Chapter 24 Myelodysplastic Syndromes
Biology and Treatment
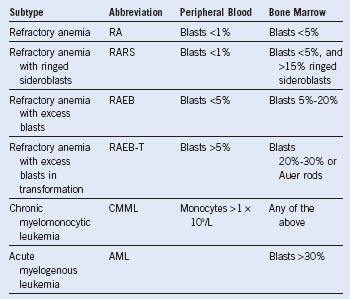
Table 24-1 French-American-British Classification Criteria*
WHO 2008 classification:
Refractory anemia with unilineage dysplasia
Refractory anemia
Refractory neutropenia
Refractory thrombocytopenia
Refractory anemia with ring sideroblasts
Refractory cytopenia with multilineage dysplasia (with or without ringed sideroblasts)
Refractory anemia with excess of blasts-1
Refractory anemia with excess of blasts-2
Myelodysplastic syndrome, unclassifiable
Myelodysplastic syndrome with deletion 5q
Table 24-2 International Prognostic Scoring System for Myelodysplastic Syndrome
| Overall Score* | Median Survival (Years) | 25% AML Evolution (Years) |
|---|---|---|
| Low (0) | 5.7 | 9.4 |
| Intermediate-1 (0.5-1.0) | 3.5 | 3.3 |
| Intermediate-2 (1.5-2.0) | 1.2 | 1.1 |
| High (≥2.5) | 0.4 | 0.2 |
AML, Acute myelogenous leukemia.
The percent of myeloblasts are scored as follows: <5% = 0; 5%-10% = 0.5: 11%-20% = 1.5: 21%-30% = 2.0.
Cytogenetic features associated with good prognosis are scored as 0 and include normal karyotype, loss of Y, 5q–, or 20q–; those associated with a poor prognosis are scored as 1.0 and include abnormalities of chromosome 7 or three or more cytogenetic changes; all other cytogenetic abnormalities are scored as 0.5 and are of intermediate prognosis.
A score of 0 refers to a patient with either zero or one cell lineage cytopenia, and a score of 0.5 is assigned to two or more lineage cytopenias. Lineage cytopenias are defined as hemoglobin <10 g/dL, absolute neutrophil count <1800/mm3, and platelet count <100,000/mm3.
*The overall score is the sum of the scores from the percent of bone marrow myeloblasts, karyotype, and cytopenias.
Data from Greenberg P, Cox C, LeBeau MM, et al: International scoring system for evaluating prognosis in myelodysplastic syndromes (published erratum appears in Blood 91:1100, 1998). Blood 89:2079, 1997.
Table 24-3 World Health Organization Classifications of MDS
The World Health Organization has reclassified chronic myelomonocytic leukemia (CMML) within the myeloproliferative disorders (MPD) and RAEB-T to “AML with multilineage dysplasia.” Acute myeloid leukemia (AML) is now classified as ≥20% myeloblasts.
The following are considered AML regardless of blast percentage: t(8;21)-AML1/ETO, t(15;17)-PML/RARA, inv(16) or t(16;16)-CBFβMYH11, and 11q23-MLL.
Table 24-4 World Health Organization–Based Prognostic Scoring System for Predicting Survival in Patients With Myelodysplastic Syndrome
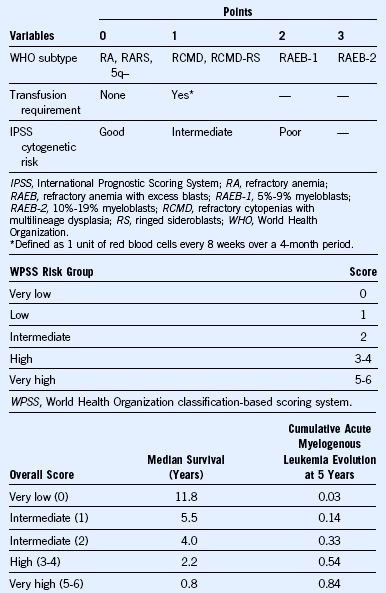
Adapted from Malcovati L, Porta MG, Pascutto C, et al: Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: A basis for clinical decision making, J Clin Oncol 23:7594, 2005.
Management Guidelines for a Newly Diagnosed Patient With Myelodysplasia
The only known curative modality for patients with MDS is SCT. Therefore, all appropriate candidates should be considered for SCT. They include patients younger than 70 years (age 75 years at some centers), with a reasonable performance status and no significant comorbidity. Retrospective data from the International Bone Marrow Transplant Registry suggest that patients with low-risk disease (IPSS low or intermediate-1) should undergo SCT only at the time of disease progression (see Table 24-5). Disease progression includes progressive, clinically significant cytopenias such as progressive RBC or platelet transfusion dependency or transformation to a high-risk disease. Transformation to high-risk MDS typically is manifested by an increase in the number of bone marrow blasts but also could represent accumulation of additional cytogenetic abnormalities.
Myelodysplastic Syndromes in Young Patients
Cutler et al1 demonstrated a superior outcome when patients in low or intermediate-1 IPSS risk groups were transplanted at the time of disease progression as opposed to the time of their initial diagnosis. Furthermore, data from the German registry revealed that 86% of patients younger than 50 years with low-risk disease were alive at 20 years, and patients with an intermediate-1 IPSS score had a median survival of 176 months. Therefore, patients with low or intermediate-1 risk MDS who are younger than 50 years should be observed, and SCT should be delayed until disease progression. Disease progression includes the development of significant transfusion requirements, progressive cytopenias, or transformation to a higher IPSS subgroup.
Erythropoietin-Stimulating Agents in Patients With Myelodysplastic Syndrome
Table 24-5 Decision Analysis of Allogeneic Bone Marrow Transplantation for Myelodysplastic Syndrome
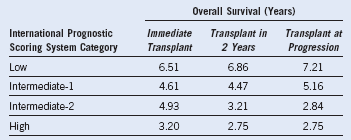
From Cutler CS, Lee SJ, Greenberg P, et al: A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 104:579, 2004.