Neonatal Ophthalmology
Normal Visual Development
The pupillary light reflex should be observable after 31 weeks’ gestational age. A blink reflex to light can often be observed a few days after birth. 123
Basic Ocular Examination
Strabismus refers to misalignment of the eyes. Intermittent strabismus is often observed in the newborn and tends to resolve. Strabismus that persists beyond the first few months of life should be referred to an ophthalmologist for further evaluation. 45
ROP
Retinal vascular development begins during the second trimester of pregnancy, and full maturation typically occurs during or after the third trimester of pregnancy. In premature babies much of this development is taking place ex utero. The abnormal retinal development seen in ROP is in response to the artificial environment experienced by the neonate after birth.
In the first phase of pathogenesis, hyperoxia leads to cessation of the normal vascular development of the peripheral retina. In the second phase increased metabolic demand causes relative hypoxia to the peripheral retina, which leads to increased production of pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF) in the eye. This in turn stimulates abnormal proliferative vascular development. The proliferation can cause traction on the retina and bleeding inside the eye, which leads to vision loss ( Fig.15-1).
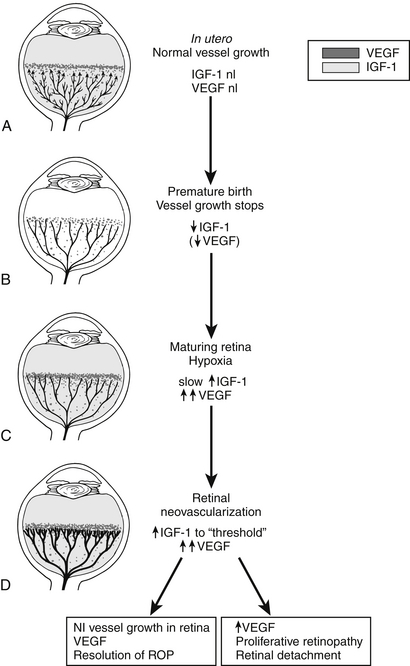
Figure 15-1 Schematic representation of IGF-1/VEGF control of blood vessel development in ROP. A, In utero, VEGF is found at the growing front of vessels. IGF-1 is sufficient to allow vessel growth. B, With premature birth, IGF-1 is not maintained at in utero levels, and vascular growth ceases despite the presence of VEGF at the growing front of vessels. Both endothelial cell survival (AKT) and proliferation (MAPK) pathways are compromised. With low IGF-1 and cessation of vessel growth, a demarcation line forms at the vascular front. High oxygen exposure (as occurs in animal models and in some premature infants) may also suppress VEGF, further contributing to inhibition of vessel growth. C, As the premature infant matures, the developing but nonvascularized retina becomes hypoxic. VEGF increases in retina and vitreous. With maturation, the IGF-1 level slowly increases. D, When the IGF-1 level reaches a threshold at 34 weeks’ gestation, with high VEGF levels in the vitreous, endothelial cell survival and proliferation driven by VEGF may proceed. Neovascularization ensues at the demarcation line, growing into the vitreous. If VEGF vitreal levels fall, normal retinal vessel growth can proceed. With normal vascular growth and blood flow, oxygen suppresses VEGF expression, so it will no longer be overproduced. If hypoxia (and elevated levels of VEGF) persists, further neovascularization and fibrosis leading to retinal detachment can occur. (Smith LEH. Pathogenesis of retinopathy of prematurity. Semin Neonatol 2003;8:469–73.)
The examination involves dilating the child’s pupils with eyedrops (e.g. Cyclomydril, which is a combination of 1% phenylephrine, a sympathomimetic, and 0.2% cyclopentolate, an anticholinergic). These drops are usually administered in each eye by the nursing staff. During the eye examination the ophthalmologist will typically look at the anterior segment (cornea, iris, and lens) of the eye with a penlight, and then examine the retina using indirect ophthalmoscopy (a headlamp with a handheld lens). The examination may include using a lid speculum to keep the eyelids open during the examination and pressing gently on the sclera using a small rod to view the peripheral retina. If retinopathy is noted, the severity (stage), extent (clock hours), location relative to the central retina (zone), and degree of vascular tortuosity (plus) are recorded. Examinations continue every 1 to 2 weeks until the peripheral retina is fully vascularized or more frequently if warranted by clinical findings ( Table 15-1).
TABLE 15-1
AGE AND TIMING OF FIRST ROP SCREENING EXAMINATION FOR INFANTS
| AGE AT BIRTH | TIMING OF FIRST ROP EXAMINATION |
| 24 weeks GA | 31 weeks GA |
| 25 weeks GA | 31 weeks GA |
| 26 weeks GA | 31 weeks GA |
| 27 weeks GA | 31 weeks GA |
| 28 weeks GA | 32 weeks GA |
| 29 weeks GA | 33 weeks GA |
| 30 weeks GA | 34 weeks GA |
| >30 weeks GA | 4 weeks after birth |
Laser treatment to the peripheral retina is currently the standard of care for the treatment of type 1 ROP. The treatment attempts to halt the production of VEGF by ablating the metabolically active, yet hypoxic peripheral retina. Ophthalmologists are divided regarding whether the infant should be intubated and anesthetized for the laser procedure or whether it may be performed at the NICU bedside with topical anesthesia and intravenous sedation. This varies depending on surgeon preference, extent of treatment, and other systemic comorbidities.
Usually yes. Although severe complications are less frequent, follow-up with a pediatric ophthalmologist is recommended for infants with ROP who do not require treatment. This is because of the increased prevalence of myopia, strabismus, and astigmatic refractive errors in this population. 678910111213
Torch Complex
TABLE 15-2
COMMON EYE MANIFESTATIONS OF TORCH INFECTIONS
| NAME | SYSTEMIC MANIFESTATIONS | COMMON EYE MANIFESTATIONS |
| Toxoplasma gondii | Hydrocephalus, intracranial calcifications | Chorioretinitis |
| Rubella | Deafness, heart disease | Cataract, retinopathy, glaucoma |
| Cytomegalovirus | Most asymptomatic | Chorioretinitis |
| Herpes simplex virus | Sepsis (natal/postnatal) | Conjunctivitis, chorioretinitis |
| Syphilis | Hepatosplenomegaly, rash | Interstitial keratitis, cataract, chorioretinitis |
Chorioretinal scar or active chorioretinitis is most characteristic. This has been reported in congenital toxoplasmosis, syphilis, cytomegalovirus, herpes simplex, lymphocytic choriomeningitis virus, varicella zoster virus, and West Nile virus. Chorioretinitis can be seen at birth but is often not evident until approximately the 10th day of life. 14
Cataract
A cataract is an opacity of the crystalline intraocular lens. Congenital cataracts can be associated with intrauterine infections, associated syndromes, metabolic disorders, genetic factors, or other ocular abnormalities. The prevalence is estimated to be between 1 and 13 cases per 10,000 births ( Table 15-3).
TABLE 15-3
CONGENITAL CATARACT ASSOCIATIONS
| Intrauterine infections | Toxoplasmosis, rubella, CMV, herpes, syphilis |
| Syndromes | Trisomy 21: bilateral cataract eventually in 13% to 21% of patients, but only 1.4% presenting in neonatal period Lowe syndrome |
| Genetics | Autosomal dominant in approximately 25% of bilateral congenital cataract, but generally sporadic in unilateral congenital cataract |
| Other ocular disorders | Aniridia, microphthalmos, anterior segment dysgenesis, persistent fetal vasculature |
| Metabolic | Galactosemia, hypoparathyroidism, mannosidosis, hypoglycemia |
Surgery is indicated if a congenital cataract is greater than 3 mm in diameter, prevents a good view of the retina, or is associated with either nystagmus or strabismus. Unilateral cataracts are often removed within the first 6 weeks after birth to prevent form-deprivation amblyopia. Bilateral cataracts are often removed within 10 weeks after birth. Postoperative visual rehabilitation often includes aphakic contact lenses, specialized glasses, and patching for an extended period of time. 15
Glaucoma
Epiphora (excess tearing), blepharospasm (spastic lid closure), and photophobia (light sensitivity) are the classic triad of congenital glaucoma. Other signs and symptoms include buphthalmos (enlarged eye), corneal clouding (due to elevated intraocular pressure causing corneal edema), and pain.
Cortical Visual Impairment
In severe cases there can be an inability to perceive light (i.e., complete blindness) in both eyes. It may be difficult to assess visual function in affected children in the perinatal period, and follow-up examinations after hospital discharge are needed to fully assess visual potential. 16
Nystagmus
Nystagmus is an involuntary, rhythmic pendular or jerking movement of the eyes.
Children with suspected nystagmus should be referred to an ophthalmologist for further characterization. 1718
Periocular Problems
Most cases of NLDO in children result from blockage at the valve of Hasner, at the junction of the distal nasolacrimal duct and the inferior meatus of the nose. Excessive tearing and mucoid eye discharge can result. Most cases resolve spontaneously and are managed conservatively with massage and topical antibiotic eyedrops. However, some cases require probing and intubation of the nasolacrimal system. If infection of the lacrimal sac (dacryocystitis) develops, administration of intravenous antibiotics, surgical intervention, or both may be required.
Congenital ptosis is an inability to raise one or both eyelids. Ptosis can cause significant astigmatism and can also cause form-deprivation amblyopia if the pupil is constantly occluded by the eyelid. A surgical procedure to raise the upper eyelids is often performed if amblyopia is present. 19
Genetics
37. What are some common genetic conditions for which eye examination is often requested in the NICU to assist in diagnosis?
TABLE 15-4
GENETIC CONDITIONS FOR WHICH EYE EXAMINATION IS COMMONLY REQUESTED IN NICU
| CONDITION | EYE FINDINGS |
| Alagille syndrome | Posterior embryotoxon |
| PHACE complex | Lid hemangioma, microphthalmia, optic nerve hypoplasia, retinal vascular abnormalities |
| Down syndrome | Prominent epicanthal folds, upward slanting palpebral fissures, cataract |
| Aicardi syndrome | Chorioretinal depigmented lesions |
| CHARGE | Coloboma |
| Moyamoya disease | Morning Glory syndrome, Coloboma |
| Septo-optic dysplasia (de Morsier) | Optic nerve hypoplasia |
1Mills MD. The eye in childhood. Am Fam Physician 1999;60(3):907–16.
3Repka MX. Ophthalmological problems of the premature infant. Mental Retardation and Developmental Disabilities Research Reviews 2002;8:249–57.
4Ramasubramanian A, Johnston S. Neonatal eye disorders requiring ophthalmology consultation. NeoReviews 2011;12(4):c216–c222.
5Buckley EJ, Ellis GS, Glaser S, for the American Academy of Pediatrics section on ophthalmology. Red reflex examination in neonates infants, and children. Pediatrics 2008;122:1401–1404.
2Hoyt CS, Good W, Petersen R. Disorders of the eye. In: Taeusch HW, Ballard RA, Avery ME, editors. Schafer and Avery’s diseases of the newborn. 6th ed. Philadelphia: Saunders; 1991.
6Chen J, Stahl A, Hellstrom A, et al. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr 2011;23(2):173–8.
7Mills MD. Evaluating the cryotherapy for retinopathy of prematurity study (CRYO-ROP). Arch Ophthalmol 2007;125(9):1276–81.
8Good WV. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233–50.
9Mintz-Hittner HA, Kennedy KA, Chuang AZ for the BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603–15.
10Darlow BA, Ells AL, Gilvert CE, et al. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed 2013;98(2):F170–4
11Chiang MF, Melia M, Buffenn AN, et al. Detection of clinically significant retinopathy of prematurity using wide-angle digital retinal photography: a report by the American Academy of Ophthalmology.Ophthalmology 2012;119(6):1272–80.
12Richter GM, Williams SL, Starren J, et al. Telemedicine for retinopathy of prematurity diagnosis: evaluation and challenges. Surv Ophthalmol 2009;54:671–85.
13Laws DE, Morton C, Weindling M, et al. Systemic effects of screening for retinopathy of prematurity. Br J Ophthalmol 1996;80:425–8.
14Mets MB, Chhabra MS. Eye manifestations of intrauterine infections and their impact on childhood blindness. Surv Ophthalmol 2008;53(2):95–111.
15Krishnamurthy R, Vanderveen DK. Infantile cataracts. Int Ophthalmol Clin 2008;48(2):175–92.
16Flanagan C, Kline L, Curè J. Cerebral blindness. Int Ophthalmol Clin 2009;49(3):15–25.
17Hertle RW. Nystagmus in infancy and childhood. Semin Ophthalmol 2008;23:307–317.
18Kline LB, Chair. Section 5: Neuro-ophthalmology. In: Basic and clinical science course 2009–2010. American Academy of Ophthalmology; 2009.
19Holds JB, Chair. Section 7: Orbit, eyelids, and lacrimal system. In: Basic and clinical science course 2009–2010. American Academy of Ophthalmology; 2009.


