CHAPTER 381 Multimodality Management of Complex Cerebrovascular Lesions
For vascular pathologies of the central nervous system, effective interventions include surgery, endovascular techniques, and stereotactic radiosurgery. Some patients have complex vascular lesions that require the use of more than one treatment modality. The term multimodality treatment has been used increasingly over the past 2 decades and refers to a variety of clinical scenarios and treatment algorithms of different complexity (Table 381-1).1–9 This chapter outlines the different concepts of treatment plans involving more than one treatment modality for complex vascular lesions, including difficult cerebral aneurysms, large arteriovenous malformations (AVMs), the coexistence of AVMs and aneurysms, and complex dural arteriovenous fistulas (DAVFs).
TABLE 381-1 Different Concepts of the Term Multimodality Treatments with Examples Mentioned in the Text
Patients With Cerebral Aneurysms
Aneurysmal subarachnoid hemorrhage accounts for more than 30,000 hospitalizations annually in the United States, with an in-hospital mortality rate of 25.3%.10 Over the past 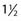 decades, management of ruptured and unruptured cerebral aneurysms has changed dramatically. Since 1995, detachable microcoils for endovascular obliteration of cerebral aneurysms have been approved and are used more frequently each year. In an international prospective randomized trial comparing coil versus clip obliteration in patients who were thought to be equally suitable for both endovascular treatment and open surgery, coil obliteration was favored over clip occlusion with regard to clinical outcome at 1 year.11 More recently, follow-up evaluation of these same patients seems to support clip ligation in patients younger than 40 years because of the lower long-term rehemorrhage rate with this procedure.12 However, neurovascular surgeons and endovascular interventionalists increasingly believe that both treatment modalities are complementary rather than competitive13 and that individualized treatment plans need to be established, especially if the clinical situation is complex. These treatment plans can include open surgery, endovascular treatment of the lesion, or a combination of these modalities in a one-step or staged fashion. In discussing the multimodality treatment of aneurysms, we focus on four distinct complex situations in which the complementary nature of clipping and coiling could best improve the clinical outcome of the patient. These situations include multiple aneurysms, surgery after failed embolization, embolization after failed surgery, and intentional combination therapy for complex and giant aneurysms.
decades, management of ruptured and unruptured cerebral aneurysms has changed dramatically. Since 1995, detachable microcoils for endovascular obliteration of cerebral aneurysms have been approved and are used more frequently each year. In an international prospective randomized trial comparing coil versus clip obliteration in patients who were thought to be equally suitable for both endovascular treatment and open surgery, coil obliteration was favored over clip occlusion with regard to clinical outcome at 1 year.11 More recently, follow-up evaluation of these same patients seems to support clip ligation in patients younger than 40 years because of the lower long-term rehemorrhage rate with this procedure.12 However, neurovascular surgeons and endovascular interventionalists increasingly believe that both treatment modalities are complementary rather than competitive13 and that individualized treatment plans need to be established, especially if the clinical situation is complex. These treatment plans can include open surgery, endovascular treatment of the lesion, or a combination of these modalities in a one-step or staged fashion. In discussing the multimodality treatment of aneurysms, we focus on four distinct complex situations in which the complementary nature of clipping and coiling could best improve the clinical outcome of the patient. These situations include multiple aneurysms, surgery after failed embolization, embolization after failed surgery, and intentional combination therapy for complex and giant aneurysms.
Multiple Modalities for Multiple Aneurysms
Intracranial aneurysms are multiple in up to 15% to 20% of patients with subarachnoid hemorrhage14 and in up to 37% of patients with cerebral aneurysms. Risk factors for multiple aneurysms include female gender and cigarette smoking15; bilateral symmetrical aneurysms (mirror aneurysms) are common. In the setting of an aneurysmal subarachnoid hemorrhage, the risk for subsequent rupture of additional incidental aneurysms is significantly higher.16
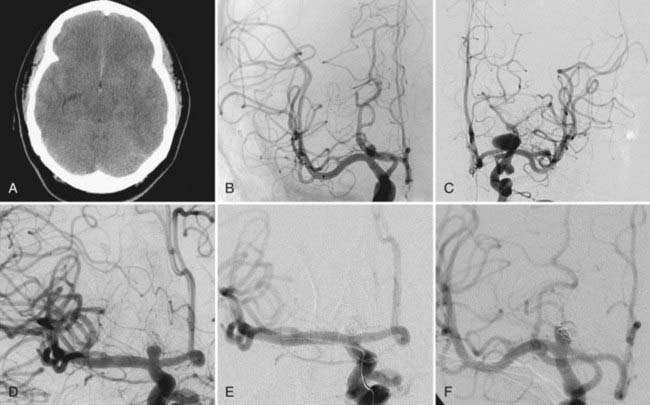
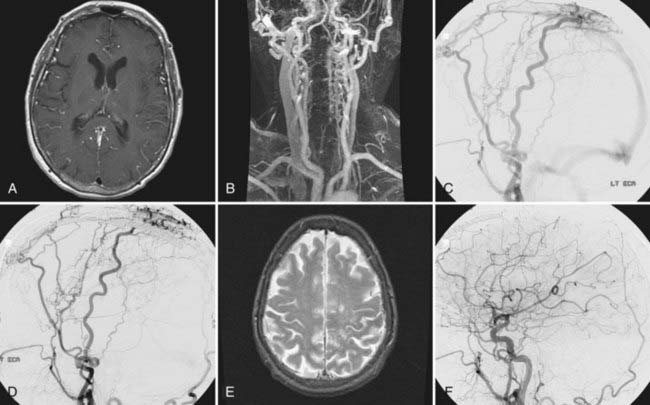
In patients with nontraumatic subarachnoid hemorrhage, it is of importance to determine which aneurysm ruptured. The location of the intracranial hemorrhage, aneurysm size, and aneurysm morphology are all important factors in deciding which aneurysm bled. If such a determination has been made, the symptomatic aneurysm should be addressed first by whatever technique is appropriate. For the remaining incidental aneurysms, treatment options include observation, simultaneous endovascular treatment if the symptomatic lesion is being coiled, simultaneous surgical obliteration if the symptomatic lesion is being clipped, delayed elective endovascular treatment, delayed elective surgical treatment, or a combination thereof (Fig. 381-1). Even subsequent endovascular and surgical treatment during the same anesthesia for two different lesions has been described. A 31-year-old patient with subarachnoid hemorrhage underwent coiling of a basilar artery aneurysm followed immediately by clipping of a right middle cerebral artery bifurcation aneurysm.17
Simultaneous treatment of all lesions in the acute or subacute phase of hemorrhage eliminates the risk of mistaking the wrong aneurysm as the site of rupture and decreases the risk for rupture of incidental aneurysms during treatment of vasospasm. Simultaneous clipping should be taken into consideration if all lesions can be approached safely through a single craniotomy and if endovascular treatment is less likely to be successful. Simultaneous endovascular treatment can be taken into consideration if the aneurysm shapes and sizes are conducive to achieving total aneurysm occlusion. This approach has been reported in particular for lesions of the posterior fossa18 and can be accomplished safely even in the acute phase of subarachnoid hemorrhage.19
One report reviewed a surgical series of 124 patients with multiple aneurysms (N = 323) and acute subarachnoid hemorrhage. In 57 patients, the index aneurysm and additional incidental aneurysms were clipped during the first operation. In 9 patients, only the index aneurysm and some of the coexisting aneurysms were treated surgically. In 55 patients, just the ruptured aneurysm was treated initially. One of the patients waiting for the additional procedure to secure an incidental aneurysm suffered a second hemorrhage. Of all patients, 78% had a Glasgow Outcome Scale score of 4 or 5.8 Another report focusing on the role of endovascular management of multiple aneurysmal lesions reviewed 38 consecutive patients with a total of 101 aneurysms. Twenty-five patients (66%) underwent embolization of more than 1 aneurysm in a single session. Of 21 patients with acute subarachnoid hemorrhage, 15 underwent embolization of all aneurysms in a single session. Excellent or good outcomes were achieved in 35 of 38 patients (92%).19
In patients who harbor multiple asymptomatic aneurysms or have recovered from an acute hemorrhagic event, the treatment plan can be elective and staged. This approach has to balance the risks and benefits of observation against the different treatment options available for all the patient’s aneurysms. The advantages of a staged procedure versus the additional strain caused by prolongation of a procedure have to be considered carefully.8 A case was reported of a 39-year-old woman with bilateral carotid occlusion and five aneurysms of the vertebrobasilar circulation who underwent clipping of a right anterior inferior cerebellar artery, a right superior cerebellar artery, and a basilar tip aneurysm and delayed coiling of a second, more posterior basilar tip aneurysm.20
Surgery after Failed Embolization of Aneurysms
Rates of failed coil embolization were found to be as high as 14.5% of attempted procedures because of an inability to place coils into the aneurysmal sac, with an intraprocedural mortality rate of 2%.21 Retreatment and rehemorrhage rates of the target aneurysm after coil embolization within the first year were 7.7% and 3.0%, respectively22; late retreatment after a mean of 20.7 months was necessary in 9.0% of patients undergoing coil embolization, with an overall retreatment rate of 17.4%.23
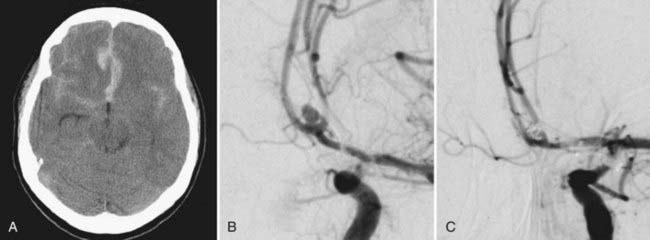
Because greater than 80% of aneurysms rupture at the dome, partial embolization and thrombosis of the dome may decrease the rupture rate until definitive treatment can be undertaken. In many cases, coil compaction over time facilitates additional coil placement, and complete obliteration of the aneurysm may be feasible. If complete obliteration by means of repeated coil embolization does not seem possible or is threatening blood flow in the parent vessel, consideration of surgery is warranted (Fig. 381-1). If coil embolization was being considered as the first option for the treatment of ruptured intracerebral aneurysms, up to 11.6% of aneurysms undergoing coil embolization were subsequently clipped24,25; multicenter trials have reported that 9.3% of coiled aneurysms will eventually require surgical clip occlusion.23 Further classification of these complex surgical cases of inadequately embolized aneurysms was attempted. Group A (65% of cases) allowed direct surgical clipping; group B (23% of cases) showed parent vessel narrowing after clip application, which required extraction of coils from the fundus and reconstruction of the neck by clip or suture; and group C (12% of cases) consisted of emergency coil removal after embolization procedures.26
A number of technical sequelae can be encountered with clip occlusion of previously coil-embolized aneurysms, in addition to the aforementioned narrowing of the parent vessel after clip placement. After a few weeks, coils can become embedded within the wall of the aneurysm and thus make coil removal difficult. This situation increases the risk of tearing the neck of the aneurysm or the parent vessel. Coils can even extrude through the aneurysm sac into the subarachnoid space27; in this case, a number of authors recommend that the coils not be extracted.28,29 Dense subarachnoid scarring around the aneurysm sac potentially endangers the preservation of exiting vessels.27 It is important to recognize that partially coiled aneurysms are frequently less mobile, which makes three-dimensional visualization more difficult at surgery.30 In contrast, some authors point out that partial coil occlusion stabilizes the dome of the aneurysm and decreases the risk for intraoperative rupture.29
Although the majority of these patients will achieve an excellent or good outcome with high occlusion rates, perioperative mortality rates as high as 7.9% have been reported.31
Unique difficulties were observed in a patient who initially underwent stent-assisted coiling of a 6-mm left posterior communicating artery aneurysm. In an attempt to surgically occlude a neck remnant, the aneurysm ripped off its base and created a hole in the internal carotid artery. The authors believe that the stent prevented the clip from gathering enough vessel tissue to close the aneurysm and hence created a tissue stress situation.29 Because stent-assisted coiling for broad-based aneurysms is becoming increasingly popular, these unique difficulties will probably be encountered more often.
Embolization after Failed Surgery for Aneurysms
Failure to completely occlude an aneurysm by surgical clipping is reported in up to 5.9% of procedures, as determined by postoperative angiography.32 Retreatment and rehemorrhage rates of the target aneurysm within the first year after clipping were found to be 1.7% and 1.3%, respectively22; late retreatment after a mean of 5.7 months was performed in 0.9% of patients undergoing clip occlusion, with an overall retreatment rate of 3.8%.23 Reoperation after incomplete clip occlusion is associated with high morbidity and mortality rates.33
In cases of incomplete clip occlusion, subsequent aneurysm regrowth, or clip slippage, coil embolization can be a useful adjunct to promote progressive thrombosis of the aneurysm (Fig. 381-2). Partial clip occlusion may also result in a smaller neck, thereby improving the likelihood of complete occlusion with subsequent coil embolization.34 A large case series (N = 21) of endovascular coil embolization after incomplete clipping reported total occlusion in 81% of patients and nearly total occlusion in the remaining patients without associated morbidity or mortality. The mean size of the aneurysm remnant before coiling was 6.4 mm. Over a mean follow-up of 22 months, no cases of subarachnoid hemorrhage or symptomatic aneurysm regrowth were noted.5 Equally promising results have been reported by other authors.35 In a review of 25 residual and recurrent previously clipped aneurysms with a mean remnant size of 11 mm, retreatment consisted of coiling (44%), reclipping (32%), parent vessel occlusion (8%), and extracranial-intracranial bypass surgery with occlusion or trapping (16%). The authors concluded that reoperation is advisable for anterior circulation aneurysm remnants less than 10 mm in size because of the superior cure rates noted on radiography.36
Intentional Multimodality Approach for the Treatment of Complex Aneurysms
Intentional staged multimodality approaches have been described for patients with acute subarachnoid hemorrhage in whom vasospasm was believed to be a relative contraindication to open surgery. After partial coil occlusion of the dome of a broad-necked middle cerebral artery aneurysm to prevent rebleeding and angioplasty of the stenotic vessel, definitive clipping was performed in delayed fashion.37
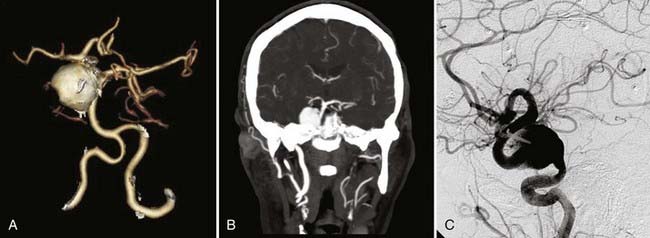
The complementary nature of endovascular and surgical approaches to the treatment of cerebral aneurysms can best be appreciated in case reports and series on intentional single-stage multimodality treatment of more complex aneurysmal lesions. A number of authors rely on intraoperative endovascular coil or balloon occlusion of proximal vessels in surgical attempts to permanently trap aneurysms,38 as well as in conjunction with complex revascularization procedures (Fig. 381-3).4,39,40 Balloon occlusion of proximal vessels has been shown to successfully facilitate surgical suction decompression of large paraclinoid aneurysms.41 In a very similar approach to these challenging lesions, suction decompression was applied endovascularly by gentle aspiration with a catheter before clip placement in 24 consecutive patients. Complete occlusion of the aneurysm was achieved in 83% of patients.1 In 4 patients with paraclinoid aneurysms, endovascular nondetachable balloons were used to temporarily stent the internal carotid artery at the neck of the aneurysm not only to gain proximal control but also to improve the accuracy of clip placement and reduce the risk for distal embolization of intraluminal thrombus.42 To perfect the clipping process of a complex anterior communicating artery aneurysm and to avoid a second staged procedure, intraoperative transaneurysmal coil placement has been reported.2
Patients With Large Arteriovenous Malformations
The incidence of AVMs of the brain is estimated to be 12.4 cases per 1 million people per year. Of these, large, Spetzler-Martin grade IV and V AVMs account for 15%.43,44 These high-grade AVMs carry an annual risk for hemorrhage of 1.5%.45 Lesions harboring all three independent risk factors for AVM hemorrhage—previous hemorrhagic manifestation, deep brain location, and exclusive deep venous drainage—have an annual hemorrhage rate of 34.4%.46 Treatment-related permanent major neurological morbidity rates are 21.9% for grade IV lesions and 16.7% for grade V lesions.47
Modalities for the treatment of AVMs include microsurgery, embolization, and radiosurgery. Multimodality treatment algorithms combining (1) embolization with surgery and possible subsequent radiosurgery, (2) embolization with radiosurgery, and (3) surgery with subsequent radiosurgery have been described. Their application, however, shows great variability among institutions.48 The fact that patients treated after 1990 with treatment plans involving microembolization procedures and radiosurgery had a lower long-term complication rate than did patients treated before 1990 led to the concept that a multimodality approach improves outcomes in selected patients with large and complex AVMs.49 A careful, individualized assessment of the risks and benefits associated with different treatment modalities along with consideration of conservative management is essential in counseling patients with high-grade AVMs.47,50
Microsurgery for Arteriovenous Malformations with Preoperative Embolization, with or without Radiosurgery
The goal of preoperative embolization of large AVMs is reduction of blood flow, reduction of the nidus,51 and elimination of deep or surgically inaccessible feeding vessels. With new liquid embolic systems (Onyx, MicroTherapeutics, Irvine, CA), a mean reduction in the nidus of 84% can be achieved.52 Preoperative embolization results in reduced operating time and less blood loss and often allows clearer delineation of the AVM margins.53,54 With presurgical embolization, five of eight high-grade AVMs were downgraded by at least 1 point on the Spetzler-Martin scale.55 The postoperative complication rate is believed to be lower because of reduced perilesional hyperemia.
A number of large case series support the use of preoperative embolization. In a report on 101 malformations, including 60 high-grade AVMs (grade IV and V), preoperative embolization achieved a 50% to 75% reduction in size in 50 patients and a 75% to 90% reduction in size in 31 patients.56 In a nonrandomized, retrospective comparison of 30 patients who underwent surgery after preoperative embolization and 41 patients who underwent surgery alone, patients in the embolization group had a significantly higher Glasgow Outcome Scale score at 1 week. At long-term follow-up, 86% of patients who underwent combined embolization and surgery were independent as compared with 66% of patients managed by surgery alone, despite patients in the latter group having smaller and lower grade AVMs.57 Preoperative embolization and resection can be performed in one anesthesia session, with the total anesthesia time averaging about 7 hours.54 The combination of preoperative embolization and surgical resection led to an angiographic cure rate of 100% at 2 years in 19 consecutive patients treated58 and was also successful in preservation of visual function in 23 of 55 patients with occipital lobe AVMs.59
Because preoperative reductive embolization is becoming more widespread, especially for high-grade, high-risk AVMs, the major risk associated with treatment of AVMs may be shifting from the resection itself to the preoperative endovascular procedure.58 In a review of 339 preoperative embolization procedures in 201 patients with AVMs of unreported grade, 9.0% of all patients had a permanent neurological deficit and 2.0% died as a direct result of the embolization procedure.60 In another retrospective series consisting of 489 procedures in 192 patients, 49.4% of whom harbored a grade IV or V AVM, the postprocedure outcomes were better, with permanent neurological deficits and death in 4.5% of patients.5 In a study reporting staged modified Rankin scores in 119 patients undergoing 240 embolizations, the combined endovascular and surgical risk for permanent disabling complication was 5%. Before embolization, 96% of the patients were considered to have modified Rankin scale scores of 0 and 1 (“no symptom” or “able to carry out all usual duties”). Before surgery, this number dropped to 90%, and late after surgery, only 80% were included in this group.61 These numbers underline the significant procedural risk associated with presurgical embolization, as well as the overall benefit of this combined approach.
Radiosurgery for Arteriovenous Malformations with Preradiation Embolization, with or without Microsurgery
Radiosurgery causes injury to the vessel walls that results in progressive luminal closure and obliteration of the malformation. The goal of AVM radiosurgery is complete obliteration with minimal morbidity. Obliteration rates of greater than 70% have been documented when the radiation dose to the AVM margin is at least 16 Gy.62 Radiosurgery as a treatment option is likely to fail if the nidus is ill defined or has been incompletely irradiated.63,64 As a result of the dose-volume relationship for AVM radiation-related complications, radiosurgery is generally recommended only for AVMs with an average maximum diameter of 3 cm or less. Therefore, the goal of preradiosurgical embolization is permanent reduction of the volume of the nidus to facilitate radiosurgery. In a series of 90 evaluable patients who underwent preradiosurgical embolization and subsequent radiosurgery, 59% exhibited complete obliteration of the AVM.65
In a recent retrospective comparison between patients undergoing preradiosurgical embolization and radiosurgery (n = 15) and patients undergoing just radiosurgery (n = 237), long-term morbidity rates were 7% and 9%, respectively. Obliteration rates were 67% and 55% and hemorrhage rates were 0% and 3%, respectively.66 Although these differences were not significant, the higher hemorrhage rate in the group undergoing just radiosurgery reflects the lack of immediate effect by radiation. This finding was demonstrated very well in a retrospective case series of 56 patients with AVMs located in the basal ganglia, thalamus, and brainstem; 7 patients (12.5%) experienced hemorrhage at a median of 12 months after treatment, 5 of whom died.67 Of 53 patients with giant AVMs with a maximum diameter of greater than 6 cm, 46 (86.8%) were treated with the multimodality approaches of embolization and radiosurgery (23 patients) and embolization, radiosurgery, and microsurgery (23 patients). After a mean follow-up of 37 months, 42 patients (79.2%) had excellent or good results, 3 patients had a poor outcome, and 8 were dead (15.1%).68 It has been reported that previously irradiated AVMs are easier to resect because they seem to be less vascular and partially thrombosed.69
Patients with Aneurysms and Arteriovenous Malformations
The coexistence of cerebral aneurysms and AVMs has been well described. The incidence of coexisting extranidal aneurysms ranges between 9.8% and 17% in patients with AVMs.70,71 the incidence of intranidal aneurysms is reported to be 8%68 or even 58% when superselective catheter-based angiography is performed.72 Extranidal aneurysms are found (1) proximally on major ipsilateral (or contralateral) vessels and contributing to the AVM in 61%, (2) distally on superficial feeders in 15.6%, (3) proximally or distally on deep feeders in 12.5%, or (4) unrelated to the AVM.70 The cause of these aneurysms is most likely related to abnormal hemodynamic stress. The association of extranidal and intranidal aneurysms with risk for hemorrhage is still a matter of debate. According to a multicenter, prospectively maintained database on 662 patients with AVMs, a hemorrhagic manifestation was not associated with any type of aneurysm.73 However, other authors reporting series of similar volume have found a high positive correlation of intranidal aneurysms and a hemorrhagic manifestation and risk for recurrent bleeding.74 Others have calculated a high and independent correlation of extranidal aneurysms and a clinical manifestation of intracranial hemorrhage.71,75,76
Aneurysms associated with cerebral AVMs are thought to arise secondary to hemodynamic stress. Therefore, not surprisingly, spontaneous regression of aneurysms after obliteration or resection of AVMs and restoration of baseline hemodynamics has been reported.77 The rate of regression of extranidal aneurysms, especially those in the midline and close to the circle of Willis, was associated with the degree of AVM occlusion.73
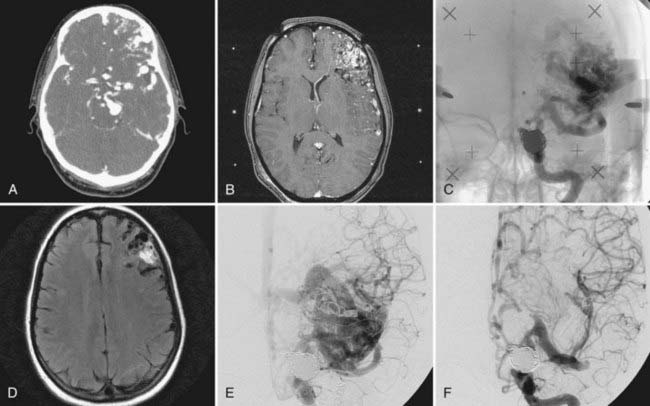
Treatment should initially be directed toward the symptomatic lesion (Fig. 381-4). As a general rule, subarachnoid hemorrhage usually arises from the aneurysm, and intraparenchymal blood arises from the AVM. Determining the site of hemorrhage can be difficult, however, because of close proximity of the lesions. In such cases, both lesions should be treated simultaneously, whenever possible.78 In elective treatment plans, recommendations differ. Some authors support treating and securing the associated aneurysms first before definitive treatment of the AVM because of the increased hemorrhage rate.76,79 On the assumption that extranidal aneurysms are secondary lesions and spontaneously disappear after obliteration of the AVM, other authors believe the AVM to be the primary treatment target.73
Microsurgery Alone for Arteriovenous Malformations with Associated Aneurysms
Patients with AVMs and aneurysms can be treated with microsurgery alone. When surgery is performed, it should be approached with the attitude of treating both lesions definitively. Such treatment is possible in select patients in whom the AVM and the aneurysm are on the same side and can be reached through a single craniotomy. For patients with aneurysmal subarachnoid hemorrhage, it may be prudent to focus on treatment of the aneurysm first by either coil embolization for more proximally located aneurysms or surgical clip occlusion for distal extranidal aneurysms, followed by treatment of the AVM after the patient has recovered. If the source of hemorrhage is the AVM and the patient does not require urgent clot evacuation, it is reasonable to delay surgical resection to permit the adjacent edema to subside. During this time, consideration should be given to securing the aneurysm before excision of the AVM. A review of 5 current patients and 22 patients from the literature with posterior fossa AVMs and associated aneurysms treated in the course of a single surgical procedure demonstrated excellent and good outcome in 82%.80
Radiosurgery for Arteriovenous Malformations and Microsurgery or Embolization for the Associated Aneurysm
For patients with deep AVMs of the basal ganglia, the thalamus, or the brainstem, radiosurgery is preferred over surgical resection to minimize neurological injury.81 Consequently, for most patients with a symptomatic extranidal aneurysm who are found to have an incidental AVM, it is reasonable to manage the aneurysm with surgery or endovascular therapy initially and perform radiosurgery for the AVM in a delayed manner.9 To determine the timing and order of management of patients with an intracerebral hemorrhage from an AVM in a location associated with high morbidity with removal and a related aneurysm, one should consider the morphology. If the aneurysm is large or if it contains any daughter sacs, strong consideration should be given to early treatment of the aneurysm before AVM radiosurgery.
The presence of intranidal aneurysms is believed to increase the likelihood of AVM hemorrhage.74 Therefore, because AVM obliteration occurs only after a latency interval, usually 1 to 4 years, consideration of particulate or liquid embolization before radiation therapy has been suggested as a means of reducing the risk for bleeding in the latency period.82 However, in a series of 14 patients with intranidal aneurysms, 10 showed obliteration of the nidus and the associated aneurysm after radiosurgery, even though the aneurysm was not always within the irradiated volume. The authors concluded that radiosurgery alone might be an option for treating both lesions at the same time.83
Patients with Dural Arteriovenous Fistulas
DAVFs, also referred to as dural AVMs, are typically acquired lesions and represent approximately 10% to 15% of intracranial vascular malformations. The cause of DAVFs is thought to be thrombosis or stenosis of a venous sinus with subsequent recruitment of shunting dural arteries.84 Patients with DAVFs often complain of pulsatile tinnitus, headaches, seizures, or ocular symptoms depending on the location of the DAVF. Intracranial hemorrhage as the initial symptom is less common in patients with DAVFs than in those with pial AVMs. The most frequent sites are the transverse and sigmoid sinus, anterior cranial base, and tentorium. Interestingly, cerebral aneurysms can be associated with DAVFs, especially those located in the anterior cranial fossa and overlying the convexities.85,86 Untreated DAVFs have an annual hemorrhage rate of 1.6%.87 A meta-analysis of the published literature on DAVFs found cortical venous drainage, venous dilation, and galenic drainage to be predisposing factors for hemorrhage.88 Treatment is typically reserved for patients with cortical venous drainage or persistent severe symptoms regardless of the nature of the venous outflow of the fistula.
Embolization of Dural Arteriovenous Fistulas
Depending on the complexity of the lesion and the clinical findings, DAVFs can be treated by embolization alone. Because many patients have a very benign natural history with spontaneous obliteration being rare but reported, the treatment options must be tailored to have an acceptably low risk. Generally speaking, transvenous embolizations are associated with a higher long-term obliteration rate than transarterial embolizations are.89 A new liquid embolic system (Onyx) has shown complete transarterial occlusion of DAVFs in five of six patients on preliminary, short-term angiographic follow-up studies.90
Microsurgery for Dural Arteriovenous Fistulas with Preoperative Embolization
Surgical resection of DAVFs is typically reserved for patients who have failed previous attempts at endovascular therapy or who are symptomatic but have poor venous access to the lesion. The choice of intraoperative techniques in these patients remains unclear; although some authors favor excision of the nidus, most believe that surgical clipping of the draining vein close to the dural lesion is safe and effective for long-term control,91 possibly in combination with presurgical embolization.92 To better define high-risk subtypes and to determine possible treatment options for DAVFs of the transverse sinus, three angiographic categories were established.93 Category 1 was defined as extremely high flow with no associated sinus occlusion or leptomeningeal retrograde venous drainage. These lesions were best treated by combined transarterial and transvenous embolization and possible surgical excision of the involved sinus if necessary. Category 2 included localized DAVFs with leptomeningeal retrograde venous drainage but not involving a sinus. Proposed treatment consisted of transarterial embolization with surgical disconnection of the leptomeningeal veins. A combined endovascular and surgical approach in this subgroup was favored by other authors as well.94,95 Category 3 DAVFs showed both sinus occlusion and cortical retrograde venous drainage. For these patients, transarterial embolization together with sinus packing and surgical resection was recommended (Fig. 381-5).
Another situation in which preoperative embolization followed by surgery may be beneficial is the management of DAVFs involving the deep cerebral venous system. DAVFs of the tentorium are traditionally difficult to obliterate with endovascular techniques because of multiple small feeding arteries, which are hard to visualize and often originate off the internal carotid arteries. A staged extracranial and intracranial transarterial embolization procedure followed by surgically assisted cannulation of the straight sinus and transvenous coil embolization of the fistula was reported in a patient with a traumatic DAVF involving the straight sinus and the tentorium.96 Similarly, in a series of seven patients with symptomatic DAVFs that were not accessible for endovascular treatment, transvenous embolization was performed without complications after surgical exposure of the junction of the transverse and the sigmoid sinus, the superior sagittal sinus, and the superior ophthalmic vein. Complete radiographic occlusion was achieved.97 For a carotid–cavernous sinus dural fistula that failed embolization, extradural exposure of the superior orbital fissure was performed and the anterior cavernous sinus cannulated for embolization under intraoperative ultrasound and angiographic guidance.3
Radiosurgery and Postradiosurgical Embolization
Staging of radiosurgery and transarterial or transvenous embolization of DAVFs provides both immediate relief of symptoms and reduction of cortical venous drainage, as well as long-term protection from intracranial hemorrhage.98,99 Typically, radiosurgery is performed before any embolization to ensure that the entire fistula is included in the irradiated volume.99
Of 23 evaluable patients with symptomatic DAVFs of the transverse and sigmoid sinuses, 20 underwent staged stereotactic radiosurgery and transarterial embolization and the 3 remaining patients received vadiosurgery alone. After treatment, the symptoms resolved completely in 20 patients of the reported series (87%); angiographically complete or nearly total occlusion was seen in 11 of 17 patients undergoing follow-up angiography.100 In another series of 18 patients with 23 DAVFs treated by radiosurgery, 10 underwent embolization procedures as well. All patients had an excellent or good clinical outcome. Of 8 patients undergoing angiographic follow-up, none showed a persistent fistula. There were 2 patients with permanent neurological deficits after embolization and 1 with temporary hemiparesis after radiosurgery.6
Brisman JL, Roonprapunt C, Song JK, et al. Intentional partial coil occlusion followed by delayed clip application to wide-necked middle cerebral artery aneurysms in patients presenting with severe vasospasm. Report of two cases. J Neurosurg. 2004;101:154-158.
CARAT investigators. Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke. 2006;37:1437-1442.
Chang SD, Marcellus ML, Marks MP, et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1-11.
Hartmann A, Mast H, Mohr JP, et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36:2431-2435.
James Ling A, D’Urso PS, Madan A. Simultaneous microsurgical and endovascular management of multiple cerebral aneurysms in acute subarachnoid haemorrhage. J Clin Neurosci. 2006;13:784-788.
Kiyosue H, Hori Y, Okahara M, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004;24:1637-1653.
Kong DS, Kwon KH, Kim JS, et al. Combined surgical approach with intraoperative endovascular embolization for inaccessible dural arteriovenous fistulas. Surg Neurol. 2007;68:72-77.
Lanzino G, Fraser K, Kanaan Y, et al. Treatment of ruptured intracranial aneurysms since the International Subarachnoid Aneurysm Trial: practice utilizing clip ligation and coil embolization as individual or complementary therapies. J Neurosurg. 2006;104:344-349.
Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46:793-800.
Rabinstein AA, Nichols DA. Endovascular coil embolization of cerebral aneurysm remnants after incomplete surgical obliteration. Stroke. 2002;33:1809-1815.
Richling B, Killer M, Al-Schameri AR, et al. Therapy of brain arteriovenous malformations: multimodality treatment from a balanced standpoint. Neurosurgery. 2006;59(5 suppl 2):S148-S157.
Wiebers DO, Whisnant JP, Huston J3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
1 Ng PY, Huddle D, Gunel M, et al. Intraoperative endovascular treatment as an adjunct to microsurgical clipping of paraclinoid aneurysms. J Neurosurg. 2000;93:554-560.
2 Krayenbühl N, Krisht AF. Intraoperative transaneurysmal coil-assisted clip occlusion of a complex anterior communicating artery aneurysm. Technical note. J Neurosurg. 2007;107:202-205.
3 Krisht AF, Burson T. Combined pretemporal and endovascular approach to the cavernous sinus for the treatment of carotid-cavernous dural fistulae: technical case report. Neurosurgery. 1999;44:415-418.
4 Kim LJ, Albuquerque FC, McDougall C, et al. Combined surgical and endovascular treatment of a recurrent A3-A3 junction aneurysm unsuitable for stand-alone clip ligation or coil occlusion. Technical note. Neurosurg Focus. 2005;18(2):E6.
5 Jayaraman MV, Marcellus ML, Hamilton S, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol. 2008;29:242-246.
6 Koebbe CJ, Singhal D, Sheehan J, et al. Radiosurgery for dural arteriovenous fistulas. Surg Neurol. 2005;64:392-398.
7 Rabinstein AA, Nichols DA. Endovascular coil embolization of cerebral aneurysm remnants after incomplete surgical obliteration. Stroke. 2002;33:1809-1815.
8 Imhof HG, Yonekawa Y. Management of ruptured aneurysms combined with coexisting aneurysms. Acta Neurochir Suppl. 2005;94:93-96.
9 Ezura M, Takahashi A, Jokura H, et al. Endovascular treatment of aneurysms associated with cerebral arteriovenous malformations: experiences after the introduction of Guglielmi detachable coils. J Clin Neurosci. 2000;7(suppl 1):14-18.
10 Shea AM, Reed SD, Curtis LH, et al. Characteristics of nontraumatic subarachnoid hemorrhage in the United States in 2003. Neurosurgery. 2007;61:1131-1137.
11 Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
12 Mitchell P, Kerr R, Mendelow AD, et al. Could late rebleeding overturn the superiority of cranial aneurysm coil embolization over clip ligation seen in the International Subarachnoid Aneurysm Trial? J Neurosurg. 2008;108:437-442.
13 Lanzino G, Fraser K, Kanaan Y, et al. Treatment of ruptured intracranial aneurysms since the International Subarachnoid Aneurysm Trial: practice utilizing clip ligation and coil embolization as individual or complementary therapies. J Neurosurg. 2006;104:344-349.
14 Ostergaard JR, Hog E. Incidence of multiple intracranial aneurysms. Influence of arterial hypertension and gender. J Neurosurg. 1985;63:49-55.
15 Qureshi AI, Suarez JI, Parekh PD, et al. Risk factors for multiple intracranial aneurysms. Neurosurgery. 1998;43:22-26.
16 Wiebers DO, Whisnant JP, Huston J3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
17 James Ling A, D’Urso PS, Madan A. Simultaneous microsurgical and endovascular management of multiple cerebral aneurysms in acute subarachnoid haemorrhage. J Clin Neurosci. 2006;13:784-788.
18 Massoud TF, Guglielmi G, Vinuela F, et al. Endovascular treatment of multiple aneurysms involving the posterior intracranial circulation. AJNR Am J Neuroradiol. 1996;17:549-554.
19 Solander S, Ulhoa A, Vinuela F, et al. Endovascular treatment of multiple intracranial aneurysms by using Guglielmi detachable coils. J Neurosurg. 1999;90:857-864.
20 Konishi Y, Sato E, Shiokawa Y, et al. A combined surgical and endovascular treatment for a case with five vertebro-basilar aneurysms and bilateral internal carotid artery occlusions. Surg Neurol. 1998;50:363-366.
21 Shanno GB, Armonda RA, Benitez RP, et al. Assessment of acutely unsuccessful attempts at detachable coiling in intracranial aneurysms. Neurosurgery. 2001;48:1066-1072.
22 CARAT investigators. Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke. 2006;37:1437-1442.
23 Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke. 2007;38:1538-1544.
24 Gurian JH, Martin NA, King WA, et al. Neurosurgical management of cerebral aneurysms following unsuccessful or incomplete endovascular embolization. J Neurosurg. 1995;83:843-853.
25 Raftopoulos C, Mathurin P, Boscherini D, et al. Prospective analysis of aneurysm treatment in a series of 103 consecutive patients when endovascular embolization is considered the first option. J Neurosurg. 2000;93:175-182.
26 Raftopoulos C, Vaz G, Docquier M, et al. Neurosurgical management of inadequately embolized intracranial aneurysms: a series of 17 consecutive cases. Acta Neurochir (Wien). 2007;149:11-19.
27 König RW, Kretschmer T, Antoniadis G, et al. Neurosurgical management of previously coiled recurrent intracranial aneurysms. Zentralbl Neurochir. 2007;68:8-13.
28 Civit T, Auque J, Marchal JC, et al. Aneurysm clipping after endovascular treatment with coils: a report of eight patients. Neurosurgery. 1996;38:955-960.
29 Minh T, Hwang PY, Nguyen KC, et al. Neurosurgical management of intracranial aneurysms following unsuccessful or incomplete endovascular therapy. Br J Neurosurg. 2006;20:306-311.
30 Veznedaroglu E, Benitez RP, Rosenwasser RH. Surgically treated aneurysms previously coiled: lessons learned. Neurosurgery. 2004;54:300-303.
31 Zhang YJ, Barrow DL, Cawley CM, et al. Neurosurgical management of intracranial aneurysms previously treated with endovascular therapy. Neurosurgery. 2003;52:283-293.
32 Sindou M, Acevedo JC, Turjman F. Aneurysmal remnants after microsurgical clipping: classification and results from a prospective angiographic study (in a consecutive series of 305 operated intracranial aneurysms). Acta Neurochir (Wien). 1998;140:1153-1159.
33 Drake CG, Friedman AH, Peerless SJ. Failed aneurysm surgery. Reoperation in 115 cases. J Neurosurg. 1984;61:848-856.
34 Marks MP, Steinberg GK, Lane B. Combined use of endovascular coils and surgical clipping for intracranial aneurysms. AJNR Am J Neuroradiol. 1995;16:15-18.
35 Bendok BR, Ali MJ, Malisch TW, et al. Coiling of cerebral aneurysm remnants after clipping. Neurosurgery. 2002;51:693-697.
36 Hoh BL, Carter BS, Putman CM, et al. Important factors for a combined neurovascular team to consider in selecting a treatment modality for patients with previously clipped residual and recurrent intracranial aneurysms. Neurosurgery. 2003;52:732-738.
37 Brisman JL, Roonprapunt C, Song JK, et al. Intentional partial coil occlusion followed by delayed clip application to wide-necked middle cerebral artery aneurysms in patients presenting with severe vasospasm. Report of two cases. J Neurosurg. 2004;101:154-158.
38 Pelz DM, Ferguson GG, Lownie SP, et al. Combined endovascular/neurosurgical therapy of blister-like distal internal carotid aneurysms. Can J Neurol Sci. 2003;30:49-53.
39 Matsumoto K, Masaki H, Hirai M, et al. Combined surgical and intraoperative endovascular approach for a giant internal carotid artery aneurysm in the high cervical region. Minim Invasive Neurosurg. 2002;45:112-113.
40 Ponce FA, Albuquerque FC, McDougall CG, et al. Combined endovascular and microsurgical management of giant and complex unruptured aneurysms. Neurosurg Focus. 2004;17(5):E11.
41 Arnautovic KI, Al-Mefty O, Angtuaco E. A combined microsurgical skull-base and endovascular approach to giant and large paraclinoid aneurysms. Surg Neurol. 1998;50:504-518.
42 Thorell W, Rasmussen P, Perl J, et al. Balloon-assisted microvascular clipping of paraclinoid aneurysms. Technical note. J Neurosurg. 2004;100:713-716.
43 Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001;95:633-637.
44 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
45 Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98:3-7.
46 Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
47 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-6.
48 Richling B, Killer M, Al-Schameri AR, et al. Therapy of brain arteriovenous malformations: multimodality treatment from a balanced standpoint. Neurosurgery. 2006;59(5 suppl 2):S148-S157.
49 Uno M, Satoh K, Matsubara S, et al. Does multimodality therapy of arteriovenous malformations improve patient outcome? Neurol Res. 2004;26:50-54.
50 Ferch RD, Morgan MK. High-grade arteriovenous malformations and their management. J Clin Neurosci. 2002;9:37-40.
51 Mizoi K, Jokura H, Yoshimoto T, et al. Multimodality treatment for large and critically located arteriovenous malformations. Neurol Med Chir (Tokyo). 1998;38(suppl):186-192.
52 Weber W, Kis B, Siekmann R, et al. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61:244-252.
53 Jafar JJ, Davis AJ, Berenstein A, et al. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78:60-69.
54 Westphal M, Cristante L, Grzyska U, et al. Treatment of cerebral arteriovenous malformations by neuroradiological intervention and surgical resection. Acta Neurochir (Wien). 1994;130:20-27.
55 Kinouchi H, Mizoi K, Takahashi A, et al. Combined embolization and microsurgery for cerebral arteriovenous malformation. Neurol Med Chir (Tokyo). 2002;42:372-378.
56 Vinuela F, Dion JE, Duckwiler G, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75:856-864.
57 DeMeritt JS, Pile-Spellman J, Mast H, et al. Outcome analysis of preoperative embolization with N-butyl cyanoacrylate in cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 1995;16:1801-1807.
58 Deruty R, Pelissou-Guyotat I, Morel C, et al. Reflections on the management of cerebral arteriovenous malformations. Surg Neurol. 1998;50:245-255.
59 Sinclair J, Marks MP, Levy RP, et al. Visual field preservation after curative multi-modality treatment of occipital lobe arteriovenous malformations. Neurosurgery. 2005;57:655-667.
60 Taylor CL, Dutton K, Rappard G, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810-812.
61 Hartmann A, Mast H, Mohr JP, et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36:2431-2435.
62 Flickinger JC, Pollock BE, Kondziolka D, et al. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873-879.
63 Gallina P, Merienne L, Meder JF, et al. Failure in radiosurgery treatment of cerebral arteriovenous malformations. Neurosurgery. 1998;42:996-1002.
64 Kwon Y, Jeon SR, Kim JH, et al. Analysis of the causes of treatment failure in gamma knife radiosurgery for intracranial arteriovenous malformations. J Neurosurg. 2000;93(suppl 3):104-106.
65 Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
66 Izawa M, Chernov M, Hayashi M, et al. Combined management of intracranial arteriovenous malformations with embolization and Gamma Knife radiosurgery: comparative evaluation of the long-term results. Surg Neurol. 2009;71:43-52.
67 Pollock BE, Gorman DA, Brown PD. Radiosurgery for arteriovenous malformations of the basal ganglia, thalamus, and brainstem. J Neurosurg. 2004;100:210-214.
68 Chang SD, Marcellus ML, Marks MP, et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1-11.
69 Steinberg GK, Chang SD, Levy RP, et al. Surgical resection of large incompletely treated intracranial arteriovenous malformations following stereotactic radiosurgery. J Neurosurg. 1996;84:920-928.
70 Cunha e Sa MJ, Stein BM, Solomon RA, et al. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1992;77:853-859.
71 Stapf C, Mohr JP, Pile-Spellman J, et al. Concurrent arterial aneurysms in brain arteriovenous malformations with haemorrhagic presentation. J Neurol Neurosurg Psychiatry. 2002;73:294-298.
72 Turjman F, Massoud TF, Vinuela F, et al. Aneurysms related to cerebral arteriovenous malformations: superselective angiographic assessment in 58 patients. AJNR Am J Neuroradiol. 1994;15:1601-1605.
73 Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46:793-800.
74 Redekop G, TerBrugge K, Montanera W, et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539-546.
75 Kim EJ, Halim AX, Dowd CF, et al. The relationship of coexisting extranidal aneurysms to intracranial hemorrhage in patients harboring brain arteriovenous malformations. Neurosurgery. 2004;54:1349-1357.
76 Thompson RC, Steinberg GK, Levy RP, et al. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery. 1998;43:202-211.
77 Stiefel MF, Al-Okaili R, Weigele JB, et al. De novo aneurysm formation and regression after brain arteriovenous malformation embolization: case report. Surg Neurol. 2007;67:99-101.
78 Liu Y, Zhu S, Jiao L, et al. Cerebral arteriovenous malformations associated with aneurysms—a report of 10 cases and literature review. J Clin Neurosci. 2000;7:254-256.
79 Nakahara I, Taki W, Kikuchi H, et al. Endovascular treatment of aneurysms on the feeding arteries of intracranial arteriovenous malformations. Neuroradiology. 1999;41:60-66.
80 Kaptain GJ, Lanzino G, Do HM, et al. Posterior inferior cerebellar artery aneurysms associated with posterior fossa arteriovenous malformation: report of five cases and literature review. Surg Neurol. 1999;51:146-152.
81 Lawton MT, Hamilton MG, Spetzler RF. Multimodality treatment of deep arteriovenous malformations: thalamus, basal ganglia, and brain stem. Neurosurgery. 1995;37:29-35.
82 Marks MP, Lane B, Steinberg GK, et al. Intranidal aneurysms in cerebral arteriovenous malformations: evaluation and endovascular treatment. Radiology. 1992;183:355-360.
83 Vymazal J, Liscak R, Novotny JJr, et al. The role of Gamma Knife radiosurgery in arteriovenous malformation with aneurysms. Stereotact Funct Neurosurg. 1999;72(suppl 1):175-184.
84 Mullan S. Reflections upon the nature and management of intracranial and intraspinal vascular malformations and fistulae. J Neurosurg. 1994;80:606-616.
85 Suzuki S, Tanaka R, Miyasaka Y, et al. Dural arteriovenous malformations associated with cerebral aneurysms. J Clin Neurosci. 2000;7(suppl 1):36-38.
86 Chen Z, Zhu G, Feng H, et al. Dural arteriovenous fistula of the anterior cranial fossa associated with a ruptured ophthalmic aneurysm: case report and review of the literature. Surg Neurol. 2008;69:318-321.
87 Brown RDJr, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994;81:531-538.
88 Awad IA, Little JR, Akarawi WP, et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839-850.
89 Kiyosue H, Hori Y, Okahara M, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004;24:1637-1653.
90 Carlson AP, Taylor CL, Yonas H. Treatment of dural arteriovenous fistula using ethylene vinyl alcohol (Onyx) arterial embolization as the primary modality: short-term results. J Neurosurg. 2007;107:1120-1125.
91 Hoh BL, Choudhri TF, Connolly ESJr, et al. Surgical management of high-grade intracranial dural arteriovenous fistulas: leptomeningeal venous disruption without nidus excision. Neurosurgery. 1998;42:796-804.
92 Pelz DM, Lownie SP, Fox AJ, et al. Intracranial dural arteriovenous fistulae with pial venous drainage: combined endovascular-neurosurgical therapy. Can J Neurol Sci. 1997;24:210-218.
93 Goto K, Sidipratomo P, Ogata N, et al. Combining endovascular and neurosurgical treatments of high-risk dural arteriovenous fistulas in the lateral sinus and the confluence of the sinuses. J Neurosurg. 1999;90:289-299.
94 Barnwell SL, Halbach VV, Dowd CF, et al. A variant of arteriovenous fistulas within the wall of dural sinuses. Results of combined surgical and endovascular therapy. J Neurosurg. 1991;74:199-204.
95 Misra M, Nijensohn E, Debrun GM, et al. Transverse sinus dural fistula: combined surgical and endovascular approach: a case report. Surg Neurol. 1996;46:129-134.
96 Zink WE, Meyers PM, Connolly ES, et al. Combined surgical and endovascular management of a complex posttraumatic dural arteriovenous fistula of the tentorium and straight sinus. J Neuroimaging. 2004;14:273-276.
97 Kong DS, Kwon KH, Kim JS, et al. Combined surgical approach with intraoperative endovascular embolization for inaccessible dural arteriovenous fistulas. Surg Neurol. 2007;68:72-77.
98 Pollock BE, Nichols DA, Garrity JA, et al. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999;45:459-466.
99 Link MJ, Coffey RJ, Nichols DA, et al. The role of radiosurgery and particulate embolization in the treatment of dural arteriovenous fistulas. J Neurosurg. 1996;84:804-809.
100 Friedman JA, Pollock BE, Nichols DA, et al. Results of combined stereotactic radiosurgery and transarterial embolization for dural arteriovenous fistulas of the transverse and sigmoid sinuses. J Neurosurg. 2001;94:886-891.