Chapter 82 Mycobacterial Infections
Introduction
Ocular mycobacterial infection represents an important form of extra pulmonary infection which encompasses tubercular (TB) as well as nontubercular mycobacterial (NTM) diseases in and around the eye. It presents with diverse clinical manifestations because of a number of factors that are related to the microbe and the host. In spite of recent revolutionary advances in diagnostic technologies, establishing the diagnosis as well as treating the disease are clinical challenges. Mycobacterial disease is known to have affected humans for more than a century and still it continues to be a global health concern. There are several challenges as far as TB is concerned. To list a few, TB stands as the most common opportunistic infection in HIV-positive patients in many developing countries. In 2009, 1.7 million people died from TB, including 380 000 people with concomitant HIV infection, which equates to about 4700 deaths a day.1 Yet another global threat is the emergence of multidrug-resistant (MDR-TB) and extensively drug-resistant strains of tuberculosis (XDR-TB). The World Health Organization has estimated there are 11.1 million patients with tuberculosis worldwide, of which 440 000 cases are due to MDR-TB. XDR-TB cases have been confirmed in 58 countries.1 A second important concern is the emergence of NTM infections, both in immune-competent and immune-compromised individuals in previously unrecognized settings and with new clinical manifestations.2 Clinical manifestations of NTM simulate typical tuberculosis. Lack of better laboratory tools for differentiation, lack of treatment guidelines, and resistance to routine antitubercular treatment challenge the early management of mycobacterial infections.
Pulmonary and extrapulmonary tuberculosis
Tuberculosis is an infection caused by a rod-shaped, non-spore-forming, aerobic bacterium, Mycobacterium tuberculosis. Bacilli spread by small airborne droplets from infected patients. Once the droplet nuclei are inhaled, the bacilli settle in the airways. If the infection is not contained by the immune system, in around 3–8 weeks, local spread and spread to regional lymph nodes in the lungs occur. Subsequent spread to other organs results in extrapulmonary tuberculosis (EPTB). EPTB is reported to be increasing over the last several years.3 Organs affected in EPTB include lymph nodes, pleura, central nervous system, eyes, musculoskeletal system, genitourinary tract and gastrointestinal tract. Symptoms and clinical presentations of EPTB are variable and depend on the organ involved. Unlike pulmonary TB patients, EPTB patients are less likely to present with cough, dyspnea, hemoptysis, abnormal chest X-ray, night sweats, weight loss, anorexia or fatigue. They may present with higher rates of abdominal pain, diarrhea, infertility, monoarticular joint pain or adenopathy depending upon the organ involved. Ocular tuberculosis represents an extrapulmonary dissemination of the bacilli primarily from lungs. However patients with ocular TB may have normal chest X-ray and negative chest complaints; alternatively they may have evidence of other forms of EPTB such as tubercular lymphadenitis. Ophthalmologists have to include appropriate questions in the history and consider extraocular systems whenever a tubercular etiology is suspected. It is essential to rule out EPTB and involvement of other systems in patients who may appear only to harbor pulmonary tuberculosis.
Ocular tuberculosis
Tuberculosis is one of the most common infectious uveitis in tropical countries.4–6 It is either unilateral or asymmetrically bilateral, characterized by a chronic and insidious course. Anatomically, tubercular uveitis may present as anterior, intermediate, posterior or pan-uveitis; it more often presents as granulomatous than nongranulomatous uveitis.
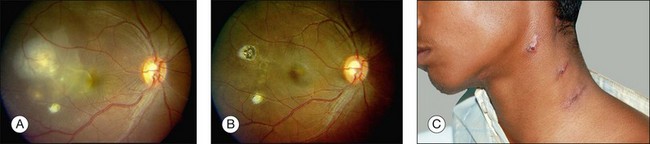
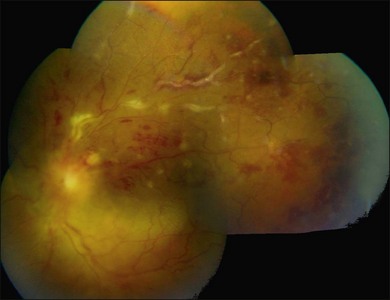
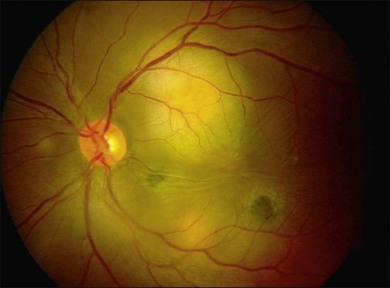
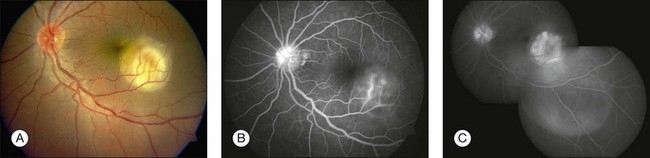
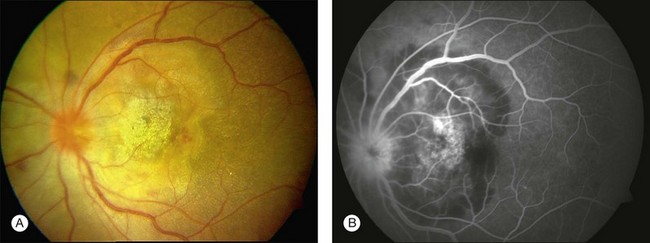
Three classic forms of ocular tuberculosis have been described. Direct ocular infection from an exogenous source may involve ocular adnexa, conjunctiva, sclera or cornea. The second form results from a hypersensitivity reaction to distant foci of infection causing episcleritis, phlyctenulosis, and occlusive retinal vasculitis of the type observed in Eales disease. The third form relates to the hematogenous spread of M. tuberculosis from pulmonary or extrapulmonary sites. The manifestations of this form of ocular tuberculosis are numerous (Figs 82.1 to 82.5), including lupus vulgaris, episcleritis, scleritis, necrotizing scleritis, posterior scleritis, interstitial keratitis, subretinal abscess, optic disc granuloma (Fig. 82.2), choroiditis, chorioretinitis, and choroidal granuloma (Fig. 82.3). Fundus fluorescein angiography may show a well-demarcated choroidal tubercle with initial hypo- and then late hyperfluorescence in such patients. Vessel wall staining, vascular leakage, disc staining and pooling of dye in the area of exudative detachment are other signs commonly seen in fluorescein angiography (Fig. 82.4B,C). Multifocal chorioretinitis with pigmented scars often indicates a tubercular etiology.4 Exudative retinal hemorrhagic periphlebitis (Fig. 82.2) in a patient with uveitis is highly suggestive of tubercular etiology. Healed periphlebitis results in sclerosed venules. Presence of perivascular healed chorioretinal scars in such patients would also suggest tubercular cause. Serpiginous-like choroiditis (SLC) of presumed tubercular etiology closely mimics classic serpiginous choroidopathy (SC) (Fig. 82.5A,B). Patients with SLC, however, are more likely to have multifocal scattered highly pigmented lesions with vitreous cells in contrast to classic SC, which is characteristically seen in the peripapillary area.4,5
Thus tubercular uveitis has a variable presentation, making the clinical diagnosis challenging. To help the clinician, a diagnostic criterion has been recommended. Diagnosis is considered as definitive TB only when the bacilli are isolated from the ocular tissues. The criterion for presumed tuberculous uveitis is reported to be presence of any one of the following clinical signs, such as choroidal granuloma, broad-based posterior synechiae, retinal vasculitis with or without choroiditis, or serpiginous-like choroiditis with a positive tuberculin skin test or QuantiFERON-TB Gold test, or any other relevant tests, such as chest radiograph and computed tomography.4 Good response to anti tubercular treatment and absence of recurrence further supports the diagnosis of presumed ocular tuberculosis.
Differential diagnosis
Granulomatous uveitis may also be seen in patients with herpes simplex or varicella zoster infection, phacoantigenic uveitis, sarcoidosis, syphilis, leprosy, Vogt–Koyanagi–Harada disease and sympathetic ophthalmia. Other causes of choroidal granulomas include syphilis, sarcoidosis, and fungal lesions.4,5
Pathogenesis
Tissue damage in ocular tuberculosis is not only a direct consequence of infection but it is also due to non-resolving inflammation that results from a sensitive balance between protective immunity and destructive pathology.7 Molecular studies and sequencing of the M. tuberculosis genome have identified specific and highly immunogenic antigens. These are the so-called 6 kDa early secretory antigenic target (ESAT-6) and the 10 kDa culture filtrate protein (CFP-10). They are capable of eliciting vigorous helper T-cell responses with resultant cytotoxicity in cellular models. They cause cell lysis and may enable the bacteria to invade and spread within the alveolar epithelium. Recent studies indicate ESAT-6 may also stimulate the trafficking of infected macrophages into the granulomas, to utilize the granulomas as foci of macrophage recruitment, infection, and subsequent bacterial dissemination.
Host genetic factors appear to play a role in disease severity. Abnormalities in the genes encoding the interferon-gamma receptor chain and genes encoding interleukin-12 are important in determining the susceptibility to disseminated mycobacterial disease.8 Pulmonary alveolar macrophages express complement and toll-like receptors and destroy the bacteria when they fuse with lysosomes. However, the mycobacteria can inhibit fusion with lysosomes, and may then thrive in the phagosome. Retinal pigment epithelium (RPE) shares several functions with the macrophages, including expression of toll-like receptors, complement, and phagocytosis of bacteria. Clinicopathologic study combined with molecular analysis revealed distribution of the mycobacteria in the RPE even though the retina and uvea were involved with the inflammatory process. The authors suggest a possibility of reactivation of sequestered organisms in cases of recurrent inflammation.9
Nontuberculous mycobacterial infections
More recently, nontuberculous mycobacterial (NTM) disease appears to be increasing.10 The genus Mycobacterium contains more than 125 species; these saprophytic mycobacteria are ubiquitous in the environment, and may be present in soil, water, and dust particles. Both rapid and slow-growing NTM have been implicated in ocular infection, M. chelonae and M. abscessus are often reported to result in severe and progressive endophthalmitis. NTM endophthalmitis has been reported after uncomplicated phacoemulsification and intraocular lens implantation, laser in situ keratomileusis, endothelial keratoplasty, scleral buckling, and intravitreal injections.11 Difficulty in management is mainly due to delay in the diagnosis and varying sensitivity to antibiotics. In many studies, initial cultures took variable periods to demonstrate growth or failed entirely to show the causative organism. Etiologies were often misdiagnosed as Propionibacterium acnes, nocardial or fungal endophthalmitis.11 NTM are resistant to most conventional antimycobacterial drugs but are sensitive to a variety of other antibiotics. Amikacin, moxifloxacin, and to a lesser extent levofloxacin and ciprofloxacin demonstrate significant effectiveness against NTM.12 Biofilm-forming, nonpigmented, rapidly growing mycobacteria have also been shown to result in endophthalmitis. These organisms show resistance to eradication strategies, resistance to innate host immune responses, and to antibiotics.13 Large gaps still exist in our knowledge on ocular NTM infection. Accurate biomarkers to predict exposure, latency, relapse, and resistant diseases are being investigated and are not yet available for clinical use.
Laboratory evaluation
A complete systemic history and examination are fundamental steps in the evaluation of all patients with ocular tuberculosis and may reveal evidence of pulmonary and other extrapulmonary tuberculosis. Recent advances in diagnostic tools for tuberculous infection, including molecular biology techniques, IFN-γ release assays, and radiodiagnostics have improved the specificity of the diagnosis. However, all these investigations have their own strengths and limitations.14 The suboptimal specificity and sensitivity of existing diagnostic tools delay the diagnosis and treatment of active ocular infection. Clear understanding of their principles and proper test selection are needed to obtain relevant clinical support.
Positive reaction after Mantoux or TST intradermal injection of tuberculin purified protein derivative (PPD) indicate a successful cellular immune response by the patient. The antigen used in TST is a mixture of more than 200 proteins derived from M. tuberculosis. They can cross-react with BCG and NTM antigens, undermining the specificity of the test. Hence TST has limited specificity and a positive result may not confirm disease. The American Thoracic Society and the United States Centers for Disease Control (CDC) consider reactions of 5 mm or more to be positive in very high-risk individuals (e.g., abnormal chest X-ray, HIV patients), 10 mm or more in high-risk patients (e.g., patients from endemic areas), and 15 mm or more in patients with no identified risk factors. In addition, a negative test does not rule out TB. In a study on patients with histopathologically proven ocular tuberculosis, 40% had negative TST results.15
To study the immune response of the patient, novel in vitro tests have been developed using ESAT-6 and CFP-10 proteins (see pathogenesis). Commercially available QuantiFERON (QFT) and TSPOT.TB are commonly known as interferon-gamma release assays (IGRA). T cells, collected from the patient are exposed to these specific tubercular antigens. The assay measures the interferon-γ (IFN-γ) released by sensitized T cells of the patient. The CDC guidelines state that IGRAs can be used in all situations in which the TST is currently used. Canada and United Kingdom national guidelines suggest using IGRAs only to confirm a positive TST.16 Although these tests were originally designed to screen for latent tuberculosis, they have been tested in active systemic and ocular tuberculosis cohorts as well. A meta-analysis on IGRA concludes that the diagnostic sensitivities of both IGRAs are higher than that of tuberculin skin tests and the specificity, lower.17 TST and QFT were found equally predictive of progression to TB disease; QFT was not found superior to TST in a cohort of adolescents in a high systemic TB burden population from South Africa,18 as well as in uveitis.19 The QFT result together with the TST may increase the sensitivity.20 Positive and negative predictive values of IGRA depend on prevalence of TB in that population. As far as systemic TB is concerned, there is increasing evidence that IGRAs perform differently in high burden compared to low burden countries. Because of the low prevalence of TB in the United States, the low pretest probability and low positive predictive value, IGRAs may not be useful as a standard part of the uveitis workup.21 The lack of a gold standard for diagnosis of TB infection prevents an effective and interpretable comparison of the IGRAs and the TST in ocular tuberculosis.21
Radiologic examination of the chest may reveal infiltrations, cavitation, hilar adenopathy, pleural effusion, or fibrotic or calcific lesions in some patients. The predominant route of infection to ocular tissues is by hematogenous spread from the lungs; however, the pulmonary focus may not be evident clinically or radiographically in all patients. In a cohort of histopathologically proven ocular TB patients, 57% of patients had negative chest radiograph results.15 Hence an initial negative workup does not rule out the infection, and repeat workup may be needed. More recently, high resolution computed tomography of the chest was found to be a useful tool in the diagnosis of granulomatous uveitis. Some centers further recommend use of positron emission tomography/CT-guided lymph node identification for biopsy to aid the diagnosis of tuberculosis-associated uveitis.22
Isolation of M. tuberculosis by culture remains the cornerstone for diagnosis; however, isolation from inflamed ocular tissue is not always possible. Whenever it is possible, the specimen is often too small for all procedures, such as Ziehl Neelsen staining, inoculation in liquid and solid media, species identification, and drug susceptibility testing. Biopsy specimens may be obtained for histopathological analysis from either the iris or by retinochoroidal biopsy. The absence of acid-fast bacilli or of caseating necrosis in the biopsy specimen does not rule out tuberculosis. In such circumstances, tests based on nucleic acid amplification are rapid and more specific for M. tuberculosis. Diagnosis based on detection of mycobacterial DNA through PCR and real time PCR is becoming the method of choice because of rapid test results and the ability to test in a very small sample.15
Resistance to anti-TB drugs may be caused by chromosomal mutations in genes encoding drug targets resulting in MDR-TB. Proper management of MDR-TB relies on early recognition of drug resistance. Clinical diagnosis of drug resistance is checked by drug susceptibility testing. It is performed on all positive mycobacterial cultures on Middlebrook 7H10 agar, with 1% proportional method for isoniazid, rifampicin, ethambutol, streptomycin, ciprofloxacin, and kanamycin. Drug susceptibility testing by conventional mycobacterial culture is slow and elaborate, requiring sequential procedures for the diagnosis. Several molecular diagnostic tests, such as the Sanger-based DNA sequencing methods, are expected to lead to faster identification of MDR and XDR strains.23
Treatment
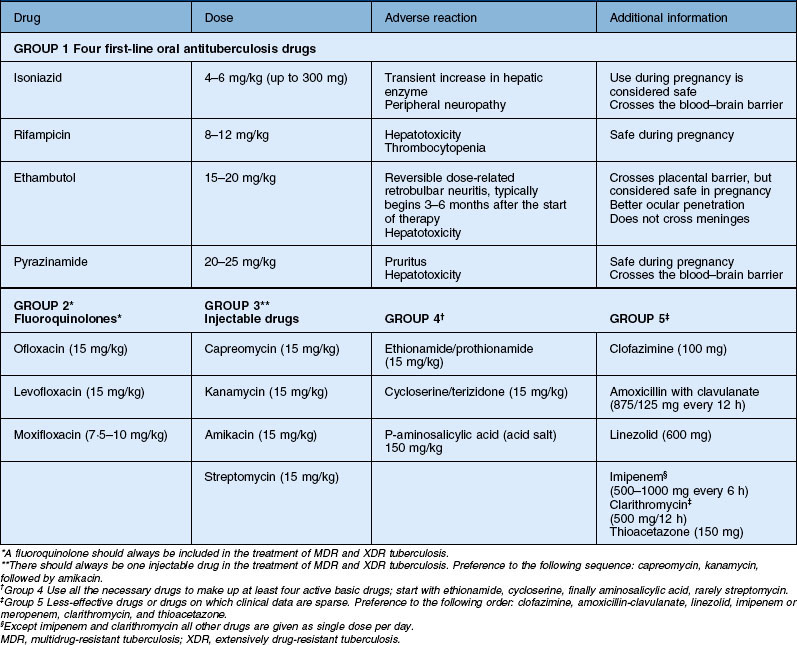
Antitubercular therapy is highly effective in reducing the recurrences of uveitis in patients with manifest TB. The systemic management of TB is complex and requires the input of infectious disease experts. The WHO recommends new patients with both pulmonary TB and extrapulmonary TB to be treated with a four-drug regimen (isoniazid 5 mg/kg/day, rifampicin 10 mg/kg/day, ethambutol 15 mg/kg/day, and pyrazinamide 20–25 mg/kg/day). Ethambutol and pyrazinamide are stopped after 2 months and isoniazid and rifampicin are continued for 4–6 months. Corticosteroids seem to have a potential benefit in patients with tubercular pericarditis and meningitis.24 Similarly, steroids are used in ocular TB as well. More evidence is required on the dose and duration of corticosteroid treatment in ocular infections. In patients with coexisting HIV and systemic tuberculosis, initiation of concomitant antitubercular and antiretroviral therapy may result in exacerbation of inflammation and clinical deterioration.25 Either addition of corticosteroids or delaying the administration of highly active antiretroviral therapy (HAART) for the first 2 months of antituberculosis treatment is advised. Hepatotoxic effects are seen with all first-line antituberculosis therapies and usually present as nausea, vomiting, and abdominal pain in the right upper quadrant. In most patients, even if hepatotoxic effects develop, severe liver injury can be avoided and antituberculosis therapy can be completed by following a comprehensive management algorithm.26 Drug dosage and adverse reactions are summarized in Table 82.1.
Drug-resistant tuberculosis
MDR tuberculosis is defined as the disease caused by organisms that are resistant to isoniazid and rifampicin, which are two first-line anti-TB drugs. XDR-TB is defined as tuberculosis that is caused by organisms resistant to isoniazid and rifampicin and to any one of the fluoroquinolones and at least one of the injectable second-line drugs. MDR and XDR tuberculosis are generally thought to have high morbidity and mortality rates and drug-resistant strains have evolved mainly due to incomplete or improper treatment of TB patients. MDR and XDR-TB are now global threats.1 In the vast majority of clinical settings in the developing world, culture and drug-susceptibility testing are not available. Most cases likely go undetected due to insufficient laboratory infrastructure for diagnosis.27,28 At present, MDR-TB is treated by a combination of 8–10 drugs with therapies lasting up to 18–24 months; only four of these drugs were actually developed to treat TB. Such suboptimal therapy leads to almost 30% of MDR-TB patients experiencing treatment failure. The current situation necessitates the immediate identification of new and more potent drugs.29 A better prognosis can be obtained, however, with the right combination and rational use of drugs chosen in a stepwise selection process through five groups on the basis of efficacy, safety, and cost.30
Detection of drug resistance is possible only in culture isolates, because of challenges in isolating the organisms from the eye; drug susceptibility testing is difficult in ocular TB. This has serious implications, specifically in uveitis. When a trial of antitubercular treatment fails in presumed ocular TB, there is a very high possibility for the uveitis specialist to assume a nontubercular etiology and to start corticosteroids or immunosuppressive treatment to control inflammation.28 In such situations, molecular diagnostic studies may be of help.
The WHO has developed a new six-point Stop TB Strategy addressing the key challenges facing TB control.1 Its goal is to dramatically reduce the global burden of tuberculosis by 2015 by ensuring all TB patients, including those co-infected with HIV and those with drug-resistant TB, benefit from universal access to high-quality diagnosis and patient-centered treatment. The strategy also supports the development of new and effective tools to prevent, detect, and treat TB. If success is achieved and quality care is provided as per the International Standard of TB Care, it is anticipated the incidence of extrapulmonary tuberculosis, including ocular TB, will decline.
1 World Health Organization. Global tuberculosis control 2010. World Health Organization. Available at www.who.int/tb/publications/global_report accessed June 6, 2011
2 Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;15:367–416.
3 Gonzalez OY, Adams G, Teeter LD, et al. Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int J Tuberc Lung Dis. 2003;7:1178–1185.
4 Gupta A, Bansal R, Gupta V, et al. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. 2010;149:562–570.
5 Vasconcelos-Santos DV, Rao PK, Davies JB, et al. Clinical features of tuberculous serpiginouslike choroiditis in contrast to classic serpiginous choroiditis. Arch Ophthalmol. 2010;128:853–858.
6 Rathinam SR, Namperumalsamy P. Global variation and pattern changes in epidemiology of uveitis. Indian J Ophthalmol. 2007;55:173–183.
7 Dorhoi A, Reece ST, Kaufmann SHE. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol Rev. 2011;240:235–251.
8 Krishnan N, Robertson BD, Thwaites G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis (Edinb). 2010;90:361–366.
9 Rao NA, Saraswathy S, Smith RE. Tuberculous uveitis: distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Arch Ophthalmol. 2006;124:1777–1779.
10 Shenai S, Rodrigues C, Mehta A. Time to identify and define non-tuberculous mycobacteria in a tuberculosis-endemic region. Int J Tuberc Lung Dis. 2010;14:1001–1008.
11 Matieli LCV, De Freitas D, Sampaio J, et al. Mycobacterium abscessus endophthalmitis: treatment dilemma and review of the literature. Retina (Philadelphia, Pa). 2006;26:826–829.
12 Caballero AR, Marquart ME, O’Callaghan RJ, et al. Effectiveness of fluoroquinolones against Mycobacterium abscessus in vivo. Curr Eye Res. 2006;31:23–29.
13 Holland SP, Pulido JS, Miller D, et al. Biofilm and scleral buckle-associated infections. A mechanism for persistence. Ophthalmology. 1991;98:933–938.
14 Vasconcelos-Santos DV, Zierhut M, Rao NA. Strengths and weaknesses of diagnostic tools for tuberculous uveitis. Ocul Immunol Inflamm. 2009;17:351–355.
15 Wroblewski KJ, Hidayat AA, Neafie RC, et al. Ocular tuberculosis: a clinicopathologic and molecular study. Ophthalmology. 2011;118:772–777.
16 Schluger NW, Burzynski J. Recent advances in testing for latent TB. Chest. 2010;138:1456–1463.
17 Sester M, Sotgiu G, Lange C, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37:100–111.
18 Mahomed H, Hawkridge T, Verver S, et al. The tuberculin skin test versus QuantiFERON TB GoldH in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS ONE. 2011;6(3):e17984. doi:10.1371/journal.pone.0017984
19 Babu K, Satish V, Satish S, et al. Utility of QuantiFERON TB gold test in a south Indian patient population of ocular inflammation. Indian J Ophthalmol. 2009;57:427–430.
20 Marcus A, Hla MH, Soon-Phaik C. Diagnosis of tuberculous uveitis: clinical application of an interferon-gamma release assay. Ophthalmology. 2009;116:1391–1396.
21 Albini TA, Karakousis PC, Rao NA. Interferon-gamma release assays in the diagnosis of tuberculous uveitis. Am J Ophthalmol. 2008;146:486–488.
22 Doycheva D, Deuter C, Hetzel J, et al. The use of positron emission tomography/CT in the diagnosis of tuberculosis-associated uveitis. Br J Ophthalmol [Internet]. Oct 8, 2010. [cited 2011 May 11]. Available from http://www.ncbi.nlm.nih.gov/pubmed/20935307
23 Campbell PJ, Morlock GP, Sikes RD, et al. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2011;55:2032–2041.
24 Kadhiravan T, Deepanjali S. Role of corticosteroids in the treatment of tuberculosis: an evidence-based update. Indian J Chest Dis Allied Sci. 2010;52:153–158.
25 Rathinam SR, Lalitha P. Paradoxical worsening of ocular tuberculosis in HIV patients after antiretroviral therapy. Eye (Lond). 2007;21:667–668.
26 Senousy BE, Belal SI, Draganov PV. Hepatotoxic effects of therapies for tuberculosis. Nat Rev Gastroenterol Hepatol. 2010;7:543–556.
27 Koul A, Arnoult E, Lounis N, et al. The challenge of new drug discovery for tuberculosis. Nature. 2011;469:483–490.
28 Rathinam SR. Treating uveitis in the developing world setting. Int Ophthalmol Clin. 2010;50:219–228.
29 Rao NA, Irfan M, Soomro MM, et al. Drug resistance pattern in multidrug resistance pulmonary tuberculosis patients. J Coll Physicians Surg Pak. 2010 Apr;20(4):262–265.
30 Caminero JA, Sotgiu G, Zumla A, et al. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–629.