CHAPTER 57 Motor, Sensory, and Language Mapping and Monitoring for Cortical Resections
Functional localization of eloquent regions of cortex is important for minimizing the morbidity associated with the removal of abnormal tissue. The techniques used for functional localization have been adapted from those that have been used for many years during epilepsy surgery for the removal of tumors and vascular malformations involving eloquent cortex and subcortical white matter. Because of our increased ability to identify functional and eloquent cortex, previously unresectable tumors and arteriovenous malformations are no longer inoperable.1–4
This chapter reviews the basics of functional mapping, including preoperative planning, the Wada test, intraoperative mapping techniques, language localization, and resection strategies. The major functional areas that can be defined during surgery are listed in Table 57-1.
TABLE 57-1 Areas Identified by Functional Mapping
| Motor pathways | Primary motor cortex, subcortical corona radiata, internal capsule, cerebral peduncle |
| Supplementary motor area | Motor cortex and descending motor pathways |
| Insula | Dominant: language localization and subcortical motor pathways |
| Nondominant: subcortical motor pathways | |
| Language localization | Dominant hemisphere: posterior frontal, perisylvian, temporal insula, subcortical arcuate fasciculus, inferior occipitofrontal fasciculus |
| Sensory pathways | Primary sensory cortex |
| Intractable seizures | Electrocorticography, grid mapping of ictal onsets |
Preoperative Planning
Magnetic resonance imaging (MRI) has been extremely helpful in predicting the relationship of motor cortex to a tumor by identifying a few constant MRI landmarks. On T2-weighted axial images near the convexity, a pair of mirror-image lines nearly perpendicular to the falx may be readily identified and represent the central sulcus.5,6 Large lesions may compress the sulcus and distort the regional anatomy, but the landmark is usually identified by comparing the hemispheres on T1- and T2-weighted images. Although less sensitive, a midline sagittal image and a lateral parasagittal image may be viewed with respect to the marginal ramus of the cingulate sulcus and a perpendicular line drawn from the posterior roof of the insular triangle to identify the rolandic cortex.
The anterior suprasylvian region can have varying sulcus topography, and a classification based on anatomic landmarks has been published by Ebeling and coworkers.7 Correlation has been shown between the structure of the frontal operculum as seen on MRI and the location of Broca’s area, which allows preoperative prediction of this location.8
Patients with dense hemiparesis are not good candidates for mapping the motor pathways intraoperatively regardless of the stimulation used. Volitional movements of the face and extremities may be stimulated by cortical and subcortical mapping intraoperatively, but children younger than 5 to 7 years often have an electrically inexcitable cortex when a direct stimulating current is applied with a bipolar electrode.9,10 Complex stimulating paradigms may still, however, bring out the excitability of pediatric motor cortex.11 With the use of somatosensory evoked potentials (SSEPs), phase reversal over the central sulcus is available if direct stimulation mapping cannot be accomplished easily.12–15 Insertion of a subdural electrode array under general anesthesia followed by extraoperative mapping may allow the mapping of motor, sensory, language, and ictal seizure onsets in children before early adolescence and in uncooperative adults. These techniques may be contraindicated in those with significant cerebral edema from malignant gliomas or metastatic tumors and may expose patients to a second craniotomy with its inherent risk of poor contact with the cortical surface as a result of blood or cerebrospinal fluid collecting underneath the electrodes. Delayed infection is also possible.16
Foundations of Language Mapping in Epilepsy Surgery
Stimulation mapping during language measurement in awake adults has shown several features of the cortical organization of language that are not anticipated from the effect of brain lesions.17
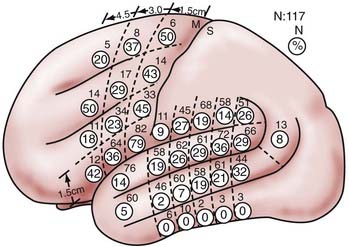
Several of these features are of major importance in planning dominant hemisphere resections, including the high degree of localization of sites with repeated evoked errors in one language measure (and thus essential for function), but with broad variance across the patient population in terms of the exact locations of these sites. In a series of 117 patients undergoing intraoperative stimulation mapping in the left dominant perisylvian cortex during naming, Ojemann and colleagues found that most patients had essential sites with surface areas of 2 cm or less, with only 16% having an area of essential language sites as large as 6 cm.18 It has been shown that if another language is acquired as a second language, more diffuse localization is seen than with the primary language (Haglund and Ojemann, unpublished observations). It has also been shown in sign language patients that localization of American sign language differs slightly from that of naming in hearing patients proficient in sign language.19 The discrete localization is evident in both the frontal and temporoparietal sites and has been demonstrated with naming and word and sentence reading as language measures. Some sites have very sharp boundaries, whereas others have a surrounding area in which occasional errors are evoked, thus suggesting a more graded transition from cortex unrelated to language to that essential for it.20,21
The considerable variance in language localization is illustrated in Figure 57-1. The percentage of essential language sites in the entire series is shown in the circles and demonstrates a range in the temporoparietal region of 2% to 36% of patients with essential language sites in a single area. Note the 14% of essential language sites in the anterior superior temporal gyrus and 5% of such sites in the anterior middle temporal gyrus, in front of the central sulcus. In the posterior language area, no local area was crucial for language in more than about a third of the patients. This variation in language localization is substantially greater than the morphologic variability in the perisylvian cortex, although this is also substantial.22,23 However, no cytoarchitectonic area seems to have a reliable relationship to language. It is the combination of discrete localization of essential language areas in the individual patient and the great variation in their location across the population that form the basis for using stimulation mapping rather than anatomic landmarks in planning resection near eloquent cortex.
Stimulation mapping in children has shown a lower frequency of sites of stimulation-induced errors than in the adult population.24 This finding implies that new language areas may still arise with maturation in children during the age range of 4 through 16 years. In children younger than 10 years, language cortex is less likely to be identified by stimulation mapping than in older children. Wada testing seems to be more likely to be successful than stimulation mapping in this younger age group.25 Extraoperative electrocortical stimulation mapping, however, is an established procedure for surgery near eloquent cortex in children.26 Because awake craniotomy is less tolerable for children, implantation of subdural stimulation electrodes allows precise extraoperative mapping of cortical function without psychological trauma.
Anatomic knowledge of subcortical white matter tract connectivity in the temporal lobe is important for preventing postoperative deficits. The uncinate fasciculus connects the uncus, amygdala, and hippocampal gyrus to the orbital and frontopolar cortex.27 No functional role has yet been attributed to the uncinate fasciculus, and resection of it is performed routinely in epilepsy surgery.
The inferior longitudinal fasciculus connects the anterior part of the temporal lobe to the occipital pole and has also been shown to play no role in language processing.28 The inferior occipitofrontal fasciculus runs from the occipital lobe laterally to the lateral wall of the temporal horn of the lateral ventricle and then continues through the external capsule to the orbitofrontal and dorsolateral prefrontal cortices. Direct stimulation of this pathway induces semantic paraphasias,29 so care should be taken to not disrupt this pathway during surgery. The superior longitudinal fasciculus is also called the arcuate fasciculus and connects Wernicke’s area in the posterior and superior temporal cortex with Broca’s area in the frontal lobe. Preservation of this pathway during surgery is mandatory because section of it produces phonemic paraphasias.30 Finally, it is important to preserve the optical pathways to prevent the development of permanent postoperative hemianopia. Intraoperative stimulation mapping can elicit a transient shadow that can be used to identify the visual pathways.31
Assessment of the effects of stimulation on an array of language-related functions (naming, reading, recent verbal memory, orofacial mimicry, and speech sound identification) in a series of 14 patients provided the basis for a model of language organization in the lateral perisylvian cortex. This model included a perisylvian area involving the superior temporal and anterior parietal as well as the posterior frontal lobes important for speech production and perception, a surrounding zone of specialized sites, some of which are related to syntax, and an even more peripheral area related to recent verbal memory.32,33 Significant interference with reading has been noted with stimulation of the lower part of the precentral and postcentral gyri; the dominant supramarginal, angular, and posterior part of the superior temporal gyri; the dominant inferior and middle frontal gyri; and the posterior part of the dominant middle temporal gyrus.34
Positive language sites in bilingual patients may or may not colocalize. In an individual who acquired both languages during infancy, anomia was demonstrated for both languages when stimulation was performed at the same sites.35 Four patients who acquired their second language during early school years (5 or 6 years of age) exhibited varying degrees of anomia in the second language.
Different tasks such as naming, reading, and responding may share the same cortical site, in which case they are referred to as multiuse sites.36 Multimodality language mapping can generate an accurate map of eloquent language cortex in bilingual patients. Single-task sites are defined as sites where one task is disrupted across both languages with stimulation. Single-use sites are sites where one task is disrupted in only one language with stimulation. Cortical mapping of a bilingual patient has shown the presence of multiuse, single-task, and single-use sites, which underlines the necessity to test for both different languages and different language modalities if optimal postoperative functional outcomes are to be achieved.37
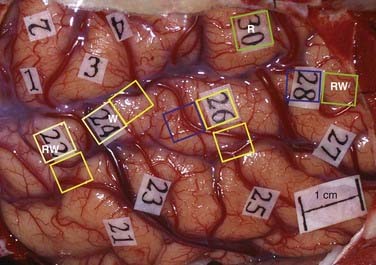
Many neurosurgical operating teams rely solely on visual naming tasks for the intraoperative testing of language function. Resection of auditory naming sites has, however, been shown to lead to postoperative word-finding difficulties.38 Preservation of auditory naming and reading sites is critical for preserving language function, and intraoperative removal of these sites may account for up to 25% of the language deficits seen postoperatively.39 Figure 57-2 illustrates that there are distinct as well as overlapping language sites that can be identified during intraoperative stimulation mapping for visual naming, auditory naming, reading, and word finding.
The extent of separation of language-related functions and the relationships of areas to one another constitute an active area of research in stimulation mapping techniques and single-unit microelectrode recordings.40–43
The Wada Test
In their original article in 1960, Juhn Wada and Theodore Rasmussen at the Montreal Neurological Institute described an invasive procedure for the lateralization of speech that has become to be known as the Wada test.44 The procedure involves the injection of 150 to 200 mg of amobarbital (Amytal) sodium into the common carotid artery. An angiogram should be performed before the injection to rule out persistent trigeminal, otic, hypoglossal, or proatlantic arteries and avoid respiratory or circulatory problems as a result of shunting of the injection solution to the brainstem. The injection is made with the patient counting, the forearms up in the air, and the fingers either moving constantly or gripping an examiner’s hands. As the injection is completed, the contralateral arm will become hemiplegic and flaccid. The patient is usually hesitant in counting on the dominant and nondominant side toward the end of the injection but then is quickly able to resume counting and naming objects while the contralateral hemiplegia still persists if the nondominant hemisphere is injected. In the case of injection into the dominant hemisphere, the patient is unable to continue counting or naming objects while the contralateral hemiplegia is complete. The patient is still able to follow commands, although on the side ipsilateral to the injection, which is tested to ensure that the aphasia is not due to confusion. At doses of 150 to 200 mg of Amytal Sodium, the contralateral hemiplegia typically lasts 1.5 to 5 minutes. Thirty to 90 seconds after the contralateral hemiplegia begins to resolve, the patient regains the ability to answer questions requiring yes and no answers. This is followed by a period of dysphasia that typically lasts for 1 to 3 minutes until normal speech is restored.
Although the Wada test has proved to be an important tool in the presurgical evaluation of epilepsy surgery patients for more than 50 years, it has lost some of its clinical significance over recent years with the advent of newer imaging techniques.45 The majority of epilepsy centers no longer conduct a Wada test on every surgical candidate. In one multicenter study, 50% of respondents stated that they used the Wada test for less than 25% of their surgical epilepsy patients.46 Functional MRI has largely replaced the Wada test for language lateralization in the preoperative evaluation of epilepsy patients to answer the question of whether language mapping should be performed during the resection. Therefore, the Wada test is now used mostly to screen for a patient’s postoperative risk for memory deficits and amnesia. The indication for Wada testing in these cases may be limited to bilateral hippocampal pathology or epileptiform activity because one normal remaining hippocampus on fine-cut MRI scans after resection can usually sustain memory. The advent of MRI technology has further diminished the importance of Wada testing over the years since bilateral hippocampal anatomic abnormalities can now be studied with high-resolution coronal MRI.
Amnesia after unilateral anterior temporal lobe resection is a very rare occurrence.47 Only about 20 patients have been reported in the literature.48 In 3 of these patients, Wada testing had been performed before surgery and accurately predicted postoperative amnesia.49–51 Therefore, surgical resection is often not performed on patients who fail the memory portion of the Wada test. However, one study involving 10 patients showed that all patients still retained memory after temporal lobe resections even if they failed the memory portion of the Wada test.52 Other investigators have also demonstrated that a failed memory portion of the Wada test does not predict an amnestic syndrome,53 and many epilepsy centers repeat the Wada test until the patient passes. Thus, use of the Wada test to evaluate for postoperative amnesia is also controversial. Noninvasive neuropsychological tests have been shown to be accurate predictors of postoperative neurocognitive and memory decline, so the Wada test does not always need to be used for this assessment as well.54,55
The Wada test is an invasive procedure with a reported complication rate of 0.5% to 10%.56 Consequently, patients should be carefully selected on a case-by-case basis to determine the appropriateness of Wada testing. Many of the patients now undergoing Wada testing have disconcordant findings on video-electroencephalographic (EEG) recordings, high-resolution fine-cut hippocampal MRI, or neuropsychological testing. Furthermore, some clinicians have completely omitted Wada testing from their decision-making algorithms for epilepsy surgery.57 Currently, the Wada test is used mostly to test for the ability to sustain memory in the setting of bilateral hippocampal pathology.
Noninvasive Brain Mapping
Functional MRI, positron emission tomography (PET), and magnetoencephalography (MEG) are noninvasive tools used to search for the location of eloquent cortex.58,59 Functional MRI is based on measurement of the real-time increase in deoxyhemoglobin in the venous structures associated with motor movement, sensory perception, or speech generation.58
The question is whether functional MRI as a noninvasive method can replace the Wada test in determining language lateralization. Although lesion and Wada studies have shown that only the left hemisphere is dominant in the majority of subjects, functional MRI typically demonstrates some degree of activity in the hemisphere contralateral to the Wada-dominant hemisphere in virtually all individuals. The language lateralization index is therefore used in functional MRI to determine hemispheric dominance. One method of calculating this index consists of defining an area of interest that has a known association with language function on MRI (Broca’s area, for example) and subsequently performing a simple bilateral suprathreshold count in the region of interest.60 Reproducibility of the language lateralization index has, however, been found to strongly vary between task analyses. Object naming has been found to be a more sensitive measure of speech localization than has number counting.61 The language lateralization index showed significant correlation between sessions in the same individual for verb generation tasks, whereas no significant correlation was found for picture naming and only a weak correlation was seen in the antonym generation task.62 Therefore, only the verb generation task allows a reliable calculation of the lateralization index across sessions. When the verb generation task was used to determine hemispheric dominance, functional MRI was concordant with invasive measures (Wada testing or cortical stimulation) in all but 1 of 23 patients in one study.63 Correlation of the results for language lateralization between functional MRI and Wada testing was found to be highly significant in another study.64 Thus, functional MRI based on the verb generation task has replaced Wada testing at many institutions in the United States for the determination of language dominance.
The inability of functional MRI to currently produce reliable results across all language tasks has raised concern about its general applicability to intraoperative surgical planning in eloquent cortex. Such concern is due to the fact that functional MRI has been shown to miss cortical sites that are found to be essential by intraoperative stimulation mapping.65 To analyze its sensitivity and specificity with respect to direct cortical mapping, functional MRI data were registered in a frameless stereotactic neuronavigational device and correlated with the results of direct brain mapping. Although the specificity of functional MRI has been shown to be high for naming and verb generation tasks,66 poor sensitivity was noted (only 22% in naming tasks and 36% in verb generation tasks).65 A combined battery of language tasks may enhance the sensitivity of functional MRI in comparison to testing individual language tasks.67 The results, however, have discouraged the use of functional MRI as a tool for making critical surgical decisions in the absence of direct brain mapping. Functional MRI cannot replace stimulation mapping for positive language sites on the basis of the current state of technology.
Functional MRI has, however, been found to be a useful clinical tool for the prediction of selective motor cortex areas. Concordance between the contours of functional MRI and intraoperative electrical cortical mapping was found in 20 of 21 patients in one study that evaluated its reliability for the implantation of an epidural electrode for chronic motor cortex stimulation.68
Functional cortex can also be mapped with PET. Speech eloquent areas can be localized with 2-[18F]-2-deoxy-D-glucose (FDG) based on the higher consumption of glucose in eloquent areas during verb generation tasks. Although integration of functional PET data into frameless navigation systems for surgical planning is possible, its low reliability has precluded it from replacing direct stimulation mapping as well. In three of seven patients, preoperative PET findings were not supported by intraoperative mapping.69
In healthy individuals, MEG has been demonstrated to be capable of delineating the somatotopic organization of the motor cortex since its results were shown to correlate with anatomic landmarks.70 Newer source reconstruction algorithms have increased its reliability in the clinical setting. In one patient with a perirolandic tumor, MEG correctly identified the hand motor area, which was subsequently confirmed by intraoperative cortical stimulation mapping.71 Somatosensory mapping with MEG can be used to guide intraoperative mapping of motor cortex. One study found a consistent quantitative relationship in the distance between the mouth motor cortex identified by electrical stimulation and the lip somatosensory cortex delineated by MEG.72 This relationship allows prediction of the location of the mouth motor cortex if the lip somatosensory cortex is detected by MEG. In one study including 15 patients, MEG depicted the central sulcus correctly in all patients and proved to be superior to functional MRI in localizing the somatosensory cortex.73 MEG has also been shown to reliably map expressive and receptive language cortex.74,75
The pyramidal tract is the major output source of fibers arising from the motor cortex. Diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) are noninvasive techniques that allow preoperative visualization of the pyramidal tract on MRI.76 The disadvantage of preoperative tractographic imaging lies in the possibility of an intraoperative shift of brain tissue secondary to opening of the dura or the administration of mannitol and dexamethasone (Decadron). Intraoperative DWI has therefore been developed to acquire more reliable data. Early experience in a small number of patients has shown that the accuracy and image quality of intraoperative DWI with an MRI scanner of low magnetic field strength (0.3 T) are sufficient for possible incorporation into an intraoperative neuronavigation system.77
Bello and coworkers,78 in contrast, were able to show that preoperatively performed motor and language DTI also allowed accurate intraoperative identification of eloquent fiber tracts through neuronavigation. They reported a sensitivity of 95% for detection of the corticospinal tract and 97% for language tracts, figures calculated by confirmation with intraoperative subcortical stimulation mapping. Another study consisting of nine patients found an average distance of 8.7 mm between positive stimulation sites and the preoperatively DTI-mapped fiber tracts and concluded that it can be used to define a safety margin around the tract.79 Because DTI has only recently been added to intraoperative neuronavigational systems, further studies are needed to validate its reliability in the clinical setting.
Preoperative Testing
To map language capabilities, the patient is tested before surgery with object naming. A baseline error rate of less than 25% is necessary for the intraoperative language mapping to reach statistical significance. Naming is used as the common test because of its involvement in many different types of language disturbances.16,18,42 If the patient’s inability to name objects is due to a mass effect, preoperative dexamethasone and mannitol may diminish the edema to the point where the patient’s language ability returns sufficiently for intraoperative mapping.
Intraoperative Mapping
The patient is brought to the operating room and carefully positioned with a small shoulder roll so that the falx is approximately parallel to the floor. The neck is checked to make sure that it is in a neutral position, and a pillow can be placed between the head and the frame of the Mayfield head holder to provide comfort. Once the patient is positioned, an intravenous infusion of propofol (Diprivan) and remifentanil is begun to induce a deep hypnotic state,80,81 and a Foley catheter is inserted. After the head is shaved and prepared, a local anesthetic (0.5% lidocaine with 1 : 100,000 to 1 : 200,000 units of epinephrine and 0.25% bupivacaine [Marcaine] with 1 : 100,000 to 1 : 200,000 units of epinephrine) is mixed in 1 : 1 fashion, and then 9.0 mL of this mixture is added to 1.0 mL of sodium bicarbonate solution. A field block is applied that initially extends anteriorly over the supraorbital nerves and in the region of the zygoma and the posterior auricular regions. The regional field block is completed with deep injections into the insertion of the temporalis muscle. If a 30-gauge needle is used, the patient can tolerate the injections well, but because propofol is not an analgesic, the patient is somewhat disinhibited during the scalp injection and the arms may have to be restrained. If the patient has become anxious or is overly sensitive to the injections, either the remifentanil can be increased or a small amount of intravenous fentanyl (25 to 50 µg) can be added. With the advent of hair-sparing incisions, the deep and field block portions of the injection are performed after the scalp opening.
Stimulation mapping to localize the rolandic cortex is performed with a bipolar electrode with 5-mm spacing between the electrodes. A constant-current generator is used to produce a train of biphasic square-wave pulses with a frequency of 60 Hz and a 1-msec single-phase duration. The maximum train duration is 4 seconds. In an asleep patient, the current required to evoke motor responses may vary between 4 and 16 mA, but when the patient is awake, a lower current (2 to 5 mA) will usually suffice, especially in the face and hand motor-sensory cortex. The current is increased in 1- to 2-mA increments until the evoked responses are demonstrated. Alternatively, motor mapping may be performed with monopolar, anodal stimulation.82 A train of 500 Hz (7 to 10 pulses) may be used for monopolar stimulation; the current intensities applied are similar to those used for bipolar stimulation. Monopolar cortex stimulation was shown to be successful in generating compound muscle action potentials during intraoperative electrocortical stimulation of the primary motor cortex in 91% of 255 patients.83 Bipolar cortical stimulation, however, has proved to be more sensitive than monopolar stimulation for mapping motor function in the premotor frontal cortex.84 Both methods were found to be equally sensitive for mapping the primary motor cortex. Special electrodes have been designed that allow both monopolar and bipolar mapping.85
In an awake patient, the sensory cortex may be identified more easily at lower stimulation amplitudes. When motor and sensory responses are elicited, the cortical site is marked with a small numbered ticket. If a seizure is elicited by stimulation mapping, the cortex is irrigated with ice-cold Ringer’s solution. The intraoperative risk for seizures induced by stimulation mapping has been found to be approximately 1.5%, and patients with symptomatic epilepsy do not have a higher risk than other patients for the intraoperative development of a seizure.86
Once the motor and sensory cortices have been identified in an awake patient, the electrocorticography equipment is attached to the skull clamp. If resection of the tumor includes an attempt to relieve the patient’s seizure disorder, strip electrodes are inserted to record epileptiform activity. Usually, four strip electrodes are placed: anterior subtemporal, middle subtemporal, posterior subtemporal, and subfrontal. Single carbon-tipped electrodes are placed on the lateral cortical surface over the temporal lobe and the perisylvian region. Recording is then started, and with the assistance of the electroencephalographer, epileptic activity is identified or afterdischarge thresholds are determined. If the patient has intractable epilepsy, a tailored resection is then performed by identifying areas of interictal activity over the lateral or mesial cortical surfaces. Especially with temporal lobe lesions, our standard practice is to resect the lateral cortex and expose the ventricle to place strip electrodes along the hippocampus and subcortically along the parahippocampal gyrus for identification of the extent of resection needed to eliminate all interictal epileptiform activity.18
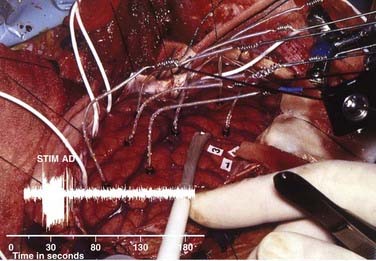
If preoperative planning did not call for identification of epileptic cortex, afterdischarge thresholds are determined. The choice of stimulating currents for language mapping depends on the selection of a current large enough to alter critical function but not so large that it will evoke seizure activity or long trains of afterdischarge activity. The long trains of afterdischarge activity may spread and thus confuse the localization being performed with stimulation. Usually, these requirements are met by setting the current 1 mA below the threshold for evoking single or small trains or afterdischarge activity. However, in cortex that involves adjacent or invasive tumor tissue, the afterdischarge thresholds may be significantly lower and several current levels for stimulation may be necessary for different cortical regions.87 For example, in Figure 57-3, the afterdischarge threshold used in the posterior temporal region was 4 mA; in the anterior temporal lobe, which was undercut by the tumor, the afterdischarge threshold was just 3 mA; and it was 7 mA in the frontal region. Language sites were identified at sites 28, 41, 44, 10, and 12. Afterdischarge thresholds vary considerably between patients and can range from 1.5 mA to greater than 10 mA.
To increase the confidence of the intraoperative mapping, stimulation should provide the information needed to know where language is localized and where it is not localized. Completely negative stimulation mapping does not always provide security that resection of the recorded sites will not cause a language deficit. In a series of 40 patients undergoing resection of temporal lobe tumors, two negative language maps resulted in language deficits in the postoperative period.41 This finding has led to the concept that the region covered by stimulation mapping should include areas where language is likely to be found, as well as the area of planned resection.
Recently, however, Sanai and coworkers did not use the traditional approach of identifying positive language sites in every awake craniotomy and demonstrated that most gliomas could still be resected without causing language deficits.88 Using a tailored approach to limit cortical exposure, 58% of 250 patients were found to have an intraoperative stimulation-induced speech arrest or anomia. Their 6-month functional outcome data, however, compared favorably with previous studies that relied on the identification of positive language sites. Only 4 of 243 surviving patients (1.6%) had a persistent language deficit 6 months after surgery, so the use of smaller, tailored craniotomies with testing for negative language sites seems to be a valid surgical strategy.
Cortical surface recordings of SSEPs can be helpful in localizing the sensorimotor cortex intraoperatively. SSEPs with approximately mirror-image waveforms can be recorded on either side of the central sulcus.89 The precentral waveform is termed P20-N30 and the postcentral waveform P30-N20, corresponding to their polarity and average peak latency across subjects. In other words, stimulation of the median nerve on average leads to a negative wave (N) 20 msec later in recordings from the postcentral gyrus, followed by a positive wave (P) 30 msec after stimulation. The opposite order of waveforms is observed in the precentral gyrus. This phenomenon is called phase reversal. Intraoperative SSEP recordings enable the surgeon to identify the motor and somatosensory cortices under general anesthesia without the use of awake stimulation techniques. For localization of the hand motor area with SSEPs, the median nerve is typically stimulated intraoperatively. Trigeminal SSEPs with stimulating electrodes in the chin, tongue, or palate can be used to identify face motor and sensory areas.90
Intraoperative infrared functional imaging has recently been developed as a means of measuring the increased neuronal heat production and capillary blood flow that are linked to greater neuronal firing.91,92 This technique represents another modality to intraoperatively map motor, sensory, and language function.
As discussed earlier, the pyramidal tract is of crucial importance for avoidance of postoperative deterioration in motor function. The pyramidal tract can also be identified by intraoperative subcortical stimulation mapping. The subcortical stimulation settings are usually identical to the ones used for cortical mapping. Initially, the cortical motor region is identified and the same current is subsequently used to identify the descending motor pathways.15 Multichannel electromyographic (EMG) recordings have been shown to be superior to mere visual observation in detecting motor responses elicited by stimulation of the internal capsule.93 In 30% of 66 operations, motor responses during mapping were noted at least once on EMG recordings, although they were not apparent on visual inspection. Because lower electrical thresholds are required to elicit an EMG response during cortical mapping, it can be performed with lower currents, which potentially decreases the incidence of intraoperative seizures. This may be useful in patients who are susceptible to stimulation-induced seizures.
Fundamentals of Cortical Stimulation
The area of cortex inactivated by bipolar cortical stimulation has been studied in a number of ways. Bipolar stimulation pulses ensure that one electrode will not become the sole source of the direction of the current and produce a lesion. Imaging during bipolar stimulation in both human and monkey cortex has shown that the area activated is confined to the region around the recording electrodes94 but that if afterdischarge activity is evoked, the spread is more diffuse.95 Therefore, stimulation of motor movements should involve a very limited focal area of cortex to ensure accuracy of the functional maps. Stimulation at the afterdischarge threshold is sufficient to disrupt activity at that area of cortex. If language fails, the area is identified as an essential language site. Following Penfield’s experience, object naming is the language measure primarily used with stimulation.16,96 Naming seems to be adequate for localization of language, and naming deficits occur in all aphasic syndromes that result from pathologic lesions. Moreover, two studies investigating the relationship of changes in stimulation to language after resection cited naming as the only measure of language during stimulation that helped predict the effects of resection on a general aphasia battery measuring many language functions and significant clinical deficits.16,97 However, with resections in the posterior temporal lobe and perisylvian area, other language functions may be assessed, such as auditory naming, reading, and memory, because these language functions have been noted to involve a somewhat wider cortical area than naming changes do.18
Strategies for Surgical Resection
Localization of language is an important factor in planning for cortical resection.98 In general, this is a concern only in the dominant hemisphere for language, and then usually in the perisylvian area. However, even in the anterior temporal lobe, a small percentage of essential language sites are identified within 3 cm of the temporal tip. The left hemisphere is commonly assumed to be dominant for language in right-handed patients, whereas in left-handed individuals, dominance is commonly established preoperatively with the intracarotid amobarbital perfusion test or functional MRI techniques, as discussed earlier in this chapter. In reality, the laterality of language may not be significantly different in left- and right-handed patients when those who are left-handed because of left hemisphere lesions are excluded.99 The left hemisphere is most likely to be essential for language in either group, with a few patients demonstrating right or bilateral language in either group, although these unusual patterns seem to be slightly more common in left-handers with a strong family history. Overall, the left hemisphere alone is essential for language in about 85% of patients and the right in 6%, with language represented bilaterally in approximately 9%.100 Within the dominant hemisphere, changes in language have been observed after lesions in a wide area. However, permanent deficits have generally been associated only with lesions in the perisylvian area; thus, localization of language in this area is particularly important. Changes in language are often evoked from the posterior superior frontal areas and often acutely follow resection there, but nearly all patients recover from the supplementary motor area syndrome.101 The same situation seems to exist with regard to the basal temporal cortex.14,102
Two different approaches can be taken to minimize the risk for language deficits with cortical resection near the dominant hemisphere’s perisylvian cortex. The traditional approach is to use anatomic landmarks that are thought to indicate areas not involved in language, for example, limiting temporal resection to the anterior 4 to 5 cm, anterior to the inferior aspect of rolandic cortex, or anterior to the vein of Labbé.41,103 Sparing of the superior temporal gyrus has also been recommended. The pterion has been considered the safe posterior limit for inferior frontal resection. However, particularly in the temporal lobe, resections within these supposed safe limits are occasionally associated with postoperative aphasia,97 and these landmarks provide no guidance for resection in the perisylvian areas, especially the posterior temporal and inferior parietal lobes. The alternative approach is to make a unique map for each individual and identify the essential language areas.
Functional cortex and subcortical white matter may be located within tumors or adjacent infiltrated brain regardless of the degree of tumor infiltration, swelling, apparent necrosis, and gross distortion by the mass. Direct stimulation mapping of the cortical and subcortical portions of tumors during resection has been shown to generate motor, sensory, or language dysfunction.104
Because of the bilateral representation of face motor function at the neocortical level, radical resection of tumors from the face motor cortex on the nondominant side may result in only transient contralateral facial weakness and apraxia, which resolves within 6 to 8 weeks after surgery.105 Because language cortex is contiguous to the face motor area on the dominant side, resection of the face motor area is controversial in the dominant hemisphere but has been accomplished without major deficits (Friedman and Haglund, unpublished observations).
Verbal memory deficits may arise after temporal lobectomies in the dominant hemisphere. Ojemann and Dodrill found a significant correlation between a decline in postoperative verbal memory scores and the lateral, but not the medial extent of the temporal lobe resection.106 Verbal memory scores measure the ability of a patient to recall a word after a short period of distraction. In their series of 14 adults undergoing left temporal lobectomy, the Wechsler verbal memory score was decreased an average of 22% at 1 month and 11% at 1 year. Thus, verbal memory is not only generated by medial temporal lobe structures such as the hippocampus but is also stored in the temporal cortex.
Functional intraoperative language mapping aims to limit the development of postoperative aphasias. Several investigators have examined the occurrence and time course of postoperative language dysfunctions after cortical mapping. A common theme of all studies is the finding that the majority of new postoperative aphasias improve or resolve over time. Ilmberger and colleagues found that in 32% of their 128 patients without preoperative deficits, a new aphasic disturbance developed within 21 days of microsurgical treatment of tumors in close proximity to or within language areas.107 Seven months after treatment, only 10.9% of the 128 patients continued to demonstrate these postoperative language disturbances. Risk factors for the development of a postoperative aphasic disturbance included preoperative aphasia, intraoperative complications, language-positive sites within the tumor, and a nonfrontal lesion location. In patients without a preoperative deficit, normal but submaximal naming performance was found to be a strong predictor of early postoperative aphasia. The only risk factors that were identified for persistent postoperative language disturbances included age older than 40 years and preoperative aphasia.
Bello and coworkers identified language tracts through subcortical stimulation in 59% of 88 patients undergoing surgical removal of gliomas.108 The identification of language tracts was associated with the development of a higher number of transient postoperative deficits (67.3% of patients), but permanent language deficits were ultimately noted in just 2.3% of their patients.
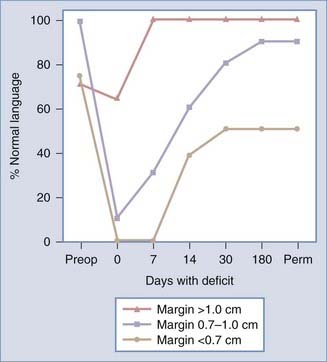
An important consideration when planning surgical resection of an intrinsic brain tumor is its proximity to positive language sites. Haglund and coworkers showed in a series of 40 patients with temporal lobe gliomas in the dominant hemisphere that the distance of the resection margin from the nearest language site is crucial for estimating the likelihood of a permanent postoperative language deficit.41 In comparing the distance from the nearest language site to the resection margin, a clear association was found between the distance from the resection margin and the postoperative development of language deficits (Fig. 57-4). Patients with no postoperative deficit had an average distance between the nearest language site and the resection margin of 2.0 ± 0.43 cm; in contrast, the distance was 1.6 ± 0.2 cm in patients with deficits lasting 1 to 7 days, 0.71 ± 0.06 cm in those with deficits lasting 8 to 30 days, and 0.67 ± 0.05 cm in patients with permanent deficits. In the subgroup of patients with normal preoperative speech and comprehension, language sites identified by cortical stimulation and resection margins more than 1 cm away from the nearest language site resulted in normal language function by the end of the first postoperative week. Thus, resection of intrinsic brain tumors that follows a 1-cm safety margin from the nearest cortical language site is considered to be the best surgical strategy for preventing the development of permanent postoperative aphasias while maximizing the extent of tumor resection.
Ojemann and Dodrill found that left anterior temporal resection within 2 cm of a site associated with repeated anomia or testing errors on naming tasks was linked to subtle increases in errors on the Wepman aphasia battery (administered 1 month postoperatively); no changes in the aphasia battery were evident when the resections avoided those sites, and changes in language were not induced with resections more than 2 cm away from such sites.109 Moreover, there was no correlation between these postoperative aphasic errors and the size of the resection, preoperative language performance, or postoperative seizure control.
In a series of 294 patients, Keles and coworkers showed that subcortical stimulation to identify descending motor pathways could achieve an acceptable rate of permanent morbidity. Sixty patients (20.4%) had an additional postoperative motor deficit.110,111 Of these patients, however, 76.7% regained their baseline function within 90 days of surgery, many of them returning to baseline in the first postoperative week.
Duffau and colleagues found that with subcortical stimulation mapping, 80% of their patients undergoing resection for low-grade gliomas experienced immediate postoperative neurological worsening.112 However, 94% of these patients recovered to their preoperative status within 3 months, and 10% even improved. The same group also showed that postoperative morbidity and the extent of gross total resection in or near eloquent cortex at their institution significantly improved after the introduction of intraoperative stimulation mapping.113 In a series of 115 patients who underwent intraoperative subcortical stimulation mapping, all but 2 (98%) had their language function return to baseline or better after resection of grade II gliomas in the left dominant hemisphere.114
Fundamentals of Surgical Resection
With most of the cortical surface being buried within sulci, it is surprising that surface cortical stimulation can predict the effects of resections. It appears that the cortical language system has a major vertical organization and preferential location of essential areas in the crowns of gyri. Essential language areas in buried cortex, well away from those in the surface, do not seem to play a major role. Otherwise, surface stimulation would not reliably predict the effect of buried cortex resections. In fact, surface sites were identified by stimulation mapping in most patients (117 of 119), and this would not be the case if the language sites were randomly distributed. On a number of occasions, sulci that have been mapped for language have not shown independent sites, although occasionally a surface site extends a short distance into the sulcus.13,26 The connections of essential language areas must also be somewhat vertically organized because surface stimulation predicts the effect of resections that remove white matter near the essential language areas. This relationship between surface stimulation effects and resection also seems to apply to frontal operculum stimulation, which can predict the results of resection of subinsular dominant hemisphere language sites (unpublished data).
There is a limited body of data on the stability of stimulation maps of language localization over time with or without an intervening brain lesion. On a few occasions, with repeated mapping after several months and without an intervening brain lesion, usually a comparison of extraoperative and intraoperative mapping shows that sites with or without changes in language function have had generally similar locations.32 Remapping of language years after a static perisylvian brain injury (trauma or stroke) associated with partially recovered aphasia has shown language sites at the edge of the damaged cortex in locations where language sites are expected in nonaphasic patients. Repeated mapping for the development of aphasia in patients with recurrent brain tumors has shown disappearance of one of the localized essential language areas when the language deficit progressed, with other areas remaining stable (Haglund, Berger, and Ojemann, unpublished observations). None of these findings suggest any significant plasticity in adult language localization. In a series of nonaphasic patients with low-grade intrinsic tumors in the left temporal lobe, fewer language sites were found in the nonaphasic patients with left temporal epileptic foci, thus raising the possibility that the tumor had slowly destroyed some of the temporal lobe language sites without causing functional deficits. There was no evidence of an excess of extratemporal sites, as might be expected with reorganization.41
Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978.
Duffau H, Capelle L, Denvil D, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98:764.
Duffau H, Gatignol P, Mandonnet E, et al. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797.
Haglund MM, Berger MS, Shamseldin M, et al. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567.
Haglund MM, Ojemann GA, Schwartz TW, et al. Neuronal activity in human lateral temporal cortex during serial retrieval from short-term memory. J Neurosci. 1994;14:1507.
Hamberger MJ, Seidel WT, McKhann GM2nd, et al. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128:2742.
Ilmberger J, Ruge M, Kreth FW, et al. Intraoperative mapping of language functions: a longitudinal neurolinguistic analysis. J Neurosurg. 2008;109:583.
Keifer JC, Dentchev D, Little K, et al. A retrospective analysis of a remifentanil/propofol general anesthetic for craniotomy before awake functional brain mapping. Anesth Analg. 2005;101:502.
Keles GE, Lundin DA, Lamborn KR, et al. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100:369.
Ojemann G, Mateer C. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science. 1979;205:1401.
Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316.
Papanicolaou AC, Simos PG, Breier JI, et al. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999;90:85.
Pouratian N, Cannestra AF, Bookheimer SY, et al. Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals. J Neurosurg. 2004;101:458.
Quinones-Hinojosa A, Ojemann SG, Sanai N, et al. Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg. 2003;99:311.
Rostomily RC, Berger MS, Ojemann GA, et al. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 1991;75:62.
Roux FE, Boulanouar K, Lotterie JA, et al. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335.
Ruge MI, Victor J, Hosain S, et al. Concordance between functional magnetic resonance imaging and intraoperative language mapping. Stereotact Funct Neurosurg. 1999;72:95.
Rutten GJ, Ramsey NF, van Rijen PC, et al. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002;80:421.
Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18.
Skirboll SS, Ojemann GA, Berger MS, et al. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678.
Wada J, Rasmussen T. Intracarotid injections of sodium Amytal for the lateralization of cerebral speech dominance. J Neurosurg. 1960;17:266.
Wood CC, Spencer DD, Allison T, et al. Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg. 1988;68:99.
Woolsey CN, Erickson TC, Gilson WE. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg. 1979;51:476.
Yingling CD, Ojemann S, Dodson B, et al. Identification of motor pathways during tumor surgery facilitated by multichannel electromyographic recording. J Neurosurg. 1999;91:922.
1 Conte V, Baratta P, Tomaselli P, et al. Awake neurosurgery: an update. Minerva Anestesiol. 2008;74:289.
2 Tonn JC. Awake craniotomy for monitoring of language function: benefits and limits. Acta Neurochir (Wien). 2007;149:1197.
3 Duffau H. Contribution of cortical and subcortical electrostimulation in brain glioma surgery: methodological and functional considerations. Neurophysiol Clin. 2007;37:373.
4 Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity—a review. J Neurooncol. 2006;79:77.
5 Berger MS, Cohen WA, Ojemann GA. Correlation of motor cortex brain mapping data with magnetic resonance imaging. J Neurosurg. 1990;72:383.
6 Berger MS. Preoperative magnetic resonance imaging localization of the primary motor cortex. Perspect Neurol Surg. 1991;2:23.
7 Ebeling U, Steinmetz H, Huang Y, et al. Topography and identification of the inferior precentral sulcus in MR imaging. AJNR Am J Neuroradiol. 1989;10:937.
8 Quinones-Hinojosa A, Ojemann SG, Sanai N, et al. Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg. 2003;99:311.
9 Berger MS, Kincaid J, Ojemann GA, et al. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery. 1989;25:786.
10 Goldring S, Gregorie EM. Surgical management of epilepsy using epidural recordings to localize the seizure focus. Review of 100 cases. J Neurosurg. 1984;60:457.
11 Jayakar P. Physiological principles of electrical stimulation. Adv Neurol. 1993;63:17.
12 Goldring S. Epilepsy surgery. Clin Neurosurg. 1983;31:369.
13 Gregorie EM, Goldring S. Localization of function in the excision of lesions from the sensorimotor region. J Neurosurg. 1984;61:1047.
14 Luders H, Lesser RP, Hahn J, et al. Basal temporal language area demonstrated by electrical stimulation. Neurology. 1986;36:505.
15 Woolsey CN, Erickson TC, Gilson WE. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg. 1979;51:476.
16 Penfield W, Roberts L. Speech and Brain Mechanisms. Princeton, NJ: Princeton University Press; 1959.
17 Ojemann GA. Cortical organization of language. J Neurosci. 1991;11:2281.
18 Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316.
19 Haglund MM, Ojemann GA, Lettich E, et al. Dissociation of cortical and single unit activity in spoken and signed languages. Brain Lang. 1993;44:19.
20 Ojemann GA, Whitaker HA. Language localization and variability. Brain Lang. 1978;6:239.
21 Ojemann GA, Whitaker HA. The bilingual brain. Arch Neurol. 1978;35:409.
22 Rubens AB, Mahowald MW, Hutton JT. Asymmetry of the lateral (sylvian) fissures in man. Neurology. 1976;26:620.
23 Steinmetz H, Ebeling U, Huang YX, et al. Sulcus topography of the parietal opercular region: an anatomic and MR study. Brain Lang. 1990;38:515.
24 Ojemann SG, Berger MS, Lettich E, et al. Localization of language function in children: results of electrical stimulation mapping. J Neurosurg. 2003;98:465.
25 Schevon CA, Carlson C, Zaroff CM, et al. Pediatric language mapping: sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia. 2007;48:539.
26 Berger MS. The impact of technical adjuncts in the surgical management of cerebral hemispheric low-grade gliomas of childhood. J Neurooncol. 1996;28:129.
27 Duffau H, Thiebaut de Schotten M, Mandonnet E. White matter functional connectivity as an additional landmark for dominant temporal lobectomy. J Neurol Neurosurg Psychiatry. 2008;79:492.
28 Mandonnet E, Nouet A, Gatignol P, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623.
29 Duffau H, Gatignol P, Mandonnet E, et al. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797.
30 Duffau H, Capelle L, Sichez N, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain. 2002;125:199.
31 Duffau H, Velut S, Mitchell MC, et al. Intra-operative mapping of the subcortical visual pathways using direct electrical stimulations. Acta Neurochir (Wien). 2004;146:265.
32 Ojemann GA. Brain organization for language from the perspective of electrical stimulation mapping. Behav Brain Sci. 1983;6:189.
33 Ojemann G, Mateer C. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science. 1979;205:1401.
34 Roux FE, Lubrano V, Lauwers-Cances V, et al. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain. 2004;127:1796.
35 Walker JA, Quinones-Hinojosa A, Berger MS. Intraoperative speech mapping in 17 bilingual patients undergoing resection of a mass lesion. Neurosurgery. 2004;54:113.
36 Ojemann JG, Ojemann GA, Lettich E. Cortical stimulation mapping of language cortex by using a verb generation task: effects of learning and comparison to mapping based on object naming. J Neurosurg. 2002;97:33.
37 Serafini S, Gururangan S, Friedman A, et al. Identification of distinct and overlapping cortical areas for bilingual naming and reading using cortical stimulation. Case report. J Neurosurg Pediatr. 2008;1:247.
38 Hamberger MJ, Seidel WT, McKhann GM2nd, et al. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128:2742.
39 Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. 2007;17:477.
40 Haglund MM, Ojemann GA, Schwartz TW, et al. Neuronal activity in human lateral temporal cortex during serial retrieval from short-term memory. J Neurosci. 1994;14:1507.
41 Haglund MM, Berger MS, Shamseldin M, et al. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567.
42 Ojemann GA. Surgical treatment of epilepsy. In: Rangachy SS, Wilkins RH, editors. Neurosurgery. New York: McGraw-Hill; 1996:4173-4183.
43 Lehman RM. A review of neurophysiological testing. Neurosurg Focus. 16(4), 2004.
44 Wada J, Rasmussen T. Intracarotid injection of sodium Amytal for the lateralization of cerebral speech dominance. J Neurosurg. 1960;17:266.
45 Baxendale S. The Wada test. Curr Opin Neurol. 2009;22:185.
46 Baxendale S, Thompson PJ, Duncan JS. The role of the Wada test in the surgical treatment of temporal lobe epilepsy: an international survey. Epilepsia. 2008;49:715.
47 Loring DW, Hermann BP, Meador KJ, et al. Amnesia after unilateral temporal lobectomy: a case report. Epilepsia. 1994;35:757.
48 Baxendale S. Amnesia in temporal lobectomy patients: historical perspective and review. Seizure. 1998;7:15.
49 Guerreiro CA, Jones-Gotman M, Andermann F, et al. Severe amnesia in epilepsy: causes, anatomopsychological considerations, and treatment. Epilepsy Behav. 2001;2:224.
50 Kubu CS, Girvin JP, McLachlan RS, et al. Does the intracarotid amobarbital procedure predict global amnesia after temporal lobectomy? Epilepsia. 2000;41:1321.
51 Wyllie E, Naugle R, Awad I, et al. Intracarotid amobarbital procedure: I. Prediction of decreased modality-specific memory scores after temporal lobectomy. Epilepsia. 1991;32:857.
52 Davies KG, Bell BD, Bush AJ, et al. Prediction of verbal memory loss in individuals after anterior temporal lobectomy. Epilepsia. 1998;39:820.
53 Baxendale S, Thompson P, Harkness W, et al. Predicting memory decline following epilepsy surgery: a multivariate approach. Epilepsia. 2006;47:1887.
54 Baxendale SA, Thompson PJ, Duncan JS. Evidence-based practice: a reevaluation of the intracarotid amobarbital procedure (Wada test). Arch Neurol. 2008;65:841.
55 Ahern GL, O’Connor M, Dalmau J, et al. Paraneoplastic temporal lobe epilepsy with testicular neoplasm and atypical amnesia. Neurology. 1994;44:1270.
56 Loddenkemper T, Morris HH, Moddel G. Complications during the Wada test. Epilepsy Behav. 2008;13:551.
57 Loddenkemper T. Quo vadis Wada? Epilepsy Behav. 2008;13:1.
58 Kucharczyk J, Moseley ME, Roberts T, et al. Functional neuroimaging. Neuroimag Clin North Am. 1995;5:161.
59 Keles GE, Berger MS. Advances in neurosurgical technique in the current management of brain tumors. Semin Oncol. 2004;31:659.
60 Chlebus P, Mikl M, Brazdil M, et al. fMRI evaluation of hemispheric language dominance using various methods of laterality index calculation. Exp Brain Res. 2007;179:365.
61 Petrovich Brennan NM, Whalen S, de Morales Branco D, et al. Object naming is a more sensitive measure of speech localization than number counting: converging evidence from direct cortical stimulation and fMRI. Neuroimage. 2007;37(suppl 1):S100.
62 Rutten GJ, Ramsey NF, van Rijen PC, et al. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002;80:421.
63 Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798.
64 Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978.
65 Roux FE, Boulanouar K, Lotterie JA, et al. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335.
66 Ruge MI, Victor J, Hosain S, et al. Concordance between functional magnetic resonance imaging and intraoperative language mapping. Stereotact Funct Neurosurg. 1999;72:95.
67 FitzGerald DB, Cosgrove GR, Ronner S, et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol. 1997;18:1529.
68 Pirotte B, Neugroschl C, Metens T, et al. Comparison of functional MR imaging guidance to electrical cortical mapping for targeting selective motor cortex areas in neuropathic pain: a study based on intraoperative stereotactic navigation. AJNR Am J Neuroradiol. 2005;26:2256.
69 Sobottka SB, Bredow J, Beuthien-Baumann B, et al. Comparison of functional brain PET images and intraoperative brain-mapping data using image-guided surgery. Comput Aided Surg. 2002;7:317.
70 Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141.
71 Pang EW, Drake JM, Otsubo H, et al. Intraoperative confirmation of hand motor area identified preoperatively by magnetoencephalography. Pediatr Neurosurg. 2008;44:313.
72 Kirsch HE, Zhu Z, Honma S, et al. Predicting the location of mouth motor cortex in patients with brain tumors by using somatosensory evoked field measurements. J Neurosurg. 2007;107:481.
73 Korvenoja A, Kirveskari E, Aronen HJ, et al. Sensorimotor cortex localization: comparison of magnetoencephalography, functional MR imaging, and intraoperative cortical mapping. Radiology. 2006;241:213.
74 Castillo EM, Simos PG, Venkataraman V, et al. Mapping of expressive language cortex using magnetic source imaging. Neurocase. 2001;7:419.
75 Papanicolaou AC, Simos PG, Breier JI, et al. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999;90:85.
76 Krings T, Reinges MH, Thiex R, et al. Functional and diffusion-weighted magnetic resonance images of space-occupying lesions affecting the motor system: imaging the motor cortex and pyramidal tracts. J Neurosurg. 2001;95:816.
77 Ozawa N, Muragaki Y, Nakamura R, et al. Intraoperative diffusion-weighted imaging for visualization of the pyramidal tracts. Part II: clinical study of usefulness and efficacy. Minim Invasive Neurosurg. 2008;51:67.
78 Bello L, Gambini A, Castellano A, et al. Motor and language DTI fiber tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. 2008;39:369.
79 Berman JI, Berger MS, Chung SW, et al. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107:488.
80 Erickson KM, Cole DJ. Anesthetic considerations for awake craniotomy for epilepsy. Anesthesiol Clin. 2007;25:535.
81 Keifer JC, Dentchev D, Little K, et al. A retrospective analysis of a remifentanil/propofol general anesthetic for craniotomy before awake functional brain mapping. Anesth Analg. 2005;101:502.
82 Maertens de Noordhout A, Born JD, Hans P, et al. Intraoperative localization of the primary motor cortex using single electrical stimuli. J Neurol Neurosurg Psychiatry. 1996;60:442.
83 Suess O, Suess S, Brock M, et al. Intraoperative electrocortical stimulation of Brodmann area 4: a 10-year analysis of 255 cases. Head Face Med. 2006;2:20.
84 Kombos T, Suess O, Kern BC, et al. Comparison between monopolar and bipolar electrical stimulation of the motor cortex. Acta Neurochir (Wien). 1999;141:1295.
85 Schekutiev G, Schmid UD. Coaxial insulated bipolar electrode for monopolar and bipolar mapping of neural tissue: technical note with emphasis on the principles of intra-operative stimulation. Acta Neurochir (Wien). 1996;138:470.
86 Szelenyi A, Joksimovic B, Seifert V. Intraoperative risk of seizures associated with transient direct cortical stimulation in patients with symptomatic epilepsy. J Clin Neurophysiol. 2007;24:39.
87 Pouratian N, Cannestra AF, Bookheimer SY, et al. Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals. J Neurosurg. 2004;101:458.
88 Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18.
89 Wood CC, Spencer DD, Allison T, et al. Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg. 1988;68:99.
90 McCarthy G, Allison T, Spencer DD. Localization of the face area of human sensorimotor cortex by intracranial recording of somatosensory evoked potentials. J Neurosurg. 1993;79:874.
91 Ecker RD, Goerss SJ, Meyer FB, et al. Vision of the future: initial experience with intraoperative real-time high-resolution dynamic infrared imaging. Technical note. J Neurosurg. 2002;97:1460.
92 Gorbach AM, Heiss J, Kufta C, et al. Intraoperative infrared functional imaging of human brain. Ann Neurol. 2003;54:297.
93 Yingling CD, Ojemann S, Dodson B, et al. Identification of motor pathways during tumor surgery facilitated by multichannel electromyographic recording. J Neurosurg. 1999;91:922.
94 Haglund MM, Ojemann GA, Blasdel GG. Optical imaging of bipolar cortical stimulation. J Neurosurg. 1993;78:785.
95 Haglund MM, Ojemann GA, Blasdel GG. Video imaging of bipolar cortical stimulation. Epilepsia. 32(suppl 3), 1991.
96 Ojemann GA. Some brain mechanisms for reading. In: Von Euler C, Lundberg I, Lennerstrand G, editors. Brain and Reading. New York: Macmillan; 1989:47.
97 Heilman KM, Wilder BJ, Malzone WF. Anomic aphasia following anterior temporal lobectomy. Trans Am Neural Assoc. 1972;97:291.
98 Black PM, Ronner SF. Cortical mapping for defining the limits of tumor resection. Neurosurgery. 1987;20:914.
99 Woods RP, Dodrill CB, Ojemann GA. Brain injury, handedness, and speech lateralization in a series of amobarbital studies. Ann Neurol. 1988;23:510.
100 Wada J, Rasmussen T. Intracarotid injections of sodium Amytal for the lateralization of cerebral speech dominance. J Neurosurg. 1960;17:266.
101 Rostomily RC, Berger MS, Ojemann GA, et al. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 1991;75:62.
102 Krauss GL, Fisher R, Plate C, et al. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476.
103 Fried I. Anatomic temporal lobe resections for temporal lobe epilepsy. Neurosurg Clin N Am. 1993;4:233.
104 Skirboll SS, Ojemann GA, Berger MS, et al. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678.
105 LeRoux PD, Berger MS, Haglund MM, et al. Resection of intrinsic tumors from nondominant face motor cortex using stimulation mapping: report of two cases. Surg Neurol. 1991;36:44.
106 Ojemann GA, Dodrill CB. Verbal memory deficits after left temporal lobectomy for epilepsy. Mechanism and intraoperative prediction. J Neurosurg. 1985;62:101.
107 Ilmberger J, Ruge M, Kreth FW, et al. Intraoperative mapping of language functions: a longitudinal neurolinguistic analysis. J Neurosurg. 2008;109:583.
108 Bello L, Gallucci M, Fava M, et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60:67.
109 Ojemann GA, Dodrill CG. Intraoperative techniques for reducing language and memory deficits with left temporal lobectomy. Adv Epilept. 1987;16:327-330.
110 Keles GE, Lundin DA, Lamborn KR, et al. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100:369.
111 Bernstein M. Subcortical stimulation mapping. J Neurosurg. 2004;100:365.
112 Duffau H, Capelle L, Denvil D, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98:764.
113 Duffau H, Lopes M, Arthuis F, et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985-96) and with (1996-2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76:845.
114 Duffau H, Peggy Gatignol ST, Mandonnet E, et al. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with grade II glioma in the left dominant hemisphere. J Neurosurg. 2008;109:461.