Myelodysplastic Syndromes
After completion of this chapter, the reader will be able to:
1. Define myelodysplastic syndromes (MDSs).
2. Explain the etiology of MDS.
3. Recognize morphologic features of dyspoiesis in bone marrow and peripheral blood.
4. Discuss abnormal functions of granulocytes, erythrocytes, and thrombocytes in MDS.
5. Correlate peripheral blood, bone marrow, and cytogenetic findings in MDS with classification systems.
6. Compare and contrast the French-American-British and the 2008 World Health Organization classifications of MDS.
7. Discuss prognostic indicators in MDS.
8. Discuss modes of management for MDS.
9. Discuss the epidemiology of MDS and apply it as a contributor in differential diagnosis.
10. Suggest laboratory tests and their results that would rule out MDS in the differential diagnosis.
11. Explain the rationale for the category of myelodysplastic/myeloproliferative neoplasms (MDS/MPN).
12. Correlate peripheral blood, bone marrow, and cytogenetic findings in MDS/MPN with disease classification.
Historically, this pattern of abnormalities was referred to as refractory anemia, smoldering leukemia, oligoblastic leukemia, or preleukemia.1–3 In 1982, the French-American-British (FAB) Cooperative Leukemia Study Group proposed terminology and a specific set of morphologic criteria to describe what are now known as myelodysplastic syndromes (MDSs).4 In 1997, a group from the World Health Organization (WHO) proposed a new classification that included molecular, cytogenetic, and immunologic criteria in addition to morphologic features.5,6 The WHO classification was revised in 2008. Both the FAB and WHO classifications are discussed in this chapter.
MDSs are a group of acquired clonal hematologic disorders characterized by progressive cytopenias in the peripheral blood, reflecting defects in erythroid, myeloid, and/or megakaryocytic maturation.7,8 The median age at diagnosis is 70. MDS rarely affect individuals younger than age 50 unless preceded by chemotherapy or radiation for another malignancy.1,9 Cases in young adults and children have been reported, however.10,11 The incidence of these disorders seems to be increasing, but this apparent increase may be attributable in part to improved techniques for identifying these diseases and to improved classification.12,13 At this time, the fastest-growing segment of the population is the group older than 60 years of age. MDSs are becoming a more common finding in the hematology laboratory, and familiarity with these disorders is an essential part of the body of knowledge of all medical laboratory professionals.
Etiology
MDS may arise de novo (primary MDS) or as a result of therapy (therapy-related MDS). Although MDSs are a group of heterogeneous diseases, all are the result of proliferation of abnormal stem cells.7,8,14,15 The initiating defect in most cases is at the level of the myeloid stem cell, because primarily the erythroid, myeloid, and megakaryocytic cells are affected. It may be that the affected hematopoietic stem cell has lost its lymphopoietic potential, because only rarely does MDS transform to acute lymphoblastic leukemia.16,17 The abnormal stem cell may be the result of the cumulative effects of environmental exposure in susceptible individuals. Mutations may be caused by chemical insult, radiation, or viral infection. There also may be an association with smoking.18 An association with inherited hematologic disorders has also be found.19 The mutated stem cell produces a pathologic clone of cells that expands in size at the expense of normal cell production.18,20 Because each mutation produces a unique clone with a specific cellular defect, MDSs have a multitude of expressions. Two morphologic findings are common to all types of MDS, however: the presence of progressive cytopenias despite cellular bone marrows and dyspoiesis in one or more cell lines.
Disruption of apoptosis may be responsible for the ineffective hematopoiesis in MDS.21–26 Apoptosis (programmed cell death) regulates cell population by decreasing cell survival. In MDS, apoptosis is increased in early disease, when peripheral blood cytopenias are evident. Later in MDS, when progression toward leukemia is apparent, apoptosis has been shown to be decreased, which allows increased neoplastic cell survival and expansion of the abnormal clone.27–30 Other important factors include the levels of antiangiogenic cytokines, tumor necrosis factor, and cellular components of the immune system, as well as the interaction between MDS clonal cells and the hematopoietic inductive microenvironment. Evidence has shown that patients with MDS have increased levels of angiogenic growth factors, including vascular endothelial growth factor.31,32
Therapy-related MDS occurs in patients who have been treated previously with chemotherapy or radiotherapy or both. Median onset of therapy-related MDS varies with the agents used and is usually 2.5 to 5.0 years after therapy was initiated,33,34 although cases occurring 7 years after initial exposure to chemotherapy or radiation have been reported.35 Therapy-related MDS often is more aggressive and may evolve quickly into acute myeloblastic leukemia (AML).20,34,35 The 2008 WHO classification places therapy-related MDSs into the AML category of therapy-related myeloid neoplasms (see Chapter 36).
Morphologic Abnormalities in Peripheral Blood and Bone Marrow
In MDS each of the three major myeloid cell lines has dyspoietic morphologic features. The following sections provide descriptions of common abnormal morphologic findings.4,9,19 These descriptions are not all inclusive because of the large number of possible cellular mutations and combinations of mutations.
Dyserythropoiesis
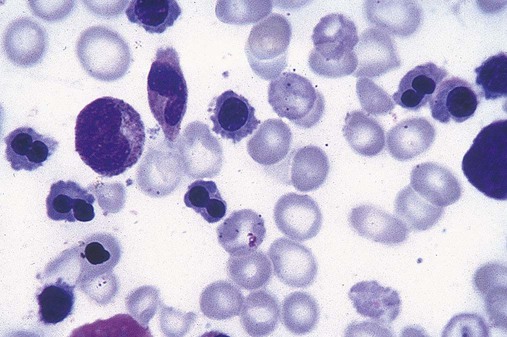
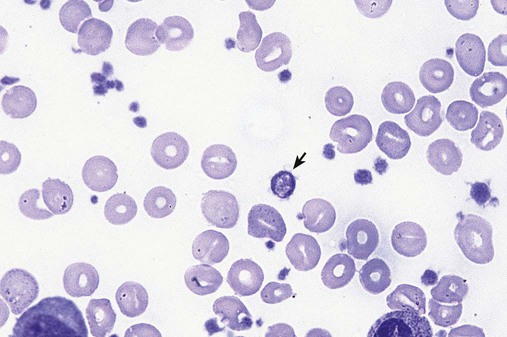
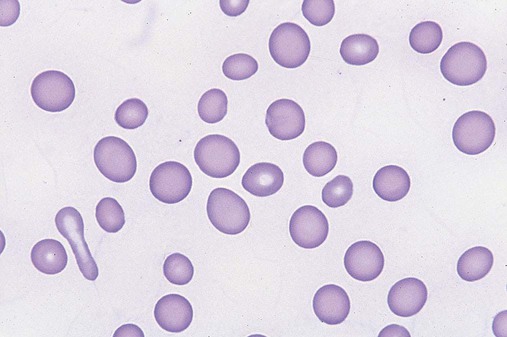
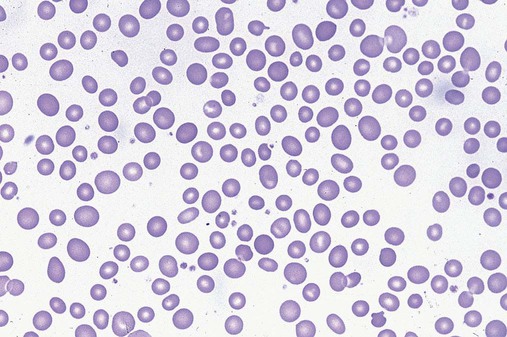
In the peripheral blood, the most common morphologic finding in dyserythropoiesis is the presence of oval macrocytes (Figure 35-1). When these cells are seen in the presence of normal vitamin B12 and folate values, MDS should be included in the differential diagnosis. Hypochromic microcytes in the presence of adequate iron stores also are seen in MDS. A dimorphic red blood cell (RBC) population (Figure 35-2) is another indication of the clonality of this disease. Poikilocytosis, basophilic stippling, Howell-Jolly bodies, and siderocytes also are indications that the erythrocyte has undergone abnormal development.36
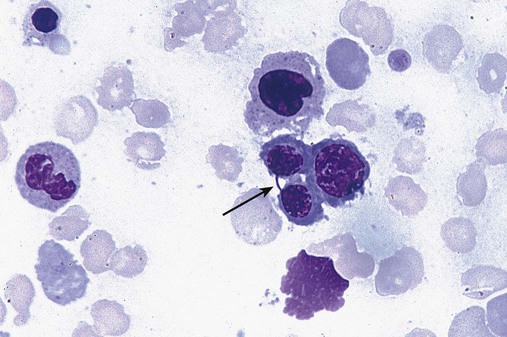
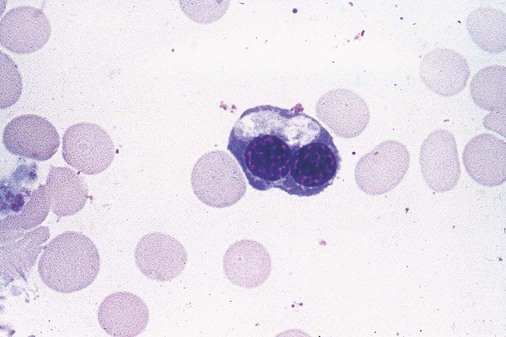
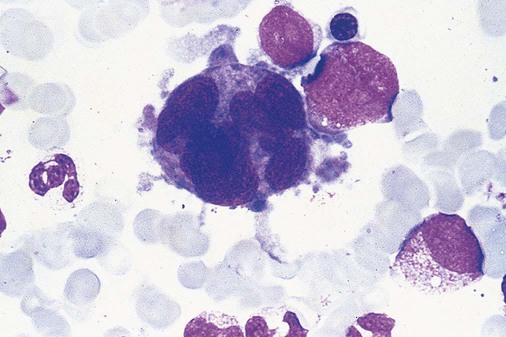
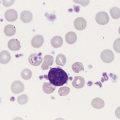
Dyserythropoiesis in the bone marrow is evidenced by RBC precursors with more than one nucleus or abnormal nuclear shapes. The normally round nucleus may have lobes or buds. Nuclear fragments may be present in the cytoplasm (Figure 35-3). Internuclear bridging is occasionally present (Figure 35-4).37 Abnormal cytoplasmic features may include basophilic stippling or heterogeneous staining (Figure 35-5). Ringed sideroblasts are a common finding. Megaloblastoid cellular development in the presence of normal vitamin B12 and folate values is another indication of MDS. The bone marrows in these cases may have erythrocytic hyperplasia or hypoplasia (Box 35-1).
Dysmyelopoiesis
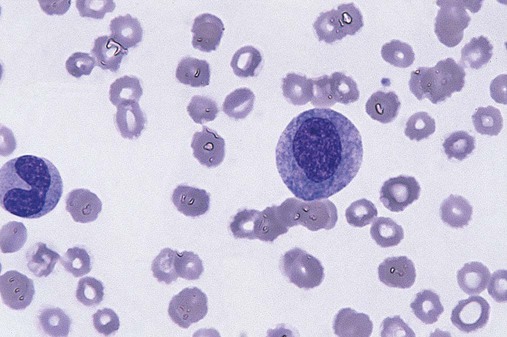
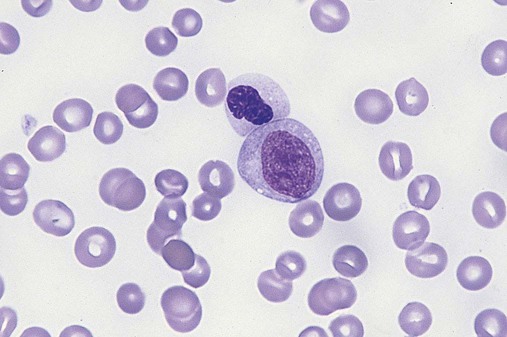
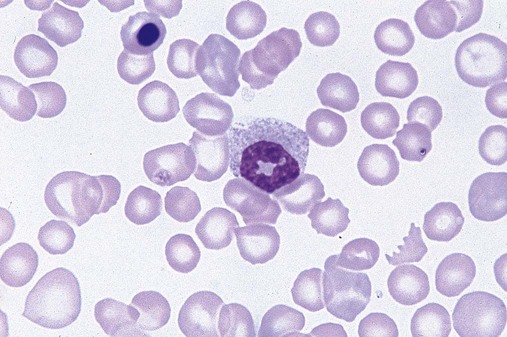
Dysmyelopoiesis in the peripheral blood is suspected when there is a persistence of basophilia in the cytoplasm of otherwise mature white blood cells (WBCs), indicating nuclear-cytoplasmic asynchrony (Figure 35-6). Abnormal granulation of the cytoplasm of neutrophils, in the form of larger than normal granules, hypogranulation, or the absence of granules, is a common finding. Agranular bands can be easily misclassified as monocytes (Figure 35-7). Abnormal nuclear features may include hyposegmentation, hypersegmentation, or nuclear rings (Figure 35-8).38
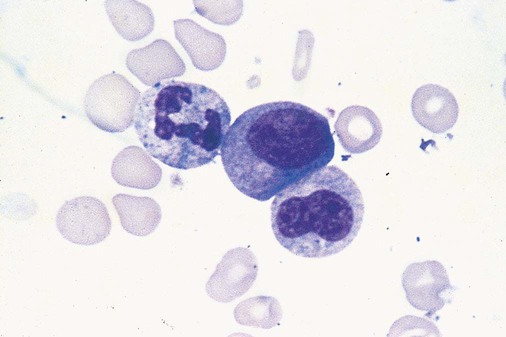
In the bone marrow, dysmyelopoiesis may be represented by nuclear-cytoplasmic asynchrony. Cytoplasmic changes include uneven staining, such as a dense ring of basophilia around the periphery with a clear unstained area around the nucleus or whole sections of cytoplasm unstained with the remainder of the cytoplasm stained normally (Figure 35-9). There may be abnormal granulation of the cytoplasm in which promyelocytes or myelocytes or both are devoid of primary granules (Figure 35-10), primary granules may be larger than normal, or secondary granules may be reduced in number or absent, and there may be an occasional Auer rod.39,40 Agranular promyelocytes may be mistaken for blasts; this could lead to misclassification of the disease in the AML scheme. Abnormal nuclear findings may include hypersegmentation or hyposegmentation and possibly ring-shaped nuclei (Box 35-2).
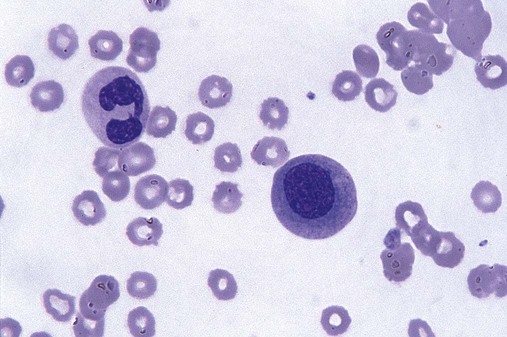
Abnormal localization of immature precursors is a characteristic finding in bone marrow biopsy specimens from patients with MDS.41 Normally, myeloblasts and promyelocytes reside along the endosteal surface of the bone marrow. In some cases of MDS, these cells tend to cluster centrally in marrow sections.
Dysmegakaryopoiesis
Platelets also exhibit dyspoietic morphology in the peripheral blood. Common changes include giant platelets and abnormal platelet granulation, either hypogranulation or agranulation (Figure 35-11). Some platelets may possess large fused granules. Circulating micromegakaryocytes may be present in peripheral blood from patients with MDS (Figure 35-12).9
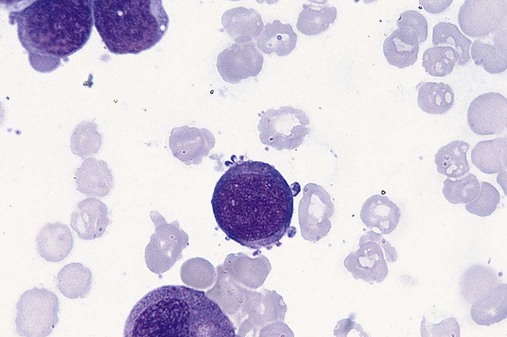
The megakaryocytic component of the bone marrow may exhibit abnormal morphology: large mononuclear megakaryocytes, micromegakaryocytes, or micromegakaryoblasts. The nuclei in these cells may be bilobed or have multiple small, separated nuclei (Figure 35-13; Box 35-3).9
Differential Diagnosis
Dysplasia by itself is not sufficient evidence for MDS, because several other conditions can cause similar morphologic features. Some examples are vitamin B12 or folate deficiency, which can cause pancytopenia and dysplasia, and exposure to heavy metals. Copper deficiency may cause reversible myelodysplasia.42 Some congenital hematologic disorders, such as Fanconi anemia and congenital dyserythropoietic anemia, may also present with dysplasia. Parvovirus B19 and some chemotherapeutic agents may give rise to dysplasia similar to that in MDS. Paroxysmal nocturnal hemoglobinuria has similar features. Therefore a thorough history and physical examination, including questioning about exposure to drugs and chemicals, is essential.9
Abnormal Cellular Function
The cells produced by abnormal maturation not only have an abnormal appearance but also have abnormal function.9,43 The granulocytes may have decreased adhesion,44,45 deficient phagocytosis,45 decreased chemotaxis,44,45 or impaired microbicidal capacity.46 Decreased levels of myeloperoxidase and alkaline phosphatase may be found.47 The RBCs may exhibit shortened survival,48 and erythroid precursors may have a decreased response to erythropoietin that may contribute to anemia.49 Patients may experience increased bleeding despite adequate platelet numbers.9,50,51 The type and degree of dysfunction depend on the mutation present in the hematopoietic stem cell.
Classification of Myelodysplastic Syndromes
French-American-British Classification
In an effort to standardize the diagnosis of MDSs, the FAB created five classes of MDS, each with a specific set of morphologic criteria. The categories were defined by the amount of dysplasia and the number of blasts in the bone marrow. The diagnosis of acute leukemia required at least 30% blasts in the bone marrow.4 The FAB classification included the following:
2. Refractory anemia with ringed sideroblasts (RARS)
3. Refractory anemia with excess blasts (RAEB)
4. Chronic myelomonocytic leukemia (CMML)
5. Refractory anemia with excess blasts in transformation (RAEB-t)
The FAB classification provided a framework for discussion of a seemingly heterogeneous group of disorders; however, its reliance on morphology alone limited its usefulness as a prognostic indicator. In addition, the FAB classification did not view MDSs in their totality, because it did not address therapy-related or hereditary forms, and the special case of childhood MDS was not considered. Advances in medical knowledge, including molecular analysis, have allowed integration of clinical, immunologic, genetic, and molecular data with morphologic features. The WHO classification retains many of the FAB features while recognizing molecular, cytogenetic, and immunologic characteristics of these disorders. The WHO classification also removed the problematic categories of CMML and RAEB-t and placed them in MDS/MPD and acute leukemia, respectively.19,52
World Health Organization Classification
The original modifications from the FAB classification of MDS included a reduction in the percentage of blasts required for diagnosis of AML from 30% to 20% and the recognition of two new classifications: refractory cytopenia with multilineage dysplasia (RCMD) and del(5q) syndrome. The 2008 revision of the WHO criteria added the category of refractory cytopenia with unilineage dysplasia (RCUD), refined some categories, and added the provisional category of childhood MDS, also called refractory cytopenia of childhood. The 2008 WHO classification is outlined in Box 35-4 and detailed in Table 35-1. The classification is extensive, and only the highlights are presented in this chapter.19
TABLE 35-1
Peripheral Blood and Bone Marrow Findings in Myelodysplastic Syndromes (MDSs)
| Disease | Blood Findings | Bone Marrow Findings |
| Refractory cytopenia with unilineage dysplasia (RCUD); refractory anemia (RA); refractory neutropenia (RN); refractory thrombocytopenia (RT) | Unicytopenia* No or rare blasts (<1%)† |
Unilineage dysplasia: ≥10% of cells in one myeloid lineage <5% blasts <15% of erythroid precursors are ringed sideroblasts |
| Refractory anemia with ringed sideroblasts (RARS) | Anemia No blasts |
≥15% of erythroid precursors are ringed sideroblasts Erythroid dysplasia only <5% blasts |
| Refractory cytopenia with multilineage dysplasia (RCMD) | Cytopenia(s) No or rare blasts (<1%)† No Auer rods <1 × 109/L monocytes |
Dysplasia in ≥10% of cells in two or more myeloid lineages (neutrophil and/or erythroid precursors and/or megakaryocytes) <5% blasts in marrow No Auer rods ±15% ringed sideroblasts |
| Refractory anemia with excess blasts 1 (RAEB-1) | Cytopenia(s) <5% blasts No Auer rods <1 × 109/L monocytes |
Unilineage or multilineage dysplasia 5-9% blasts† No Auer rods |
| Refractory anemia with excess blasts 2 (RAEB-2) | Cytopenia(s) 5-19% blasts ± Auer rods‡ <1 × 109/L monocytes |
Unilineage or multilineage dysplasia 10-19% blasts† ± Auer rods‡ |
| Myelodysplastic syndrome, unclassified (MDS-U) | Cytopenia(s) ≤1% blasts† |
Unequivocal dysplasia in <10% of cells in one or more myeloid cell lines when accompanied by a cytogenetic abnormality considered as presumptive evidence for a diagnosis of MDS <5% blasts |
| MDS associated with isolated del(5q) | Anemia Usually normal or increased platelet count No or rare blasts (<1%) |
Normal to increased megakaryocytes with hypolobulated nuclei <5% blasts Isolated del(5q) cytogenetic abnormality No Auer rods |
*Bicytopenia may occasionally be observed. Cases with pancytopenia should be classified as MDS-U.
†If the marrow myeloblast percentage is <5% but there are 2-4% myeloblasts in the blood, the diagnostic classification is RAEB-1. Cases of RCUD and RCMD with 1% myeloblasts in the blood should be classified as MDS-U.
‡Cases with Auer rods and <5% myeloblasts in the blood and <10% myeloblasts in the marrow should be classified as RAEB-2.
From Swerdlow SH, Campo E, Harris NL, et al, editors: WHO classification of tumours of haematopoietic and lymphoid tissues, ed 4, Lyon, France, 2008, IARC Press.
Refractory Cytopenia with Unilineage Dysplasia
Presenting symptoms of RCUD are related to the cytopenia: namely, fatigue or shortness of breath if anemia is present, increased infections from neutropenia, and petechiae, bruising, or bleeding if thrombocytopenia is present. This category includes MDS cases with fewer than 1% blasts in the peripheral blood and fewer than 5% blasts in the bone marrow. Dysplasia must be present in more than 10% of a single myeloid lineage. Included in RCUD is refractory anemia with only dyserythropoiesis (but fewer than 15% ringed sideroblasts), refractory neutropenia, and refractory thrombocytopenia.52 Although cytogenetic abnormalities may be seen in up to 50% of cases of refractory anemia, none are specific to the diagnosis.
Median survival is generally 2 to 5 years with only a 2% risk of transformation to acute leukemia.52,53
Refractory Anemia with Ringed Sideroblasts
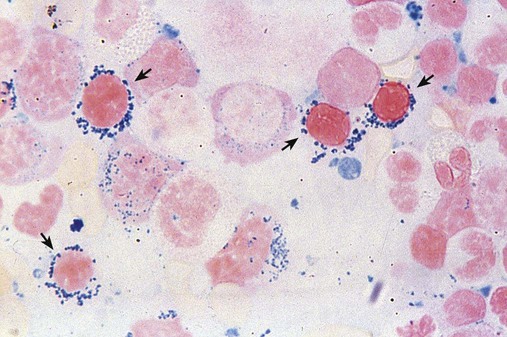
In RARS, anemia and dyserythropoiesis are present, and more than 15% of the bone marrow erythroid precursors are ringed sideroblasts. To be considered a ringed sideroblast, an erythroid precursor must contain more than five iron granules per cell, and these iron-containing mitochondria must circle at least one third of the nucleus (Figure 35-14).54,55 In the peripheral blood there may be a dimorphic picture, with a population of hypochromic cells along with a majority of normochromic cells. RARS occurs primarily in the older population. Mean survival is 69 to 108 months.56 Acquired sideroblastic anemia, which is not considered MDS, is discussed in Chapter 19.
Refractory Cytopenia with Multilineage Dysplasia
RCMD is categorized by one or more cytopenias, dysplasia in two or more myeloid cell lines, fewer than 1% blasts in peripheral blood, and fewer than 5% blasts in the bone marrow. In RCMD, the myeloblasts do not contain Auer rods; if Auer rods are noted, the disorder is classified as RAEB-2.57 Some cases of RCMD have more than 15% ringed sideroblasts, but the dyspoiesis in more than the erythroid line places them in the RCMD, rather than the RARS, category.53 This distinction is important, because RCMD has a more aggressive course than RARS.33
Refractory Anemia with Excess Blasts
RAEB-1—5% to 9% blasts in the bone marrow or 2% to 4% blasts in the peripheral blood
RAEB-2—10% to 19% blasts in the bone marrow and 5% to 19% blasts in the peripheral blood
The presence of Auer rods, regardless of blast count, qualifies a case as RAEB-2.
RAEB with greater than 10% myeloblasts has a more aggressive course, with a greater percentage of cases transforming to AML.53,57
Myelodysplastic Syndrome with Isolated del(5q) (5q– Syndrome)
In patients who have only the deletion of 5q (5q–), MDS represents a fairly well-defined syndrome, affecting predominantly women and occurring at a median age of 67. These patients typically have refractory anemia without other cytopenias and/or thrombocytosis, hypolobulated megakaryocytes, and erythroid hypoplasia.58–60 There are fewer than 1% blasts in the peripheral blood and Auer rods are not seen.61 Patients with MDS with isolated del(5q) have long-term stable disease (median survival, 145 months). Recently the thalidomide analogue lenalidomide has proven to be effective in patients with isolated del(5q) as well as in those with del(5q) and additional cytogenetic abnormalities.58–60
Myelodysplastic Syndrome, Unclassifiable
The category of myelodysplastic syndrome, unclassifiable refers to subtypes of MDS that initially lack the specific changes necessary for classification into other MDS categories. If characteristics of a specific subtype develop later, the case should be reclassified into the appropriate group.62
Childhood Myelodysplastic Syndromes
De novo MDS in children is very rare, and although some of the characteristics of adult MDS are present, there are also some distinct differences. The 2008 WHO classification introduced a provisional category of refractory cytopenia of childhood. Several authors have addressed this provisional category in detail.10,52,58
Myelodysplastic/Myeloproliferative Neoplasms
The MDS/MPN category includes myeloid neoplasms with clinical, laboratory, and morphologic features that are characteristic of both MDS and MPN. Included in this classification are chronic myelomonocytic leukemia, atypical chronic myeloid leukemia, juvenile myelomonocytic leukemia, and MDS/MPN, unclassifiable, with a provisional subtype of refractory anemia with ringed sideroblasts and thrombocytosis (Box 35-5).52
Chronic Myelomonocytic Leukemia
CMML is characterized by a persistent monocytosis of more than 1.0 monocytes × 109/L, absence of the BCR/ABL1 fusion gene, fewer than 20% blasts and promonocytes in the peripheral blood and bone marrow, and dysplasia in one or more myeloid cell line. Patients usually have an increased leukocyte count with absolute monocytosis. Dysgranulopoiesis is evident, but neutrophil precursors make up fewer than 10% of the total leukocytes.63 Splenomegaly may be present due to infiltration of leukemic cells. Although cytogenetic abnormalities are found in up to 40% of patients, there are none specific for CMML. Prognosis varies depending on the number of blasts plus promonocytes. If there are fewer than 5% blasts and promonocytes in the peripheral blood and fewer than 10% in the bone marrow, the disease is classified as CMML-1 and the prognosis is better than in those cases in which there are 5% to 19% blasts and promonocytes in the peripheral blood or 10% to 19% in the bone marrow (classified as CMML-2).52,63
Atypical Chronic Myeloid Leukemia, BCR/ABL1 Negative
Atypical CML, BCR/ABL1 negative (aCML), is characterized by leukocytosis with morphologically dysplastic neutrophils and their precursors. Basophilia may be present, but is not a prominent feature. Multilineage dysplasia is common. The BCR/ABL1 fusion gene is not present, but a variety of other karyotypic abnormalities may be seen. Dyspoiesis may be seen in all cell lines, but is most remarkable in the neutrophils, which may exhibit Pelger-Huët–like cells, hypogranularity, and bizarre segmentation.64,65 The prognosis is poor for patients with aCML, who either progress to AML or succumb to bone marrow failure.66
Juvenile Myelomonocytic Leukemia
Juvenile myelomonocytic leukemia is a clonal disorder characterized by proliferation of the granulocytic and monocytic cell lines and affects children from 1 month to 14 years of age. There is a strong association with neurofibromatosis type 1.67 Allogeneic stem cell transplantation is effective in about 50% of patients.68
Cytogenetics and Molecular Genetics
Cytogenetics
Chromosome abnormalities are found in about 50% of cases of de novo MDS.70 Karyotype has a major effect on prognosis in MDS patients, and specific karyotypes can be used cautiously to predict response to certain treatments.70 Balanced translocations, which are common among patients with AML, are found only rarely in cases of de novo MDS.19 Except for del(5q), no cytogenetic abnormality is specific to subtype. The most common abnormalities involve chromosomes 5, 7, 8, 11, 13, and 20.9,19 The most common single abnormalities are trisomy 8 and monosomy 7.71,72 Less common abnormalities in MDS are 12p–, iso 17, –22, and loss of the Y chromosome.55,73
Molecular Alterations
Several molecular aberrations have been identified in MDS, but these features have not been used in predicting prognosis, because previously such testing was not readily available. Advances in molecular genetic testing have made testing more available for routine use, and there is the potential that such information could be used to strengthen other prognostic indicator schemes.74 Likewise, identification of genetic defects may allow development of targeted therapies.75 Although not specific to MDS, the most common mutations include those in the TP53 gene74 and RUNX1/AML1.76,77 NRAS has been detected in a small percentage of MDS patients.78
Prognosis
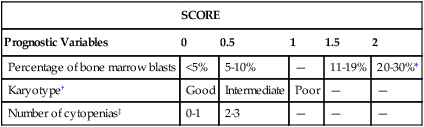
Subtypes of MDS are divided into three risk groups based on morphologic subtype and length of survival and incidence of transformation to AML. RCUD and RARS are considered low risk. The intermediate-risk group includes RCMD and RAEB-1. Cases of RAEB-2 comprise the high-risk group. Cytogenetic features have been recognized as an important prognostic indicator in MDS, and the International Myelodysplastic Syndrome Working Group identified three major risk categories. Patients with a normal karyotype, isolated 5q–, isolated 20q–, and –Y belong to the low-risk group. The high-risk group includes patients with complex abnormalities, that is, three or more abnormalities or an abnormality of chromosome 7. The intermediate-risk group includes those with all other abnormalities.79 Three criteria (percentage of bone marrow blasts, cytopenias, and cytogenetic features) were combined to produce the International Prognostic Scoring System (IPSS) for MDSs (Table 35-2). Median survival predicted by the IPSS is as follows:
TABLE 35-2
International Prognostic Scoring System for Myelodysplastic Syndromes
| SCORE | |||||
| Prognostic Variables | 0 | 0.5 | 1 | 1.5 | 2 |
| Percentage of bone marrow blasts | <5% | 5-10% | — | 11-19% | 20-30%* |
| Karyotype† | Good | Intermediate | Poor | — | — |
| Number of cytopenias‡ | 0-1 | 2-3 | — | — | — |
Scores for risk groups are as follows:
*This group is recognized as acute myeloid leukemia in the World Health Organization classification (see Chapter 36).
†Karyotype categories are defined as follows:
Good: normal, −Y, del(5q), del(20q) (as single abnormalities)
Poor: complex (≥3 abnormalities) or chromosome 7 abnormalities
‡Cytopenias are defined as follows:
Adapted from Greenberg P, Cox E, LeBeau M, et al: International scoring system for evaluating prognosis in myelodysplastic syndromes, Blood 89:2079-2088, 1997.
Treatment
Recently, however, three drugs (lenalidomide, azacitidine, and decitabine) have been approved by the U.S. Food and Drug Administration (FDA) that show promise when used either alone or in combination with other therapies.80 Therapies are usually stratified into those used in low-risk disease and those in higher-risk MDS cases.
Treatment for patients with low-risk MDS is aimed at maintaining residual function of the bone marrow through the use of hematopoietic growth factors such as erythropoietin, thrombopoietin, and granulocyte colony-stimulating factor. Although levels of these growth factors are often normal in MDS patients, there is a subset of patients who respond to their use.80–82
In patients with low-risk MDS, immunosuppressive therapy with drugs such as antithymocyte globulin and cyclosporine has resulted in decreased risk of leukemic tranformation.82–84
Lenalidomide (Revlimid; Celgene, Summit, N.J.), a thalidomide analogue that is less toxic than thalidomide, was approved by the FDA in 2005 for use in patients with low- or intermediate-risk MDS.85–87 It has shown remarkable promise, especially in patients with the 5q chromosome arm deletion. Transfusion independence was achieved in 64% of patients, and a median increase of 3.9 g/dL of hemoglobin was achieved in patients taking the drug. Complete cytogenetic remission was seen in 55% of MDS patients taking lenalidomide, whereas in MDS patients taking erythropoietin cytogenetic remission is rare.85,86 Lenalidomide has immunomodulatory and antiangiogenic effects.80,82 The apparent efficacy of lenalidomide must be weighed against its ability to cause significant myelosuppression.80,85
Ras is mutated in about 20% of MDS patients. Farnesyltransferase inhibitors interfere with this process.82,88 Patients with high-risk MDS benefit from treatment with hypomethylating agents such as azacitidine and, to a lesser extent, decitabine.80,89–91
The only cure is hematopoietic stem cell transplantation. Patients with an IPSS score of intermediate 2 or higher and patients with more than 10% blasts should be considered for stem cell transplantation. Stem cell transplantation is most successful in patients younger than age 70 with no comorbidity.9
Future Directions
As research addressing the role of apoptosis in MDS continues, future therapies may be aimed at controlling apoptosis, with or without the use of chemotherapeutic agents. Because effective treatment for MDS remains limited, it has been suggested that patients be provided with information on the prognosis for their type of MDS, available therapies, and success rates, and take part in making decisions regarding their treatment. As more is learned about the molecular biology of MDS, it may be possible to develop customized treatment plans for individual patients.92
Summary
• MDSs are a group of clonal disorders characterized by progressive cytopenias and dyspoiesis of the myeloid, erythroid, and megakaryocytic cell lines.
• The dyspoiesis is evidenced by abnormal morphologic appearance and abnormal function of the cell lines affected.
• The WHO classification of MDSs is based on morphologic, molecular, cytogenetic, and immunologic characteristics of blood cell lines.
• Prognosis in MDS depends on several factors, including subtype, percentage of bone marrow blasts, cytopenias, and karyotypic abnormalities.
• Treatment of MDS depends on the prognosis. If the prognosis is favorable, patients may receive only supportive therapy.
• Other treatments that have met with limited success include chemotherapeutic agents and biologic response modifiers.
• Currently, the only cure for MDS is bone marrow or hematopoietic stem cell transplantation.
• Future treatment possibilities include the use of apoptosis-controlling drugs.
Review Questions
1. MDSs are most common in which age group?
2. What is a major indication of MDS in the peripheral blood and bone marrow?
3. An alert hematologist should recognize all of the following peripheral blood abnormalities as diagnostic clues in MDS except:
4. For an erythroid precursor to be considered a ringed sideroblast, the iron-laden mitochondria must encircle how much of the nucleus?
5. According to the WHO classification of MDS, what percentage of blasts would constitute transformation to an acute leukemia?
6. A patient has anemia, oval macrocytes, and hypersegmented neutrophils. Which of the following tests would be most efficient in differential diagnosis of this disorder?
7. A 60-year-old woman comes to the physician with fatigue and malaise. Her hemoglobin is 8 g/dL, hematocrit is 25%, RBC count is 2.00 × 1012/L, platelet count is 550 ×109/L, and WBC count is 3.8 × 109/L. Her WBC differential is unremarkable. Bone marrow shows erythroid hypoplasia and hypolobulated megakaryocytes; granulopoiesis appears normal. Ringed sideroblasts are rare. Chromosome analysis reveals the deletion of 5q only. Based on the classification of this disorder, what therapy would be most appropriate?
a. Supportive therapy; lenalidomide if the disease progresses
c. Bone marrow transplantation
d. Low-dose cytosine arabinoside, accompanied by cis-retinoic acid
8. Which of the following is least likely to contribute to the death of patients with MDS?
9. Into what other hematologic disease does MDS often convert?
10. Chronic myelomonocytic leukemia is classified in the WHO system as: