CHAPTER 137 Neoplastic Meningitis
The survival time of patients with NM is short, an average of 3.5 to 6 months. Longer survival rates are occasionally observed in patients with breast cancer (13% at 1 year and 6% at 2 years).1 In retrospective series, prognostic factors that correlate with longer survival times include young age, a Karnofsky Performance Scale (KPS) score higher than 70, a long duration of related symptoms, controlled systemic disease, lack of encephalopathy or cranial nerve deficits, low cerebrospinal fluid (CSF) protein levels, the presence of breast cancer, and in neuroimaging studies, the lack of bulky leptomeningeal deposits.1–7
Incidence
NM occurs in approximately 4% to 8% of patients with solid tumors, in 5% to 15% of patients with hematologic malignancies, and in less than 2% of those with primary brain tumors.1 The frequency of NM is also dependent on the histology and grade of the tumor and the duration of the disease. In approximately 1% to 7% of patients with NM, the primary tumor has not been located at the patient’s initial evaluation.8 NM is more likely to develop in patients with high-risk lymphomas (diffuse large B-cell lymphoma and Burkitt’s lymphoma), ALL, melanoma, breast adenocarcinoma, and small cell lung carcinoma.9–11
Pathogenesis
Metastasis to the leptomeninges is thought to result from hematogenous dissemination, but direct extension to the meninges can occur from parameningeal or contiguous bony structures (e.g., the base of the skull or vertebrae, regional lymph nodes, soft tissues) or by retrograde growth along the adventitia of blood vessels or perineurium of the spinal and cranial nerve roots.10,12 After entering the meninges, the CSF circulation provides an effective conduit for dissemination of tumor cells to the distant surfaces of the brain, spinal cord, nerve roots, and the ventricular ependymal surfaces. Late in the disease, invasion of the brain and spinal cord parenchyma by tumor from the meninges produces edema, microinfarction, and striking neurological dysfunction. Symptoms may also result from secondary complications such as hydrocephalus and increased intracranial pressure. Although this paradigm is accepted, it does not completely explain why NM does not develop in some patients with extensive systemic metastases, and little is known regarding the initial molecular and cellular mechanisms involved in the pathogenesis of NM.
Clinical Features
The typical clinical manifestations of NM are dependent on the level of nervous system involvement. Most commonly, patients have altered mental status, headache, diplopia, seizures, back pain, lower extremity weakness or numbness, or other cranial neuropathies. Positional headache, intractable nausea with vomiting, and visual obscurations may reflect increased intracranial pressure from CSF obstruction. The classic meningeal sign of nuchal rigidity has been reported in less than 20% of patients.8 Occasional patients, in particular those with breast cancer, may have more indolent courses. However, most patients rapidly progress at an alarming rate and experience neurological and systemic decline despite treatment efforts.
Diagnosis
Examination of Cerebrospinal Fluid
Definitive diagnosis of NM is made by identification of malignant cells in CSF. The relative sensitivity of this test depends on the number and volume of CSF specimens examined, the state of preservation of the CSF cells, and the source of the specimen (lumbar or cisternal being more reliable than intraventricular fluid). In one study, the sensitivity of the initial CSF examination was approximately 50% to 60% and improved to about 80% with a second specimen, and then each subsequent sample increased sensitivity by 2% to 5%.13 In another study, the yield of a single CSF analysis improved if the CSF volume studied exceeded 10.5 mL and was removed from the location (lumbar, cisternal, ventricular) nearest the symptomatic site or the area of greatest involvement, as seen on neuroimaging studies.14 Most cytopathologists prefer to assess serial samples obtained on different dates rather than a single large-volume specimen.
Although nonspecific, CSF protein determination has historically been the most sensitive indicator of NM because this protein level is abnormal (>45 mg/dL) in approximately 80% to 90% of patients.13 Conversely, finding a normal CSF protein reading is relatively strong (but not absolute) evidence against this diagnosis. Lumbar CSF is more likely than ventricular fluid to have elevated protein and malignant cells. Cisternal fluid has also been proposed to be a more reliable indicator, but its acquisition may carry greater risk.15 If high elevations (>500 mg/dL) of CSF protein are found in lumbar fluid, either NM is advanced or there is a partial or complete blockage of CSF flow from cephalad locations. Approximately 30% to 57% of patients have lumbar CSF pressure higher than 150 mm H2O, and approximately 31% to 55% have a reduction in CSF glucose levels. In patients with subsequently verified NM, less than 5% have completely normal CSF profiles.8
Neuroimaging
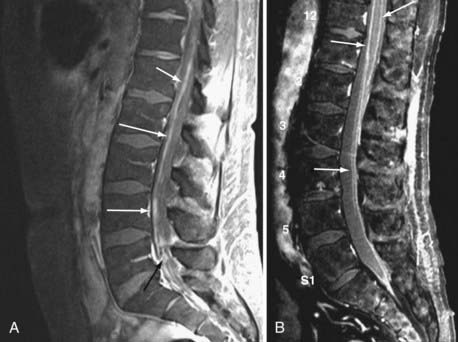
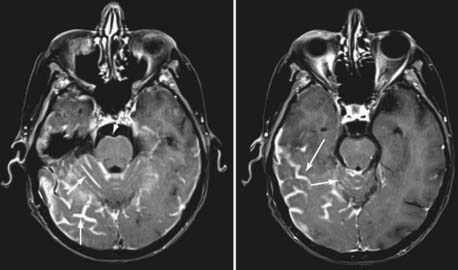
Currently, magnetic resonance imaging (MRI) of the brain and spine with gadolinium contrast enhancement is the procedure of choice. Findings on contrast-enhanced MRI will appear abnormal during the course of the disease in approximately 70% of patients with NM.16 High-resolution MRI is necessary for proper evaluation of the meninges of the spine. In general, lower resolution “open” MRI is less reliable for identification of NM unless florid abnormalities are present. Patchy nerve root enhancement, matting of nerve roots of the cauda equina, nodular deposits, and linear, continuous enhancement of the pia-arachnoid of the conus medullaris, brainstem, and cerebrum are hallmarks of the diagnosis (Fig. 137-1). Patchy, asymmetric enhancement of the leptomeninges over the cerebral convexities and in the sulci, basilar cisterns, insulae, pituitary stalk, cranial nerve roots, or superior cerebellar folia (or any combination of these sites) may be observed (Fig. 137-2). Indirect signs include communicating hydrocephalus, bilateral transependymal edema, and effacement of convexity sulci. Contrast enhancement of the meninges can be associated with many non-neoplastic conditions, including prior or current infections, previous subarachnoid hemorrhage, previous lumbar puncture or intrathecal chemotherapy, prior neurosurgical procedures (e.g., placement of an intraventricular reservoir), chronic CSF leak syndrome, and other neurological conditions.16 Nevertheless, in a patient with a high KPS score and active systemic cancer in whom symptoms or signs and neuroimaging findings strongly suggest NM, some physicians believe that treatment is justifiable even if the CSF cytologic study is negative.17,18
Cerebrospinal Fluid Flow Studies
Some investigators have proposed that CSF flow dynamics should be evaluated before initiating treatment of NM. CSF flow is usually assessed by nuclear medicine techniques with either 111In-diethylenetriamine pentaacetic acid or, less commonly, 99Tc-labeled albumin. Partial or total blockage of CSF flow has been identified in up to 30% to 70% of patients with NM.19,20 The stated rationale for performing flow studies includes identifying CSF flow blockage before treatment, preventing accumulation of high concentrations of administered drug in areas of CSF loculation, and conversely, identifying areas that would not receive adequate drug concentration beyond the areas of blockage. Retrospective studies have suggested that outcome is poorer in patients with CSF blocks or those in whom blocks cannot be opened with focal radiation therapy (RT).19,20 To date, routine use of flow studies has not gained widespread acceptance among practitioners.
Meningeal Biopsy
Occasional patients are appropriate candidates for meningeal biopsy, but most are not because of poor performance status and comorbid conditions. The best candidates are patients with high clinical suspicion of NM but whose CSF cytologic studies are repetitively negative. Nevertheless, the sensitivity and specificity of this technique have proved to be low, particularly when there is no acceptable target showing MRI contrast enhancement, and the procedure carries added risks for the patient.21
Prognosis
Most patients with NM from solid tumors survive only 3 to 4 months after diagnosis.8 Patients with NM from breast cancer may live longer, with reports of 1-year survival rates varying from 11% to 25%.22 Several factors appear to correlate with improved prognosis, including young age, good performance status, long duration of symptoms, no cranial nerve deficits or encephalopathy, and controlled systemic disease. It should be stated that these factors have not yet been validated by multivariate analysis in controlled prospective studies. To date, no specific factors have predicted a survival benefit for these patients after therapy.
Treatment
Surgery
Neurosurgical procedures may include placement of intraventricular (e.g., Ommaya) reservoirs for CSF access, ventriculoperitoneal shunting for hydrocephalus, and the occasional meningeal biopsy. Justifications stated for Ommaya placement include the ease of repetitive access to CSF, decreased patient discomfort in comparison to lumbar access, and improved drug distribution within the CSF pathways after intraventricular administration.23 Except for patients with childhood ALL, clinical studies have not shown statistically significant improvement in overall survival when comparing intraventricular with intralumbar drug administration. The intraventricular reservoir is typically placed below the galea in the right frontal region (in left hemisphere–dominant individuals), with the catheter located within the right frontal horn of the lateral ventricle.24 Complications of reservoir placement occur in approximately 10% of patients and include aseptic meningitis (43%), malpositioning (2% to 12%), obstruction of the catheter (6%), and bacterial infection (2% to 13%). Bacteria isolated from infected systems are usually Staphylococcus epidermidis or Propionibacterium, although more pathogenic organisms, including Staphylococcus aureus, Streptococcus pyogenes, and gram-negative species, can be found.25
Chemotherapy
Many patients in whom NM is diagnosed are too ill to receive aggressive therapy, and they opt for supportive or hospice care. However, there remain certain younger patients with high KPS scores and controlled systemic disease who many believe are more appropriate candidates for drug therapy. Many systemically administered agents exhibit incomplete penetration of the blood-brain barrier. Penetration (CSF concentration/systemic blood concentration) is reasonable for thiotepa (90%), topotecan (30%), temozolomide (30%), and cytarabine (ara-C; 20% to 28%) but low for MTX (3%).26 Some response to systemically administered capecitabine has been reported in patients with NM from breast adenocarcinoma.27,28 Temozolomide has produced responses in NM accompanying malignant gliomas.29 In a small study of 31 patients, cytologic clearing of tumor cells from CSF was as frequent with systemic administration of high-dose MTX as with intrathecal administration of the conventional dose. Survival time was also significantly longer (13.8 versus 2.3 months, P = .003) in patients receiving systemic MTX than intrathecal MTX.30 In another small study, 104 patients were randomized to receive either RT plus systemic chemotherapy (various agents) and intrathecal chemotherapy or RT plus systemic chemotherapy alone. There were no differences in outcome, with both groups having a median overall survival interval of 4 months, and more toxicity was observed in the patients receiving intrathecal chemotherapy.31
Theoretically, intrathecal administration can provide adequate CSF drug delivery and thus obviate the need for the high, potentially toxic systemic doses necessary to achieve the same concentrations. However, for most neoplasms, survival has not yet been shown to be superior after intrathecal treatment. There are several potential barriers to intrathecal treatment with respect to chemotherapeutic agents, including their short half-life, cell cycle specificity, CSF compartmentalization, and inadequate penetration of the CNS parenchymal surfaces. Previous studies have reported that only 55% of CSF tumor cells cycle in a 10-day span, with most cells in G0, only 0.1% in S phase, and 1% in mitosis.23 In contrast, the half-life of most intrathecal agents is measured in only minutes or a few hours at most.32 Some agents are not converted to active metabolites within CSF; for example, triethylenephosphoramide (TEPA), the active metabolite of thiotepa, is not measurable in CSF after intrathecal administration.
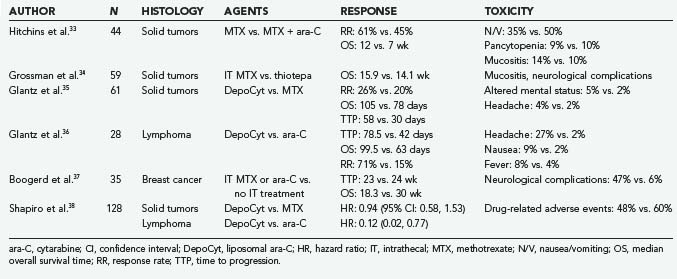
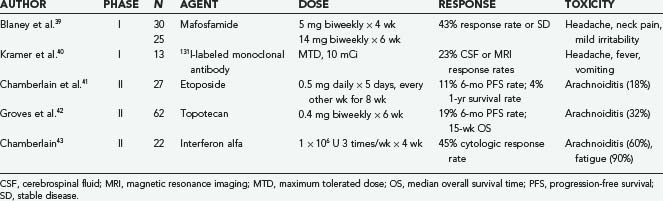
Nevertheless, agents that have been administered intrathecally, either in anecdotal reports or in clinical trials, include MTX, ara-C, sustained-release ara-C (depo-ara-C), thiotepa, mafosfamide, etoposide, rituximab, interferon alfa, and topotecan. The first four of these agents have been used most often in clinical practice. It should be stressed that many of these agents have not yet been approved by the Food and Drug Administration (FDA) for this specific indication. Depo-ara-C has received FDA approval for the treatment of lymphomatous meningitis, and MTX and ara-C are indicated for the treatment of lymphomatous and leukemic meningitis. The results of phase II studies and randomized controlled clinical trials involving intrathecal agents are summarized in Tables 137-1 and 137-2.
Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53:626-632.
Berg SL, Chamberlain MC. Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy, and symptom management. In: Abrey LE, Chamberlain MC, Engelhard HH, editors. Leptomeningeal Metastases. New York: Springer; 2005:121-146.
Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomized study. Eur J Cancer. 2004;40:2726-2733.
Chamberlain MC. Carcinomatous meningitis. Arch Neurol. 1997;54:16-17.
DeAngelis LM. Current diagnosis and treatment of leptomeningeal metastasis. J Neurooncol. 1998;38:245-252.
Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561-1567.
Glantz MJ, LaFollette S, Jaeckle KA, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999;17:3110-3116.
Glass JP, Melamed M, Chernik NL, et al. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29:1369-1375.
Grossman SA, Finkelstein DM, Ruckdeschel JC, et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously treated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11:561-569.
Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103-119.
Jayson GC, Howell A. Carcinomatous meningitis in solid tumours. Ann Oncol. 1996;7:773-786.
1 Chamberlain MC. Carcinomatous meningitis. Arch Neurol. 1997;54:16-17.
2 Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103-119.
3 Chamberlain MC, Kormanik PA. Prognostic significance of coexistent bulky metastatic central nervous system disease in patients with leptomeningeal metastases. Arch Neurol. 1997;54:1364-1368.
4 Chamberlain MC, Kormanik PR. Carcinomatous meningitis secondary to breast cancer: predictors of response to combined modality therapy. J Neurooncol. 1997;35:55-64.
5 Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53:626-632.
6 Fizazi K, Asselain B, Vincent-Salomon A, et al. Meningeal carcinomatosis in patients with breast carcinoma. Clinical features, prognostic factors, and results of a high-dose intrathecal methotrexate regimen. Cancer. 1996;77:1315-1323.
7 Jayson GC, Howell A. Carcinomatous meningitis in solid tumours. Ann Oncol. 1996;7:773-786.
8 Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49:759-772.
9 Shapiro WR, Posner JB, Ushio Y, et al. Treatment of meningeal neoplasms. Cancer Treat Rep. 1977;61:733-743.
10 Rosen ST, Aisner J, Makuch RW, et al. Carcinomatous leptomeningitis in small cell lung cancer: a clinicopathologic review of the National Cancer Institute experience. Medicine (Baltimore). 1982;61:45-53.
11 Amer MH, Al Sarraf M, Baker LH, et al. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42:660-668.
12 Gonzalez-Vitale JC, Garcia-Bunuel R. Meningeal carcinomatosis. Cancer. 1976;37:2906-2911.
13 Glass JP, Melamed M, Chernik NL, et al. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29:1369-1375.
14 Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82:733-739.
15 Rogers LR, Duchesneau PM, Nunez C, et al. Comparison of cisternal and lumbar CSF examination in leptomeningeal metastasis. Neurology. 1992;42:1239-1241.
16 Chamberlain MC, Sandy AD, Press GA. Leptomeningeal metastasis: a comparison of gadolinium-enhanced MR and contrast-enhanced CT of brain. Neurology. 1990;40:435-438.
17 Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38:51-57.
18 Chamberlain MC. Cytologically negative carcinomatous meningitis; usefulness of CSF biochemical markers. Neurology. 1998;50:1173-1175.
19 Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol. 1998;38:135-140.
20 Glantz MJ, Hall WA, Cole BF, et al. Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer. 1995;75:2919-2931.
21 Cheng TM, O’Neill BP, Scheithauer BW, et al. Chronic meningitis: the role of meningeal or cortical biopsy. Neurosurgery. 1994;34:590-595.
22 DeAngelis LM. Current diagnosis and treatment of leptomeningeal metastasis. J Neurooncol. 1998;38:245-252.
23 Kuo AH, Yataganas X, Galicich JH, et al. Proliferative kinetics of central nervous system (CNS) leukemia. Cancer. 1975;36:232-239.
24 Berweiler U, Krone A, Tonn JC. Reservoir systems for intraventricular chemotherapy. J Neurooncol. 1998;38:141-143.
25 Chamberlain MC, Kormanik PA, Barba D. Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J Neurosurg. 1997;87:694-699.
26 Berg SL, Chamberlain MC. Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy, and symptom management. In: Abrey LE, Chamberlain MC, Engelhard HH, editors. Leptomeningeal Metastases. New York: Springer; 2005:121-146.
27 Rogers LR, Remer SE, Tejwani S. Durable response of breast cancer leptomeningeal metastasis to capecitabine monotherapy. Neuro Oncol. 2004;6:63-64.
28 Giglio P, Tremont-Lukats IW, Groves MD. Response of neoplastic meningitis from solid tumors to oral capecitabine. J Neurooncol. 2003;65:167-172.
29 Friedman HS. Temozolomide in early stages of newly diagnosed malignant glioma and neoplastic meningitis. Semin Oncol. 2000;27:35-40.
30 Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561-1567.
31 Siegal T. Leptomeningeal metastases: rationale for systemic chemotherapy or what is the role of intra-CSF-chemotherapy? J Neurooncol. 1998;38:151-157.
32 Blasberg RG, Patlak C, Fenstermacher JD. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J Pharmacol Exp Ther. 1975;195:73-83.
33 Hitchens RN, Bell DR, Woods RL, et al. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987;5:1655-1662.
34 Grossman SA, Finkelstein DM, Ruckdeschel JC, et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously treated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11:561-569.
35 Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (Depocyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3349-3351.
36 Glantz MJ, LaFollette S, Jaeckle KA, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999;17:3110-3116.
37 Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomized study. Eur J Cancer. 2004;40:2726-2733.
38 Shapiro WR, Schmid M, Glantz M, et al. A randomized phase III/IV study to determine benefit and safety of cytarabine liposome injection for treatment of neoplastic meningitis [abstract]. J Clin Oncol. 2006;24(18S):1528.
39 Blaney SM, Balis FM, Berg S, et al. Intrathecal mafosfamide: a preclinical pharmacology and phase I trial. J Clin Oncol. 2005;23:1555-1563.
40 Kramer K, Humm JL, Souweidan MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancer using intra-Ommaya 131-I-3F8. J Clin Oncol. 2007;25:5465-5470.
41 Chamberlain MC, Tsoa-Wei DD, Groshen S. Phase II trial of intracerebrospinal fluid etoposide in the treatment of neoplastic meningitis. Cancer. 2006;106:2021-2027.
42 Groves MD, Glantz MJ, Chamberlain MD, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10:208-215.
43 Chamberlain MC. A phase II trial of intra-cerebrospinal fluid alpha interferon in the treatment of neoplastic meningitis. Cancer. 2002;94:275-280.