CHAPTER 36 Motor Proteins
Molecular motors use ATP hydrolysis to power movements of subcellular components, such as organelles and chromosomes, along the two polarized cytoskeletal fibers: actin filaments and microtubules. No motors are known to move on the apolar intermediate filaments. Motor proteins also produce force locally within the network of cytoskeletal polymers, which transmits these forces to determine the shape of each cell and, ultimately, the architecture of tissues and whole organisms. Chapters 37 to 39 and 44 illustrate how motors move cells and their parts.
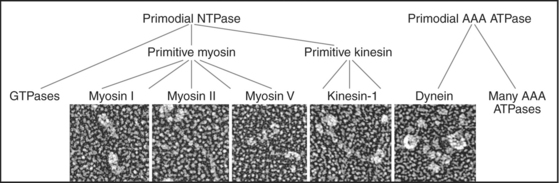
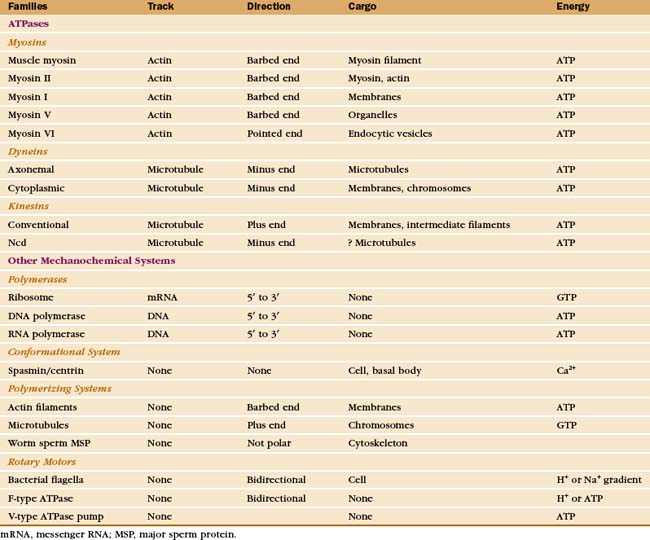
Just three families of motor proteins—myosin, kinesin, and dynein—power most eukaryotic cellular movements (Fig. 36-1 and Table 36-1). During evolution myosin, kinesin, and Ras family GTPases appear to have shared a common ancestor (Fig. 36-1), whereas dynein is a member of the AAA ATPase family (Box 36-1). Although the ancestral genes appeared in prokaryotes, and prokaryotes have homologs of both actin and tubulin, none of these motor proteins has been found in prokaryotes. Over time, gene duplication and divergence in eukaryotes gave rise to multiple genes for myosin, dynein, and kinesin, each encoding proteins with specialized functions. Even the slimmed down genome of budding yeast includes genes for five myosins, six kinesins, and one dynein. Table 36-1 lists other protein machines that produce molecular movements during protein and nucleic acid synthesis, proton pumping, and bacterial motility.
BOX 36-1 AAA ATPases
The common ancestor of life on the earth had a gene for a versatile ATP-binding domain. Through gene duplication and divergence this progenitor gave rise to the AAA family of ATPases in all branches of the phylogenic tree. Given the remarkable variety of functions of the contemporary proteins, the name “ATPases Associated with Diverse Activities” is apt. The family now includes regulatory subunits of proteasomes (see Fig. 23-5); proteases from prokaryotes, chloroplasts, and mitochondria; Hsp100 protein folding chaperones; dynein microtubule motors (Fig. 36-14); the microtubule severing protein katanin (see Fig. 34-9); activators of origins of replication (including ORC1, 4, and 5 and Mcm-7 [see Fig. 42-7]); clamp loader proteins for DNA polymerase processivity factors (see Fig. 42-11); two proteins required for peroxisome biogenesis (see Appendix 18-1); and proteins involved in vesicular traffic such as NSF (the N-ethylmaleimide-sensitive factor [see Fig. 21-12]).
Motor proteins have two parts: a motor domain that utilizes adenosine triphosphate (ATP) hydrolysis to produce movements and a tail that allows the motors to self-associate or to bind particular cargo. Within the three families, the tails are more diverse than the motor domains, allowing for specialized functions of each motor isoform.
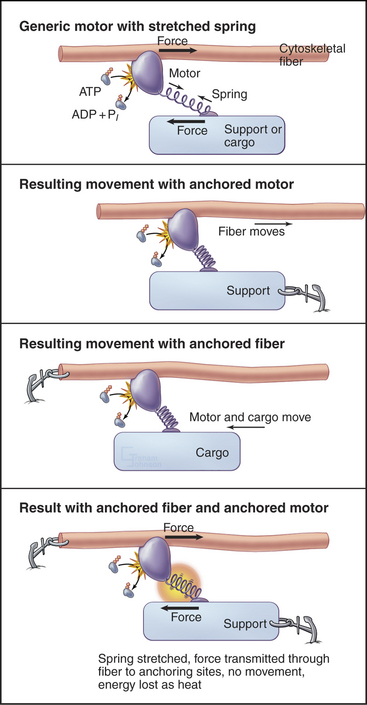
All motor proteins are enzymes that convert chemical energy stored in ATP into molecular motion to produce force upon an associated cytoskeletal polymer (Fig. 36-2). If the motor is anchored, the polymer may move. If the polymer is anchored, the motor and any attached cargo may move. If both are anchored, the force stretches elastic elements in the molecules transiently, but nothing moves, and the energy is lost as heat. Cells use all of these options (see Chapters 37to3839).
Biochemists originally discovered and purified these motors by means of enzyme activity or in vitro motility assays (Fig. 36-11). With the prototype enzymes identified, investigators found further examples and variant isoforms of each motor by purification of the proteins, molecular cloning of DNAs, genomewide DNA sequencing, or genetic screening.
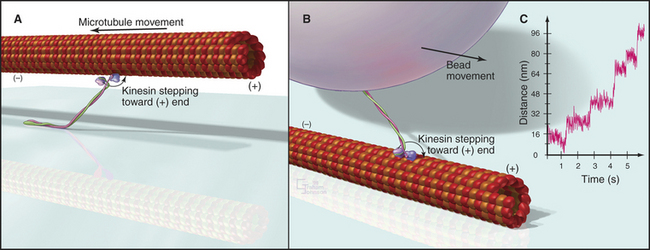
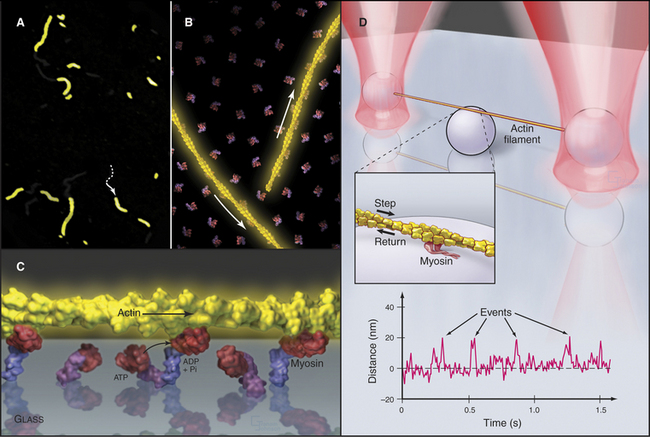
Figure 36-11 in vitro motility assays for microtubule motors. A, Gliding assay. Kinesin or dynein that is attached to a microscope slide uses ATP hydrolysis to move microtubules over the surface. A single kinesin-1 molecule can move a microtubule in this assay. Microtubules can be imaged by video-enhanced differential interference contrast microscopy (see Fig. 34-7).B, Bead assay. Kinesin or dynein that is attached to a plastic bead uses ATP hydrolysis to move the bead along a microtubule attached to the microscopic slide. C, Experimental measurement of the kinesin-1 step size using the bead assay. The bead is held in a laser optical trap so that 8-nm steps can be recorded, as a single, two-headed kinesin-1 moves a bead processively along a microtubule, as in B. The position of the bead is recorded with nanometer precision by interferometry.
(Reference: Svoboda K, Schmidt CF, Schnapp BJ, Block SM: Direct observation of kinesin stepping by optical trapping interferometry. Nature 365:721–727, 1993.)
Myosins
Myosins are the only motors that are known to use actin filaments as tracks. As is discussed in the section entitled “The Myosin Superfamily,” the members of this diverse family arose from a common ancestor and share a common motor unit called a myosin “head” that produces force on actin filaments (Fig. 36-3). Myosins have one or two heads attached to various types of tails that are adapted for diverse purposes, including polymerization into filaments, binding membranes, and interacting with various cargos.
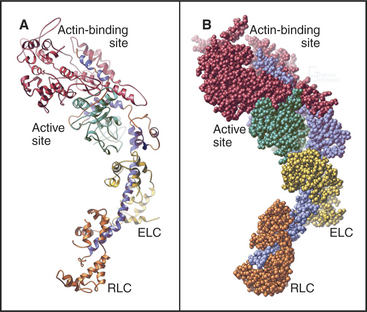
Myosin heads consist of two parts. A catalytic domain at the N-terminus of the myosin heavy chain binds and hydrolyzes ATP and interacts with actin filaments. Light chain domains consist of an α-helical extension of the heavy chain from the catalytic domain associated with one to seven light chains. Calmodulin (see Fig. 3-12) serves as a light chain for some cytoplasmic myosins, but many light chains are specialized relatives of calmodulin.
Myosin Mechanochemistry
Studies of skeletal muscle myosin established general principles that apply, with some interesting variations, to energy transduction by all myosins. This founding member of the myosin family is responsible for the forceful contraction of skeletal muscle. Like other types of myosin-II, it has two heads on a long tail formed from an α-helical coiled-coil. These tails polymerize into bipolar filaments (see Figs. 5-7 and 39-6).
The head of muscle myosin was originally isolated as a proteolytic fragment called subfragment-1 (Fig. 36-3). The N-terminal 710 residues of the heavy chain form the globular catalytic domain. The nucleotide binding site in the core of the catalytic domain is formed by a β-sheet flanked by α-helices with a topology similar to Ras GTPases (see Fig. 4-6) despite little sequence similarity. The γ-phosphate of ATP inserts deeply into the nucleotide-binding site with the adenine exposed on the surface. Actin binds more than 4 nm away from the nucleotide on the other side of the head. The light-chain domain has an essential light chain and a regulatory light chain wrapped around and stabilizing a long α-helix formed by the heavy chain (Fig. 36-3). The interaction of light chains with the heavy chain α-helix is similar to calmodulin binding its target proteins (see Fig. 3-12).
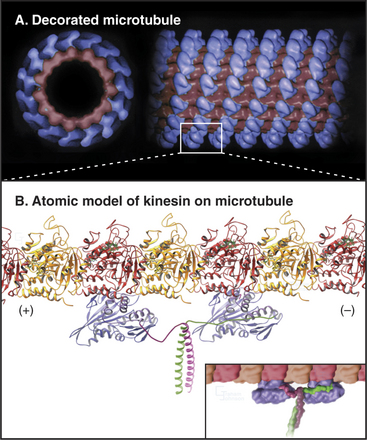
Myosin heads bind tightly and rigidly to actin filaments in the absence of ATP. This is called a rigor complex because it forms in muscle during rigor mortis when ATP is depleted after death. Myosin heads bound along an actin filament form a polarized structure, resembling a series of arrowheads when viewed from the side (Fig. 36-4). The heads bind at an angle and wrap around the filament. Their orientation defines the barbed and pointed ends of the actin filament (see Fig. 33-8). All known myosins except myosin-VI move toward the barbed end of the filament.
The atomic structures of the myosin head and actin filament fit nicely into the three-dimensional structure of the decorated filament determined by electron microscopy, providing a reasonable model of the complex at near atomic resolution (Fig. 36-4). The model shows each head in contact with two adjacent actins but does not reveal important details, including atomic contacts, between the proteins. This model is the structural starting point for understanding the mechanics of force production.
Actomyosin ATPase Cycle
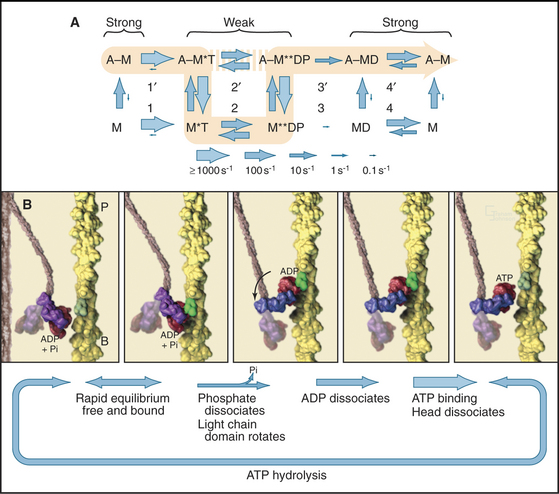
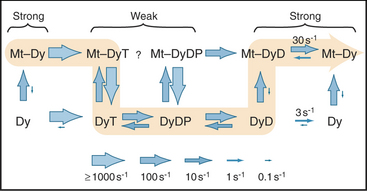
Myosin uses energy from ATP hydrolysis to move actin filaments, so an appreciation of the mechanism requires an understanding of the steps in the biochemical reaction. Figure 36-5A looks intimidating, but working through it one step at a time reveals its logic and simplicity. Note that the mechanism consists of two parallel lines of chemical intermediates. This series of reactions explains why myosin alone turns over ATP remarkably slowly, at a rate of only about 0.02 s−1. First, consider the bottom line, which shows how myosin hydrolyzes ATP in the absence of actin:
Now focus on the upper line in Figure 36-5A, where myosin is associated with an actin filament. The chemical intermediates are the same, but some of the key rate constants differ for the actin-bound and free enzymes. Steps 1 and 2 are similar to those of free myosin, but step 3—the dissociation of phosphate—is much faster when a head is bound to an actin filament. As a result, myosin bound to actin traverses the ATPase cycle about 200 times faster than myosin free in solution, and ATP hydrolysis becomes the rate-limiting step. This effect of actin is referred to as “actin activation of the myosin ATPase.” A practical advantage of this mechanism is that the ATPase cycle is essentially turned off unless a head interacts with an actin filament.
Finally, consider the vertical arrows representing transitions between bound and free states of each myosin chemical intermediate. All myosin intermediates bind rapidly to actin filaments, but the dissociation rate constants vary over a wide range depending on the nucleotide that is bound to the active site of the myosin. Myosin with no nucleotide or with bound ADP alone dissociates very slowly and therefore binds tightly to actin filaments. Myosin with bound ATP or ADP+Pi dissociates rapidly from actin, so these states bind actin weakly.
These rapid binding and dissociation reactions allow myosin intermediates (MT and MDP) to hop on and off actin filaments on a millisecond time scale, a key feature of muscle contraction (see Chapter 39). As a result of this rapid equilibrium, a single pathway cannot be drawn through the reaction mechanism of ATP, myosin, and actin. One cycle of ATP hydrolysis takes about 50 ms. Starting with AM, ATP binds very rapidly and sets up a rapid, four-way equilibrium including AMT, MT, AMDP, and MDP—the major intermediates during steady-state ATP turnover. Because the products of ATP hydrolysis dissociate much more rapidly from AMDP than from MDP, the favored pathway out of this four-way equilibrium is through AMDP to AMD and back to AM. Because the fraction of myosin heads bound to actin in the AMDP state depends on the actin concentration, the overall ATPase rate depends on the actin concentration. At the high actin concentrations in cells, a significant fraction of myosin heads are associated with actin (about 10% in contracting muscle), but each molecule continues to exchange on and off actin filaments.
Transduction of Chemical Energy into Molecular Motion
Establishing the structural basis for the conversion of free energy into force has been the most challenging question in this field of research for 50 years. A combination of mechanical measurements, static atomic structures of myosin heads with various bound nucleotides, and spectroscopic observations of contracting muscle have revealed the most likely mechanism: a dramatic conformational change in the myosin head associated with phosphate dissociation (Fig. 36-5B).
One approach has been to measure the size of the mechanical step produced by a myosin during one cycle of ATP hydrolysis. Elegant mechanical experiments on live muscles first suggested that each cycle of ATP hydrolysis moves an actin filament about 5 to 10 nm relative to myosin. Now light microscopy makes it possible to observe myosin moving single actin filaments. An array of myosin heads attached to a microscope slide can utilize ATP hydrolysis to push actin filaments over the surface (Fig. 36-6A-C). More complicated assays with single myosin molecules show that each cycle of ATP hydrolysis can move an actin filament up to 5 to 15 nm and develops a force of about 3 to 7 pN (Fig. 36-6D). At low ATP concentrations, the interval between the force-producing step and the binding of the next ATP is relatively long, so single steps can be observed.
Further insights emerged from biophysical studies of muscle and purified proteins using X-ray diffraction, electron microscopy, electron spin resonance spectroscopy, and fluorescence spectroscopy. These experiments showed that the light-chain domain pivots around a fulcrum just within the catalytic domain, which is stationary relative to the actin filament. For example, spectroscopic probes on light chains reveal a change in orientation when muscle is activated to contract, whereas the same probes on the catalytic domain do not rotate. Crystal structures of myosin heads with various bound nucleotides and nucleotide analogs show that the light-chain domain can pivot up to 90° (Fig. 36-4D). The light-chain domain is bent more acutely in the AMT and AMDP intermediates and pivots to a more extended orientation, when phosphate dissociates. ADP dissociation extends this rotation of some classes of myosin. Consistent with this concept of rotation of the light-chain domain, the rate of actin filament gliding in an in vitro assay is proportional to the length of the light-chain domain. The observed range of orientations of the light-chain domain relative to the catalytic domain can account for the observed step size of 10 nm for muscle myosin. This conformational change on phosphate release depends on rearrangements in the polypeptide chain around the γ-phosphate of ATP, similar to the changes in the Ras family of GTPases (see Fig. 4-6), but many mechanistic details remain to be resolved.
Rotation of the light-chain domain is believed to produce movement indirectly in the sense that force-producing intermediates stretch elastic elements in the system. This mechanism is represented by a spring in Figure 36-2. The elastic elements in the myosin-actin complex are most likely to be mainly in the myosin head, with small contributions from the actin and myosin filaments. Movement of the light-chain domain tensions the spring transiently in the AMD and AM states. Dissociation of ADP and rebinding of ATP to the AM intermediate reverts the system to the rapid equilibrium of weakly bound intermediates, including dissociated heads. Any force left in the spring is lost as soon as the head dissociates from the actin filament.
The actual motion depends on the mechanical resistance in the system (Fig. 36-2). If both myosin and actin are fixed, elastic elements are stretched for the life of the force-producing states (AMD and AM), and the energy is lost as heat when the head dissociates. This happens when one tries to lift an immovable object. If the resistance is less than the force in the stretched elastic elements, the actin filament moves relative to myosin, as in muscle contraction. The distance moved in each step depends on the resistance, as the spring stops shortening when the forces are balanced.
The Myosin Superfamily
All 18 classes of myosin arose from a common genetic ancestor in an early Eukaryote more than a billion years ago (Fig. 36-7). Gene duplication and divergence produced myosins specialized for particular biological functions owing to variations of the mechanochemical ATPase cycle and acquisition of diverse tails to interact with cargo. Extreme examples include myosin-V, which takes giant processive steps; myosin-VI, the only myosin that is capable of moving toward the pointed end of an actin filament; and plant myosins, which move at very high speeds (see Fig. 37-9). Within a myosin class, the tails are similar to each other, but between classes, tails are diverse in terms of their ability to polymerize and interact with other cellular components including membranes and ribonucleoprotein particles.
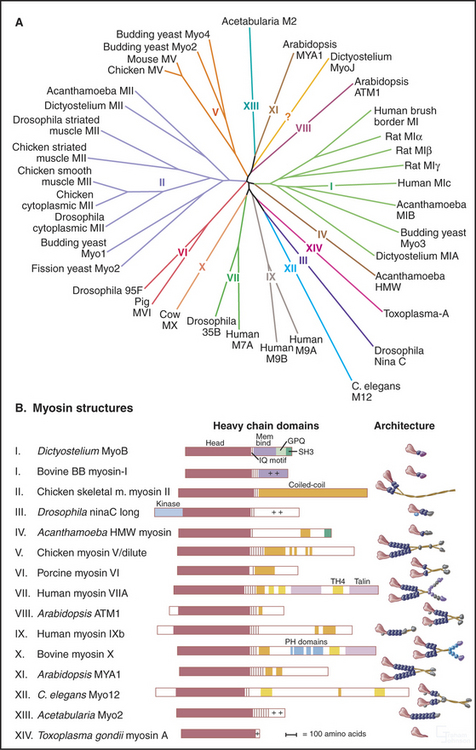
(Redrawn with permission from Mermall V, Post P, Mooseker MS: Unconventional myosins in cell movement, membrane traffic and signal transduction. Science 279:527–533, 1998. Copyright 1998 AAAS. See also Myosin Home Page, available at http://www.mrc-lmb.cam.ac.uk/myosin/myosin.html.)
No organism has genes for all 18 classes of myosin. Humans have 40 myosin genes from 12 classes. Yeast have five myosin genes, including types I, II, and V, which are widely dispersed among eukaryotes. Plants have lost the genes for these ancient myosins and are the only organisms with the highly diverged relatives of myosin-V, called myosin-VIII and myosin-XI. Gene duplications gave rise to multiple isoforms within most classes of myosin. For instance, vertebrate smooth muscle myosin genes arose from duplication of a gene for a cytoplasmic myosin-II.
Myosin-I was the first “unconventional myosin” discovered—unconventional in the sense that it differed from the type II myosin originally isolated from skeletal muscle. These myosins have one head and short tails with various types of domains, including a basic domain with affinity for acidic phospholipids. Those with an SH-3 domain (see Fig. 25-11) can bind proline-rich sequences in other proteins. Those with an actin filament–binding domain separate from the motor domain can cross-link actin filaments. Some types of myosin-I have ATPase cycles similar to skeletal muscle myosin, but others differ considerably. With duty cycles less than 10%, multiple myosin heads must work together to move membranes. Mutations show that myosin-I participates in endocytosis, as expected from its concentration at sites of phagocytosis and macropinocytosis. In microvilli of intestinal epithelial cells, myosin-I links actin filaments laterally to the plasma membrane (see Fig. 33-2B). Heavy chain phosphorylation activates myosin-I from lower eukaryotes, whereas calcium binding to calmodulin light chains regulates myosin-I from the intestinal brush border.
The myosin-II class includes various muscle myosins and cytoplasmic myosins that also have two heads and long coiled-coil tails. Assembly of tails into bipolar filaments (see Figs. 5-7 and 6-4) allows myosin-II to pull together oppositely polarized actin filaments during muscle contraction (see Chapter 39) and cytokinesis (see Fig. 44-23). As in smooth muscle (see Fig. 39-21), phosphorylation of the regulatory light chain activates myosin-II in animal nonmuscle cells. In addition, phosphorylation of the heavy chain regulates the polymerization of some myosin-IIs. Lower eukaryotes use both light-chain phosphorylation to activate myosin-II and heavy-chain phosphorylation to inhibit myosin-II.
Myosin-V participates in the movement of pigment granules and other cellular components (see Fig. 37-11). Myosin-V takes long, processive steps along an actin filament by virtue of the fact that it has a long light-chain domain and each of its two heads spends most of each ATPase cycle attached to the filament (Fig. 36-8). This is accomplished by very slow ADP dissociation from the AMD intermediate. This rate-limiting step allows plenty of time for the other head to take a long step, binding to an actin subunit 36 nm along the barbed end. Mechanical strain after the step may modestly increase the rate of ADP dissociation from the trailing head. This cooperation between the heads initiates ATP binding and the next ATPase cycle, as the motor walks deliberating along the filament. These features of myosin-V provide strong support for the light-chain domain serving as the lever arm for movements of the whole myosin family.
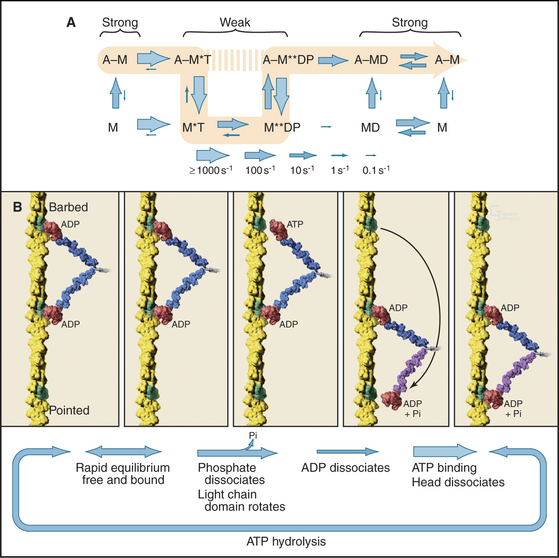
Figure 36-8 myosin-v mechanism. A, ATPase cycle with ADP release as the rate-limiting step rather than phosphate dissociation as for muscle myosin (Fig. 36-5A).B, Relationship of mechanical steps to the ATPase cycle. Shown are actin filament (A), myosin head (M), ATP (T), ADP (D), and inorganic phosphate (P).
(Reference: De La Cruz EM, Ostap EM: Relating biochemistry and function in the myosin superfamily. Curr Opin Cell Biol 16:61–67, 2004.)
Microtubule Motors
The kinesin and dynein families of molecular motors are responsible for movements of vesicles, membrane-bound organelles, chromosomes, and other cargo along microtubules in cells (see Fig. 37-1). Dynein also powers bending motions of eukaryotic flagella and cilia (see Fig. 38-14). Dyneins move themselves and any cargo toward the minus end of microtubules. Most kinesins move in the opposite direction, toward the plus end, but some kinesin family members are minus-end-directed motors (Table 36-2). Like myosins, microtubule motors have heads with ATPase activity and tails that serve as adapters for interacting with cargo.
Kinesins
Kinesins use ATP hydrolysis to move along microtubule tracks. Some can move processively along a microtubule, using cooperation between two heads to maintain physical contact with the microtubule. Processive movement allows single kinesin molecules to move cargo, such as an organelle, toward the plus end of a microtubule.
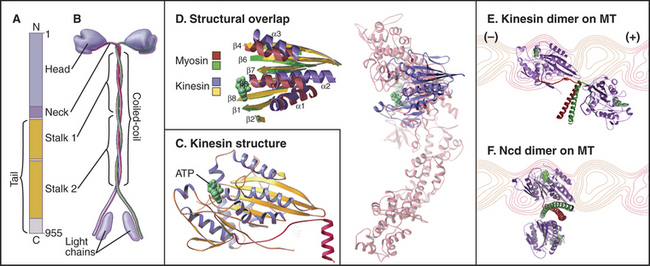
Classic kinesin or kinesin-1 has two heads at the N-terminus of an α-helical coiled-coil tail, much like myosin-II, except both the heads and coiled-coil are smaller (Fig. 36-9). The tail of kinesin-1 is called the stalk. Each head, consisting of about 340 residues, is a motor unit that binds microtubules and catalyzes ATP hydrolysis. With some variation in amino acid sequence, this motor unit is common to the whole kinesin family. In kinesin-1, light chains are associated with the C-terminal bifurcation of the tail. Other members of the kinesin family have the motor do-main (Fig. 36-13) attached to a variety of tails that are believed to interact with cargo. Most kinesin motor domains are located at the N-terminus of the polypeptide chain, but a few are at the C-terminus or even in the middle.
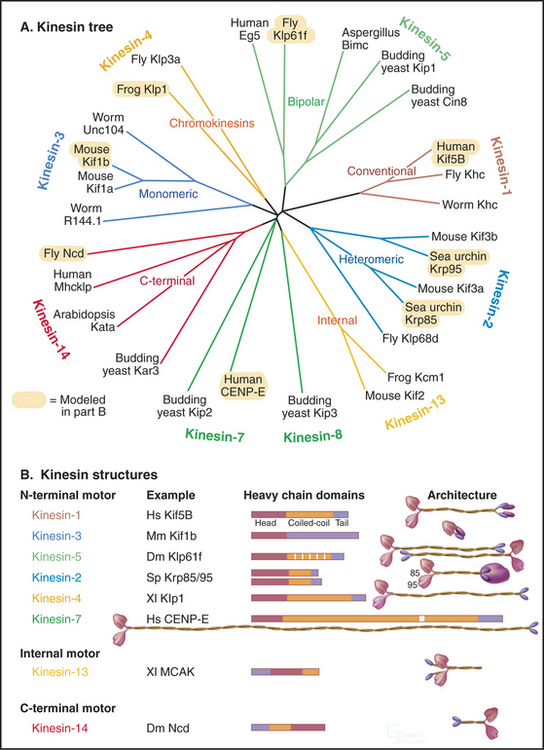
(Based on data of R. Case and R. Vale, University of California, San Francisco. References: Lawrence CJ, Dawe RK, Christie KR, et al: Standardized kinesin nomenclature. J Cell Biol 167:19–22, 2004; Dagenbach EM, Endow S: A new kinesin tree. J Cell Sci 117:3–7, 2004; see also the Kinesin Home Page at http://www.proweb.org/kinesin.)
Because the kinesin head is less than half the size of a myosin head and because the proteins lack appreciable sequence homology, determination of the atomic structure of kinesin-1 (Fig. 36-9) revealed a major surprise: The small kinesin head is folded like the core of the catalytic domain of myosin! In fact, this core consisting of a central, mixed β-sheet flanked by helices is similar to the considerably smaller Ras family GTPases (see Fig. 4-6). This provided strong evidence that all three families of nucleoside triphosphatases evolved from a common ancestor. ATP binds to a site on kinesin that is homologous to the GTP-binding site of Ras, but the enzyme mechanisms differ in important ways. The microtubule-binding site is some distance from the ATP-binding site, as seen by fitting the atomic model of the head into three-dimensional reconstructions of kinesin-1 bound to microtubules (Fig. 36-10).
Kinesin Mechanochemistry
In vitro motility assays (Fig. 36-11) revealed that a two-headed kinesin-1 can move along a single (or two parallel) microtubule protofilaments for long distances at 0.8 mm/s. Kinesin-1 moves in discrete steps of 8 nm, the spacing of successive tubulin dimers in a microtubule, so the motor takes a step every 10 ms when moving at full speed. This large step of 8 nm is remarkable for the small (<10 nm) kinesin heads linked together at the neck region. Processive movement depends on the ability of kinesin to remain associated with the microtubule as it moves.
Single kinesin-1 heads, produced experimentally by expression of truncated complementary DNAs (cDNAs), traverse a microtubule-stimulated ATPase cycle much like myosin (Fig. 36-12A). Like myosin, kinesin-1 binds and hydrolyzes ATP rapidly followed by slower release of phosphate and ADP. Some kinesin head preparations dissociate from the microtubule during each cycle of ATP hydrolysis, but others (differing in the length of the polypeptide) appear to remain bound to the microtubule (presumably a single tubulin dimer) through multiple cycles of ATP hydrolysis.
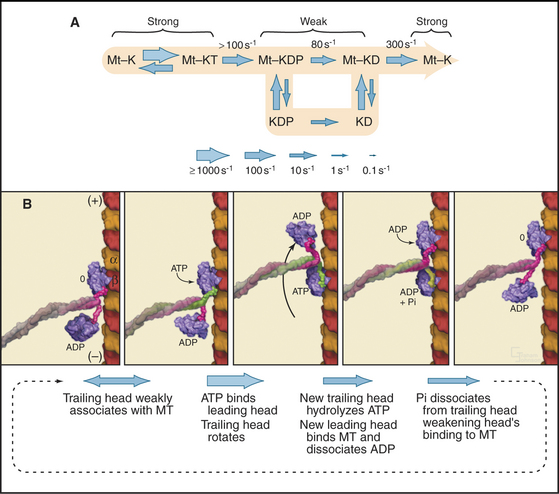
Kinesin-1 with two heads moves processively along a microtubule, remaining attached through more than a hundred cycles of ATP hydrolysis (Fig. 36-12B). The presence of a second head introduces a key feature: The two heads strongly influence each other, leading to reciprocal affinities of the heads for nucleotide (either ATP or ADP) and microtubules. One head binds nucleotide strongly and microtubules weakly; the other does the opposite. Hence, one head tends to bind the tubule and to dissociate its bound nucleotide rapidly. For example, if kinesin-1 with ADP bound to both heads is mixed with microtubules, only one of the two heads binds the microtubule and dissociates its ADP. Given an excess of ATP over ADP in cells, ATP will bind to this open site on the head associated with the microtubule, starting a processive cycle of stepping, each step coupled to one ATP turnover. ATP binding drives the conformational change or switch that propels the rearward head forward to bind the next tubulin subunit toward the plus end of the microtubule. ATP hydrolysis on the rearward head leads to tight binding of the forward head, whose active site is now empty. Phosphate release from the rearward head weakens its affinity for the microtubule, resulting in detachment of the rearward head.
Cooperation between the two heads ensures that at least one head is bound to the microtubule at every point in the ATPase cycle (Fig. 36-12B). The reciprocal affinities for nucleotide and microtubules allows the two heads to alternate between microtubule binding and dissociation. During this interchange, the trailing ADP head steps past the bound ATP head and binds to the tubulin dimer 8 nm beyond the ATP head. A simple mechanism might have the trailing head always step around the same side of the leading head, resulting in a 360° rotation every two steps. However, microtubules moved by kinesin-1 attached to a slide do not rotate. This result together with several types of experiments with single kinesin-1 heads labeled with fluorescent dyes showed that kinesin walks asymmetrically, alternating steps on the right and left sides of the microtubule like a person walking on a beam.
The mechanism of stepping is postulated to be the folding and unfolding of a segment of the kinesin-1 heavy chain linking the motor domain to the coiled-coil neck/stalk. When ATP is bound to the motor domain, this “neck-linker” peptide is postulated to associate tightly with the motor domain, as in the X-ray structure of dimeric kinesin (Fig. 36-9). When no nucleotide or ADP is bound to the motor domain, the neck-linker peptide is thought to be flexible and only loosely bound to the motor domain.
The Kinesin Superfamily
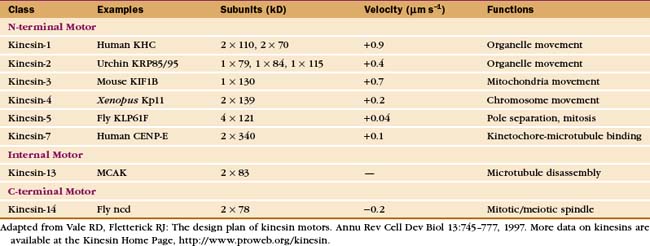
Early in evolution, gene duplication and recombination produced at least 14 families of kinesins having motor domains associated with a variety of coiled-coil stalks and tails (Fig. 36-13 and Table 36-2). Classification systems based on the sequences of motor domains turn out to group kinesins that also have similar coiled-coil stalks and tails, and functions. Most kinesins are dimeric, with two polypeptides joined in a coiled-coil. Most are homodimers, but the kinesin-2 class consists of two different polypeptides with motor domains plus another large subunit. Most kinesins move along microtubules toward their plus ends, but C-terminal kinesin-14 motors, such as Ncd, move toward the minus end, and internal motor kinesins-13 might not move at all but function to destabilize microtubules (see Fig. 34-9). Whether located at the N- or C-terminus, the motor domains are similar in structure; peptide sequences that link the head to the neck/stalk determine the direction of movement on microtubules.
Kinesins transport a variety of cargo, including chromosomes and organelles, along microtubules. A variety of evidence implicates kinesin-1 in the movement of membrane vesicles toward the plus end of microtubules in nerve axons and other cells (see Fig. 37-1). Chromokinesins or kinesin-4 motors have DNA-binding sites that allow them to bind to the surface of mitotic chromosomes and carry them toward the metaphase plate (see Fig. 44-7). Kinesin-7 or CENP-E is concentrated at kinetochores. Bipolar kinesin-5 motors form an antiparallel tetramer of two dimeric kinesins that can interact with a pair of oppositely polarized microtubules and push apart the poles of the mitotic spindle (see Fig. 44-7).
Some kinesins, such as the Drosophila kinesin-14, Ncd, move backward, toward the minus rather than the plus end of microtubules. This difference is not explained by the structural architecture of the motor domain, as Ncd is nearly identical to plus-end kinesin-1 (Fig. 36-9). The Ncd ATPase mechanism is similar to kinesin-1, although there is less cooperation between the heads and no processivity. The most obvious difference is that the Ncd motor domain is attached to the coiled-coil stalk by its N-terminus, rather than C-terminus, as in kinesin-1. However, this does not explain backward movement, because transplantation of kinesin-1 heads to the C-terminus of a coiled-coil stalk or Ncd heads to the N-terminus of a coiled-coil stalk does not necessarily reverse their motor activity. Experiments with more complicated chimeric proteins suggest that the neck-linker peptide and the proximal part of the Ncd coiled-coil stalk determine the direction of movement. Interactions of the heads with the neck/stalk are important in directing the motor toward the microtubule minus end.
Dyneins
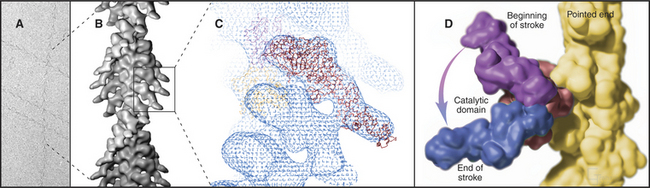
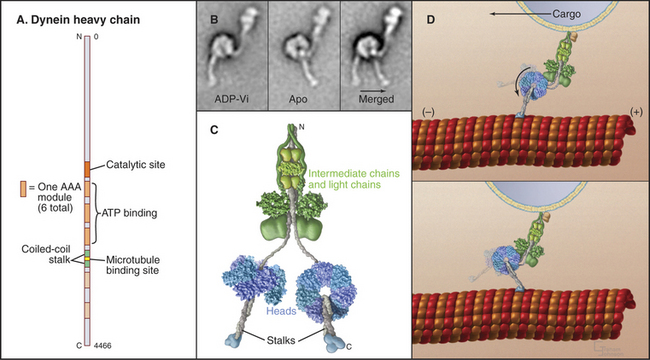
Dynein microtubule-based motors are AAA ATPases (Box 36-1), so they have a different evolutionary origin than myosins and kinesins. Unique in the AAA family, dyneins consist of six ATPase domains concatenated in a giant heavy chain of nearly 500 kD (Fig. 36-14). The globular head is formed from the six AAA domains, each predicted to be folded like the known crystal structures of other AAA ATPases, and a seventh domain of un-known structure. The N-terminal half of the heavy chain forms a tail that interacts with accessory polypeptides (called light and intermediate chains) and cargo molecules. The segment of dynein heavy chain between the fourth and fifth AAA domains forms a coiled-coil stalk. The globular end of the stalk binds to a site on microtubules similar to the kinesin bind-ing site.
Dynein Mechanochemistry
AAA domain 1 binds and hydrolyzes ATP during force-producing interactions with microtubules. Full motor function requires ADP or ATP binding to domains 2 to 4, but ATP hydrolysis by these domains is not coupled directly to motility. The dynein ATPase cycle of AAA domain 1 resembles the actomyosin ATPase mechanism in broad outline but differs in important details, particularly the rate-limiting reactions (Fig. 36-15). Remarkably, ATP binding to AAA domain 1 dissociates the stalk from microtubules that are more than 20 nm distant. The dynein-ADP-Pi intermediate also binds weakly to microtubules. After rapid dissociation of inorganic phosphate, the dynein-ADP complex rebinds to the microtubule. Binding to a microtubule stimulates the rate of ADP dissociation from dynein about 10-fold, from about 3 s−1 to about 33 s−1, by accelerating a rate-limiting conformational change. Consequently, microtubules stimulate the dynein ATPase to levels required for the rapid beating of cilia at up to 100 cycles per second. Free dynein turns over ATP relatively rapidly (3 s−1). However, in cilia and flagella, control mechanisms keep dynein turned off except during beating, as ATP hydrolysis is tightly coupled to the production of motion (see Fig. 38-14).
If dynein is immobilized on a surface in an in vitro motility assay, the plus end of a microtubule moving on the bound motors will lead as the dynein “walks” toward the minus end of the microtubule.
Crystal structures of the various chemical intermediates will be required to learn how the ATPase cycle drives the motion of dynein toward the minus end of microtubules. An attractive hypothesis that is consistent with electron micrographs of isolated dynein (Fig. 36-14B) is that the microtubule-binding stalk is used as a lever arm to amplify conformational changes in the globular domain during the ATPase cycle. However, it is not understood how ATP binding to the active site dissociates a distant binding site from the microtubule or how phosphate release moves the stalk, particularly since the whole molecule appears to be quite flexible. The size of the mechanical step associated with each ATP hydrolysis in most often 8 nm, but cytoplasmic dynein can take larger steps when the load is low.
The Dynein Superfamily
Tissues express these dynein isoforms differentially and target them to specific cellular locations. In axonemes of cilia and flagella, at least seven different dynein isoforms bind to unique sites on the outer doublets (see Fig. 38-16). Cytoplasmic dynein isoforms associate with organelles for transport along microtubules toward the cell body in nerve axons and with ER vesicles for transport to the Golgi apparatus located at the cell center. A null mutation in the gene for a mouse cytoplasmic dynein heavy chain leaves the Golgi apparatus dispersed throughout the cytoplasm and is lethal during embryogenesis. During mitosis, dyneins in the cell cortex and bound to kinetochores of chromosomes apply forces to microtubules of the mitotic spindle (see Fig. 44-7). A temperature-sensitive mutation in Caenorhabditis elegans dynein heavy chain results in defects in mitosis at the restrictive temperature.
Calcium and a cyclic adenosine monophosphate (cAMP)–dependent protein kinase (see Fig. 25-3D) regulate dynein in cilia and flagella, but little is known about the regulation of cytoplasmic dynein.
Asbury CL. Kinesin: World’s tiniest biped. Curr Opin Cell Biol. 2005;17:89-97.
Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780-794.
Burgess SA, Knight PJ. Is the dynein motor a winch? Curr Opin Struct Biol. 2004;14:138-146.
Buss F, Spudich G, Kendrick-Jones J. Myosin VI: Cellular functions and motor properties. Annu Rev Cell Dev Biol. 2004;20:649-676.
De La Cruz EM, Ostap EM. Relating biochemistry and function in the myosin superfamily. Curr Opin Cell Biol. 2004;16:61-67.
Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu Rev Biophys Biomol Struct. 2006;35:93-114.
Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem. 1999;68:687-728.
Hackney DD. The kinetic cycles of myosin, kinesin and dynein. Annu Rev Physiol. 1996;58:731-750.
Hirokawa N, Takemura R. Biochemical and molecular characterization of diseases linked to motor proteins. Trends Biochem Sci. 2003;28:558-565.
Kull FJ, Endow SA. A new structural state of myosin. Trends Biochem Sci. 2004;29:103-106.
Mandelkow E, Johnson KA. The structural and mechanochemical cycle of kinesin. Trends Biochem Sci. 1998;23:429-433.
Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 2005;15:467-476.
Oiwa K, Sakakibara H. Recent progress in dynein structure and mechanism. Curr Opin Cell Biol. 2005;17:98-103.
Spudich JA. Motors take tension in stride. Cell. 2006;126:242-244.
Tyska MJ, Mooseker MS. Myosin-V motility: These levers were made for walking. Trends Cell Biol. 2003;13:447-451.
Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467-480.