25. Neonatal Nephrology*
Mackenzie S. Frost, Lucy Fashaw, Jacinto A. Hernandez and M. Douglas Jones Jr
In utero, the fetal kidney is not necessary for toxin removal or fluid and electrolyte homeostasis; that is primarily the placenta’s function. By contributing to amniotic fluid, the fetal kidney instead has an essential role in the normal development of the fetus. After birth, as the infant adapts to the external milieu, the kidney gradually assumes its role as regulator of fluid and electrolyte homeostasis. At birth, renal function changes dramatically, complicating clinical assessment. Assessment is an even greater challenge in the premature infant.
The more complicated an organ is in its development, the more subject it is to maldevelopment. In this aspect, the kidney outranks most other organs. Abnormalities of the genitourinary system constitute up to 30% of all anomalies diagnosed prenatally.116 Anomalies may cause problems during the neonatal period, but they may also not be clinically apparent until the infant is an older child or adult.
Neonatal renal disease is important not just during the neonatal period but also as it may affect adult renal pathology. Congenital renal dysplasias, renal obstructive disorders, and cystic diseases account for a substantial percentage of patients with end-stage renal failure. Furthermore, a growing body of data supports a link between prenatal and neonatal events and later hypertension in adolescents and adults. 21,89,109
NORMAL DEVELOPMENT
Anatomic Development of the Kidney115,116
The mammalian embryo progressively develops three sets of excretory organs, all of which might be termed the “embryonic kidney.” The pronephros and mesonephros regress in the human but induce the metanephros, the direct precursor of the adult kidney (Figure 25-1). The pronephros, a solid mass of cells along the nephrogenic cord, is located at the cervical level at approximately 3 weeks’ gestation. Degeneration of the pronephros begins soon after its formation, and regression has completely occurred by week 5. The pronephros has no excretory function but plays an important role in the formation of the mesonephros. The primitive ureter of the pronephros forms the wolffian, or mesonephric, duct via fusion of the pronephric tubular buds. The mesonephric duct then induces the formation of the second kidney, the mesonephros, at approximately 4 weeks of gestation. The mesonephros develops from the nephrogenic cord and forms 40 pairs of thin-walled tubules and glomeruli with excretory function. Portions of the mesonephric duct system are retained in the male fetus and form the ducts of the epididymis, the ductus deferens, and the ejaculatory duct. The remainder of the mesonephric duct system in the male infant has degenerated by the 4th month of gestation as the metanephric kidney develops. In the female, near-complete degeneration has occurred by the 3rd month of gestation.
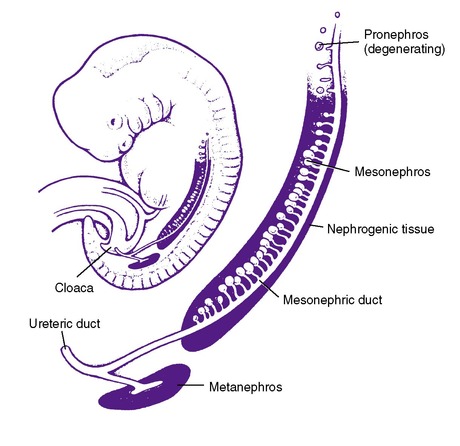 |
| FIGURE 25-1
(From Holliday MA: Developmental abnormalities of the kidney in children, Hosp Pract 13:101, 1978.)
|
The metanephros appears at 4½ to 5 weeks’ gestation. The metanephric kidney is the product of a series of inductive interactions between the metanephric mesenchyme and epithelial ureteric bud. Initially, the ureteric bud grows from the mesonephric duct into the mesenchymal portion of the urogenital ridge; concomitantly, the metanephric mesenchyme changes, becoming histologically distinct from the surrounding tissue. When the metanephric mesenchyme and ureteric bud make contact, a condensation of cells begins along the surface of the bud. These cells are the beginnings of pretubular aggregates that undergo mesenchymal-to-epithelial transformation to become the segmented nephron. The condensed mesenchyme is also thought to produce a number of stem cells, which remain undifferentiated and proliferative. These cells serve to maintain a supply of precursor cells until the completion of nephron development. Thus the epithelial portion of the adult kidney is derived from both the metanephric mesenchyme, via the stem cells ultimately responsible for individual nephron formation, and the ureteric bud, whose migration and division determine the pattern of formation of the urinary collecting system via its pretubular aggregates. The ureteric bud migrates to the most caudal end of the nephrogenic cord and finally to the lumbar region by week 8 of gestation. The ureteric bud also rotates 90 degrees medially along the longitudinal axis. Abnormalities in ascent or rotation can lead to pelvic kidneys, horseshoe kidneys, or crossed fused ectopia. Anomalies of the kidney often accompany anomalies of the ureter, as well as other portions of the urinary tract. Congenital anomalies of the kidney and urinary tract (CAKUT) are a family of diseases with a diverse anatomic spectrum of kidney anomalies (agenesis, dysplasia, hypoplasia) and ureteropelvic anomalies (megaureter, agenesis, hydronephrosis, vesicoureteric reflux, posterior urethral valves, and ureteral duplications).48
Nephrogenesis is the process of nephron formation via growth and differentiation of multiple cell types and leads to formation of the overall renal architecture. The process begins in the renal cortex closest to the medulla (juxtamedullary nephrons) and proceeds in a dichotomous branching centrifugal pattern with the outermost (superficial cortical) nephrons forming last. There are multiple phases of growth and structural reorganization following the interactions between the mesonephric mesenchyme and the ureteric bud. The formation of the collecting system is controlled by the branching pattern of the ureteral bud, and this occurs at the same time as the formation of functional nephron units. Four progressive phases of nephrogenesis occur during which the nephron proceeds through several intermediate forms. By the fourth stage, there is a definitive glomerulus with highly differentiated visceral and parietal epithelial cells. The vascular system development occurs in concert with nephron formation. The surrounding major vessels and spinal ganglia grow into the metanephros to complete the remaining cell types, and vessel architecture is similar to the newborn kidney by 15 weeks of gestation.
Physiologic Development and Clinical Assessment5,21,38,59
Although newborn kidneys are usually described as “immature,” they are perfectly suited to their usual responsibilities. During the latter part of gestation, their primary role is maintenance of amniotic fluid volume. This requires a large volume of urine with a relatively high concentration of sodium. Thus fetal urine output is on the order of 10 mL/kg/hr of sodium-rich urine. Fetal fractional excretion of sodium (FENa) (i.e., the fraction of sodium in glomerular filtrate that appears in urine) is especially high, approximately 15%. This compares with less than 1% in a growing infant born after a full-term pregnancy.
The next major responsibility is during the first week of life. Fetuses have a large amount of extracellular fluid (ECF). ECF as a percentage of body weight progressively diminishes throughout gestation: (1) approximately 65% of body weight at 26 weeks of gestation; (2) 40% at full-term; and (3) 25% by 1 year of age. Most of the postnatal reduction occurs in the first week of life and is the primary reason that body weight may decrease by up to 10% in breast-fed term infants and even more in premature infants. The newborn kidney can handle this challenge without difficulty. Finally, in subsequent weeks, the kidney has no trouble retaining the electrolytes needed for growth and no trouble producing dilute urine to accommodate the large water load presented by breast milk. Growth itself is a powerful homeostatic ally. A substantial portion of carbohydrates, electrolytes, and nitrogenous wastes from protein absorbed from breast milk are never presented to the kidney for excretion. They are incorporated into the growing body.
Only when the neonatal kidney has to cope with unexpected derangements of water, electrolyte, or acid-base status secondary to premature birth or illness, especially illness accompanied by cessation of growth, does its relative lack of ability to concentrate urine, excrete extra sodium and potassium loads, conserve sodium (in preterm infants), and regulate acid-base status become problematic. In older children and adults, normal kidneys can correct for substantial errors in clinical judgment as to water and electrolyte administration or creation and correction of acid-base abnormalities. This is not so with neonatal kidneys, especially in smaller preterm infants.
With that in mind, it is helpful to review specific aspects of neonatal renal function.
Nephron Development21,89
The process of forming the adult complement of approximately 600,000 nephrons in each kidney is complete by 34 to 36 weeks gestational age (GA). Development proceeds in centrifugal fashion, with juxtamedullary nephrons developing first and superficial cortical nephrons last. 5In general, nephron development continues at approximately the same rate even if the infant is born prematurely. In other words, development continues whether in utero or ex utero. For example, a premature infant born at 28 weeks of gestation will not complete nephrogenesis for another 6 to 8 weeks (Figure 25-2). Despite continued nephrogenesis, infants with intrauterine growth restriction and those born with extremely low birth weights may never achieve a normal number of nephrons. This has been termed congenital oligophrenia. Compromised renal function and elevated blood pressures have been reported on long-term follow-up of small preterm infants.
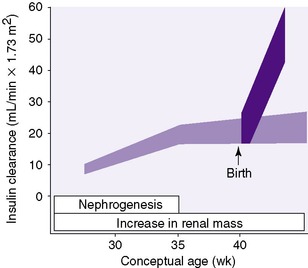 |
| FIGURE 25-2
(From Guignard JP: Neonatal nephrology. In Holliday MA, Barratt TM, Vernier RL, editors: Pediatric nephrology, ed 2, Baltimore, 1987, Williams & Wilkins.)
|
Glomerular Filtration Rate*
Glomerular filtration rate (GFR) is the rate at which filtrate of renal blood, or more precisely of renal plasma, appears in proximal renal tubules. A primary physiologic limitation of the neonatal kidney, increasingly so with decreasing gestational age, is limited GFR. For the fetus, the placenta serves to maintain fluid and electrolyte composition and clearance of metabolic wastes. Thus renal arterial blood flow is approximately 5% of fetal cardiac output as compared with 25% later on. After full-term birth, GFR doubles to triples in the first weeks of life (see Figure 25-2) and then further increases to adult levels between 1 and 2 years of life.
The situation is different in infants born before 34 to 36 weeks GA. For example, GFR is approximately 5 mL/min/1.73 m2 or approximately 0.5 mL/kg/min (30 mL/kg/hr) in a 24-week infant. That increases little in absolute terms until 34 to 36 weeks of gestation (see Figure 25-2). Thereafter GFR increases rapidly, as it does in full-term infants although, as just mentioned, it may never reach normal adult values.
In clinical settings, GFR may be estimated using the clearance of creatinine. For this to be accurate, serum creatinine concentration must be constant, creatinine in the urine must represent creatinine in glomerular filtrate with no creatinine added or taken away during passage through renal tubules, and urine collection must be carefully timed and complete. Because serum creatinine concentrations change after birth, filtered creatinine is reabsorbed by tubules, especially in small preterm infants, and urine collection in newborns is difficult without bladder catheterization; therefore determination of creatinine clearance is uncommon in neonatal intensive care units (NICUs).
Under ideal steady-state conditions, serum creatinine concentrations should provide an accurate indirect indication of GFR, eliminating the need to collect urine. Creatinine production rate is roughly constant. In a steady state, creatinine excretion in urine is equal to creatinine production and likewise constant. The equation for measurement of GFR with creatinine is as follows:
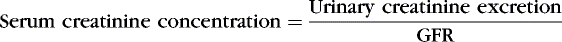
Serum creatinine concentration is thus equal to a constant divided by GFR. Therefore a true increase in creatinine concentration from 0.4 to 0.5 mg/dL, a 25% increase, indicates a reduction in GFR of 20%; the inverse of 1.25 is 0.80.
As mentioned, strict steady-state conditions are often absent in the neonatal period. Nevertheless, serum creatinine concentration is useful as a general indicator of renal function. In full-term infants, as GFR increases, creatinine concentration falls during the first week of life from 0.8 to 1.2 mg/dL, reflecting maternal creatinine concentrations, to neonatal levels of 0.2 to 0.3 mg/dL. The rate of decrease depends on hydration and clinical status. Rising or stable serial serum creatinine concentrations or an isolated value exceeding 0.5 mg/dL after 1 week of age indicates renal dysfunction.
In preterm infants, the steep increase in GFR does not occur until nephrogenesis is complete at 34 to 36 weeks postmenstrual age (PMA). Furthermore, filtered creatinine is reabsorbed along the tubule. This increases with decreasing gestational age and PMA. As a result, creatinine concentrations often rise in the first 24 to 48 hours and are then slow to fall. Gestational-age– and postnatal-age–based graphs are needed to identify abnormal values. 6,107 After the initial increase in creatinine concentration, concentration should slowly fall. A secondary rise indicates renal dysfunction.
Tubular Function5,49
Urine flow depends on both GFR and tubular reabsorption. Fetal GFR is approximately 30 mL/kg/hr, yet fetal urine output is 10 mL/kg/hr. GFR in full-term infants is approximately 90 mL/kg/hr, yet urine output is 2 to 3 mL/kg/hr. The difference is the activity of the renal tubule.
Oliguria is ordinarily defined as urine output of less than 1 mL/kg/hr. However, urine output may transiently decrease immediately after birth to less than 1 mL/kg/hr because tubular reabsorption of water increases because of an increase in fetal antidiuretic hormone (ADH) during labor. Nevertheless, 50% of full-term infants void by 12 hours, 92% by 24 hours, and 99% by 48 hours of life. Causes for prolonged failure to void include poor cardiac output or blood pressure, primary renal dysfunction, and obstruction to urine flow. After transient oliguria/anuria, urine flow rate increases as the newborn excretes his or her physiologically expanded fetal extracellular fluid volume as described earlier.
Proximal Tubular Function*
The proximal tubule is responsible for reabsorbing glucose, amino acids, and most of the bicarbonate, sodium chloride, and water in glomerular filtrate. In smaller preterm infants, tubular transport mechanisms are insufficient to prevent spillage of each of these in varying degrees.
Sodium8,15,23,30,99
Physiologic diuresis in the first week of life is accompanied by physiologic natriuresis. The kidney is then responsible for conserving sufficient dietary sodium for growth. This is a challenge for preterm infants (Figure 25-3).Thus premature infants often require extra sodium intake to compensate for what amounts to obligatory sodium wastage. Conversely, in the presence of a sodium load (e.g., from administration of large amounts of sodium chloride), the neonatal kidney cannot compensate with a rapid increase in FENa. The result is edema and possibly circulatory overload.
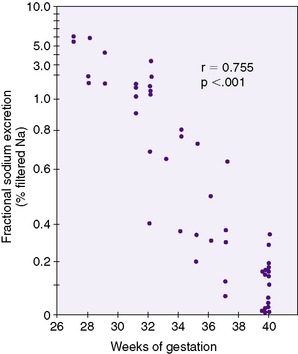 |
| FIGURE 25-3
(From Siegel S, Oh W: Renal function as a marker of human fetal maturation, Acta Paediatr Scand 65:481, 1976.)
|
Potassium5,41
The kidney is an important site for regulation of potassium balance. In the adult, it is responsible for maintaining zero balance. In contrast, to sustain the neonate, the kidney must maintain positive potassium balance. In this context, it is less surprising that mechanisms for potassium excretion are underdeveloped at birth.
Serum potassium concentrations tend to be high in neonates (5.5 to 6 mEq/L).59 The levels are not of pathologic significance and perhaps play a role in supporting growth. This serves to point out the importance of growth as a homeostatic mechanism. Some clinicians have the impression that nonoliguric hyperkalemia in small preterm infants is less common since routine institution of early parenteral protein administration.
Acid-Base Balance5,59,84
By adult standards, serum bicarbonate concentrations are low in full-term newborns (19-21 mEq/L) and even lower (16-20 mEq/L) in premature infants. Lower serum bicarbonate concentrations reflect limited ability to cope with the higher acid load from high protein intake and acid generated with formation of new bone. The capacity of the neonatal proximal tubule to reabsorb filtered bicarbonate is one-third that of an adult. Proximal tubular bicarbonate reabsorption is further compromised if ECF is over-expanded with crystalloid solutions; because proximal tubular sodium and bicarbonate reabsorption are closely linked, bicarbonate is wasted as sodium reabsorption decreases to rid the body of excess sodium chloride. The capacities of the collecting duct to secrete hydrogen ions and of the proximal tubule to make ammonia to buffer secreted hydrogen ion are also limited. The net result is limited capacity to correct metabolic acidosis. Limited ability to achieve minimal urine pH values is relative. If serum bicarbonate is low enough (e.g., 14 to 15 mEq/L), the kidney can completely reabsorb the smaller amount of filtered bicarbonate and achieve a urine pH of 5. Serum bicarbonate concentrations increase to adult levels of 24 to 26 mEq/L by the end of the first year.
Metabolic acidemia (see Chapter 8) with low bicarbonate and high chloride concentrations is seen in premature infants with transient proximal renal tubular acidosis and in infants who have received excessive amounts of chloride in normal saline. It can also be seen during recovery from acute renal failure and with renal vein thrombosis and nephrocalcinosis.
Uric Acid101
Serum uric acid concentrations are elevated in the newborn because production from nucleotide breakdown is increased just after birth, especially in premature infants. This is accompanied by increased uric acid excretion. High urinary uric acid concentrations leave reddish uric acid crystals in the diaper and may be mistaken for blood.
CONGENITAL AND ACQUIRED RENAL ABNORMALITIES
Chromosomal Disorders115,116
Although lower urinary tract and renal anomalies are seldom the presenting feature of chromosomal disorders, they frequently form part of a multisystem malformation syndrome caused by chromosomal anomalies. Renal disorders seen with chromosomal disturbance include fused kidneys, duplication defects, renal agenesis or hypoplasia, hydronephrosis and hydroureter, renal dysplasia or cystic disease, hypospadias, micropenis, and cryptorchidism.
The overall pattern of malformation with individual chromosomal disorders is usually sufficient for diagnosis; however, variation can be seen from one individual to another, even for patients with aneuploidy. Although certain renal anomalies are characteristic of certain chromosomal disorders, no one renal malformation is unique to any particular chromosomal disorder.
Consequences of obstruction of the developing nephron unit include hydronephrosis, hydroureter, and cortical cysts. Severe obstruction leads to renal dysplasia or agenesis. Dysplasia and agenesis may also be secondary to developmental growth failure and may be unilateral or bilateral. In the case of the multicystic dysplastic kidney, there may be no evidence of obstruction, whereas in other cases, dysplasia may be secondary to lower tract dysfunction and obstruction. Typically, the dysplastic kidney does not keep up with somatic growth and gradually shrinks and disappears.
Acquired Disorders*
Three drugs commonly used in neonates may cause renal damage and dysfunction: furosemide, aminoglycosides, and nonsteroidal anti-inflammatory drugs (NSAIDs). GFR changes with gestational and postnatal age, making it difficult to relate toxicity to dosage.
Furosemide may cause electrolyte and acid-base disturbances, including hyponatremia, hypochloremia, hypokalemia, and metabolic alkalosis. It increases calcium excretion and may be associated with nephrocalcinosis and less commonly with nephrolithiasis, secondary hyperparathyroidism, and osteopenia. Although nephrocalcinosis can occur without furosemide, there is little doubt that furosemide increases the risk. Calcification and the additional complication of renal tubular acidosis may be ameliorated or reversed by promotion of calcium reabsorption by addition of a thiazide diuretic. 93,97 The long-term effects of nephrocalcinosis in preterm infants are not clear. Ototoxicity is another complication of furosemide, especially when used in combination with aminoglycosides.
Aminoglycosides have long been one of the commonest causes of drug-induced nephrotoxicity. Pharmacokinetic monitoring can achieve desired concentrations (peak 6 to 8 mcg/mL and trough <2 mcg/mL) and reduce risk. The neonate may be at less risk for nephrotoxicity from aminoglycosides than is the mature kidney. However, gentamicin-induced renal toxicity was recently confirmed in the neonatal kidney without any relationship to peak and trough serum levels. In fact, the long-term effects of neonatal aminoglycoside exposure on renal development have yet to be adequately evaluated. Ototoxicity is the second main adverse effect of aminoglycosides and, in contrast to nephrotoxicity, is irreversible.
The nephrotoxicity induced by aminoglycosides manifests clinically as nonoliguric renal failure, with a slow rise in serum creatinine and a hypo-osmolar urine developing after several days of treatment. The nephrotoxicity of the aminoglycosides is believed to be secondary to a small percentage of retained drug within the kidney’s proximal epithelial cells. At low or appropriate doses, tubular alterations can generate proteinuria, hypo-osmotic urine, and increases in blood urea nitrogen (BUN) and creatinine reflecting a decrease in GFR. At higher doses of aminoglycosides, tubular wasting of potassium, magnesium, and calcium, along with decreased water reabsorption, bicarbonate, and glucose concomitantly with tubular necrosis, can be seen.
Since the 1970s, premature infants with symptomatic patent ductus arteriosus (PDA) have been treated with indomethacin, a nonspecific prostaglandin inhibitor. Indomethacin, as well as other NSAIDs, has been shown to have various side effects including hemodynamic changes in cerebral, mesenteric, and renal circulations. The renal side effects seen with indomethacin appear to be related to three phenomena, as follows:
1. Intrauterine cyclooxygenase (COX) inhibition may induce renal dysplasia and dysgenesis and alter renal maturation by slowing glomerular maturation.
2. Oligohydramnios may be the end result of fetal indomethacin exposure with concomitant decline in renal blood flow and glomerular filtration.
3. Indomethacin given for closure of PDA may induce and exacerbate renal failure by changing the balance of cortical juxtamedullary nephron perfusion.
The fragile balance of vasoconstrictor (mediated by angiotensin II, endothelin) and vasodilatory (atrial natriuretic peptide, nitric oxide, prostaglandins, kallikrein-kinin) forces is now altered in favor of vasoconstriction and further reduction of the already low GFR.
For preterm infants and newborns, the administration of NSAIDs should be done with care and frequent monitoring of renal function, even though these changes often are reversible. When a change or decline in GFR is noted (e.g., plasma creatinine increase), then the administration of NSAIDs should be halted. Indomethacin has been shown to have clinically important renal side effects including proteinuria, oliguria, renal failure, hyperkalemia, and hyponatremia. Patients at higher risk include those with persistent patent ductus arteriosus, dehydration, and simultaneous administration of other nephrotoxic drugs. Unfortunately, the combined use of furosemide and indomethacin does not improve outcome. At this time, in the absence of large randomized and controlled trials, guidelines for NSAID administration must rely on animal studies. In addition, there are no studies on the effect of selective COX inhibitors on PDA closure.
The neonatal patient, particularly the low-birth-weight infant, is now exposed to an increased use of invasive procedures and broad-spectrum antimicrobial therapy and therefore is at a higher risk for systemic fungal sepsis. Agents for therapy include amphotericin B, which is associated with adverse effects, including infusion reactions with hemodynamic instability and nephrotoxicity with electrolyte disturbances.
Multiple studies have indicated that maintenance of adequate fluid and electrolyte balance before amphotericin B administration may prevent nephrotoxicity. In particular, two strategies, use of a liposomal amphotericin system and salt-loading before amphotericin B administration, are employed. To date, no definitive controlled data exist that show an ameliorated risk for liposomal amphotericin B. Salt-loading, on the other hand, before amphotericin B therapy, of greater than 4 mEq/kg/day may reduce nephrotoxicity; the exact mechanism by which sodium reduces the incidence and severity of amphotericin B–induced nephrotoxicity has not been shown. Suggestions have been made that amphotericin B–enhanced tubuloglomerular feedback is reversed by high sodium intake.
GENERAL DATA COLLECTION38,55,66,109
History
A complete family history of renal disease or syndromes that involve the kidneys is important. Prenatal exposures to maternal infection, drugs, toxin, or medication intake are risk factors. Paternal smoking and advanced age may also be associated with an increased risk for urinary tract anomalies.
The quantity of amniotic fluid is an indicator of fetal renal function since fetal urination is responsible for most of the amniotic fluid volume beginning in the second trimester of pregnancy. Normally, amniotic fluid volume increases during gestation, peaking at 34 weeks of gestation. Severe fetal genitourinary abnormalities result in oligohydramnios (Table 25-1). Severe urinary concentrating defects (e.g., diabetes insipidus and Bartter syndrome) have been associated with polyhydramnios. Perinatal asphyxia is a risk factor for renal damage.
| VATER,Vertebral defects, imperforate anus, tracheo esophageal fistula, and radial and renal dysplasia. | |
| Finding | Suspected Abnormality |
|---|---|
| Oligohydramnios |
Bilateral renal agenesis, polycystic kidney disease, or dysplasia
Amnion nodosum
|
| Polyhydramnios | Nephrogenic diabetes insipidus, trisomy 18 or 21, anencephaly, esophageal or duodenal obstruction, Klippel-Feil syndrome, Bartter syndrome |
| Enlarged placenta (>25% of infant birth weight) | Congenital nephrotic syndrome |
| Velamentous insertion of umbilical cord | Increased congenital anomalies |
| Asphyxia neonatorum | Renal failure |
| PHYSICAL EXAMINATION | |
| Hypertension | See text |
| SKIN | |
| Hemangioma | Hemangioma of kidney or bladder |
| Edema | Congenital nephrotic syndrome, hydrops fetalis |
| Adenoma sebaceum | Tuberous sclerosis—cystic kidneys |
| HEAD | |
| Encephalocele | Meckel’s or Meckel-Gruber syndrome—polycystic kidney disease |
| Cleft lip and palate | Urinary tract anomalies |
| Macroglossia |
Beckwith-Wiedemann syndrome—renal dysplasia
Johanson-Blizzard syndrome—hydronephrosis, orofacial-digital syndrome—renal microcystic disease
|
| EYES | |
| Phakoma (tubular sclerosis) | Angiomyolipoma of the kidney |
| Retinitis pigmentosa | Medullary cystic disease of the kidney |
| Cataracts | Cystic disease, Lowe syndrome, Wilms’ tumor, congenital rubella |
| Aniridia | Wilms’ tumor |
| EARS | |
| Low-set or malformed | Increased risk for renal abnormalities, Potter syndrome |
| Ear tags | Branchio-oto-renal (BOR) syndrome |
| Preauricular pits | |
| SKELETON | |
| Hemihypertrophy | Wilms’ tumor |
| Spina bifida | Neurogenic bladder |
| Arthrogryposis | Oligohydramnios, Potter syndrome |
| Dysplastic nails | Nail patella syndrome |
| Vertebral anomalies | VATER syndrome—renal dysplasia |
| Polydactyly | Meckel’s or Meckel-Gruber syndrome—polycystic kidney disease |
| ABDOMEN | |
| Absence of abdominal musculature | Prune-belly syndrome |
| Single umbilical artery | Increased congenital anomalies of the urinary tract |
| Umbilical discharge | Patent urachus |
| Abdominal mass | See Table 25-6 |
| Hepatomegaly | Storage diseases—renal tubular dysfunction, Beckwith-Wiedemann syndrome, Zellweger syndrome |
| PULMONARY | |
| Spontaneous pneumothorax | Increase in renal anomalies |
| Pulmonary hypoplasia | Oligohydramnios |
| GENITOURINARY—MALE | |
| Undescended testes | Prune-belly syndrome, Noonan syndrome, Lawrence-Moon-Biedl syndrome |
| Congenital absence of vas deferens | Renal agenesis or ectopia |
| Hypospadias | Increase in renal anomalies |
| Abnormal urinary stream | Bladder dysfunction or urethral outlet obstruction |
| GENITOURINARY—FEMALE | |
| Enlarged clitoris | Adrenogenital syndrome |
| Cystic mass in urethral region |
Ectopic ureterocele, paraurethral cyst
Sarcoma botryoides
|
| Bulging in vagina | Hydrometrocolpos |
| Abnormal urinary stream or dribbling | Bladder dysfunction, urethral obstruction |
| Common cloaca | Urinary tract abnormalities |
| URINALYSIS | See text |
| RECTAL | |
| Deficient anal sphincter tone | Neurogenic bladder dysfunction |
| Dilated prostatic urethra | Posterior urethral valves, prune-belly syndrome |
| Masses | Tumor |
| Anal atresia | VATER syndrome—renal dysplasia (see text) |
Signs and Symptoms
Physical findings that are indicators of genitourinary tract abnormalities are outlined in Table 25-1.
Laboratory Data
IMAGING STUDIES87,109,115
Fetal ultrasound can provide (1) estimation of amniotic fluid volume, (2) information on the appearance and echogenicity of kidneys, and (3) evidence of renal and/or lower tract dilation. Prenatal ultrasonography can define anatomy but does not accurately predict function. Mild dilation does not necessarily mean obstruction. More severe dilation and reduced amniotic fluid volume are more likely to mean obstruction and compromised renal function. The more severe the dilation (>7 mm after 32 weeks of gestation), the more likely the infant will need either follow-up or even surgical intervention. The later in pregnancy that dilation is found, the more likely hydronephrosis will be confirmed postnatally.
Nuclear scans are most useful when abnormalities are severe (e.g., lack of renal perfusion). A voiding cystourethrogram evaluates the lower urinary tract and is typically reserved for more mature infants.
URINALYSIS
Specific Gravity
Specific gravity in term infants ranges from 1.001-1.005 to 1.015-1.020. Specific gravity is useful as an indicator of urine osmolality and thus of the ability of the kidney to concentrate and dilute. However, it can be altered by the presence of glucose, protein, and urinary contrast agents. In that case, osmolality must be measured directly and compared with serum osmolality.
Glucosuria
Trace quantities of glucose may be found occasionally in term infants and more frequently in premature infants. Even minor elevations of plasma glucose concentrations may cause glucosuria. Large glucose loads may cause osmotic diuresis.
Urinary pH
Urinary pH is typically around 6, although most neonates can achieve a urine pH of 5. Urine pH is frequently 7 or greater in premature infants with proximal renal bicarbonate wasting.
Hematuria19,76
Hematuria is defined as more than 5 to 6 red blood cells per high-power field (hpf). A positive dipstick test occurs with hemoglobinuria from hemolysis and with myoglobinuria from muscle breakdown, usually from asphyxia. Hematuria may occur if kidneys are damaged during delivery, especially with an enlarged kidney (e.g., cystic disease, obstruction). Hematuria is common in perinatal asphyxia. Other conditions associated with hematuria are renal vein thrombosis, urinary tract infections, sepsis, renal artery embolization (especially from umbilical artery catheters), renal necrosis, hypercalciuria, coagulopathies, and, rarely, congenital glomerulonephritis or nephrosis. Factitious hematuria may occur as a result of blood from circumcision, perineal irritation, and uterine bleeding caused by withdrawal from maternal hormones. If hematuria is persistent, it should be evaluated with urine culture, assessment of proteinuria and urine calcium excretion, assessment of GFR, and an anatomic evaluation of the kidneys.
Pyuria38
Pyuria is common in newborns, especially females. As many as 25 to 50 white blood cells (WBCs) per hpf may be observed in the first days of life. Pyuria may indicate infection, and a urine culture should be obtained if clinically indicated. However, pyuria also may indicate noninfectious renal injury.
Proteinuria77
A positive dipstick test for protein indicates the amino groups of proteins. Although convenient, dipstick testing is subject to limitations. Because albumin and low-molecular-weight proteins give positive results, dipstick testing cannot distinguish between glomerular and tubular proteinuria. An alkaline urine (pH of ≈8) may give a false-positive result. The test may also be confounded by prolonged immersion of the strip and by the presence of detergents, WBCs, or bacteria in the urine. If urine is concentrated, small amounts of protein can give a falsely elevated reading; conversely, if the urine is dilute, important amounts of protein will go undetected.
ACUTE RENAL FAILURE
Pathophysiology
Acute renal failure (ARF) in the newborn is a relatively common problem. Although the precise incidence and prevalence of acute renal failure in the NICU is unknown, several studies have shown an incidence between 6% and 24%. 3,4,103ARF is defined as the sudden deterioration of the kidney’s baseline function and is usually characterized by an increase in the blood concentration of creatinine and nitrogenous waste products, by a decrease in the GFR, and by the inability of the kidney to appropriately regulate fluid and electrolyte homeostasis.
After birth, the serum creatinine in the newborn is a reflection of maternal renal function and cannot be used as a measure of renal function in the newborn shortly after birth. 4,20,22,96In full-term healthy newborns, the serum creatinine declines to about 0.4 to 0.6 mg/dL at about 2 weeks of age. In premature infants, this postnatal decline in serum creatinine is at a slower rate. As a general rule, the more premature the infant, the higher the serum creatinine. Any rising serum creatinine from initial baseline or a serum creatinine greater than 1.5 mg/dL with normal maternal function should be investigated.
A decline in urine output is a common clinical manifestation of ARF (e.g., prerenal failure, hypoxic-ischemic insults, or cortical necrosis), but many forms of ARF are associated with normal urine output (e.g., nephrotoxic renal insults).
Etiology
There are many different causes of renal failure in the newborn (Box 25-1). These causes are typically classified as prerenal, intrinsic renal disease including vascular insults, and obstructive uropathy. The preponderance of factors causing ARF in the newborn are prerenal in nature (e.g., hypoxia, hypovolemia, hypotension); primary intrinsic renal disease and obstructive uropathy are much less common.
BOX 25-1
Prerenal Failure
Decreased True Intravascular Volume
• Dehydration
• Gastrointestinal losses
• Salt-wasting renal or adrenal disease
• Central nephrogenic diabetes insipidus
• Third space losses (sepsis, traumatized tissue)
Decreased Effective Intravascular Blood Volume
• Congestive heart failure
• Pericarditis, cardiac tamponade
Intrinsic Renal Disease
Acute Tubular Necrosis
• Ischemic/hypoxic insults
• Drug induced
• Aminoglycosides
• Intravascular contrast
• Nonsteroidal anti-inflammatory drugs
• Toxin mediated
• Endogenous toxins
• Rhabdomyolysis, hemoglobinuria
• Interstitial nephritis
• Drug induced—antibiotics, anticonvulsants
• Idiopathic
• Vascular lesions
• Cortical necrosis
• Renal artery thrombosis
• Renal venous thrombosis
• Infectious causes
• Sepsis
• Pyelonephritis
• Obstructive uropathy
• Obstruction in a solitary kidney
• Bilateral ureteral obstruction
• Urethral obstruction
Congenital Renal Diseases
• Dysplasia/hypoplasia
• Cystic renal diseases
• Autosomal recessive polycystic kidney disease
• Autosomal dominant polycystic kidney disease
• Cystic dysplasia
From Andreoli SP: Acute renal failure in the newborn, Sem Perinatol 28:112, 2004.
In prerenal failure, renal function is decreased because of decreased renal perfusion and the kidney is intrinsically normal. Renal hypoperfusion results from true volume contraction (e.g., hemorrhage, dehydration, third space losses) or from a decreased effective blood volume (e.g., congestive heart failure, cardiac tamponade).
Timely correction of the underlying disturbance and restoration of normal perfusion will return renal function to normal. Alternatively, profound and prolonged hypoperfusion can lead to intrinsic kidney damage. However, the evolution of prerenal failure to intrinsic renal failure is not sudden, and a number of compensatory mechanisms work together to maintain renal perfusion when it is otherwise compromised. 4,55
Acute tubular necrosis (ATN) can evolve from prerenal failure if the insult is severe and sufficient enough to result in vasoconstriction and patchy tubular necrosis. The prognosis of ATN is good, except when the severity of the insult leads to the development of cortical necrosis. The recovery of the renal function depends on the underlying events that precipitated the ischemic/hypoxic insult. The length of time before recovery is quite variable (few days to several weeks). Return of renal function may be accompanied by a diuretic phase with excessive urine output. During this phase, close attention to fluid and electrolyte balance is very important to ensure adequate fluid management to promote recovery and prevent additional renal damage.
In the newborn, some forms of renal failure may have a prenatal onset in congenital diseases, such as renal dysplasia with or without obstructive uropathy, and in genetic diseases, such as autosomal recessive polycystic kidney. Acute renal failure in the newborn is also commonly acquired in the postnatal period because of hypoxic ischemic injury and toxic insults. In fact, asphyxia is the most common cause of acute tubular necrosis in the term neonate (65%), both oliguric and nonoliguric. 82 In the premature infant, sepsis is the most common cause (35%). Patients with congenital heart disease appear to be especially vulnerable to tubular necrosis after cardiac catheterization and cardiac surgery. Nephrotoxic ARF in newborns is commonly associated with the administration of aminoglycoside antibiotics, NSAIDs, intravascular contrast media, and amphotericin B. Indomethacin therapy to promote closure of the patent ductus arteriosus in premature neonates is associated with renal dysfunction in approximately 40% of exposed infants. These alterations are usually reversible. 2,9,39,45 Nephrotoxic ARF from exposure to endogenous compounds such as hemoglobinuria or myoglobinuria is very rare in the newborn.
Renal artery thrombosis and renal venous thrombosis will result in renal failure if bilateral or if either occur in a solitary kidney. In addition to acute renal failure, infants may demonstrate hypertension, gross or microscopic hematuria, thrombocytopenia, and oliguria.
Diagnosis
The diagnosis of acute renal failure in the newborn is not an easy one since oliguria is not a consistent finding and serum creatinine is an unreliable predictor of glomerular filtration in neonates. However, serum creatinine values consistently above the 99th percentile, prolonged oliguria, or failure to achieve a diuresis is clinically significant.
The urine osmolality, urine sodium concentration, fractional excretion of sodium, and renal failure index have been proposed for use to help differentiate prerenal failure from ATN. This differentiation is based on the premise that the tubules are working appropriately in prerenal failure and therefore can conserve salt and water appropriately, whereas in ATN, the injured tubules cannot conserve sodium appropriately. 63,68,69,102 However and of importance, because the renal tubules in newborns and premature infants are relatively immature, the distinction between prerenal failure and ATN is not as clear-cut as we would like to see. In the newborn, values suggestive of hypoperfusion are urine osmolality greater than 350 mOsm/L, urine sodium less than 20 to 30 mEq/L, and a fractional excretion of sodium of less than 2%. Alternatively, values suggestive of ATN are urine osmolality less than 350 mOsm/L, the urine sodium greater than 30 to 40 mEq/L, and the fractional excretion of sodium greater than 2.5%. Similarly, a urine creatinine–to–serum creatinine ratio of greater than 40 implies water conservation and a prerenal cause, whereas a ratio of less than 20 suggests intrinsic renal damage. These values vary greatly, according to gestational age and maturity. Some newborns, particularly premature infants, may have prerenal failure with urinary indices suggestive of ATN.17 Therefore it is important to recognize the limitations of these indices in assessing renal failure in the newborn period (Table 25-2).
| Pretubular: Hypotension/sepsis, shock, hypovolemia/dehydration, hemorrhage, hypoproteinemia, cardiac failure, renal artery stenosis, hypoxemia, asphyxia, glomerulonephritis, mechanical ventilation, pressor agents. | |||||
| Renal parenchymal (tubular): Acute tubular necrosis, corticomedullary necrosis, asphyxia neonatorum, pyelonephritis, interstitial nephritis, polycystic kidney disease, renal parenchymal/aplasia/hypoplasia, intrauterine infection, endogenous toxins (uric acid, hemoglobinuria, myoglobinuria), exogenous toxins (aminoglycosides, indomethacin, contrast media), renal vein thrombosis, disseminated intravascular coagulation, congenital nephrotic syndrome. | |||||
| Obstruction: Ureteral obstruction, urethral obstruction. | |||||
| Cre, Creatinine (mg/dL); FENa, fractional excretion of sodium; Osm, osmolarity (mOsm/L); P, plasma concentration; RFI, renal failure index (U na × P/U creatinine); U, urine concentration. | |||||
| Urinary Indexes of Acute Renal Failure | |||||
|---|---|---|---|---|---|
| U na (mEq/L) | FENa (%) | RFI | U/P cre | U/P osm | |
| Pretubular | 31.4 ± 19.5 | 0.95 ± 0.55 | 1.29 ± 0.82 | 29.2 ± 15.6 | >1.3 |
| Renal parenchymal (tubular) obstruction | 63.4 ± 34.7 | 4.25 ± 2.2 | 11.6 ± 9.6 | 9.6 ± 3.6 | >1 |
A renal ultrasound examination should be performed in all neonates with suspected ARF to assess for possible urinary tract obstruction, renal vein thrombosis, and congenital renal abnormalities such as dysplasia, polycystic disease, and aplasia. 34
Prevention
The prevention of ARF in the preterm and term infant is a complicated discussion. Nonetheless, the following are some recommendations:
• Minimization of perinatal asphyxia
• Avoidance of maternal and infant ACE-inhibitor use
• Aggressive management of hypoxemia, hypovolemia, hypotension, acidosis, and hypothermia
• Early detection and treatment of infections
• Careful attention to agents with vasoactive or nephrotoxic properties, which can exacerbate renal injury (e.g., diuretics, aminoglycosides, NSAIDs)
Management/Treatment
Once the diagnosis of ARF has been established, management of its metabolic derangements needs to be initiated promptly and involves appropriate management of fluid balance, electrolyte status, acid-base balance, and nutrition, as well as initiation of renal replacement therapy when appropriate. 4,13,35
WATER BALANCE
Prerenal causes require increasing perfusion of the kidney by fluid therapy and restoring cardiac output and blood pressure to normal. A fluid challenge of 10 mL/kg of body weight of crystalloid for the small preterm infants and up to 20 mL/kg of body weight for the term infant should be attempted. With no signs of congestive heart failure and continuing oliguria or anuria, fluid administration continues now with the administration of colloid, 5% albumin, in a similar amount. Central venous pressure (CVP) is an important, underutilized parameter in measuring the appropriateness of fluid therapy. Use of CVP is especially important in infants with capillary leak syndrome or third spacing of fluid postoperatively. These infants appear fluid overloaded but may be intravascularly depleted.
Diuretic therapy has some potential benefits (removal of fluid), but the conversion of oliguric to nonoliguric ARF has not been shown to alter the course of the acute renal failure. When using diuretics in newborns with ARF, potential risks and benefits need to be considered. In fact, diuretics may cause dehydration and further exacerbation of the failure. Mannitol (0.5 to 1.0 g/kg over several minutes) may increase intratubular urine flow and may limit cell damage. However, in neonates (particularly premature infants), mannitol should be avoided because of its hyperosmolarity and increased risk for intraventricular hemorrhage (IVH).
ELECTROLYTE AND ACID-BASE DISTURBANCES
Mild hyponatremia is very common in acute renal failure and usually is the result of fluid overload with dilutional hyponatremia. This level of hyponatremia responds very well to fluid restriction or water removal by dialytic therapy. In severe cases (serum sodium <120 mEq/L), there is a greater risk for seizures and correction to a sodium level of approximately 125 mEq/L with hypertonic saline should be considered.
Hyperkalemia is a common and potentially life-threatening complication. The risk for disturbances of the cardiac rhythm secondary to hyperkalemia increases with the presence of acidosis and hypocalcemia. Severe hyperkalemia requires prompt therapy with sodium bicarbonate, intravenous glucose and insulin, and intravenous calcium gluconate.4,67 Severe hyperkalemia in some cases of ARF is an indication for dialysis or hemofiltration.
Hypocalcemia and acidosis are very common in ARF. Severe acidosis can be treated with intravenous or oral sodium bicarbonate, oral sodium citrate solutions, and/or dialysis therapy. When considering treatment of acidosis, it is important to consider the serum ionized calcium level. Correction of acidosis would decrease the amount of ionized calcium and may precipitate tetany and/or seizures. Finally, hyperphosphatemia is a very common electrolyte abnormality noted during ARF. Hyperphosphatemia should be treated with dietary phosphorus restriction and with oral calcium carbonate.
In many instances, ARF is associated with marked catabolism, and malnutrition can develop rapidly, leading to delayed recovery from ARF. Prompt and proper nutrition is essential in the management of the newborn with ARF.
RENAL REPLACEMENT THERAPY
The purpose of acute renal replacement therapy is to remove endogenous and exogenous toxins and to maintain fluid, electrolyte, and acid-base balance until renal function returns. Indications for this type of therapy include fluid overload, severe acidosis, hyperkalemia with electrocardiogram (ECG) changes, symptomatic uremia, hyperuricemia, hyperammonemia, and drug overdose (e.g., theophylline, gentamicin, vancomycin). Renal replacement therapy may be provided by peritoneal dialysis (PD), intermittent hemodialysis (HD), and hemofiltration (HF) or continuous renal replacement therapy (CRRT) with or without a dialysis circuit. Despite the preferential use of hemofiltration by pediatric nephrologists for neonates and small infants with ARF, PD, and HD still remain important therapeutic modalities for ARF in neonates. 31,46,64
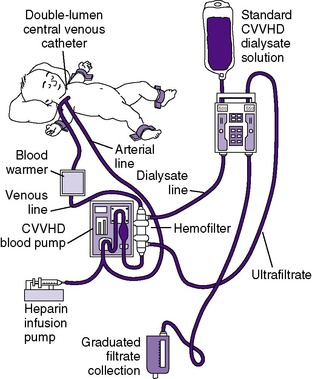
CONTINUOUS RENAL REPLACEMENT THERAPY (HEMOFILTRATION) 33,36,65
Over the past several years, renal replacement therapy with hemofiltration, including continuous venovenous hemofiltration (CVVH) or with the addition of a dialysis circuit to the hemofilter (continuous venovenous hemodiafiltration [CVVHD]), has become increasingly popular in the treatment of ARF (Figure 25-4) . The advantages of hemofiltration include that it can result in rapid fluid removal, does not require the patient to be hemodynamically stable, and is administered continuously, avoiding rapid solute and fluid shifts as occurs in hemodialysis. The disadvantages include that hemofiltration may require constant heparinization. 90
In recent years, improvements in the technologies of CRRT have made it more suitable for use in neonates. 26,32 For some centers, CRRT has become the standard of care for neonatal acute dialysis. CVVHD offers a great alternative, especially in the infant with labile hemodynamic status, in whom HD and PD are not feasible. 98,113
HEMODIALYSIS
Hemodialysis (HD) has the advantage of rather quickly correcting metabolic abnormalities, and hypervolemia can be corrected by ultrafiltration as well. 26 The disadvantages include the need for heparinization, the need for skilled nursing personnel, and the need for vascular access. Relative contraindications include hemodynamic instability or severe hemorrhage.
PERITONEAL DIALYSIS
Traditionally, acute peritoneal dialysis (PD) has been a major modality of therapy for ARF in the neonate when vascular access may be difficult to maintain. 46,47 Advantages of PD include that it is relatively easy to perform, it does not require heparinization, and the newborn does not need to be hemodynamically stable to undergo PD. The disadvantages include a slower correction and the potential for peritonitis.
As a renal replacement therapy, PD is useful for both the acute and chronic setting; therefore it remains the intervention of choice for the neonate with end-stage renal disease (Figure 25-5andTable 25-3). The goal of long-term PD is ideally to permit normal growth and development up to the time of transplantation, if needed. Although technically challenging, long-term PD has been performed in very-low-birth-weight (VLBW) infants with birth weight as low as 930 grams. 4,111 Currently, despite the fact that there has been a clear improvement in the availability of infant catheters and dialysis tubing, chronic dialysis remains extremely time consuming, challenging, and demanding for the infant, the family, and medical personnel. Ultimately, this is a home-based therapy ostensibly provided by parent(s). Although renal replacement therapy for infants has become a standard-of-care therapy, the final decision to begin chronic dialysis remains in the hands of both the parents and the multidisciplinary team. Mortality remains high for the infant group, with 20% to 50% dying within the first year of life.
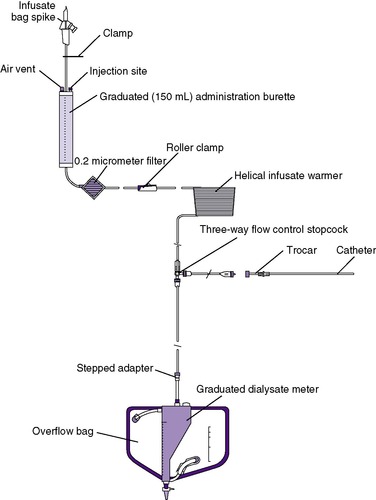 |
| FIGURE 25-5
(Courtesy Utah Medical Products, Midvale, Utah.)
|
| HOB, Head of bed; PD, peritoneal dialysis. | |
| Problems | Nursing Actions |
|---|---|
| Potential peritonitis |
1. Sterile technique to be used at all tubing connections and bag spikes; all connections clamped and taped.
2. Assess PD effluent with each drain for color, turbidity, and the presence of fibrin.
3. If turbidity exists:
a. Obtain cell count, differential, and Gram stain and culture PD fluid
b. Administer antibiotics as ordered
4. Occlusive dressing at catheter site.
|
| Potential fluid overload or dehydration |
1. Measure and record the exact amount of inflow and outflow of dialysate with each exchange.
2. Weigh neonate at regular intervals during drain to determine his or her real weight.
3. Assess fluid reabsorption:
a. Peripheral and dependent edema
b. Weight gain
c. Failure to drain out all of dwell volume
4. Assess for dehydration:
a. Weight loss
b. Poor skin turgor and sunken eyes
c. Hypotension
5. Notify physician of weight discrepancies or other symptoms.
|
| Potential temperature maintenance problems |
1. Warm all PD fluid to body temperature by blood warmer or heating pad immediately before inflow.
|
| Inflow or outflow obstruction |
1. Check for kinks in line.
2. Reposition patient, inflow and/or drain bags.
3. Plain radiograph of the abdomen to check position of catheter—should be toward pelvis.
4. Add heparin to dialysate if fibrin is present.
|
| Potential respiratory compromise |
1. Use smaller exchange volumes.
2. Position patient with HOB elevated to reduce pressure from the abdomen on the diaphragm.
3. If distress exists after drain, obtain chest x-ray film to rule out pneumonia or hydrothorax.
|
Outcome and Prognosis
In the newborn infant, the prognosis and recovery from acute renal failure highly depends on the underlying etiology of the ARF.3,7,32,56Factors that are associated with mortality include multiorgan failure, hypotension, need for pressors, hemodynamic instability, and need for mechanical ventilation and dialysis.28,35
Newborns who have suffered substantial loss of nephrons as may occur in cortical necrosis, hypoxic/ischemic injury, and nephrotoxic injury are at significant risk for late development of chronic renal failure long after the initial insult. 80,114 Newborns with ARF need lifelong monitoring of their renal function, blood pressure, and urinalysis. Typically, the late development of chronic renal failure will first become apparent with the development of hypertension, proteinuria, and eventually an elevated BUN and creatinine. 4
HYPERTENSION*
Hypertension is a significant clinical problem in the neonate cared for in a NICU setting. The incidence of hypertension in healthy term infants appears to be quite low, and the majority of hypertensive infants have a definable etiology. Nearly universal blood pressure monitoring in nurseries with established normal blood pressure ranges enables more frequent diagnosis. A hypertensive infant may be quite ill, with symptoms similar to those of an infant with sepsis or heart or lung disease. If the infant is properly diagnosed and treated, the outcome is favorable.
Blood pressures (BPs) vary by gestational age, body weight, cuff size, and state of alertness. Normal values have been developed by body weight and postnatal age. BP increases by 1 to 2 mm Hg/day for the first 3 to 8 days after birth and by 1 mm Hg/week for 5 to 7 weeks. It reaches a steady value for the first year of life by 2 months of age. How the percentile ranking for an infant’s BP will track into later childhood or adulthood is still unclear. Normal values for BPs in infants are shown in Table 25-4.
| Age | n | State | Measured Pressures (mm Hg) | ||
|---|---|---|---|---|---|
| Systolic | Diastolic | Mean | |||
| 1 hour62 | 17 | 70 | 44 | 53 | |
| 12 hours62 | 17 | 66 | 41 | 50 | |
| 1 day106 | 46 | Asleep | 70 ± 9 | 42 ± 12 | 55 ± 11 |
| Awake | 71 ± 9 | 43 ± 10 | 55 ± 9 | ||
| 3 days106 | 46 | Asleep | 75 ± 11 | 48 ± 10 | 59 ± 9 |
| Awake | 77 ± 12 | 49 ± 10 | 63 ± 13 | ||
| 6 days106 | 46 | Asleep | 76 ± 10 | 46 ± 12 | 58 ± 12 |
| Awake | 76 ± 10 | 49 ± 11 | 62 ± 12 | ||
| 2 weeks118 | 566 | 78 ± 10 | 50 ± 9 | ||
| 3 weeks118 | 77 | 79 ± 8 | 49 ± 8 | ||
| 4 weeks118 | 642 | 85 ± 10 | 46 ± 9 | ||
Etiology
The causes of hypertension can be seen inBox 25-2. All infants with hypertension must first be assumed to have a specific etiology.
BOX 25-2
Vascular
• Renal artery stenosis
• Renal artery thrombosis
• Coarctation of the aorta
• Hypoplastic abdominal aorta
• Renal vein thrombosis
• Idiopathic arterial calcification
Renal
• Renal dysplasia or hypoplasia
• Polycystic kidney disease (autosomal dominant or recessive)
• Renal failure
• Obstructive uropathy
• Reflux nephropathy
• Pyelonephritis
• Glomerulonephritis
Tumors
• Wilms’ tumor
• Neuroblastoma
Endocrine
• Adrenogenital syndrome
• Cushing disease
• Hyperaldosteronism
• Thyrotoxicosis
Other
• Closure of abdominal wall defects
• Fluid overload
• Genitourinary surgery
• Hypercalcemia
• Increased intracranial pressure
• Medications
• Phenylephrine
• Corticosteroids
• Theophylline
• Deoxycorticosterone
• Seizures
• Chronic lung disease/bronchopulmonary dysplasia
Data from Adelman RD: Neonatal hypertension. In Loggie JMH, Horan MJ, Hohn AR et al, editors: NHLBI workshop on juvenile hypertension, New York, 1983, Biomedical Information Corporation; Gulgnard JP: Neonatal nephrology. In Holliday MA, Barratt TM, Vernier RL, editors: Pediatric nephrology, ed 2, Baltimore, 1987, Williams & Wilkins.
Diagnosis
Accurate, reliable measurements of BP are critical to prevent falsely elevated (or depressed) values. Under study conditions, the best measurement of BP is the direct arterial measurement, usually through an umbilical artery catheter (UAC). Older techniques such as auscultation, palpation, and flush blood pressure measurements have been replaced by Doppler measurements and oscillometry. These latter two techniques correlate well with direct arterial measurements for systolic BP but not diastolic BP. Cuff selection is also important, because cuffs that are too small give falsely high values. The cuff should completely encircle the extremity and be the largest cuff possible without impinging on the joints. The position for measuring BP is always supine. BPs in extremities elevated above the level of the heart will be erroneously low; the converse is true of pressures taken below the level of the heart. BP can vary greatly with state of alertness and crying.
Frequently a sick infant will have a BP measured both directly through an arterial catheter and indirectly by oscillometry. Discrepancies between these measurements are often difficult to resolve. Oscillometric pressure may be inaccurate because of improper cuff size or equipment problems, or arterial blood pressure may be so low that oscillometry is difficult. Direct measurements may be inaccurate because of equipment malfunction, improper placement of the transducer in relation to the heart, or partial catheter occlusion.
Data Collection
HISTORY*
Many infants who develop hypertension have had a UAC. Although ultrasound imaging has shown that the incidence of associated aortic and/or renal artery thrombosis is high and therefore the potential for renal artery embolism as a cause for hypertension is likewise high, documented renal infarction is relatively uncommon. It is possible that embolism in some infants may be sufficient to cause hypertension but too small to be identified without, or even with, arteriography. It is also possible that in many such infants, the UAC was simply part of management rather than causative. Hypertension is common in infants with severe bronchopulmonary dysplasia for reasons that are unclear; this usually occurs long after a UAC has been removed, even after discharge.
SIGNS AND SYMPTOMS
An infant with blood pressures above the 95th percentile for similar gestational age and post-conceptual age (PCA) (on three measurements 3 days in a row [unless severe, meaning more than 30% above expected for age]) should be considered hypertensive. In general, a term infant with blood pressures consistently exceeding 95 mm Hg systolic or 75 mm Hg diastolic should be considered hypertensive. In premature infants, the definition varies with gestational and postnatal age and is defined in reference to graphically displayed normal data. 25,112BP measurements should be taken in all extremities to rule out coarctation of the aorta.
Severe hypertension may present as congestive heart failure with respiratory distress and hepatomegaly and with neurologic symptoms (seizures, tremor, and abnormalities in tone). Infants with mild and moderate hypertension are usually asymptomatic. In long-standing hypertension, funduscopic examination may show typical changes of hypertensive retinopathy.
LABORATORY DATA25,91,112
Diagnosis of hypertension requires full evaluation. This should include gray-scale ultrasound examination to evaluate renal anatomy and look for aortic and renal artery thrombi. Color-flow Doppler should be used to look for flow abnormalities. Renal scintigraphy can identify side-to-side differences in renal function. Magnetic resonance angiography (MRA) adds information about the anatomy of larger vessels, but MRA resolution is insufficient to identify disorders in small vessels. Classic contrast angiography is seldom performed in newborns.
Serum creatinine concentrations are usually normal. Urinalysis is also usually normal, but hematuria and proteinuria also may be noted either as a sign of a cause of hypertension or as a result of hypertension. Plasma renin activity, thyroid function studies, plasma and urinary steroids, and urinary catecholamines will be indicated in selected patients. The effects of hypertension should be sought, including a cardiac evaluation and funduscopic examination by an ophthalmologist.
Treatment60,88,112
There are no firm indications for treatment of hypertension in infancy. The long list of available antihypertensive medications has not been systematically studied. Therefore treatment relies on case-series data, older clinical trials, expert opinion, and personal experience.
There is general agreement that hypertension should be treated if systolic blood pressure exceeds 100 to 110 mm Hg, although rapid normalization of long-standing hypertension may be detrimental and must be avoided. A definable cause, such as urinary tract obstruction, abdominal tumor, or coarctation, should be treated surgically. Nephrectomy may be necessary in medically unmanageable, severe hypertension.
Medical management should begin with correction of salt or fluid overload if either exists. Drugs and dosages commonly used in the neonate are shown inTable 25-5. Captopril and enalapril are commonly used in NICUs and after discharge. They should be started with extreme caution if bilateral renal artery obstruction is suspected; dramatic, prolonged decreases in blood pressure with renal failure and neurologic abnormalities have been reported. Calcium channel blockers are also used. Within recommended dosing, they do not typically cause fluid retention or reflex tachycardia. For an acute hypertensive crisis, intravenous (IV) hydralazine, labetalol, nicardipine, and nitroprusside are used.
| BID, Twice a day; CLD/BPD, chronic lung disease/bronchopulmonary dysplasia; IV, intravenous; kg, kilogram; mcg, microgram; mg, milligram; PO, per os, orally; QID, four times a day; TID, three times a day. | ||||
| *No reported experience in the newborn with nifedipine, clonidine, labetalol, or verapamil. Furosemide (Lasix) and thiazides are not antihypertensive medications but are used for volume overload. |
||||
| Medications | Dose | Schedule | Route | Comments |
|---|---|---|---|---|
| Propranolol | 1-4 mg/kg/dose | BID-TID | PO | Contraindicated in heart failure, possibly in CLD/BPD, sedation |
| 0.025-1 mg/kg/dose | BID | IV | ||
| Hydralazine | 0.25-1.5 mg/kg/dose (max 4.5 mg/kg/dose) | BID-QID | PO | Tachycardia, sodium retention |
| 0.1-0.5 mg/kg/dose with a beta blocker | q 6 hr | IV | ||
| 0.4-0.8 mg/kg/dose (sole agent) | ||||
| Captopril | 0.1-2 mg/kg/dose | TID | PO | Leukopenia, rash, proteinuria, hyperkalemia, acute renal failure, seizures |
| Enalapril | 0.1-0.3 mg/kg/dose | q 12-24 hr | PO | Hypotension |
| Enalaprilat | 0.005-0.05 mg/kg/dose | q 12-24 hr | IV | Hypotension |
| Diazoxide | 1-5 mg/kg/dose | q 4-24 hr | IV | Hyperglycemia, fluid retention, hyperuricemia |
| Sodium nitroprusside | 0.5-10 mcg/kg/min | Continuous infusion | IV | Keep covered in foil, careful observation for infiltration of IV or varying rate of administration |
Prognosis25,27,112
Prognosis for these patients is excellent if BP is controlled medically or cured surgically. These infants have normal somatic growth and development. Antihypertensive medications are usually unnecessary after 1 to 2 years of follow-up. Poor renal growth may occur on the side of a renal artery lesion, and renal function scans tend to be persistently abnormal. Creatinine clearance is usually normal.
ABDOMINAL MASSES
Abdominal masses in neonates reflect a wide spectrum of pathologies, ranging from small lesions found incidentally to large ones occupying the entire peritoneal cavity; from unilocular cysts to complex solid ones; from lesions that can cause significant morbidity and mortality to entities that may be safely observed. This spectrum is further broadened by the variety of organs that can give rise to such masses. 14,16,24,38
In the era of almost universal prenatal ultrasound, many such masses are identified, and some are even treated, before delivery. Others are discovered during the course of a thorough routine examination of the neonate. Although most of these babies are otherwise healthy, the news is likely to disturb the new parents. It is incumbent upon the infant’s physician to determine the nature of the mass in a timely, safe, and cost-effective manner.14,40,43
Diagnosis
Slightly more than 50% of abdominal masses present during the newborn period are of renal origin.61,71 The literature offers no consistent data on frequency of abdominal masses in infants, but there is general agreement about the urgent need to evaluate these infants quickly and thoroughly and to reach an accurate diagnosis before planning intervention.
PHYSICAL EXAMINATION
To examine the abdomen, the infant should be in the supine position. Inspection of the abdomen before manual exploration enables the examiner to note a mass that may be missed on a tense abdomen. The shape of the abdomen should be noted. The position of the umbilicus and the presence of any hernias should be assessed. Bimanual palpation using the flat surface of the fingers while supporting the infant’s flank with the other hand permits the exploration of the abdomen during deep palpation. Characteristics of the mass to note include location, size, shape, texture, mobility, and tenderness. The mass should be categorized as solid, cystic, or air-filled; however, the differentiation between solid and cystic masses can be difficult on physical examination. Percussion may be used to outline the suspected area, and transillumination is sometimes helpful.
If gastric distention or intestinal obstruction is suspected, a nasogastric tube is inserted and air and fluid evacuated. If there is a question of urinary retention, the infant should be re-examined after placement of a urinary catheter or after inducing voiding with a Credé maneuver. Rectal examination, applied judiciously, may provide useful information, such as in a suspected intra-pelvic or intra-abdominal mass. Upon the recognition of an abdominal mass, findings of the entire physical examination should be reviewed in this light. Clues to the nature of the lesion may be external or distant to the mass.
LABORATORY DATA
Radiographic imaging is usually the next step. Plain films may provide a surprising amount of information, such as organomegaly and calcifications in a number of tumors or displacement of the intestines, as a subtle clue to the nature or even presence of a mass.
Additional information can be expected from ultrasonography (US). This modality, with ever-increasing image resolution, is an excellent screening tool. It is noninvasive, accessible for bedside studies, radiation-free, and painless and can provide detailed information on the location, nature, and vascularity of the mass and adjacent structures. When this diagnostic tool is incapable of differentiating dysplasia and hydronephrosis, renal scintigraphy is indicated to better assess renal function. Rarely a percutaneous nephrostogram is performed to determine whether cysts result from obstruction or dysplasia. A voiding cystourethrogram is the method of choice to diagnose vesicoureteral reflux. Computed tomography (CT) or magnetic resonance imaging (MRI) is occasionally indicated, especially in differentiating renal masses and extent of the disease.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis in the infant with an abdominal mass is shown inTable 25-6. The workup of most abdominal masses requires only a thorough physical examination and few (goal-oriented) studies. Usually, the location of the mass is a very useful clue to the possible organ involved and the most likely diagnosis14:
| Type of Mass | Percent of Total |
|---|---|
| RENAL MASSES | |
|
Hydronephrosis
Multicystic dysplastic kidney
Polycystic kidney disease
Mesoblastic nephroma
Renal ectopia
Renal vein thrombosis
Nephroblastomatosis
Wilms’ tumor
|
55 |
| GENITAL MASSES | |
|
Hydrometrocolpos
Ovarian cyst
|
15 |
| GASTROINTESTINAL MASSES | |
|
Duplication
Volvulus
Complicated meconium ileus
Mesenteric-omental cyst
“Pseudocyst” proximal to atresia
|
15 |
| NONRENAL RETROPERITONEAL MASSES | |
|
Adrenal hemorrhage
Neuroblastoma
Teratoma
|
10 |
| HEPATOSPLENOBILIARY MASSES | |
|
Hemangioendothelioma
Hepatoblastoma
Hepatic cyst
Splenic hematoma
Choledochal cyst
Hydrops of gallbladder
|
5 |
1. Flank: The most common causes of flank masses are of renal origin, hydronephrosis or multicystic kidney. Other flank masses of importance are the solid tumors of the kidney, such as the benign congenital mesoblastic nephroma (the most common) and Wilms’ tumor. Another group of flank masses are of juxtarenal origin: neuroblastoma, adrenal hemorrhage, various necrotic lesions, bronchogenic cyst, and infradiaphragmatic (extralobar) pulmonary sequestration. Renal vein thrombosis is an unusual cause of flank mass.
2. Right upper quadrant (RUQ): Most RUQ masses usually involve the liver and biliary tract. In fact, the typical presentation of the most common benign hepatic tumor, infantile hepatic hemangioma (hemangioendothelioma), is a palpable RUQ mass. Other masses include the benign mesenchymal hamartomas, the hepatoblastoma (the only significant primary hepatic malignancy in neonates), and the choledochal cyst.
3. Left upper quadrant (LUQ): Splenic cysts are very rarely diagnosed.
4. Mid-abdominal: Abdominal masses in the mid-abdomen usually involve the intestine. Duplications of the gastrointestinal (GI) tract occur anywhere from the esophagus to the anus and are either cystic (the most common) or tubular. Usually, they are present as an asymptomatic palpable mass but may also cause pain, intestinal obstruction, GI bleeding, or even volvulus.
Other mid-abdominal masses may include intestinal lymphatic malformations, meconium diseases, mid-abdominal wall defects, and omphalomesenteric remnants. Failure of the vitelline duct to resorb completely may lead to a variety of related entities, including Meckel’s diverticulum and omphalomesenteric sinus, cyst, or fistula.
5. Pelvic: A residual pelvic mass in a female infant after voiding may represent an enlarged vagina (hydrocolpos) or uterus (hydrometrocolpos). Such a mass should trigger a close look at the perineum and vaginal introitus. Other pelvic masses may represent ovarian masses, urachal cyst, and teratomas. Cystic ovarian tumors are more common than solid ones, and the majority are benign; however, every cystic ovarian mass needs to be investigated. Some malignancies have been reported.
INTRINSIC RENAL PARENCHYMAL ABNORMALITIES79,81
Renal abnormalities can be classified by the amount of tissue, differentiation of tissue, and position of the kidneys.
Congenital absence or agenesis of renal tissue can occur unilaterally or bilaterally. Unilateral renal agenesis is seen more frequently (1:1000 live births) and may manifest as a solitary kidney on examination with enlargement caused by compensatory hypertrophy. Unilateral agenesis has been associated with Turner, Poland, and VATER ( vertebral defects, imperforate anus, tracheo esophageal fistula, and radial and renal dysplasia) syndromes. Bilateral agenesis, also known as Potter disease, is seen rarely, with an incidence of 1 per 4000 births.
Hypoplasia is a deficiency in the amount of renal tissue expressed as an abnormally small kidney. Morphologically, the kidney is normal, and renal function is unaffected in the neonatal period. Later in life, patients can sometimes “outgrow” their renal function.
Signs and Symptoms
In unilateral agenesis, patients are often asymptomatic and are diagnosed inadvertently on ultrasound or based on the significant association with malformations of the lower genitourinary tract. There is no need for long-term follow-up if only a solitary kidney without additional involvement is found.
In bilateral agenesis, the majority of affected infants are male and small for gestational age, with a history of maternal oligohydramnios. The characteristic facial features accompanying Potter syndrome include wide-set eyes, parrot-beak nose, receding chin, and large, low-set ears with little cartilage. Other associated malformations include pulmonary hypoplasia, hydrocephalus, meningocele, multiple skeletal anomalies, and imperforate anus. Death usually occurs within hours to several days.
Differentiation of Tissue79,85,86
Abnormalities in renal tissue differentiation are most commonly expressed as dysplastic kidneys. Renal dysplasia is a failure of the metanephric tissue to mature appropriately, frequently because of obstruction of the urinary tract early in gestation. The result is a persistence of immature structures and very little normal functioning renal tissue.
Renal dysplasia may be seen in one or both kidneys and involving the entire kidney, segments of the kidney, or microscopic areas (foci) of a kidney. Dysplasia is most commonly expressed as cyst formation. Bilateral multicystic dysplastic kidneys (MCDKs) are nonfunctional and not compatible with life. Unilateral MCDK involvement is both the most common cystic lesion of the neonatal kidney and one of the most frequently palpated abdominal masses in the newborn. Unilateral MCDK shows no predilection for males or females or for involvement of right or left kidney. Usually the ureter is absent, atretic, or stenotic. No orifice is found in the bladder. The kidneys are extremely hypoplastic, enlarged, diffusely cystic with almost complete loss of the reniform configuration. The histopathologic landmark of MCDK is nests of cartilage and mesenchymal mantles surrounding primitive tubules. Renal function and structure may be normal in the remaining kidney of infants with unilateral dysplasia; however, frequently vesicorectal reflux or ureteropelvic junction (UPJ) obstruction is present in the contralateral kidney. Therefore a voiding cystourethrogram should be performed on every patient. In addition, hypertension is a potential complication (estimates of 20% have been made) of MCDK and requires treatment or long-term follow-up.
Renal dysplasia is usually sporadic, but some familial cases have been reported. A lack of blood flow on 99mTc DPTA nuclear renal scan confirms the diagnosis of dysplasia.
Treatment
Generally, these kidneys involute with time; therefore a conservative rather than surgical approach is recommended. The association between renal dysplasia and neoplasia has not been confirmed. However, removal of the kidney is sometimes indicated if its size prevents adequate nutrition.
POLYCYSTIC KIDNEY DISEASE18,37,51,53,83
Pathophysiology
Polycystic kidney disease (PKD) may present as one of two types in the infant: (1) autosomal recessive polycystic kidney disease (ARPKD); and (2) autosomal dominant polycystic kidney disease (ADPKD). Traditionally, ADPKD has not been associated with onset during the first year of life, but recent studies have confirmed both presentations in the infant and, conversely, ARPKD has been reported in the older child.
ARPKD manifests with varied severity, but it is always bilateral. The kidneys become enlarged with a proliferation of renal tubules and dilated collecting tubules. These are not true “cysts,” and the kidney has a reniform shape. Various combinations of cystic renal disease and hepatic disease occur in ARPKD including dilation of collecting tubules in the kidney, congenital hepatic fibrosis because of ductal plate malformation, and nonobstructive dilation of intrahepatic bile ducts (Caroli disease). Autosomal dominant disease involves cyst formations in any portion of the nephron, Bowman’s space, and liver. Of affected individuals, 50% have cysts in other visceral organs including the liver, pancreas, spleen, and lung. There is a strong association between ADPKD and cerebral artery aneurysms.
Data Collection
HISTORY
Criteria for making a definitive diagnosis for both diseases have been developed. Autosomal recessive disease includes infants with the following: (1) congenital hepatic fibrosis on liver biopsy or evidence of portal hypertension; (2) renal histologic studies consistent with collecting tubule ectasis; or (3) a sibling with the disease. Infants diagnosed with ARPKD have either a positive parental history or known liver cysts or berry aneurysm.
SIGNS AND SYMPTOMS
Both types of PKD can manifest initially with an abdominal mass. The infant may present with bilateral flank masses, hepatic enlargement, Potter facies caused by oligohydramnios, oliguria, acute renal failure, hypoplastic lungs, respiratory distress, and spontaneous pneumothorax. Hypertension is common in both types of the disease.
LABORATORY DATA
Differentiation of ADPKD versus ARPKD may be difficult, even with ultrasound, because radiographic studies are not consistently accurate in discerning differences. Indeed, retrospectively, it is not uncommon to find infants misclassified.
Treatment
Management consists of serial monitoring of blood pressure, renal function, and urine cultures. Neonates with either form of PKD need aggressive treatment of hypertension with captopril as the drug of choice, treatment of any urinary tract infection, and aggressive nutritional management.
HYDRONEPHROSIS70,72,78
Physiology
The collecting system of the kidney is composed of the ureter, pelvis, and calyces, all of which function as a system for removing urine from the kidney. Hydronephrosis, one of the most common abdominal masses in the newborn, involves a dilation of the pelvis and calyces, most often as a result of congenital obstruction. The impaired movement of urine from severe or chronic obstruction may lead to dysplastic and cystic changes that further impair kidney function if the obstruction occurs early in gestation.
The most common ureteral site of obstruction is at the ureteropelvic junction (UPJ). The infant presents with a ballooning of the renal pelvis. Obstruction at the ureterovesical junction (UVJ), also known as congenital megaureter in its primary form, occurs more often in the male infant. UVJ obstruction more frequently affects the left ureter. Posterior urethral valves (PUVs) in males are the major cause of urethral obstruction. This distal obstruction may result in bladder hypertrophy, hydroureter, and hydronephrosis if severe. Dysplastic changes can be seen if the obstruction occurs early in gestation. The neonate with PUV is at risk for developing an ascending infection and subsequent renal damage. Prune-belly syndrome, also known as Eagle-Barrett syndrome, is a less common cause of obstruction and dilation of the pelvis and calyces. There is a strong male predominance. This triad of anomalies includes (1) absence or hypoplasia of the abdominal wall muscles, (2) bilateral cryptorchidism, and (3) urinary tract abnormality. The loose, shriveled abdomen is responsible for the “prune belly” appearance, which diminishes with age and does not require surgical correction. Renal dysplasia is usually seen in prune-belly syndrome and may range from mild to severe involvement. The enlarged bladder may be seen in conjunction with a patent urachus draining urine. The prostatic urethra is usually hypoplastic.
Etiology
The etiology of most types of hydronephrosis remains unclear. Primary prune-belly syndrome may be a result of a mesenchymal developmental arrest. A variant of the syndrome also can be seen as a sequela of an intrauterine distention of the abdomen by an obstructed urinary system. The existence of this secondary cause of prune-belly syndrome is controversial. Another cause of calyceal dilation not associated with obstruction is vesicoureteral reflux, as discussed further in the “Urinary Tract Infection” section.
Infants may have few if any symptoms, and there are usually no physical findings unless a bladder or kidney is palpated on routine examination. These infants can present with a poor urinary stream and frequently with failure to thrive.
Treatment70
Mild to moderate unilateral obstruction does not require immediate treatment. Close follow-up is indicated for monitoring of kidney growth and obstruction as surgery may be a postnatal consideration; bilateral dilation with normal amounts of amniotic fluid is managed with close observation. Treatment of bilateral dilation with decreased amniotic fluid depends on the gestational age of the fetus.
A viable fetus with dilated collecting systems, initial normal amount of amniotic fluid, and evidence of decreasing amniotic fluid should be delivered early. Other conditions such as bilateral vesicoureteral reflux, prune-belly syndrome, and primary megaureter may present with dilated collecting systems and are not amenable to in utero surgery. Surgical intervention in utero is very controversial and center dependent, and the morbidity of this therapy is very high.
Complete obstruction at the UPJ is surgically corrected. For uterovesical obstructions, surgical correction involves excising the stenotic segment in the obstructed megaureter, as well as ureteric reimplantation, and is successful in the large majority of infants. Management of obstruction secondary to PUV depends on the age at presentation and infant’s condition. After initial stabilization, relief of obstruction with a catheter provides quick decompression. Permanent repair consists of removal of the obstructing valves. The use of a vesicostomy versus a higher diversion is controversial.
RENAL VEIN THROMBOSIS57,75,94
Renal vein thrombosis (RVT) can be an acute life-threatening condition or insidious with the development of microhematuria and inflammation. RVT is associated with conditions that cause circulatory collapse and decreased oxygenation within the kidney.
Etiology
Perinatal causes of neonatal RVT include maternal diabetes, toxemia, maternal thiazide therapy, polycythemia, placental insufficiency, birth asphyxia, prematurity, respiratory distress syndrome (RDS), and sepsis. Angiography has also been associated with RVT. Thrombosis most often occurs in the smaller renal veins rather than in the main renal vein.
Data Collection
SIGNS AND SYMPTOMS
The involved kidney may enlarge secondary to obstruction to blood flow and forms a palpable flank mass. Other clinical symptoms may include hematuria (60% of cases), anemia, oliguria, and thrombocytopenia (<75,000 platelets).
LABORATORY DATA
Positive blood on dipstick testing, urine output of less than 1 mL/kg/hr, and a low platelet count may indicate RVT.
Treatment
Management includes treatment of the underlying illness, treatment of sepsis if suspected, fluid therapy, and possibly dialysis in select cases. Heparin therapy remains controversial for RVT. Surgical excision of the thrombus is not usually indicated during the acute phase but may be appropriate at a later time. Rarely is nephrectomy necessary. Renal tubular dysfunction is often observed after recovery from RVT.
MISCELLANEOUS CAUSES OF ABDOMINAL MASS
Wilms’ tumor, also known as nephroblastoma, is the most common intraabdominal tumor seen in children and occurs at a rate of 8 to 9 per 100,000 per year in the United States; two thirds of patients present in the first 3 to 6 months of life. The tumor is described as firm, smooth, and confluent with the kidney or attached to the organ. Both kidneys are involved in 10% of cases. This condition has an excellent prognosis with treatment. Surgical removal of the tumor is followed by irradiation for most patients and chemotherapy.
Neuroblastoma, on the other hand, is the most common malignant tumor in infancy. The primary site of the tumor may be any area of neural crest tissue, with the most common site identified in the adrenal gland. Presenting in the neonate as a palpable abdominal mass, this tumor may also cause urinary obstruction. Prognosis is related to the site of the primary tumor, histologic appearance of the tumor, staging of the disease, and age of the patient.
RENAL TUBULAR DISORDERS5,104,108
Although most of the renal tubular disorders are congenital, they rarely manifest clinically during the newborn period. However, in sick infants admitted to the intensive care unit, these tubular abnormalities can lead to severe and frequently life-threatening electrolyte disorders.
Etiology
With the advent of routine prenatal ultrasound, a number of newborns referred for evaluation of polyhydramnios and polyuria have been diagnosed with diabetes insipidus (central or nephrogenic) and Bartter syndrome. In addition, obstruction of the urinary tract, which is frequently diagnosed prenatally, is commonly associated with renal tubular acidosis (RTA), particularly the hyperkalemic type (type IV).
Data Collection
HISTORY/SIGNS AND SYMPTOMS
Infants with Fanconi syndrome and distal RTA most commonly present after the neonatal period with the complaint of failure to thrive. Frequently a history of previous admissions to the hospital for evaluation of sepsis or dehydration is obtained.
LABORATORY DATA
The diagnosis of Fanconi syndrome is confirmed by demonstration of a generalized dysfunction in the proximal tubule, evidenced by the presence of glycosuria, proteinuria (low-molecular-weight proteins), bicarbonaturia, phosphaturia, and uricosuria. Distal RTA is diagnosed by demonstrating a decreased urinary excretion of ammonium; if the result is a negative number (less than zero), then distal RTA is ruled out. A positive urine net charge (higher than zero) is consistent with RTA. However, because of the presence of other organic anions in the urine during the first 2 weeks of life, the validity of this test during the neonatal period has been questioned. Disorders of vitamin D metabolism or phosphate reabsorption (rickets) usually manifest by the end of the first year of life, after the child starts walking. Infants with diabetes insipidus typically present during the first 2 months of life with dehydration and a sepsis-like picture.
Complications
Thus, although most of the tubular disorders are not clinically evident at birth, the clinician should keep a high index of suspicion in those infants with prenatal diagnosis of urologic abnormalities or serious abnormalities in water and electrolyte metabolism. Early evaluation and treatment of renal tubular disorders may prevent catastrophic complications such as life-threatening episodes of dehydration and delayed growth and development.
URINARY TRACT INFECTION44,105
Urinary tract infections (UTIs) affect approximately 1% of full-term infants and 3% of premature infants. Male infants are affected 5 times more frequently than females. Vesicoureteral reflux is a common radiographic finding in infants. Primary reflux is seen in abnormalities of the vesicoureteral junction, ureteral duplication, and ureterocele. Secondary reflux is associated with infection, PUV, and neurogenic diseases.
Etiology
Abnormalities of the urinary tract are responsible for a large number of UTIs in the neonate. Whether the infection is ascending from the bladder or hematogenously spread is a matter of debate. The high association of reflux with UTI makes determining the etiology of reflux a priority for planning appropriate treatment. Reflux is graded on a four-point scale, with grade IV denoting massive hydronephrosis and hydroureter.
Maternal urinary infections also have been associated with neonatal UTIs. Symptomatic manifestations include abnormal weight loss during the first days of life, decreased feeding, dehydration, irritability, lethargy, cyanosis, jaundice, and septicemia. In some cases, the affected kidneys are palpable. Infected infants also may be asymptomatic.
Data Collection
LABORATORY DATA
Evaluation of a neonate with suspected UTI includes immediate urine and blood cultures and a complete blood count (CBC). The optimum method of obtaining urine for culture is suprapubic aspiration of the bladder or catheterization. Catheterization may not be recommended in the neonate because of possible urethral stricture formation in the male and frequent culture contamination in the female. Urine obtained in a urine bag should not be used for cultures because it is easily contaminated. With diagnosis of UTI, radiographic evaluation should be undertaken to rule out anatomic abnormality. This should include ultrasound and voiding cystourethrogram (VCUG). Grades of reflux are diagnosed by voiding cystourethrogram; thus sterile urine is necessary before a VCUG is undertaken. Renal scintigraphy and CT scan may be needed to evaluate renal scarring and damage.
Treatment
Pyuria (10 to 15 WBC per hpf) can be observed in the neonate normally. Treatment for UTI is indicated when an organism is cultured from the urine. Any growth in a urine specimen obtained by suprapubic aspiration should be considered to represent an infection if the procedure was cleanly done. Any aspiration of bowel contents must affect the interpretation of culture results. Traditional antibiotic coverage consists of both ampicillin and an aminoglycoside. The advent of third-generation cephalosporins has allowed for excellent gram-negative coverage without the nephrotoxicity of the aminoglycosides. Escherichia coli is the organism most often implicated in neonatal UTIs, followed by Klebsiella. Sulfonamides are contraindicated in the neonate because of their potential to complicate hyperbilirubinemia.
Antibiotic therapy should continue for 10 to 14 days, with a follow-up urine culture 3 days after therapy is discontinued. Many practitioners recommend antibiotic prophylaxis until significant reflux or anatomic abnormality is ruled out.
NEUROGENIC BLADDER92,105
Neurogenic bladder is an anatomic interruption of the micturition reflex normally triggered by a full bladder. The bladder may be flaccid and unable to empty urine or spastic and hyperreflexive and unable to store urine. Infants with lumbosacral spinal malformations commonly have a urinary tract dysfunction known as neurogenic bladder. Lower motor neuron deficit causes bladder atony, and upper motor neuron deficit can cause spasticity.
Data Collection
SIGNS AND SYMPTOMS
Often there is a mixed presentation of symptoms. The flaccid bladder requires aggressive intervention in the neonate. Diagnosis begins immediately at the bedside when the newborn has no apparent voiding stream or the urine flow rate falls below expectations without other explanations. Further clarification of the diagnosis can be made by VCUG and by cystometric studies.
Treatment
Surgical intervention is indicated in the neonate with neurogenic bladder when there is severe reflux with renal damage present or recurrent UTI. The urologist creates a vesicostomy to allow the free flow of urine into diapers.
Complications
Early diagnosis and intervention for infants with neurogenic bladder can decrease the risks of the complications associated with this problem. Long-term complications of neurogenic bladder include UTI and vesicoureteral reflux leading to hydronephrosis, electrolyte imbalances, and permanent damage to the kidney.
PARENT TEACHING
Because many renal problems are secondary to abnormalities, parents should be assured that nothing they did or did not do caused the anomaly. Grief work over the loss of the perfect infant is necessary before attachment and care giving are possible (see Chapters 29 and 30). Genetic counseling enables parents to make informed choices about subsequent pregnancies (see Chapter 27).
Most infants with renal problems require accurate intake and output measurement. The importance and necessity of measuring intake and not overfeeding must be stressed to parents, as well as the necessity of saving and weighing diapers. Infants who are fluid restricted may be “difficult” for care providers and parents because they are fussy and irritable. Adherence to the prescribed formula or breast milk is very important to regulate sodium intake and fluid retention.
Long-term complications that parents may have to recognize or manage must be explained and written instructions given. Because abnormalities in renal function and anatomy may be sequelae of renal diseases, follow-up by a pediatric nephrologists or urologist for urinalysis, cultures, and other diagnostic tests is important. General health maintenance is also important, because growth failure may be a manifestation of ongoing or recurring renal problem.
The importance of administering antihypertensive medications must be stressed to parents. Because hypertension is often a silent condition, the need for continuation of medications must be thoroughly explained. Side effects of hypertensive medications such as sedation, tachycardia, and excessive weight gain, as well as the necessity of medical follow-up, also must be emphasized.
REFERENCES
1. Adelman, R.D., Neonatal hypertension, In: (Editors: Loggie, J.M.H.; Horan, M.J.; Hohn, A.R.; et al.) NHLBI workshop on juvenile hypertension, ( 1983)Biomedical Information Corporation, New York.
2. Adelman, R.D.; Wirth, F.; Rubio, T., A controlled study of the nephrotoxicity of methicillin and gentamicin plus ampicillin in the neonate, J Pediatr 111 (1987) 888.
3. Andreoli, S.P., Acute renal failure in the newborn, Curr Opin Pediatr 17 (2002) 713.
4. Andreoli, S.P., Acute renal failure in the newborn, Semin Perinatol 28 (2004) 112.
5. Arant, B.S., Renal and genitourinary diseases, In: (Editors: McMillan, J.; Feigen, R.D.; DeAngelis, C.D.; et al.) Oski’s pediatrics, ( 2006)Lippincott Williams & Wilkins, Philadelphia.
6. Auron, A.; Mhanna, M.J., Serum creatinine in very low birth weight infants during their first days of life, J Perinatol 26 (2006) 755.
7. Basile, D.P.; Donohoe, D.; Roethe, K.; et al., Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function, Am J Physiol 281 (2001) F887.
8. Baum, M., Developmental changes in proximal tubule NaCl transport, Pediatr Nephrol 23 (2008) 185.
9. Brion, L.P.; Campbell, D.E., Furosemide in indomethacin-treated infants: systematic review and meta-analysis, Pediatr Nephrol 13 (1999) 212.
10. Brion, L.P.; Campbell, D.E., Furosemide for symptomatic patent ductus arteriosus in indomethacin-treated infants, Cochrane Database Syst Rev 3 (2001); CD001148.
11. Brion, L.P.; Primhak, R.A., Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease, Cochrane Database Syst Rev 1 (2002); CD001453.
12. Brion, L.P.; Soll, R.A., Diuretics for respiratory distress syndrome in preterm infants, Cochrane Database Syst Rev 3 (2008); CD001454.
13. Bunchman, T.E., Infant dialysis: the future is now, J Pediatr 136 (2000) 1.
14. Chandler, J.C.; Gauderer, M.W.L., The neonate with an abdominal mass, Pediatr Clin N Am 51 (2004) 979.
15. Chevalier, R.L., The moth and the aspen tree: sodium in early postnatal development, Kidney Int 59 (2001) 1617.
16. Chevalier, R.L., Perinatal obstructive nephropathy, Semin Perinatol 28 (2004) 124.
17. Choker, G.; Gouyon, J.B., Diagnosis of acute renal failure in very preterm infants, Biol Neonate 86 (2004) 212.
18. Cole, B.R.; Conley, S.B.; Stapleton, S.B., and Southwest Pediatric Nephrology Study Group: Polycystic kidney disease presenting in the first year of life, J Pediatr 111 (1987) 693.
19. Cruz, C.; Spitzer, A., When you find protein or blood in the urine, Contemp Pediatr ( 1998); September.
20. Drukker, A.; Guignard, J.P., Renal aspects of the term and preterm infants: a selective update, Curr Opin Pediatr 14 (2002) 175.
21. Drukker, A.; Mosig, D.; Guignard, J.P., The renal hemodynamics effect of aspirin in newborn and young adult rabbits, Pediatr Nephrol 16 (2001) 113.
22. Engle, W.D., Evaluation of renal function in acute renal failure in the neonate, Pediatr Clin North Am 33 (1986) 129.
23. Ertl, T.; Hadzsiev, K.; Vincze, O.; et al., Hyponatremia and sensorineural hearing loss in preterm infants, Biol Neonate 79 (2001) 109.
24. Farmer, D.L., Urinary tract masses, Semin Pediatr Surg 9 (2000) 109.
25. Flynn, J.T., Neonatal hypertension: diagnosis and management, Pediatr Nephrol 14 (2000) 332.
26. Forni, L.G.; Hilton, P.J., Continuous hemofiltration in the treatment of acute renal failure, N Engl J Med 336 (1997) 1303.
27. Friedman, A.L.; Hustead, V.A., Hypertension in babies after discharge from a neonatal intensive care unit, Pediatr Nephrol 1 (1987) 30.
28. Gallego, N.; Perez-Caballero, C.; Estepo, R.; et al., Prognosis of patients with acute renal failure without cardiomyopathy, Arch Dis Childhood 84 (2001) 258.
29. Galley, H.F., Renal dose dopamine: will the message get through?Lancet 356 (2000) 2112.
30. Gallini, F.; Maggio, L.; Romagnoli, C.; et al., Progression of renal function in preterm neonates with gestation age less than or equal to 32 wks, Pediatr Nephrol 15 (2000) 119.
31. Geary, D.F., Initiation of renal replacement therapy in infancy, In: (Editors: Warady, B.A.; Fine, R.N.; Alexander, S.R.; et al.) Pediatric dialysis, ( 2004)Kluwer, Netherlands.
32. Goldstein, S.L.; Currier, H.; Graf, C.D.; et al., Outcome in children receiving continuous venous hemofiltration, Pediatrics 107 (2001) 1309.
33. Gong, W.K.; Tan, T.H.; Foong, P.P.; et al., Eighteen years in pediatric acute dialysis: analysis of predicted outcome, Pediatr Nephrol 16 (2001) 212.
34. Gordon, I.; Barratt, T.M., Imaging the kidneys and urinary tract in the neonate with acute renal failure, Pediatr Nephrol 1 (1987) 321.
35. Gouyon, J.B.; Guignard, J.P., Management of acute renal failure in newborns, Pediatr Nephrol 14 (2000) 1037.
36. Gregory, M.; Bunchman, T.E.; Brophy, P.D., Continuous renal replacement therapies for children with acute renal failure and metabolic disorders, In: (Editors: Warady, B.A.; Fine, R.N.; Alexander, S.R.; et al.) Pediatric dialysis, ( 2004)Kluwer, Netherlands.
37. Guay-Woodford, L.; Desmond, R., Autosomal recessive polycystic kidney disease: the clinical experience in North America, Pediatrics 111 (2003) 1072.
38. Guignard, J.P., Neonatal nephrology, In: (Editors: Holliday, M.A.; Barratt, T.M.; Vernier, R.L.) Pediatric nephrology,ed 2 ( 1987)Williams & Wilkins, Baltimore.
39. Guignard, J.P.; Gouyan, J.B., Adverse effects of drugs on the immature kidney, Biol Neonate 53 (1988) 243.
40. Gunn, T.R.; Mora, J.D.; Pease, P., Antenatal diagnosis of urinary tract abnormalities by ultrasonography after 28 week gestation: incidence and outcome, Am J Obstet Gynecol 172 (1995) 479.
41. Gurkan, S.; Estilo, G.K.; Wei, Y.; et al., Potassium transport in the maturing kidney, Pediatr Nephrol 22 (2007) 915.
42. Hein, G.; Richter, D.; Manz, F.; et al., Development of nephrocalcinosis in very low birth weight infants, Pediatr Nephrol 19 (2004) 616.
43. Helin, I.; Persson, P.H., Prenatal diagnosis of urinary tract abnormalities by ultrasound, Pediatrics 78 (1986) 879.
44. Hellstrom, M.; Jacobsson, B.; Jodal, U.; et al., Renal growth after neonatal urinary tract infection, Pediatr Nephrol 1 (1987) 269.
45. Holler, B.; Omar, S.A.; Farid, M.D.; et al., Effects of fluid and electrolyte management on amphotericin B-induced nephrotoxicity among extremely low birth weight infants, Pediatrics 113 (2004) 608.
46. Holmberg, C.; Ronnholm, K., Maintenance peritoneal dialysis during infancy, In: (Editors: Warady, B.A.; Fine, R.N.; Alexander, S.R.; et al.) Pediatric dialysis ( 2004)Kluwer, Netherlands.
47. Hölttä, T.; Rönnholm, K.; Jalanko, H.; et al., Clinical outcomes of pediatric patients on peritoneal dialysis under adequacy control, Pediatr Nephrol 14 (2000) 889.
48. Ichikawa, I.; Kuwayama, F.; Pope IV, J.C.; et al., Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT, Kidney Int 61 (2002) 889.
49. Jones, D.P.; Chesney, R.W., Tubular function, In: (Editors: Avner, E.D.; Harmon, W.E.; Niaudet, P.) Pediatric nephrologyed 5 ( 2004)Lippincott Williams & Wilkins, Philadelphia.
50. Jones, J.E.; Jose, P.A., Neonatal blood pressure regulation, Semin Perinatol 28 (2004) 141.
51. Kalia, A.; Brouhard, B.H.; Travis, L.B.; et al., Renal transplantation in the infant and young child, Am J Dis Child 143 (1988) 47.
52. Kao, L.C.; Warburton, D.; Cheng, M.H.; et al., Effect of oral diuretics on pulmonary mechanics in infants with chronic bronchopulmonary dysplasia: results of a double-blind crossover sequential trial, Pediatrics 74 (1984) 37.
53. Kaplan, B.S.; Kaplan, P.; Ruchelli, E., Inherited and congenital malformations of the kidneys in the neonatal period, Clin Perinatol 19 (1992) 197.
54. Kapur, G.; Mattoo, T.; Aranda, J.V., Pharmacogenomics and renal drug disposition in the newborn, Semin Perinatol 28 (2004) 132.
55. Karlowicz, G.M.; Adelman, R.D., Nonoliguric and oliguric acute renal failure in asphyxiated neonates, Pediatr Nephrol 9 (1995) 718.
56. Katz, A.; Bock, G.H.; Mauer, M., Improved growth velocity with intensive dialysis: consequence or coincidence, Pediatr Nephrol 14 (2000) 710.
57. Keidan, I.; Lotan, D.; Gazit, G.; et al., Early neonatal renal venous thrombosis: long-term outcome, Acta Paediatr 83 (1994) 1225.
58. Kellum, J.A.; Decker, J.M., Use of dopamine in acute renal failure: a meta-analysis, Crit Care Med 29 (2001) 1526.
59. Kelly, L.K.; Seri, I., Renal developmental physiology: relevance to clinical care, NeoReviews 9 (2008) e150.
60. Killian, K., Hypertension in neonates: causes and treatments, J Perinat Neonatal Nurs 17 (2003) 65.
61. Kirks, D.R.; Merten, D.F.; Grossman, H.; et al., Diagnostic imaging of pediatric abdominal masses: an overview, Radiol Clin North Am 19 (1981) 527.
62. Kitterman, J.A.; Phibbs, R.H.; Tooley, W.H., Aortic blood pressure in normal newborn infants during the first 12 hours of life, Pediatrics 44 (1969) 959.
63. Kleinman, L.I.; Stewart, C.L.; Kaskel, F.J., Renal disease in the newborn, In: (Editor: Edelmann, C.M.) Pediatric nephrology,ed 2 ( 1992)Little, Brown, Boston.
64. Ledermann, S.E.; Scanes, M.E.; Fernando, O.N.; et al., Long-term outcome of perinatal dialysis in infants, J Pediatr 136 (2000) 24.
65. Lieberman, K., Continuous arteriovenous hemofiltration in children, Pediatr Nephrol 1 (1987) 330.
66. Lindemann, R., Congenital renal tubular dysfunction associated with maternal sniffing of organic solvents, Acta Pediatr Scand 80 (1991) 882.
67. Malone, T.A., Glucose and insulin versus cation-exchange resin for the treatment of hyperkalemia in very low birth weight infants, J Pediatr 118 (1991) 121.
68. Mathew, O.P.; Jones, A.S.; James, E.; et al., Neonatal renal failure: usefulness of diagnostic indices, Pediatrics 65 (1980) 57.
69. Matos, V.; Drukker, A.; Guignard, J.P., Spot urine samples for evaluating solute excretion in the first week of life, Arch Dis Child Fetal Neonatal Ed 80 (1999) F240.
70. McLean, R.H.; Gearhart, J.P.; Jeffs, R., Neonatal obstructive uropathy, Pediatr Nephrol 2 (1988) 48.
71. McVicar, M.; Margouleff, D.; Chandra, M., Diagnosis and imaging of the fetal and neonatal abdominal mass: an integrated approach, Adv Pediatr 38 (1991) 135.
72. Mesrobian, H.O., Urologic problems of the neonate: an update, Clin Perinatol 34 (2007) 667.
73. Mingeot-Leclercq, M.P.; Tulkens, P.M., Aminoglycosides: nephrotoxicity, Antimicrob Agents Chemother 43 (1999) 1003.
74. Mirmiran, M.; Kok, J.H.L., Circadian rhythms in early human development, Early Hum Dev 26 (1991) 121.
75. Mocan, H.; Beattie, T.J.; Murphy, A.V., Renal vein thrombosis in infancy: long term follow-up, Pediatr Nephrol 5 (1991) 45.
76. Moxey-Mims, M., Hematuria and proteinuria, In: (Editors: Kher, K.K.; Schnaper, H.W.; Makker, S.P.) Clinical pediatric nephrology,ed 2 ( 2007)Informahealthcare, Abingdon, Oxon, United Kingdom.
77. Moxey-Mims, M.; Stapleton, F.B., Renal tubular disorders in the neonate, Clin Perinatol 19 (1992) 159.
78. Murphy, J.I.; Kaplan, G.W.; Packer, M.G.; et al., Prenatal diagnosis of severe urinary tract anomalies improves renal function and growth, Child Nephrol Urol 9 (1988) 290.
79. Murugasu, B.; Cole, B.R.; Hawkins, E.P.; et al., Familial renal adysplasia, Am J Kid Dis 18 (1991) 490.
80. Polito, C.; Papale, M.R.; La Manna, A.L., Long-term prognosis of acute renal failure in the full term newborn, Clin Pediatr 37 (2001) 381.
81. Pope, J.C.; Brock, J.W.; Adams, M.C.; et al., How they begin and how they end: classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT, J Am Soc Nephrol 10 (1999) 2018.
82. Portman, R.J.; Carter, B.S.; Gaylord, M.S.; et al., Predicting neonatal morbidity after perinatal asphyxia: a scoring system, Am J Obstet Gynecol 162 (1990) 174.
83. Potter, E.L., Normal and abnormal development of the kidney. ( 1972)Year Book, Chicago.
84. Quigley, R.; Baum, M., Neonatal acid base balance and disturbances, Semin Perinatol 28 (2004) 97.
85. Rabelo, E.; Oliveira, E.A.; Diniz, J.S.; et al., Natural history of multicystic kidney conservatively managa prospective study, Pediatr Nephrol 19 (2004) 1102.
86. Reznik, V.M.; Kaplan, G.W.; Murphy, J.L.; et al., Follow-up of infants with bilateral renal disease detected in utero, Am J Dis Child 142 (1988) 453.
87. Richards, D.S., Ultrasound for pregnancy dating, growth and diagnosis of fetal malformation, In: (Editors: Gabbe, S.G.; Niebyl, J.R.; Simpson, J.L.) Obstetrics: normal and problem pregnanciesed 5 ( 2007)Churchill Livingstone, Philadelphia.
88. Robinson, R.F.; Nahata, M.C.; Batisky, D.L.; et al., Pharmacologic treatment of chronic pediatric hypertension, Pediatr Drugs 7 (2005) 27.
89. Rodríguez-Soriano, J.; Aguirre, M.; Oliveros, R.; et al., Long-term renal follow-up of extremely low birth weight infants, Pediatr Nephrol 20 (2005) 579.
90. Ronco, C.; Brendolan, A.; Bragantini, L.; et al., Treatment of acute renal failure in newborns by continuous arteriovenous hemofiltration, Kidney Int 29 (1986) 908.
91. Roth, C.G.; Spottswood, S.E.; Chan, J.C.M.; et al., Evaluation of the hypertensive infant: a rational approach to diagnosis, Rad Clin N Am 41 (2003) 91.
92. Roussan, M.S., Neurogenic bladder dysfunction, Med Times 109 (1981) 43.
93. Schell-Feith, E.A., Etiology of nephrocalcinosis in preterm neonates: association of nutritional intake and urinary parameters, Kidney Int 58 (2000) 2102.
94. Schmidt, B.; Andrew, M., Neonatal thrombosis: report of a Prospective Canadian and International Registry, Pediatrics 96 (1995) 939.
95. Seeman, T.; John, U.; Blahova, K.; et al., Ambulatory blood pressure monitoring in children with unilateral MCDK, Eur J Pediatr 160 (2001) 78.
96. Sertel, H.; Scopes, J., Rates of creatinine clearance in babies less than one week of age, Arch Dis Child 48 (1973) 717.
97. Shankaran, S.; Liang, K.C.; Ilagen, N.; et al., Mineral excretion following furosemide compared with bumetanide therapy in premature infants, Pediatr Nephrol 9 (1995) 159.
98. Shooter, M.; Watson, M., The ethics of withholding and withdrawing dialysis therapy in infants, Pediatr Nephrol 14 (2000) 347.
99. Siegel, S.; Oh, W., Renal function as a marker of human fetal maturation, Acta Paediatr Scand 65 (1976) 481.
100. Sitka, U.; Weiner, T.D.; Berle, K.; et al., Investigation of the rhythmic function of heart rate, blood pressure, and temperature in neonates, Eur J Pediatr 153 (1994) 117.
101. Sniderman, S.; Charlton, V.E., Routine postnatal care and observation, In: (Editors: Rudolph, C.D.; Rudolph, A.M.) Rudolph’s pediatricsed 21 ( 2003)McGraw Hill, New York.
102. Springate, J.E.; Fildes, R.D.; Feld, L.G., Assessment of renal function in newborn infants, Pediatr Rev 9 (1987) 51.
103. Stapleton, F.B.; Jones, D.P.; Green, R.S., Acute renal failure in neonates: incidence, etiology and outcome, Pediatr Nephrol 1 (1987) 314.
104. Sulyok, E.; Guigard, J.P., Relationship of urinary anion gap to urinary ammonium excretion in the neonate, Biol Neonate 57 (1990) 98.
105. Swinford, R.D.; Bonilla-Felix, M.; Cerda, R.D.; et al., Neonatal nephrology, In: (Editors: Merenstein, G.B.; Gardner, S.L.) Handbook of neonatal intensive careed 6 ( 2006)Mosby, St Louis.
106. Tan, K.L., Blood pressure in full-term healthy newborns, Clin Pediatr 26 (1987) 21.
107. Thayyil, S.; Sheik, S.; Kempley, S.T.; et al., A gestation- and postnatal age-based reference chart for assessing renal function in extremely premature infants, J Perinatol 28 (2008) 226.
108. Vainio, J.S.; Uvsitalo, M.S., A road to kidney tubules in the WNT pathway, Pediatr Nephrol 15 (2000) 151.
109. Vanderheyden, T.; Kumar, S.; Fisk, N.M., Fetal renal impairment, Semin Neonatol 28 (2003) 279.
110. Wahlig, T.M.; Thompson, T.R.; Sinaiko, A.R., Drug use in the newborn: effects on the kidney, Clin Perinatol 19 (1992) 251.
111. Warady, B.A.; Bunchman, T., Dialysis therapy for children with acute renal failure: survey results, Pediatr Nephrol 15 (2000) 61.
112. Watkinson, M., Hypertension in the newborn baby, Arch Dis Child Fetal Neonatal Ed 86 (2002) F78.
113. Watson, A.R.; Shooter, M., The effects of withholding and withdrawing dialysis, In: (Editors: Warady, B.A.; Fine, R.N.; Alexander, S.R.; et al.) Pediatric dialysis ( 2004)Kluwer, Netherlands.
114. Wood, E.G., Risk factors for mortality in infants and children on dialysis, Am J Kid Dis 37 (2001) 573.
115. Woolf, A.S., Developmental anatomy and physiology, In: (Editors: Morgan, S.H.; Grunfeld, J.P.) Inherited disorders of the kidney, ( 1998)Oxford University Press, Oxford.
116. Woolf, A.S.; Winyard, P.J.D., Molecular mechanisms of human embryogenesis: developmental pathogenesis of renal tract malformations, Pediatr Dev Pathol 5 (2002) 108.
117. Wu, J.; Cornelissen, G.; Tarquini, B., Circaseptan and circannual modulation of circadian rhythms in neonatal blood pressure and heart rate, In: (Editors: Hayes, D.; Pauly, J.; Reiter, R.) Chronobiology: its role in clinical medicine, general biology and agriculture, ( 1990)Wiley-Liss, New York.
118. Zinner, S.H.; Rosner, B.; Oh, W.; et al., Significance of blood pressure in infancy: familial aggregation and predictive effect on later blood pressure, Hypertension 7 (1985) 411.