am J Clin Pathol. 1987;88(5):570-577.
38. S. Biesterfeld, B. Bernhard, S. Bamborschke, et al. DNA single cell cytometry in lymphocytic pleocytosis of the cerebrospinal fluid. Acta Neuropathol (Berl). 1993;86(5):428-432.
39. R.J. Van Oostenbrugge, A.H. Hopman, J.W. Arends, et al. The value of interphase cytogenetics in cytology for the diagnosis of leptomeningeal metastases. Neurology. 1998;51(3):906-908.
40. C.H. Rhodes, M.J. Glantz, L. Glantz, et al. A comparison of polymerase chain reaction examination of cerebrospinal fluid and conventional cytology in the diagnosis of lymphomatous meningitis. Cancer. 1996;77(3):543-548.
41. T.M. Cheng, B.P. O’Neill, B.W. Scheithauer, et al. Chronic meningitis: the role of meningeal or cortical biopsy. Neurosurgery. 1994;34(4):590-595.
42. M.C. Chamberlain, A.D. Sandy, G.A. Press. Leptomeningeal metastasis: a comparison of gadolinium-enhanced MR and contrast-enhanced CT of the brain. Neurology. 1990;40(3 Pt 1):435-438.
43. M. Schumacher, M. Orszagh. Imaging techniques in neoplastic meningiosis. J Neuro Oncol. 1998;38(2–3):111-120.
44. G. Sze, S. Soletsky, R. Bronen, et al. MR imaging of the cranial meninges with emphasis on contrast enhancement and meningeal carcinomatosis. AJR Am J Roentgenol. 1989;153(5):1039-1049.
45. B. Schuknecht, P. Huber, B. Buller, et al. Spinal leptomeningeal neoplastic disease. Evaluation by MR, myelography and CT myelography. Eur Neurol.. 1992;32(1):11-16.
46. M.C. Chamberlain. Comparative spine imaging in leptomeningeal metastases. J Neuro Oncol. 1995;23(3):233-238.
47. R.J. Freilich, G. Krol, L.M. DeAngelis. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38(1):51-57.
48. R.L. MittlJr, D.M. Yousem. Frequency of unexplained meningeal enhancement in the brain after lumbar puncture. AJNR Am J Neuroradiol. 1994;15(4):633-638.
49. M.J. Glantz, W.A. Hall, B.F. Cole, et al. Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer. 1995;75(12):2919-2931.
50. S.A. Grossman, C.L. Trump, D.C.P. Chen, G. Thompson, E. Camargo. Cerebrospinal flow abnormalities in patients with neoplastic meningitis. Am J Med. 1982;73:641-647.
51. M.C. Chamberlain, P.A. Kormanik. Prognostic significance of 111indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology. 1996;46(6):1674-1677.
52. W.P. Mason, S.D. Yeh, L.M. DeAngelis. 111Indium-diethylenetriamine pentaacetic acid cerebrospinal fluid flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology. 1998;50(2):438-444.
53. M.C. Chamberlain, J. Corey-Bloom. Leptomeningeal metastases: 111indium-DTPA CSF flow studies. Neurology. 1991;41(11):1765-1769.
54. M.C. Chamberlain, P. Kormanik, K.A. Jaeckle, et al. 111Indium-diethylenetriamine pentaacetic acid CSF flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology. 1999;52(1):216-217.
55. J. Hildebrand. Prophylaxis and treatment of leptomeningeal carcinomatosis in solid tumors of adulthood. J Neuro Oncol. 1998;38(2–3):193-198.
56. M.C. Chamberlain, P.A. Kormanik. Prognostic significance of coexistent bulky metastatic central nervous system disease in patients with leptomeningeal metastases. Arch Neurol. 1997;54(11):1364-1368.
57. M.C. Chamberlain. Neoplastic meningitis-related encephalopathy: prognostic significance. Neurology. 2003;60(5):A17-A18.
58. W.R. Shapiro, D.F. Young, B.M. Mehta. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975;293(4):161-166.
59. U. Berweiler, A. Krone, J.C. Tonn. Reservoir systems for intraventricular chemotherapy. J Neuro Oncol. 1998;38(2–3):141-143.
60. D.I. Sandberg, M.H. Bilsky, M.M. Souweidane, et al. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery. 2000;47(1):49-54.
61. W.T. Sause, J. Crowley, H.J. Eyre, et al. Whole brain irradiation and intrathecal methotrexate in the treatment of solid tumor leptomeningeal metastases–a Southwest Oncology Group study. J Neuro Oncol. 1988;6(2):107-112.
62. M.C. Chamberlain, P. Kormanik. Carcinoma meningitis secondary to non-small cell lung cancer: combined modality therapy. Arch Neurol. 1998;55(4):506-512.
63. T. Siegal. Leptomeningeal metastases: rationale for systemic chemotherapy or what is the role of intra-CSF-chemotherapy? J Neuro Oncol. 1998;38(2–3):151-157.
64. L. Giannone, F.A. Greco, J.D. Hainsworth. Combination intraventricular chemotherapy for meningeal neoplasia. J Clin Oncol. 1986;4(1):68-73.
65. R.N. Hitchins, D.R. Bell, R.L. Woods, et al. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987;5(10):1655-1662.
66. S.A. Grossman, D.M. Finkelstein, J.C. Ruckdeschel, et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. J Clin Oncol. 1993;11:561-569.
67. M.J. Glantz, K.A. Jaeckle, M.C. Chamberlain, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394-3402.
68. M.J. Glantz, S. LaFollette, K.A. Jaeckle, W. Shapiro, L. Swinnen, J.R. Rozental, et al. Randomized Trial of a Slow Release Versus a Standard Formulation of Cytarabine for the Intrathecal Treatment of Lymphomatous Meningitis. J Clin Oncol. 1999;17:3110-3116.
69. K.A. Jaeckle, T. Batchelor, S.J. O’Day, S. Phuphanich, P. New, G. Lesser, et al. An open trial of sustained-release cytarabine (Depocyt) for the intrathecal treatment of solid tumor neoplastic meningitis. J Neuro Oncol. 2002;57(3):231-239.
70. T. Siegal, M.R. Pfeffer, I. Steiner. Antibiotic therapy for infected Ommaya reservoir systems. Neurosurgery. 1988;22(1 Pt 1):97-100.
71. J.Z. Kerr, S. Berg, S.M. Blaney. Intrathecal chemotherapy. Crypt Rev Oncol Hematol. 2001;37(3):227-236.
72. W.A. Bleyer, J.C. Drake, B.A. Chabner. Neurotoxicity and elevated cerebrospinal-fluid methotrexate concentration in meningeal leukemia. N Engl J Med. 1973;289(15):770-773.
73. F. Bokstein, A. Lossos, T. Siegal. Leptomeningeal metastases from solid tumors: a comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer. 1998;82(9):1756-1763.
74. M.J. Glantz, B.F. Cole, L. Recht, et al. High-Dose Intravenous Methotrexate for Patients with Nonelukemic Leptomeningeal Cancer: Is Intrathecal Chemotherapy Necessary? J Clin Oncol. 1998;16(4):1561-1567.
75. W. Boogerd, L.DA. Dorresteijn, J.J. van der Sande, et al. Response of leptomeningeal metastases from breast cancer to hormonal therapy. Neurology. 2000;55(1):117-119.
76. S.M. Blaney, D.G. Poplack. New cytotoxic drugs for intrathecal administration. J Neuro Oncol. 1998;38:219-223.
77. J.H. Sampson, G.E. Archer, A.T. Villavicencio, et al. Treatment of Neoplastic Meningitis with Intrathecal Temozolomide. Clin Cancer Res. 1999;5:1183-1188.
78. U. Herrlinger, M. Weller, M. Schabet. New aspects of immunotherapy of leptomeningeal metastasis. J Neuro Oncol. 1998;38:233-239.
79. H.B. Coakham, J.T. Kemshead. Treatment of neoplastic meningitis by targeted radiation using 131I-radiolabelled monoclonal antibodies. J Neuro Oncol. 1998;38:225-232.
80. F.D. Vrionis. Gene Therapy of Neoplastic Meningiosis. J Neuro Oncol. 1998;38:241-244.
81. J.I. Rubenstein, J. Fridlyand, L. Abrey, et al. Phase 1 study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25(11):1350-1356.
82. M.C. Chamberlain, DD. Wei-Tao, S. Groshen. A Phase 2 trial of intra-CSF etoposide in the treatment of neoplastic meningitis. Cancer. 2006;31(9):2021-2027.
83. M.C. Chamberlain. Alpha-Interferon in the Treatment of Neoplastic Meningitis. Cancer. 2002;94:2675-2680.
84. W.R. Shapiro, M. Schmid, M. Glantz, J.J. Miller. A randomized phase III/IV study to determine benefit and safety of cytarabine liposome injection for treatment of neoplastic meningitis. J Clin Oncol. 2006;24(June 6 Suppl):1528. (abstract)
85. W. Boogerd, M.J. van den Bent, P.J. Koehler, J.J. Heimans, J.J. Van der Sande, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomized study. Eur J Cancer. 2004;40:2726-2733.
86. M.C. Chamberlain, P.A. Kormanik, D. Barba. Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J Neurosurg. 1997;87:694-699.