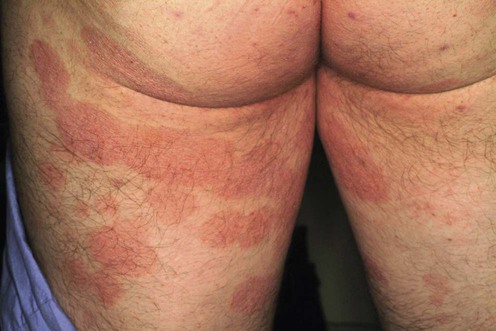
Mycosis fungoides
Diagnosis and staging
Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC).
Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Blood 2007; 110: 1713–22.
Natural history
Specific investigations
 Adequate skin biopsy with histology to be reviewed by a pathologist expert in cutaneous lymphoma. Immunophenotype, and TCR gene analysis are recommended but not essential
Adequate skin biopsy with histology to be reviewed by a pathologist expert in cutaneous lymphoma. Immunophenotype, and TCR gene analysis are recommended but not essential
 A complete physical examination, with assessment of percentage body surface area and pruritus
A complete physical examination, with assessment of percentage body surface area and pruritus
 Comprehensive metabolic panel, LDH, hematology, differential white blood cell count, Sézary cell count (if available) or peripheral blood flow cytometry (preferred), and TCR gene analysis if flow is abnormal or SS is suspected
Comprehensive metabolic panel, LDH, hematology, differential white blood cell count, Sézary cell count (if available) or peripheral blood flow cytometry (preferred), and TCR gene analysis if flow is abnormal or SS is suspected
 Imaging with CT or PET/CT scans is suggested in patients with tumor lesions and palpable lymph nodes
Imaging with CT or PET/CT scans is suggested in patients with tumor lesions and palpable lymph nodes
 Lymph node biopsy with histology, immunophenotype, and TCR gene analysis, if palpable lymph nodes are noted
Lymph node biopsy with histology, immunophenotype, and TCR gene analysis, if palpable lymph nodes are noted


 Emollients
Emollients Topical corticosteroids
Topical corticosteroids UVB or PUVA
UVB or PUVA Topical chemotherapy
Topical chemotherapy Local radiotherapy
Local radiotherapy Topical bexarotene and other retinoids
Topical bexarotene and other retinoids Oral bexarotene and other retinoids
Oral bexarotene and other retinoids Interferons
Interferons Denileukin diftitox
Denileukin diftitox Extracorporeal photopheresis
Extracorporeal photopheresis Low-dose methotrexate
Low-dose methotrexate PUVA ± retinoids
PUVA ± retinoids PUVA ± interferons
PUVA ± interferons Retinoids plus PUVA
Retinoids plus PUVA Interferons
Interferons Total skin electron beam radiotherapy
Total skin electron beam radiotherapy Oral bexarotene
Oral bexarotene Low-dose methotrexate
Low-dose methotrexate Total skin electron beam radiotherapy
Total skin electron beam radiotherapy Vorinostat
Vorinostat Depsipeptide
Depsipeptide Alemtuzumab
Alemtuzumab Liposomal doxorubicin
Liposomal doxorubicin Gemcitabine
Gemcitabine Pralatrexate
Pralatrexate Purine analogs
Purine analogs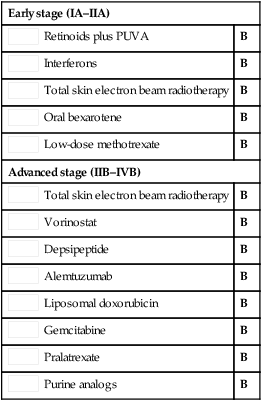
 Clinical trials
Clinical trials Topical bexarotene gel and other retinoids
Topical bexarotene gel and other retinoids Single-agent chemotherapy
Single-agent chemotherapy Combination chemotherapy
Combination chemotherapy Allogeneic bone marrow transplantation
Allogeneic bone marrow transplantation Liposomal doxorubicin
Liposomal doxorubicin Gemcitabine
Gemcitabine Pralatrexate
Pralatrexate Purine analogs
Purine analogs
