51
Mosaic Skin Conditions
• A mosaic organism is composed of ≥2 genetically distinct cell populations derived from a homogeneous zygote.
– Genomic mosaicism results from alteration in the DNA sequence (affecting genes or chromosomes).
• Clinical findings in mosaic skin conditions depend not only on the underlying genetic alteration but also on the timing of its origin (with earlier onset generally leading to more widespread involvement) and the cells or tissues affected (cutaneous ± extracutaneous).
• The accessibility of the skin allows visualization of mosaic patterns.
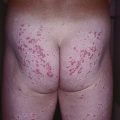
– Blaschko’s lines are streaks and swirls that represent pathways of epidermal cell (e.g. keratinocyte or melanocyte) migration during embryonic development (Fig. 51.1).
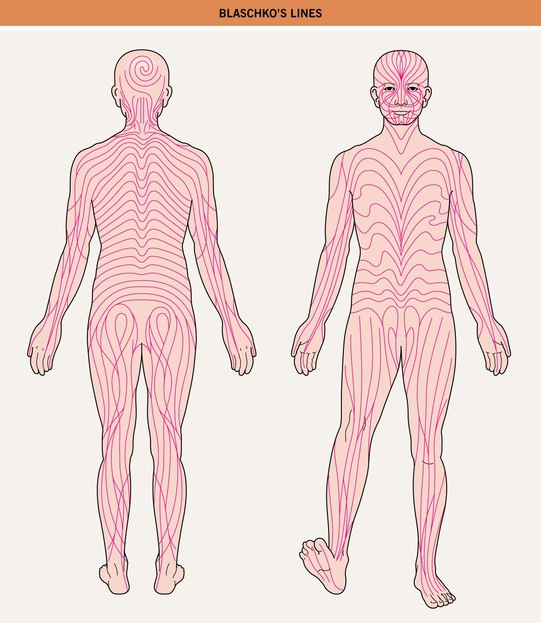
Fig. 51.1 Blaschko’s lines. Note the S-shape of lines on the abdomen, the V-shape on the central back, perpendicular lines on the face, and swirls on the posterior scalp.
• Types of cutaneous lesions that can follow Blaschko’s lines or have a block-like/segmental pattern are outlined in Tables 51.1 and 51.2, respectively (Fig. 51.2).
Table 51.1
Skin findings that can occur along Blaschko’s lines.
* Inflammatory manifestations occur primarily during infancy.
** In addition to conditions with inflammatory or verrucous papulonodules noted above.
GVHD, graft-versus-host disease; IP, incontinentia pigmenti; PEODDN, porokeratotic eccrine ostial and dermal duct nevus.
Table 51.2
Skin findings that can have a block-like or segmental pattern that reflects mosaicism.
Hypopigmentation – e.g. nevus depigmentosus, segmental vitiligo (see Chapter 54)
Hyperpigmention ± hypertrichosis – e.g. CALM, Becker’s nevus/smooth muscle hamartoma (see Table 50.3 and Chapter 55)
Hypertrichosis – e.g. X-linked congenital generalized hypertrichosis (female ‘carriers’)
Vascular lesions – e.g. port wine stain,† CMTC, unilateral nevoid telangiectasia, venous malformation,* plaque-type glomuvenous malformation,** segmental infantile hemangioma (see Chapter 85)
Papulonodular lesions – e.g. segmental leiomyomas** or neurofibromas**
† Associated with mosaic activating mutations in the GNAQ gene encoding the Q-class G protein α-subunit; mutations in this gene have also been associated with dermal melanocytosis, which occurs as ‘twin spots’ with port wine stains in phakomatosis pigmentovascularis.
CALM, café-au-lait macule; CMTC, cutis marmorata telangiectatica congenita.
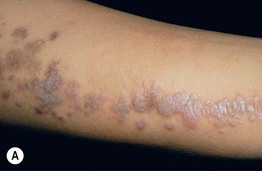
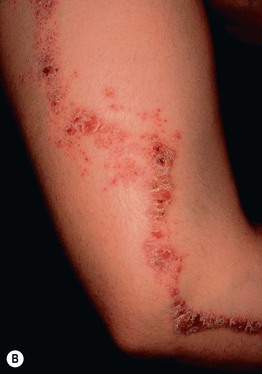
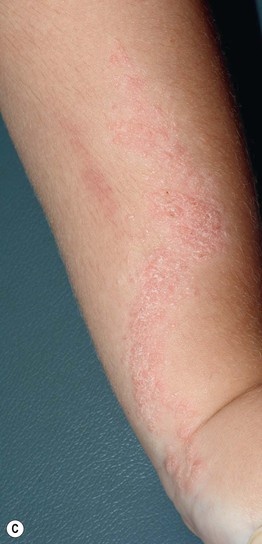
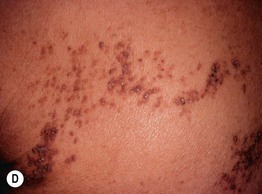
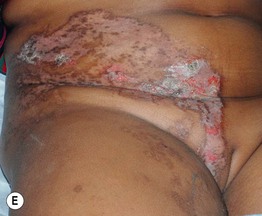
Fig. 51.2 Inflammatory lesions along Blaschko’s lines. A Linear lichen planus presenting as a band of coalescing violaceous papules and plaques with Wickham’s striae on an extremity. Note the postinflammatory hyperpigmentation proximally. B Inflammatory linear verrucous epidermal nevus (ILVEN) featuring persistent, extremely pruritic, scaly psoriasiform plaques. C Linear psoriasis with erythema and scale that responded to treatment with a high-potency topical CS. D Linear Darier disease presenting with keratotic papules in an adult, representing type 1 mosaicism (see Fig. 51.5A). E Linear Hailey–Hailey disease presenting with recurrent blistering and erosions in a young girl, representing type 2 mosaicism with earlier and more severe involvement in the affected region due to a ‘second hit’ mutation (see Fig. 51.5B). A, Courtesy, Joyce Rico, MD; C, E, Courtesy, Julie V. Schaffer, MD.
Epidermal Nevi and ‘Epidermal Nevus Syndromes’
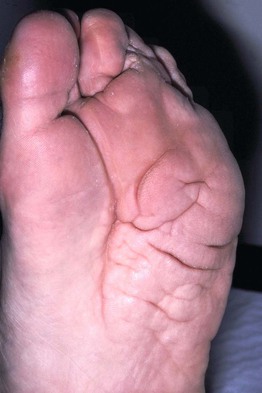
• Epidermal nevi (see Chapter 89) present as streaks and swirls of thickened (e.g. verrucous, hyperkeratotic, or velvety) skin along Blaschko’s lines, usually with hyperpigmentation and sometimes with adnexal involvement (e.g. in a nevus sebaceus or nevus comedonicus) (Fig. 51.3).
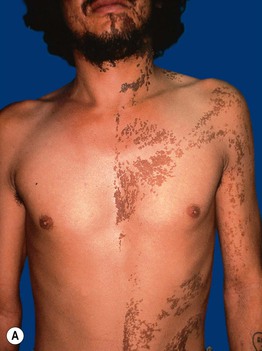
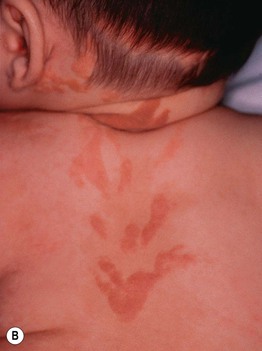
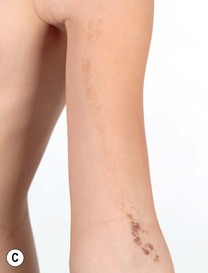
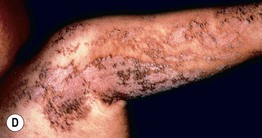
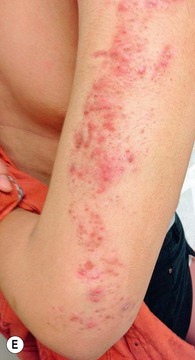
Fig. 51.3 Epidermal nevi. A Extensive lesion with especially verrucous areas on the neck. B V-shaped pattern on the mid back. C Lesion due to a FGFR3 mutation, representing a mosaic counterpart of the acanthosis nigricans in thanatophoric dysplasia, an autosomal dominant neuroskeletal syndrome. This explains the flexural accentuation of the epidermal nevus. D Epidermolytic epidermal nevus. Note the shedding of scale and associated hypopigmentation. E Nevus comedonicus with inflammatory papulonodules as well as comedones in an adolescent. The lesion initially presented as a congenital hypopigmented streak. C, Courtesy, Celia Moss, MD; E, Courtesy, Julie V. Schaffer, MD.
• The heterogeneous genetic etiologies and potential systemic associations (‘epidermal nevus syndromes’) of various types of epidermal nevi are presented in Table 51.3 (Fig. 51.4).
Table 51.3
Variants of epidermal nevi (EN) and possible associated findings.
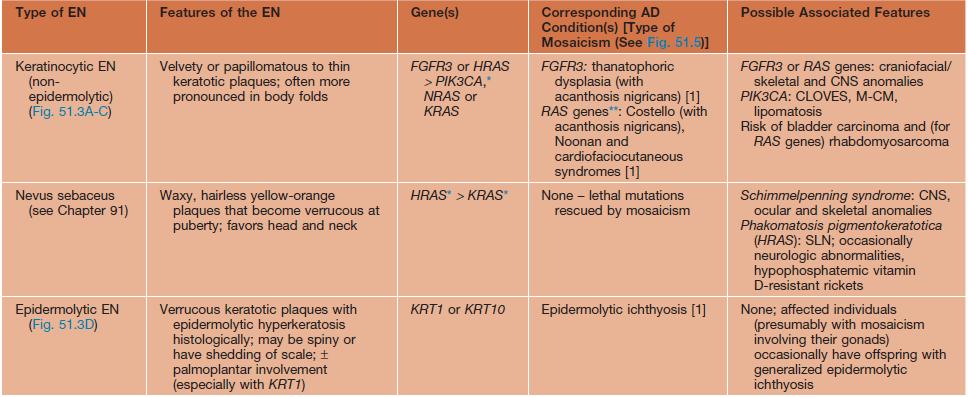
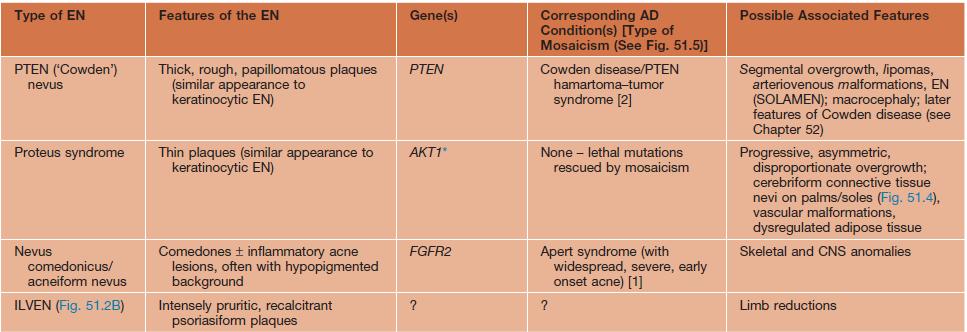
* The underlying mosaic mutations are generally not compatible with life if present in all the cells of the body.
** Usually but not always different (e.g. more severe) mutations within the EN than in the AD disorder.
AD, autosomal dominant; CLOVES, congenital lipomatous overgrowth with vascular, epidermal, and skeletal anomalies; FGFR, fibroblast growth factor receptor; ILVEN, inflammatory linear verrucous epidermal nevus; KRT, keratin; M-CM, megalencephaly (macrocephaly)–capillary malformation syndrome; PIK3CA, phosphoinositide-3-kinase, catalytic α-polypeptide; SLN, speckled lentiginous nevus.
Mosaicism in Autosomal Dominant Skin Conditions
• Type 1, type 2, and revertant forms of mosaicism occurring in autosomal dominant skin disorders are summarized in Fig. 51.5.
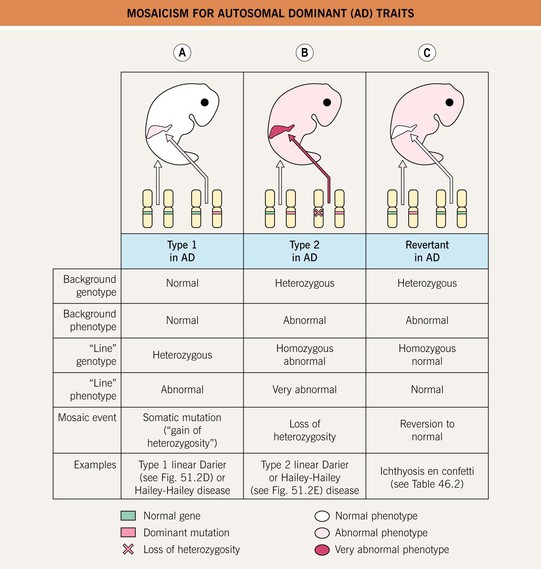
Fig. 51.5 Mosaicism for autosomal dominant (AD) traits. A Type 1 mosaicism features a linear distribution of the skin lesions that characterize an AD genodermatosis in the absence of involvement elsewhere on the body. This results from a postzygotic heterozygous mutation in the underlying gene that is only present in the affected region. B Type 2 mosaicism presents with a more severe linear lesion superimposed on the background of a generalized AD genodermatosis. This reflects a constitutional (in all the cells of the body) heterozygous mutation plus a ‘second hit’ in the other allele of the gene within the more severely affected region. C In revertant mosaicism, an area of normal-appearing skin occurs in the background of a generalized AD genodermatosis due to a reversion to normal or other correction of the underlying heterozygous mutation in that region.
Mosaicism in X-Linked Conditions
• In conditions traditionally referred to as X-linked recessive, male patients have generalized disease and female patients or ‘carriers’ are affected to a variable (usually lesser) degree, often in a mosaic pattern; an example is hypohidrosis and hyperpigmentation following Blaschko’s lines in female ‘carriers’ of X-linked hypohidrotic ectodermal dysplasia (Fig. 51.6).
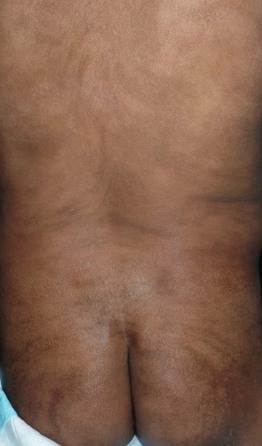
Fig. 51.6 Female ‘carrier’ of X-linked hypohidrotic ectodermal dysplasia. The skin within the hyperpigmented streaks and swirls following Blaschko’s lines is extremely smooth. Starch-iodide testing on the back disclosed a patchy distribution of active sweat glands. This 2-year-old girl also had whorled areas of sparse hair on the posterior scalp and several cone-shaped teeth. Courtesy, Julie V. Schaffer, MD.
Incontinentia Pigmenti (IP)
• X-linked dominant disorder caused by mutations in the NF-κB essential modulator (NEMO) gene, which encodes a protein that protects against TNF-α-induced apoptosis; male patients with milder NEMO mutations may present with hypohidrotic ectodermal dysplasia with immune deficiency (see Table 52.5).
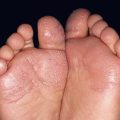
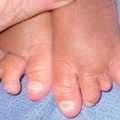
• IP has four stages, which may be absent or overlapping, of cutaneous findings that follow Blaschko’s lines (with the exception of stage 4) (Fig. 51.7; see Fig. 28.9):
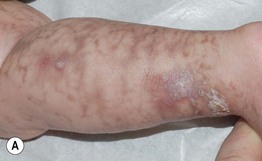
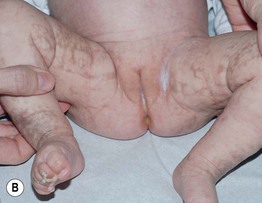
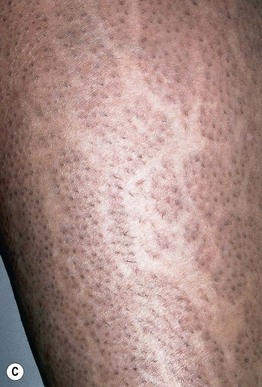
Fig. 51.7 Incontinentia pigmenti. A, B Stages 2 (verrucous) and 3 (hyperpigmented). Note the erythematous background of the keratotic papules and plaques on the leg (A) and the remaining keratotic lesions on the toes (B). The scalloped edges of the hyperpigmented streaks reflect growth of normal keratinocytes into areas of apoptosis. C Stage 4 (atrophic and hypopigmented). Note the absence of hairs within the streaks on the calf. A and B, Courtesy, Julie V. Schaffer, MD.
Goltz Syndrome (Focal Dermal Hypoplasia)
• Skin lesions following Blaschko’s lines are characterized by vermiculate dermal atrophy, outpouchings of fat, telangiectasias, and hypo- > hyperpigmentation (Fig. 51.8).
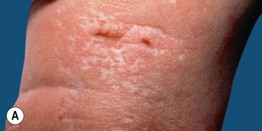
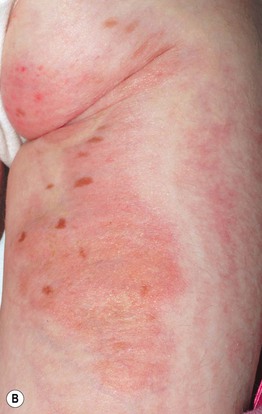
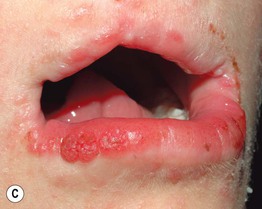
Fig. 51.8 Goltz syndrome. A Hypopigmented streaks of vermiculate atrophy and fat ‘herniation.’ B Bands of telangiectatic erythema, dermal atrophy with fat ‘herniation,’ and hyperpigmented macules on the posterior thigh. C Raspberry-like papules on the lower lip. Similar lesions can occur in the anogenital region and may be confused with warts. B, C, Courtesy, Julie V. Schaffer, MD.
X-Linked Dominant Ichthyosiform Conditions
Lethal Disorders Rescued by Mosaicism
• Examples include Proteus and Schimmelpenning syndromes presenting with epidermal and sebaceous nevi (see Table 51.3), Sturge–Weber syndrome (see Table 51.2 and Chapter 85), and forms of ‘pigmentary mosaicism’ including McCune–Albright syndrome (see Table 50.3 and Chapters 54 and 55).