Chapter 127 Minimally Invasive Spinal Decompression and Stabilization Techniques II
Thoracic and Lumbar Endoscopic Approaches
History
Endoscopic spine surgery refers to a rapidly evolving set of techniques potentially offering equivalent surgical outcomes with lower surgical morbidity. This includes traditional endoscopic access to a hollow cavity such as the thoracic cavity and “manufactured” cavities for spinal access. Endoscopic spine surgery does not refer to a single technique but rather to a set of tools that may be used during the approach to the spine. The philosophy behind spine endoscopy is to target the pathology and apply a therapeutic intervention while minimizing damage to surrounding nonpathologic tissues.1–3 These endoscopic techniques are part of a trend toward less (“minimally”) invasive interventions.
Endoscopic inspection of the thoracic cavity was initially conceived by Bozzini in 1806.4 Jacobaeus provided its first clinical application for diagnosing and treating tuberculosis in 1910.5 In 1991, Lewis popularized video-assisted thorascopic surgery (VATS) for pulmonary diseases. Orthopaedic applications of endoscopic principles began with the advent and acceptance of knee arthroscopy. Tagaki was the first to describe and Watanabe was the first to advance diagnostic knee arthroscopy in 1918 and 1957, respectively. Cascells in 1970 and Jackson in 1972 are credited with promoting the Japanese experience with arthroscopy in North America. Over a short time, endoscopic techniques have become standard for many abdominal and knee procedures, such as cholecystectomy and meniscectomy.6 Endoscopic spine surgery was first performed in the lumbar spine in the 1990s and, currently, experience in the lumbar spine outweighs that of thoracic endoscopic spine surgery.7 The first description of VATS for thoracic spinal diseases was published by Mack et al. in 1993.2 An endoscope (usually a 30- or 45-degree endoscope) may be placed during dorsal approaches to the thoracic and lumbar spine to improve visualization of the ventral spinal cord.
Thoracic Ventral Endoscopic Approach
Although no direct, randomized trial has compared endoscopic techniques with traditional open thoracotomy (ventral) or costotransversectomy (dorsolateral), there are many theoretical and apparent advantages to an endoscopic approach1,3,6–24 (Box 127-1). A quality endoscopic video system affords the surgeon improved visualization through outstanding illumination and up to 15× magnification. By manipulating the endoscopic portal placement, scope angle, and camera trajectory, a parallel approach to disc space can be maintained even with significant kyphotic or coronal plane deformity, making the technique useful for thoracic scoliosis correction.
BOX 127-1 Advantages and Disadvantages of Thoracic Endoscopic Spine Surgery
Advantages
Disadvantages
Surgical novelty (learning curve)
Monocular visualization (triangulation, depth perception)
Loss of tactile feedback (increased working distance)
Technical limitations in treating intraoperative complications
Anesthetic demands of double-lumen intubation and single-lung ventilation
Currently limited ability to perform endoscopic reconstruction
VATS requires less muscle dissection and no rib spreading, and, therefore, it probably decreases incisional pain. Decreased soft tissue/paraspinal muscle injury also allows more cosmetically acceptable scars, lower risk of postoperative infection, and decreased compromise of respiratory and shoulder mechanics. With prone positioning, simultaneous ventral and dorsal procedures may be undertaken, obviating the need for repositioning. Simultaneous lumbar and thoracic corrections can also potentially be performed. These advantages, taken together, may reduce ICU and hospital stays, as well as operative morbidity.
On the other hand, VATS has several disadvantages over open surgery.1,3,6–24 First, these procedures require substantially different technical skill sets than traditional, open approaches. Although the word “steep” has been associated with the learning curve for this surgical novelty, the procedure more correctly represents a “flat” learning curve in that a significant amount of time must be spent with animal, cadaveric, and proctored cases before proceeding with independent VATS spine surgery. Endoscopy changes the surgeon’s binocular vision to monocular video-assisted vision. This loss of depth perception is compounded with a loss of tactile feedback associated with the long-handled instruments needed to pass through endoscopic portals. Working distance and instrument excursion increases from 4 to 30 cm. Visualization and manipulation of sensitive spinal structures also requires triangulation from widely separated starting points on the chest wall to a small thoracic disc space. Prior to embarking on endoscopic thoracic spine surgery, the surgeon must be familiar with open ventral spinal anatomy and surgical techniques. Ultimately, the surgical procedures are the same, but the methods are different enough to challenge even the most experienced spine surgeon. Other disadvantages are more technical in nature. In some centers, VATS is performed with a second, experienced thoracoscopic surgeon. In many circumstances when a spine surgeon is working with a thoracic surgeon, there is an increase in manpower during the VATS procedure. This commonly can increase operative times.
Thoracic Dorsal and Dorsolateral and Combined Open and Endoscopic Approaches
The majority of open thoracic and lumbar spine procedures continue to be performed from posterior approaches. The approaches offer relatively direct access to the bony elements and the spinal canal. However, canal exposure may result in symptomatic epidural fibrosis. The dissection and retraction of the paraspinal muscles may lead to dead space formation and extensor muscle disruption. Such disruption has been referred to as “fusion disease”25 and may be associated with early fatigability and other long-term symptoms. Endoscopic techniques may allow for less disruption of the posterior musculature and a smaller laminotomy. Dorsal and dorsolateral approaches are also limited by the surgeon’s ability to visualize the ventral dura mater. Use of an angled endoscope may also greatly improve visualization while decreasing soft tissue dissection and rib resection.
Combined approaches are being increasingly described in the literature. These approaches include simultaneous ventral and dorsal surgery for tumors and deformity.20,26 Combined approaches may also refer to combining endoscopic and open techniques in “mini-open” or endoscopically assisted spine procedures to exploit the advantages of both techniques.17,19
Anatomic Considerations for the Video-Assisted Thoracoscopic Surgery Approach
The rib cage and chest wall form a rigid open space in which endoscopic surgery may be performed. Unlike the abdomen, CO2 insufflation is not required to maintain a working space. Through most of the thoracic spine, ribs articulate at the disc space level. The rib number corresponds to the lower vertebral body at the disc space (e.g., the sixth rib comes off the T5-6 disc space). Because the rib comes directly off the disc space from demifacets arising on the vertebral body just above and below the disc, rib resection is required for adequate access to the posterolateral corner of the disc. In the lower thoracic region, T11 and T12, the rib head may be well caudal to the disc space, permitting unobstructed access.
The segmental vessels lie in the waist of the vertebral bodies, entering the epidural space via the foramina. When approaching the spine endoscopically, it is important not to inadvertently lacerate these vessels. Some spine procedures, including corpectomy and instrumentation, require unilateral and occasionally bilateral sacrifice of the segmental vessels. Discectomy and ventral release procedures, on the other hand, usually do not require vessel sacrifice. One cadaver study demonstrated adequate discectomy without sacrifice of the intercostal or segmental vessels once an adequate mobilization of the esophagus and azygos vein had been carried out. The authors concluded that the segmental vessels ought to be preserved to reduce the risk of spinal cord ischemia.10 In a two-phase goat study, thoracoscopic discectomy and fusion were undertaken both with and without sacrifice of the segmentals.27 In the first phase, the area of disc excision was slightly higher in the vessel-ligated group, but the investigators did not believe this to be significant. Operative times were the same. In the second phase, biomechanical testing of the resulting fusion was undertaken, and the vessel-spared group revealed less flexibility in lateral bending. The authors concluded that the segmental vessels in the thoracic spine can be effectively spared without injury during disc excision and fusion. They noted that while slightly more disc area was excised with ligation of the vessels, sparing the segmental vessels may provide a blood supply that aids in fusion. They recommended sparing the segmental vessels in patients with a higher risk for cord perfusion–related neurologic injury, such as revision surgery, severe kyphosis, paraparesis, and congenital anomalies.
These studies have been criticized for not adequately modeling the intraoperative conditions of spine deformity.3 Many authors report that sacrificing the segmental vessels provides better visualization through improved pleural reflection and more complete discectomy.21,22,28,29 In a report of 1197 procedures in which more than 6000 vessels were sacrificed, there were no adverse neurologic consequences.30 It may be reasonable to use both vessel-sparing and vessel-sacrificing techniques as a function of the clinical situation. For example, in congenital deformity cases in which spinal cord blood supplies may be anomalous, vessel sparing may be more important.21 Also, if the intervention requires the sacrifice of one or more segmental arteries in the mid to lower thoracic region, especially T9 to T11, a right-sided approach should be considered to avoid ligation of the artery of Adamkiewicz. The artery of Adamkiewicz can be visualized with either CT or MRI angiography, and this is recommended if a left-sided approach is being considered.
Other surgically important structures include the superior intercostal veins and the sympathetic chain. The veins empty into the azygos circulation at or about the T3-4 interspace.1 Branches from the sympathetic chain run over the rib heads, just below the parietal pleura.31 Over the 5th through 10th ribs, these coalesce as the greater splanchnic nerve that courses into the abdomen.
There are critical regional differences in thoracoscopic anatomy that dictate different exposure techniques. In the upper thoracic region, for example, the surgeon should elevate and support the ipsilateral arm to rotate the scapula away. Here, unless there are apical adhesions, the collapsed lung readily falls away from the spine, allowing excellent visualization.32 In the midthorax, there is more available space that allows more variation in placement of the camera and retractor ports. On the other hand, a fan retractor or strategically placed sponges are typically needed to keep the collapsed lung out of the operative field. Similarly, a second retracting port may be needed to move aside a bullous or stiff lung.32 Tilting the operating table forward may improve visualization with less forceful lung retraction. In the lower thoracic region, it may be necessary to retract the diaphragm, but here, as at the apices, lung retraction is not usually a problem.
Indications and Contraindications
Indications
The majority of endoscopic thoracic spine surgery is directed ventrally to avoid larger incisions and postthoracotomy pain. A traditional thoracotomy requires a large incision, division of the shoulder girdle musculature, rib resection, and forcible rib retraction. This approach can result in desiccation of the exposed lungs and vessels, measurable reduction in pulmonary and shoulder girdle function, postthoracotomy intercostal pain syndrome, and an unsightly scar.2,20 On the other hand, thoracoscopic approaches visualize the ventral spinal elements from the T1-2 to L1-2 disc spaces from the side of approach to the midline.14 VATS affords easier exposure of the extremes of the thoracic spine than open thoracotomy.33 For example, a T12 corpectomy can be performed without diaphragmatic incision, and T3 can be accessed without mobilizing the scapula as would be required with an open technique. However, the great vessels, heart, and lungs remain major considerations for either open or endoscopic surgical egress.
Currently, VATS may be used for a number of pathologies affecting the anterior and middle columns of the thoracic spine (Box 127-2). These include infections of the vertebral bodies, paraspinal gutter, discs, and epidural space, which can be biopsied, debrided, or drained. Thoracoscopic access is also highly suitable for tumor biopsy or piecemeal excision. A number of authors have also described the use of VATS in patients with degenerative conditions of the thoracic spine, including excision of herniated thoracic discs and fusion of painful degenerated segments. In trauma, corpectomy/decompression and stabilization procedures have been performed.1 The largest early experience with VATS has been for the correction of deformity.1 Here, a thoracoscope can be used to assist or perform ventral release surgery in moderate kyphotic or scoliotic deformities. VATS has included the pediatric patient population as well as those with neuromuscular scoliosis and adult degenerative scoliosis. Thoracic discectomy for ventral myelopathy has also become an established technique.
Contraindications
Contraindications to thoracoscopic spinal surgery include the inability to tolerate single-lung ventilation in patients with severe or acute respiratory insufficiency.24 However, VATS should be considered as an alternative for patients who are at high pulmonary risk for thoracotomy.24 VATS may decrease many of the detrimental physiologic sequelae of thoracotomy. For example, postthoracotomy rib splinting leads to atelectasis and decreased functional residual capacity.34 Thus, although VATS is ideally avoided in patients with severe lung disease, its less deleterious effect on pulmonary mechanics may make it a better option than open thoracotomy. Typical patient groups include those with chronic obstructive pulmonary disease; pulmonary interstitial fibrosis; or significant restrictive lung disease from thoracic spinal deformity, such as children with neuromuscular scoliosis.
Anesthesia
Cooperation between the anesthesia team and a thoracoscopic spine surgeon begins with a careful preoperative evaluation, followed by meticulous room and intubation setup. An experienced anesthesiologist with expertise in thoracic surgery is strongly recommended. Selective double-lumen endotracheal intubation allows collapse of the lung on the operative side. The tube may easily migrate, and frequent bronchoscopic assessment of tube position is mandatory.35 In small patients (<45 kg), even the smallest double-lumen endotracheal tube may not fit. In these cases, bronchial blockers are required.3,21 Blockers are technically more difficult to use and have a higher rate of incomplete lung deflation, which may seriously impair visualization.3 Prone positioning in deformity cases may allow single-lumen intubation.36
General anesthetic options include total intravenous technique, isolated volatile agent, or a combination of volatile and intravenous agents. Initially, intravenous technique was recommended because of the potential risk of hypoxic pulmonary vasoconstriction with inhalational agents during single-lung ventilation. Recently, however, a series of 85 patients found no difference among these techniques.35 Hemodynamic monitoring includes a double-lumen central venous catheter. Alternatively, pulmonary artery catheterization may be undertaken.35 Hypotensive anesthesia should be avoided in myelopathic patients or in those undergoing segmental artery sacrifice.23
Once the tube position has been confirmed, the ipsilateral lung is deflated by clamping the lumen of its endotracheal tube. Once the chest has been entered with the first portal, the lung should collapse. If inadequate collapse is noted, a short period of positive pressure CO2 insufflation may assist in collapsing the lung. Usually, this insufflation is not necessary.37
Patient and Operating Room Positioning
The patient is belted into place so that the table may be tilted into the Trendelenburg or reverse Trendelenburg position or even tilted right or left as necessary to improve intraoperative visualization. It is important to ensure that the patient stays in a strict lateral position during the initial approach to the spine to maintain surgeon orientation. Some surgeons prefer to “airplane” the table up for portions of the procedure, but given the loss of tactile feedback and three-dimensional information associated with endoscopy, a strict knowledge of the patient’s body position in space is necessary. In some settings, particularly in the lower thoracic spine, it may be useful to “jackknife” the table to improve access to the lateral body wall. However, in patients with significant spinal cord compression, excessive “jackknifing” may increase the risk of iatrogenic neurologic progression.37
Prone positioning may be used as part of a simultaneous ventral/dorsal approach.20,36 Simultaneous surgery eliminates the need to stage the procedures, as well as the added time and costs for repositioning, redraping, and a new operating room setup. Prone position is particularly advantageous in cases of marked instability. For example, in ankylosing spondylitis or trauma, the spine may suddenly translate at the osteotomy site during repositioning. Prone position also allows a gravity reduction of hyperkyphosis.36 Finally, prone positioning confers the additional advantage of allowing the lungs to fall away from the spine, decreasing the need for retraction.
A large operating room is typically needed to accommodate the special equipment required in VATS cases.33 The gowned and sterile team typically includes two surgeons, one assistant, and one scrub nurse. Additional personnel include the anesthesia team, somatosensory-evoked potential monitoring personnel, cell saver transfusion technicians, and circulating nursing staff. Beyond the usual complement of anesthesia machines and back tables for instruments and implants, endoscopic spine surgery requires two video monitors, a fluoroscope, and neurologic monitoring equipment (usually sensory-evoked and motor-evoked potentials). The endoscopic surgeon and spine surgeon typically work on the same side of the patient (facing the patient in the lateral decubitus position). Alternatively, they can work opposite one another. Video mixers can convert image orientation so that no mirror image instrument manipulation is required.1 More and more frequently, voice-activated robotics are being used to replace the assistant surgeon in camera positioning. Robotics may improve visualization by providing a steady image.
Instruments for an open thoracotomy should be readily available for emergency use. In the prone position, open access can be achieved with an extended costotransversectomy approach. Because the lung has already been mobilized, it is easy to enter the chest. Once in, the surgeon is readily able to access, identify, and control the major vascular structures.20 Immediate potential access to a thoracic surgeon is also recommended.
Equipment
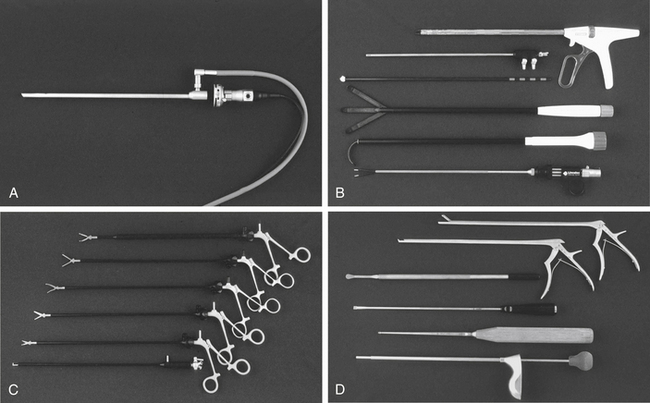
The workhorse of spinal endoscopy is the video equipment. This begins with the endoscope itself. A number of standard 10-mm endoscopes are used, most commonly 0-degree and 30-degree scopes. Occasionally, 70-degree scopes are needed. The 0-degree scope is standard for thoracoscopy, but a 30-degree scope decreases instrument crowding and allows better visualization around bony corners.24,38 Typically, a 10-mm, 15-inch end-viewing scope is used; however, in pediatric cases and, with increasing frequency in adults, a 5-mm scope may be preferred.24 The smaller scope provides less illumination, but with improvements in high-resolution, three-chip technology is adequate in most cases. Three-dimensional endoscopes are becoming increasingly available and help visualize landmarks and render improved depth perception.24
Instruments are introduced into the chest through trocars. Initially, these trocars were hard. Hard trocars may protect the thoracoscope against the rigid fulcrum of the rib cage.15 More recently, soft trocars have been developed that may be less traumatic to the neurovascular bundle on the inferior rib undersurface. Standard trocars are either 5 mm or 10 mm and are selected based on the size of instrument to be passed and the intercostal distance, which in adults is less than 12 mm.37
Typical spine instruments are customized for endoscopic applications by creating an extended shank of uniform diameter to match standard trocar sizes (Fig. 127-1). These include long-handled curets, Cobb elevators, pituitary rongeurs, disc shavers, nerve hooks, and Penfield dissectors. High-speed burs with long extenders are often required, as are more specialized types of endoscopic equipment such as Endo Shears, a bipolar endoscopic electrocautery, the harmonic scalpel, endovascular clips and loop ligatures, as well as endoscopic fan retractors.
Surgical Technique and Avoidance of Complications
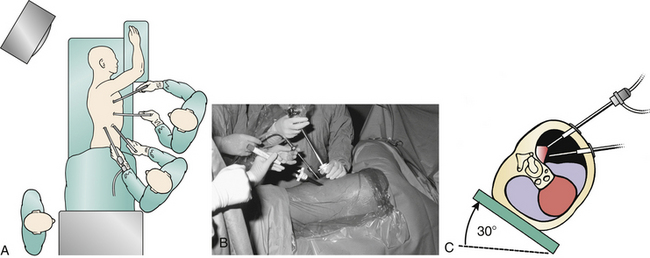
A left-sided or right-sided approach to the thoracic spine may be used depending on the eccentricity of the pathology (Fig. 127-2). With a left-sided approach, the thick resilient aorta is less prone to injury than the large friable tortuous veins of the azygos system. Some authors prefer a right-sided approach when the pathology is not lateralized, because there is a greater spinal surface area lateral to the azygos vein than the aorta.14 This difference can be assessed with preoperative axial CT or MRI images. Below T9, one may consider a left-sided approach to avoid the more cranial diaphragm reflection on the right.
The initial trocar creates the main viewing portal and is placed in the anterior axillary line at the sixth or seventh intercostal space, giving an unobstructed view of the entire hemithorax. The trocar is inserted in the manner of a tube thoracostomy with blunt dissection with a Kelly forceps just over the top of the rib to avoid damage to the neurovascular bundle or deep structures. A digital exploration is undertaken to exclude adhesions and avoid parenchymal lacerations of the lung. Some recommend Bovie dissection through the musculature to prevent bleeding around portals, which can obscure the camera image.1
The camera assistant must maintain the camera orientation and keep the operative field centered in view with a steady hand. The camera and instruments should be in the same 180-degree arc to avoid working in a mirror image. The camera assistant and the operating surgeon should work in unison with small movements. The camera should be removed for cleaning at various intervals. It should be reinserted carefully, because the lung may have partially reinflated, and injury to the lung parenchyma is possible.37 Self-cleaning and defogging arthroscopes are very helpful in this regard.24
It is necessary to manipulate only one object at a time. The camera operator zooms into the operative site. Then, as new instruments are introduced, the operator pans the camera out to follow the instrument into the operative field, preferably without changing the camera angle. Similarly, retractors and other instruments should be removed under direct visualization. Fan retractors should be removed in the fully closed position only. Levering instruments on the rib cage should be avoided to decrease pressure on the intercostal nerve.39
Once in the chest, surgical levels are identified. Operating at the wrong level is always a concern in the thoracic spine. It is important to screen for variant anatomy, such as accessory ribs, with preoperative radiographs, then find the level by counting down from the first rib. The first rib may be difficult to identify. It is necessary to mark the disc space and obtain radiographic confirmation. A Steinmann pin can be passed directly through the chest wall into the pathologic level.33 A radiograph confirms level localization, and the pin also demonstrates the optimal location for the working portal. Only quality radiographic images are acceptable. Anteroposterior radiographs are typically more helpful than lateral radiographs.31 In some centers, marking beads are placed at each spinal level, and radiographs or fluoroscopy are obtained prior to entering the operating suite. The appropriate level bead is maintained on the patient for intraoperative confirmation.32
To manipulate the spinal tissues, additional instruments need to be inserted into the chest through additional trocars. Typically, two working portals are created under direct video visualization with the lung protected. The chest is percussed, and by visualizing the percussions from within the chest, additional sites are chosen. As an alternative, 18-gauge spinal needles can be placed through the interspace to verify the level and trajectory. It is necessary to organize the remaining portals to center the instruments at the level of pathology. A number of portal patterns have been described and are covered in more detail in subsequent sections. Typically, an L or V shape at the dorsal axillary line two interspaces cephalad and caudad to the viewing portal is created, depending on the chest wall morphology and the intended spinal level. Portals should be spaced far enough apart that instruments do not “fence” with each other. The final viewing portal should be far enough away from the lesion to allow a panoramic view and to allow room to manipulate instruments.3 During the procedure, the instruments and scope are interchanged between the portals to facilitate work at different levels. It is preferable to make another portal perpendicular to the operative level than to operate at an acute angle to the pathology.32
When the appropriate portals have been placed and the correct level identified, the surgeon incises the parietal pleura with monopolar cautery. Alternatively, he or she uses the harmonic scalpel to dissect with less smoke and char.12,40 It is necessary to avoid monopolar cautery over the inferior margin of the rib head, where electrocautery injury to the intercostal nerve may occur.37 The degree of pleural dissection depends on the extent of the intended surgery but may include longitudinal approaches parallel to the spine or transverse approaches parallel to each disc space. If the segmental vessels are to be preserved, smaller pleural incisions are created parallel to the disc space. Bluntly dissect the incised parietal pleura rostrally, caudally, and ventrally to expose as much of the vertebral margins as is necessary.
At the conclusion of the procedure, it is necessary to irrigate out any disc or bony debris. Most authors do not attempt to close the parietal pleura. Some recommend an intercostal bupivacaine block to decrease postoperative pain.9
A chest tube is then selected—20F, 24F, or 28F—depending on the patient’s chest size. One inserts the tube through the inferiormost portal and runs it cranially along the vertebral column. Rosenthal uses two chest tubes: one, an apical tube for air and second, a basilar tube for effusions.41 Depending on the nature of the procedure, the tube can be maintained at water seal or at 20 cm of water suction. Typically, the tube can be removed 1 to 2 days postoperatively.
In the postoperative period, most patients are extubated immediately. A chest radiograph is obtained in the recovery room to verify full inflation of the lungs.7 A brief period of postoperative ICU monitoring is recommended. Aggressive respiratory care and physical therapy are required to prevent “down lung” atelectasis and pneumonia.
Results and Complications
Anesthesia Issues
Liske et al.35 described the anesthesia outcomes in their series of 82 patients who underwent thoracoscopic spine surgery under single-lung ventilation. The authors found that the anesthesia time was not significantly different than that for their series of patients who underwent open thoracotomy. They noted that VATS required extremely long single-lung ventilation times (mean, 270.2 minutes), a significantly longer period compared with open procedures. Also, the oxygenation index decreased significantly after initiation of single-lung ventilation. Despite these physiologic stressors, the authors concluded that VATS was a reasonable alternative to open thoracotomy from the anesthesia perspective because of the clinical benefits of accelerated return to activity as well as decreased ICU and hospital stays.
Other Complications
The types of complications associated with thoracoscopically assisted spine surgery in humans are essentially the same as with an open thoracotomy approach and can be categorized as incomplete operation, neurologic injury, lung injury, and vascular injury.16,37,41 As in any spinal cord level procedure, dural laceration, cord injury, or ischemia is possible. Most common, however, is intercostal neuralgia. This may be seen in up to 21% of patients, but it is usually transient.37 Flexible portals may reduce its incidence, but intercostal neuralgia still occurred in two cases in a series of 17 patients in which these portals were used.16 Transection of the sympathetic chain causes little or no morbidity, but the surgeon should inform the patient and family of possible temperature and skin color changes below and ipsilateral to the level of surgery.
A variety of pulmonary complications have been reported. Longer periods of lung collapse increase pulmonary morbidity. To decrease the rate of prolonged atelectasis, the deflated lung should be reinflated for 5 to 10 minutes for every hour of operating time.7 Trocars or instruments may cause direct trauma to the lungs. Larger air leaks should be repaired with an endoscopic suture ligature. Other common postoperative lung problems include pleural effusions and diaphragmatic injury.
More unusual pulmonary complications may stem from anesthesia or single-lung ventilation mishaps. Sucato and Girgis42 reported a case of an 11-year-old patient with severe scoliosis who developed air in both chest cavities, mediastinum, peritoneum, retroperitoneum, and subcutaneous tissue after intubation with a double-lumen endotracheal tube. Although the patient remained hemodynamically stable, bilateral chest tubes were required. The authors note that just as for the surgeon, there is a significant learning curve for the anesthesiologist to become adept at obtaining and maintaining single-lung ventilation.
McAfee et al. reported their complications with VATS in a prospective series of 78 cases. Transient intercostal neuralgias were noted in six patients and atelectasis in five patients. One case was converted to an open procedure for extensive pleural adhesions. One case of partial spinal cord neurologic deficit was noted.37 Huang et al.39 reported their complications in a series of 90 consecutive patients treated with thoracoscopic techniques for a variety of pathologies, including infection, fracture, deformity, and degenerative disease. A total of 30 complications was noted in 22 patients (24.4%). Two of these complications were fatal, including one case of massive blood loss and another of pneumonia. One graft dislodgement required revision surgery. The other complications were transient and included four cases of intercostal neuralgia, three superficial wound infections, three cases of pharyngeal pain, two cases of atelectasis, and two cases of residual pneumothorax. Four cases were converted to open thoracotomy.
Video-Assisted Thorascopic Surgery in Deformity and Scoliosis Surgery
Indications and Contraindications
Endoscopic surgery may directly or indirectly address several of the goals of surgery for spine deformity, which include arrest of curve progression, maximization and maintenance of curve correction, improvement in fusion rate, and decompression and protection of the neural elements43–46 (Box 127-3). In scoliosis, morphologic studies demonstrate that the anterior longitudinal ligament migrates to the concavity of the curve. The anterior longitudinal ligament and costotransverse ligaments on the concave side form a structural tether that must be released to gain maximum mobility of the spine.44 Typically, ventral approaches to the spine in deformity are indicated to release these tethers to allow correction of coronal and sagittal plane deformities.
BOX 127-3 Indications and Contraindications to Thoracoscopic Spine Surgery in Deformity
Indications
The indications are the same as for thoracotomy:
• Curves greater than 60 degrees with less than 50% correction on bending
• Curves greater than 75 degrees
• Curves requiring rebalancing into the stable zone in either the coronal or sagittal plane
• Crankshaft prevention in skeletally immature patients with curves greater than 50 degrees
• Kyphotic deformities greater than 70 degrees
• Progressive congenital deformities requiring epiphysiodesis
• Patients at high risk for pseudarthrosis from dorsal fusion alone
• Patients for whom bone grafting will achieve an interbody fusion
Indications for endoscopic techniques in spine deformity are the same as those for thoracotomy.1,47 The surgeon should consider ventral release in large scoliotic curves greater than 75 degrees and in rigid curves with less than 50% correction on bending films. Ventral epiphysiodesis is typically required to prevent the crankshaft phenomena in skeletally immature children with curves greater than 50 degrees or in those with progressive congenital deformities in the thorax. Patients with kyphotic deformities greater than 70 degrees or curves that require rebalancing into the stable zone in the coronal or sagittal plane are also candidates for ventral surgery. Interbody fusion techniques are often added to dorsal stabilization procedures to minimize pseudarthrosis risk.
Relative contraindications to the use of spinal endoscopy to treat patients with spine deformity include all of the contraindications to endoscopy in general.20 Also, special consideration needs to be paid to the relationship of the spine to the thoracic cage. A narrow anterior-posterior chest diameter, significant vertebral rotation at the apex, or thoracic scoliosis curves greater than 75 degrees may preclude safe visualization and instrumentation of the spine. For example, in curves greater than 75 degrees, the chest cavity on the concave side and the rib interspaces are too small to accommodate the 10-mm-diameter endoscopic portals and instruments. Also, with spinal rotation, the mediastinal organs begin to obstruct exposure. In certain cases, these variables may be overcome by adding more working portals. However, for the novice spine endoscopist, formal open thoracotomy may be more prudent. Consultation with an experienced thoracic surgeon is usually helpful.
Operative Technique and Avoidance of Complications
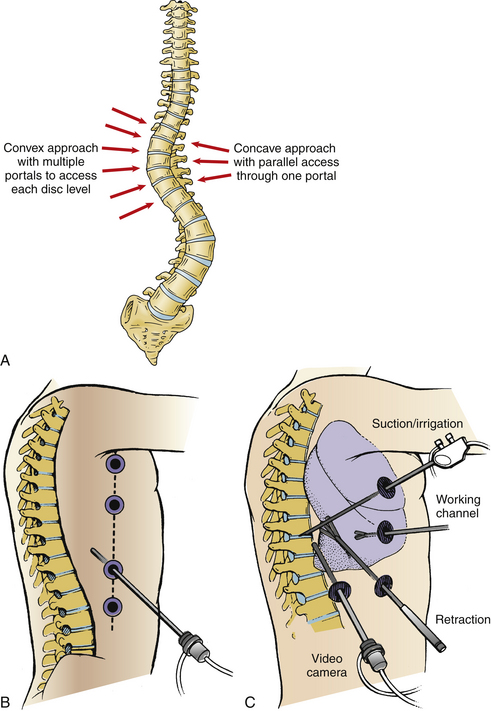
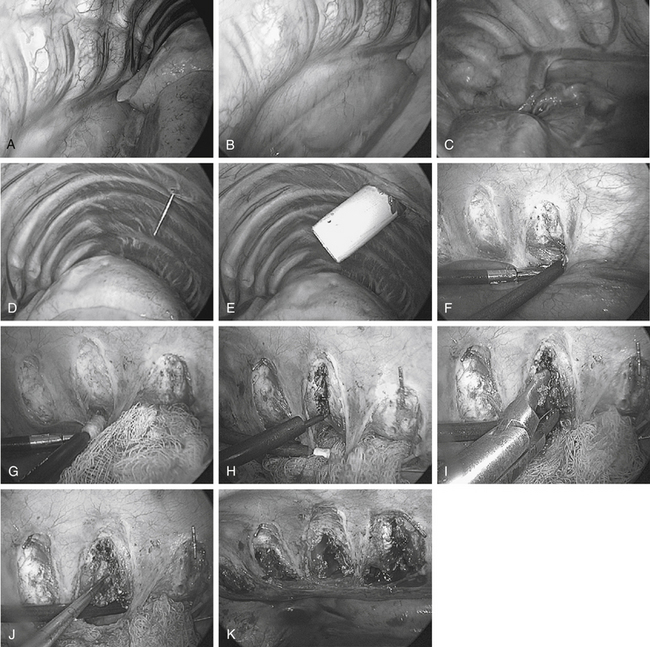
During scoliosis correction, the spine may be exposed on the curve’s convexity or concavity, depending on clinical circumstances or the surgeon’s preference (Fig. 127-3). Historically, because the structural tether resides in the concavity, ventral releases through a concave side thoracotomy were recommended by Stagnara.20 This approach has not gained popularity because of difficulty working deep in the concave portion of the deformity between the narrowed rib spaces. Moreover, the segmental vessels are clumped together and are more likely to be injured. The mediastinal structures, including the aorta, unfold into the concavity of the curve and must be meticulously dissected and mobilized. On the other hand, working in the concavity allows access to more disc spaces with fewer portals and a direct approach to the structural tether in the dorsolateral corner of the disc space.
Release of the convexity typically allows easier access to the apex of the curve and has become more common overall. However, complete release and exposure of the dorsolateral corner on the concave side is occasionally difficult at the most proximal and distal levels.44 Working from the convexity may require more portals to gain parallel access to each disc space. If thoracoplasty is planned, a convex side approach is required. For kyphosis correction, the spine can be approached from either side at the surgeon’s discretion.
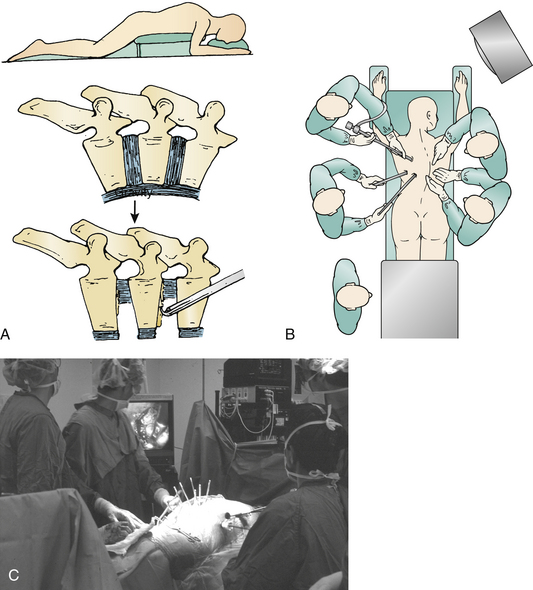
The lateral decubitus position, mimicking open thoracotomy, is typically selected for spine deformity procedures.1–3,12,24 However, if a subsequent dorsal stabilization is also required, lateral positioning requires repositioning and draping.48 Simultaneous prone ventral thoracoscopic release with dorsal instrumentation20,36 is another option (Fig. 127-4).
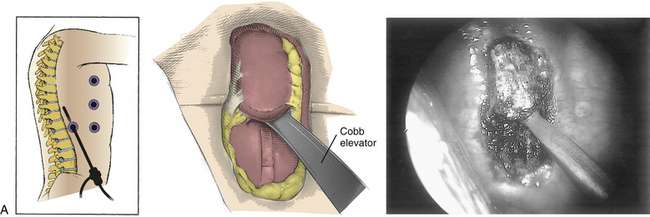
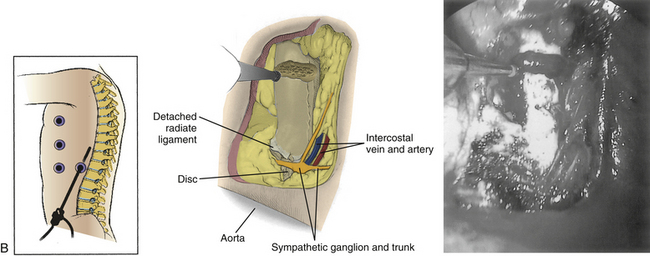
In gaining access to the spine, the first portal is created opposite the apex of deformity at the midaxillary line. The lung is retracted, and then the sympathetic chain is bluntly dissected out of the operative field. The surgeon incises the anterior longitudinal ligament and anulus. In scoliosis the aorta may need to be mobilized with blunt dissection, because it frequently lies in the acute angle between the rib head and the lateral vertebral body. Once it has been mobilized, a small sponge or peanut retractor is placed in the interval between the vertebral body and the aorta to protect the great vessels during the preparation of the disc space.
Unlike cord decompression procedures, formal resection of the pedicle is not necessary. Rather, the disc space is incised and the nucleus pulposus is evacuated with rongeurs and curets down to bleeding subchondral bone end plates to the posterior longitudinal ligament (Fig. 127-5). For the scoliosis cases approached from the concavity, the dorsolateral corner, costotransverse ligaments, and rib heads on the concave side are released under direct view to optimize correction. The convex lateral anulus is left intact as a pivot point to prevent overdistraction during the dorsal correction. If working on the convexity, the concave dorsolateral corner must be released to achieve a complete release. For kyphosis releases, the entire anterior longitudinal ligament and anulus must be incised. Once all levels are released, a periosteal elevator is inserted and rotated slightly to ensure that proper release has been achieved.15
Internal thoracoplasty can be performed just after ventral endoscopic release or as an independent procedure, as described by Mehlman et al.49 The rib deformity associated with idiopathic scoliosis often represents a significant cosmetic concern to the patient but may not improve significantly after dorsal fusion.50 Preoperatively, ribs to be resected are identified radiographically and by physical examination. From the lateral decubitus position, thoracoplasty is performed on the convex side (Fig. 127-6). Occasionally, ribs are cut but left in place to ensure adequate thoracic cage stability.49
Results
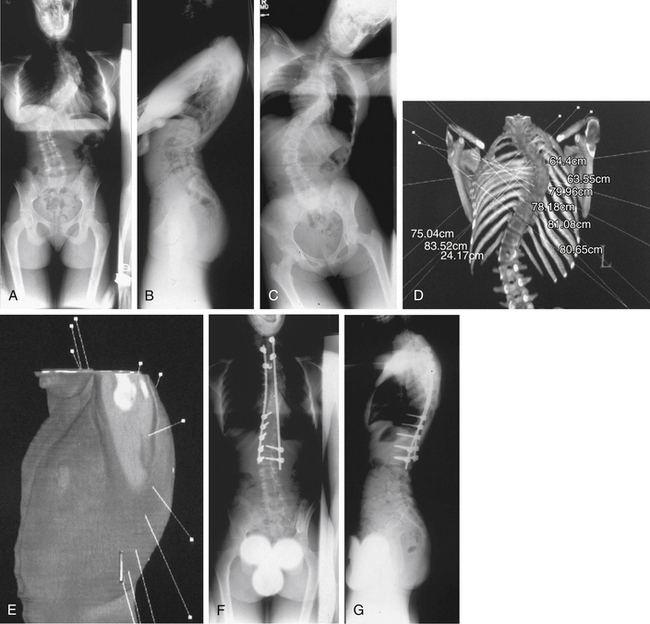
Wall et al.51 and Newton et al.52 independently reported their results with endoscopic discectomy in an animal model. These authors concluded that the extent of discectomy and quality of release are comparable to those of open techniques. Published studies describing results of open ventral releases by Kostuik et al.,45 Simmons et al.,46 and Byrd et al.43 reveal that average curve correction ranged from 36% to 48%. The average correction achieved with thoracoscopic techniques appears to be at least equivalent to those reports.20,21 However, Arlet28 performed a meta-analysis of the use of VATS in spine deformity surgery. He identified 10 articles that involved 151 procedures. He reported that most authors selected a convex side approach from a lateral decubitus position with four or more ports in the midaxillary line. Four to seven discs were excised and grafted over 21⁄2 to 4 hours. Most procedures were followed by same-day dorsal stabilization. The mean scoliotic curve was corrected from 65 to 35 degrees, and the mean kyphosis was corrected from 78 to 44 degrees. He noted that 7 years after the first report, the literature still only showed 151 patients and no long-term follow-up or significant outcome data in terms of spinal balance, fusion rate, rib hump correction, cosmesis, pain, and satisfaction. Arlet concludes that a more complete discectomy is possible with open technique and that animal studies documenting equivalent discectomies do not account for the visualization difficulties encountered in deformity patients. In his view, the data do not yet support widespread implementation, and the individual surgeon must consider whether he or she treats enough of the relevant pathologies to make learning the technique worthwhile.
Alternatively, Niemeyer et al.53 evaluated the 2-year clinical results, radiologic correction, and morbidity of ventral thoracoscopic surgery followed by dorsal instrumentation and fusion in their series of 29 patients with idiopathic scoliosis. A mean preoperative Cobb angle of 65.1 degrees was corrected to a mean 34.4 degrees at final follow-up. In nine hyperkyphotic patients, the mean preoperative Cobb angle of 81 degrees was corrected to 65 degrees. The average duration of the thoracoscopic procedure was 188 minutes, and this time decreased as the series progressed. No neurologic or vascular complications occurred. Postoperative complications included four recurrent pneumothoraces, one surgical emphysema, and one respiratory infection. The authors concluded that thoracoscopic ventral surgery is a safe and effective technique for the treatment of pediatric spine deformity but that a randomized controlled trial, comparing open with thoracoscopic methods, is required.
Newton et al.21 reported a prospective series of 65 consecutive cases of thoracoscopic ventral release with discectomy and fusion performed by one surgeon for the treatment of pediatric spine deformity. This patient group was 14 ± 3 years of age and included patients with idiopathic scoliosis (13), Scheuermann kyphosis (9), neuromuscular spine deformity (35), congenital scoliosis (4), and tumor/syrinx (4). The average operative time for the thoracoscopic procedure was 161 ± 41 minutes (range, 50–240), with a slight decrease in the average operative time occurring as the series progressed. The average number of discs excised was 6.5 ± 1.5 (range, 3–10), and this number increased as the series progressed. The average operative time per disc decreased as the series progressed. Initial postoperative scoliosis time was 29.3 ± 7.7 minutes in the first 30 patients, compared with 22.3 ± 4.7 minutes in the next 35 patients (P < .01). The average blood loss during the thoracoscopic procedure was 301 ± 322 mL (range, 25–2000) but it did not decrease as the series progressed. Initial postoperative scoliosis and kyphosis correction were 59% (from 62 to 25 degrees) and 92% (from 78 to 44 degrees), respectively. Complications occurred in six patients and were evenly distributed throughout the series. Complications included chest tube reinsertion for pleural effusion or chylothorax (three patients), conversion to thoracotomy (two patients), and incorrect levels of fusion (one patient). The authors concluded that the learning period for thoracoscopy is substantial but not prohibitive and that the technique provides a safe and effective alternative to thoracotomy in the treatment of pediatric spine deformity.
In another series, Newton et al.22 compared their early outcomes and costs between a series of 14 consecutive thoracoscopic releases with 18 open thoracotomies performed the previous year. The percentage of curve correction was similar between the groups, with 56% correction in the thoracoscopic scoliosis group versus 60% in the open group and 88% in the thoracoscopic kyphosis group versus 94% in the open group. Blood loss and complication rates were the same in both groups, but chest tube output was greater in the thoracoscopic group. The length of hospital stay was not reduced in the open group, and costs of the open procedure were 29% less than for the thoracoscopic procedure.
Holcomb and et al.54 reported on their first seven patients with deformities undergoing ventral discectomy followed by dorsal fusion. These included patients with congenital deformities with hemivertebrae. They performed a mean of four discectomies in an average of 174 minutes. There was only one complication related to excessive bleeding from an intercostal vessel that was immediately controlled with vessel clips.
Rothenburg34 reported a series of 20 ventral releases performed thoracoscopically in children from 8 to 17 years of age. The thoracoscopic portion of the procedure lasted a mean of 106 minutes. All patients underwent subsequent dorsal instrumentation and fusion. Corrections were noted to be acceptable and equivalent to those of the open technique. Earlier extubation, shorter ICU stays, and lower morbidity were reported.
Crawford and colleagues1 reviewed their experience with VATS in the treatment of children with severe spine deformities. The authors noted that surgical time was reduced from that of open thoracotomy. More importantly, tissue damage, blood loss, postoperative pain, and ICU and hospital stays were all reduced. Respiratory and shoulder function were less affected. The authors list possible complications from the procedure, but these are not different from similar complications encountered in open procedures.
The prospective series of Lieberman et al.20 followed 15 adult patients with spine deformity who underwent simultaneous ventral endoscopic and dorsal instrumentations for an average of 31 months (Fig. 127-7). There were no intraoperative technical problems with the endoscopic equipment or instruments, and visualization of the ventral spinal column was excellent. No vessel or organ injuries were encountered, and no cases were converted to open thoracotomy. No patient complained of postoperative radicular symptoms or chest pain consistent with a postthoracotomy syndrome, and there were no wound complications at the portal or dorsal incisions. There were no immediate, 6-month, or 2-year postoperative complications related to the endoscopic component of the procedure. In the scoliosis patients, the average correction was 60%. In the kyphosis patients, the average correction was 39%. Total operative time varied from 6 to 10 hours, depending primarily on the complexity of the dorsal instrumentation. Average time per disc space evacuation was 20 minutes, with a range of 15 to 35 minutes. Blood loss averaged 1200 mL for the entire series of ventral and dorsal procedures. Average hospital stay was 6.5 days (range, 5–8).
King et al.36 describe 27 patients undergoing VATS in a prone position for scoliosis or kyphosis. No conversions to open technique were required. Only discs that did not correct to neutral or beyond on bending films were incised. A mean of 3.5 disc spaces were treated. In the scoliosis patients, the mean preoperative Cobb angle of 70.2 degrees was corrected to 22.9 degrees. For kyphosis patients, the mean Cobb angle improved from 82.3 to 48.1 degrees. Complications related to the ventral approach included one case of atelectasis and three persistent pneumothoraces after removal of the chest tube.
Video-Assisted Thorascopic Surgery for Thoracic Spinal Degenerative Disease
Although the majority of thoracoscopic procedures have been performed for deformity correction, implementation of this technique in patients with tumors or herniated thoracic discs is becoming increasingly common.2,3,55,56 Overall, the incidence of clinical significant thoracic disc herniation is as low as 1 per million,57 or 0.25% to 0.75% of all disc ruptures.58
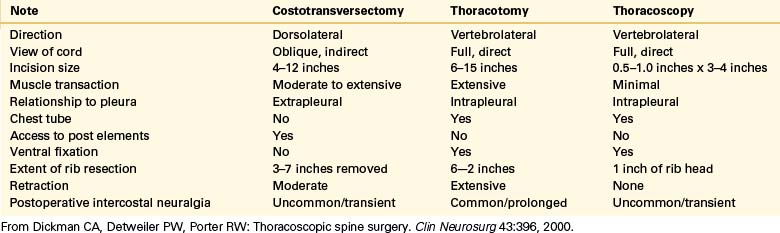
For these uncommon lesions, a number of surgical approaches have been described, including laminectomy, pediculectomy, costotransversectomy, lateral extracavitary, transverse arthropediculectomy, and transthoracic approaches.9 Laminectomy alone is ineffective for dorsal and dorsolateral canalicular disc herniations and may be associated with neurologic decline in up to 50% of patients.59 Although costotransversectomy and lateral extracavitary approaches have the advantages of being able to decompress and stabilize the spine through a single incision in one stage, they are limited by their time-consuming and technically demanding nature.60 Each of these has significant potential morbidity (Table 127-1). Moreover, they confer only a limited view of the ventral spinal cord. In one report, ventral approaches were found to have a complication rate ranging from 14% to 31%, whereas dorsal-approach–related complications ranged from 17% to 51%7 (see Table 127-1). The first application of VATS to degenerative spinal disease was in 1993.2
Operative Technique and Avoidance of Complications
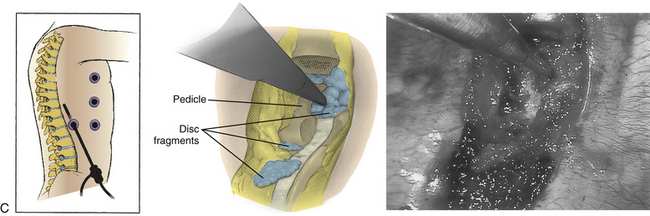
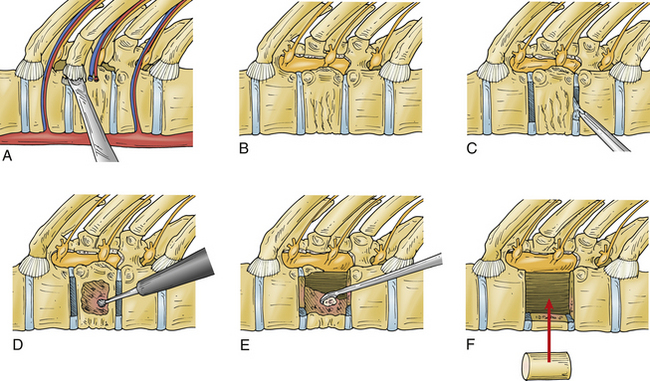
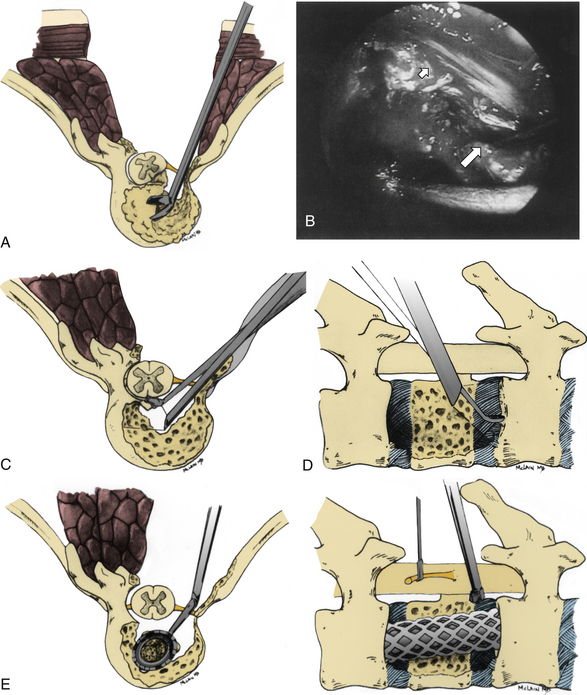
Symptomatic thoracic disc herniations amenable to endoscopic transthoracic decompression are typically approached from the side of the herniation. In the context of previous surgery, a contralateral approach may be used. The first trocar is inserted at the sixth or seventh interspace toward the anterior axillary line. Then two to three additional portals are inserted under direct vision. Usually one to two portals are inserted at the anterior axillary line and one at the posterior axillary line. A 30-degree angled rigid scope may be used to see into the disc space and around the bony edges. It is necessary to identify the appropriate level and resect the proximal 2 cm of rib head if it is above T11 (Fig. 127-8). Resection of the superior part of the pedicle exposes the dura. Some authors excise the entire pedicle, particularly for larger and more central herniations.56
Next, with the spinal cord continuously visualized to prevent inadvertent entry into the canal, a cavity is created in the disc space and vertebral bodies ventrally. The size of this space is increased for larger or more central herniations. The cavity should be wide enough that the surgeon can visualize normal dura above and below the herniation.56 The cavity must be deep enough to expose the entire width of the ventral dura to the contralateral pedicle. A corpectomy may be required for larger or ossified herniations (Fig. 127-9).
If a total corpectomy was required for adequate decompression, concomitant fusion should be considered. Typically, a trough is made with a side-cutting bur, and a rib fragment or iliac crest wedge is tamped into position across the disc space. Alternatively, Regan et al.61 describe placement of an interbody cage into the disc space after decompression.
Results
Only a few centers are currently using endoscopic techniques to decompress thoracic disc herniations, and only a few clinical reports are published. In one series of thoracic endoscopic discectomies, the 14% complication rate was comparable with rates reported for open approaches.3 Horowitz et al.62 documented their experience with cadavers and a porcine model. At the time of their report, the authors were successful in decompressing the cord in five of seven cadaver disc spaces and two of three porcine spaces. One dural violation occurred. The authors thought that improvement in instrumentation would allow safer and more complete disc space access.
Rosenthal and Dickman56 reported outcomes of 55 consecutive patients undergoing video-assisted thoracoscopic discectomy. In their group, 65% presented with myelopathic signs and symptoms caused by spinal cord compression and the remainder had severe thoracic radiculopathy. After surgery, 60% of the myelopathic patients recovered neurologically (22 completely and 5 with improvement but residual myelopathic symptoms). Seventy-nine percent of the radiculopathic patients recovered completely, and 21% improved. None worsened. The investigators compared their thoracoscopic patients with patients treated by costotransversectomy or thoracotomy and found that thoracoscopy was associated with 1 hour less time in the operating room and a reduction in blood loss by half. Postoperative chest tube output and narcotic usage were significantly lower in the thoracoscopic group.
Regan et al.23 reported 1-year follow-up data on their first 29 patients undergoing video-assisted thoracoscopic excision of herniated discs at 32 levels. In this series, the mean operating time was 175 minutes and the estimated blood loss ranged from 75 to 2500 mL. The mean hospitalization time was 3.9 days. The investigators found that 75.8% of patients were satisfied with the procedure, 20.1% were unchanged, and 3.4% were worse. Significant improvements in Oswestry scores occurred in radiculopathic and myelopathic patients. There was a 13.8% complication rate but no long-term sequelae from any of the complications.
Subsequently, Anand and Regan9 reported their results after 117 thoracoscopic discectomy procedures in 100 consecutive patients with an average follow-up of 4 years. Patient presentation ranged from pure axial pain to pure radiculopathy to pure myelopathy, with various combinations of these complaints. The mean operating time was 173 minutes, and blood loss averaged 259 mL. The average ICU stay was less than 1 day, and the average hospital stay was 4 days. Minor complications occurred in 21 patients, all of which resolved with no untoward effect. No patient’s neurologic status worsened. Clinical success was defined as a modest 20% improvement in Oswestry score at final follow-up. Overall, objective clinical success was observed at final follow-up in 70% of these patients. The greatest gains were noted in myelopathic patients, followed by the radiculopathic patients. Axial pain patients exhibited the least postoperative improvement. The authors concluded that VATS appears to be a safe and efficacious method for the treatment of refractory symptomatic thoracic disc herniations.
McAfee37 reported complications related to a thoracoscopic decompression, in a series of 77 patients, as excessive epidural bleeding in 1 patient and transient paraparesis in another. A number of other case reports and small series are also available with similar results.63,64
Video-Assisted Thoracoscopic Surgery in the Treatment of Spinal Tumors
Much as with thoracic disc herniations, the previous standard approach to cord compression from spinal tumors was laminectomy. However, for lesions ventral to the cord, the rate of neurologic improvement after surgery has been poor.29 Over time, more aggressive and more highly morbid approaches such as costotransversectomy and thoracotomy have been espoused for more complete cord decompression. VATS techniques have been typically used in intralesional or “piecemeal” tumor debulking procedures, or for biopsy. Solitary tumor en bloc resections typically require open approaches.
The technique of decompression in patients with spinal tumors is similar to that in degenerative disease (Fig. 127-10). Preoperatively, it is necessary to consider the vascularity of the lesion. In many cases, preoperative embolization should be considered, particularly for vascular tumors such as renal cell carcinoma. Reconstruction techniques in malignancy may be liberalized depending on the anticipated life span of the patient. To avoid the morbidity of graft harvest and to achieve early stability, Rosenthal described reconstruction by injection of semiliquid polymethylmethacrylate (PMMA).65 The PMMA is injected through a tube into the cavity and allowed to polymerize in situ. For patients with longer anticipated survival, a fusion should be performed. Ventral instrumentation options are improving. In Rosenthal’s series, special equipment was used to dilate the skin incision to allow insertion of a ventral plate. Special instruments are used to handle the plates and screws in the chest cavity.
There are few published series describing the results of endoscopic decompression of spine tumors. Rosenthal et al. described outcomes in four patients with malignancies of the thoracic spine and progressive neurologic deficits treated with thoracoscopic decompression.41 At an average of 11 months of follow-up, all patients were free of pain and neurologically improved. In this small series, there were no complications or hardware failures. All patients were also externally braced, and adjuvant chemotherapy or radiation therapy was used in several patients. Dickman et al.16 reported on the outcomes of seven patients undergoing thoracoscopic vertebrectomy for tumor. They found that the operative time and blood loss were similar to that of a similar group undergoing open thoracotomy. Narcotic use, ICU stay, and hospital length of stay were all dramatically reduced in the thoracoscopic group.
One subgroup of spinal tumors that has received special emphasis is the so-called dumbbell tumor groups. Up to 10% of neurogenic tumors in the dorsal mediastinum extend into the spinal canal.26 These lesions have previously required resection through open thoracotomy, often with a staged laminectomy. Heltzer et al.26 describe resection of such a dumbbell lesion through a staged laminectomy followed by a thoracoscopic approach. Subsequently, Konno et al.66 reported on a series of five patients treated with a similar technique and followed for at least 3 years. In their series, postoperative instability did not develop in any patient.
Citow et al.11 described a single-stage, combined laminectomy and thoracoscopic resection of a 4 × 5 × 5 cm mass filling 60% of the spinal canal at the T3 level. The lesion was first detached from the spinal cord by way of laminectomy with medial facetectomy. Then, a three-portal thoracoscopic approach was undertaken in which the parietal pleura was incised and the tumor bluntly dissected and removed through an expanded ventral portal in a specimen bag. The authors noted that a potential limitation to this approach was possible communication between the subarachnoid space with the low-pressure pleural cavity, which would increase the risk of cerebrospinal fluid fistula. They recommended an endoscopic suture of the parietal pleura. Further, because of the potential for malignant lesions to encase or invade the mediastinal structures, they suggested distinguishing between benign and malignant lesions prior to proceeding with the endoscopic approach.
Van Dijk et al.67 described another combined technique for solitary spinal tumor resection in which thoracoscopically assisted ventral releases were followed by a dorsal en bloc spondylectomy and reconstruction. This approach allowed thoracoscopic access and release of the involved spinal segments to achieve surgical and histopathologic wide margins while avoiding the disadvantages inherent to thoracotomy.
McLain’s technique for decompression of thoracic metastases is similar but is undertaken through a longitudinal incision.8,68 The initial approach mimics costotransversectomy, wherein the proximal rib is removed with the entire pedicle. Here, too, a cavity is created ventrally, and ventral compressive pathology is collapsed into this cavity by using Epstein curets. After complete decompression, a corpectomy defect is created, and the space can be reconstructed by using titanium mesh cages followed by dorsal, segmental instrumentation (Fig. 127-11).
Video-Assisted Thoracoscopic Surgery in the Treatment of Thoracic Spine Trauma
Because a significant portion of thoracic trauma occurs at the thoracolumbar junction, the extended manipulating channel method described by Huang et al.69 may be useful. For these injuries, an approach from the left is recommended because the aorta lies just left of the midline and there is more space available next to the vertebral surface. After the initial portal has been made at the seventh intercostal space, an extended portal 5 to 6 cm in length is made at the injured level or slightly behind the posterior axillary line at the T9-10 interspace. A similar length of underlying rib is removed, and a small, self-retaining rib spreader is then placed, allowing introduction of larger instruments and direct palpation of the spine. The diaphragm is pushed down with a sponge forceps introduced through the manipulating channel. The approach-side pedicles are key landmarks and are removed at the vertebrectomy level and the next caudal level.7 The dura mater is exposed, and discectomy of the superior and inferior disc spaces is undertaken.
Reconstruction after trauma also includes morselized or structural ventral grafting followed by ventral or dorsal instrumented stabilization. Morselized bone placed into partial corpectomy defects is typically stabilized dorsally with short-segment transpedicular instrumentation.17 Alternatively, a corpectomy reconstruction can be completed by negotiating allograft struts, or mesh cages, into the defect, after inserting them into the chest through one of the portals. Then, either ventral or dorsal instrumentation is used to stabilize the construct to extension, rotation, and side flexion.
Hertlein et al.17 describe eight cases of staged, ventral thoracic discectomy and bone grafting after dorsal transpedicular stabilization. Short-segment dorsal instrumentation is used to reduce the kyphotic deformity. Then tomograms or sagittal CT reconstructions are obtained to assess the size of the anterior column defect. If large, this ventral defect is directly bone grafted by using thoracoscopic means.
Dickman et al.16 reported on the outcomes of six patients undergoing thoracoscopic vertebrectomy for fracture. They found that the operative time and blood loss were similar to that of a similar, open thoracotomy group. On the other hand, narcotic use, ICU stay, and hospital length of stay were all dramatically reduced in the thoracoscopic group.
Huang et al.70 described their series of ventral decompressions and stabilization in eight elderly patients when using a modified two-portal technique with a 5-cm minithoracotomy incision. Over the mean 30-month follow-up period, no injuries to the great vessels, internal organs, or spinal cord occurred. Complications included one screw migration with graft displacement and transient problems with iliac crest donor site pain and wound hypesthesias. Average neurologic recovery was 1.1 grade on the Frankel scale. The authors concluded that this minimal-access technique with thoracoscopic assistance is an ideal alternative in treating patients with osteoporotic vertebral fractures and neurologic deficits.
Video-Assisted Thoracoscopic Surgery in the Treatment of Spine Infection
Spine infections are typically divided anatomically into vertebral osteomyelitis, discitis, or epidural abscess.33 Treatment decisions are based on the sensitivity of the organism, the response of the infection to antibiotic management, the presence of abscess, the presence of neurologic involvement, the progression of spinal instability or deformity, involved spinal levels, and host factors such as age and medical comorbidities. Increased age and cephalad level of infection predispose to neurologic decline and paralysis.71
Treatment begins with identification of the offending organism through blood culture or biopsy. When clear identification of the organism is not obtained with percutaneous biopsy, surgical biopsy can be considered. Endoscopic techniques are an ideal, less invasive means to obtain an adequate tissue sample. Tan et al.72 reported the case of an 18-month-old infant with increasing back pain and gait difficulty. Low-grade fever was noted, as was irregularity of the end plates at T7-8. CT scan demonstrated a soft tissue mass, but attempts at percutaneous needle biopsy were unsuccessful. A standard thoracoscopic approach was undertaken, and a 4-mm pediatric biopsy forceps was used to take several samples from the affected site.
After the organism has been identified, appropriate antibiotic treatment can commence. Surgery is indicated in any patient with progressive neurologic deficits, failure to improve with medical management, or continued vertebral collapse and instability. In most cases, when spinal cord compression occurs, the pathology is ventral, and wide approaches such as thoracotomy or costotransversectomy are required. Unfortunately, these approaches are highly morbid in this compromised patient population.33 VATS can be used in patients with spine infections in a manner similar to that of tumor patients. That is, VATS can be used for biopsy, debridement, cord decompression, and reconstruction.
The technical details of VATS for spine infection are similar to those of other indications. Surgical goals include confirmation of tissue diagnosis with biopsy, radical debridement of all necrotic debris, correction of any secondary deformity, and stabilization with autogenous bone grafting.33 Typically, a four-portal technique starting with an initial viewing portal at the T7 level is used. There may be a higher rate of pleurodesis secondary to the inflammation that requires either meticulous thoracoscopic adhesion dissection or a higher rate of conversion to open procedures. For similar reasons, the risk of postoperative air leak is higher as well.
Huang et al.19 reported their experience with VATS in managing tuberculous spondylitis in 10 patients. At a mean 24-month follow-up, average neurologic recovery was 1.1 Frankel grade. Complications included one lung atelectasis, pleural adhesions requiring conversion to an open procedure in one case, and four transient postoperative air leaks. The authors concluded that thoracoscopic techniques were a useful adjunct in the management of patients with tuberculous spondylitis for either biopsy or formal decompression and reconstruction. For debridement and reconstruction, the authors recommended a combination of thoracoscopic visualization and minithoracotomy for debridement and instrumentation.
Dickman et al.16 reported on the outcomes of three patients undergoing thoracoscopic vertebrectomy for infection. They found that the operative time and blood loss were similar to that of an open thoracotomy group. On the other hand, narcotic use, ICU stay, and hospital length of stay were all dramatically reduced in the thoracoscopic group.
Thoracoscopic Instrumentation
With some difficulty, existing rod and screw implant systems may be inserted in an endoscopic or endoscopically assisted fashion. These systems have also been modified by using, for example, cannulated screws and combinations of endoscopic and fluoroscopic insertion techniques.73
Hertlein et al.18 described their first two cases of thoracoscopic osteosynthesis. After decompression and grafting as described previously, dynamic compression plates were brought into the wound through the working trocar. The plate was preliminarily fixed to the spine by using two Kirschner wires. A Cardan drill was then inserted into the trocar, and 3.2-mm holes of 2-cm depth were prepared. Next, 6-cm screws were inserted into the plate with a Cardan screwdriver. These patients were not braced postoperatively.
Crawford1 and Picetti et al.40 have described similar endoscopic instrumentation techniques in the deformity setting. The authors used circumferential C-arm access to the patient along with a tricannulated pitchfork. The tricannulated pitchfork was used to place guide pins and subsequently screws. The device allows the surgeon to line up the best coronal location for the screw and place a Steinmann pin through the cannula under image control. The pin is placed in the midcoronal plane of the vertebra and driven from slightly dorsal to ventral starting just ventral to the rib head. Starting at the rib head prevents the surgeon from starting the screw too far dorsally. The surgeon stands at the patient’s back and places the instrumentation from back to front, aiming away from the spinal cord. Once the Steinmann pin has been placed, its external portion is secured to prevent penetration through the body into the opposite hemithorax. A cannulated tap prepares the site for a bone screw. When the screws have been placed, a measuring device for rod length is advanced through the inferior portal. The rod is inserted through the inferior portal and seated into the screw heads. Capture screws are seated into screw heads to secure the rod. The bottom screw is tightened first so that all compression projects superiorly toward the top of the thoracic cage rather than inferiorly toward the diaphragm. Once the rod has been fully seated, a compressor is introduced, each set of screws is individually compressed, and the capture screw is tightened and torqued. With present designs, a true rotational maneuver is not possible.
Recently, Assaker et al.74,75 studied the feasibility of adding computer image guidance to endoscopic spine procedures. In this setting, three-dimensional assessment of instrumentation position would be possible through video monitors by using an extracorporeal fiducial and a CT-based navigational system. This may improve the speed and accuracy of endoscopically performed decompression and instrumentation.
Huang et al.76 describe a combination thoracoscopic and “mini-open” approach to decompress and stabilize the spine in a series of four patients with thoracic myelopathy. A 3- to 4-cm manipulating channel is created for both endoscopic instrumentation and subsequent tumor removal and reconstruction. Standard reconstructive instrumentation may then be inserted.
These techniques are not without potential complications. The greatest of these is malposition of the instrumentation into the spinal canal or vascular structures because of the loss of three-dimensional vision and direct palpation. Roush et al.73 also reported a case of tension pneumothorax during fluoroscopic guide pin placement for a video-assisted ventral scoliosis stabilization procedure.
Spine Endoscopy in the Thoracic Spine: Dorsal Approach
Direct dorsal approaches to midline neurocompressive thoracic pathology have largely been abandoned because laminectomy alone does not adequately decompress a kyphotic spinal segment. Moreover, attempts to indirectly decompress central pathologies have been unsuccessful, or worse, have resulted in neurologic decline.77,78 On the other hand, costotransversectomy and lateral extracavitary approaches are associated with large incisions, increased postoperative morbidity, wound healing problems, and difficulty with visualization.1,7 Smaller, transpedicular dorsal approaches can use small incisions and a 70-degree endoscope to better visualize the ventral dura and avoid the need for postoperative chest tube drainage required of either thoracoscopy or thoracotomy.
The role of endoscopic and minimally invasive techniques in treatment of metastatic and degenerative disease is evolving. Although ventral approaches to metastatic disease are favored overall, the use of an endoscope to assist dorsolateral decompression may obviate the need for a second, ventral surgery in patients undergoing dorsal stabilization. This approach is particularly useful in patients with radioresistant metastases of the upper thoracic spine, where thoracotomy is difficult and highly morbid.8,68 Similarly, dorsal vertebrectomy and decompression techniques must be considered in patients unable to tolerate single-lung ventilation or thoracotomy. Contraindications to the currently available techniques include failed prior open surgery and large lesions. Dorsal transpedicular instrumentation is another area in which endoscopic assistance may allow for smaller incisions and decreased muscle injury. These resection techniques are intralesional and are therefore not indicated in patients with primary neoplasms.8
Anatomy and Technique
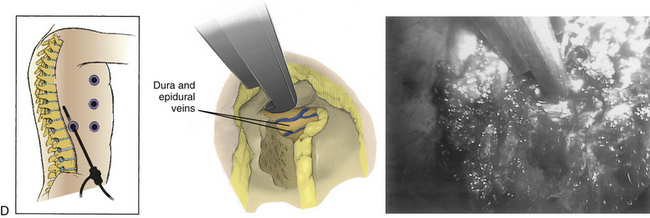
Osman and Marsolais79 described a dorsal endoscopic approach to the thoracic disc space in a cadaver. The authors found that above T10, the rib neck was an ideal guide to the disc space and prevented lateral excursion into the lung. The shoulder girdle and transition from thoracic kyphosis to cervical lordosis made accurate insertion at the T1-2 and T2-3 levels difficult. They concluded that this approach would be technically feasible for soft lateral discs. With further development of endoscopic instrumentation, even calcified or adherent central discs could be approached in this manner.
Jho80 described a minimally invasive dorsal approach to thoracic disc herniations using a 2-cm-long transverse paramedian incision at the pedicle level of the involved vertebra. Patients are positioned 60 degrees forward, inclined to keep the lesion side facing upward. The paraspinal muscles are dissected from the spinous process, lamina, and transverse processes by using a periosteal elevator. A tubular retractor is passed into the wound over the facet and lamina. The medial portion of the facet, the lateral portion of the lamina, and the rostral third of the pedicle are removed with a high-speed bur to gain access to the disc space and to expose the very lateral margin of the spinal cord dura. A 2-mm bur removes the bone spurs rostral and caudal to the herniated disc and creates a cavity into which material from the decompression is moved. When an appropriate 1.5-cm cavity has been created, a 4-mm-diameter rigid endoscope with a 70-degree lens is mounted to a custom-made endoscope holder. Surgical decompression of the ventral cord can then be performed by using 90-degree curved surgical instruments. For example, a down-biting curet can be used to push more osteophyte away from the cord and into the created cavity. Material can be removed from the cavity with a curved pituitary rongeur.
Results
Jho80 reported on a consecutive series of 25 patients undergoing minimal-access thoracic discectomy. Seven patients were myelopathic, 6 presented with myeloradiculopathy, 10 presented with radicular complaints, and 2 were believed to have segmental pain. He reports the perioperative morbidity of this procedure to be similar to that of lumbar microdiscectomy, and radiculopathic patients are allowed to go home the same day. In his series, the 2 patients with segmental pain did not note relief of symptoms despite MRI documentation of complete decompression. Of the radiculopathic patients, 9 of 10 had complete relief of symptoms. Twelve of 13 myelopathic or myeloradiculopathic patients had significant relief of symptoms.
McLain8 described the successful use of an endoscope to complete ventral decompression from a dorsal approach in five patients by using an endoscope to increase visualization. The mean operative time was 7.25 hours, and the mean blood loss was 1800 mL. Neurologic recovery was judged excellent.
Dorsal Spine Endoscopy in the Lumbar Spine
This phrase may refer to the tubular dilation/endoscopic camera system used for minimal access to the lumbar spine or to endoscopic laparoscopy used to gain an approach to the ventral lumbar region.
The ventral laparoscopic approach can be used for ventral fusion and stabilization procedures, resection of neoplasms, and spinal reconstruction.81 Laparoscopic skills are not part of the repertoire of most neurosurgeons, and often a cosurgeon is necessary.
The dorsal spinal approach using a dilator is a tried, tested, and effective technique.82 Robust clinical data suggest that the complication rates, length of stay, and cost are comparable to those of open techniques. The dorsal spinal endoscopic approach may be used for access for dorsal fusion and stabilization, nerve and canal decompression, tumor and infection removal, and intradural lesion surgery.82
Technique
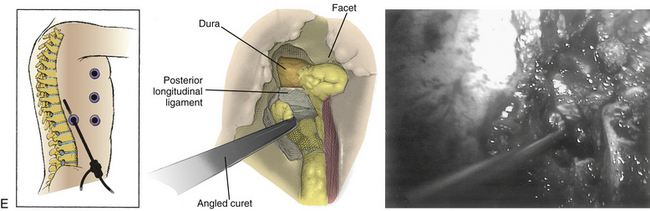
Our technique was described by Khoo and Fessler in 2002.83 The patient is usually positioned prone on a radiolucent spinal table (Jackson or similar). The level is confirmed on a lateral radiograph, and an incision is performed to allow access to the ipsilateral facet by a Steinmann pin, which is then followed by serial dilators and attachment of the dilators to a flexible arm retractor. The working tube is then docked and attached to a 30-degree endoscope, and the ipsilateral facet, interlaminar space, and caudal edge of the superior lamina are identified after removal of soft tissue. Access to the interlaminar space is gained with an angled curet, and the lamina is removed using a drill, punches, and rongeurs. The tube can then be angled to achieve medial, lateral, rostral, and caudal access. Ipsilateral or contralateral nerve decompression, disc access and sequestrectomy, canal decompression, and dural access can be achieved using this technique. The incision can be widened to achieve multilevel decompression or dural access as required.
Results
Khoo and Fessler83 found a 68% improvement in symptoms following endoscopic decompression for lumbar canal stenosis, comparable to open techniques. Both endoscopic canal decompression and endoscopic microdiscectomy show statistically significant reduction in blood loss and hospital stay compared with open techniques.83 Recent data show a comparable improvement in canal decompression area with endoscopic compared to open techniques.84
Lateral decompressions can also be combined with minimally invasive fusion and instrumentation, including dorsal osteotomies, dorsolateral vertebrectomy, and interbody cage fusion and combinations of these for tumor, trauma, infection, and degeneration.83
However, Sairyo et al.,85 in an unselected series of 138 patients, suggest that the initial complication rates may be higher than in open procedures, highlighting the importance of appropriate training in these techniques. In a randomized multicenter trial of lumbar microdiscectomy, Arts et al.86 propose that the rates of back pain and sciatica were higher in the endoscopy group compared with those of open techniques. Unfortunately, the experience of the treating surgeons in these techniques was not specifically mentioned. Three other randomized trials of microdiscectomy (Righesso et al.,87 Ryang et al.,88 and Mayer and Brock89) did not show a significant difference in complication rates. Recent papers also suggest a significant reduction in the incidence of postfusion infection rates.90
There has also been interest in recent years in endoscopic dorsal decompression of the cervical spine, which has also gradually become an established technique.91,92
Summary
Endoscopic spine surgery confers many proven and potential advantages to both the surgeon and patient, including improved surgical visualization through magnification and lighting, decreased perioperative morbidity, and shorter hospital stays. But these advantages must be counterbalanced with disadvantages of these approaches, including lack of familiarity, decreased three-dimensional perspective, and a loss of tactile sense. Initial experience with these procedures should include cadaveric and animal laboratory work, followed by visits to active centers or proctoring.7 Every surgeon undertaking a VATS procedure should be able to convert to an open procedure or have a thoracic surgeon available. Early cases may be associated with higher complication rates and operating times. Ultimately, a less efficacious technique should not be used merely because it is endoscopic.
Lumbar endoscopic spine surgery is a more established field but also has a steep learning curve, and either fellowship training or proctoring is strongly recommended. The dorsal endoscopic approach to the thoracic and lumbar spine has a good experience and good safety profile.
Crawford A.H., Wall E.J., Wolf R. Video-assisted thoracoscopy. Orthop Clin North Am. 1999;30(3):367-385.
Dickman C.A., Karahalios D.G. Thoracoscopic spine surgery. Clin Neurosurg. 2000;43:392-422.
Regan J.J. Percutaneous endoscopic thoracic discectomy. Neurosurg Clin North Am. 1996;7(1):87-98.
Rosenthal D., Marquardt G., Lorenz R., et al. Anterior decompression and stabilization using a microsurgical endoscopic technique for metastatic tumors of the thoracic spine. J Neurosurg. 1996;84:565-572.
1. Crawford A.H., Wall E.J., Wolf R. Video-assisted thoracoscopy. Orthop Clin North Am. 1999;30(3):367-385.
2. Mack M.J., Regan J.J., Bobechko W.P., Acuff T.E. Application of thoracoscopy for diseases of the spine. Ann Thorac Surg. 1993;56:736-738.
3. Regan J.J. Percutaneous endoscopic thoracic discectomy. Neurosurg Clin North Am. 1996;7(1):87-98.
4. Bloomberg A.E. Collective reviews: thoracoscopy in perspective. Surg Gynecol Obstet. 1978;147:433-443.
5. Kuklo T.R., Lenke L.G. Thoracoscopic spine surgery: current indications and techniques. Orthop Nurs. 2000;19(6):15-22.
6. Regan J.J., Yuan H., McCullen G. Minimally invasive approaches to the spine. Instr Course Lect. 1997;46:127-141.
7. Dickman C.A., Detweiler P.W., Porter R.W. Endoscopic spine surgery. Clin Neurosurg. 2000;46:526-553.
8. McLain R.F. Endoscopically assisted decompression for metastatic thoracic neoplasms. Spine (Phila Pa 1976). 1998;15(10):1130-1135.
9. Anand N., Regan J.J. Video-assisted thoracoscopic surgery for thoracic disc disease: classification and outcome study of 100 consecutive cases with a 2-year minimum follow-up period. Spine (Phila Pa 1976). 2002;27(8):871-879.
10. Birnbaum K., Pieper S., Prescher A., Siebert C.H. Thoracoscopically assisted ligamentous release of the thoracic spine: a cadaver study. Surg Radiol Anat. 2000;22(3-4):143-150.
11. Citow J.S., Macdonald R.L., Ferguson M.K. Combined laminectomy and thoracoscopic resection of a dumbbell neurofibroma. Technical case report. Neurosurgery. 1999;45(5):1263-1266.
12. Crawford A.H., Wolf R.K., Wall E.J. Pediatric spinal deformity. In: Regan J.J., McAfee P.A., Mack M.J., editors. Atlas of endoscopic spine surgery. St Louis: Quality Medical, 1995.
13. Cunningham B.W., Kotani Y., McNulty P.S., et al. Video-assisted thoracoscopic surgery versus open thoracotomy for anterior thoracic spinal fusion. A comparative radiographic, biomechanical, and histologic analysis in a sheep model. Spine (Phila Pa 1976). 1998;23(12):1333-1340.
14. Dickman C.A., Karahalios D.G. Thoracoscopic spine surgery. Clin Neurosurg. 2000;43:392-422.
15. Dickman C.A., Mican C.A. Multilevel anterior thoracic discectomies and anterior interbody fusion using a microsurgical thoracoscopic approach. Case report. J Neurosurg. 1996;84:104-109.
16. Dickman C.A., Rosenthal D., Karahalios D.G., et al. Thoracic vertebrectomy and reconstruction using a microsurgical thorascopic approach. Neurosurgery. 1996;38:279-293.
17. Hertlein H., Hertl W.H., Dienemann H., et al. Thoracoscopic repair of thoracic spine trauma. Eur Spine J. 1995;4:302-307.
18. Hertlein H., Hartl W.H., Piltz S., et al. Endoscopic osteosynthesis after thoracic spine trauma: a report of two cases. Injury. 2000;31(5):333-336.
19. Huang T.J., Hsu R.W., Chen S.H., Liu H.P. Video-assisted thoracoscopic surgery in managing tuberculous spondylitis. Clin Orthop Relat Res. 2000;379:143-153.
20. Lieberman I.H., Salo P.T., Orr R.D., Kraetschmer B.G. Prone position endoscopic transthoracic release with simultaneous posterior instrumentation for spinal deformity: a description of the technique. Spine (Phila Pa 1976). 2000;25(17):2251-2257.
21. Newton P.O., Shea K.G., Granlund K.F. Defining the pediatric spinal thoracoscopy learning curve: sixty-five consecutive cases. Spine (Phila Pa 1976). 2000;25(8):1028-1035.
22. Newton P.O., Wenger D.R., Mubarak J.S., Meyer R.S. Anterior release and fusion in pediatric spinal deformity. A comparison of early outcome and cost of thoracoscopic and open thoracotomy approaches. Spine (Phila Pa 1976). 1997;22(12):1398-1406.
23. Regan J.J., Ben-Yishay A., Mack M.J. Video-assisted thoracoscopic excision of herniated thoracic disc. Description of technique and preliminary experience in the first 29 cases. J Spinal Disord. 1998;11(3):183-191.
24. Regan J.J., Guyer R.D. Endoscopic techniques in spinal surgery. Clin Orthop Relat Res. 1997;225:122-139.
25. Zdeblick T.A. A prospective, randomized study of lumbar fusion. Preliminary results. Spine (Phila Pa 1976). 1993;18(8):983-991.
26. Heltzer J.M., Krasna M.J., Aldrich F., McLaughlin J.S. Thoracoscopic excision of a posterior mediastinal dumbbell tumor using a combined approach. Ann Thorac Surg. 1995;60:431-433.
27. Sucato D.J., Welch R.D., Pierce B., et al. Thoracoscopic discectomy and fusion in an animal model: safe and effective when segmental blood vessels are spared. Spine (Phila Pa 1976). 2002;27(8):880-886.
28. Arlet V. Anterior thoracoscopic spine release in deformity surgery. A meta-analysis and review. Eur Spine J. 2000;9(Suppl 1):S17-S22.
29. Hall A.J., MacKay N.N. The results of laminectomy for compression of the cord and cauda equina by extradural malignant tumor. J Bone Joint Surg [Br]. 1973;55:497-505.
30. Winter R.B., Lonstein J.E., Denis F., et al. Paraplegia resulting from vessel ligation. Spine (Phila Pa 1976). 1996;21(10):1232-1233.
31. Vissochi M., Masferrer, Sonntag V.K.H., Dickman C.A. Thoracoscopic approaches to the thoracic spine. Acta Neurochir (Wien). 1998;140:737-744.
32. Ikard R.W., McCord D.H. Thoracoscopic exposure of intervertebral discs. Ann Thorac Surg. 1996;61:1267-1268.
33. Parker L.M., McAfee P.C., Fedder I.L., et al. Minimally invasive surgical techniques to treat spine infections. Orthop Clin North Am. 1996;27(1):183-199.
34. Rothenberg S., Erickson M., Eilert R., et al. Thoracoscopic anterior spinal procedures in children. J Pediatric Surg 33(7). 1168–1171, 1998.
35. Lischke V., Westphal K., Behne M., et al. Thoracoscopic microsurgical technique for vertebral surgery. Anesthetic considerations. Acta Anaesthesiol Scand. 1998;42:1199-1204.
36. King A.G., Mills T.E., Loe W.A., et al. Video-assisted thoracoscopic surgery in the prone position. Spine (Phila Pa 1976). 2000;25(18):2403-2406.
37. McAfee P.C., Regan J.J., Zdeblick T., et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine (Phila Pa 1976). 1995;14:1624-1632.
38. Regan J.J., Mack M.J., Picetti G.D. A technical report on video-assisted thoracoscopy in thoracic spinal surgery. Preliminary description. Spine (Phila Pa 1976). 1995;20:831-837.
39. Huang T.J., Hsu R.W., Sum S.W., Lie H.P. Complications in thoracoscopic spinal surgery. Surg Endoscopy. 1999;13:346-350.
40. Picetti G.III, Blackman R.G., O’Neal K., Luque E. Anterior endoscopic correction and fusion of scoliosis. Orthopedics. 1998;21(12):1285-1287.
41. Rosenthal D., Marquardt G., Lorenz R., Nichtweiss M. Anterior decompression and stabilization using a microsurgical endoscopic technique for metastatic tumors of the thoracic spine. J Neurosurg. 1996;84:565-572.
42. Sucato D.J., Girgis M. Bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, pneumoretroperitoneum, and subcutaneous emphysema following intubation with a double-lumen endotracheal tube for thoracoscopic anterior spinal release and fusion in a patient with idiopathic scoliosis. J Spinal Disord Tech. 2002;15(2):133-138.
43. Byrd J.A.3rd, Scoles P.V., Winter R.B., et al. Adult idiopathic scoliosis treated by anterior and posterior spinal fusion. J Bone Joint Surg [Am]. 1987;69:843-850.
44. Johnson J.R., Holt R.T. Combined use of anterior and posterior surgery for adult scoliosis. Orthop Clin North Am. 1998;19:361-370.
45. Kostuik J.P., Israel J., Hall J.E. Scoliosis surgery in adults. Clin Orthop Relat Res. 1973;93:225-234.
46. Simmons E.D.Jr., Kowalski J.M., Simmons E.H. The results of surgical treatment for adult scoliosis. Spine (Phila Pa 1976). 1993;18:718-724.
47. Gonzalez-Barrios I., Fuentes Caparrios S. Avila Jurado MM: Anterior thoracoscopic epiphysiodesis in the treatment of crankshaft phenomenon. Eur Spine J. 1995;4:343-346.
48. Shufflebarger H.L., Grimm J.O., Bui V., Thomson J.D. Anterior and posterior spinal fusion. Staged versus same day surgery. Spine (Phila Pa 1976). 1991;16:930-933.
49. Mehlman C.T., Crawford A.H., Wolf R.K. Video assisted thoracoscopic surgery (VATS), endoscopic thoracoplasty technique. Spine (Phila Pa 1976). 1997;22(18):2178-2182.
50. Weatherly C.R., Draycott V., O’Brien J.F., et al. The rib deformity in adolescent idiopathic scoliosis. J Bone Joint Surg [Br]. 1987;69:179-182.
51. Wall E.J., Bylski-Austrow D.I., Shelton F.S., et al. Endoscopic discectomy increases flexibility as effectively as open discectomy. Spine (Phila Pa 1976). 1998;23(1):9-16.
52. Newton P.O., Cardelia J.M., Farnsworth C.L., et al. A biomechanical comparison of open and thoracoscopic anterior spinal release in a goat model. Spine (Phila Pa 1976). 1998;23(5):530-536.
53. Niemeyer T., Freeman B.J., Grevitt M.P., Webb J.K. Anterior thoracoscopic surgery followed by posterior instrumentation and fusion in spinal deformity. Eur Spine J. 2000;9(6):499-504.
54. Holcomb G.W., Mencio G.A., Green N.E. Video assisted thoracoscopic discectomy and fusion. J Pediatr Surg. 1997;32(7):1120-1122.
55. Regan J.J., Mack M.J., Picetti G.D., et al. A comparison of video-assisted thoracoscopy surgery (VATS) with open thoracotomy in the thoracic spinal surgery. Today’s Therapeutic Trends. 1994;11:203-218.
56. Rosenthal D., Dickman C.A. Thoracoscopic microsurgical excision of herniated thoracic discs. J Neurosurg. 1998;89(2):224-235.
57. Carson J., Gumpert J., Jefferson A. Diagnosis and treatment of thoracic disc protrusions. J Neuro Neurosurg Psychiatry. 1971;34:68-77.
58. Arce C.A., Dohrman G.J. Thoracic disc herniation. Improved diagnosis with CT scanning and a review of the literature. Surg Neurol. 1985;23:356-361.
59. Arseni C., Nash F. Thoracic intervertebral disc protrusion. A clinical study. J Neurosurg. 1960;17:418-430.
60. Resnick D.K., Benzel E.C. Lateral extracavitary approach for thoracic and thoracolumbar spine trauma. Operative complications. Neurosurgery. 1998;43:796-803.
61. Regan J.J., Mack M.J. Endoscopic thoracic fusion cage. In: Regan J.J., McAfee P.J., Mack M.J., editors. Atlas of endoscopic spine surgery. St Louis: Quality Medical; 1995:130.
62. Horowitz M.B., Moossy J.J., Julian T., et al. Thoracic discectomy using video assisted thoracoscopy. Spine (Phila Pa 1976). 1994;19:1082-1086.
63. Oskouian R.J.Jr., Johnson J.P., Regan J.J. Thoracoscopic microdiscectomy. Neurosurgery. 2002;50(1):103-109.
64. Waisman M., Saute M. Thoracoscopic spine release before posterior instrumentation in scoliosis. Clin Orthop Relat Res. 1997;336:130-136.
65. Rosenthal D. Endoscopic internal fixation of the thoracic spine. In: Regan J.J., McAfee P.J., Mack M.J., editors. Atlas of endoscopic spine surgery. St Louis: Quality Medical; 1995:333.
66. Konno S., Yabuki S., Kinoshita T., Kikuchi S. Combined laminectomy and thoracoscopic resection of dumbbell-type thoracic cord tumor. Spine (Phila Pa 1976). 2001;26(6):E130-E134.
67. van Dijk M., Cuesta M.A., Wuisman P.I. Thoracoscopically assisted total en bloc spondylectomy: two case reports. Surg Endosc. 2000;14(9):849-852.
68. McLain R.F. Spinal cord decompression. An endoscopically assisted approach for metastatic tumors. Spinal Cord. 2001;29:482-487.
69. Huang T.J., Scu R.W., Hsu K.Y., et al. Video-assisted thoracoscopic treatment of spinal lesions in the thoracolumbar junction. Surg Endosc. 1997;11:1189-1193.
70. Huang T.J., Hsu R.W., Chen Y.J. Minimal-access surgery in managing osteoporotic vertebral fractures with neurological deficits: a preliminary report. Changgeng Yi Xue Za Zhi. 2000;23(9):542-549.
71. Eismont F.J., Bohlman H.H., Soni P.L., et al. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg [Am]. 1983;65:19-29.
72. Tan H.L., McMurrick P.J., Merriman T.E., Torode I.P. Thoracoscopic biopsy of a pathological vertebral body. Aust NZ J Surg. 1994;64:726-728.
73. Rouch T.F., Crawford A.H., Berlin R.E., Wolff R.K. Tension pneumothorax as a complication of video-assisted thoracoscopic surgery for anterior correction of idiopathic scoliosis in an adolescent female. Spine (Phila Pa 1976). 2001;26(4):448-450.
74. Assaker R., Cinquin P., Cotten A., Lejeune J.P. Image-guided endoscopic spine surgery: Part I. A feasibility study. Spine (Phila Pa 1976). 2001;26(15):1705-1710.
75. Assaker R., Reyns N., Pertruzon B., Lejeune J.P. Image-guided endoscopic spine surgery. Part II: clinical applications. Spine (Phila Pa 1976). 2001;26(15):1711-1718.
76. Huang T.J., Hsu W.W., Liu H.P., et al. Analysis of techniques for video-assisted thoracoscopic internal fixation of the spine. Arch Orthop Trauma Surg. 1998;117:92-95.
77. Hulme A. The surgical approach to thoracic intervertebral disc protrusions. J Neuro Neurosurg Psychiatry. 1960;23:133-137.
78. Larson S.J., Holst R.A., Hemmy D.C., et al. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
79. Osman S.G., Marsolais E.B. Posterolateral arthroscopic discectomies of the thoracic and lumbar spine. Clin Orthop Relat Res. 1994;304:122-129.
80. Jho H.D. Endoscopic transpedicular thoracic discectomy. J Neurosurg (Spine). 1999;91:151-156.
81. Liu J.C., Ondra S.L., Angelos P., et al. Is laparoscopic anterior lumbar interbody fusion a useful minimally invasive procedure? Neurosurgery Nov. 2002;51(Suppl 5):S155-S158.
82. Smith J.S., Ogden A.T., Shafizadeh S., et al. Clinical outcomes after microendoscopic discectomy for recurrent lumbar disc herniation. J Spinal Disord Tech. 2010;23(1):30-34.
83. Khoo L.T., Fessler R.G. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(Suppl 5):S146-S154.
84. Bresnahan L., Ogden A.T., Natarajan R.N., et al. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976). 2009;34(1):17-23.
85. Sairyo K., Sakai T., Higashino K., et al. Complications of endoscopic lumbar decompression surgery. Minim Invasive Neurosurg. 2010;53(4):175-178.
86. Arts M.P., Brand R., van den Akker M.E., et al. Tubular diskectomy vs conventional microdiskectomy for sciatica: a randomized controlled trial. Leiden, The Hague Spine Intervention Prognostic Study Group (SIPS). JAMA. 2009;302(2):149-158.
87. Righesso O., Falavigna A., Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: results of a randomized controlled trial. Neurosurgery. 2007;61(3):545-549.
88. Ryang Y.M., Oertel M.F., Mayfrank L., et al. Standard open microdiscectomy versus minimal access trocar microdiscectomy: results of a prospective randomized study. Neurosurgery. 2008;62(1):174-181. discussion 181–182
89. Mayer H.M., Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg. 1993;78(2):216-225.
90. O’Toole J.E., Eichholz K.M., et al. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine. 2009;11(4):471-476.
91. Gala V.C., O’Toole J.E., Voyadzis J.M., et al. Posterior minimally invasive approaches for the cervical spine. Orthop Clin North Am. 2007;38(3):339-349.
92. Santiago P., Fessler R.G. Minimally invasive surgery for the management of cervical spondylosis. Neurosurgery. 2007;60(1 Supp1 1):S160-S165.