Chapter 153 Minimally Invasive Posterior Cervical Foraminotomy and Microdiscectomy
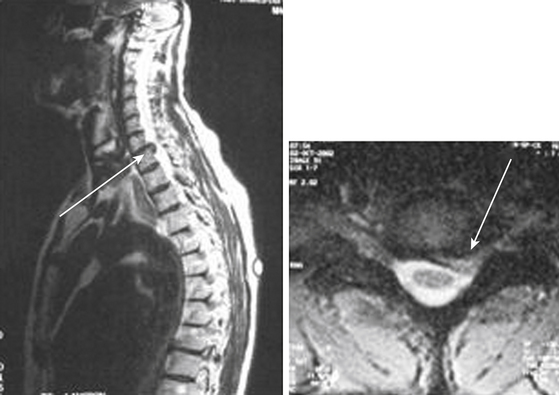
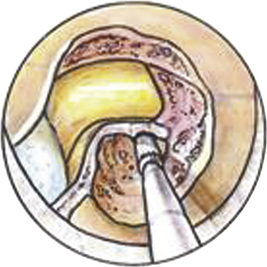
Posterior cervical foraminotomy and microdiscectomy is a safe and effective procedure that can be used in the treatment of clinically significant foraminal stenosis resulting from lateral disc herniation or osteophytes. Symptoms improve in more than 90% of patients who undergo the procedure (Fig. 153-1).1–8 Until the late 1950s, this was the predominant approach used in the treatment of herniated cervical discs. In 1958, however, Cloward9 introduced an anterior approach to cervical discectomy, and popularity with this technique grew as modifications to the technique made it safe and easier to perform. In addition, patients experienced less postoperative pain and muscle spasm, they had shorter hospital stays, and surgery could be performed through a smaller incision with less muscle trauma.
Numerous series show that patients achieve good relief from their symptoms with the anterior approach.9–12 However, despite these advantages, the anterior approach is associated with the risk of numerous complications, including recurrent laryngeal nerve injury,13–16 dysphagia and speech dysfunction,17 esophageal injury,18 and airway compromise,19–21 as well as injury to the carotid artery22 and jugular vein.23 In addition, anterior cervical discectomy has been implicated in both radiographic24 and clinically significant adjacent segment degenerative disease,25 as well as altering the native biomechanics of the cervical spine that may result from discectomy and fusion.26,27
Because the posterior approach avoids a complete discectomy and fusion, it remains a viable option in the treatment of lateral soft discs or osteophytes in the cervical region, and superb decompression of the lateral recess and neural foramen has been well documented using posterior laminoforaminotomy.1–5 This approach offers direct visualization of the exiting nerve root and avoids fusion, as is the case with anterior surgery.
Enthusiasm for the posterior approach is tempered by technical limitations that limit visualization of the distal foramen, in addition to generous bony and epidural venous bleeding. Significant muscle dissection also is necessary to obtain adequate surgical exposure, which increases intraoperative bleeding, as well as postoperative pain, muscle spasm, and hospital stay. Also, muscle denervation and retraction injury, as a result of stripping muscles from their point of attachment to the spine, recently have been shown to result in compromised biomechanical stability in the posterior cervical region.28 Negative long-term effects similar to those that may occur with muscle injury during lumbar spine surgery29–32 may also occur with muscle dissection during the approach to the posterior cervical spine.
As a result, posterior microendoscopic foraminotomy (MEF) was developed by Roh et al. to address cervical nerve root compression by direct visualization of pathology while minimizing tissue destruction upon exposure.33 Muscle and ligamentous attachments to the spine are preserved, thus decreasing postoperative pain and spasm and helping maintain long-term stability. Finally, fusion and its associated sequelae are avoided.
Initial cadaveric studies using this technique demonstrated that the average vertical and transverse diameter of the laminotomy defect was identical for MEF or open techniques.33 The average amount of facet removed and the extent of neural decompression was greater with MEF than with the open approach. Initial clinical results were quite favorable for the MEF procedure. Overall, the operative time, blood loss, length of hospitalization, and need for postoperative pain medications were all reduced with the MEF group compared with a similar group of patients who underwent the open procedure.34 Within the MEF group, although some surgeons35 advocate the prone position, we have found that changing from a prone to a semisitting position further improved these variables; compared with the open group, average operative time was reduced from 171 to 90 minutes and average blood loss was lowered from 246 to 28 ml. Moreover, the average hospital stay was reduced from 68 hours in the open group to 8 hours in the sitting MEF group.34 Recently, many series of MEF have shown similar results.33,36–40
We previously described our technique for performing a minimally invasive posterior cervical foraminotomy.41 As described here, lateral soft disc herniations can be treated using the MEF technique by performing a small resection of the superior medial pedicle, thereby allowing access to the herniated disc fragment. Over the last several years, we have preferred to use a microscope instead of an endoscope, because the microscope affords three-dimensional visualization of the anatomy as opposed to the endoscope, which is two dimensional. Other advantages of using the microscope over the endoscope include familiarity among neurosurgeons and improved optics for enhanced visualization of the operative field.
Positioning
Initially in the supine position, the patient’s head is affixed to the Mayfield head holder, and the patient’s body is gradually brought to a semisitting position such that the knees are level with the heart. The Mayfield adaptor is attached to the table between the hips and the knees and arched back as necessary to accommodate the patient’s body habitus, connecting to the Mayfield head holder. Care is taken to maintain the neck in a comfortable, neutral position. The surgeon should be able to pass two fingers below the chin to assure adequate venous drainage via the jugular veins. The arms are folded and secured across the chest or supported at the sides; pressure points are padded to prevent nerve palsies and pressure sores (Fig. 153-2). Precordial Doppler monitoring is used to monitor air emboli in the right atrium; a central venous catheter is usually not necessary because of the brevity and minimal blood loss associated with the procedure.
Localization
The fluoroscope is positioned to obtain a lateral view of the cervical spine. The midline is marked before drapes are applied by palpation of the spinous processes. The back of the neck is then prepped and draped in a routine surgical fashion. An 18-gauge spinal needle is used to identify the proper level approximately 1.5 cm lateral to the midline. A skin incision is then made over the level of interest. A small retractor is placed to visualize the fascia, and the fascia is cut in a rostral to caudal direction parallel to the spinous processes to expose the underlying muscle. In individuals with large necks, the spinous process can be palpated with a finger to help orient the location of the midline structures. In heavy patients or those with muscular shoulders, the lower levels of the cervical spine can be difficult to visualize. In these cases, the shoulders can be taped down to aid in visualization.
Approach
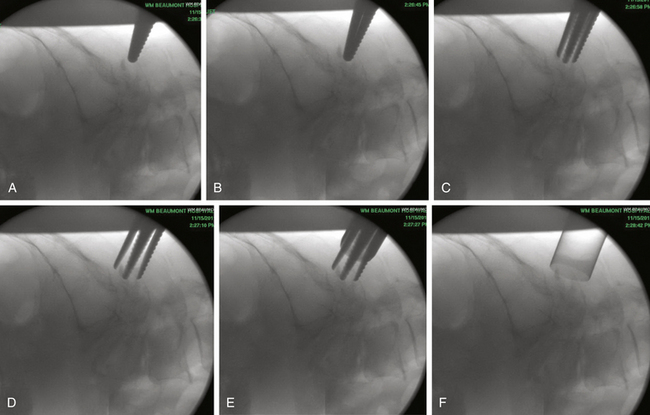
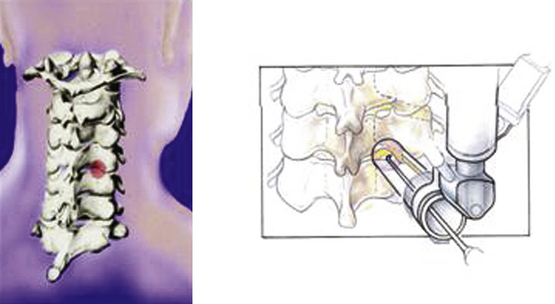
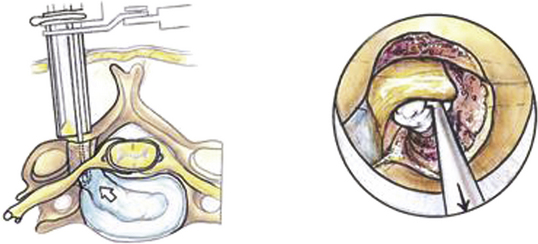
Under fluoroscopic guidance, a Kirschner (K) wire is carefully advanced toward the laminar facet junction. Care needs to be taken so that the K wire is not directed medially, where it can enter the interlaminar space and cause a spinal cord injury. It is therefore imperative to visualize the K wire and dilators as they approach the spine to prevent neurologic injury. In large, muscular individuals, we often stop advancing the K wire before docking on the facet and then pass the first muscle dilator, remove the K wire, and place subsequent muscle dilators and finally the tubular retractor, as shown in Fig. 153-3. Recent developments have resulted in minimal invasive muscle dilators that do not require a K-wire and subsequent muscle dilators to approach the spine (Fig. 153-4). This retractor system might help reduce iatrogenic neural injury.
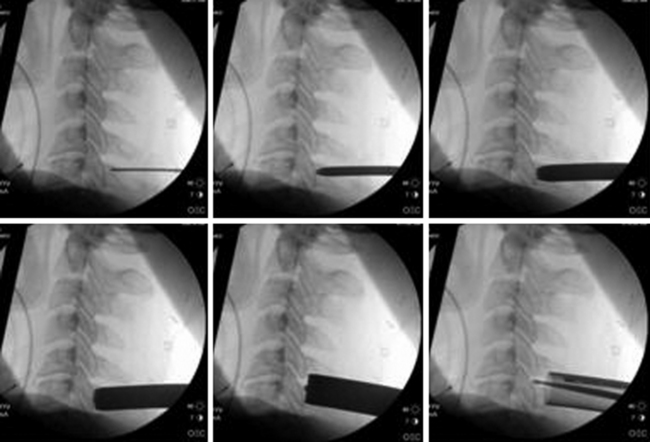
FIGURE 153-3 These images illustrate progressive dilation of the soft tissue in order to create a working channel. This process begins with insertion of a K-wire (top left), and ends with the placement of the tubular retractor (bottom left). A straight curette is seen in the tubular retractor in the bottom left image, and is used to assist in docking onto the lamino-facet complex as described in the text and as seen in Figure 153-4.
Once the tubular retractor has been placed, a straight curette can be used to dissect through the muscles to the level of the facet complex. After the facet is palpated, the first and subsequent muscle dilators are used again and the working corridor is “redilated,” over which the tubular retractor is advanced again to dock closer to the facet complex. The tubular retractor must be positioned directly over the facet complex to facilitate dissection, as shown in Fig. 153-5. We typically use an 18-mm port, which we find provides sufficient exposure of the facet complex and ample working space. If needed, confirmatory anteroposterior views are also obtained with a K wire to ensure that the laminofacet junction of the desired level is adequately localized.35
Once the tubular retractor is adjusted to the desired position under fluoroscopy, it is then fixed to a flexible arm mounted to the operating table. The microscope is then brought into the field, and the rest of the procedure is performed under high magnification.
Surgical Technique
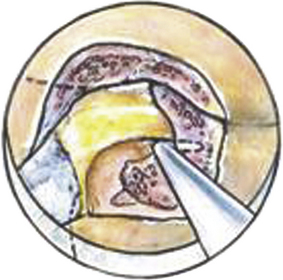
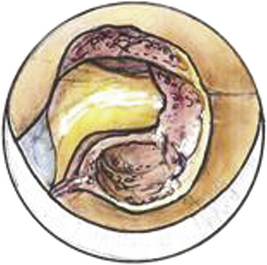
A Bovie cautery instrument on a long handle is used to circumferentially remove muscle from the operative field. It is best to work from lateral to medial and always be cognizant of the interlaminar space. The bony anatomy is further defined with a curette to appreciate the interlaminar space and facet relationship, as shown in Fig. 153-6. A 1- or 2-mm Kerrison punch is used to begin the laminotomy; this is extended laterally to complete the foraminotomy. It is often necessary to use a high-speed drill to help facilitate the foraminotomy. Epidural bleeding in this region is common but can be controlled with Gelfoam and cotton patties. Once the nerve root is visualized, a 45-degree, angled nerve hook can be used to palpate the neural foramen and confirm that the decompression is adequate, as shown in Fig. 153-7.
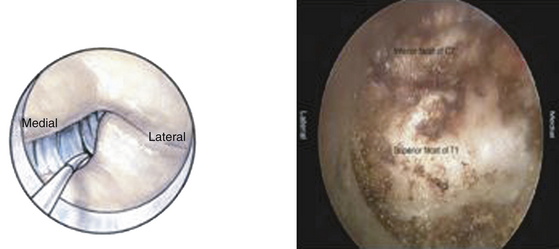
FIGURE 153-6 After removal of soft tissue, a curette is used to define the anatomy of the lamino-facet complex.
When performing a posterior discectomy, drilling approximately 2 mm of the superior medial pedicle facilitates safe removal of herniated disc fragments while limiting nerve root manipulation (Figs. 153-8 to 153-10). The space created by drilling the pedicle can also be used to reduce anterior osteophytes contributing to foraminal stenosis with the aid of a down-angled curette. Radiographic and tactile confirmation of the extent of discectomy or foraminotomy is obtained before closing.
Complications
Although many potential complications are possible, our experience has been associated with few complications. One of the complications that may result is a dural tear, which can easily be managed by local application of a dural regeneration matrix such as Duragen (Integra, Plainsboro, NJ) and fibrin glue and/or with short-term lumbar subarachnoid drain placement.35,36 In our experience, such management has been sufficient for repair, with no patient having recurrent cerebrospinal fluid leaks. Blood loss has been reduced to minimal levels by changing to the sitting position. Due to the negligible amount of bleeding, the risk of air emboli as a result of the sitting position is insignificant. Cord or root injury during dilation is avoided by dividing the cervical fascia before passing the dilators and removing the K wire after inserting the first dilator. At no time is the K wire pushed forcefully toward the spine; visualization of the K wire is done with spot fluoroscopic images. There have been cases of K wires causing spinal cord injury—as a result, patients should be informed of this complication during the preoperative visit.
Conclusion
Minimally invasive techniques have been used to perform posterior cervical foraminal decompression with good success. These approaches have several obvious advantages over more invasive open procedures: (1) reduced operative time, (2) decreased hospital stay, (3) decreased postoperative pain and muscle spasm, (4) decreased postoperative need for narcotic pain medication, (5) earlier return to work and normal activity, and most importantly, (6) minimal disruption of the integrity of the musculoligamentous structures of the posterior cervical spine. The decision of whether to utilize a minimally invasive posterior approach partly depends on the surgeon’s familiarity with the technique. There is a learning curve associated with the implementation of any new technique. We believe that posterior minimally invasive foraminotomy and microdiscectomy is a highly effective means of treating cervical radiculopathy while preserving the motion segment.
Adamson T.E. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95:51-57.
Aldrich F. Posterolateral microdisectomy for cervical monoradiculopathy caused by posterolateral soft cervical disc sequestration. J Neurosurg. 1990;72:370-377.
Apfelbaum R.I., Kriskovich M.D., Haller J.R. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25:2906-2912.
Aryan H.E., et al. Relaxation of forces needed to distract cervical vertebrae after discectomy: a biomechanical study. J Spinal Disord Tech. 2009;22:100-104.
Baba H., et al. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18:2167-2173.
Cloward R.B. The anterior approach for removal of ruptured cervical disks. J. Neurosurg. 1958;15:602-617.
Fager C.A. Management of cervical disc lesions and spondylosis by posterior approaches. Clin Neurosurg. 1977;24:488-507.
Henderson C.M., et al. Posterior–lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
Hilton D.L. Minimally invasive tubular access for posterior cervical foraminotomy with three-dimensional microscopic visualization and localization with anterior/posterior imaging. Spine J. 2007;7:154-158.
Holly L.T., et al. Minimally invasive 2-level posterior cervical foraminotomy: preliminary clinical results. J Spinal Disord Tech. 2007;20:20-24.
Jagannathan J., et al. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: a single-surgeon experience with a minimum of 5 years’ clinical and radiographic follow-up. J Neurosurg Spine. 2009;10:347-356.
Khoo L.T., Perez-Cruet M.J., Laich D.T., et al. Posterior cervical microendoscopic foraminotomy. In: Perez-Cruet M.J., Fessler R.G. Outpatient Spinal Surgery. St. Louis: Quality Medical Publishing, Inc.; 2006:71-93.
Krupp W., Schattke H., Müke R. Clinical results of the foraminotomy as described by Frykholm for the treatment of lateral cervical disc herniation. Acta Neurochir (Wien). 1990;107:22-29.
Murphey F., Simmons J.C., Brunson B. Surgical treatment of laterally ruptured cervical disc. Review of 648 cases, 1939 to 1972. J. Neurosurg. 1973;38:679-683.
Nolan J.P., Sherk H.H. Biomechanical evaluation of the extensor musculature of the cervical spine. Spine. 1988;13:9-11.
Olsewski J.M., Garvey T.A., Schendel M.J. Biomechanical analysis of facet and graft loading in a Smith-Robinson–type cervical spine model. Spine. 1994;19:2540-2544.
O’Toole J.E., et al. Endoscopic posterior cervical foraminotomy and discectomy. Neurosurg. Clin N Am. 2006;17:411-422.
Roh S.W., et al. Endoscopic foraminotomy using MED system in cadaveric specimens. Spine. 2000;25:260-264.
Ruetten S., et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine. 2008;33:940-948.
Ruetten S., et al. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: prospective 2-year results of 87 patients. Minim Invasive Neurosurg. 2007;50:219-226.
Russell S.M., Benjamin V. Posterior surgical approach to the cervical neural foramen for intervertebral disc disease. Neurosurgery. 2004;54:662-665. discussion 665-666
Sandhu F.A., Perez-Cruet M.J., Fessler R.G. Microendoscopic cervical foraminotomy and discectomy. In: Perez-Cruet M.J., Khoo L.T., Fessler R.G. An Anatomic Approach to Minimally Invasive Spine Surgery. St. Louis, Missouri: Quality Medical Publishing; 2006:337-347.
Smith G.W., Robinson R.A. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40-A:607-624.
Wu W., et al. Degenerative changes following anterior cervical discectomy and fusion evaluated by fast spin-echo MR imaging. Acta Radiol. 1996;37:614-617.
Zeidman S.M., Ducker T.B. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery. 1993;33:356-362.
1. Henderson C.M., et al. Posterior–lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
2. Krupp W., Schattke H., Müke R. Clinical results of the foraminotomy as described by Frykholm for the treatment of lateral cervical disc herniation. Acta Neurochir (Wien). 1990;107:22-29.
3. Aldrich F. Posterolateral microdisectomy for cervical monoradiculopathy caused by posterolateral soft cervical disc sequestration. J Neurosurg. 1990;72:370-377.
4. Russell S.M., Benjamin V. Posterior surgical approach to the cervical neural foramen for intervertebral disc disease. Neurosurgery. 2004;54:662-665. discussion 665-666
5. Jagannathan J., et al. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: a single-surgeon experience with a minimum of 5 years’ clinical and radiographic follow-up. J Neurosurg Spine. 2009;10:347-356.
6. Murphey F., Simmons J.C., Brunson B. Surgical treatment of laterally ruptured cervical disc. Review of 648 cases, 1939 to 1972. J Neurosurg. 1973;38:679-683.
7. Fager C.A. Management of cervical disc lesions and spondylosis by posterior approaches. Clin Neurosurg. 1977;24:488-507.
8. Zeidman S.M., Ducker T.B. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery. 1993;33:356-362.
9. Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
10. Smith G.W., Robinson R.A. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40-A:607-624.
11. Bailey R.W., Badgley C.E. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg Am. 1960;42-A:565-594.
12. Clements D.H., O’Leary P.F. Anterior cervical discectomy and fusion. Spine. 1990;15:1023-1025.
13. Bulger R.F., Rejowski J.E., Beatty R.A. Vocal cord paralysis associated with anterior cervical fusion: considerations for prevention and treatment. J Neurosurg. 1985;62:657-661.
14. Apfelbaum R.I., Kriskovich M.D., Haller J.R. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25:2906-2912.
15. Jung A., et al. Recurrent laryngeal nerve palsy during anterior cervical spine surgery: a prospective study. J Neurosurg Spine. 2005;2:123-127.
16. Daniels A.H., et al. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg. 2008;16:729-738.
17. Frempong-Boadu A., et al. Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech. 2002;15:362-368.
18. Lu D.C., et al. Esophageal erosion 9 years after anterior cervical plate implantation. Surg Neurol. 2008;69:310-312. discussion 312-313
19. Kuhn J.E., Graziano G.P. Airway compromise as a result of retropharyngeal hematoma following cervical spine injury. J Spinal Disord. 1991;4:264-269.
20. Sagi H.C., et al. Airway complications associated with surgery on the anterior cervical spine. Spine. 2002;27:949-953.
21. Andrew S.A., Sidhu K.S. Airway changes after anterior cervical discectomy and fusion. J Spinal Disord Tech. 2007;20:577-581.
22. Pollard M.E., Little P.W. Changes in carotid artery blood flow during anterior cervical spine surgery. Spine. 2002;27:152-155.
23. Karim A., Knapp J., Nanda A. Internal jugular venous thrombosis as a complication after an elective anterior cervical discectomy: case report. Neurosurgery. 2006;59:E705. discussion E705
24. Wu W., et al. Degenerative changes following anterior cervical discectomy and fusion evaluated by fast spin-echo MR imaging. Acta Radiol. 1996;37:614-617.
25. Baba H., et al. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18:2167-2173.
26. Olsewski J.M., Garvey T.A., Schendel M.J. Biomechanical analysis of facet and graft loading in a Smith-Robinson–type cervical spine model. Spine. 1994;19:2540-2544.
27. Aryan H.E., et al. Relaxation of forces needed to distract cervical vertebrae after discectomy: a biomechanical study. J Spinal Disord Tech. 2009;22:100-104.
28. Nolan J.P., Sherk H.H. Biomechanical evaluation of the extensor musculature of the cervical spine. Spine. 1988;13:9-11.
29. Sihvonen T., et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575-581.
30. Kawaguchi Y., Matsui H., Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 1: histologic and histochemical analyses in rats. Spine. 1994;19:2590-2597.
31. Kawaguchi Y., Matsui H., Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 2: histologic and histochemical analyses in humans. Spine. 1994;19:2598-2602.
32. Hyun S.J., et al. Postoperative changes in paraspinal muscle volume: comparison between paramedian interfascial and midline approaches for lumbar fusion. J Korean Med Sci. 2007;22:646-651.
33. Roh S.W., et al. Endoscopic foraminotomy using MED system in cadaveric specimens. Spine. 2000;25:260-264.
34. Khoo L.T., Perez-Cruet M.J., Laich D.T., et al. Posterior cervical microendoscopic foraminotomy. In: Perez-Cruet M.J., Fessler R.G. Outpatient Spinal Surgery. St. Louis: Quality Medical Publishing, Inc.; 2006:71-93.
35. Hilton D.L. Minimally invasive tubular access for posterior cervical foraminotomy with three-dimensional microscopic visualization and localization with anterior/posterior imaging. Spine J. 2007;7:154-158.
36. Adamson T.E. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95:51-57.
37. O’Toole J.E., et al. Endoscopic posterior cervical foraminotomy and discectomy. Neurosurg Clin N Am. 2006;17:411-422.
38. Holly L.T., et al. Minimally invasive 2-level posterior cervical foraminotomy: preliminary clinical results. J Spinal Disord Tech. 2007;20:20-24.
39. Ruetten S., et al. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: prospective 2-year results of 87 patients. Minim Invasive Neurosurg. 2007;50:219-226.
40. Ruetten S., et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine. 2008;33:940-948.
41. Sandhu F.A., Perez-Cruet M.J., Fessler R.G. Microendoscopic cervical foraminotomy and discectomy. In: Perez-Cruet M.J., Khoo L.T., Fessler R.G. An Anatomic Approach to Minimally Invasive Spine Surgery. St. Louis, Missouri: Quality Medical Publishing; 2006:337-347.