Chapter 126 Minimally Invasive Spinal Decompression and Stabilization Techniques I
The evolution of minimally invasive spinal surgery for decompression of the neural structures began with the uniportal procedures, using the arthroscope for decompression of contained disc herniations. The first laparoscopic lumbar discectomy was reported by Obenheim in 1991.1 The efficacy of different endoscopic surgical procedures has been documented, leading to the development of more complex and biportal arthroscopic procedures for treatment of noncontained herniations.
The use of minimally invasive surgery for fusion of motion segments of the spine was introduced at a later date. Magerl introduced this technique for percutaneous external transpedicular fixation of the thoracic and lumbar spine in the 1980s.2 Percutaneous dorsolateral interbody fusion also was performed successfully by Leu and Schreiber, who reported on the procedure in 1991.3 Drawbacks of these procedures included the likelihood of screw tract infection and discomfort associated with externally placed implants.
Leu4 was one of the first to use endoscopy for spinal fusion, both ventrally and dorsolaterally. Endoscopic spinal fusion was performed first in the lumbar spine. Interest in the use of minimally invasive surgery for thoracic spine disease has increased recently. The initial results, using video-assisted thoracoscopic surgery (VATS), are encouraging, because this procedure is characterized by less pain and shorter hospital stays.5–7
Regan et al.8–10 reported their results in thoracic spinal pathology using ventral and dorsal interbody grafting, with and without instrumentation. Rosenthal11 reported the use of VATS for ventral decompression and stabilization in patients with metastatic tumors or scoliotic deformities of the thoracic spine. His technique involves endoscopic microsurgical decompression, combined with reconstructive techniques and instrumentation placed through thoracoscopic portals.
Advantages and Difficulties
The most significant disadvantage of endoscopic stabilization is that it is time consuming. This aspect can be overcome, but there is a considerable learning curve. The technology and equipment costs for this approach also create a large “up front” investment requirement. All endoscopic approaches, especially thoracoscopic approaches, can be converted to open procedures, if necessary, to control bleeding or to deal with excessive adhesions.
Indications and Contraindications
Contraindications to endoscopic dorsolateral lumbar spine fusion and stabilization include (1) considerable loss of intervertebral disc height, preventing decortication of the end plates; (2) severe spinal deformity associated with distorted neural and pedicular anatomy; (3) infection; (4) failed previous operation for interbody fusion; and (5) very large tumors requiring extensive resection.12
Contraindications to VATS include (1) inability to tolerate deflation of one lung, (2) significant respiratory disease, and (3) previous open thoracotomy.13
Contraindications to laparoscopic transperitoneal lumbar fusion and stabilization include (1) significant abdominal trauma, (2) previous transabdominal lumbar operation, and (3) previous lower abdominal laparoscopic procedure (e.g., hysterectomy).14
Thoracic Spine
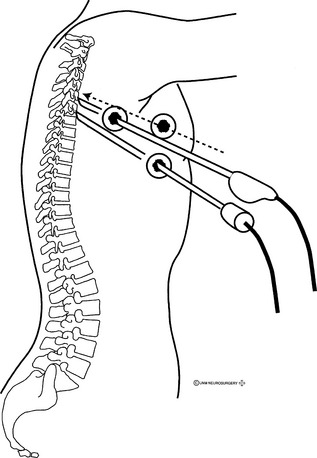
The most important minimally invasive technique for decompression and stabilization of the thoracic spine is VATS. This technique has been applied to a variety of thoracic spine disorders, including tumor, infection, disc disease, and deformity. VATS is performed using a double-lumen tube for deflation of the ipsilateral lung with the patient under general anesthesia and in the lateral decubitus position (Fig. 126-1).
Method
The surgical levels are identified by counting ribs, preferably with fluoroscopy, and by marking in the disc space. Alternatively, ribs may be counted endoscopically from the first rib down. The rib number corresponds to the lower vertebral body at the disc space (e.g., sixth rib at T5-6). Adequate exposure of the disc space usually requires resection of the rib head, except in the lower thoracic region where the rib head may be well caudal to the disc space, permitting unobstructed access. Attention to the segmental vascular branches in the mid-bodies is advised. Stabilization across the vertebral body requires the careful division of these vascular structures. The sympathetic chain also may be identified in the surgical field through the parietal pleura. Varying anatomy of the regions of the thoracic spine dictates different exposure techniques. For the upper thoracic region, it may be necessary to elevate and support the ipsilateral arm to rotate the scapula away. In the lower thoracic region, it may be necessary to retract the diaphragm.
On completion of the decompression, fusion can be performed using bone chips obtained from a rib or harvested from the iliac crest. Regan et al.10 described the placement of an interbody cage into the disc space after decompression.
Rosenthal11 described reconstruction by homologous bone or by injection of semiliquid methylmethacrylate. He used a ventral plate and screw system (Z-plate, Sofamor-Danek, Memphis, TN) for fixation and special equipment for dilation of the skin incision during the insertion of the plate, and for insertion of instruments for handling the plates and screws in the chest cavity. These techniques allow the surgeon to address pathology resulting from degenerative disease, trauma, or metastatic disease, and then to stabilize the spine with methylmethacrylate struts, cages, or plates.11,15–17
Complications
As with laparoscopic procedures, there is an entire complement of risks associated with the intrathoracic approach. Reported complications include prolonged atelectasis, pleural effusions, intercostal neuralgia, and diaphragmatic injury.15–17 Time of lung collapse (i.e., length of operation) is related to the pulmonary morbidity associated with chest procedures. Therefore, until the operating surgeon has become familiar with the thoracoscopic spine procedures, he or she may expect longer operating times and some increased morbidity. In one series of thoracic endoscopic discectomies, the complication rate was 14%, which was compatible with the reported complication rate with open approaches.18 The use of flexible portals may reduce the incidence of intercostal neuralgia, although this still occurred in 2 of 17 patients in whom the flexible portals were used.15 Complications related to the decompression, in a series of 77 patients, were excessive epidural bleeding in one patient and transient paraparesis in another.16 In a comparative study, Mangione et al. reported less blood loss and a shorter hospital stay, as well as a similar rate of complication after VATS, when compared with open thoracoscopic thoracic spine decompression.19
Lumbar Spine
Fusion and Instrumentation
Laparoscopic Transperitoneal Surgery for the Lumbar Spine
Despite limited enthusiasm for laparoscopic ventral discectomy for the treatment of simple disc disease,10 interbody fusion techniques have received a warm welcome, as evidenced by the number of meeting presentations and papers on the topic.16,20–26 The procedure has evolved from the placement of bone graft in the interspace to the use of titanium interbody distraction cages.
Technique
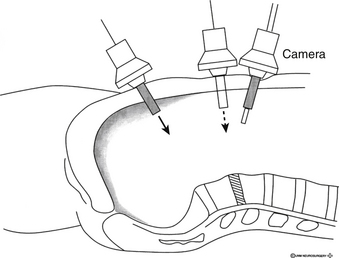
The ventral exposure of the lumbar spine from the peritoneal cavity is limited to L5-S1 and variably to L4-5. Laparoscopic exposure is performed in the routine manner, similar to an intra-abdominal procedure. This includes a bowel preparation, insertion of a Foley catheter and a nasogastric tube, and preincisional prophylactic antibiotics. The patient is supine in the Trendelenburg position, with the back extended using large rolls under the lumbosacral junction (Fig. 126-2). Insufflation techniques are standard, but a gasless technique also is described, which does not require specially designed instruments.27 Assistance from a general surgeon comfortable with laparoscopy is advised.
Discectomy is performed by sharp incision of the ventral anulus fibrosus and by radical removal of disc material with curets and pituitary forceps. Fusion proceeds by distraction of the disc space and insertion of bone graft under compression. Currently popular is the use of a threaded titanium cage, which has been cleared by the U.S. Food and Drug Administration and is commercially available for open procedures. It also is cleared for endoscopic placement from the ventral approach. With the endoscopic placement, the procedure is outlined as follows: an estimate of graft size is made on the basis of the preoperative radiographs and specific templates provided by the manufacturer of the device. The sizes are checked in situ to confirm that adequate exposure is available. Next, a disc space distractor is placed on one side of the midline. On the other side, a circular hole is drilled (under fluoroscopic guidance to ensure a trajectory parallel to the vertebral end plates) to a depth of approximately two thirds of the diameter of the disc space. The hole is then tapped, and the titanium implant, filled with autologous bone chips, is screwed into the disc space. The distractor is then removed, and the tapping/implant procedure is repeated at the site previously occupied by the distractor. The implants should be sufficiently countersunk so that no aspect comes into contact with the overlying vascular structures. The peritoneum is then reapproximated over the site. The exposure and anatomy may dictate that only one graft be placed. However, this is not as biomechanically sound as two cages, placed side by side, especially in lateral flexion.20 Currently, other materials such as threaded allograft dowels also are available for transperitoneal endoscopic fusion operations.
A retroperitoneal approach to the ventral aspect of the spine at levels L4-5 and L5-S1 with interbody fusion also is technically feasible. This reduces the chance of postoperative adynamic ileus and intra-abdominal adhesions. This approach also provides access to the lateral aspects of level L1-4, although the great vessels prevent midline access at these levels. Previous abdominal surgery is a relative contraindication for the transperitoneal exposure. This is not an issue with the retroperitoneal approach. An experience with 20 cases has been reported.24
Complications
Complications associated with transperitoneal exposure are uncommon. However, they require immediate management by experienced abdominal surgeons. An experience with 17 cases included significant ileus in four patients, which required, in two patients, an open laparotomy for bleeding. In addition, there were two graft donor site infections.26 In another series of 22 cases, 1 iliac vein laceration was encountered, as well as 2 bone donor site infections.16 In the small series of ventral retroperitoneal fusions, no significant complications were reported.24 Sexual dysfunction is a dreaded complication of this approach, resulting from disruption of the sacral autonomic plexus. Several cases of transient (3 weeks) retrograde ejaculation were reported in a series using an open anterior lumbar interbody fusion technique.20 It is recommended that, in men, reflection of the parietal peritoneum over the ventral disc space be performed with the aid of bipolar rather than monopolar electrocautery to prevent the spread of current and reduce the risk of damage to the autonomic plexus.20,27 If Steinmann pins are used, care must be taken during removal, as well as insertion, so that the sharp tip does not lacerate a vessel, particularly the iliac vein. The hazards of the approach and drilling the disc space adjacent to the iliac vessels are ameliorated with special instruments to protect the vessels during drilling. Familiarity with these instruments is imperative.
Results
Although the total experience with minimally invasive techniques in spine surgery is small in comparison with the vast experience with open procedures, the early results are encouraging (Table 126-1). Follow-up of these patients, to date, has been relatively short. Mathews et al.21 reported successful fusion in all of their first five cases. McAfee et al.,28 reporting the complications in the first 50 endoscopic procedures in a multicenter study, did not observe any great vessel complications. The complications reported by other authors include cage extrusion, inferior vena cava laceration, dorsal disc extrusion, prolonged ileus, and atelectasis. Zucherman and Zdeblick29 and Novotny et al.23 reported that 2 of 23 (9%) and 2 of 8 (25%) operated cases, respectively, were converted to open surgery.
TABLE 126-1 Recently Reported Series of Patients Undergoing Fusion and Stabilization via Endoscopic Procedures
| Author | No. of Patients | Procedure |
|---|---|---|
| Mathews et al. (1991)39 | 4 | ALIF |
| Novotny et al. (1994)23 | 8 | TP-ALIF with cage or femoral allograft |
| McAfee et al. (1994)22 | 10 | TP fusion |
| Mathews et al. (1994)21 | 5 | TP with iliac crest |
| Kambin and Schaffer (1996)12 | 25 | Lumbar posterolateral |
| Zuckerman et al. (1996)14 | 23 | TP with cage implant |
| Rosenthal et al. (1996)17 | 12 | Thoracoscopic with ventral plate |
| Regan (1996)18 | 7 | Thoracoscopic with anterior instrumentation |
| Dickman et al. (1996)5 | 1 | Thoracoscopic fusion |
ALIF, anterior lumbar interbody fusion; TP, transperitoneal.
Note that cases involving decompression only are excluded from the table.
The first results of minimally invasive surgery for spine decompression and stabilization are encouraging. However, some problems persist, including long operation time, the requirement for expensive equipment, and the steep learning curve.30 With increased experience, the rate of complications should decrease. Technologic advances should improve the safety of these operations and also may broaden the indications for the application of these techniques.
Percutaneous Translaminar Facet Screw Fixation
Percutaneous translaminar facet screw fixation using fluoroscopy was performed successfully.31,32 Shim et al.32 reported that this technique was a safe and useful minimally invasive dorsal augmentation method following ALIF or axiALIF.
Robotic Instrumentation
Robotic systems currently are used in a few spine centers for transpedicular and translaminar screw fixations. The results show an average accuracy level of within 1 mm relative to the surgeon’s plan in both pedicle screw and translaminar facet screw applications in most of the procedures. Pechlivanis et al.33 reported a deviation of less than 2 mm in 91.0% of cases. The major disadvantage of robotic systems is the increased cost; the major advantages include reduced radiation exposure and increased accuracy.
Extreme Lateral Approach for Lumbar Interbody Fusion
The extreme lateral approach for lumbar interbody fusion (XLIF), a modification of the retroperitoneal approach to the lumbar spine, was first presented in 2001 by Pimenta, who has performed more than 100 lateral trans-psoas surgeries since 1998. The equipment currently used in this procedure does not require additional capital expenditure. An operative microscope may be used but is not required. The XLIF technique can be safely used for interbody fusion of L3-4 and L4-5. Because of the location of the iliac bone, however, this technique cannot be applied to the L5-S1 level. Ozgur et al.34 reported successful results using the XLIF technique.
Transforaminal Lumbar Interbody Fusion
Transforaminal lumbar interbody fusion (TLIF) also is used for interbody fusion of the lumbosacral spine. The technique requires a lumbar paramedian incision, paravertebral dissection, facet resection, discectomy, and cage placement. The upper and lower lumbar vertebrae are commonly instrumented by ipsilateral transpedicular screws.21,35,36
Axial Interbody Fusion
Axial interbody fusion (AxiALIF), recently developed for L5-S1 interbody fusion, uses a percutaneous paracoccygeal approach. It is indicated for minor instabilities of the L5-S1 level, for L5-S1 discogenic pains, and for pseudarthrosis of L1 screws. It can be used alone or in combination with minimally invasive L5-S1 translaminal facet screw fixation or L5-S1 transpedicular instrumentation. Early results are encouraging.37,38
Lumbar Discectomy
With or without laser nucleotomy, lumbar discectomy is the most commonly used minimally invasive technique.39 Three major pathways are used to perform lumbar discectomy: dorsolateral uni- or biportal discectomy, transforaminal endoscopic discectomy, and dorsal midline endoscopic discectomy.40–42 Dorsolateral uni- or biportal discectomy is the earliest technique, using a small incision 8 cm lateral to the midline and almost 45 degrees of working canal. A midline approach is similar to the microdiscectomy technique, whereas the transforaminal technique approaches the neural foramen first. Endoscopic discectomy provides better pain relief, shorter hospitalization stays, earlier return to work, and less paraspinal muscle atrophy.43,44 Ruetten at al.,40 using a transforaminal technique, reported that patients had a lower rate of low back pain after the endoscopic procedure when compared with microdiscectomy.
Complications of Endoscopic Discectomy
Complications associated with percutaneous lumbar discectomy are few, but include a 1% to 2% risk of discitis and a 1% to 2% risk of a symptomatic psoas muscle hematoma. Anecdotal evidence indicates that there is a risk of injury to the nerve roots or surrounding vascular structures.41,45–47 As is emphasized elsewhere in this textbook, careful placement of the guidewire is crucial for the avoidance of complications with this approach. Theoretically, the risks of infection and injury to the great vessels are increased by the manipulations of bone grafting. However, this has not been demonstrated clinically.
Cervical Spine Decompression
Despite advances in available technology and interest in minimally invasive stabilization procedures in the thoracic and lumbar spine, relatively few reports on the use of these methods in the cervical spine have been published. Percutaneous discectomy and chemonucleolysis of the cervical spine reportedly have been effective in selected patients in uncontrolled trials.48,49 Percutaneous disc access requires a ventrolateral approach. The needle entry is at the medial border of the sternocleidomastoid muscle. With the fingers of one hand acting to separate the carotid sheath and esophagus, the needle is passed under fluoroscopic control into the desired disc space. Although percutaneous decompression has been described, concomitant fusion has not been reported to date.
Microendoscopic technique has been popularized during recent years. Using a small incision for a mini-keyhole foraminotomy, nerve root can be exposed and decompression for both spondylotic changes and disc herniation can be performed.50,51 Fessler and Khoo50 reported less blood loss, shorter hospitalization stays, less use of narcotics, and better pain relief after cervical microendoscopic foraminotomy when compared with the open technique.
Percutaneous surgical techniques have been recently popularized in craniovertebral junction surgery. The first results after percutaneous odontoid screw fixation and C1-2 fixation are encouraging.52,53
Dickman C.A., Rosenthal D., Karahalios D.G., et al. Thoracic vertebrectomy and reconstruction using a microsurgical thoracoscopic approach. Neurosurgery. 1996;38:279-293.
Fessler R.G., Khoo L.T. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51(Suppl 5):S37-S45.
Mac M.J., Regan J.J., Bobechko W.P., Acuff T.E. Application of thoracoscopy for disease of the spine. Ann Thorac Surg. 1993;56:736-738.
Ruetten S., Komp M., Merk H., Godolias G. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop. 2009;33:1677-1682.
Sachs B.L., Schwaitzberg S.D. Lumbosacral (L5-S1) discectomy and interbody fusion technique. In: Regan J.J., McAfee P.C., Mack M.J., editors. Atlas of endoscopic spine surgery. St Louis: Quality Medical Publishing; 1995:275.
Shim C.S., Lee S.H., Jung B., et al. Fluoroscopically assisted percutaneous translaminar facet screw fixation following anterior lumbar interbody fusion: technical report. Spine. 2005;30:838-843.
1. Obenheim T.G. Laparoscopic lumbar discectomy: case report. J Laparoendosc Surg. 1991;1:145-149.
2. Magerl F.P. Stabilization of the lower thoracic and lumbar spine with external skeletal fixation. Clin Orthop Relat Res. 1984;189:125-141.
3. Leu H.J., Schreiber A. Percutaneous lumbar restabilization. In: Kambin P., editor. Arthroscopic microdiscectomy: minimal intervention in spinal surgery. Baltimore: Urban & Schwarzenberg; 1991:p 123.
4. Leu H., Hauser R., Schreiber A. Percutaneous lumbar spine fusion. Acta Orthop Scand. 1993;64(suppl 251):116-119.
5. Dickman C.A., Mican C.A. Multilevel anterior thoracic discectomies and anterior interbody fusion using a microsurgical thoracoscopic approach: a case report. J Neurosurg. 1996;84:104-109.
6. Mac M.J., Regan J.J., Bobechko W.P., Acuff T.E. Application of thoracoscopy for disease of the spine. Ann Thorac Surg. 1993;56:736-738.
7. Rosenthal D., Rosenthal R., de Simone A. Removal of a protruded thoracic disc using microsurgical endoscopy. A new technique. Spine. 1994;19:1087-1091.
8. Regan J.J. Endoscopic applications of the BAK system (L4-5). In: Regan J.J., McAfee P.J., Mack M.J., editors. Atlas of endoscopic spine surgery. St. Louis: Quality Medical Publishing; 1995:321.
9. Regan J.J., Mack M.J. Endoscopic thoracic fusion cage. In: Regan J.J., McAfee P.J., Mack M.J., editors. Atlas of endoscopic spine surgery. St. Louis: Quality Medical Publishing; 1995:350.
10. Regan J.J., Mack M.J., Picetti G.D. A technical report on video-assisted thoracoscopy in thoracic spinal surgery. Preliminary description. Spine. 1995;20:831-837.
11. Rosenthal D. Endoscopic internal fixation of the thoracic spine. In: Regan J.J., McAfee P.J., Mack M.J., editors. Atlas of endoscopic spine surgery. St Louis: Quality Medical Publishing; 1995:333.
12. Kambin P., Schaffer J.L. Arthroscopic fusion of the lumbosacral spine. In: Margulies J.Y., Floman Y., Farcy J.P., Neuwirth M.G., editors. Lumbosacral and spinopelvic fixation. Philadelphia: Lippincott-Raven; 1996:565.
13. Horowitz M.B., Moossy J.J., Julian T., et al. Thoracic discectomy using video assisted thoracoscopy. Spine. 1994;19:1082-1086.
14. Zuckerman J.F., Hsu K., Implacito D. Instrumented transperitoneal laparoscopic fusion. In: Margulies J.Y., Floman Y., Farcy J.P., Neuwirth M.G., editors. Lumbosacral and spinopelvic fixation. Philadelphia: Lippincott-Raven; 1996:579.
15. Dickman C.A., Rosenthal D., Karahalios D.G., et al. Thoracic vertebrectomy and reconstruction using a microsurgical thoracoscopic approach. Neurosurgery. 1996;38:279-293.
16. McAfee P.C., Regan J.R., Zdeblick T., et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine. 1995;20:1624-1632.
17. Rosenthal D., Marquardt G., Lorenz R., Nichtweiss M. Anterior decompression and stabilization using a microsurgical endoscopic technique for metastatic tumors of the thoracic spine. J Neurosurg. 1996;84:565-572.
18. Regan J.J. Percutaneous endoscopic thoracic discectomy. Neurosurg Clin North Am. 1996;7:87.
19. Mangione P., Vadier F., Sénégas J. Thoracoscopy versus thoracotomy in spinal surgery: comparison of 2 paired series. Rev Chir Orthop Reparatrice Appar Mot. 1999;85:574-580.
20. Kuslich S.D., McAfee P.C., Regan J.J. Spinal instrumentation. In: Regan J.J., McAfee P.C., Mack M.J., editors. Atlas of endoscopic spine surgery. St Louis: Quality Medical Publishing; 1995:293.
21. Mathews HH, Evans MT, Kyles MK, et al: Laparoscopic discectomy with fusion. In Proceedings of the 9th Annual Meeting of the North American Spine Society, Minneapolis, October 1994, p 63.
22. McAfee PC, Fedder IL, Geis P, et al: Laparoscopic lumbar spine surgery. In Proceedings of the 9th Annual Meeting of the North American Spine Society, Minneapolis, October 1994, p 63.
23. Novotney SR, Guyer RD, Regan JJ, Ohnmeiss DD: Laparoscopic-assisted anterior lumbar interbody fusion. In Proceedings of the 9th Annual Meeting of the North American Spine Society, Minneapolis, October 1994, p 61.
24. Ominus M., Papin P., Gangloff S. Extraperitoneal approach to the lumbar spine with video assistance. Spine. 1996;21:2491-2494.
25. Sachs B.L., Schwaitzberg S.D. Lumbosacral (L5-S1) discectomy and interbody fusion technique. In: Regan J.J., McAfee P.C., Mack M.J., editors. Atlas of endoscopic spine surgery. St Louis: Quality Medical Publishing; 1995:p 275.
26. Zucherman J.F., Zdeblick T.A., Bailey S.A., et al. Instrumented laparoscopic spinal fusion: preliminary results. Spine. 1995;20:2029-2034.
27. Transfeldt E.E., Schultz L. Approach to the lumbar spine without insufflation. In: Regan J.J., McAfee P.C., Mack M.J., editors. Atlas of endoscopic spine surgery. St Louis: Quality Medical Publishing; 1995:137.
28. McAfee PC, Regan J, Picetti G: The incidence of complications in endoscopic anterior thoracic spinal reconstructive surgery. A prospective multicenter study comprising the first 50 consecutive cases. In Proceedings of the 9th Annual Meeting of the North American Spine Society, Minneapolis, October 1994, p 64.
29. Zuckerman JF, Zdeblick T: Instrumented laparoscopic spinal fusion. In Proceedings of the 9th Annual Meeting of the North American Spine Society, Minneapolis, October 1994, p 66.
30. Leu H.F., Hauser R.K. Percutaneous endoscopic lumbar spine fusion. Neurosurg Clin North Am. 1996;7:107-117.
31. Rajasekaran S., Naresh-Babu J. Translaminar facetal screw (Magerl’s) fixation. Neurol India. 2005;53:520-524.
32. Shim C.S., Lee S.H., Jung B., et al. Fluoroscopically assisted percutaneous translaminar facet screw fixation following anterior lumbar interbody fusion: technical report. Spine. 2005;30:838-843.
33. Pechlivanis I., Kiriyanthan G., Engelhardt M., et al. Percutaneous placement of pedicle screws in the lumbar spine using a bone mounted miniature robotic system: first experiences and accuracy of screw placement. Spine. 2009;34:392-398.
34. Ozgur B.M., Aryan H.E., Pimenta L., Taylor W.R. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
35. Jagannathan J., Sansur C.A., Oskouian R.J.Jr., et al. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64:955-963.
36. Peng C.W., Yue W.M., Poh S.Y., et al. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34:1385-1389.
37. Aryan H.E., Newman C.B., Gold J.J., et al. Percutaneous axial lumbar interbody fusion (AxiaLIF) of the L5-S1 segment: initial clinical and radiographic experience. Minim Invasive Neurosurg. 2008;51:225-230.
38. Marotta N., Cosar M., Pimenta L., Khoo L.T. A novel minimally invasive presacral approach and instrumentation technique for anterior L5-S1 intervertebral discectomy and fusion: technical description and case presentations. Neurosurg Focus. 2006;20(1):E9.
39. Mathews HH: Presented at the First International Symposium on Lasers in Orthopaedics, San Francisco, September 1991.
40. Ruetten S., Komp M., Merk H., Godolias G. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop. 2009;33:1677-1682.
41. Schaffer J.L., Kambin P. Percutaneous posterolateral lumbar discectomy and decompression with a 6.9 millimeter cannula. Analysis of operative failures and complications. J Bone Joint Surg [Am]. 1991;73:822-831.
42. Schreiber A., Leu H. Percutaneous nucleotomy: technique with discoscopy. Orthopedics. 1991;14:439-444.
43. Kambin P. Posterolateral percutaneous lumbar interbody fusion. In: Kambin P., editor. Arthroscopic microdiscectomy: minimal intervention in spinal surgery. Baltimore: Urban & Schwarzenberg; 1991:117.
44. Sihvonen T., Herno A., Paljarvi L. Local denervation atrophy of paraspinal muscles in post-operative failed back syndrome. Spine. 1993;18:575-581.
45. Epstein N.E. Surgically confirmed cauda equina and nerve root injury following percutaneous discectomy at an outside institution: a case report. J Spinal Disord. 1990;3:380-382.
46. Onik G., Mooney V., Maroon J.C., et al. Automated percutaneous discectomy: a prospective multi-institutional study. Neurosurgery. 1990;26:228-232.
47. Savitz M.H. Same-day microsurgical arthroscopic lateral-approach laser-assisted (SMALL) fluoroscopic discectomy. J Neurosurg. 1994;80:1039-1045.
48. Gómez-Castresana F., Vázquez Herrero C., Baltés Horche J.L. Rodriguez-Navia IM: Cervical chymopapain nucleolysis: MR imaging assessment of chymopapain efficacy. Neurosurg Clin North Am. 1996;7:1-16.
49. Hoogland T., Scheckenbach C. Low-dose chemonucleolysis combined with percutaneous nucleotomy in herniated cervical disks. J Spinal Disord. 1995;8:228-232.
50. Fessler R.G., Khoo L.T. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51(Suppl 5):S37-S45.
51. Kim C.H., Chung C.K., Kim H.J., et al. Early outcome of posterior cervical endoscopic discectomy: an alternative treatment choice for physically/socially active patients. J Korean Med Sci. 2009;24:302-306.
52. Börm W., König R.W., Albrecht A., et al. Percutaneous transarticular atlantoaxial screw fixation using a cannulated screw system and image guidance. Minim Invasive Neurosurg. 2004;47:111-114.
53. Sucu H.K., Akkol I., Minoğlu M., Gelal F. Percutaneous anterior odontoid screw fixation. Minim Invasive Neurosurg. 2008;51:106-108.