CHAPTER 392 Natural History of Cavernous Malformations
Before the availability of modern imaging technology, cavernous malformations were considered rare lesions. In 1976, Voigt and Yasargil described their clinical experience with one patient and thoroughly reviewed the world literature, finding only 126 reported cases.1 Soon after the publication of this article, computed tomography (CT) became widely available; however, CT lacked both the sensitivity and specificity for identifying cavernous malformations. Only partially calcified or recently hemorrhagic lesions could be readily visualized, and diagnosis still required pathologic confirmation.
The subsequent introduction of magnetic resonance imaging (MRI) in the mid-1980s revolutionized our understanding of these lesions. The MRI characteristics of cavernous malformations are sufficiently unique to allow diagnosis of the majority of these lesions on the basis of the MRI findings alone (Fig. 392-1).2–6 The widespread availability of MRI has increased the recognition of cavernous malformations in both symptomatic and asymptomatic patients and the number of requests for neurosurgical consultation. Appropriate management of these patients requires a thorough understanding of the epidemiology and natural history of these lesions. The goals of this chapter are to provide the reader with an in-depth review of the available literature on this topic and to examine the implications related to the treatment of these patients.
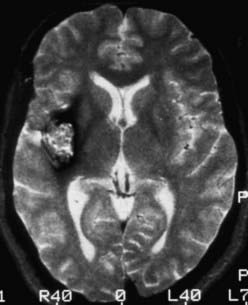
FIGURE 392-1 Axial T2-weighted magnetic resonance image demonstrating the classic appearance of a cavernous malformation. The core of the lesion has a reticulated “salt and pepper” pattern and is surrounded by a halo of low signal intensity. Histologically, these lesions are composed of loculated areas of hemorrhage and thrombosis of various age surrounded by gliotic, hemosiderin-stained brain tissue. Note the absence of mass effect and edema. This pattern of hemorrhage (type II) is pathognomonic for a cavernous malformation. See Table 392-3 for the classification of lesion types.
Epidemiology
Cavernous malformations are more common than generally suspected. Postmortem studies from the 1980s (Table 392-1) demonstrated that cavernous malformations affect 0.37% to 0.5% of the population.7,8 Remarkably similar results were reported by two groups reviewing more than 22,000 MRI examinations, for which incidence rates of 0.4% to 0.5% were calculated.9,10 Based on these studies, it is estimated that 1 in every 200 to 250 people, or approximately 30 million individuals worldwide, are affected by cavernous malformations.
| REFERENCE | NO. PATIENTS STUDIED | NO. LESIONS/INCIDENCE RATE OF CAVERNOUS MALFORMATIONS |
|---|---|---|
| McCormick,7 1984 | 5,734 | 19/0.34% |
| Otten et al.,8 1989 | 24,535 | 131/0.53% |
| del Curling et al.,9 1991 | 8,131 | 32/0.39% |
| Robinson et al.,10 1991 | 14,035 | 66/0.47% |
| Total | 52,435 | 248/0.47% |
Cavernous malformations occur in two forms: spontaneous and familial. The spontaneous form occurs as an isolated event, most commonly with a single lesion. The familial form is characterized by multiple lesions (Fig. 392-2) and an autosomal dominant mode of inheritance.11–14 At least three distinct genetic loci have been identified with this form of the disease.15–17 A detailed review of the genetics of familial cavernous malformations is beyond the scope of this chapter; interested readers are referred to Chapter 393.
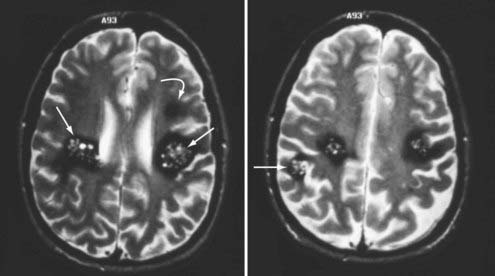
FIGURE 392-2 Axial T2-weighted magnetic resonance images in a patient with a new onset of seizures and familial cavernous malformations. Four distinct lesions are seen at these two levels, including three type II lesions (straight arrows) and one type III lesion (curved arrow). A fifth lesion was identified in the cerebellum. Multiple lesions and a family history of seizures are characteristic of the familial form of this disease. See Table 392-3 for the classification of lesion types.
The presence of three or more lesions and a family history of seizures are essentially pathognomonic for the familial form of this disease. In a recent series of 132 patients with familial cavernous malformations, Denier and coworkers reported that 80% had multiple lesions detected on T2-weighted MRI and 90% had multiple lesions on gradient-echo images, with an average of 5 lesions per patient on T2-weighted images and 20 per patient on gradient-echo sequences.18 A thorough family history is indicated for any patient with multiple cavernous malformations.
Cavernous malformations occur throughout the central nervous system in rough proportion to the volume of the various compartments: supratentorial, 80%; brainstem and basal ganglia, 15%; and spinal cord, 5%. They have been well described in infants and children but are seldom symptomatic until the second and third decades of life. Large population studies have demonstrated that cavernous malformations occur with equal frequency in both sexes.9,10 Some data suggest that women may more commonly be seen initially with symptomatic hemorrhage, but the evidence is far from conclusive. The issues of gender and pregnancy are covered later in this chapter.
Not all patients with cavernous malformations become clinically symptomatic. Twenty percent to 30% of sporadic lesions are incidental findings discovered during evaluation for headache or other unrelated symptoms.9,10 Approximately 40% of patients with the familial form of the disease remain asymptomatic despite the presence of multiple lesions.13,19
Clinical Findings
Evidence of previous hemorrhage is a constant feature of cavernous malformations regardless of whether the lesions are symptomatic. Hemorrhages of various ages, combined with the deposition of hemosiderin in cerebral tissue surrounding the cavernous malformation, produce the unique MRI characteristics of these lesions (see Figs. 392-1 and 392-2). Increases in size may result from repeated small hemorrhages within the lesion and from spontaneous thrombosis of the blood-filled caverns. Organization and endothelialization within these hemorrhagic/thrombotic cavities create the potential for further growth. Rarely, these lesions rupture outside their capsule and produce “overt” hemorrhage into the surrounding brain tissue (Figs. 392-3 and 392-4). Because cavernous malformations are low-flow, low-pressure lesions, hemorrhage (even “overt” hemorrhage outside the lesion) usually displaces and compresses adjacent neural tissue rather than destroying it.
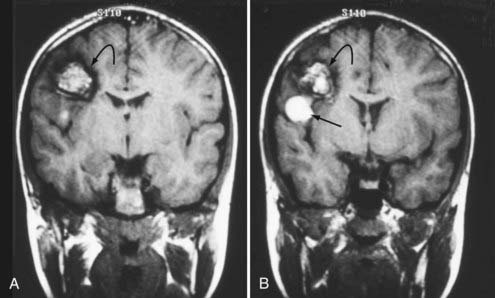
FIGURE 392-3 A and B, Coronal T1-weighted magnetic resonance images in a 10-year-old boy with a cavernous malformation (curved arrows). His past history was remarkable for an episode of seizure activity at 4 years of age and a negative computed tomographic study. The patient had a sudden onset of mild weakness and decreased sensation in his left upper extremity. Note the focal area of subacute blood (straight arrow) extending outside the capsule of the lesion and producing “overt” extralesional (type IA) hemorrhage. See Table 392-3 for the classification of lesion types.
(From Zabramski JM. Cavernous malformations. In: Aminoff M, Daroff RB, eds. Encyclopedia of the Neurological Sciences. San Diego, CA: Academic Press; 2003. Used with permission.)
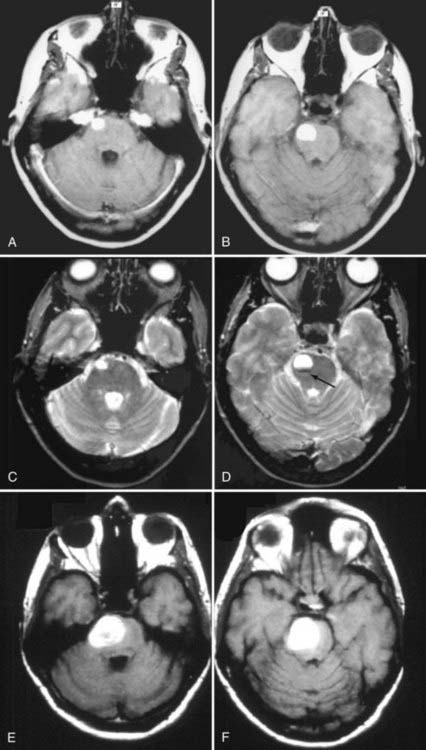
FIGURE 392-4 This 38-year-old woman was seen at an outside emergency department with complaints of severe headache and nausea. A basic head computed tomographic scan revealed a subtle area of increased density in the right pons. T1-weighted (A and B) and T2-weighted (C and D) magnetic resonance imaging (MRI) revealed a 1.6-cm area of subacute blood consistent with recent “overt” extralesional (type IA) hemorrhage from a small cavernous malformation (arrow), which is best seen on the T2-weighted image (D). The patient was seen in neurosurgical consultation approximately 1 month after this episode when all symptoms had resolved. She initially declined surgical intervention. Two months later, she had an acute onset of left-sided weakness and hemisensory deficits. Repeated T1-weighted MRI (E and F) revealed a dramatic increase in the size of the lesion, which was composed almost entirely of acute and subacute blood. Surgical pathologic evaluation confirmed the diagnosis of cavernous malformation. After resection of this lesion, she made a complete recovery. See Table 392-3 for the classification of lesion types.
(C and D, From Zabramski JM, Spetzler RF. Management of brainstem cavernous malformations. In: Batjer HH, Loftus CM, eds. Textbook of Neurological Surgery. Philadelphia: Lippincott, Williams & Wilkins; 2003. Used with permission; E and F, from Robinson JR, Zabramski JM. Cavernous malformations of the cervicomedullary junction. In: Dickman CA, Spetzler RF, Sonntag VKH, eds. Surgery of the Craniovertebral Junction. New York: Thieme; 1998.)
Supratentorial Lesions
Seizures are the most common manifestation of supratentorial cavernous malformations and account for 40% to 80% of the initial symptoms.9,10,12,13,20–22 Despite the prevalence of seizures in this population, remarkably few data are available regarding the annual risk for the onset of seizures. Only four studies directly addressed this issue and reported rates for new onset of seizures of 1.4% to 2.4% per person-year.9,19,23,24 The onset or exacerbation of seizure activity in these patients is often associated with MRI evidence of acute/subacute hemorrhage (Fig. 392-5). The exact mechanism that leads to the seizure activity in these lesions is unknown. Cavernous malformations do not typically contain neuronal tissue and are therefore not intrinsically epileptogenic. They induce seizures through their effect on surrounding brain tissue. Such effects may include focal gliosis, hemosiderin deposition, and cellular and humoral inflammatory responses. Pathologically, cavernous malformations are surrounded by a yellow-brown–stained border that is heavily infiltrated with hemosiderin-laden macrophages and iron (Fig. 392-6). Iron is a well-known epileptogenic material used to induce seizures in laboratory models of epilepsy.25,26 Focal neurologic deficits secondary to mass effect are rarely associated with supratentorial lesions unless the lesion is located in the basal ganglia or thalamus.
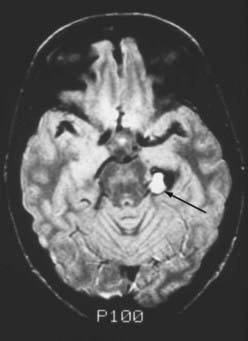
FIGURE 392-5 Intermediate-weighted, axial, spin echo magnetic resonance image in a 23-year-old man with acute exacerbation of temporal lobe seizure activity. Note the focal area of high-signal intensity (arrow) compatible with subacute hemorrhage in the region of the left hippocampus. A ring of low signal intensity surrounds the entire lesion, consistent with an intralesional (type IB) hemorrhage. Surgical pathologic evaluation confirmed the diagnosis of cavernous malformation. See Table 392-3 for classification of lesion types.
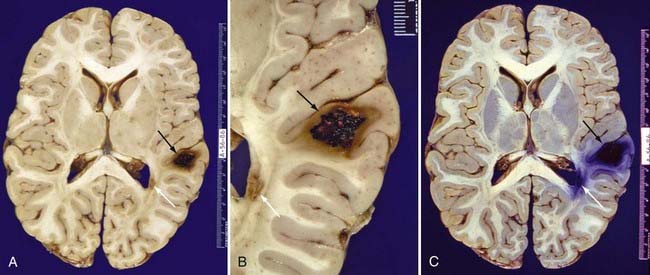
FIGURE 392-6 Whole-brain sections from a 26-year-old Hispanic man who died of septic complications. A, An unstained formalin-fixed section reveals a type II cavernous malformation (black arrow; see Table 392-3 for definition) in the right posterior frontal lobe and a small type III cavernous malformation (white arrow; see Table 392-3 for definition) adjacent to the right occipital horn. B, Close-up view of the same lesions. C, The same section stained with Prussian blue for iron emphasizes the extent of hemosiderin and iron deposition. The hemosiderin and iron surrounding these lesions are responsible for the areas of low signal intensity (known as blooming artifact) seen on T2-weighted and gradient-echo magnetic resonance images.
(A and B, From Zabramski JM. Cavernous malformations. In: Aminoff M, Daroff RB, eds. Encyclopedia of the Neurological Sciences. San Diego, CA: Academic Press; 2003. Used with permission; C, used with permission from Barrow Neurological Institute.)
Brainstem Lesions
The sudden onset of focal neurological deficits is the most frequent finding in patients with brainstem cavernous malformations.27–30 Porter and coauthors reported 100 surgical cases of brainstem cavernous malformations. In their series, 97% of patients had focal neurological deficits from hemorrhage.30 The development of symptoms in patients with hemorrhage from brainstem cavernous malformations is characteristically acute and maximal at onset (see Fig. 392-4). However, the neurological deficits from the first episode of clinically symptomatic hemorrhage tend to resolve completely as the hemorrhage is organized and absorbed. In contrast, recurrent episodes of hemorrhage are apt to be associated with progressively more severe deficits and an increased risk for permanent neurological impairment (see Fig. 392-4). This stuttering clinical course with improvement of symptoms between episodes may lead to a misdiagnosis of multiple sclerosis if the evaluation does not include MRI. Occasionally, large brainstem lesions develop that are associated with only minimal deficits, particularly in the pons, where the mass can gradually displace the densely packed ascending and descending fiber tracts (Fig. 392-7). Death is rare without multiple episodes of symptomatic hemorrhage.
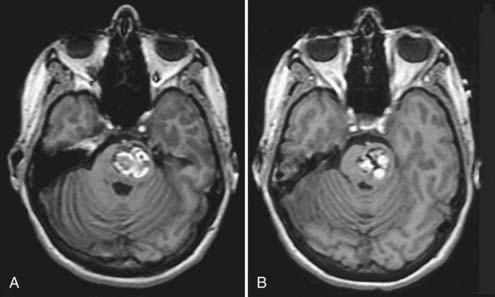
FIGURE 392-7 A and B, T1-weighted magnetic resonance images in a 39-year-old woman with a history of an acute onset of mild right-sided weakness that had completely resolved over a period of 3 to 4 weeks. The hyperintense regions represent areas of subacute hemorrhage within the more chronic matrix of this pontine cavernous malformation. This lesion reached considerable size with only minimal deficits by gradually displacing the dense fiber tracts within the pons. Compare this pattern with the “overt” pontine hemorrhage sustained by the patient shown in Figure 392-4.
Spinal Cord Lesions
Ogilvy and associates described four types of manifestations in patients with symptomatic intramedullary spinal cord lesions31: (1) acute onset of symptoms with a rapid decline, (2) acute onset of mild symptoms with a subsequent gradual decline, (3) acute episodes of stepwise neurological deterioration, and (4) slow progression of neurological deterioration. Sandalcioglu and colleagues noted that the first three of these categories all seem to be related to an acute event leading to neurological deficits of varying severity and course.32 Consequently, they recommended that patients be separated into only two subgroups. One group is typified by major hemorrhage and the sudden onset of symptoms and neurologic deficits, the gravity of which is related to the exact location of the lesion (level and position within the cord) and the extent of hemorrhage (intralesional or extralesional volume, or both). The second group is characterized by slowly progressive myelopathic or radicular symptoms (or both), probably related to minor bleeding episodes and gradual growth of the cavernous malformation. Combinations of these two manifestations occur frequently.
Both radicular and myelopathic symptoms are common at initial evaluation. Pain is a significant component in 40% to 50% of patients with spinal cord lesions31,33–37 and was the only symptom in approximately 25% of patients in a series from our institution.38
It is important to note that patients with spinal cord cavernous malformations appear to be at significantly increased risk of harboring intracranial lesions. Vishteh and colleagues reported that 47% of patients with symptomatic spinal cord lesions who undergo MRI of the complete neuraxis will be found to have intracranial lesions.39 Cohen-Gadol and coworkers recently confirmed this finding and reported a 40% incidence of intracranial lesions in a series of 33 patients with spinal cord cavernous malformations who underwent both cranial and spinal imaging studies.40 These reports suggest that it may be prudent to perform MRI of the brain in this population.
Natural History
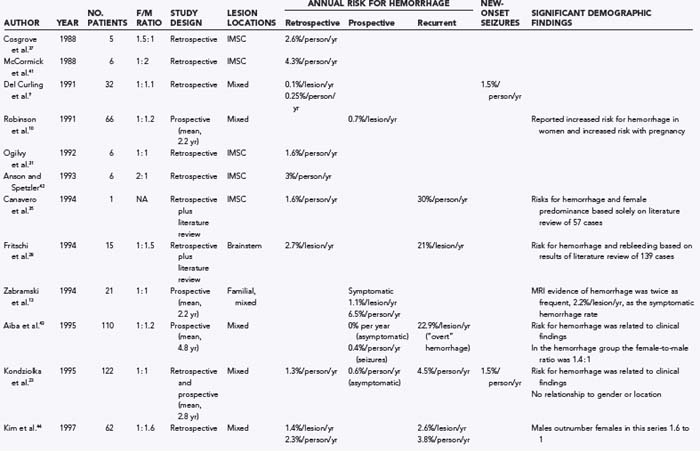
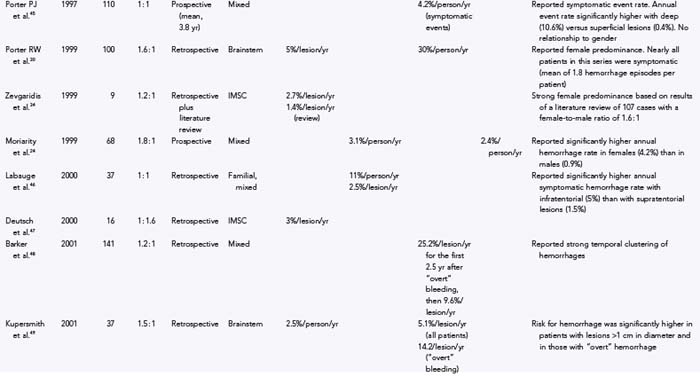
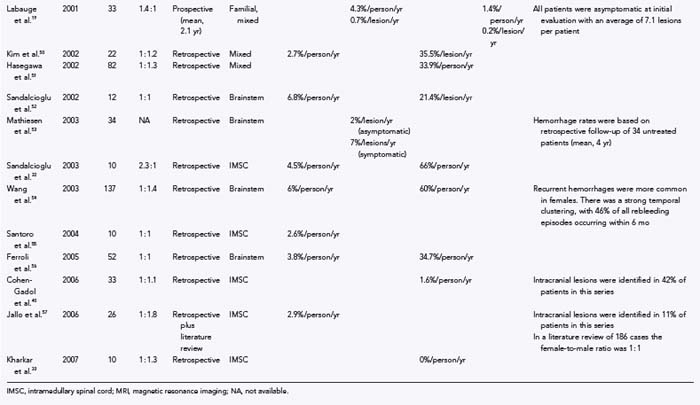
Numerous studies have been published on the natural history of cavernous malformations (Table 392-2).* Hemorrhage rates vary widely from series to series depending on the authors’ definition of hemorrhage and the population being studied. Not surprisingly, hemorrhage rates tend to be higher in surgical series because patients with symptomatic lesions are more likely to be referred for neurosurgical intervention. In addition, there are essentially four different methods of calculating hemorrhage rates, including retrospective and prospective methods, either of which can be reported as risk for hemorrhage per patient or per lesion. The retrospective method assumes that all lesions have been present since birth. Using this assumption, Del Curling and collaborators calculated an annual hemorrhage rate of 0.25% per patient.9 Kondziolka and coauthors reported a rate of 1.3% per patient per year,23 and Kim and associates calculated a rate of 2.3% per patient per year.44 This method of calculation, which depends on the patient’s recall to define episodes of hemorrhage and assumes that all lesions are present from birth, is likely to underestimate the actual risk of significant bleeding. Once considered congenital in origin, there is increasing evidence that new lesions may appear de novo in both the sporadic and familial forms of the disease.13,14,58–68
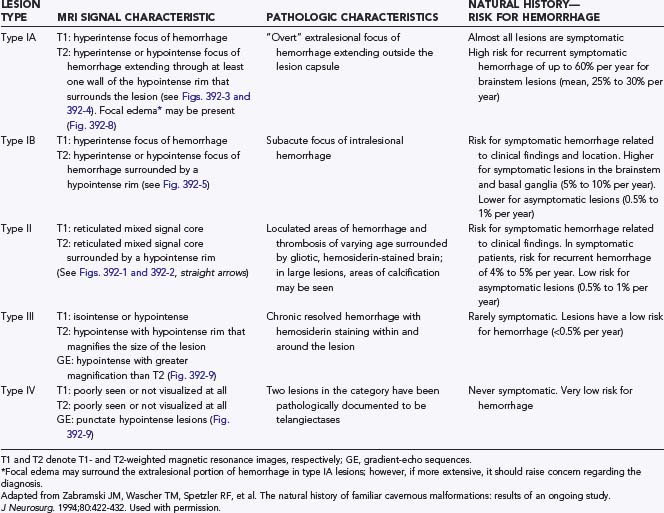
Another confounding factor is the varying nature of these lesions. Zabramski and colleagues classified cavernous malformations into four subtypes based on MRI characteristics (Table 392-3).13 These authors and others have found that the risk for hemorrhage appears to be greatest with type I and type II lesions, which are more likely to be symptomatic.10,19,43,46,48,69 Most clinical and surgical series are heavily biased to these two subtypes of lesions, which are readily identified on MRI studies because they do not require gradient-echo sequences. These uncertainties emphasize the need to rely on prospective natural history data in this population.
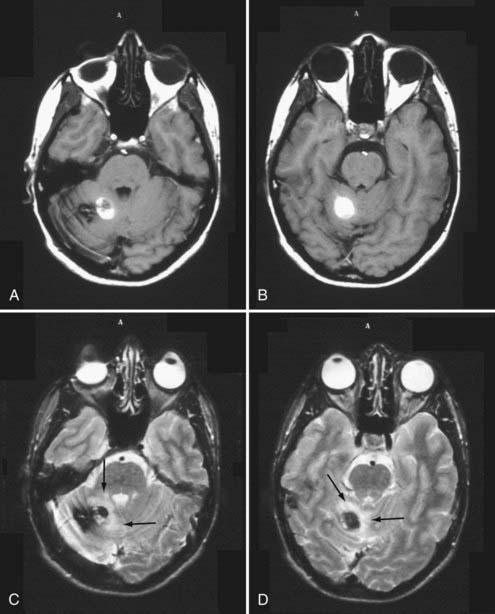
FIGURE 392-8 This 27-year-old man went to the emergency department after a 1-week history of headache and nausea. Computed tomography (CT) demonstrated an area of increased density in the right cerebellum consistent with possible hemorrhage. Magnetic resonance imaging confirmed the CT findings. T1-weighted (A and B) and T2-weighted (C and D) images in the posterior fossa demonstrate a right cerebellar cavernous malformation with a focal area of subacute “overt” hemorrhage (type 1A) extending outside the lesion capsule. Note the presence of focal edema (arrows) surrounding the hemorrhage on the T2-weighted images. See Table 392-3 for the classification of lesion types.
(Used with permission from Barrow Neurological Institute.)
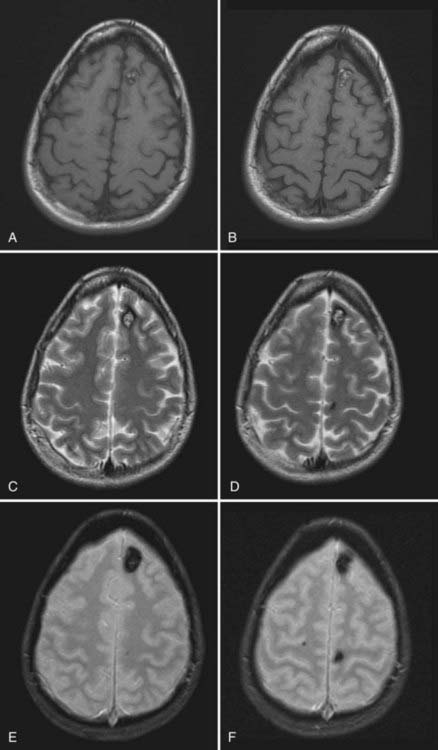
FIGURE 392-9 Magnetic resonance images from an asymptomatic 32-year-old woman with a known history of familial cavernous malformations. T1-weighted (A and B), T2-weighted (C and D), and gradient-echo (E and F) images demonstrate three distinct cavernous malformations, including a classic-appearing, small type II lesion in the left anterior frontal lobe, a tiny type III lesion in the left posterior frontal lobe, and a punctate type IV lesion in the right posterior frontal lobe (seen only on the gradient-echo images). See Table 392-3 for the classification of lesion types.
(Used with permission from Barrow Neurological Institute.)
Robinson and coworkers prospectively monitored 57 patients with serial clinical and MRI studies for an average of 26 months and found a risk for symptomatic hemorrhage of 0.7% per lesion per year.10 Kondziolka and coauthors reported a slightly higher hemorrhage rate of 2.6% per year but noted that the risk for hemorrhage was strongly related to the patient’s signs and symptoms.23 They prospectively monitored 122 patients with cavernous malformations (mean, 34 months) and found that the hemorrhage rate was significantly lower in patients with incidental lesions, 0.6% per year (n = 61), than in those with a history of previous symptomatic hemorrhage, 4.5% per year (n = 61). Aiba and associates observed 110 patients with cavernous malformations for a mean of 4.5 years and reported a 0% hemorrhage rate in patients with incidental lesions versus 0.4% per year in those with seizures.43
Porter and coauthors reported a somewhat higher annual event rate of 4.2%.45 This group defined an event as any neurological worsening of symptoms with or without radiologically proven hemorrhage. The authors observed 110 patients with cavernous malformations for an average of 46 months, which represented 427 years of observation. In this study, location was the most important factor for predicting future events, with significantly higher rates for deeply located lesions (i.e., brainstem and basal ganglia), 10.6% per patient-year, versus 0% per patient-year for superficially located lesions.
Hemorrhage rates appear to be particularly high in patients initially seen after bleeding episodes that violate the lesion capsule and produce a so-called overt, extralesional hemorrhage (type Ia lesion; see Table 392-2 and Figures 392-3 and 392-4) into the surrounding brain. Aiba and colleagues monitored a group of 62 such patients for a mean of 3.12 years and noted a risk for recurrent symptomatic hemorrhage of 22.3% per lesion-year.43 Barker and colleagues reported a similar experience in 141 patients selected for intervention who had “overt” hemorrhages.48 In this series, 63 patients experienced a second hemorrhage before treatment. Hemorrhages clustered around the initial event, with a repeat hemorrhage rate of 25.2% per year for the first 28 months. Comparable rates of rebleeding have been reported after incomplete resection of cavernous malformations, presumably because of interruption of the lesion capsule, thus stressing the importance of complete resection during the initial surgical procedure.28,53,70,71
Brainstem Cavernous Malformations
In general, the natural history of brainstem cavernous malformations parallels that of lesions elsewhere in the central nervous system. However, because of the eloquence of surrounding structures, episodes of hemorrhage (even relatively small hemorrhages) are much more likely to be symptomatic. A conservative estimate, based on the assumption that lesions are present from birth to the first symptom, places the risk for symptomatic hemorrhage in the range of 2.5% to 6.8% per year (mean, 4.5% per lesion-year).28,30,49,52,54,56 This estimate is similar to the 2% per year risk reported by Mathiesen and associates in a group of 11 patients with asymptomatic brainstem cavernous malformations who were monitored prospectively for a mean of 4 years.53 For patients with a history of previous symptomatic hemorrhage, the risk of rebleeding ranged from 5.1% to 60% (mean, 28.7% per lesion-year),28,30,49,52–54,56 with higher rates reported in those with recent hemorrhage48,54 or evidence of extralesional hemorrhage on MRI.43,49
Spinal Cord Cavernous Malformations
Cavernous malformations of the spinal cord are rarely diagnosed before the onset of symptoms. Data on the natural history of these lesions are primarily derived from surgical series. The risk for symptomatic hemorrhage based on the age at symptom onset and the assumption that lesions are present from birth ranges from 1.4% to 4.5% per patient-year.32,34,35,72 Early reports suggested that once lesions become symptomatic, patients tended to experience progressive neurological deterioration, with prospective rehemorrhage rates as high as 66% per patient-year.32,72 More recently, however, several groups have published the results of conservative management of spinal cord lesions, and prospective symptomatic hemorrhage rates were determined to be 0% to 1.6% per patient-year.33,40 The marked variation in recurrent hemorrhage rates reported by these different groups emphasizes the influence that selection bias can play in retrospective studies. In the latter two reports, approximately a third of the patients (those with rapidly progressive deficits and recent hemorrhages) underwent surgical resection, which left a separate cohort to manage conservatively. Nevertheless, these two studies demonstrate that nonoperative treatment is an acceptable option in selected patients with intramedullary spinal cord cavernous malformations.
Familial
The natural history of the familial form of cavernous malformations is analogous to that of its spontaneous counterpart. Clinical penetrance is highly variable, with 40% to 60% of patients reported being free of symptoms.13,19,46 The most common manifestations are seizures and headaches with supratentorial lesions and focal neurological deficits with those involving the brainstem, basal ganglia, and spinal cord.
Several prospective studies have examined the natural history of this population. Zabramski and colleagues identified 59 members of six families with the familial form of cavernous malformations.13 From this cohort, 21 patients harboring a total of 128 lesions (mean, 6.5 lesions per patient) were prospectively monitored for a mean of 2.2 years by clinical examinations and serial MRI studies at 6- to 12-month intervals. Clinically silent hemorrhages were common. MRI evidence of hemorrhage (defined as signal changes consistent with acute/subacute hemorrhage) occurred in 28% of patients, but only half of these episodes were associated with symptoms. The overall hemorrhage rate was 2% per lesion-year, with a rate of symptomatic hemorrhage of 1.1% per lesion-year. This report also documented the ongoing development of new lesions. During the follow-up period, new lesions developed in 29% of the patients. Altogether, 17 de novo lesions were identified, for a rate of 0.4 new lesions per patient-year.
Labauge and coworkers identified 264 patients in 51 families with familial cavernous malformations.46 Forty of these patients, who had a total 232 lesions (mean, 5.9 lesions per patient), underwent at least two clinical and MRI studies. The mean follow-up was 3.2 years. New lesions were noted in 27% of patients, for a rate of 0.2 new lesions per patient per year. The authors observed 21 acute hemorrhages in 14 patients, for an overall hemorrhage rate of 2.5% per lesion-year. The rate was higher for infratentorial lesions, 5% per lesion-year, than for those in the supratentorial compartment, 1.9% per lesion-year. A third of the hemorrhages were symptomatic. Hemorrhage was most common with type I and type II lesions (see Table 392-3): 32% of type I and 14% of type II lesions demonstrated evidence of hemorrhage on follow-up MRI. In contrast, only 2.8% of type III lesions and 0% of type IV lesions showed evidence of hemorrhage.
In a subsequent study by Labauge and colleagues, serial clinical and MRI examinations were used to prospectively monitor 33 asymptomatic patients with familial cavernous malformations for a mean of 2.1 years.19 On initial evaluation, 234 lesions were identified for a mean of 7.1 lesions per patient. New lesions occurred in 30% of the patients during follow-up, for an average rate of 0.4 new lesions per patient per year. MRI evidence of hemorrhage was noted in 3 patients (9%) but was symptomatic in only 2 (6%), for a symptomatic hemorrhage rate of 0.4% per lesion-year and 2.8% per patient-year. Hemorrhage was detected only in patients with type II lesions.
Pregnancy and Gender
Although it is widely believed that pregnancy and the puerperium are associated with an increased risk for hemorrhage and aggressive behavior of cavernous malformations, quantitative data supporting this assumption are scarce.73 Cavernous malformations have been reported to increase in size during pregnancy,74,75 and exacerbation of other symptoms related to acute hemorrhage has been documented.10,30,35,67,75–77 Overall, however, the number of reports in the literature documenting such events is small. More importantly, there have been no reports documenting an increased risk for symptomatic hemorrhage associated with pregnancy in women with the familial form of this disease, in which the average patient harbors five to seven lesions.
Management of cavernous malformations during pregnancy should be based on the time when symptoms develop, the severity of the episode, and the imaging characteristics of the lesion. Fortunately, the need for emergency neurosurgical treatment during pregnancy has been rare.78,79 Most cases have been successfully managed after cesarean section or vaginal delivery. The method of delivery should be based on obstetric considerations.
In addition to pregnancy, some authors have suggested that female hormonal factors may be associated with an increased risk for hemorrhage. Several groups have reported a marked female preponderance in patients with symptomatic hemorrhage, particularly in those with brainstem and spinal cord lesions, with female-to-male ratios as high as 1.6 : 1 and 2.3 : 1, respectively.30,32 In other reports, however, the ratios are reversed and males predominate by similar ratios.54,57 Such disparity suggests that referral bias may be playing a significant, although unappreciated role at these various institutions. We reviewed the gender of all patients in the reports listed in Table 392-2, excluding the literature reviews and the three publications dealing exclusively with familial cavernous malformations. Altogether, 1466 patients were identified, including 718 females and 748 males, for a female-to-male ratio of 1 : 1.04. Restricting the review to the reports in Table 392-2 dealing exclusively with brainstem lesions, we identified 421 cases, with the female-to-male ratio being 1 : 1. Likewise, restricting the review to the reports of intramedullary spinal cord cavernous malformations yielded 175 cases, including 83 females and 92 males, for a ratio of 1 : 1.1 Nearly all patients in these reports were symptomatic. These results support the hypothesis that hemorrhage rates are equal in both sexes and argue against a significant hormonal effect.
Conclusion
The risk for recurrent symptomatic hemorrhage is higher in patients with symptomatic lesions and varies with the type of hemorrhage and the interval from the bleeding episode. The recurrent hemorrhage rate approaches 30% per year in patients with acute episodes of “overt” or extralesional hemorrhage that disrupts the lesion capsule (type IA, see Table 392-2). Patients with acute intralesional hemorrhage (type IB, see Table 392-2) or with symptomatic type II lesions (see Table 392-2) have an intermediate risk of rebleeding (range, 5% to 10% per patient-year). Reasonable evidence suggests that the risk for rebleeding after an episode of extralesional hemorrhage is increased for 2 to 3 years and then gradually declines.
Aiba T, Tanaka R, Koike T, et al. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83:56-59.
Awad I, Jabbour P, Awad I, et al. Cerebral cavernous malformations and epilepsy. Neurosurgery. 2006;21(1):e7.
Barker FG, Amin-Hanjani S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49:15-24.
Canavero S, Pagni CA, Duca S, et al. Spinal intramedullary cavernous angiomas: a literature meta-analysis. Surg Neurol. 1994;41:381-388.
Cohen-Gadol AA, Jacob JT, Edwards DA, et al. Coexistence of intracranial and spinal cavernous malformations: a study of prevalence and natural history. J Neurosurg. 2006;104:376-381.
Del Curling OJr, Kelly DLJr, Elster AD, et al. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
Detwiler PW, Porter RW, Zabramski JM, et al. De novo formation of a central nervous system cavernous malformation: implications for predicting risk of hemorrhage. Case report and review of the literature. J Neurosurg. 1997;87:629-632.
Fritschi JA, Reulen HJ, Spetzler RF, et al. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir (Wien). 1994;130:35-46.
Jallo GI, Freed D, Zareck M, et al. Clinical presentation and optimal management for intramedullary cavernous malformations. Neurosurg Focus. 2006;21(1):e10.
Kharkar S, Shuck J, Conway J, et al. The natural history of conservatively managed symptomatic intramedullary spinal cord cavernomas. Neurosurgery. 2007;60:865-872.
Kim LJ, Klopfenstein JD, Zabramski JM, et al. Analysis of pain resolution after surgical resection of intramedullary spinal cord cavernous malformations. Neurosurgery. 2006;58:106-111.
Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
Labauge P, Brunereau L, Laberge S, et al. Prospective follow-up of 33 asymptomatic patients with familial cerebral cavernous malformations. Neurology. 2001;57:1825-1828.
Labauge P, Brunereau L, Levy C, et al. The natural history of familial cerebral cavernomas: a retrospective MRI study of 40 patients. Neuroradiology. 2000;42:327-332.
Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg. 2003;99:31-37.
New PF, Ojemann RG, Davis KR, et al. MR and CT of occult vascular malformations of the brain. AJR Am J Roentgenol. 1986;147:985-993.
Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006;21(1):e4.
Porter PJ, Willinsky RA, Harper W, et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
Rigamonti D, Drayer BP, Johnson PC, et al. Familial cerebral cavernous malformations. N Engl J Med. 1988;319:343-347.
Rigamonti D, Drayer BP, Johnson PC, et al. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987;67:518-524.
Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709-714.
Sandalcioglu IE, Wiedemayer H, Gasser T, et al. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26:253-256.
Vishteh AG, Zabramski JM, Spetzler RF. Patients with spinal cord cavernous malformations are at an increased risk for multiple neuraxis cavernous malformations. Neurosurgery. 1999;45:30-32.
Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 1994;80:422-432.
1 Voigt K, Yasargil MG. Cerebral cavernous haemangiomas or cavernomas. Incidence, pathology, localization, diagnosis, clinical features and treatment. Review of the literature and report of an unusual case. Neurochirurgia (Stuttg). 1976;19:59-68.
2 Rigamonti D, Drayer BP, Johnson PC, et al. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987;67:518-524.
3 Gomori JM, Grossman RI, Goldberg HI, et al. Occult cerebral vascular malformations: high-field MR imaging. Radiology. 1986;158:707-713.
4 Lemme-Plaghos L, Kucharczyk W, Brant-Zawadzki M, et al. MRI of angiographically occult vascular malformations. AJR Am J Roentgenol. 1986;146:1223-1228.
5 New PF, Ojemann RG, Davis KR, et al. MR and CT of occult vascular malformations of the brain. AJR Am J Roentgenol. 1986;147:985-993.
6 Schorner W, Bradac GB, Treisch J, et al. Magnetic resonance imaging (MRI) in the diagnosis of cerebral arteriovenous angiomas. Neuroradiology. 1986;28:313-318.
7 McCormick WF. Pathology of vascular malformations of the brain. In: Wilson CB, Stein BM, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:44-63.
8 Otten P, Pizzolato GP, Rilliet B, et al. A propos de 131 cas d’angiomes caverneux (cavernomes) du S.N.C. reperes par l’analyse retrospective de 24,535 autopsies [131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies]. Neurochirurgie. 1989;35:82-83.
9 Del Curling OJr, Kelly DLJr, Elster AD, et al. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
10 Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709-714.
11 Hayman LA, Evans RA, Ferrell RE, et al. Familial cavernous angiomas: natural history and genetic study over a 5-year period. Am J Med Genet. 1982;11:147-160.
12 Rigamonti D, Drayer BP, Johnson PC, et al. Familial cerebral cavernous malformations. N Engl J Med. 1988;319:343-347.
13 Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 1994;80:422-432.
14 Labauge P, Laberge S, Brunereau L, et al. Hereditary cerebral cavernous angiomas: clinical and genetic features in 57 French families. Societe Francaise de Neurochirurgie. Lancet. 1998;352:1892-1897.
15 Labauge P, Denier C, Bergametti F, et al. Genetics of cavernous angiomas. Lancet Neurol. 2007;6:237-244.
16 Dashti SR, Hoffer A, Hu YC, et al. Molecular genetics of familial cerebral cavernous malformations. Neurosurgery. 2006;21(1):e2.
17 Johnson EW, Zabramski JM. Genetics of cerebral cavernous malformations. In: Lanzino G, Spetzler RF, editors. Cavernous Malformations of the Brain and Spinal Cord. New York: Thieme; 2008:26-29.
18 Denier C, Labauge P, Brunereau L, et al. Clinical features of cerebral cavernous malformations patients with KRIT1 mutations. Ann Neurol. 2004;55:213-220.
19 Labauge P, Brunereau L, Laberge S, et al. Prospective follow-up of 33 asymptomatic patients with familial cerebral cavernous malformations. Neurology. 2001;57:1825-1828.
20 Alvarez-Sabin J, Montalban J, Tintore M, et al. Pure sensory stroke due to midbrain haemorrhage [letter]. J Neurol Neurosurg Psychiatry. 1991;54:843.
21 Giombini S, Morello G. Cavernous angiomas of the brain. Account of fourteen personal cases and review of the literature. Acta Neurochir (Wien). 1978;40:61-82.
22 Awad I, Jabbour P, Awad I, et al. Cerebral cavernous malformations and epilepsy. Neurosurgery. 2006;21(1):e7.
23 Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
24 Moriarity JL, Clatterbuck RE, Rigamonti D. The natural history of cavernous malformations. Neurosurg Clin N Am. 1999;10:411-417.
25 Willmore LJ, Sypert GW, Munson JB. Recurrent seizures induced by cortical iron injection: a model of posttraumatic epilepsy. Ann Neurol. 1978;4:329-336.
26 Chusid JB, Kopelott LM. Epileptogenic effects of pure metals implanted in motor cortex of monkeys. J Appl Physiol. 1962;17:697-700.
27 Zimmerman RS, Spetzler RF, Lee KS, et al. Cavernous malformations of the brain stem. J Neurosurg. 1991;75:32-39.
28 Fritschi JA, Reulen HJ, Spetzler RF, et al. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir (Wien). 1994;130:35-46.
29 Weil SM, Tew JMJr. Surgical management of brain stem vascular malformations. Acta Neurochir (Wien). 1990;105:14-23.
30 Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
31 Ogilvy CS, Louis DN, Ojemann RG. Intramedullary cavernous angiomas of the spinal cord: clinical presentation, pathological features, and surgical management. Neurosurgery. 1992;31:219-229.
32 Sandalcioglu IE, Wiedemayer H, Gasser T, et al. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26:253-256.
33 Kharkar S, Shuck J, Conway J, et al. The natural history of conservatively managed symptomatic intramedullary spinal cord cavernomas. Neurosurgery. 2007;60:865-872.
34 Zevgaridis D, Medele RJ, Hamburger C, et al. Cavernous haemangiomas of the spinal cord. A review of 117 cases. Acta Neurochir (Wien). 1999;141:237-245.
35 Canavero S, Pagni CA, Duca S, et al. Spinal intramedullary cavernous angiomas: a literature meta-analysis. Surg Neurol. 1994;41:381-388.
36 Cristante L, Hermann HD. Radical excision of intramedullary cavernous angiomas. Neurosurgery. 1998;43:424-430.
37 Cosgrove GR, Bertrand G, Fontaine S, et al. Cavernous angiomas of the spinal cord. J Neurosurg. 1988;68:31-36.
38 Kim LJ, Klopfenstein JD, Zabramski JM, et al. Analysis of pain resolution after surgical resection of intramedullary spinal cord cavernous malformations. Neurosurgery. 2006;58:106-111.
39 Vishteh AG, Zabramski JM, Spetzler RF. Patients with spinal cord cavernous malformations are at an increased risk for multiple neuraxis cavernous malformations. Neurosurgery. 1999;45:30-32.
40 Cohen-Gadol AA, Jacob JT, Edwards DA, et al. Coexistence of intracranial and spinal cavernous malformations: a study of prevalence and natural history. J Neurosurg. 2006;104:376-381.
41 McCormick PC, Michelsen WJ, Post KD, et al. Cavernous malformations of the spinal cord. Neurosurgery. 1988;23:459-463.
42 Anson JA, Spetzler RF. Surgical resection of intramedullary spinal cord cavernous malformations. J Neurosurg. 1993;78:446-451.
43 Aiba T, Tanaka R, Koike T, et al. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83:56-59.
44 Kim DS, Park YG, Choi KH, et al. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997;48:9-18.
45 Porter PJ, Willinsky RA, Harper W, et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
46 Labauge P, Brunereau L, Levy C, et al. The natural history of familial cerebral cavernomas: a retrospective MRI study of 40 patients. Neuroradiology. 2000;42:327-332.
47 Deutsch H, Jallo GI, Faktorovich A, et al. Spinal intramedullary cavernoma: clinical presentation and surgical outcome. J Neurosurg. 2000;93:65-70.
48 Barker FG, Amin-Hanjani S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49:15-24.
49 Kupersmith MJ, Kalish H, Epstein F, et al. Natural history of brainstem cavernous malformations. Neurosurgery. 2001;48:47-53.
50 Kim DG, Choe WJ, Paek SH, et al. Radiosurgery of intracranial cavernous malformations. Acta Neurochir (Wien). 2002;144:869-878.
51 Hasegawa T, McInerney J, Kondziolka D, et al. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197.
52 Sandalcioglu IE, Wiedemayer H, Secer S, et al. Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry. 2002;72:351-355.
53 Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg. 2003;99:31-37.
54 Wang CC, Liu A, Zhang JT, et al. Surgical management of brain-stem cavernous malformations. Surg Neurol. 2003;59:444-454.
55 Santoro A, Piccirilli M, Frati A, et al. Intramedullary spinal cord cavernous malformations: report of ten new cases. Neurosurg Rev. 2004;27:93-98.
56 Ferroli P, Sinisi M, Franzini A, et al. Brainstem cavernomas: long-term results of microsurgical resection in 52 patients. Neurosurgery. 2005;56:1203-1212.
57 Jallo GI, Freed D, Zareck M, et al. Clinical presentation and optimal management for intramedullary cavernous malformations. Neurosurg Focus. 2006;21(1):e10.
58 Zabramski JM, Henn JS, Coons S. Pathology of cerebral vascular malformations. Neurosurg Clin N Am. 1999;10:395-410.
59 Brunken M, Sagehorn S, Leppien A, et al. [De novo formation of a cavernoma in association with a preformed venous malformation during immunosuppressive treatment.]. Zentralbl Neurochir. 1999;60:81-85.
60 Maraire JN, Abdulrauf SI, Berger S, et al. De novo development of a cavernous malformation of the spinal cord following spinal axis radiation. Case report. J Neurosurg. 1999;90:234-238.
61 Rosahl SK, Vorkapic P, Eghbal R, et al. Ossified and de novo cavernous malformations in the same patient. Clin Neurol Neurosurg. 1998;100:138-143.
62 Larson JJ, Ball WS, Bove KE, et al. Formation of intracerebral cavernous malformations after radiation treatment for central nervous system neoplasia in children. J Neurosurg. 1998;88:51-56.
63 Houtteville JP. Brain cavernoma: a dynamic lesion. Surg Neurol. 1997;48:610-614.
64 Detwiler PW, Porter RW, Zabramski JM, et al. De novo formation of a central nervous system cavernous malformation: implications for predicting risk of hemorrhage. Case report and review of the literature. J Neurosurg. 1997;87:629-632.
65 Pozzati E, Giangaspero F, Marliani F, et al. Occult cerebrovascular malformations after irradiation. Neurosurgery. 1996;39:677-682.
66 Tekkok IH, Ventureyra EC. De novo familial cavernous malformation presenting with hemorrhage 12.5 years after the initial hemorrhagic ictus: natural history of an infantile form. Pediatr Neurosurg. 1996;25:151-155.
67 Pozzati E, Acciarri N, Tognetti F, et al. Growth, subsequent bleeding, and de novo appearance of cerebral cavernous angiomas. Neurosurgery. 1996;38:662-669.
68 Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006;21(1):e4.
69 Yoon PH, Kim DI, Jeon P, et al. Cerebral cavernous malformations: serial magnetic resonance imaging findings in patients with and without gamma knife surgery. Neurol Med Chir (Tokyo). 1998;38(suppl):255-261.
70 Jain KK, Robertson E. Recurrence of an excised cavernous hemangioma in the opposite cerebral hemisphere. Case report. J Neurosurg. 1970;33:453-456.
71 Wakai S, Ueda Y, Inoh S, et al. Angiographically occult angiomas: a report of thirteen cases with analysis of the cases documented in the literature. Neurosurgery. 1985;17:549-556.
72 Hsu FPK, Clatterbuck RE, Kim LJ, et al. Intramedullary spinal cord cavernous malformations. Oper Tech Neurosurg. 2003;6:32-40.
73 Safavi-Abbasi S, Feiz-Erfan I, Spetzler RF, et al. Hemorrhage of cavernous malformations during pregnancy and in the peripartum period: causal or coincidence? Neurosurgery. 2006;21(1):E12.
74 Yamasaki T, Handa H, Yamashita J, et al. Intracranial and orbital cavernous angiomas. A review of 30 cases. J Neurosurg. 1986;64:197-208.
75 Katayama Y, Tsubokawa T, Maeda T, et al. Surgical management of cavernous malformations of the third ventricle. J Neurosurg. 1994;80:64-72.
76 Awada A, Watson T, Obeid T. Cavernous angioma presenting as pregnancy-related seizures. Epilepsia. 1997;38:844-846.
77 Lopate G, Black JT, Grubb RLJr. Cavernous hemangioma of the spinal cord: report of 2 unusual cases. Neurology. 1990;40:1791-1793.
78 Ondra SL, Doty JR, Mahla ME, et al. Surgical excision of a cavernous hemangioma of the rostral brain stem: case report. Neurosurgery. 1988;23:490-493.
79 Flemming KD, Goodman BP, Meyer FB. Successful brainstem cavernous malformation resection after repeated hemorrhages during pregnancy. Surg Neurol. 2003;60:545-547.