Chapter 27 Modern Concepts in Intrauterine Devices
INTRODUCTION
The intrauterine device (IUD) represents a unique approach to birth control and was the first woman-initiated contraceptive device to become widely available. From the beginning, it had the advantages of reversibility and relative effectiveness. In the past, these benefits were offset by the disadvantage of requiring placement by a healthcare provider, as well as by a spectrum of side effects and serious risks. Over the years, technological improvements have resulted in the evolution of the IUD into a highly efficacious and safe contraceptive method. Worldwide, it has become the most widely used form of reversible contraception. In Western Europe, 7% to 18% of women using contraception have an IUD.1 In the United States, less than 1% of women use an IUD for contraception, primarily because of reports of infectious complications resulting from IUDs no longer in use.2
HISTORY
It has long been alleged that the idea for a human IUD arose from Arab traders’ use of stones in the uterine cavities of camels to prevent unplanned pregnancies. Regretfully, this fascinating story appears to have no basis in fact.3 In actuality, the IUD appears to have evolved in the early 1800s from the stem pessary. This cup-shaped device had a stem that fit into the cervical canal and was designed to be placed in the vagina to support the uterus or rectum. It was soon found to have some contraceptive effectiveness, and in 1902 Hallwig designed a version with a stem that extended into the uterine cavity. This device, sold without prescription for self-insertion, was associated with a high infection rate and was not endorsed by the medical community.
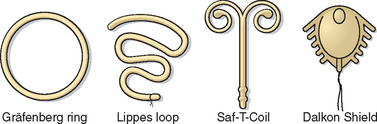
In 1930 in Germany, Gräfenberg introduced a silver ring IUD without the tail common to earlier versions (Fig. 27-1). This contraceptive device had the drawback of a high expulsion rate. In 1934 in Japan, Ota modified this design slightly by adding a supportive structure to the ring. Both of these pioneers were ostracized by their respective medical communities. Regardless of their personal lack of success, their innovative work proved to be the first effective female-initiated contraceptive technique with a reasonably low risk of side effects and complications. Based on these attributes and a lack of equivalent alternative methods, IUD use gradually spread throughout the world.
Intrauterine devices gained wide popularity in the 1960s and 1970s with the introduction of such models as the Margulies Spiral, the Lippes Loop, the Saf-T-Coil, the Birnberg bow, and the Dalkon Shield (see Fig. 27-1).4 In 1962, the Population Council convened the first International IUD Conference in New York City to analyze IUD data. In 1970, the Population Council published a report that concluded that IUDs were generally safe and an efficacious method of birth control.5 With this endorsement, IUD utilization increased rapidly. By early 1970, there were more than 70 IUDs on the market, which together made up 10% of the contraceptives used in the United States.
Inert IUDs
The IUDs approved by the Food and Drug Administration (FDA) in the 1960s and 1970s (e.g., Lippes Loop, Saf-T-Coil) were made of plastic (see Fig. 27-1). The pregnancy rates associated with the use of these IUDs were almost 20% per year. Another problem with this generation of IUD was that their relatively large size and shapes resulted in an unacceptable rate of increased menstrual bleeding and discomfort.
The Dalkon Shield
The Dalkon Shield was a uniquely designed IUD, whose associated complications resulted in the diminished popularity of IUDs in the United States that continues to this day. The Dalkon Shield, introduced by the A. H. Robbins Company in 1971, was a plastic ring to which had been added lateral spikes to decrease the expulsion rate, a central membrane, and a braided multifilament tailstring for removal (see Fig. 27-1). By 1974, more than 2.8 million women in the United States were using the Dalkon Shield. By coincidence, the early 1970s witnessed a period of increased sexual freedom referred to as the Sexual Revolution, which was associated with a corresponding increase in the prevalence of sexually transmitted diseases and pelvic inflammatory disease (PID).
The first indications of a problem were case reports of adverse IUD-related events, including PID, ectopic pregnancy, and septic abortions, some of which were fatal.6 Systematic investigations ultimately concluded that the Dalkon Shield, in particular, was associated with an increased risk of infectious complications. When tailstrings from different IUD models were cultured, the braided Dalkon Shield tailstrings were found to grow several types of bacteria, whereas monofilament tailstring from other IUDs had no positive cultures.7 This information supported the hypothesis that the increased risk of pelvic infection associated with the Dalkon Shield was the result of a wicking action of the braided tailstring that facilitated the ascent of bacteria into the upper genital tract. After only 3 years in production, the Dalkon Shield was taken off the U.S. market in 1974.
Copper-containing IUDs
Since the introduction of modern IUDs, research has focused on ways to increase their contraceptive efficacy, first by altering their configuration and then by including some pregnancy-preventing substance in their design. Studies in rabbits indicate that intrauterine placement of either copper or zinc could prevent implantation.8 Based on this information, copper-containing IUDs were developed for humans.
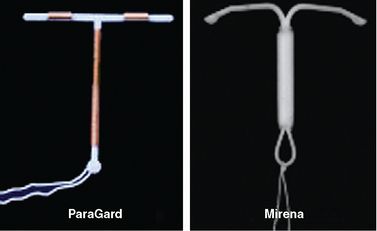
These first “medicated” IUDs had copper wire wrapped around the vertical stem of a T-shaped IUD. Earlier copper IUDs contained from 30 to 200 mm2 of copper.9 Animal studies indicated that adding higher amounts of copper dramatically enhanced contraceptive efficacy. The copper-containing ParaGard IUD, introduced in 1988, contains 300 mm2 of copper on the vertical arm and 40 mm2 on each of the horizontal arms, for a total of 380 mm2 (Fig. 27-2). This amount of copper decreased the pregnancy rate to less than 1% with copper. For the first time, the failure rate of an IUD was comparable to oral and injectable contraceptive methods and rivaled that of surgical sterilization. The ParaGard IUD is one of two IUDs currently available in the United States today.
The First Progestin-impregnated IUD
Hormone-impregnated IUDs were also evaluated to determine if they increase contraceptive efficacy. In 1976, the first progestin-releasing IUD was introduced to the market as the Progestasert. This T-shaped IUD had a vertical stem containing 38 mg of progesterone, which was released at a rate of 65 μg/day. In addition to contraception, it offered the additional benefit of diminished menstrual bleeding and discomfort. However, due to a failure rate of 2.9% and need for annual replacement, it was never widely utilized and its production was discontinued in 2001. The efficacy of this approach was greatly improved by the use of the more potent progestin levonorgestrel. The levonorgestrel-containing Mirena IUD is the second of two IUDs available in the United States today (see Fig. 27-2).
MODERN INTRAUTERINE DEVICES
Two IUDs are currently available in the United States; the copper T 380A (ParaGard, Ortho Pharmaceutical Corp., Raritan, N.J.) and the levonorgestrel intrauterine system (Mirena, Berlex Pharmaceuticals, Montville, N.J.) (see Fig. 27-2). Both are highly effective for preventing pregnancy with acceptable side effect profiles. The Mirena has the added benefit of decreasing both menstrual flow and discomfort in many patients.
The Copper-containing ParaGard IUD
The ParaGard IUD, introduced in 1988, has a flexible plastic T frame (see Fig. 27-2). A copper wire is wound around the stem, and copper sleeves are attached to the horizontal bars for a total copper surface area of 380 mm2. A monofilament tail-string is attached to the end of the vertical stem. The pregnancy failure rate for the ParaGard IUD is less than 1%, and it was originally approved by the FDA for 4 years of use before removal and replacement. However, subsequent studies showed that the ParaGard IUD could be used much longer without a decline in efficacy, and it is currently approved for 10 years of use. It has withstood the test of time with proven safety and patient tolerance.
The Levonorgestrel-containing Mirena IUD
The Mirena IUD was introduced in the United States in 2001. Also referred to as the levonorgestrel intrauterine system, the Mirena has a reservoir that contains 52 mg of levonorgestrel in the polyethylene T-shaped frame measuring 32 mm × 32 mm and has a monofilament string attached to the end of its vertical stem (see Fig. 27-2). The failure rate of this product is less than 0.1% per year. It has the added benefit of decreasing both menstrual bleeding and dysmenorrhea in most women.
Mechanisms of Action
The two IUDs currently available in the United States, the copper-containing ParaGard and the levonorgestrel-containing Mirena, both work primarily by inhibiting fertilization and secondarily by inhibiting implantation.10,11 Because they work before implantation, IUDs are not considered to be abortifacients. Both IUDs share a foreign body mechanism of action, but each has a second mechanism of action related to the substance they release locally.
Foreign Body Effect
Like earlier inert IUDs, both the ParaGard and Mirena IUDs produce a strong foreign body inflammatory reaction in the endometrium. The presence of the plastic T-shaped IUD causes the endometrial lining to release white blood cells, prostaglandins, and enzymes.12 In addition to making the endometrium unfavorable for implantation, this inflammatory reaction is toxic for sperm. As a result, IUD users have fewer sperm reaching the site of fertilization in the ampullary region of the fallopian tube.13 Based on studies of the earlier inert IUDs, it can be extrapolated that this foreign body reaction prevents pregnancy with a failure rate of approximately 20% per year. The addition of copper and progestin is responsible for further reducing the failure rate with modern IUDs to less than 1% per year.
Copper Effects
The primary contraceptive effect of the copper released from the ParaGard IUD appears to be toxicity to sperm and oocytes before fertilization in various locations in the uterus and tubes. In the cervix, a high copper concentration in the mucus decreases both sperm motility and the ability of sperm to penetrate the mucus.14–16 Copper has the curious ability to cause the heads and tails of sperm to separate.17
In the uterine cavity, copper ions enhance the inflammatory intrauterine response in the endometrium, adding to the spermicidal effect.10 In the fallopian tubes, high copper concentrations interfere with transportation and function of both sperm and oocytes.18 When the uterine tubes of women with a copper IUD in place were flushed, only half as many oocytes were present compared to controls, and none of these oocytes showed normal development.19
Copper also appears to affect fertilization and implantation. Although copper IUDs do not prevent ovulation, oocyte fertilization is decreased by half and those embryos that do form rarely implant.12,13 Further evidence of the preimplantation nature of copper IUD effects is the finding that serum levels of β-human chorionic gonadotropin (hCG) are uniformly undetectable in these women.20 To date, there has been no evidence to indicate that copper IUDs act as abortificients after implantation.
Levonorgestrel Effects
The levonorgestrel in the Mirena IUD has some contraceptive effects that are similar to copper but other effects that are unique. In the cervix, levonorgestrel, like copper, decreases sperm motility and the ability of sperm to penetrate the mucus, apparently by increasing mucus viscosity.11 This appears to be both a direct effect of the progestin on cervical mucus production as well as an indirect effect through alterations in ovarian function.
Levonorgestrel affects ovarian function.21 Part of the effect on cervical mucus appears to be secondary to the ability of levonorgestrel to suppress ovarian estrogen production. In some women, follicular development and ovulation is diminished, although most women remain ovulatory after the first year of use.22 Like copper, levonorgestrel also prevents fertilization by inhibiting transport of the sperm through the fallopian tube.
In contrast to copper, levonorgestrel prevents implantation by directly suppressing the endometrial lining. In addition to foreign body reaction, endometrial biopsies from levonorgestrel IUD users reveal atrophic endometrial glands and decidualized stroma consistent with a progestin effect.23 As with copper IUDs, evidence of the ability of levonorgestrel IUDs to prevent implantation is the finding that serum levels of β-hCG is uniformly undetectable in these women.24 Once again, there has been no evidence to indicate that levonorgestrel IUDs act as abortificients after implantation.24
Contraceptive Efficacy of Modern IUDs
The annual and long-term pregnancy rates of the copper and levonorgestrel IUDs are among the lowest for available reversible contraceptive measures (Table 27-1). The 1-year pregnancy rate for the copper-containing ParaGard IUD is approximately 0.5 per 100 women.12 The 1-year pregnancy rate for the levonorgestrel-containing Mirena IUD is 0.2 per 100 women, but the overall 5-year pregnancy rate averages 0.7 per 100 women.25 These pregnancy rates do not appear to be affected by parity.
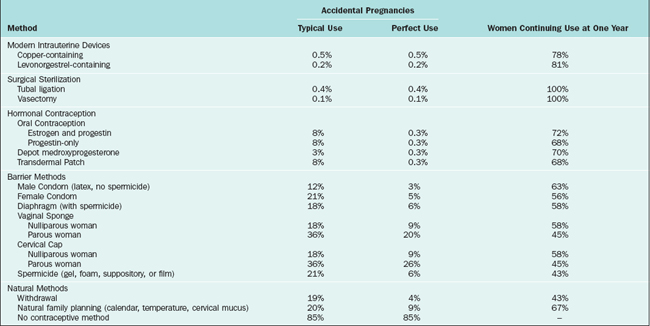
Table 27-1 Contraceptive Failure Rate for Various Methods During the First Year of Typical and Perfect Use, and the Percentage Continuing Use at the End of the First Year72,73
In practice, the cumulative pregnancy rates for both IUDs are impressively low. In a clinical study of use of these IUDs by 4000 women, the World Health Organization (WHO) reported the cumulative pregnancy rate for the Mirena IUD after 6 years to be only 0.6%, which was significantly lower than the rate for the ParaGard IUD, 2.0% (Table 27-2).26
Table 27-2 The Cumulative Six-year Probability of a Woman Discontinuing the Copper-containing ParaGard IUD and Levonorgestrel-releasing Mirena IUD26
| Name | ParaGard (copper) | Mirena (levonorgestrel) |
|---|---|---|
| Cumulative Pregnancy Rate | 2.0% | 0.6% |
| Intrauterine pregnancy | 1.8% | 0.6% |
| Ectopic pregnancy | 0.1% | 0.0% |
| Expulsion | ||
| Complete expulsion | 1.7% | 3.0% |
| Partial expulsion | 6.6% | 4.9% |
| Pelvic Inflammatory Disease | 0.0% | 0.3% |
| Menstrual Reasons | 10.7% | 36.2% |
| Amenorrhea | 0.6% | 23.8% |
| Reduced bleeding | 3.0% | 11.2% |
| Increased bleeding | 7.0% | 5.6% |
| Pain | 5.9% | 5.2% |
Use of Copper-containing IUDs for Emergency Contraception
Placement of the copper-containing ParaGard IUD in women after unprotected sexual intercourse is relatively effective in preventing subsequent conception. When used in this way for emergency contraception, the ParaGard IUD is most effective when placed within 72 hours after intercourse, but is still effective when placed within 5 days. In a meta-analysis of 20 studies of emergency contraception, the ParaGard IUD, with a subsequent 0.1% pregnancy rate, was found to be more effective than the standard oral contraceptive regimen, which had a 1.5% pregnancy rate.27
Discontinuation Rates
Discontinuation rates are important, because women who discontinue their birth control method for side effects are at high risk for pregnancy. In the first year, more patients continue using IUDs than any other reversible contraceptive method. Although studies of modern IUDs have shown general patient satisfaction and safety, the reported discontinuation rate after 7 to 8 years of use varies from 28% to 73%, depending on the population28–31
For both types of modern IUDs, menstrual reasons are listed as the most common side effect resulting in IUD discontinuation (see Table 27-2).26 For the ParaGard IUD, this was most commonly increased bleeding, whereas for the Mirena IUD, this was most likely amenorrhea or decreased bleeding. Because of the high rate of amenorrhea for the Mirena compared to the ParaGard IUD (23.8 vs. 0.6%, respectively), the 6-year continuation rate was significantly lower for the Mirena (43.8%) compared to the ParaGard (66.6%).26
Pregnancies and pelvic inflammatory disease (PID) are very uncommon for both types of IUDs. Although the study described in Table 27-2 showed a higher cumulative pregnancy rate for ParaGard compared to Mirena (2.0% vs. 0.6%, respectively), a multicenter prospective 7-year randomized study of more than 7000 women reported equal pregnancy rates (0.2 per 100 women-years) and PID rates (0.6 to 0.7 per 100 women-years) for both types of IUDs.28 Rates for both of these complications were highest in the first year after insertion.
Relative Cost
Modern IUDs are among the most cost-effective contraceptive methods.32 When calculating the total cost of a method, an economic analysis must include the initial costs, including contraceptive medication or device plus the placement fee; costs of treating side effects (e.g., surgical complications, deep venous thrombosis, amenorrhea, or urinary tract infections); and the cost of unintended pregnancies.
According to one analysis, over a 5-year period the copper-containing ParaGard IUD was calculated to be the least expensive at only $540, whereas oral contraceptives cost approximately $1784 over this same time period.32 Although vasectomy and tubal ligation would certainly be less expensive over the entire reproductive life of a woman, surgical sterilization has the obvious disadvantage of limited reversibility. The extremely low initial costs of many nonmedical approaches are more than offset by the cost of unintended pregnancies.
Return to Fertility
After removal of an IUD for reasons other than PID, there is no measurable residual effect on fertility no matter how long the IUD was used.33 This is in contrast to depot medroxyprogesterone, where fertility is usually delayed a matter of months until the medication has been cleared from the woman’s system.34
NONCONTRACEPTIVE BENEFITS OF IUDS
Inert and Copper-containing IUDs
A reduced risk of endometrial cancer was an unexpected finding in women who used either inert or copper-containing IUDs.35 Multiple studies performed throughout the world suggest that the risk of endometrial cancer in IUD users is reduced by 30% to 50%. It has been proposed that IUDs exert their protective effect through local structural and biochemical changes in the endometrium that may decrease endometrial sensitivity to estrogen or increase sensitivity to progesterone. Some authors suggest that this association could be a reflection of selection bias, because inert and copper-containing IUDs are less likely to be used in women with menstrual abnormalities who may be at increased risk for endometrial cancer. However, the data appears convincing that the incidence of endometrial cancer is decreased in women who have used either an inert or copper-containing IUD.
Levonorgestrel-containing IUD
Physiology
Continuous levonorgestrel exposure significantly suppresses the endometrium. Light microscopy shows that the endometrium becomes thin because of glandular cell atrophy and stromal cell decidualization. In addition, there is visible reduction in the size and number of endometrial blood vessels. Electron microscopy reveals that levonorgestrel causes thickening of the basal lamina between the endometrial epithelial and persistence of the complex intercellular junctions between the epithelial cells, which normally loosen around the time of implantation.36
Abnormal Uterine Bleeding and Dysmenorrhea
Excessive or too frequent uterine bleeding impairs the quality of life for many otherwise healthy women. After infection and uterine malignancies are excluded, the most common medical treatment for excessive uterine bleeding is progestin administration. Unfortunately, progestins given systemically do not always resolve these problems because of inconsistent effects on the uterus or because patients do not tolerate them because of systemic side effects or the risk of complications (including increased breast cancer). As a result abnormal uterine bleeding or dysmenorrhea remain some of the most common indications for hysterectomy in the United States.37
Excessive Menstruation
The Mirena IUD effectively decreases uterine bleeding in women with no demonstrable uterine pathology, regardless of parity. In normal women, the average menstrual blood loss is 30 to 40 mL. In women with a Mirena in place, the average menstrual blood loss is as little as 5 mL. More than 20% of Mirena users will have amenorrhea after the first year of use. This is in contrast to the copper-containing ParaGard IUD, for which the average menstrual bleeding is slightly increased to 40 to 50mL. Controlled, randomized trials comparing treatment of heavy menstrual bleeding with hysterectomy versus medical therapy with the Mirena IUD have shown that hysterectomy is more effective in controlling menstrual bleeding, but the Mirena IUD appears to be equally beneficial in improving quality of life.38
Endometriosis-associated Dysmenorrhea
The Mirena IUD appears to be an effective treatment for dysmenorrhea associated with endometriosis. In this randomized, controlled study of 40 patients with laparoscopically diagnosed endometriosis, insertion of the Mirena IUD after laparoscopic surgery decreased the return of moderate to severe dysmenorrhea after 1 year to only 10%, compared to 45% in the control group.39 The effect on recurrence of endometriosis was not determined.
Adjuvant to Estrogen Replacement Therapy
Menopausal women on estrogen replacement therapy appear to benefit from the use of the levonorgestrel-containing Mirena IUD. Although the use of the Mirena IUD with estrogen replacement is associated with bleeding during the first few months, after 1 year the majority of patients (73%) had no spotting or bleeding.40 Likewise, in a study of 40 postmenopausal women randomized to receive either transdermal estrogen and a Mirena IUD or daily oral estrogen plus the progestin norethisterone, the IUD group experienced more bleeding in the first 3 months, but both groups had an equivalent amount of bleeding for the remainder of the year.41 Approximately 80% of the postmenopausal women taking oral estrogen with a Mirena can be expected to have amenorrhea within a year of starting therapy.
Adjuvant to Tamoxifen Therapy
Therapy for breast cancer with the selective estrogen receptor modulator tamoxifen is associated with an increased risk of endometrial hyperplasia, polyps, and carcinoma. A randomized, controlled study of the Mirena IUD in more than 124 women taking tamoxifen for 1 year showed a uniform endometrial decidualization and fewer polyps and fibroids in women with an IUD compared to controls.42 Unfortunately, the women using the Mirena IUD had excessive bleeding that often took 6 months or more to resolve. This study was too short to assess the effect of the Mirena IUD on breast cancer recurrence; thus, further studies need to be done to assess if there is any benefit to this regimen.
Uterine Leiomyomata and Menorrhagia
The Mirena IUD is not as effective in treating menorrhagia related to uterine leiomyomata. In a study of 19 women with menorrhagia secondary to uterine leiomyomata, over the 12 months after a Mirena IUD was placed, subjective menstrual blood loss decreased.43 However, 14 of these 19 women had persistent menorrhagia, and the average hemoglobin dropped from 10.9 g/dL to 9.9 g/dL during this same period. At the time of IUD insertion, these women’s enlarged uteruses measured an average of 13 cm, and it was hypothesized that the amount of progestin was inadequate to produce endometrial atrophy because of increased endometrial surface area. Larger studies will be required to determine the efficacy of the Mirena IUD in women with leiomyomata.
IUD SIDE EFFECTS
Dysmenorrhea and Increased Menstrual Flow
The levonorgestrel-containing Mirena IUD is associated with decreased dysmenorrhea and flow. In contrast, the copper-containing ParaGard IUD still results in dysmenorrhea and increased menstrual flow in many patients, although it represents an improvement compared to larger inert IUDs.44 Physiologically, copper ions increase endometrial inflammation and prostaglandin production, resulting in both dysmenorrhea and increased flow. Although use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) often ameliorates these side effects, 5% to 15% of patients request removal of the ParaGard IUD as a result of these side effects.
Hormonal Side Effects
The progestins released from the levonorgestrel-containing Mirena IUD have no known systemic effects. However, these progestins do alter ovarian folliculogensis for as long as 12 months after insertion. As a result of these changes in ovarian function, some patients complain of headache, nausea, mood changes, mastalgia, acne flareup, and irregular bleeding. The Mirena IUD also causes a temporary increase in functional ovarian cysts in 12% of users, which is attributed to a decrease in follicular atresia.45
Expectant management is recommended for both the hormonal symptoms and the ovarian cysts, because both of these problems will resolve spontaneously within 3 to 6 months. Fortunately, the Mirena IUD does not appear to alter or inhibit ovulation after the first year of use.46
Irregular Bleeding
After the first 3 months, most Mirena IUD users will have decreased menstrual bleeding, with the average menstrual bleeding decreasing from 35 mL to 5 mL per cycle, and more than 20% of these women will have amenorrhea.37 It is important to prepare women for this possibility because up to 4% of Mirena IUD users will request removal of their IUD due to amenorrhea.
Bleeding abnormalities other than amenorrhea are more common with the copper-containing ParaGard IUD. In some studies, as many as 2% of ParaGard users discontinued their IUD due to bleeding abnormalities. Usually, this bleeding will be the result of unrelated anovulation or chronic endometritis. However, in women using an IUD over age 40, more than 10% of cases of abnormal uterine bleeding will be related to endometrial hyperplasia.47 Consequently, endometrial biopsy should be performed in IUD users over age 40 presenting with abnormal uterine bleeding.
Pregnancy
IUD Management with an Intrauterine Pregnancy
When pregnancy is diagnosed with an IUD in place, the first step is the immediate removal of the IUD, even if the patient ultimately desires termination. Based on studies of inert IUDs, immediate removal of the IUD can potentially decrease the risk of spontaneous abortion from 50% to 25%.48
Removal of the IUD also appears to reduce the risk of second- and third-trimester pregnancy complications.49 If the IUD cannot be removed because of a “lost” string, the risk of prematurity is as high as 37.5%. Unfortunately, attempts to remove a “lost” IUD using ultrasound guidance and intrauterine instrumentation further increase the risk of pregnancy loss.50
Ectopic Pregnancy
The absolute risk of having an ectopic pregnancy is much lower for women using an IUD than for women using no birth control. The ectopic pregnancy rate for women using the copper-containing ParaGard IUD is approximately 0.25 per 100 women-years, and for women using the levonorgestrel-containing Mirena IUD is 0.02 per 100 women-years. This is much less than the ectopic pregnancy rate in women using no contraception of 1.6 per 100 women-years. However, tubal pregnancies appear to be more likely to occur among IUD pregnancies than among women using oral contraceptives or barrier methods, because these methods are more likely to prevent ovulation or fertilization.51
Pelvic Inflammatory Disease
Postinsertion PID
In women in the United States, the overall risk of PID for both the ParaGard and Mirena IUDs is 0.6 to 0.7 per 100 women-years of use; these rates are highest in the first year after insertion.52 A worldwide study conducted by the World Health Organization (WHO) found that the risk of PID within 20 days after insertion was 10 in 1000 women-years, and the majority of these cases occur in women at high risk of PID who had multiple sexual partners.53 For a patient in a monogamous relationship, the incidence of PID from the insertion procedure is extremely low.52
In the absence of an STD, any case of PID that occurs within 20 days of IUD insertion is most likely to be due to contamination of the uterus with lower genital tract bacteria that cannot be cleared by the uterus. Even though IUDs are placed using aseptic technique, the uterine cavity is unavoidably contaminated with bacteria from the vagina or cervix during insertion. Unfortunately, the addition of prophylactic antibiotics has not been shown to be useful, at least in part because the background incidence is so low.54
Actinomyces
Actinomyces are gram-positive anaerobic bacilli characterized by filamentous growth and mycelia-like colonies that have a striking resemblance to fungi. These organisms, which are a part of the normal vaginal flora, can result in pelvic actinomycosis, a rare type of granulomatous PID that has an incidence of less than 0.001% of women discharged from the hospital.55
Actinomyces or Actinomyces-like organisms are detected on Pap smears only 0.13% of the time, and almost always in women using an IUD.56,57 It is important to know that the presence of Actinomyces on a Pap smear does not diagnose or predict pelvic actinomycosis. For this reason, it is recommended that the IUD should be left in place when Actinomyces are found on Pap smear in an asymptomatic woman.58 Antibiotics are not recommended because there is no evidence that they are of any value in preventing subsequent pelvic actinomycosis. The woman should be informed of the finding and instructed to return for any symptoms of pelvic infection and evaluated in 6 months. Subsequent Pap tests can be done at the normal interval. Should subsequent Pap smears show Actinomyces, removal and replacement of the IUD is a reasonable option. Alternative options include a repeated Pap test in 4 to 6 weeks and treatment with antibiotics for 2 weeks if still positive or removal of the IUD, antibiotics for 2 weeks, and then a repeated Pap smear.
If pelvic actinomycosis is suspected in a women with PID symptoms such as abdominal pain, fever, vaginal discharge, or weight loss and has Actinomyces on Pap smear, her IUD should be removed, scraped for cytology, and sent for culture.58,59 The woman should receive an appropriate antibiotic treatment with intravenous penicillin G (10 to 20 million U/day) for 4 to 6 weeks until serial cultures confirm the absence of Actinomyces. In serious cases, metronidazole can be added to treat any concomitant anaerobic infection.60,61 Oral penicillin V (1 to 2 g, twice daily) is continued for 6 to 12 months after the intravenous antibiotics.
IUD INSERTION AND REMOVAL
Patient Selection
The current recommendation is that IUDs are best suited for women of any parity who are in a mutually monogamous relationship. Although the risk of PID does not appear to be increased in IUD users, the occurrence of PID in IUD users continues to be problematic, because the IUD foreign body is usually removed for more effective treatment. Furthermore, both condoms and oral contraceptives might be more appropriate contraception for women at high risk of contracting an STD because they both appear to decrease the risk of PID. For these reasons, it is recommended that IUDs be recommended to patients who are at the lowest risk of contracting STDs.
Contraindications
IUD contraindications can be divided into conditions that are either exacerbated by the presence of an IUD or make IUD placement difficult (Table 27-3). Obviously, known or suspected pregnancy is an absolute contraindication, because IUD placement will usually, but not predictably, terminate the pregnancy and put the patient at increased risk for pregnancy complications.
Human Immunodeficiency Virus
Patients infected with human immunodeficiency virus (HIV) represent special cases. Fortunately, there has been no evidence to suggest that an IUD increases viral shedding or HIV transmission.62,63 A concern that HIV patients with decreased immunity might be at higher risk of PID near the time of insertion is unfounded. However, it is recommended that IUDs be placed in patients with AIDS only after antiviral therapy has been started. If a patient with an IUD develops an HIV infection, the IUD does not need to be removed, but the patient should be counseled and closely monitored for a pelvic infection. An HIV-positive woman with an IUD who develops AIDS may continue to use this contraception but should be closely monitored for infection.
Preplacement Examination and Laboratory Evaluation
A routine pelvic examination should be performed before IUD insertion to determine uterine position and size and to detect any previously unidentified abnormalities. A Pap smear should be obtained and cervical abnormalities evaluated and treated before IUD placement. In areas with a high background risk of STDs, it is reasonable to exclude gonorrhea and chlamydia infections before placement. A pregnancy test is also a reasonable precaution in any patient who is not using highly effective contraception.
Premedication
Prophylactic Antibiotics
The routine use of prophylactic antibiotics at the time of IUD placement to decrease the chance of PID is not recommended, because they have not been found to be effective.54 In women at low risk of sexually transmitted infection, the risk of PID is negligible after IUD insertion, with or without the administration of prophylactic antibiotics.
Timing of Insertion
Postpartum Insertion
Postpartum IUD insertion, although rare in the United States, is a common technique is in many parts of the world. There is no increased risk of bleeding, infection, or perforation with this approach, but the risk of expulsion is increased, ranging from 9% to 37%, compared with 13% for insertions delayed until 6 weeks postpartum.64–66
Ideally, the IUD should be inserted within 10 minutes of delivery of the placenta but can be delayed up to 24 hours. Expulsion increases from 9% when the IUD is placed immediately after delivery to 37% when placed between 24 and 48 hours after the delivery. Insertion is not recommended more than 48 hours after delivery, because both perforation and expulsion rates are unacceptably high.64–66
IUD Removal
Up to 2% of attempted IUD removals will prove to be difficult. If the IUD does not come out easily, a tenaculum can be used to align the cervical canal and uterine cavity. If removal is still difficult, an attempt can be made to dilate the cervix, or the patient can be scheduled to return during menstruation, when the cervix is usually softer.
COMPLICATIONS OF REMOVAL AND LOCATION
Missing Strings
Sometimes the strings are not visible protruding through the cervix or break during removal. In many cases, the strings will be found within the cervical canal by probing with a cotton-tipped applicator or a narrow forceps. If this fails, either the strings have retracted into the uterine cavity or the IUD is no longer in the uterine cavity because it has perforated the uterine wall or has been unknowingly expelled. In a 2003 review of misplaced IUDs, 80% were found in the uterine cavity and 15% in the cervical canal, 10% had been expelled, and 5% were located in the peritoneal cavity.67
An IUD hook can be used to remove an IUD with missing strings (Fig. 27-3). This instrument is gently placed through the cervix into the fundus and rotated 180 to 360 degrees. In many cases, the IUD hook will loop around the vertical stem of the IUD and literally hook the lowest portion of the stem that broadens into a ball tip. The IUD can then be removed through the cervical os.
Perforation
Uterine perforation at the time of IUD insertion occurs in fewer than 3 in 1000 cases, and the risk is inversely related to clinician experience.68–70 Another risk factor for perforation is insertion of the IUD between 0 and 6 months’ postpartum.
Management
1 Mosher W. Design and operation of the 1995 National Survey of Family Growth. Fam Plan Perspect. 1998;30:1-9.
2 Wilcox LS, Marks JS, editors. From Data to Action: CDC’s Public Health Surveillance for Women, Infants, and Children. Atlanta: U.S. Centers for Disease Control and Prevention, 1994.
3 Thomsen RJ. Camels and the IUD: It was a good story. Contemp Ob/Gyn. 1988;31:152-161.
4 Rioux JE. The intrauterine device today. J SOGC. 1993;15:921-924.
5 Tietze C. Evaluation of intrauterine devices. Ninth progress report of the cooperative statistical program. Stud Fam Plann. 1970;1:1-4.
6 Cates W. The intrauterine device and deaths from spontaneous abortion. NEJM. 1976;295:1155-1159.
7 Tatum HJ, Schmidt FH, Phillips D, et al. The Dalkon Shield controversy. Structural and bacteriological studies of IUD tails. JAMA. 1975;231:711-717.
8 Zipper J, Medel M, Prager R. Suppression of fertility by intrauterine copper and zinc in rabbits: A new approach to intrauterine contraception. Am J Obstet Gynecol. 1969;105:529-534.
9 World Health Organization. Special programme of research, development and research training. Contraception. 1990;42:141-158.
10 World Health Organization. Mechanism of action, safety and efficacy of intrauterine devices. Report of a WHO scientific group. Technical Report Series 753. Geneva: World Health Organization, 1987;1-91.
11 American College of Obstetricians and Gynecologists. Statement on Contraceptive Methods. Washington, D.C.: ACOG, 1998.
12 ParaGard Prescribing Information. Raritan, N.J.: Ortho Pharmaceutical Corp., 1995.
13 Ortiz ME, Croxatto HB, Bardin CW. Mechanism of action of intrauterine devices. Obstet Gynecol Survey. 1996;51:542-551.
14 Ullmann G, Hammerstein J. Inhibition of sperm motility in vitro by copper wire. Contraception. 1972;6:71-76.
15 Kesseru E, Camacho-Ortega P. Influence of metals on in vitro sperm migration in human cervical mucus. Contraception. 1972;6:231-240.
16 Tatum HI. Intrauterine contraception. Am J Obstet Gynecol. 1972;112:1000-1023.
17 Ortiz ME, Croxatto HB, Bardin CW. The mode of action of IUDs. Contraception. 1987;36:37-53.
18 Videla-Rivero L, Etchepareborda JJ, Kesseru E. Early chorionic activity in women bearing inert IUD, copper IUD, levonorgestrel-releasing IUD. Contraception. 1987;36:217-226.
19 Alvarez F, Brache V, Fernandez E, et al. New insights in the mode of action of intrauterine contraceptive devices in women. Fertil Steril. 1988;49:768-773.
20 Segal SJ, Alvarez-Sanchez F, Adejuwon CA, et al. Absence of chorionic gonadotropin in sera of women who use intrauterine devices. Fertil Steril. 1985;44:214-218.
21 Barbosa I, Olsson SE, Odlind V, et al. Ovarian function during use of a levonorgestrel-releasing IUD. Contraception. 1990;42:51-66.
22 Nilsson CG, Lahteenmaki PL, Luukkainen T. Ovarian function in amenorrheic and menstruating users of a levonorgestrel-releasing intrauterine device. Fertil Steril. 1984;41:52-55.
23 Phillips V, Graham CT, Manek S, McCluggage WG. The effects of the levonorgestrel intrauterine system (Mirena) on endometrial morphology. J Clin Pathol. 2003;56:305-307.
24 Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: Update and estimation of postfertilization effects. Am J Obstet Gynecol. 2002;187:1699-1708.
25 Mirena (levonorgestrel-releasing intrauterine system) Prescribing Information. Montville, N.J.: Berlex Laboratories, 2000.
26 Department of Reproductive Health and Research, World Health Organization. The intrauterine device (IUD)—worth singing about. Progr Reprod Health Res. 2002;60:1-8.
27 Trussell J, Ellertson C. Efficacy of emergency contraception. Fertil Contracept Rev. 1995;4:8-11.
28 Sivin I, Stern J. Health during prolonged use of levonorgestrel 20 μg/d and the copper TCu380Ag intrauterine contraceptive devices: Amulticenter study. Fertil Steril. 1994;61:70-77.
29 Ronnerdag M, Odlind V. Health effects of long term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78:716-721.
30 Backman T, Huhtala S, Tuominen J, et al. Sixty thousand woman-years of experience on the levonorgestrel intrauterine system: An epidemiological survey in Finland. Eur J Contracept Reprod Health Care. 2001;6(Suppl 1):23-26.
31 Backman T, Huhtula S, Blom T, et al. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: A nationwide study of 17,360 users. BJOG. 2000;107:335-339.
32 Trussell J, Leveque JA, Koening JD, et al. The economic value of contraception: A comparison of 15 methods. Am J Public Health. 1995;85:494-503.
33 Nelson A. The intrauterine contraceptive device. Obstet Gynecol Clin North Am. 2000;27:723-740.
34 Fotherby K, Howard G. Return of fertility in women discontinuing injectable contraceptives. J Obstet Gynaecol. 1986;6(Suppl 2):S110-S115.
35 Castellsague X, Thompson WD, Dubrow R. Intra-uterine contraception and the risk of endometrial cancer. Int J Cancer. 1993;54:911-916.
36 Pakarinen PI, Lahteenmaki P, Lehtonen E, Reima I. The ultrastructure of human endometrium is altered by administration of intrauterine levonorgestrel. Hum Reprod. 1998;13:1846-1853.
37 Andersson JK, Rybo G. Levonorgestrel-releasing intrauterine device in the treatment of menorrhagia. BJOG. 1990;97:694-970.
38 Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Sys Rev. 2004. CD02839.
39 Vercellini P, Frontino G, DeGiorgi O, et al. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: A pilot study. Fertil Steril. 2003;80:305-309.
40 Suhonen SP, Allonen HO, Lahteenmaki P. Sustained-release estradiol implants and a levonorgestrel-releasing intrauterine device in hormone replacement therapy. Am J Obstet Gynecol. 1995;172:562-567.
41 Raudaskoski TH, Lahti EI, Kauppila AJ, et al. Transdermal estrogen with a levonorgestrel-releasing intrauterine device for climacteric complaints: Clinical and endometrial responses. Am J Obstet Gynecol. 1995;172:114-119.
42 Gardner FJE, Konje JC, Abrams KR, et al. Endometrial protection from tamoxifen-stimulated changes by a levonorgestrel-releasing intrauterine system: A randomized controlled trial. Lancet. 2000;356:1711-1717.
43 Mercorio F, De Simone R, Di Spiezio Sardo A, et al. The effect of a levonorgestrel-releasing intrauterine device in the treatment of myoma-related menorrhagia. Contraception. 2003;67:277-280.
44 Fortier L, Lefebvre Y, Larose M, Lanctot R. Canadian experience with a copper-covered intrauterine contraceptive device. Am J Obstet Gynecol. 1973;115:291-297.
45 Robinson GE, Bounds W, Kubba AA, et al. Functional ovarian cysts associated with the levonorgestrel releasing intrauterine device. Br J Fam Plann. 1989;14:131-132.
46 Nilson CG, Lahteenmaki PL, Luukkainen T. Ovarian function in amenorrheic and menstruating uses of a levonorgestrel releasing IUD. Fertil Steril. 1984;41:52-55.
47 Ozalp S, Kabukcuoglu S, Tanir HM. Should endometrial hyperplasia be regarded as a reason for abnormal uterine bleeding in users of the intrauterine contraceptive device? Eur J Contracept Reprod Health Care. 2003;8:17-20.
48 Pollack AE, Girvin S. When should an IUD be removed and replaced? Med Aspect Hum Sexual. 1992;26:46-58.
49 Grimes DA. Whither the intrauterine device? Clin Obstet Gynecol. 1989;32:369.
50 Stubblefield PG, Fuller AF, Foster SC. Ultrasound guided intrauterine removal of interine contraceptive devices in pregnancy. Obstet Gynecol. 1981;72:961.
51 Rossing MA, Daling JR, Voigt LF, et al. Current use of an intrauterine device and risk of tubal pregnancy. Epidemiology. 1993;4:252-258.
52 Farley TMM, Rosenberg MJ. Intrauterine device and pelvic inflammatory disease: An international perspective. Lancet. 1992;339:785-788.
53 Lee NC, Rubin GL, Borucki R. The intrauterine device and pelvic inflammatory disease revisited: New results from the Women’s Health Study. Obstet Gynecol. 1988;72:1-6.
54 Walsh T, Grimes D, Frezieres R, et al. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. IUD Study Group. Lancet. 1998;351:1005-1008.
55 Lippes J. Pelvic actinomycosis: A review and preliminary look at prevalence. Am J Obstet Gynecol. 1999;180:265-269.
56 Petitti DB, Yamamoto D, Morgenstern N. Factors associated with Actinomyces-like organisms on Papanicolaou smear in users of intrauterine contraceptive devices. Am J Obstet Gynecol. 1983;145:338-341.
57 Pearlman M, Frantz AC, Floyd WS, et al. Abdominal wall Actinomyces abscess associated with an intrauterine device: A case report. J Reprod Med. 1991;36:398.
58 Fiorino AS. Intrauterine contraceptive device-associated actinomycotic abscess and Actinomyces detection on cervical smear. Obstet Gynecol. 1996;87:142-149.
59 Traynor RM, Parratt D, Duguid HLD, Duncan ID. Isolation of actinomycetes from cervical specimens. J Clin Pathol. 1981;34:914-916.
60 Persson E, Holmberg K, Dahlgren S, Nilsson L. Actinomyces israelii in the female genital tract. Acta Obstet Gynecol Scand. 1984;63:207-216.
61 Peipert JF. Actinomyces: Normal flora or pathogen? Obstet Gynecol. 2004;104:1132-1133.
62 Bonacho I, Pita S, Gome-Besteiro MI. The importance of the removal of the intrauterine device in genital colonization by actinomyces. Gynecol Obstet Invest. 2001;52:119-123.
63 Richardson BA, Morrison CS, Sekadde-Kigondu C, et al. Effect of intrauterine device use on cervical shedding of HIV-1 DNA. AIDS. 1999;13:2091-2097.
64 European Study Group on Heterosexual Transmission of HIV. Comparison of female to male and male to female transmission of HIV in 563 stable couples. BMJ. 1992;304:809-813.
65 Finger WR. IUD insertion timing vital in postpartum use. Network. 1996;16:21-24.
66 Grimes D. Timing of IUD insertion. Contracept Rep. 1998;9:6-8.
67 Grimes D, Schulz K, van Vliet H, et al. Immediate post-partum insertion of intrauterine devices. Cochrane Database Sys Rev. 2004. CD02069.
68 Barsaul M, Sharma N, Sangwan K. 324 cases of misplaced IUCD—a 5-year study. Trop Doct. 2003;33:11-12.
69 Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: Is the incidence higher than previously reported? Contraception. 2003;67:53-56.
70 Caliskan E, Öztürk N, Dilbaz BÖ, Dilbaz S. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracep Reprod Health Care. 2003;8:150-155.
71 Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: Is the incidence higher than previously reported? Contraception. 2003;67:53-56.
72 Espey E, Ogburn T. Perpetuating negative attitudes about the intrauterine device: Textbooks lag behind the evidence. Contraception. 2002;65:389-395.
73 Trussell J, Hatcher RA, Cates W, et al. Contraceptive efficiency. Contraceptive Technology Update. 2004;18:13.
74 Sonnenberg FA, Burkman RT, Speroff L, et al. Cost-effectiveness and contraceptive effectiveness of the transdermal contraceptive patch. Am J Obstet Gynecol. 2005;192:1-9.