6 Musculoskeletal causes and the contribution of sport to the evolution of chronic lumbopelvic pain
Assessment of the movement system
Lumbopelvic cylinder and chronic pelvic pain
Assessment and rehabilitation of muscles of the lumbopelvic cylinder
Assessment and rehabilitation of muscles of the lumbopelvic cylinder
The neural system and chronic pelvic pain
Sporting activities and chronic pelvic pain
The effect of aerobic exercise on chronic pelvic pain
Cycling and genitourinary symptoms in men and women
Introduction
Although it is generally accepted that movement changes with pain, there is little agreement regarding the processes that underlie movement changes and their relevance to rehabilitation of musculoskeletal pain (Hodges & Moseley 2003, Tsao et al. 2010). Additionally, one of the main difficulties in the diagnosis of pain in the pelvis is the overlap of signs and symptoms in various conditions, including medical conditions not directly related to musculoskeletal injuries (Nam & Brody 2008). As discussed in Chapters 1, 2, 3, 8 and 12, these can include urinary tract infection, prostatitis and other urinary, bowel and sexual dysfunctions. The urological, neurological, muscular, skeletal and fascial systems may all have a role to play to varying degrees, and consideration of each is necessary to provide a more accurate diagnosis, and a more valid paradigm on which to base treatment. Furthermore, as discussed in Chapter 2, pain perceived to be within the pelvis can be referred from the thoracolumbar spine, sacroiliac joint and the hip (Lee 2004), and since the body operates as a complete movement system, each component of the system is capable of influencing distal and proximal regions.
In this chapter, the authors consider the concept of movement as a physiological system, and describe a method of assessment based on analysis of a patient’s movement (dys)function, combined with an assessment of their injury history and pain presentation (Sahrmann 2002). Rehabilitation is then focused upon restoring efficient movement patterns, improving function, and less on treating the pain per se. Manual therapists have traditionally used the client’s pain patterns and physical findings, such as pain provocation tests, to identify structures contributing to mechanical pain. However, in the presence of central sensitization and neurogenic pain mechanisms, the reliability of such a pathoanatomical assessment to identify the sources of pain can be limited, particularly as the relationship between pain and the state of the tissues becomes weaker as pain persists (Moseley 2007). Classification of movement patterns, used to identify the mechanical causes of pain, may be similarly limited in the presence of central sensitization, where any mechanical stimulus may be sufficient to generate a painful response. At all times then, current pain physiology, and other contributing factors to chronic pelvic pain (CPP), as discussed in Chapters 1, 2 and 3, need to be considered when assessing the movement system. The role of clinical reasoning is further described in Chapter 7.
Assessment of the movement system
As discussed in Chapters 1 and 2, the movement system is modulated by many factors from across somatic, psychological and social domains (Moseley 2007). It is recognized that the nervous system is likely to coordinate muscle activity to meet the demands for stable movement, so it will not only be affected by the task, posture or movement direction, but potentially the real or perceived risk of injury (Hodges & Cholewicki 2007). Motor changes have been documented to occur throughout the movement system, at the motoneuron level and coordination of muscle behaviour, to changes in organization of the motor cortex (for a review see Tsao et al. 2010). Strategies adopted during pain and injury can increase protection of injured or painful parts, but can have mechanical consequences that may prolong pain states, or result in a higher incidence of recurrence (Hodges & Moseley 2003, van Dieen et al. 2003). In controlled clinical trials, rehabilitation of these motor changes has been linked to clinical improvement, resulting in improvements in pain and dysfunction (Cowan et al. 2003, Ferreira et al. 2006). More recently such improvements have also been shown to be associated with recovery of plastic changes at the motor cortex (Tsao et al. 2010).
Various classifications for the analysis of movement and the sub-classification of movement dysfunction have been proposed (McGill 2002, Sahrmann 2002, McKenzie & May 2003, O’Sullivan 2005) and in some instances are gaining good evidence of validity and reliability (Van Dillen et al. 1998, 2003, Dankaerts et al. 2006, Harris Hayes et al. 2009, 2010). It is beyond the scope of this chapter to describe the benefits and limitations of each classification system; however it is reasonable to suggest that the multidimensional problem of chronic low back and pelvic pain should also encompass biopsychosocial principles (Linton 2000, Waddell 2004, Woby et al. 2004, 2007) which are discussed in Chapter 7. The following section describes the evaluation, classification, and subsequent rehabilitation of lumbopelvic movement disorders based upon the work of Sahrmann (2002).
Sahrmann (2002) suggested a classification system for the analysis of lumbopelvic movement and the subsequent prescription of treatment based on clinically assessed movement system dysfunction. A central tenant of the system is that ‘faulty movement can induce pathology, not just be a result of it, and musculoskeletal pain syndromes are seldom caused by isolated events but are the consequence of habitual imbalances in the movement system’ (Sahrmann 1993). Therefore, specific postures and movements that produce pain need to be identified and corrected, as misalignment and aberrant movement patterns might result in further pain and future recurrences (Van Dillen et al. 2003).
The objective examination has two major components:
1. The patient reports the response of symptoms to the movement pattern tested, i.e. whether there is an increase or decrease in symptoms;
2. Assessment of bony and/or joint alignment, in various positions, as outlined in Table 6.1.
Table 6.1 Items from original examination (Van Dillen et al. 1998) proposed as important for classification of mechanical low back pain organized by test position, symptom behaviour with variations of the test position or movements within the test position, and clinical judgements of quality of alignment or movement ![]()
| Test position | Symptom behaviour with | Judgement of alignment or movement |
|---|---|---|
| Standing |
* A judgement of relative flexibility refers to a judgement made by the examiner about the relative timing of movement of the lumbar region and the proximal joints when the patient performs a trunk or a limb movement. In general, a patient exhibits a relative flexibility impairment if the lumbar region moves in the first 50% of the range of the overall movement or excessively during the overall movement.
† Corrected test items are follow-up items in which the lumbar region is repositioned to achieve a neutral alignment, or movement of the lumbar region is restricted relative to what was observed with the previous symptomatic test item. The effect of the changes in alignment and movement on symptoms is assessed.
‡ Because rotation and side-bending are coupled motions in the lumbar region, items that refer to judgements of alignment and movement of lumbar region rotation or pelvic rotation include side-bending alignment or movement.
There are several unique components to this examination system:
1. The effect of active limb movements on spinal movements and symptoms;
2. The relative timing of movements of the spine and proximal joints during limb and trunk movements;
3. The effect on symptoms of modifying lumbar alignment or movement during repetition of a previously symptomatic test (Van Dillen et al. 2003).
The information obtained from this assessment allows the patient to be categorized into one of five different categories, named after the type of mechanical factors hypothesized to be factors in the contribution of mechanical low back pain (MLBP) (Van Dillen et al. 2003):
Although the classifications are termed lumbar movement impairments, the analysis is technically of the lumbopelvic region. Additionally as discussed in Chapters 1, 2 and 3, not only do proximal regions of dysfunction affect distal segments of the movement system, the lumbar spine refers pain to the pelvis and hip and CPP definitions involve the pelvis, anterior abdominal wall, lower back and/or buttocks (ACOG 2004, Fall et al. 2010).
The musculoskeletal factors that need to be considered include the passive and active stiffness of the lumbopelvic spine and hips which will be influenced by muscle and fascial systems, all under neuronal control. Assessment of the neural system is therefore also critical, as irritability of, and the ability of the nerve trunk to glide along its neural canal, will affect the perceived length of the muscle (Hall et al. 1998; Walsh et al. 2009). Structural differences such as bony variation of the pelvis and femur, imbalances of strength, length, timing and magnitude of recruitment of the trunk and hip muscles will also need to be assessed during test movements, as well as specific functional activities.
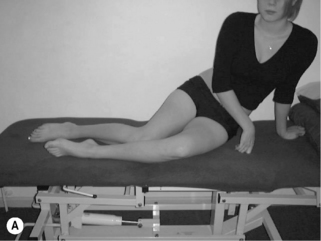
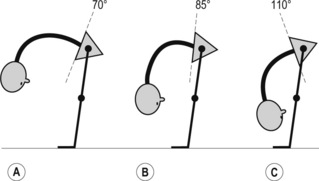
1. Lumbar flexion syndrome: the primary dysfunction in this syndrome is that the lumbar spine range of motion into flexion is more flexible than the hip range of motion into flexion. The habitual movement of the individual is towards flexion with spinal movements and movements of the extremities. The spinal alignment tends to be relatively flexed in different postures (Figure 6.1) and symptoms are elicited and/or aggravated when the patient moves towards the flexed position. When movement into flexion is restricted the symptoms are abolished or diminished. The syndrome is more common in individuals between the ages of 18 and 45 and is more common in males. Habitually flexed work postures or repeated forward bending are often reported, and there is increased relative stiffness in the hip extensors (particularly the hamstrings) relative to the erector spinae (Sahrmann 2002).
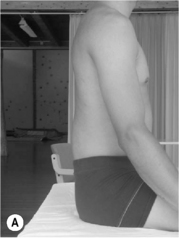
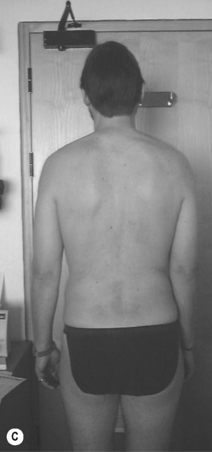
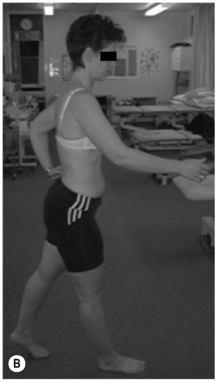
2. Lumbar extension syndrome: the primary dysfunction in this syndrome is that the lumbar spine range of motion into extension is more flexible than the hip range of motion into extension. Functionally this means that the lumbar spine can extend more readily than the hip extensors can extend the hip (Sahrmann 2002). The hip flexor muscles therefore exert an anterior shear force on the lumbar spine, as well as a forward rotation moment on the innominates bilaterally. The patient’s habitual movement is towards extension with spinal movements and movements of the extremities (Figure 6.2). When movement into extension is restricted the symptoms are abolished or diminished (Sahrmann 2002). The patients are usually over 55 years of age, and there is no reported gender bias. This pattern is often found in patients with acute recurrent low back pain or chronic ongoing low back pain (Sahrmann 2002).
3. Lumbar rotation syndrome: this syndrome describes a three-dimensional dysfunction of a spinal segment. The primary dysfunction is increased lumbar segmental rotation, side-flexion and translation, relative to other spinal segments (Sahrmann 2002) (Figure 6.3). These three motions occur together, due to the complex interaction of the shape of the Z-joint articular surface, the control of the ligamentous restraint systems, and the flexibility of segmental and global muscle system (White & Punjabi 1990). Clinical spinal instabilities usually involve increased arthrokinematic glides, and fit into this group. The rotational stress on the lumbar spine can be produced directly via lumbar spinal rotation/side-flexion/translation or, indirectly, rotation of the pelvis can produce a relative rotation of the lumbar spine segments (White & Punjabi 1990). In certain patients there is an observable rotation of the lumbar spine whilst in others there is no obvious dysfunction (Sahrmann 2002.) The signs and symptoms are similar to those described for lumbar flexion syndrome; however, they are elicited with spinal rotation, and diminished when lumbar rotation is restricted (Van Dillen et al. 2003).
4. Lumbar rotation with flexion: in response to spinal or extremity movement, the patient moves the lumbar spine in the direction of rotation and flexion. The lumbar spine tends to assume a flexed and rotated position in habitual postures. Symptoms are often unilateral, are aggravated by postures involving flexion and rotation and are eased by limitation of flexion and rotation. Symptoms are often aggravated when sitting to standing and functional movements that involve more than one plane (Sahrmann 2002).
5. Lumbar rotation with extension: in response to spinal or extremity movement, the patient moves the spine in the direction of rotation with extension. The lumbar spine tends to assume an extended and rotated position with habitual postures. Symptoms are often unilateral and elicited by extension rotation movements of the lumbar spine (Sahrmann 2002). This syndrome is more common in patients over the age of 55 and with a history of chronic low back and pelvic pain. It is also more common in those individuals who participate in sports that involve significant amounts of torsion in the lumbar spine, e.g. golf, squash, tennis and racquetball (Harris-Hayes et al. 2009).
Common postural types (see Kendall et al. 2005, Sahrmann 2002)
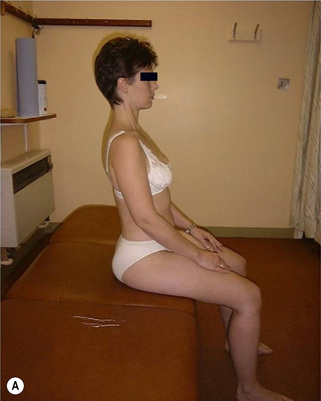
Ideal standing posture (Figure 6.4) can be described as a position where:
• A marker on the lateral tip of the acromion, the midpoint of the greater trochanter, the tip of the lateral malleolus, all line up to form an angle of approximately 180°
• The lumbar lordosis, thoracic kyphosis, and pelvic tilt are in neutral
• Lumbar neutral: the spinal curve should be 20–30°, there should be less than 1.5 cm (1/2″) difference in prominence of the (L) and (R) region 5 cm (2″) lateral from the spinous process
• Pelvis neutral: the line between anterior superior iliac spine (ASIS) and posterior superior iliac spine (PSIS) is within 15° of horizontal line (may vary in women). No lateral tilt or rotation
• Hip neutral: no angle between peak of iliac crest, to greater trochanter to line along thigh
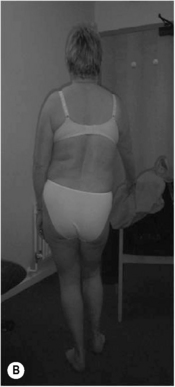
Kyphotic lordotic standing posture (see Figure 6.2B) can be described as a position where:
• A marker on the lateral tip of the acromion, the midpoint of the greater trochanter, the tip of the lateral malleolus, all line up to form an angle greater than 195°, the back is swayed back, the hips swayed forward
• Lumbar lordosis: inward curve increased in depth, greater than 30°
• Thoracic kyphosis: often increased in depth
• Pelvis anterior pelvic tilt: ASIS is 20° lower than PSIS
• Hips flexed: Hip angle of flexion, between peak of iliac crest to greater trochanter, to line along thigh is more than 10°
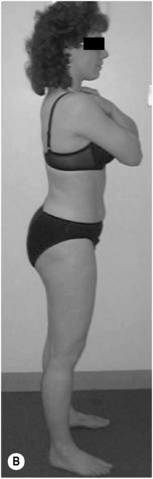
Sway standing posture (Figure 6.5) can be described as a position where:
• A marker on the lateral tip of the acromion, the midpoint of the greater trochanter, the tip of the lateral malleolus, all line up to form an angle less than 165°; the back is swayed back or the hips swayed forward
• Lumbar lordosis: often decreased in length.
• Thoracic kyphosis: often increased in length, shoulders more than 5 cm posterior to greater trochanter
• Pelvis neutral to posterior pelvic tilt: for posterior pelvic tilt, ASIS is 20° higher than PSIS
• Hips extended: hip angle of extension, between peak of iliac crest, to greater trochanter to line along thigh is more than 10°
• Knees hyperextended: backward bowing of knee joint; tibia may be posterior to femur
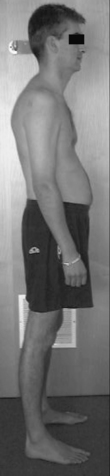
Flat back standing posture (Figure 6.6) can be described as a position where:
• A marker on the lateral tip of the acromion, the midpoint of the greater trochanter, the tip of the lateral malleolus, all line up to form an angle of approximately 180°
• Lumbar lordosis: flat, absent inward curve (may be normal in men)
• Thoracic kyphosis: flat, absent outward curve
• Pelvis neutral to posterior pelvic tilt: for posterior pelvic tilt, ASIS is 20° higher than PSIS
• Hips neutral to extended: hip angle of extension, between peak of iliac crest, to greater trochanter to line along thigh is more than 10°
• Knees neutral to hyperextended: Backward bowing of knee joint, tibia may be posterior to femur
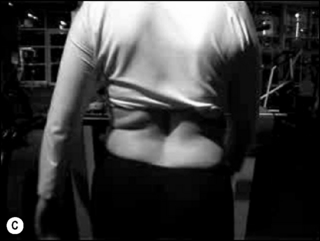
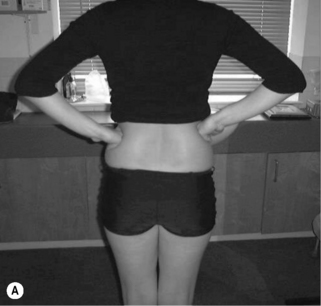
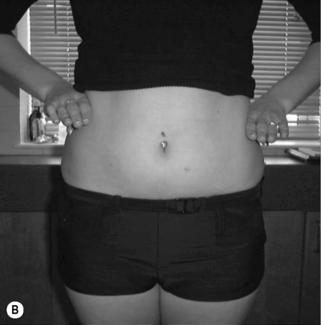
Asymmetry (Figure 6.7): any of the postural shapes above with:
• Paraspinal asymmetry: (L) and (R) paraspinal regions from lumbar spinous processes to 5 cm lateral are greater than 1.5 cm difference in prominence
• Scoliosis/rib hump: ribs more prominent on one side
• Lateral tilt: one iliac crest is more than 1.5 cm higher than other iliac crest (Figure 6.7A,B)
• Rotation: ASIS on one side is anterior to ASIS on other side
• Lateral shift: the pelvis is shifted away from the midline (Figure 6.7C)
Table 6.2 shows the proposed MLBP categories with the associated signs, symptoms and general treatment guidelines. Although it should be stressed that this movement assessment system has not yet been fully validated, the construct validity of the movement impairment-based classification system provided support for three out of the five categories (Van Dillen et al. 2003) and recently the inter-tester reliability based on this classification system has been shown to be substantial (Harris-Hayes & Van Dillen 2009).
Table 6.2 Proposed mechanical low back pain categories with associated signs and symptoms and general treatment guidelines (taken from Van Dillen et al. 2003)![]()
1. Bed positioning/mobility: Position a pillow(s) under your knees when back lying or under your abdomen when face lying. Slide your legs up so your hips and knees are bent. Avoid allowing your back to arch when you move your legs. Roll to your side moving your trunk and legs together. Drop your legs over the side of the bed as you push yourself up to sitting or lower yourself to side lying
2. Sitting: Sit with your back and feet supported and your hips and knees level. Relax your back against the chair. Don’t sit on the edge of the chair
3. Sit to stand: Come forward in chair by pushing with your hands while keeping your back slightly rounded. Lean forward. Push with your legs. As you straighten up don’t arch your back; pull in your abdominals. Lean forward when you lower yourself into the chair to sit
4. Standing: Occasionally lean up against a wall with your knees and hips bent slightly. Pull in your abdominals and relax your back against the wall
Exercise:
1. Training of trunk muscles to work isometrically with performance of limb movements (particularly abdominals)
2. Training to perform hip extension without increased lumbar region extension (e.g. perform return-from-forward bending emphasizing hip extension over lumbar extension)
3. Exercise to increase flexibility of any muscles contributing to lumbar region extension (e.g. bend knee in prone while keeping lumbar region stationary to stretch hip flexors)
4. Exercise to shorten muscles that may assist in reducing lumbar spine extension alignment (e.g. pull in lower abdominals while standing with back against wall and knees and hips flexed slightly)
Symptoms are decreased with restriction of lumbar rotation
1. Bed positioning/mobility: When side lying, place a pillow(s) between your knees and a towel roll in the area between your ribs and pelvis on the side you are sleeping. Slide your legs up so your hips and knees are bent. Roll to your side moving your trunk and legs together. Drop your legs over the side of the bed as you push yourself up to sitting or lower yourself to side lying. Don’t side bend or rotate your trunk as you get up or down from bed
2. Sitting: Sit with your back supported. Don’t rotate or side bend your trunk to one side. Don’t lean on an elbow for support. Don’t cross your legs or sit on one leg. Don’t shift from side to side as you sit for prolonged periods
3. Sit to stand: Don’t move forward by rotating one hip forward at a time
4. Standing: Don’t stand on one leg. Stand with your weight evenly distributed over both legs
Exercise:
1. Training of trunk muscles (particularly lateral abdominals) to work isometrically with performance of limb movements (e.g. lift one arm in quadruped while holding trunk stationary)
2. Training to isolate hip rotation and hip abduction and adduction without lumbar region rotation or side bending (e.g. laterally rotate and abduct hip in side lying while holding trunk stationary)
3. Exercise to increase flexibility of any muscles contributing to lumbar region rotation or side bending (e.g. laterally rotate one hip in prone while holding pelvis stationary)
4. Exercise to shorten muscles contributing to lumbar region rotation or sidebending on one side (e.g. laterally rotate and abduct a hip in side lying with pillows between the knees while holding pelvis stationary)
Support:
Lumbar spine alignment tends to be flexed and rotated relative to neutral with the assumption of postures. Symptoms (often unilateral) occur or increase with positions and movements associated with rotation and flexion of the lumbar spine. Symptoms are decreased with restriction of lumbar rotation and flexion
Symptoms (often unilateral) are decreased with restriction of lumbar rotation and extension
The aim of the assessment is to identify the postural alignment strategies and the habitual movement patterns which may contribute to the individual’s presenting condition. Each syndrome has specific key tests, which help identify the alignment strategies assumed by the individual and confirm the direction of the movement patterns. The patient is then observed during performance of symptom-provoking functional activities, to determine if the same strategies are repeated. Functional instruction is then directed toward modifying the patient’s preferred strategies. Exercise prescription is directed toward correcting the patient-preferred movement and alignment strategies identified on examination. Emphasis is on modifying the strategies that (1) are symptom-provoking and (2) can be modified to decrease the patient’s symptoms during the examination (Van Dillen et al. 2003). This systematic approach is repeated in order to classify movement impairment syndromes of the hip and is described later in this chapter (Table 6.3). It should also be emphasized that, in the authors’ opinion, if the client holds negative beliefs and attitudes associated with pain, such as exhibiting fear-avoidance behaviours without an understanding of current pain biology, the way in which these corrective exercises are explained could contribute to their fear of movement and potentially make their pain problem worse. In this instance, addressing the patients’ attitudes, beliefs and understanding needs to be the therapists’ main priority (O’Sullivan 2005).
Lumbopelvic cylinder and chronic pelvic pain
In the presence of postural changes, respiratory demands, lumbopelvic pain (LPP) and stress incontinence, muscles of the lumbopelvic cylinder (LPC) are altered (Hemborg et al. 1985, Hodges & Richardson 1996, 1998, Hides et al. 1996, Moseley et al. 2002, Hodges & Gandevia 2000, O’Sullivan et al. 2002, Jones et al. 2006, Hodges et al. 2007, Dickx et al. 2008). However, it appears that skilled voluntary activation of muscles with altered motor recruitment can reduce pain, disability and recurrence rate for musculoskeletal conditions (Hides et al. 2001, Cowan et al. 2003, Ferreira et al. 2006), restore motor co-ordination including automatic postural adjustments (Cowan et al. 2003, Tsao & Hodges 2007, 2008), reversing cortical reorganization in people with recurrent pain (Tsao et al. 2010). These findings suggest then, that in addition to the five categories of movement dysfunction described above, muscles of the LPC should be evaluated and rehabilitated, when appropriate, in patients with CPP, although to date there is no scientific trial that has evaluated this clinical approach in this sub-group of chronic pain patients.
The pelvic floor muscles (PFM) form the bottom of the LPC, with the respiratory diaphragm its top, and transversus abdominis (TrA) the sides. The spinal column is part of this cylinder and runs through the middle, supported posteriorly by segmental attachments of lumbar multifidus and anteriorly by segmental attachments of psoas (posterior fasciculii) to the abdominal muscles (Jones 2001). Intra-abdominal pressure (IAP) is generated by co-activation of the PFM, the diaphragm and the abdominal muscles (Hemborg et al. 1985, Hodges & Gandevia 2000), implying that co-ordinated co-activation of the muscles of the LPC is necessary in order to balance the functional demands of continence, respiration and lumbopelvic stability (Hemborg et al. 1985, Hodges & Gandevia 2000, Pool-Goudzwaard et al. 2004, Hodges et al. 2007). Muscles of the LPC work at low levels at all times and increase their activity when the central nervous system can predict timing of increased load, such as occurs in coughing, lifting or limb movements (Constantinou & Govan 1982, Moseley et al. 2002, Barbic et al. 2003). Further details regarding the diaphragm (Chapter 9) and the PFM (Chapter 13) will be found in the appropriate chapters, while the next section describes the assessment and rehabilitation of muscles of the LPC.
Assessment and rehabilitation of muscles of the lumbopelvic cylinder
Voluntary activation of TrA independently from other trunk muscles (Richardson et al. 1999)
• Palpate approximately two fingers’ breadth down from the anterior superior iliac spine (ASIS) along the inguinal ligament, and one finger’s breadth medially
• Ask the patient to breathe in, then on the out-breath to relax and let go of the stomach. Ask the patient to then draw in the low lateral abdominal wall (below the umbilicus) as if to pull the stomach away from the pant (knicker) elastic and breathe normally (Figure 6.8)
• Palpate for a symmetrical tensioning, without excessive bulging, underneath the palpating fingertips, or verify with real-time ultrasound imaging to observe thickening and shortening of the abdominal muscle (Hodges et al. 2003)
• Once the patient can activate TrA with minimal activity of the abdominal muscles, they should hold the contraction, whilst breathing normally, for up to 10 seconds
• Three sets of ten repetitions, twice a day, has been shown to be effective (Tsao & Hodges 2008)
Common substitution patterns or faults
• Movement of the trunk or pelvis out of a neutral position
• Breath holding, apical breathing
• Drawing in of the upper abdominal wall
• Rib cage depression with a decrease of the infrasternal angle
• Lateral flaring or bulging of the waist
• Excessive superior, inferior or lateral movement of the umbilicus
Facilitation techniques for TrA
• Alternative starting positions such as crook lying, 4-point kneeling, side lying, standing, sitting forward lean, prone
• Visualize the lower abdomen as an old-fashioned corset, and as the stays are drawn in, the waist narrows and flattens, into an hourglass shape
• Ensure that the abdomen is relaxed to start with, often easier in sitting or standing forward lean, 4-point kneeling, prone
• Tactile feedback: ask the patient to put one hand above the umbilicus and one hand below, as soon as movement is felt above the umbilicus, drawing in should cease. Tactile feedback: ask patient to palpate you, noting the difference between bulging and tensioning, then to self-palpate
• Facilitation using the PFM which can be particularly useful for asymmetry of contraction (Chapter 13) as can psoas activation (described later)
Assessment and rehabilitation of muscles of the lumbopelvic cylinder
Voluntary activation of pelvic floor muscles (Laycock & Jerwood 2001, Messelink et al. 2005)
Activation and facilitation of PFM
• In a supine, crook lying position with a pillow under the head and legs supported, the International Continence Society (ICS) guidelines to facilitate a PFM contraction are ‘squeeze the muscles of the pelvic floor as if attempting to stop the flow of urine or prevent wind or flatus escaping’ (Messelink et al. 2005)
• Recent research and clinical experience have suggested that the addition of ‘squeeze around the back passage, as if you were trying to prevent breaking wind (flatus), bring that feeling forward towards the urethra/pubic bone and then lift, as if you were elevating the PFM, whilst breathing normally’ elicits a consistent PFM contraction, resulting in a cranioventral lift of the PFM and pelvic organs (Lovegrove Jones 2010)
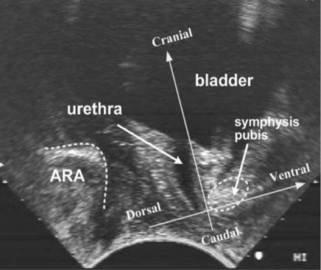
• With consent, confirm the ability of the volunteer to perform an elevating, sustained PFM contraction by vaginal or rectal palpation, real-time perineal (Figure 6.9) or transabdominal ultrasound (Figure 6.10). The patient should have a sensation of a ‘lifting’ rather than ‘bearing down’ contraction, which can also be verified by observation of the perineum ‘lifting’. If verification is not possible in any of the ways described, palpating abdominally, approximately two fingers’ breadth down from the ASIS along the inguinal ligament and one finger’s breadth medially, as in the evaluation of a TrA contraction above, will give an indication of whether co-activation of TrA and the PFM has occurred. Recent evidence has indicated the presence of co-activation in healthy continent and incontinent women (Sapsford et al. 2001, Urquhart et al. 2005, Jones et al. 2006)
• Once the patient can activate the PFM they should hold the contraction whilst breathing normally for up to 10 seconds. The co-ordination of a pelvic floor contraction and normal relaxed breathing seems to be very difficult for some subjects. If this is the case then repeated practice is advised, using low effort. The ability to voluntarily relax the PFM after contraction (Messelink et al. 2005), particularly with PFM overactivity, is essential and the presence of pain on voluntary contraction should be noted (see Chapter 13)
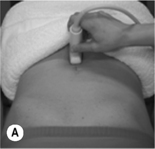
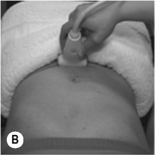
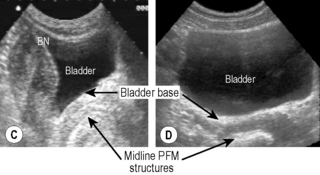
Figure 6.10 • Transabdominal ultrasound transducer placement for (A) sagittal and (B) transverse ultrasound imaging of the bladder. Reproduced from Whittaker (2007) Ultrasound Imaging for Rehabilitation of the Lumbopelvic Region: A Clinical Approach. Typical sagittal (C) and transverse (D) plane transabdominal view of the bladder, bladder base and midline pelvic floor (PF) structures. BN, bladder neck
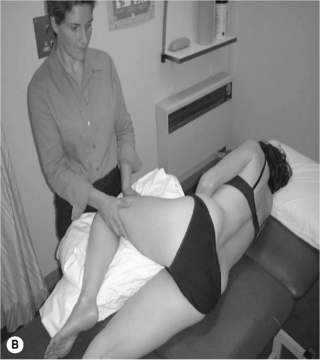
Voluntary activation of deep segmental lumbar multifidus (Richardson et al. 1999)
• In prone lying (often over a pillow is helpful), palpate the medial paraspinal muscles at each segmental level in turn, just to the side of the lumbar vertebrae and let them sink firmly into the muscle
• Instruct the patient to take a breath in, then on the breath out to relax and let go of the stomach. Slowly and gently hollow and pull up the lower stomach towards the spine, and gently swell the muscles into the palpating fingertips
• Palpate for a symmetrical tensioning, underneath the palpating fingertips or verify with real-time ultrasound imaging to observe thickening of the muscle (Hides et al. 1992)
• Once the patient can activate multifidus they should hold the contraction whilst breathing normally for up to 10 seconds
Facilitation techniques for multifidus
• Visualization of the muscle as deep triangles that extend down and out from each of the spinous processes
• Instruct the patient to feel the contraction on you first, to understand the concept of an isometric swelling contraction
• Other starting positions such as side lying, standing or sitting
• In standing, move from a position of upright standing to sway, palpating the differences in tension of the medial paraspinals
• In standing, palpate the dysfunctional multifidus with one hand and lift the opposite arm forward and away from the body from 0° to 90°, palpating the superficial fibres changes in tension. Sustain the contraction when multifidus activity decreases. Maintain active muscle tension during slow, repetitive arm flexion. Maintain this multifidus contraction and keep tension in the muscle during slow arm movements with relaxed breathing (Figure 6.11A)
• In walk stance, with full weight on the rear foot, palpate the tension in the dysfunctional multifidus muscle on the rear foot side, and move the body weight forward onto the front foot. Multifidus should activate just after heel lift. Try to sustain the contraction during slow weight transfer with relaxed breathing (Figure 6.11B)
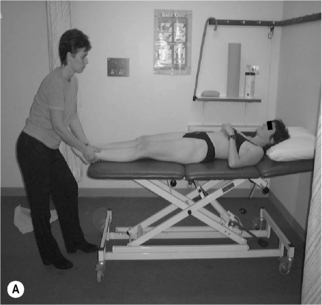
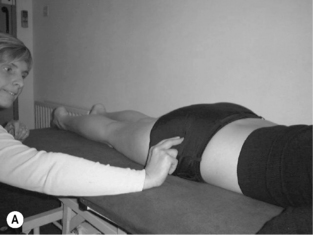
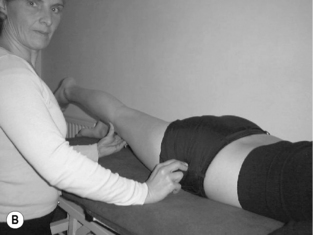
Voluntary activation of the posterior fasciculii of psoas (Gibbons et al. 2002)
Activation and facilitation of the posterior fasciculii of psoas
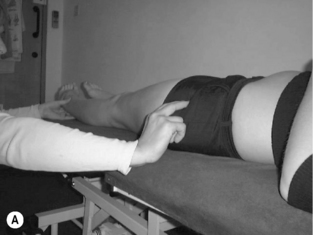
• With both the lumbar spine and hip in neutral, gently distract the femur in supine or side lying (Figure 6.12). Maintain the distraction and instruct the patient to take a breath in, then on the out-breath to relax and let go of the stomach, whilst slowly and gently drawing in or shortening the hip into the acetabulum without moving the pelvis or lumbar spine. It may be helpful to push the femur into the acetabulum several times to give the sensation of the action required
• Then palpate both the anterior superior iliac spine (ASIS) and the soft tissue below in the anterolateral groin (5 cm below the ASIS). The upper finger assesses for control of movement of the pelvis and maintenance of the lumbopelvic neutral position while the lower finger checks for excessive activity in rectus femoris and sartorius
• Once the therapist is confident that the patient is able to activate the muscle, the patient lies in prone on the plinth with one leg firmly extended to help maintain balance, and the other leg hanging freely over the edge with the foot on the floor. The side with the leg hanging freely is the side to be assessed. In pelvis and lumbar neutral with the trunk muscles relaxed, each lumbar vertebral level is manually palpated to assess the relaxed joint play displacement in the transverse and anterior directions. When the patient then facilitates the psoas contraction, each vertebral level is repalpated to assess the contracted joint play in the transverse and anterior directions. Ideally, there should be a significant palpable increase in resistance to manual displacement (stiffness) at each level
Common substitution patterns or faults
• Movement of the trunk or pelvis out of a neutral position
• Pushing down with the contralateral leg to provide stability for the trunk
• Dominant or excessive activation of rectus femoris, tensor fascia lata or trunk muscles indicated by a resistance to passive rotation of the hip or pelvis. Suggest that the patient uses less effort
Integration of voluntary activation of the lumbopelvic cylinder into function
When muscles of the LPC are working efficiently, voluntary and involuntary activation of any one muscle, should elicit a co-contraction with the others. Although this has been shown with TrA, multifidus and the PFM (Sapsford et al. 2001, Richardson et al. 2002, Urquhart et al. 2005, Jones et al. 2006), to date this has not been scientifically verified with posterior fasciculii of psoas muscle. However, in the authors’ clinical opinion, particularly when asymmetry of contraction is observed in the PFM or TrA contraction, using the methods to elicit a psoas contraction has been effective to facilitate activation (of TrA or PFM) on the deficient side. Once the patient is able to elicit a satisfactory co-contraction of the muscles of the LPC, the activation of these muscles should be incorporated into previously aggravating static postures and functional tasks (O’Sullivan et al. 1997). The ability to immediately perform a task without pain with the addition of a voluntary activation of any of the muscles of the LPC can be a powerful rehabilitation tool, significantly reduce fear of movement, and change attitudes and beliefs about what the pain means to the patient. However, skilled training of sustained voluntary activation of TrA in supine, without integrating into function, was sufficient to reduce pain, disability and create reorganization of the motor cortex in individuals with recurrent low back pain (Tsao & Hodges 2008, Tsao et al. 2010).
The neural system and chronic pelvic pain
As discussed in Chapters 2, 3 and elsewhere, the nervous system is likely to coordinate muscle activity to meet the demands for stable movement, so it will not only be affected by the task, posture, movement direction, but potentially by high real, or perceived, risk of injury (Hodges & Cholewicki 2007). The assessment of the relative stiffness of the global pelvic and hip musculature and muscles of the LPC will therefore allow the current relative stiffness between the proximal and distal movement systems to be evaluated, so that treatment can then be targeted at the specific dysfunction. Another potential confounding variable in the assessment of muscle length and relative stiffness is the range of motion of joints associated with the muscle (Kendall et al. 2005). Hence in order to accurately assess the length of a muscle, knowledge of the underlying joint range of motion is essential. Muscle tissue consists of both contractile and non-contractile components, and a shortened muscle may be due to decreased length in the non-contractile component, increased tone in the contractile component, or neurogenic/neuropathic components. As mentioned earlier, assessment of the neural system is crucial to the full understanding of pelvic dysfunction as the ability of the nerve trunk to glide along its neural canal, will influence the perceived length of the muscle (Hall et al. 1998, Walsh et al. 2009). As described in Chapter 2, there are a number of significant nerves around the pelvis which are associated with CPP, and some of them are amenable to direct assessment, such as the sciatic, femoral and pudendal nerves. For example in lumbar-pelvic flexion dysfunction, the hips will be relatively stiff in flexion compared to the lumbopelvic spine. Whether this is due to, for example, short, stiff hamstrings, long erector spinae, or an irritable sciatic nerve will need to be assessed by the examiner. Similarly, in lumbopelvic extension dysfunction, one or more of the hip flexors could be stiff relative to the abdominal muscles, or the client may have restricted hip motion due to joint degeneration, or the femoral nerve may also restrict hip extension, and will need to be evaluated. To differentiate muscle stiffness from increased neural mechanosensitivity limiting the apparent length of a muscle, the use of differentiation via slump testing, cervical spine movement or ankle dorsiflexion will be essential (Hall et al. 1998, Walsh & Hall 2009, Walsh et al. 2009).
Additionally, as discussed in Chapter 14, patients with CPP experience changes in the fascial system which may result in restrictions of neural or soft tissue movement. Assessment of this system will rely upon skilled observation of the tissues surrounding the pelvic region: especially the areas of the anterior thigh, inguinal region, anterior abdominal and trunk region, posterior trunk region, peri-perineal area and transperineal area. Clinical reasoning is explored further in Chapter 7.
Sporting activities and chronic pelvic pain
As discussed above, musculoskeletal causes of CPP are many and varied, but sport and leisure activities can result in an increased risk of sustaining an injury, particularly when the activities in question are excessively pursued early in life (Antolak et al. 2002). Failure to recognize and diagnose musculoskeletal injuries in difficult-to-access regions of the pelvis and pelvic floor myofascial system can potentially result in an acute impairment becoming a chronic disability. People with LPP who regularly participate in sports requiring repeated rotation of the trunk and hips have less overall passive hip rotation motion and more asymmetry of rotation between sides, than people without LPP (Van Dillen et al. 2008). These findings suggest that the specific directional demands imposed on the hip and trunk during regularly performed activities may be an important consideration in prevention and intervention of sporting injuries involving the lumbopelvic area.
In general though, studies suggest a therapeutic effect from aerobic exercise in CPP (Giubilei et al. 2007) with inactivity associated with negative long-term effects (Orsini et al. 2006). However there remains some controversy regarding the role of cycling and urogenic disorders (Taylor III et al. 2004, Sommer et al. 2010). Some researchers suggest a significant relationship between cycling-induced perineal compression leading to vascular, endothelial and neurogenic dysfunction with the development of erectile dysfunction (ED) (Sommer et al. 2010), and others imply that the overall prevalence of ED in the cycling community does not appear to be greater than that of historical controls (Taylor III et al. 2004). It should be emphasized that exercise in general and non-impact aerobic-cardiovascular exercise in particular have extensive support in the medical literature over a period of greater than five decades (Brock 2005) and should be encouraged.
The effect of aerobic exercise on chronic pelvic pain
A sedentary lifestyle has been shown to be a risk factor for CPP (Orsini et al. 2006). Orsini et al. (2006) analysed surveys from 30 000 Swedish men aged between 45 and 70 years of age and concluded that those men who were physically active at work and pursued an active lifestyle in their leisure time showed a 50% reduction in their risk of severe lower urinary tract symptoms, compared to inactive men. Inactivity of greater than 5 hours a day (at age 30 plus) was associated with a twofold increased risk in developing symptoms. The authors concluded that physical activity in young and late adulthood appears to be associated with a lower risk of moderate and severe urinary tract symptoms. In a double-blind, randomized controlled trial, Giubilei et al. (2007) compared the effects of an aerobic exercise programme (n = 52) versus placebo/stretching and motion exercises (n = 51) on a group of previously sedentary men. The cohort was recruited from a volunteer sample of 231 men who had at least a 12-month diagnosis of chronic prostatitis/CPP syndrome, who had not responded to conventional treatment for CPP and had no contraindications to moderately intense physical exercise. The aerobic exercise protocol included:
Specific groin injuries
Sports which include kicking, side-to-side cutting, interval sprinting, rapid or sudden changes of direction, quick accelerations and decelerations, repetitive hip and pelvic girdle rotation with axial loading such as running, golfing, ice hockey, figure skating, football, baseball, ballet, martial arts and gymnastics have a high incidence of groin injuries (Cowan et al. 2003, Verral et al. 2002, Pizzari et al. 2008, Shindle et al. 2007). According to Lovell (1995) in Nam et al. (2008), determining a differential diagnosis is essential, as 27–90% of patients who present with groin pain present with more than one injury. These co-existing injuries are thought to arise due to an initial injury altering the complicated biomechanics of the hip and groin, leading to secondary overuse injuries and/or the close proximity of anatomical elements in the region, predisposing one insult to involve adjacent structures (Morelli & Weaver 2005). Furthermore, in the sporting arena, the primary source of specialist consultation is the orthopaedic surgeon who may perform a wide-ranging assessment of the musculoskeletal system with no real evaluation of pelvic girdle mobility or pelvic floor musculature. The patient is unlikely to be asked about urinary, bowel or sexual dysfunction, and often the patient does not volunteer this information unless prompted. Likewise the urological specialist will provide a thorough assessment and examination of the pelvic floor, bladder and bowel, with no musculoskeletal component to the assessment. As described in previous chapters, many patients with CPP have a complex presentation and may well fit in to more than one diagnostic category, resulting in a ‘best fit’ diagnosis. If the signs and symptoms do not fit neatly into a diagnostic category then a more creative approach to the assessment of the patient’s condition is warranted.
The common types of groin injuries are listed below (see also Table 6.4):
Ligament and muscle strain
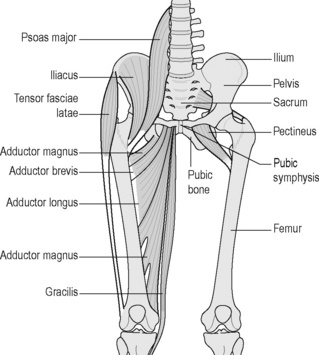
The most common site of strain is the musculotendinous junction of the adductor longus or gracilis muscle and is the most common cause of groin pain in the athlete (Reid 1992). The incidence among soccer players is between 10% and 18% (Nielsen & Yde 1989). Muscular and tendinous assessment can be performed using Cyriax’s Soft Tissue Tension Differentiation Tests, which involve palpation of the muscle over the area of strain and the elicitation of pain on resisted adduction and passive stretch into abduction (Ombregt et al. 2002). The history of the injury usually provides information about the biomechanical forces involved and the likely tissues involved, and the use of real time ultrasound imaging can help confirm or exclude the diagnosis of tendonosis/tendonitis or muscle tear (Heyde et al. 2005). Care must be taken to differentiate muscle strains and tendonoses/itis from osteitis pubis, sports hernias and nerve entrapment (described below), which can present with similar symptoms (Morelli & Weaver 2005). In cases where athletes recall a traumatic event, which results in the acute onset of symptoms, the diagnosis is more straightforward (Reid 1992). When the onset of symptoms is insidious a definitive diagnosis is difficult to make and often patients in this category are difficult to treat with orthodox protocol for adductor strains. It is these patients who present with ambiguous signs and symptoms that appear to fit into the CPP category as soft tissue differentiation tests can often be equivocal. For example, during active contraction of the adductors, which insert directly into the ramus of the pubis, stress on the symphyseal tissue can reproduce the patient’s symptoms and produce a false-positive test. This is usually confirmed by a lack of insertional tenderness and absence of pain on passive stretch (Ombregt et al. 2002). If an adductor strain is suspected, the tear has to be accurately located and this requires accurate and precise knowledge of functional anatomy (Figure 6.13). There is a need to know whether the strain has occurred in the belly of the muscle or the musculotendinous junction rather than the insertion into the pubic bone. This will in part determine how aggressive the treatment programme can be (Fricker 1997), as insertional strains will require an initial period of rest before active rehabilitation can begin (Reid 1992). Decreased abductor range of motion and decreased adductor strength are associated with an increased incidence of adductor strains (Ekstrand & Gillquist 1983, Tyler et al. 2001). Biomechanical abnormalities of the lower limb, imbalance of the surrounding hip musculature, and muscular fatigue have also been hypothesized to increase the risk of adductor strain (Holmich et al. 1999). Prevention programmes focused on eliminating some of these abnormalities have been shown to be effective in professional hockey players, although there have been no controlled clinical studies proving these latter elements to be causative (Tyler et al. 2002).
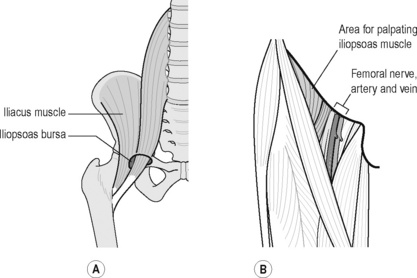
Iliopsoas originates from the transverse processes and anterior vertebral bodies of the lumbar vertebrae passing inferiorly to insert on the lesser trochanter of the femur. It traverses the pelvic rim, and crosses the hip joint and it is at this point that the iliopsoas bursa is interposed between the muscle and the underlying bone. The iliopsoas bursa (Figure 6.14) is the largest bursa in the body and has a direct communication with the hip joint in 15% of patients (Standring 2008) and is best visualized on MRI scan (Reid 1992). The bursa and tendon can become irritated as they rub over the iliopectineal eminence of the pubis, particularly in sports requiring a significant use of the hip flexors, so is common amongst football players, ballet dancers, hurdlers and martial arts experts (Hackney 1993). Bursitis can be characterized by a deep groin pain, difficult for patients to localize and to reproduce, which occasionally radiates to the anterior hip. Due to poor localization and reproducibility of the pain, the average time from onset of symptoms to diagnosis is 32–41 months (Johnston et al. 1998). There is tenderness below the lateral inguinal ligament over the femoral triangle, adjacent to the femoral artery, where the musculotendinous junction of the iliopsoas muscle can be palpated (Figure 6.15). The pain can be reproduced by stretching the iliopsoas; however, care must be taken to differentiate the pain in psoas bursitis from femoral nerve irritation and conditions of the hip such as anterior hip impingement syndrome. Pain may also be reproduced when the flexed, abducted, externally rotated hip is extended and brought back into a neutral position (extension test) or have the supine athlete raise his or her heels off the table to about 15° (Morelli & Weaver 2005).
Trigger points associated with the muscles around the pelvis and hip are discussed in Chapter 14.
Acetabular tears and impingements of the hip
Acetabular labral tears are a recently recognized source of hip pain, particularly in the anterior hip or groin region (Lewis & Sahrmann 2006). Femoro-acetabular impingement of the hip is a soft tissue impingement of the acetabular-labrum complex, which may or may not include the capsule, psoas tendon or bursa. Upon further evaluation, studies have indicated that 22% of athletes with groin pain (Narvani et al. 2003) and 55% of patients with mechanical hip pain of unknown aetiology (McCarthy et al. 2001) have a labral tear. Except in cases of specific trauma, the aetiology of labral tears is often difficult to determine and can evade detection by non-invasive means. The pain and disability can be severe, with a sudden onset of symptoms, but an acetabular labral tear should also be suspected when a patient with normal radiographs complains of a long duration of anterior hip pain and clicking, with minimal to no restriction in range of movement (ROM). A wide range of provocative tests have been reported, but typically it is confirmed with pain on passive hip flexion combined with adduction and medial (internal) rotation, and pain with a resisted active straight leg raise (Farjo et al. 1999, Hase & Ueo 1999, McCarthy et al. 2001, Mason 2001, Binningsley 2003). Other reported tests are:
• Flexion with medial rotation alone or combined with adduction or axial compression;
Whether these other tests were performed actively or passively was not specified and the sensitivity and specificity of these tests have yet to be published. In six professional soccer players with anterior labral tears, Saw & Villar (2004) found that all of the players had significant pain with combined hip flexion, medial rotation and adduction. Mitchell et al. (2003) reported that the flexion–abduction–external rotation (FABER) test elicited pain in 88% of patients (15 of 17) with intra-articular pathology, but they did not find any correlation between a positive FABER test result and different types of hip joint pathology.
The wide range of provocative tests may be attributable to differences in the location of the tear. In 56 hips of 55 patients, Fitzgerald (1995) used two different clinical tests that provoked symptoms in 54 patients, depending on tear location. To identify an anterior labral tear, the patient’s leg was brought into full flexion, lateral rotation and full abduction and then extended with medial rotation and adduction. To identify a posterior labral tear, the patient’s leg was brought into extension, abduction and lateral rotation and then flexed with medial rotation and adduction. If a labral tear is present, these manoeuvres will result in sharp pain with or without a click (Fitzgerald 1995).
Once a labral tear is diagnosed, to date conservative medical treatment has not proven to be effective, and the appropriate physical therapy intervention has yet to be established. Surgical treatment results in short-term improvement, but the long-term outcomes are still unknown (Tyler & Slattery 2010). As labral tears have been associated with a higher risk for joint degeneration, this area warrants further investigation, especially with regard to prevention, early detection, appropriate physical therapy and medical treatment (Lewis & Sahrmann 2006).
Osteitis pubis
The name osteitis pubis (OP) suggests inflammation, however it appears to involve a degenerative rather than an inflammatory process, characterized by symphysis pubis (SP) pain, with occasional referral along the adductor muscles to the hip, superiorly to the lower abdominal region and posteriorly towards the perineum and scrotum in men (Hackney 1993). OP can produce symptoms of exercise-induced pain in the inner thigh and abdominal area which come on gradually and worsen as the activity progresses. Examination reveals tenderness over the SP and this usually needs to be present to confirm a diagnosis (Reid 1992). Confusingly resisted hip adduction or trunk flexion may also reproduce the symptoms, which is usually indicative of muscular lesions. Plain film radiographs commonly reveal sclerosis of the pubic bones; with occasional widening of the symphysis, with laxity on stork views >2 mm (Harris & Murray 1974). However, X-rays have poor construct validity as often changes on X-ray do not correlate with symptoms and there are positive radiographic findings found in asymptomatic individuals (Hackney 1993). Bone and MRI scans correlate better with symptoms than radiographic appearance, with the ability of MRI scans to show bone marrow oedema into the pubic bones and detachment of anterior fascial layer, which is continuous with fascia overlying adductor muscles and the inguinal ligament (Karlsson & Jerre 1997). OP is generally thought of as the end result of an overuse continuum, resulting in excessive and repetitive strain of the SP and pelvis (Cunningham et al. 2007, Pizzari et al. 2008). There is limited evidence of proven risk factors for OP in the literature although greater hip abductor to adductor muscle strength ratios and decreased total rotation range of hip motion have been implicated (Maffey & Emery 2007, Verrall et al. 2001).
Lower-quadrant biomechanical abnormalities such as hypermobility, intrapelvic asymmetry and technique deficits are also said to play a role in the onset of OP, but to date there are no published trials to support this clinical observation (Reid 1992, Pizzari et al. 2008). Some authors also cite mechanical traction of the pelvic muscles (Ashby 1994). Although there is no convincing evidence that steroid injections are of any benefit (Hackney 1993) treatment typically includes general modified activity, physiotherapy in the form of correction of biomechanical abnormalities and muscle stretching, NSAIDs and corticosteroid injections (Holt et al. 1995). It occurs commonly in runners, footballers and in grass hockey goalkeepers and can be difficult to distinguish from adductor strains, with the two conditions frequently occurring simultaneously.
Athletic pubalgia or sports hernias
The diagnosis of a sports hernia or athletic pubalgia (AP) is controversial as there is frequently no clinically detectable inguinal hernia on physical examination and there is currently no consensus as to what specifically constitutes this diagnosis (Swan Jr & Wolcott 2007, Caudill et al. 2008). It has been defined as a set of pelvic injuries involving the abdominal and pelvic musculature outside the ball-and-socket hip joint and on both sides of the pubic symphysis (Meyers et al. 2008, Omar et al. 2008). AP occurs more often in men, although female proportion, age, numbers of sports and soft tissue structures involved have all increased recently (Meyers et al. 2008). Provocative sports usually include activities that involve quick turns whilst the foot is planted, cutting, pivoting, kicking and sharp turns, such as those that occur during soccer, ice hockey, rugby or football or high knee lift action such as in the martial arts, sprinters and hurdlers. Although with focused questioning a specific inciting incident may be identified (Caudill et al. 2008), it usually has no specific traumatic cause and comes on insidiously, with some correlation with OP, weakness of the posterior inguinal wall, a stretched external ring and generalized distension of the anterior abdominal wall (Nam & Brody 2008). Posterior inguinal wall weakening is said to occur from excessive or high repetition shear forces applied through the pelvic attachments of poorly balanced hip adductor and abdominal muscle activation (Caudill et al. 2008). The pain is felt deep in the groin and gradually worsens over time, which may spread along the inguinal ligament into the perineum, rectus abdominis muscles and to the testicles in about 30% of symptomatic individuals (Zimmerman 1988). Further investigations such as MRI, diagnostic ultrasonography and isotope scans are said not to provide any useful data in the assessment of sports hernias except to exclude other conditions (Caudill et al. 2008, Nam & Brody 2008). However, a recent review concluded that large-field-of-view MRI survey of the pelvis, combined with high-resolution MRI of the pubic symphysis provides excellent information about the location, causes and severity of the condition (Omar et al. 2008). MRI depicts patterns of findings in patients with AP including rectus abdominis insertional injury, thigh adductor injury and OP (Zoga et al. 2008). The range of possible pathologies or injuries is very wide and an in-depth knowledge of the pelvic regional anatomy is essential in the diagnosis of this condition. Conservative management consisting of soft tissue and joint mobilization and manipulation, neuromuscular re-education, manual stretching and therapeutic exercise is a viable option (Kachingwe & Grech 2008). A final course of action is surgical exploration of the posterior abdominal wall for defects, which are subsequently repaired. Surgery seems to be more effective than conservative treatment, and laparoscopic techniques generally enable a quicker recovery time than open repair (Brown et al. 2008).
Stress fractures
Although stress fractures are not common, if they do occur the two most frequent sites for these to occur are the femoral neck and the pubic ramus (Morelli & Smith 2001). Stress fractures can be caused by overtraining, which can cause repetitive strain on the underlying bone structure. They are often seen in long distance runners and military recruits, who may be required in their training to cover long distances with less than adequate footwear (Morelli & Smith 2001). Dancers are also subject to stress fractures especially classical ballet dancers, who concurrently may also present with nutritional imbalances (Howse & Hancock 1992). In addition, there are risk factors which predispose individuals to stress fractures: osteoporosis, muscle fatigue, excessive increase in training intensity or duration as well as running on cambered or uneven surfaces (Reid 1992). The pain with stress fractures is exacerbated by activity and diminished by rest; however, pain at rest is indicative of advanced disease. Confirmation of a stress fracture is usually made by MRI scan which has been show to be accurate and reliable in the imaging of these injuries (Ahovuo et al. 2002).
Nerve compression
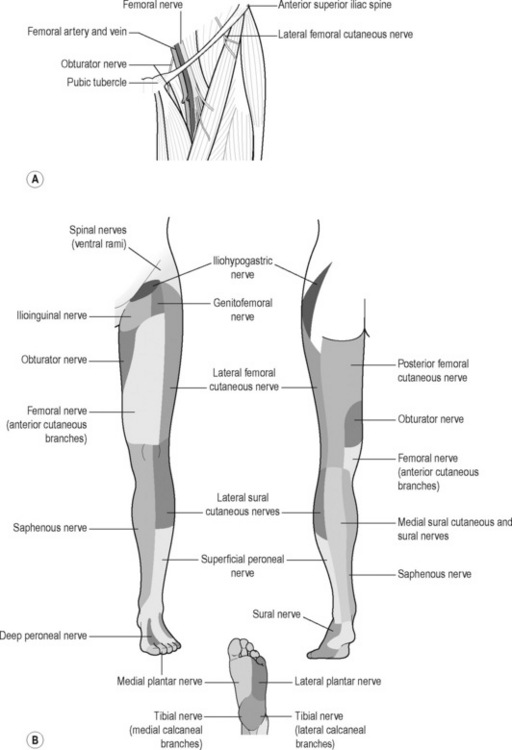
Several nerves around the groin and pelvis are vulnerable to compression: ilioinguinal; iliohypogastric; lateral femoral cutaneous nerve of the thigh; genitofemoral; obturator (Figure 6.16) and pudendal.
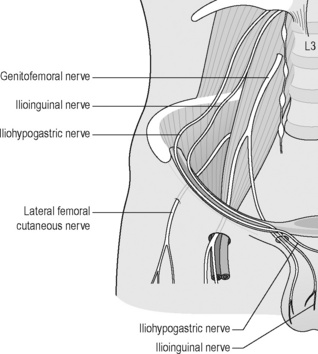
Figure 6.16 • Schematic drawing of the iliohypogastric, ilioinguinal, genitofemoral and lateral femoral cutaneous nerve of the thigh
The hip joint itself is supplied by the femoral nerve (which also innervates the iliofemoral ligament and the superior capsule), the obturator nerve (also supplying the pubofemoral ligament), the superior gluteal nerve (which supplies the superior and lateral part of the joint capsule and also the gluteus medius and minimus), and by the nerve to the quadratus lumborum, which supplies the posterior capsule and the ischiofemoral ligament (O’Brian & Delaney 1997). The spinal nerves L2, L3 and L4 can also refer pain to the groin or anterior thigh (Morelli & Weaver 2005).
The ilioinguinal, iliohypogastric and genitofemoral nerves originate from the first lumbar nerve (L1) but the genitofemoral receives additional input from L2 or L3. There is considerable anatomic variation in the origin and course of these nerves as well as anoverlap of their cutaneous distributions (Aszmann et al. 1997, Akita et al. 1999, Rab et al. 2001). The ilioinguinal branch passes through the inguinal canal, becoming fairly superficial near the superficia inguinal ring, and continues to supply the root of the penis (or labia majora), anterior scrotum, and medial thigh, as does the genitofemoral nerve.The iliohypogastric further divides into two branches: the anterior cutaneous, which carries cutaneous sensation from the lower abdominal and groin region medial to the ASIS; and the lateral cutaneous branch, which receives sensation from the lateral thigh and gluteal region (Morelli & Weaver 2005). In addition to these sensory distributions, the ilioinguinal and iliohypogastric nerves provide motor innervations to the lower abdominal musculature.
Entrapment of the ilioinguinal, iliohypogastric and genitofemoral nerves may result in groin pain or lower abdominal pain that can radiate to the genitals. These nerves can be injured by direct trauma including abdominal surgery such as caesarean section, transvaginal tape for stress incontinence surgery and hernia repairs for overzealous training of the abdominals (Starling & Harms 1989, al-Dabbagh 2002, Murovic et al. 2005, Whiteside & Barber 2005, Vervest et al. 2006, van Ramshorst et al. 2009). Tenderness is often noted 2–3 cm inferior-medial to the ASIS, and hip extension usually produces increased pain or hypoaesthesia in the nerve’s distribution (Morelli & Weaver 2005).
Compression of the lateral femoral cutaneous nerve of the thigh or meralgia paraesthetica can occur as it passes under or through the inguinal ligament resulting in a persistent burning sensation, tingling or aching pain, and hypersensitivity or hyposensitivity in the anterolateral aspect of the thigh (Moucharafieh et al. 2008). In addition to sportsmen such as squatting rifle team members and athletes who sustain acute trauma to the area, it has been noted in women who sit for prolonged periods with the involved leg underneath the body (Morelli & Weaver 2005) and more recently in wearers of tight low-cut trousers (Moucharafieh et al. 2008).
The obturator nerve supplies the adductor muscles and the skin over the inner thigh and is increasingly being reported as a source of chronic groin pain in athletes, and usually becomes entrapped in the obturator foramen or by thickened fascia surrounding the adductor muscles – usually adductor brevis (Morelli & Weaver 2005). Symptoms can include deep aching over the adductors or at the pubic bone, but its anatomy makes it difficult to distinguish between adductor strain and obturator nerve entrapment (Morelli & Weaver 2005). Classically the deep ache near the adductor origin of the pubic bone is exacerbated by exercise, subsides with rest, but often resumes with activity, and may radiate down the medial thigh toward the knee. Spasm, weakness and paraesthesia in the area may also occur (Brukner et al. 1999).
Conservative management of nerve compressions, including changes in training regimens, ice, NSAIDs, pharmacological management, local corticosteroid injections and nerve blocks, have been suggested with surgical referral in resistant cases (Brown et al. 2008, Suresh et al. 2008).
The pudendal nerve is commonly seen as a source of CPP due to its course through the levator ani muscle. The treatment and evaluation of pudendal nerve entrapment will be dealt with in Chapter 11.
Similar to the classification of mechanical LPP described earlier, classification of movement-impairment syndromes of the hip provides a systematic process with which to examine and select exercises to provide appropriate prescription and pathology-specific modification of exercise for hip pain (Lewis et al. 2006). A summation of hip classifications organized by test position, symptom behaviour with variations of the test position or movements within the test position, and clinical judgements of quality of alignment or movement are summarized in Table 6.3.
Table 6.3 Items for classification of hip organized by test position, symptom behaviour with variations of the test position or movements within the test position, and clinical judgements of quality of alignment or movement (taken from Sahrmann 2002) ![]()
| Test position | Symptom behaviour with | Judgement of femoral alignment or movement |
|---|---|---|
| Standing |
Normal: Knee flexion 45° with heel staying in contact with floor. Knee in line with second toe. Foot pronates (Figure 6.17A)
Hip medial rotation: Knee moves in line medial to big toe. Suggests poor stability of posterior gluteus medius or overactivity of tensor fascia latae (TFL) (Figure 6.17B)
Hip lateral rotation: Knee moves in line lateral to fourth toe
Normal: No change in hip joint rotation
Hip adduction: Downward tilting of opposite side of pelvis. Suggests stance side hip abductors weak/long (Figure 6.18)
Pelvic rotation: towards stance leg. Suggests short hip medial rotators
Hip rotation: Femur rotates medially. Suggests short long/weak hip lateral rotators
Normal: Hip medial and lateral rotation symmetrical and approximately 30°
Medial rotation ROM > lateral rotation. Suggests structural variation hip antetorsion when also observed in hip extension.
Lateral rotation ROM > medial rotation. Suggests structural variation hip retrotorsion when also observed in hip extension
Normal: Extended thigh lies on table with lumbar spine flat. Femur in midline without hip rotation or abduction. Knee flexed to 80° without abduction of tibia or lateral tibial rotation. Hip extended 10°
Short hip flexors: Thigh does not reach table (Figure 6.21). Abduct hip and extension range increases suggests TFL-ITB (ITB, iliotibial band) short. Passive extension knee range increases, suggest rectus femoris short. Iliopsoas short if hip abducted, knee extended and thigh does not lie on table
Femoral head glides anteriorly: Suggests iliopsoas long and/or anterior capsule stretched
Normal: Greater trochanter (GT) maintains constant axis of rotation (AoR) during passive and active SLR, hip flexes to 80°
Femoral anterior glide: GT moves anterior and superior (medial rotation and insufficient posterior glide) during active SLR. Suggests stiff posterior structures and/or hamstrings short (Figure 6.22). During passive SLR, GT maintains relatively constant position but examiner needs to control AoR with thumb placed in inguinal crease. Active SLR may produce anterior hip pain, whilst passive SLR pain-free
Femoral medial rotation: Iliopsoas long, and/or long stiff hip lateral rotators
Normal: 35° medial rotation without pelvic rotation. Hip ROM is very variable and does not necessarily imply muscle shortness. However <30° check obturators, quadratus femoris, gracilis, piriformis, gemelli, posterior gluteus medius
Structural variation: <10° medial rotation suggests retroversion of femur. >50° medial rotation suggests antetorsion of femur (Figure 6.24A). Check range in supine to confirm
Normal: 35° lateral rotation without pelvic rotation. Hip ROM is very variable and does not necessarily imply muscle shortness. However <30° check medial rotators; TFL/ITB, anterior gluteus medius, gluteus minimus
Structural variation: <10° lateral rotation suggests antetorsion of femur. >50° lateral rotation suggests retroversion of femur (Figure 6.24B). Check range in supine to confirm
Femoral anterior glide: GT moves anteriolateral, making wide arc of movement. Suggests TFL/ITB short
Normal: 10° hip extension with slight lumbar extension. Short rectus femoris/TFL if hip extension <5°. If unable to maintain hip extension when maximum resistance applied, implies weak/long gluteus
Femoral anterior glide: GT moves anteriorly. Suggests overactivity hamstrings, and/or stretched anterior capsule
Normal: Hips flex to heels, no pain. Decreased hip flexion or pelvic rotation implies short/stiff gluteus maximus/piriformis (rotation implies asymmetric stiffness) (Figure 6.26B). Confirm by abducting and/or lateral rotating hips which increases hip flexion
Cycling and genitourinary symptoms in men and women
The reported incidence of bicycling-related urogenital symptoms varies considerably (Leibovitch & Mor 2005) and some question the existence of a relationship between bicycle riding and urogenital symptoms, suggesting that larger case-control studies are required before conclusions can be drawn (Taylor III et al. 2004, Brock 2005). Yet in a recent comprehensive review of the literature, Sommer et al. (2010) conclude that there is a significant risk in relationship to cycling-related urogenital symptoms in both men and women, emphasizing the requirement for further research on bicycle and bicycle seat design. Rather than discourage cycling as an activity this section aims to describe the urogenital symptoms most commonly attributed to cycling, including the hypothesized mechanisms and inform the reader of the potential adjustable bicycle factors to assist the rider who complains of cycling-related urogenital dysfunction.
Symptoms
The most common bicycling-associated urogenital problems are genitalia numbness, followed by ED (Ricchiuti et al. 1999, Leibovitch & Mor 2005, Sommer et al. 2010). Other less common symptoms include CPP, priapism, penile thrombosis, infertility, haematuria, dysuria, difficulty in achieving orgasm, lymphoedema of the labia majora or ‘bicyclist’s vulva’, torsion of spermatic cord, prostatitis, hardened perineal nodules and elevated serum prostate-specific antigen (PSA) (Doursounian et al. 1998, LaSalle et al. 1999, Baeyens et al. 2002, Leibovitch & Mor 2005, Sommer et al. 2010). A summary of important epidemiological studies assessing the impact of bicycle riding on sexual function is shown in Table 6.4.
Potential mechanisms
Compression of the pudendal nerve between the symphysis pubis and the bicycle seat (Goodson 1981) or at its course through Alcock’s canal (Amarenco et al. 1987) have been proposed, although several authors have attributed numbness transient ischaemia caused by pressure on the vascular supply of the perineum (Oberpenning et al. 1994, Ricchiuti et al. 1999, Ramsden et al. 2003, Gemery et al 2007) As discussed in Chapters 2 and 11, Alcock’s canal is bordered laterally by the ischial spine and medially by the fascial layer of obturator internus muscle. The pudendal nerve leaves the canal ventrally below the ischiopubic ramus and it is thought that pressure on the ramus compresses neural and vascular tissue in Alcock’s canal, resulting in penile and perineal paraesthesia often called Alcock’s syndrome (Amarenco et al. 1987, Oberpenning et al 1994). Oberpenning et al. (1994) hypothesized that the onset of temporary perineal numbness, which can last 10–20 minutes or more, is due to compression on the perineum, which in turn causes compression on the pudendal nerve and artery. Ricchiuti et al. (1999) reported electromyographic (EMG) evidence of bilateral pudendal nerve injury associated with excessive cycling. They reported that the transient ischaemic episodes to the pudendal nerve and subsequent measurable delays in conduction were rapidly reversible if the ischaemia was of relatively short duration. However, ischaemia of longer than 8 hours duration resulted in significant deterioration of nerve function and would take several weeks for recovery to occur. Although acknowledging that the cause of perineal numbness and ED resulting from bicycle riding is not fully understood, it is suggested to be a result of continuous compression and strain on the pudendal nerve and arteries leading to nerve entrapment and vascular occlusion (Gemery et al. 2007, Sommer et al. 2010).
Leibovitch et al. (2005) postulated that the movements of the pedalling legs in the forward sitting position could result in stretching of the pudendal nerves over the sacrospinal and the sacrotuberous ligaments. This may cause increased tensile and compressive stress on the nerve trunk and a loss of the normal gliding movement of the nerve relative to the adjacent soft tissue and bony structures of the pelvic floor. The gliding movement of nerves in general has been described by Shacklock (2005) as being an essential aspect of the mechanical function of neural tissue, which serves to disperse the tension applied at one point of a nerve to the whole length of a nerve, reducing the forces on the nerve tissue. Neural mechanosensitivity is discussed further in Chapter 14.
Nanka et al. (2007) proposed an alternative mechanism of urogenital dysfunction in cyclists. They suggested that, as the pudendal nerve is protected by a thick layer of fatty tissue extending below the extent of the pubic body in the floor of Alcock’s canal, compression of the posterior dorsal nerve of the penis (PDNP) in the sulcus nervi dorsalis penis (SNDP) is the main cause of Alcock’s syndrome. They hypothesize that the position of the PDNP close to the pubic ramus and its proximity to the fibres of the suspensory ligament of the penis and the ischiocavernosus body make it more vulnerable to mechanical insult (Nanka et al. 2007). Since a compression neuropathy depends upon mechanical and ischaemic insult they suggest that the PDNP is the mostly likely nerve to be affected, particularly as the PDNP is the only nerve supplying the glans penis, hence able to be the cause of diminished glandular and penile sensitivity (Nanka et al. 2007).
Irrespective of the causative mechanisms, Labat et al. (2008) stated that despite these examples of pudendal nerve compression, very few cyclists go on to develop pudendal neuralgia.
Therapeutic options regarding adjustable bicycle factors
As discussed earlier there remains controversy as to whether alterations in riding habits actually change the prevalence of urogenital symptoms among cyclists (Taylor III et al. 2004). However, in addition to modifying training schedules and rising from the saddle for a brief time periodically to relieve pressure and help re-establish blood flow (Huang et al. 2005), the factors that have been evaluated are adjustments of the saddle, bicycle and body position. There are areas of agreement and inconsistencies in the literature; these factors are addressed in turn.
Saddle design
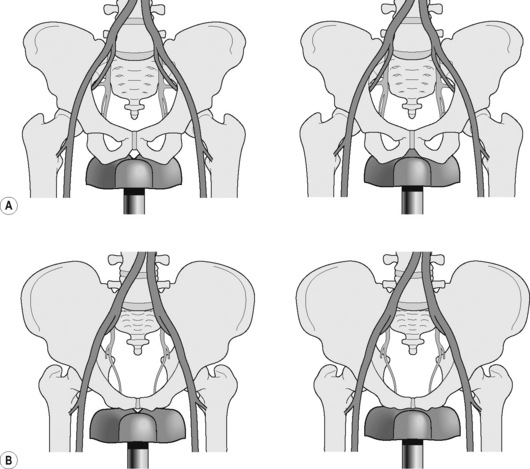
The design of the bicycle saddle is thought by a number of authors to be a major factor in the aetiology of perineal compression (Rodano et al. 2002, Seong et al. 2002, Sommer et al. 2010). Some saddles have a deep groove or hole connecting the anterior to the posterior part of the saddle and there is disagreement as to whether they are more or less likely to cause neurovascular compression (Rodano et al. 2002, Gemery et al. 2007, Sommer et al. 2010) (Figure 6.27). Saddles without a narrow protruding nose or with a large hole and a shape that allows for proper seating of the ischial tuberosities significantly reduce pressure distributed in the perineal region of cyclists (Schrader et al. 2008, Sommer et al. 2010). However, Rodano et al. (2002) argued that there is a greater likelihood of perineal numbness with holed or deep-grooved saddles as the load is transferred from the ischiopubic ramus to the perineum, particularly as the edge of the groove frequently corresponds anatomically to the location of the pudendal nerve and artery. Holed saddles present edges in the region where the hole is projected and these edges in saddle design can affect saddle pressure due to the contact of small areas under an elevated compressive load (Carpes et al. 2009). Gemery et al. (2007) stated that a grooved seat allows better preservation of the seat/symphysis space and reduces the compression on the pudendal artery more than a standard saddle. They constructed 3D models from computed tomography scans of one adult male pelvis (a healthy volunteer) and three bicycle seats to assess the influence of the rider’s saddle position on vascular compression (Figure 6.29). These models were correlated with lateral radiographs of a seated rider in order to determine potential vascular compression between the bony pelvis and cycle seats at different angles of rider positioning as well as saddle type. They concluded that the rider’s position is more important for reducing compression than is seat design alone (see section below on postural adjustment).
Posture and type of bike
Rodano et al. (2002) suggest that, in competitive road and mountain biking, 30–40% of a cyclist’s body weight will be loaded through the saddle, but because the body weight is distributed through the pedals, handlebar and saddle the combination of the position of the cyclist on the bicycle, the pedalling action and the body weight distribution will determine the effect on the muscles, ligaments and other structures around the pelvis, including the prostate and neurovascular bundle. Disagreement remains about whether the upright or forward lean posture is most suitable to minimize urogenital distress whilst cycling (Rodano et al. 2002, Gemery et al. 2007, Carpes et al. 2009, Potter et al. 2008, Sommer et al. 2010). Sommer et al. (2010) stated that in the upright-seated position, a reduction in penile blood flow of up to 70% occurs due to compression of the dorsal penile arteries in the perineum. Whereas Gemery et al. (2007) suggested that with the rider leaning forward, greater compression of the internal pudendal artery occurs, immediately below the pubic symphysis (Figures 6.27, 6.28). Rodano et al. (2002) indicated that when the cyclist is in the bent forward race position (Figure 6.29) the pudendal nerve is more likely to be compromised as the greatest pressure is transferred to the anteroposterior section of the saddle.
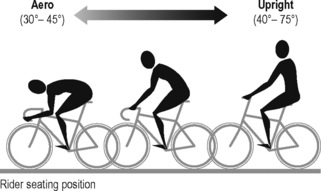
Figure 6.29 • Rider seating position. This figure shows the different seating positions on a bicycle
A study to verify the effect of trunk position and saddle design on saddle pressure in both men and women concluded that it is the masculine anatomy that mainly influences saddle pressure during riding (Carpes et al. 2009). For men the trunk forwards position lowers the values of saddle pressure only for men using the ‘holed’ saddle, whereas there were no statistical differences comparing saddle pressure between the two trunk positions for women. Differences in centre of gravity, perineal anatomy and pelvic bone shape of men and women may therefore result in gender differences in pressure values from bicycle seats (Potter et al. 2008).
Use of a recumbent cycle has also been shown to produce a smaller decrease in genital oxygen tension; however, in some situations it can be a more difficult and less practical bike to ride (Schrader et al. 2002, Dettori & Norvell 2006). Mountain bicycles have been associated with a greater risk of ED as compared with road bicycles (Dettori et al. 2004); however, the study is weakened by a small cohort size of impotent bicyclists and a high non-responder rate (>20%).
Saddle width
It has been suggested that endothelial injury of the penile artery can occur due to the compressive trauma caused by the pressure of sitting on a bicycle saddle, either by chronic compression of the neurovascular structures in the perineum or when a cyclist slips on to the top bar of a bicycle and land directly on the perineum or the ischiopubic ramus (Sommer et al. 2010). Most cyclists sit on a saddle which is narrower than the space between the ischial tuberosities, causing the load to be borne on the ischiopubic ramus, close to the Alcock’s canal which may lead to a compromised blood supply to the penis (Richciutti et al. 1999, Seong et al. 2002, Rodano et al. 2002, Sommer et al. 2010). Seong et al. (2002) also conclude that a narrower saddle significantly reduces penile blood flow and could be the cause of blunt trauma to the perineum, although the small number of subjects (n = 20) and the indirect method used to measure penile blood flow reduces the validity of the study. Weiss (1985) and Bond (1975) both have proposed that transient ischaemia can be caused by compression of structures between the saddle and the symphysis pubis.
Sommer et al. (2010) conclude that the width of the saddle must span at least the distance between the ischial tuberosities, and the saddle must be wide enough for the ischial tuberosities to be situated on the flat back region of the saddle so that they are positioned higher than the soft tissue.
Saddle padding
Cycling on a gel saddle resulted in 37% more loss of penile oxygenation than cycling on an unpadded saddle (Sommer et al. 2010). They hypothesized that this was due to the gel being pressed into the perineal region until the ischial tuberosities encountered resistance as the rider sinks into the saddle. Furthermore, the wider saddle showed 57% better penile oxygenation than the narrow saddle when comparing the same seat position and padding material.
Conclusions
Particularly given the cardiovascular benefits from this low-impact activity, practitioners are urged to balance the risk–benefit ratio of cycling, as they would any intervention in medicine (Brock 2005). Rather than discourage cycling, the authors suggest the reader should emphasize to their patients with urogenital dysfunction strategies that may minimize the potential adverse effects of cycling, such as:
• Rising from the saddle for a brief time periodically, which can relieve pressure and help re-establish blood flow;
• Modifying training schedules;
• Adjusting the posture on the bike or using a different type of bike;
• Modification of the saddle, such as an unpadded, wide, no-nosed saddle; but consideration must be taken regarding the location of the groove or hole.
Running
Whilst running has many beneficial effects such as improving cardiovascular health and improving bone density, there is a risk of injury to the musculoskeletal system due to poor training technique and overtraining (Harrast & Colonno 2010).
Geraci & Brown (2005) report that the most common causes of hip pain in runners are muscle strains and tendinitis, which can be attributable to changes in running speed, sudden change of direction and a sudden increase in weekly or monthly mileage. The athlete presents with acute localized pain, which is found over the muscle–tendon unit on examination. Assessment reveals weakness on resisted testing and pain on passive stretching. There are underlying risk factors for the development of a stress fracture, including increase in frequency, duration or intensity (Harrast et al. 2010). Periodization of training is a coaching technique which builds in rest days to a training programme, after higher-intensity training sessions, and reduces risk of injury. Training in running shoes older than 6 months is a risk factor for stress fracture (Gardner et al. 1988). Stress fractures of the pelvis account for approximately 1–7% of all stress fractures and the most common site for a fracture is the pubic ramus (Frederickson et al. 2006). Patients may present with pubic ramus stress fractures, which may be initially thought of as soft tissue injury to the adductors. The history of onset and analysis of the athlete’s training programme should help to avoid this. Hreljac (1999) reported that up to 70% of runners sustain overuse injuries during any one year.
Football
Ekstrand & Gillquist (1983) reported that the incidence of groin pain in soccer players, over a period of one year, was 8%. Other authors have reported the incidence of groin injury in professional footballers is as high as 22% (Werner et al. 2009). They investigated the incidence, pattern and severity of hip and groin injuries in professional footballers over seven consecutive seasons. A total of 628 hip/groin injuries were recorded, accounting for 12–16% of all injuries per season. More than half of the injuries (53%) were classified as moderate or severe (absence of more than a week), the mean absence per injury being 15 days. Re-injuries accounted for 15% of all registered injuries. In the 2005/6 to 2007/8 seasons, 41% of all diagnoses relied solely on clinical examination (Werner et al. 2009). They concluded that hip/groin injuries are common in professional football, and the incidence over consecutive seasons is consistent. They further noted that hip/groin injuries are associated with long absences and many hip/groin diagnoses are based only on clinical examination. A qualitative study by Pizzari et al. (2008) looking at the prevention and management of osteiitis pubis in the Australian football league, reported all clubs involved in the study showed a high awareness of the condition and had identified a number of management strategies to combat it, such as rest-modified training, correction of predisposing factors, as well as early detection of onset. As discussed previously in this chapter, there is much debate about the aetiology of adductor-related groin pain.
Ice hockey
Ice hockey is an aggressive contact sport, where the players have to move at great speed on the ice, whilst displaying a very high level of skating ability. The combination of speed, rapid acceleration and deceleration and contact makes for significant potential for injury. Kai et al. (2010) state that groin injuries in hockey make up for 5–7% of all hockey injuries, whilst National Hockey League data revealed that 13–20% of players would suffer a groin injury (Caudill et al. 2008). As in other sports, groin pain in hockey players has multiple aetiologies, which often do not present with unequivocal signs and symptoms. OP is common due to the repetitive changes in direction in combination with bursts of acceleration and deceleration (Kai et al. 2010). Whilst skating the adductor muscles function in adduction and external rotation and adductor pain is often reported as being worse on skating and shooting the puck (Kai et al. 2010). Repetitive motions during hockey, for example during a slapstick manoeuvre, involve ipsilateral hip extension with contralateral trunk rotation and will also predispose the adductor muscles to injury. A slap shot manoeuvre requires rapid twisting motions of the body and abdominal muscle tears occur during these rapid torque-producing movements.
Sports involving repetitive flexion of the hip
Antolak et al. (2002) concluded that CPP may in part be explained by compression of the pudendal nerve, especially when the activities included continued flexion of the hip as occurs during many sports such as American football, weightlifting and wrestling (Antolak et al. 2002). Antolak et al. (2002) hypothesize that as many athletes begin participation in their sport as teenagers, hypertrophy of the muscles of the pelvic floor during these developmental years can cause elongation and remodelling of the ischial spine. This results in rotation of the sacrospinous ligament, causing the sacrotuberous and sacrospinous to be superimposed over each other. Furthermore, during squatting activities or during sitting and rising activities, the pudendal nerve may be stretched over the sacrospinous ligament or the ischial spine, which may produce shearing force on the nerve (Antolak et al. 2002). This can be made worse by the actions of gluteus maximus and the abduction and extension of the hip, for example rising from the squatting position of the baseball catcher or in the rugby scrum or during a ruck.
Case study 6.1
Assessment revealed a significant shift of the pelvis to the left, with associated apparent leg length discrepancy. There was a visible rotoscoliosis to the right in the lumbar spine, with an apex at L3. Forward flexion was limited by pain and stiffness in the lumbar spine. Left rotation was also limited by pain and stiffness in the lumbar spine. Right rotation reproduced the anterior pubic pain. Arthrokinematic assessment of the mobility segments in the lumbar spine revealed reduced neutral zone motion at the L5–S1 segment, with a blocked articular end feel. There were bony alignment changes in the pelvis with the left innominate positioned in anterior rotation. Arthrokinematic assessment of the sacroiliac joints (SIJ) revealed a significantly diminished neutral zone motion especially in the left-sided SIJ (Lee 2004). There was associated diminished multifidus bulk and active voluntary recruitment. There was also delayed recruitment time in the left gluteus maximus and increased tone in the left hamstrings. Osteokinematic assessment of the hip revealed a fixed flexion deformity of the left hip flexors, and palpable hypertonicity in the hip flexor group and adductor group of muscles. Treatment consisted of a high-velocity manipulation to L5–S1, myofascial release and deep tissue mobilization. Additionally, following treatment to reduce the hip flexor and adductor group hypertonicity, it was assessed that there was underlying diminished length of the flexor and adductor group. These muscle groups were stretched passively and the athlete was prescribed a stretching programme to follow independently. Trigger points were discovered in the proximal third of adductor magnus and the distal third of iliopsoas. These were treated using a modified treatment protocol as described by Travell & Simons (1993).
A unique aspect of the assessment of this athlete was the per rectal assessment of the pelvic floor. This involves a sophisticated digital analysis of the tone, length and function of the pelvic floor muscles and the mobility of the pudendal nerve. This is outlined in detail in Chapters 11 and 13. In this case pubococcygeus (PC) and iliococcygeus (IC) were both found to be hypertonic and short. Trigger points were found in the anterior third of both PC and IC, which reproduced the anterior groin pain reported by the athlete. Assessment of the mobility of the three branches of the pudendal nerve was carried out, and diminished mobility was found in the anterior and perineal branches of the nerve (Chapter 11). Prolonged sustained pressure techniques including Thiele massage and trigger point techniques were applied to the hypertonic PC and IC (Chapter 13). Finally mobilization of the pudendal nerve was carried out, with an emphasis on the perineal and anterior branches (Chapter 11).
On assessment of breathing patterns, the athlete had poor lateral expansion of her lower ribs and a hypertonic diaphragm. The importance of the assessment of an athlete’s breathing is discussed fully in Chapter 9. Breathing re-education included awareness and relaxation of upper thorax, lateral costal breathing, teaching inhalation via nasopharyngeal breathing and exhalation via oropharyngeal breathing.
Case study 6.2
Treatment consisted of high-velocity traction manipulation to the right sacroiliac joint and high-velocity thrust manipulation to the L5–S1 mobility segment. Soft tissue release, via myofascial techniques and trigger point techniques as described in Chapter 11, were performed on the hamstrings, adductors, QL, P and OI and pelvic floor. The athlete was instructed in self-treatment of the external hypertonic muscles and the external trigger points. He was also instructed in techniques to lengthen the adductors, hamstrings and piriformis muscles and QL.
ACOG Practice Bulletin No. 51. Chronic pelvic pain. Obstet. Gynecol.. 2004;103(3):589-605.
Ahovuo J.A., Kiuru M.J., Kinnunen J.J., Haapamäki V., Pihlajamäki H.K. MR imaging of fatigue stress injuries to bones: Intra- and interobserver agreement. Magn. Reson. Imaging. 2002;20:401-406.
Akita K., Niga S., Yamato Y., et al. Anatomic basis of chronic groin pain with special reference to sports hernia. Surg. Radiol. Anat.. 1999;21(1):1-5.
al-Dabbagh A.K. Anatomical variations of the inguinal nerves and risks of injury in 110 hernia repairs. Surg. Radiol. Anat.. 2002;24:102-107.
Amarenco G., Lanoe Y., Perrigot M., Goudal H. Un nouveau syndrome canalaire: la compression du nerf honteux interne dans le canal d’Alcock ou paralysie perineale du cycliste. Presse Med.. 1987:160.
Andersen K.V., Bovim G. Impotence and nerve entrapment in long distance amateur cyclists. Acta Neurol. Scand.. 1997;95:233-240.
Antolak S.J.Jr., Hough D., Pawlina W., Spinner R.J. Anatomical basis of chronic pelvic pain syndrome: compresssion; the ischial spine and pudendal nerve entrapment. Med. Hypotheses. 2002;59(3):349-353.
Ashby E.C. Chronic obscure groin pain is commonly caused by enthesopathy: ‘tennis elbow’ of the groin. Br. J. Surg.. 1994;81:1631-1634.
Aszmann O.C., Dellon E.S., Dellon A.L. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast. Reconstr. Surg.. 1997;100:600-604.
Baeyens L., Vermeersch E., Bourgeois P. Bicyclist’s vulva: Observational study. Br. Med. J.. 2002;325:138.
Barbic M., Kralj B., Cor A. Compliance of the bladder neck supporting structures: importance of activity pattern of levator ani muscle and content of elastic fibers of endopelvic fascia. Neurourol. Urodyn.. 2003;22:269-276.
Battaglia C., Nappi R.E., Mancini F., et al. Ultrasonographic and Doppler findings of subclinical clitoral microtraumatisms in mountain bikers and horseback riders. J. Sex. Med.. 2009;6:464-468.
Binningsley D. Tear of the acetabular labrum in an elite athlete. Br. J. Sports Med.. 2003;37:84-88.
Bond R.E. Distance bicycling may cause ischaemic neuropathy of the penis. Physician Sportsmed.. 1975;3:54.
Brock G.B. Editorial comment. Eur. Urol.. 2005;47:286-287.
Brown R.A., Mascia A., Kinnear D.G., et al. An 18-year review of sports groin injuries in the elite hockey player: clinical presentation, new diagnostic imaging, treatment, and results. Clin. J. Sport Med.. 2008;18:221-226.
Brukner P., Bradshaw C., McCrory P. Obturator neuropathy. Phys. Sportsmed.. 1999;27(5):1-5.
Carpes F.P., Dagnese F., Kleinpaul J.F., Martins E.A., Mota C.B. Bicycle saddle pressure: effects of trunk position and saddle design on healthy subjects. Urol. Int.. 2009;82:8-11.
Caudill P., Nyland J., Smith C., Yerasimides J., Lach J. Sports hernias: a systematic literature review. [Review] [104 refs]. Br. J. Sports Med.. 2008;42:954-964.
Constantinou C.E., Govan D.E. Spatial distribution and timing of transmitted and reflexly generated urethral pressures in healthy women. J. Urol.. 1982;127(5):964-969.
Cowan S.M., Bennell K.L., Hodges P.W., Crossley K.M., McConnell J. Simultaneous feedforward recruitment of the vasti in untrained postural tasks can be restored by physical therapy. J. Orthop. Res.. 2003;21:553-558.
Cunningham P.M., Brennan D., O’Connell M., et al. Patterns of bone and soft tissue injury at the symphysis pubis in soccer players: observations at MRI. Am. J. Roentgenol.. 2007;188:291-296.
Dankaerts W., O’Sullivan P.B., Straker L.M., Burnett A.F., Skouen J.S. The inter-examiner reliability of a classification method for non-specific chronic low back pain patients with motor control impairment. Man. Ther.. 2006;11(1):28-39.
Dettori J.R., Koepsell T.D., Cummings P., Corman J.M. Erectile dysfunction after a long-distance cycling event: Associations with bicycle characteristics. J. Urol.. 2004;172:637-641.
Dettori N.J., Norvell D.C. Non-traumatic bicycle injuries : a review of the literature. [Review] [51 refs]. Sports Med.. 2006;36:7-18.
Dickx N., Cagnie B., Achten E., et al. Changes in lumbar muscle activity because of induced muscle pain evaluated by muscle functional magnetic resonance imaging. Spine. 2008;33:E983-E989.
Doursounian M., Catney-Kiser J., Salimpour P., et al. Sexual and urinary tract dysfunction in bicyclists. J. Urol.. 1998;159(Suppl.):30.
Ekstrand J., Gillquist J. The avoidability of soccer injuries. Int. J. Sports Med.. 1983;4(2):124-128.
Fall M., Baranowski A., Elneil S., et al. EAU guidelines on chronic pelvic pain. Eur. Urol.. 2010;57:35-48.
Farjo L.A., Glick J.M., Sampson T.G. Hip arthroscopy for acetabular labral tears. Arthroscopy. 1999;15:132-137.
Ferreira P.H., Ferreira M.L., Maher C.G., Herbert R.D., Refshauge K. Specific stabilisation exercise for spinal and pelvic pain: a systematic review. [Review] [42 refs]. Aust. J. Physiother.. 2006;52:79-88.
Fitzgerald R.H.Jr. Acetabular labrum tears: diagnosis and treatment. Clin. Orthop.. 1995;311:60-68.
Frederickson M., Jennings F., Beaulieu C., et al. Stress fractures in athletes. Top. Magn. Reson. Imaging. 2006;17(5):309-325.
Fricker P.A. Management of groin pain in athletes. Br. J. Sports Med.. 1997;31:97-101.
Gardner L.I., Dziados J.E., Jones B.H., et al. Prevention of lower extremity stress fractures: a controlled trial of shock absorbent insole. Am. J. Public Health. 1998;78:1563-1567.
Gemery J.M., Nangia A.K., Mamourian A.C., Reid S.K. Digital three-dimensional modelling of the male pelvis and bicycle seats: impact of rider position and seat design on potential penile hypoxia and erectile dysfunction. BJU Int.. 2007;99:135-140.
Geraci M., Brown W. Evidence-based treatment of hip and pelvic injuries in runners. Phys. Med. Rehabil. Clin. N. Am.. 2005;16:711-747.
Gibbons S.G.T., Comerford M.J., Emerson P. Rehabilitation of the stability function of psoas major. Orthopaedic Division Review. 2002(Jan/Feb):7-16.
Giubilei G., Mondaini N., Minervini A., et al. Physical activity of men with chronic prostatitis/chronic pelvic pain syndrome not satisfied with conventional treatments. Could it represent a valid option? The Physical Activity and Male Pelvic Pain Trial: A double-blind, randomized study. J. Urol.. 2007;177:159-165.
Goodson J.D. Pudenadal neuritis from biking. N. Engl. J. Med.. 1981;304:365.
Guess M.K., Connell K., Schrader S., et al. Genital sensation and sexual function in women bicyclists and runners: Are your feet safer than your seat? J. Sex. Med.. 2006;3:1018-1027.
Hackney R.G. The sports hernia: a cause of chronic groin pain. Br. J. Sports Med.. 1993;27:58-62.
Hall T., Zusman M., Elvey R. Adverse mechanical tension in the nervous system? Analysis of straight leg raise. Man. Ther.. 1998;3:140-146.
Harrast M.A., Colonno D. Stress fractures in runners. Clin. Sports Med.. 2010:399-416.
Harris-Hayes M., Van Dillen L.R. The inter-tester reliability of physical therapists classifying low back pain problems based on the movement system impairment classification system. Pm & R. 2009;1:117-126.
Harris-Hayes M., Holtzman G.W., Earley J.A., Van Dillen L.R. Development and preliminary reliability testing of an assessment of patient independence in performing a treatment program: standardized scenarios. J. Rehabil. Med.. 2010;42:221-227.
Harris N.H., Murray R.O. Lesions of the symphysis in athletes. BMJ. 1974;4:211.
Hase T., Ueo T. Acetabular labral tear: arthroscopic diagnosis and treatment. Arthroscopy. 1999;15:138-141.
Hemborg B., Moritz U., Lowing H. Intra-abdominal pressure and trunk muscle activity during lifting. IV. The causal factors of the intra-abdominal pressure rise. Scand. J. Rehabil. Med.. 1985;17:25-38.
Heyde C.E., Mahheld K., Stakel P.F., Kayser R. Ultrasonography as a reliable diagnostic tool in old quadriceps tendon ruptures: a prospective multi-centre study. Knee Surg. Sports Traumatol. Arthrosc.. 2005;13:564-568.
Hides J.A., Cooper D.H., Stokes M.J. Diagnostic ultrasound imaging for measurement of the lumbar multifidus muscle in normal young adults. Physiother. Theory Pract.. 1992;8:19-26.
Hides J.A., Richardson C.A., Jull G.A. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21:2763-2769.
Hides J.A., Jull G.A., Richardson C.A. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine. 2001;26:E243-E248.
Hodges P.W., Cholewicki J. Functional control of the spine. In: Vleeming A., Mooney V., Stoeckart R., editors. Movement, Stability and Lumbopelvic Pain. Elsevier; 2007:489-512.
Hodges P.W., Gandevia S.C. Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J. Appl. Physiol.. 2000;89:967-976.
Hodges P.W., Moseley G.L. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J. Electromyogr. Kinesiol.. 2003;13:361-370.
Hodges P.W., Richardson C.A. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21:2640-2650.
Hodges P.W., Richardson C.A. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. J. Spinal Disord.. 1998;11:46-56.
Hodges P.W., Pengel L.H., Herbert R.D., Gandevia S.C. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve. 2003;27:682-692.
Hodges P.W., Sapsford R., Pengel L.H. Postural and respiratory functions of the pelvic floor muscles. Neurourol. Urodyn.. 2007;26:362-371.
Holmich P., Uhrskou P., Ulnits L., et al. Effectiveness of active physical training as treatment for long-standing adductor-related groin pain in athletes: randomised trial. Lancet. 1999;353(9151):439-443.
Holt M., Keene J., Graf B., et al. Treatment of osteitis pubis in athletes: Results of corticosteroid injections. Am. J. Sports Med.. 1995;23:601-606.
Howse J., Hancock S. Dance Technique and Injury Prevention. 1992. A&C Black
Hreljac A. Evaluation of lower extremity overuse injury potential in runners. Med. Sci. Sport Exerc.. 1999;32:1653-11641.
Huang V., Munarriz R., Goldstein I. Bicycle riding and erectile dysfunction: an increase in interest (and concern). [Review] [43 refs]. J. Sex. Med.. 2005;2:596-604.
Johnston C.A., Wiley J.P., Lindsay D.M., et al. Iliopsoas bursitis and tendinitis: A review. Sports Med.. 1998;25(4):271-283.
Jones R.C. Pelvic floor muscle rehabilitation. Urol. News. 2001;5:1-4.
Jones R.C., Peng Q., Shishido K., Constantinou C.E. 2D ultrasound imaging and motion tracking of pelvic floor muscle activity during abdominal manoeuvres in stress urinary incontinent women. Neurourol. Urodyn.. 2006. Abstract
Kachingwe A.F., Grech S. Proposed algorithm for the management of athletes with athletic pubalgia, (sports hernia): A case series. J. Orthop. Sports Phys. Ther.. 2008;38:768-781.
Kai B., Lee K.D., Andrews G., Wilkinson M., Forster B.B. Puck to pubalgia: imaging of groin pain in professional hockey players. Can. Assoc. Radiol. J.. 2010;6:74-79.
Karlsson J., Jerre R. The use of radiography, MRI and ultrasound in the diagnosis of hip, pelvis and groin injuries. Sports Med. Arthrosc Rev.. 1997;5:268-273.
Kendall F., McCreary E., Provance P., Rodgers M., Romani R. Muscles: Testing and Function with Posture and Pain, fifth ed. Williams & Wilkins; 2005.
Labat J.J., Riant T., Robert R., et al. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol. Urodyn.. 2008;27(4):306-310.
LaSalle M., Salimpour P., Adelstein M., Mourtzinos A., Wen C., Renzulli J., et al. Sexual and urinary tract dysfunction in female bicyclists. J. Urol.. 1999;161:269.
Laycock J., Jerwood D. Pelvic floor muscle assessment: The PERFECT scheme. Physiotherapy. 2001;87(12):631-642.
Lee D. The Pelvic Girdle. Edinburgh: Churchill Livingston; 2004.
Leibovitch I., Mor Y. The vicious cycling: bicycling related urogenital disorders. [Review] [62 refs]. Eur. Urol.. 2005;47:277-286.
Lewis C.L., Sahrmann S.A. Acetabular labral tears. Phys. Ther.. 2006;86:110-121.
Linton S.J. A review of psychological risk factors in back and neck pain. Spine. 2000;25(9):1148-1156.
Lovegrove Jones R.C. Dynamic Evaluation of Female Pelvic Floor Muscle Function Using 2D Ultrasound and Image Processing Methods. University of Southampton, Faculty of Medicine, Health and Life Sciences; 2010. Thesis/Dissertation
Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. Aust. J. Sci. Med. Sport. 1995;27:76-79.
Maffey L., Emery C. What are the risk factors for groin strain injury in sport? A systematic review of the literature. Sports Med.. 2007;37:881-894.
Marceau L., Kleinman K., Goldstein I., McKinlay J. Does bicycling contribute to the risk of erectile dysfunction? Results from the Massachusetts Male Aging Study (MMAS). Int. J. Impot. Res.. 2001;13:298-302.
Mason J.B. Acetabular labral tears in the athlete. Clin. Sports Med.. 2001;20:779-790.
McCarthy J.C., Noble P.C., Schuck M.R., et al. The Otto E. Aufranc Award: the role of labral lesions to development of early degenerative hip disease. Clin. Orthop.. 2001;393:25-37.
McGill S. Low Back Disorders. Evidence-Based Prevention and Rehabilitation. Champaign, IL: Human Kinetics; 2002.
McKenzie R., May S. The Lumbar Spine: Mechanical Diagnosis & Therapy, second ed. Orthopedic Physical Therapy Products; 2003.
Messelink B., Benson T., Berghmans B., Bo K., Corcos J., Fowler C., et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol. Urodyn.. 2005;24:374-380.
Meyers W.C., McKechnie A., Philippon M.J., Horner M.A., Zoga A.C., Devon O.N. Experience with “sports hernia” spanning two decades. Ann. Surg.. 2008;248:656-665.
Mitchell B., McCrory P., Brukner P., et al. Hip joint pathology: clinical presentation and correlation between magnetic resonance arthrography, ultrasound, and arthroscopic findings in 25 consecutive cases. Clin. J. Sport Med.. 2003;13:152-156.
Morelli V., Smith V. Groin injuries in athletes. Am. Fam. Physician. 2001;64:1405-1414.
Morelli V., Weaver V. Groin injuries and groin pain in athletes: Part 1. Prim. Care Clin. Office Pract.. 2005;32:163-183.
Moseley G.L. Reconceptualising pain according to modern pain science. Phys. Ther. Rev.. 2007;12:169-178.
Moseley G.L., Hodges P.W., Gandevia S.C. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine. 2002;27:E29-E36.
Moucharafieh R., Wehbe J., Maalouf G. Meralgia paresthetica: a result of tight new trendy low cut trousers (‘taille basse’). Int. J. Surg.. 2008;6:164-168.
Murovic J.A., Kim D.H., Tiel R.L., Kline D.G. Surgical management of 10 genitofemoral neuralgias at the Louisiana State University Health Sciences Center. Neurosurgery. 2005;56:298-303.
Nam A., Brody F. Management and therapy for sports hernia. [Review] [63 refs]. J. Am. Coll. Surg.. 2008;206:154-164.
Nanka O., Sedy J., Jarolim L. Sulcus nervi dorsalis penis: Site of origin of Alcock’s syndrome in bicycle riders. Med. Hypothesis. 2007;69:1040-1045.
Narvani A., Tsiridis E., Tai C., Thomas P. Acetabular labrum and its tears. Br. J. Sports Med.. 2003;37:207-211.
Nielsen A.B., Yde J. Epidemiology and traumatology of injuries in soccer. Am. J. Sports Med.. 1989;17(6):803-807.
O’Brian M., Delaney M. The anatomy of the hip and groin. Sports Med. Arthrosc.. 1997(5):252-267.
Ombregt L., Bisschop P., ter Veer H.J. A System of Orthopaedic Medicine, second ed. Churchill Livingstone; 2002.
O’Sullivan. Diagnosis and classification of chronic low back pain disorders: Maladaptive movement and motor control impairments as underlying mechanism. Man. Ther.. 2005;10:242-255.
O’Sullivan P.B., Phyty G.D., Twomey L.T., Allison G.T. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997;22:2959-2967.
O’Sullivan P.B., Beales D.J., Beetham J.A., Cripps J., Graf F., Lin I.B., et al. Altered motor control strategies in subjects with sacroiliac joint pain during the active straight-leg-raise test. Spine. 2002;27:E1-E8.
Oberpenning F., Roth S., Leusmann D.B., van Ahlen H., Hertie L. The Alcock Syndrome. Temporary penile insensitivity due to compression of the pudendal nerve within the Alcock canal. J. Urol.. 1994;151:423-425.
Omar I.M., Zoga A.C., Kavanagh E.C., Koulouris G., Bergin D., Gopez A.G., et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. [Review] [75 refs]. Radiographics. 2008;28:1415-1438.
Orsini N., RashidKhani B., Andersson S.O., Karlberg L., Johansson J.E., Wolk A. Long-term physical activity and lower urinary tract symptoms in men. J. Urol.. 2006;176:2546-2550.
Pizzari T., Coburn P.T., Crow J.F. Prevention and management of osteitis pubis in the Australian Football League: A qualitative analysis. Phys. Ther. Sport. 2008;9:117-125.
Pool-Goudzwaard A., van Dijke G.H., van Gurp M., Mulder P., Snijders C., Stoeckart R. Contribution of pelvic floor muscles to stiffness of the pelvic ring. Clin. Biomech.. 2004:564-571.
Potter J.J., Sauer J.L., Weisshaar C.L., Thelen D.G., Ploeg H.L. Gender differences in bicycle saddle pressure distribution during seated cycling. Med. Sci. Sports Exerc.. 2008;40:1126-1134.
Rab M., Ebmer A.J., Dellon A.L. Anatomic variability of the ilioinguinal and genitofemoral nerve: implications for the treatment of groin pain. Plast. Reconstr. Surg.. 2001;108:1618-1623.
Ramsden C.E., McDaniel M.C., Harmon R.L., Renney K.M., Faure A. Pudendal nerve entrapment as a source of intractable perineal pain. Am. J. Phys. Med. Rehabil.. 2003;82:479-484.
Reid D.C. Sports Injury Assessment and Rehabilitation. Edinburgh: Churchill Livingstone; 1992.
Ricchiuti V.S., Haas C.A., Seftel A.D., Chelimsky T., Goldstein I. Pudendal nerve injury associated with avid bicycling. J. Urol.. 1999;162:2099-2100.
Richardson C., Jull G., Hodges P., et al. Therapeutic Exercise for Spinal Segmental Stabilization in Low Back Pain. London: Churchill Livingstone; 1999.
Richardson C.A., Snijders C.J., Hides J.A., Damen L., Pas M.S., Storm J. The relation between the transversus abdominis muscles, sacroiliac joint mechanics, and low back pain. Spine. 2002;27:399-405.
Rodano R., Squadrone R., Sacchi M., Marzegan. Pressure distribution on bicycle saddles (a comparrison between saddles with a “hole” in the perineal area). Proceedings of the Symposium of the International Society of Biomechanics in Sports. 2002. Milan
Sahrmann S.A. Movement science and physical therapy. J. Phy. Ther. Educ.. 1993;7:4-7.
Sahrmann S.A. Diagnosis and Treatment of Movement Impairment Syndromes. Harcourt Health Sciences; 2002.
Sapsford R.R., Hodges P.W., Richardson C.A., Cooper D.H., Markwell S.J., Jull G.A. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol. Urodyn.. 2001;20:31-42.
Saw T., Villar R. Footballer’s hip: a report of six cases. J. Bone Joint Surg. Br.. 2004;86:655-658.
Schrader S.M., Breitenstein M.J., Clark J.C., Lowe B.D., Turner T.W. Nocturnal penile tumescence and rigidity testing in bicycling patrol officers. J. Androl.. 2002;23:927-934.
Schrader S.M., Breitenstein M.J., Lowe B.D. Cutting off the nose to save the penis. J. Sex. Med.. 2008;5:1932-1940.
Seong S., Park K., Moon J., Ry S. Bicycle saddle shape affects penile blood flow. Int. J. Impotence Res.. 2002;14:513-551.
Shacklock M. Clinical neurodynamics: A new system of musculoskeletal treatment. Elsevier-Butterworth-Heinemann; 2005.
Shindle M.K., Domb B.G., Kelly B.T. Hip and pelvic problems. Oper. Tech. Sports Med.. 2007;15:195-203.
Sommer F., Goldstein I., Korda J.B. Bicycle riding and erectile dysfunction: a review. J. Sex. Med.. 2010;7:2346-2358.
Standring S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. In Expert Consult – Online and Print [Hardcover]. Edinburgh: Churchill Livingstone; 2008.
Starling J.R., Harms B.A. Diagnosis and treatment of genitofemoral and ilioinguinal neuralgia. World J. Surg.. 1989;13(5):586-591.
Suresh S., Patel A., Porfyris S., Ryee M.Y. Ultrasound-guided serial ilioinguinal nerve blocks for management of chronic groin pain secondary to ilioinguinal neuralgia in adolescents. Paediatr. Anaesth.. 2008;18:775-778.
Swan K.G.Jr., Wolcott M. The athletic hernia: a systematic review. [Review] [44 refs]. Clin. Orthop. Relat. Res.. 2007;455:78-87.
Taylor J.A.III, Kao T.C., Albertsen P.C., Shabsigh R. Bicycle riding and its relationship to the development of erectile dysfunction. J. Urol.. 2004;172:1028-1031.
Travell J.G., Simons D.G. Myofascial pain and dysfunction. Am. Pain Soc.. 1993;2:116-121.
Tsao H., Hodges P.W. Immediate changes in feedforward postural adjustments following voluntary motor training. Exp. Brain Res.. 2007;181:537-546.
Tsao H., Hodges P.W. Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J. Electromyogr. Kinesiol.. 2008;18:559-567.
Tsao H., Galea M.P., Hodges P.W. Driving plasticity in the motor cortex in recurrent low back pain. Eur. J. Pain. 2010;14(8):832-839.
Tyler T.F., Nicholas S.J., Campbell R.J., et al. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am. J. Sports Med.. 2001;29(2):124-128.
Tyler T.F., Nicholas S.J., Campbell R.J., et al. The effectiveness of a preseason exercise program to prevent adductor muscle strains in professional ice hockey players. Am. J. Sports Med.. 2002;30(5):680-683.
Tyler T., Slattery A. Rehabilitation of the hip following sports surgery. Clinics Sports Med.. 2010;29(1):107-126.
Urquhart D.M., Hodges P.W., Allen T.J., Story I.H. Abdominal muscle recruitment during a range of voluntary exercises. Man. Ther.. 2005;10:144-153.
van Dieen J.H., Selen L.P., Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. [Review] [141 refs]. J. Electromyogr. Kinesiol.. 2003;13:333-351.
Van Dillen L.R., Sahrmann S.A., Norton B.J., Caldwell C.A., Fleming D.A., McDonnell M.K., et al. Reliability of physical examination items used for classification of patients with low back pain. Phys. Ther.. 1998;78:979-988.
Van Dillen L.R., Sahrmann S.A., Norton B.J., Caldwell C.A., McDonnell M.K., Bloom N.J. Movement system impairment-based categories for low back pain: stage 1 validation. J. Orthop. Sports Phys. Ther.. 2003;33:126-142.
Van Dillen L.R., Bloom N.J., Gombatto S.P., Susco T.M. Hip rotation range of motion in people with and without low back pain who participate in rotation-related sports. Physical Therapy in Sport. 2008;9:72-81.
van Ramshorst G.H., Kleinrensink G.J., Hermans J.J., Terkivatan T., Lange J.F. Abdominal wall paresis as a complication of laparoscopic surgery. Hernia. 2009;13:539-543.
Verrall G.M., Slavotinek J.P., Fon G.T. Incidence of pubic bone marrow oedema in Australian rules football players: relation to groin pain. Br. J. Sports Med.. 2001;35(1):28-33.
Vervest H.A., Bongers M.Y., van der Wurff A.A. Nerve injury: an exceptional cause of pain after TVT. Int. Urogynecol. J.. 2006;17:665-667.
Waddell G. The Back Pain Revolution, second ed. Edinburgh: Churchill Livingstone; 2004.
Walsh J., Hall T. Reliability, validity and diagnostic accuracy of palpation of the sciatic, tibial and common peroneal nerves in the examination of low back related leg pain. Man. Ther.. 2009;14:623-629.
Walsh J., Ther M., Hall T. Agreement and correlation between the straight leg raise and slump tests in subjects with leg pain. J. Manipulative Physiol. Ther.. 2009;32:184-192.
Weiss B.D. Non-traumatic injuries among amateur long distance bicyclists. Am. J. Sports Med.. 1985;13:187-192.
Werner J., Haggland M., Walden M., Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br. J. Sports Med.. 2009;43:1036-1040.
White A.A., Punjabi M.M. Clinical Biomechanics of the SpIne. Lippincott & Co; 1990.
Whiteside J.L., Barber M.D. Ilioinguinal/iliohypogastric neurectomy for management of intractable right lower quadrant pain after cesarean section: a case report. J. Reprod. Med.. 2005;50:857-859.
Whittaker J.L. Ultrasound Imaging for Rehabilitation of the Lumbopelvic Region: A Clinical Approach. London: Churchill Livingstone; 2007.
Woby S.R., Watson P.J., Roach N.K., Urmston M. Adjustment to chronic low back pain – the relative influence of fear-avoidance beliefs, catastrophizing, and appraisals of control. Behav. Res. Ther.. 2004;42:761-774.
Woby S.R., Roach N.K., Urmston M., Watson P.J. The relation between cognitive factors and levels of pain and disability in chronic low back pain patients presenting for physiotherapy. Eur. J. Pain. 2007;11:869-887.
Zimmerman G. Groin pain in athletes. Am. Fam. Physician. 1988;17(12):1046-1052.
Zoga A.C., Kavanagh E.C., Omar I.M., Morrison W.B., Koulouris G., Lopez H., et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247:797-807.