Chapter 18 Minimally Invasive Intradiscal Procedures for the Treatment of Discogenic Lower Back and Leg Pain
 Better selection criteria improve results of annuloplasty and disc decompression procedures for lower back and discogenic leg pain.
Better selection criteria improve results of annuloplasty and disc decompression procedures for lower back and discogenic leg pain. Patients with evidence of one or two levels of disc degeneration on MRI and one or two levels positive on provocative discography are desired candidates for annuloplasty.
Patients with evidence of one or two levels of disc degeneration on MRI and one or two levels positive on provocative discography are desired candidates for annuloplasty. Outcomes of the percutaneous disc decompression procedures were largely positive when used for the treatment of contained lumbar disc herniations. However, more randomized, prospective studies are needed to confirm their effectiveness.
Outcomes of the percutaneous disc decompression procedures were largely positive when used for the treatment of contained lumbar disc herniations. However, more randomized, prospective studies are needed to confirm their effectiveness. Optimal patient positioning, which includes correction of lumbar lordosis using either a soft roll or pillow placed under the mid-abdomen, will facilitate needle placement for any of the intradiscal procedures.
Optimal patient positioning, which includes correction of lumbar lordosis using either a soft roll or pillow placed under the mid-abdomen, will facilitate needle placement for any of the intradiscal procedures. During provocative discography, excessive amounts of local anesthetic injected deep, closer to the disc and foramina just prior to intradiscal needle placement, may decrease the ability of patient and operator to detect the nerve root impalement.
During provocative discography, excessive amounts of local anesthetic injected deep, closer to the disc and foramina just prior to intradiscal needle placement, may decrease the ability of patient and operator to detect the nerve root impalement. During intradiscal biacuplasty, although the temperature is set to 50° C on the radiofrequency generator, tissue temperature reaches about 70° C because of distal ionic heating. During this time, the patient should be awake and communicate with the physician.
During intradiscal biacuplasty, although the temperature is set to 50° C on the radiofrequency generator, tissue temperature reaches about 70° C because of distal ionic heating. During this time, the patient should be awake and communicate with the physician. Puncturing an intervertebral disc with a needle may potentially lead to progressive disc disruption. Greater progression of degenerative disc disease has been suggested in post-discography discs, and it seems to be worse in patients who had larger diameter needles inserted in their intervertebral discs.
Puncturing an intervertebral disc with a needle may potentially lead to progressive disc disruption. Greater progression of degenerative disc disease has been suggested in post-discography discs, and it seems to be worse in patients who had larger diameter needles inserted in their intervertebral discs.Introduction
Low back pain remains one of the biggest resource-consuming problems in medicine. At least 40% of the U.S. population at one time or another will use medical resources for the treatment of low back pain. Frequent sources of mechanical lower back pains are myofascial, discogenic, and facetogenic, from sacroiliac joint, compression fractures, and lumbar canal stenosis.1,2
Low back pain is the one of the most common causes of lost work time in the United States,1 and discogenic pain is one of the main causes of chronic lower back pain.3
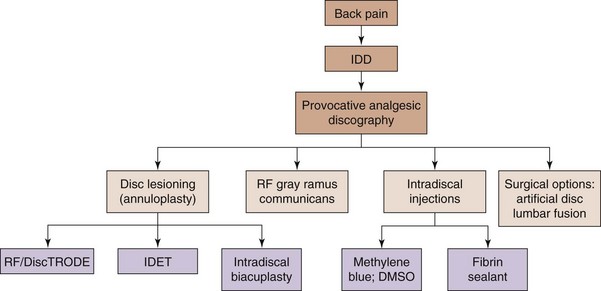
It is the aim of this chapter to briefly review new and developing interventional and minimally invasive spinal treatments for discogenic low back (Figs. 18-1 to 18-3) and leg pain caused by contained disc herniation. Also, a simple algorithm is offered in Fig. 18-4 adopted and modified for discogenic back pain4 and should only be used as a rough intervention guide. This chapter highlights some interesting, novel interventional therapeutic approaches and is not intended to be a complete guide for the treatment of patients with discogenic lower back pain. A comprehensive approach with involvement of multiple specialties and adjunct therapies, such as physical therapy and occupational interventions, are frequently required to produce significant improvement in functional capacity and pain scores of patients with chronic lower back pain.
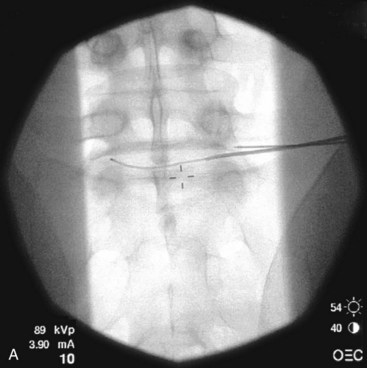
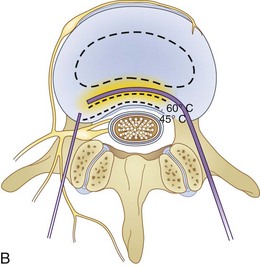
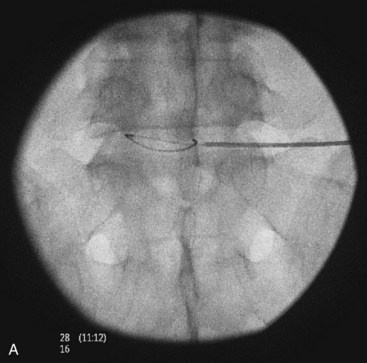
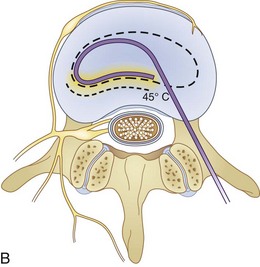
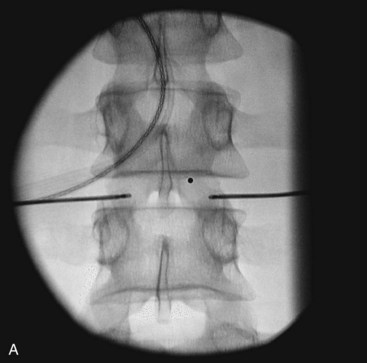
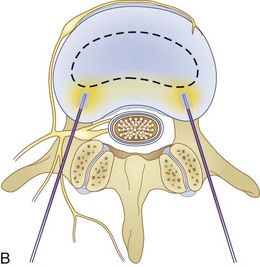
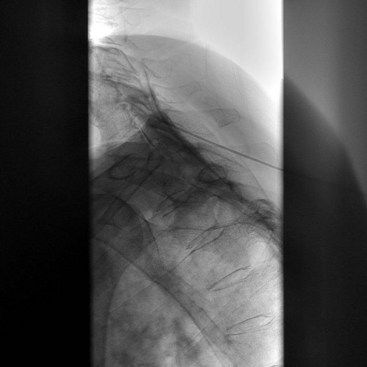
Fig. 18-1 Figs. 18-1 to 18-3 show fluoroscopic views of the final electrode positions during three different intervertebral disc heating procedures used for the treatment of discogenic pain head to head with schematic drawings of the ideal electrode placement. All three fluoroscopic views are anterior-posterior and the schematic drawings are illustrated transverse cuts through the targeted disc.
Discogenic Lower Back Pain
When evaluating patients with the main complaint of long-lasting low back pain, with or without leg pain, it is necessary to investigate the pain generator causing debilitation. More typical features of discogenic source of pain include unrelenting nociceptive low back discomfort or groin or leg pain that worsens with axial loading that improves with recumbency. These signs and reported symptoms alone are usually inadequate to confirm an accurate diagnosis.1–4 Although MRIs are helpful in visualizing such pathology as disc degeneration, desiccation, high intensity zones, and loss of disc height, these changes frequently correlate poorly with clinical findings and the presence of chronic pain, leaving open critical questions of causality.1–4 Many practitioners use provocative or analgesic diagnostic discography as a way to substantiate their clinical diagnosis of discogenic pain. Provocative or analgesic discography is the only available method to relate anatomical abnormalities seen on MRIs of the lumbar spine with clinically observed lower back pain. However, the predictive value of this test is repeatedly questioned, mainly as a consequence of potentially high false-positive rates.5–7
After provisional diagnosis of discogenic pain is introduced, an effective treatment is desired. Several commonly used minimally invasive intradiscal therapies involve careful heating of the annulus fibrosus (so-called annuloplasty procedures) (Fig. 18-4). Historically, such therapeutic modalities have been used regardless of the unclear relationship between positive therapeutic effects and absence of the histological changes expected within the annulus of the disc after heat is used.8–12 Currently, denervation of the annulus by heat destruction of the nociceptors is a plausible mechanism of pain relief. There is no evidence that collagen fibers in the annulus are significantly affected by denaturation and coalescence, possibly suggesting that the collagen alteration is an additional therapeutic mechanism for pain relief.9–12 The minimally invasive approach, low cost, and relative simplicity of these procedures are the key advantages compared with surgical procedures such as lumbar fusion and disc replacement. IDET (Smith and Nephews, London, UK), DiscTRODE (Valleylab, Boulder, CO), and intradiscal biacuplasty (Baylis Medical, Montreal, Canada) (Figs. 18-1 to 18-3) are several annuloplasty methods using heat to treat discogenic pain.
Mechanisms of Pain Relief by Annuloplasty
Dehydration of the intervertebral disc and loss of nuclear material with increasing age are associated with disc degeneration. Consequent delamination and tearing of the lamellar layers are just physical changes that can be associated with biochemical and cellular changes within the disc. Inside the degenerating disc, production of inflammatory cytokines, including tumor necrosis factor-α (TNF-α), nitric oxide, and matrix metalloproteinases are greatly altered.13,14 Neural elements that are normally limited to the outer third of the annulus penetrate farther into the degenerated disc along the vasculature and fissures.15–18 Immunohistochemical studies have shown that such nerves in growth is of nociceptive origin (C- and A-δ fibers) and likely responsible for transmitting pain responses.14,17 Elimination of these nociceptors may disrupt the transmission of pain signals.
During the application of RF, alternating flow of electrical current causes ions in the tissue to move back and forth. This alternating movement by the ions causes molecular vibration within the tissue and results in frictional heating.19,20 This effect is called ionic heating, and it can lead to thermal injury of the cells when tissue temperature reaches greater than 42° C.21 The extent of cellular damage usually depends on the amount of temperature and duration of heating.22 Increase in tissue temperature is a function of current density, or the amount of current per unit area. Current density is greatest at the proximity of the electrode and decreases with increasing distance from the electrode. However, by increasing the power output, current density around the electrode is increased, and thus the lesion size produced by ionic heating is limited by the current density.
One method of increasing lesion size or volume is by cooling the RF electrode internally. This technique was initially developed for tumor and cardiac ablation23–25 and is currently used in intradiscal biacuplasty procedure.26–28 Cooled RF probes have hollow lumens that extend to the tip of the electrode. The cooling fluid circulates in a closed loop through the hollow lumens to the tip of the electrode and back to a pump. The coolant acts as a heat sink that removes heat from the tissue adjacent to the electrode. Consequently, larger lesion volumes can be produced by increasing power deposition and the duration at which current is delivered without causing tissue charring around the electrode.23 A larger lesion volume can be produced by using two internally cooled RF electrodes in a bipolar arrangement at the lower temperature.
Intradiscal Electrothermal Therapy
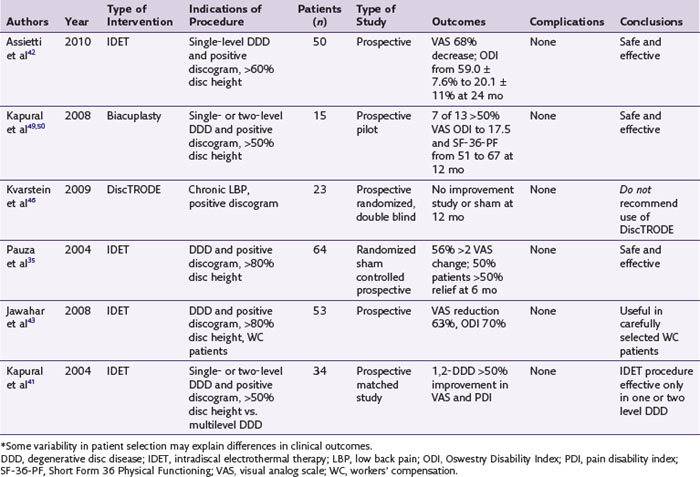
IDET technology (Figs. 18-2 and 18-4) relies on elongated resistive coil of very small diameter to deliver surrounding heat over the limited area of the posterior annulus. Possible mechanisms of pain relief were discussed above. The only difference between the IDET and other disc annuloplasty procedures is that the temperatures attained during the IDET are very high just around the electrode itself, and they dissipate relatively quickly at 2 to 4 mm radius away from the coil. The IDET procedure requires from the proceduralist a relatively long learning curve, and although it seems relatively easy to position resistive coil within the posterior annulus of the disc, multiple attempts may be required or it may be necessary to place another coil from the opposite side of the posterolateral disc to achieve optimal position within the interface between the annulus and nucleus (Fig. 18-2). This may contribute to further damage of the intervertebral disc, and sometimes placement of the tip of the coil within the posterior annular fissure may extend too close to the posterior edge of the disc. Indications for IDET include persistent discogenic low back pain despite comprehensive conservative treatments, including physical therapy, a directed home exercise program, and fluoroscopically guided epidural corticosteroid injections. The Saal brothers, inventors of the IDET catheter,29–31 used initially some additional criteria for the selection of patients, which include those with normal neurological examination results, negative results on straight-leg raise test, absence of any inflammatory arthritic or nonspinal condition that may impersonate lumbar pain, and the absence of prior surgery at the symptomatic intervertebral disc level.29–31 Provocative discography should replicate the concordant pain at low disc pressurization at up to three intervertebral disc levels. The above selection criteria disparities used in subsequent studies evaluating the effectiveness of IDET are thought to account for divergence seen in clinical results (Table 18-1).29–43
The IDET results seem to improve if additional patient selection criteria are used.33,41 Multilevel disc degeneration in patients with discogenic pain was an important predictor of treatment failure compared with a group of patients with one or two degenerated discs as shown on the MRI. Unfortunately, single disc level disease is less frequent, and the majority of the patients present with discogenic pain and multilevel degeneration present on MRI.41 Overweight patients44 and patients receiving workers’ compensation benefits40,45 represent additional patient subsets that have a low probability of achieving desired results from IDET.
Other Annuloplasty and Nucleoplasty Procedures
IDET is not the only minimally invasive annuloplasty procedure (Fig. 18-4). The DiscTRODE is a radiofrequency (RF) method in which heat is applied to the posterior annulus. This treatment is generally termed percutaneous intradiscal RF thermocoagulation.46,47 Kvarstein et al46 used appropriate inclusion criteria, which listed disc height reduction less than 30% and disc protrusion of less than 4 mm, as well as positive one-level pain provocation discography. A disappointing performance resulted because patients reported only modest or no improvements in pain scores and functional capacity.46,47 Furthermore, the mean reduction of Oswestry Disability Index scores did not reach statistical significance when compared with baseline.46 This technology proved to be unsuccessful in improving functional capacity and visual analog score (VAS) versus IDET during another study in which strict patient selection criteria were used.47
Intradiscal Biacuplasty
Intradiscal biacuplasty is the latest minimally invasive posterior annulus heating technique (Fig. 18-4). This technology uses bipolar cooled RF electrodes called transdiscal electrodes (Kimberly Clark, Atlanta, GA). Reviewing the pain scores and functional capacity improvement ratings in patients with discogenic pain, biacuplasty is comparable or better than other minimally invasive annuloplasty methods.48–52 Internally cooling electrodes provides for even heating over the wider area of the posterior annulus (Fig. 18-3, B).26–28
The procedure itself is fluoroscopy guided with the patient lying in the prone position. Electrodes are inserted bilaterally in the posterior annulus of the intervertebral disc as shown in Fig. 18-3, B. The generator controls delivery of RF energy by monitoring the temperature measured by a thermocoupler near the tip of the electrode. The temperature increases gradually over a period of 7 to 8 minutes to 50° C with final heating for another 7 minutes. During this time, the patient should be awake and communicating with the physician to decrease the probability of neurological injury.
Currently, clinical biacuplasty data from two case series of eight and 15 patients are available for critical review. Both studies demonstrated significant pain relief after the disc biacuplasty procedure at 3, 6, and 12 months.50–52 European case series suggested improvement in pain scores greater than 50% at 3 months with general good patient satisfaction. A U.S. pilot study involving 15 patients described reduction in the median VAS pain score from 7 to 3 cm at 6 and 12 months of follow-up, respectively; improvement in Oswestry Disability Index from 23.3 to 16.5 points; and significant increase in the Short Form 36 Physical Functioning scores (Table 18-1).50,52 The sham, prospective randomized study is currently being conducted, and data may be available as soon as mid-2011. Intradiscal biacuplasty may offer several advantages over the earlier techniques. There is negligible disruption to the native tissue architecture, and thus the biomechanics of the spine is likely unchanged, and the relative ease of electrode placement abolishes the need to thread a long-heating catheter (e.g., IDET).
Complications of Annuloplasty Procedures
The incidence of various complications related to annuloplasty could be as high as 10%.53,54 If the RF electrode or resistive coil is positioned in the close proximity of the neural elements, high temperatures delivered may cause nerve injury possibly manifested as a radicular pain or transient palsy. Transient radiculopathy with the resolution in less than 6 weeks and, rarely, motor deficit with prominent foot drop were previously reported after the IDET procedure.54
Catheter breakage,54 vertebral osteonecrosis,55 and cauda equina56 syndrome all have been reported as rare complications of the IDET procedure. The duration of back pain, obesity, smoking, history of leg pain, and diabetes may not be associated with the higher incidence of complications.53 Compared with nucleoplasty (disc decompressive procedure) alone, the frequency of the complications was not higher in patients who received IDET combined with nucleoplasty procedure.57
Discitis is rare complication of any intradiscal procedure.58–62 Appropriate timing of intravenous antibiotic seems to be effective in preventing discitis after provocative discography and any minimally invasive intradiscal procedure.58–62 The incidence of disc herniation after annuloplasty could be as high as 0.3%, and it was speculated to be caused by thermally mediated loss of tensile strength of the collagen fibers.63 At least one case report documented disc herniation with clear increase in the size of disc protrusion after IDET procedure.53,54
Mechanisms of Disc Herniation and Pain Relief from Percutaneous Disc Decompression
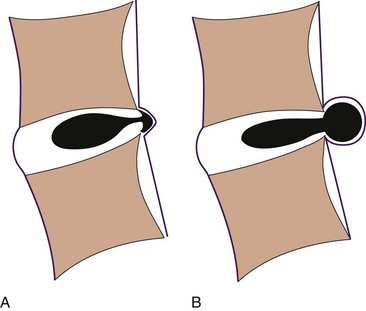
A herniated disc can cause radicular pain or referred pain in the extremity involved. Whereas radicular pain follows appropriate dermatomal area, referred pain can be elsewhere in the extremity. With an internal disc disruption, nucleus of the disc has altered weight-bearing loads and could cause a change in shape or complete disruption of the annular fibers, which in turn may produce contained disc herniation or extrusion of the disc. Nerve root compression can result from either of those two mechanical events (Fig. 18-5).64
Occasionally, radicular or referred pain in the extremity can be significant with or without significant mechanical pathology as described above. This is because various inflammatory mediators are present in the intravertebral disc, including phospholipase A2, prostaglandin E2, interleukin-1α, interleukin-1β, interleukin-6, TNF-α, and nitric oxide.65 Exposure of the neural structures to the nuclear material, therefore, may cause chemical painful radiculitis. MRI remains the modality of choice in diagnosing lumbar disc herniations. Intervertebral disc can be well described, as well as the surrounding nerves and bony structures.
The Dekompressor Device
The percutaneous decompression (Dekompressor) technology extracts nuclear disc material by an auger within a cannula that ends inside the nucleus. A significant change in intradiscal pressure follows the reduction of nuclear volume within the closed hydraulic space. It is imperative that the annular wall be intact for this technique to retract the bulging section; therefore provocative discography may occasionally be needed to confirm the affected level and to rule out any annular disruption.66–68
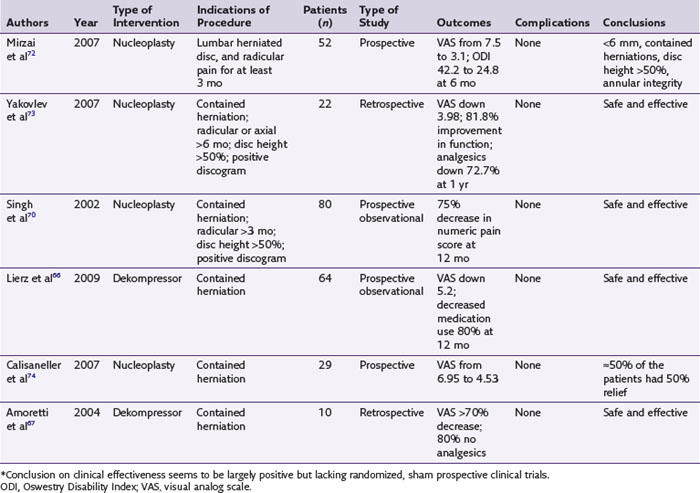
Studies published on the efficacy of the Dekompressor device when used for treatment of contained, symptomatic disc herniation are few and observational. In 64 patients, Lierz et al66 reported an average decrease in pain scores from 7.3 to 2.1 after 12 months with 80% of the patients being able to reduce their pain medications. Amoretti et al67 reported in 50 patients a more than 70% decrease in pain in 72% of cases, and Alò and colleagues68 reported an 80% success rate using the same technique. There are still no controlled studies published on Dekompressor efficacy.
Coblation Nucleoplasty
Coblation technology has been used in various arthroscopic procedures and relies on partial ablation of the nuclear tissue followed by coagulation. Similarly, Nucleoplasty Coblation (ArthroCare Corporation, Austin, TX) can ablate and coagulate the nucleus pulposus to decompress the disc and thermally alter disc tissue.69 It seems that decompression is minimal or nonexistent in degenerated discs and more likely in nondegenerated ones.70–74 An access to the disc is accomplished through a canula with the obturator stylet using fluoroscopy. It is followed by an RF electrode called the SpineWand. Tissue ablation creates channels through the nucleus to the opposite side. The canula is then slowly withdrawn to the starting position five or six times.69
Radicular pain should be greater than axial pain, and patients should have already failed conservative treatments. Less favorable outcomes are seen with large disc protrusions and disc extrusions. A patient with a contained disc protrusion of less than 6 mm whose annular integrity is documented by discography and who has consistent radicular symptoms confirmed by selective nerve root blocks represents the ideal candidate for annuloplasty.69–75 Improvements in both functional capacity and pain relief were seen with nucleoplasty during observational studies (Table 18-2).70–74 A recent systematic review, although strongly supporting therapeutic efficacy of this procedure, repeated necessity of randomized, prospective sham study to acquire level 1 evidence for the procedure.75
Complications of Percutaneous Decompressive Procedures
At least one study found a statistically significant higher prevalence of leg pain and increased weakness in patients who received nucleoplasty compared with those receiving just conservative treatment. However, the most common side effect was soreness at the site of the catheter insertion.76 Although side effects with the Dekompressor are rare, so is the volume of the patients reported receiving this procedure (at least when compared with the number of patients in studies on nucleoplasty). The probe break within the disc nucleus has been described where the tip of the probe had to be removed surgically.77
1 Andersson GBJ. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581-585.
2 Kaaria S, Kaila-Kangas L, Kirjonen J, et al. Low back pain, work absenteeism, chronic back disorders, and clinical findings in the low back as predictors of hospitalization due to low back disorders. Spine. 2005;10:1211-1218.
3 Schwartzer AC, Aprill CN, Derby R, et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20:1878-1883.
4 Kapural L, Goldner JD. Interventional pain management: when/what therapies are best for low back pain. Current Opin Anesthesiol. 2005;18:569-575.
5 Carragee EJ, Tanner CM, Khurana S, et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2000;25:1373-1380.
6 Walsh TR, Weinstein JN, Spratt KF, et al. Lumbar discography in normal subjects. A controlled, prospective study. J Bone Joint Surg Am. 1990;72:1081-1088.
7 Derby R, Howard MW, Grant JM, et al. The ability of pressure-controlled discography to predict surgical and nonsurgical outcomes. Spine. 1999;24:364-371.
8 Freeman BJ, Walters RM, Moore RJ, et al. Does intradiscal electrothermal therapy denervate and repair experimentally induced posterolateral annular tears in an animal model? Spine. 2003;28:2602-2608.
9 Kleinstueck FS, Diederich CJ, Nau WH, et al. Temperature and thermal dose distributions during intradiscal electrothermal therapy in the cadaveric lumbar spine. Spine. 2003;28:1700-1708.
10 Shah RV, Lutz GE, Lee J, et al. Intradiskal electrothermal therapy: a preliminary histologic study. Arch Phys Med Rehabil. 2001;82:1230-1237.
11 Smith HP, McWhorter JM, Challa VR. Radiofrequency neurolysis in a clinical model. J Neurosurg. 1981;55:248-253.
12 Obrzut SL, Hecht P, Hayashi K, et al. The effect of radiofrequency energy on length and temperature properties of the glenohumeral joint capsule. Arthroscopy. 1998;4:395-400.
13 Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol (Noisy-le-grand). 2007;53(5):4-18.
14 Ashton IK, et al. Neuropeptides in the human intervertebral disc. J Orthop Res. 1994;12(2):186-192.
15 Johnson WE, Evans H, Menage J, et al. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine. 2001;26(23):2550-2557.
16 Melrose J, Roberts S, Smith S, et al. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine. 2002;27(12):1278-1285.
17 Palmgren T, Grönblad M, Virri J, et al. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine. 1999;24(20):2075-2079.
18 Jackson HC2nd, Winkelmann RK, Bickel WH. Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg Am. 1966;48(7):1272-1281.
19 Noe CE, Racz GB. Radiofrequency. In: Raj P, editor. Pain medicine: a comprehensive review. St. Louis: Mosby; 1996:305-308.
20 Organ LW. Electrophysiologic principles of radiofrequency lesion making. Appl Neurophysiol. 1976;39(2):69-76.
21 Dickson JA, Calderwood SK. Temperature range and selective sensitivity of tumors to hyperthermia: a critical review. Ann NY Acad Sci. 1980;335:180-205.
22 Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10(4):338-347.
23 Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol. 3(7), 1996. 556-556
24 Wittkamp FHM, Hauer RN, Robles de Medina EO. Radiofrequency ablation with a cooled porus electrode catheter [abstract]. J Am Coll Cardiol. 11(17), 1988.
25 Goldberg SN, Gazelle GS, Solbiati L, et al. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3(8):636-644.
26 Pauza K. Cadaveric intervertebral disc temperature mapping during disc biacuplasty. Pain Physician. 2008;11(5):669-676.
27 Kapural L, Mekhail N, Sloan S, et al. Histological and temperature distribution studies in the lumbar degenerated and non-degenerated human cadaver discs using novel transdiscal radiofrequency electrodes. Pain Med. 2008;9(1):68-75.
28 Petersohn JD, Conquergood LR, Leung M. Acute histologic effects and thermal distribution profile of disc biacuplasty using a novel water-cooled bipolar Electrode system in an in vivo porcine model. Pain Med. 2008;9(1):26-32.
29 Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: prospective outcome study with a minimum 2-year follow-up. Spine. 2002;27:966-973.
30 Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: a prospective outcome study with minimum 1-year follow-up. Spine. 2000;25:2622-2627.
31 Saal JS, Saal JA. Management of chronic discogenic low back pain with a thermal intradiscal catheter. A preliminary report. Spine. 2000;25:382-388.
32 Appleby D, Andersson G, Totta M. Meta-analysis of the efficacy and safety of intradiscal electrothermal therapy (IDET). Pain Med. 2006;7:308-316.
33 Bogduk N, Karasek M. Two-year follow-up of a controlled trial of intradiscal electrothermal anuloplasty for chronic low back pain resulting from internal disc disruption. Spine J. 2002;2:343-350.
34 Endres SM, Fiedler GA, Larson KL. Effectiveness of intradiscal electrothermal therapy in increasing function and reducing chronic low back pain in selected patients. WMJ. 2002;101:31-34.
35 Pauza KJ, Howell S, Dreyfuss P, et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
36 Wetzel FT, McNally TA, Phillips FM. Intradiscal electrothermal therapy used to manage chronic discogenic low back pain: new directions and interventions. Spine. 2002;27:2621-2626.
37 Derby R, Eek B, Chen Y, et al. Intradiscal electrothermal annuloplasty (IDET): a novel approach for treating chronic discogenic back pain. Neuromodulation. 2000;3:82-88.
38 Lutz C, Lutz GE, Cooke PM. Treatment of chronic lumbar diskogenic pain with intradiskal electrothermal therapy: a prospective outcome study. Arch Phys Med Rehabil. 2003;84:23-28.
39 Lee MS, Cooper G, Lutz GE, et al. Intradiscal electrothermal therapy (IDET) for treatment of chronic lumbar discogenic pain: a minimum 2-year clinical outcome study. Pain Physician. 2003;6:443-448.
40 Mekhail N, Kapural L. Intradiscal thermal annuloplasty of discogenic pain: an outcome study. Pain Pract. 2004;4:84-90.
41 Kapural L, Korunda Z, Basali AH, et al. Intradiscal thermal annuloplasty for discogenic pain in patients with multilevel degenerative disc disease. Anesth Analg. 2004;99:472-476.
42 Assietti R, Morosi M, Block JE. Intradiscal electrothermal therapy for symptomatic internal disc disruption: 24-month results and predictors of clinical success. J Neurosurg Spine. 2010;12(3):320-326.
43 Jawahar A, Brandao SM, Howard C, et al. Intradiscal electrothermal therapy (IDET): a viable alternative to surgery for low back pain in workers’ compensation patients? J La State Med Soc. 2008;160(5):280-285.
44 Cohen SP, Larkin T, Abdi S, et al. Risk factors for failure and complications of intradiscal electrothermal therapy: a pilot study. Spine. 2003;28:1142-1147.
45 Webster BS, Verma S, Pransky GS. Outcomes of workers’ compensation claimants with low back pain undergoing intradiscal electrothermal therapy. Spine. 2004;29:435-441.
46 Kvarstein G, Mawe L, Indahl A, et al. A randomized double-blind controlled trial of intra-annular radiofrequency thermal disc therapy-A 12-month follow-up. Pain. 2009;145:279-286.
47 Kapural L, Hayek S, Malak O, et al. Intradiscal thermal annuloplasty versus intradiscal radiofrequency ablation for the treatment of discogenic pain: a prospective matched control trial. Pain Med. 2005;6:425-431.
48 Kapural L, Mekhail N. novel transdiscal biacuplasty for the treatment of lumbar discogenic pain: a case report. Pain Pract. 2007;7(2):130-134.
49 Kapural L, De la Garza M, Ng A, et al. Novel transdiscal biacuplasty for the treatment of lumbar discogenic pain: a 6 months follow-up. Pain Med. 2008;9(1):60-67.
50 Kapural L. Intervertebral disc cooled bipolar radiofrequency (intradiscal biacuplasty) for the treatment of lumbar discogenic pain: a 12 month follow-up of the pilot study. Pain Med. 2008;9(4):464.
51 Kapural L, Cata JP, Narouze S. Successful treatment of lumbar discogenic pain using intradiscal biacuplasty in previously discectomized disc. Pain Pract. 2009;9(2):130-134.
52 Cooper AR: Disc biacuplasty for treatment of axial discogenic low back pain: initial case series. In Glasgow, Scotland, 2007, British Pain Society Annual General Meeting.
53 Cohen SP, Larkin T, Abdi S, et al. Risk factors for failure and complications of intradiscal electrothermal therapy: a pilot study. Spine. 2003;28:1142-1147.
54 Kapural L, Cata J. Complications of minimally invasive procedures for discogenic pain. Tech Reg Anesth Pain Med. 2007;11(3):157-163.
55 Djurasovic M, Glassman SD, Dimar JR, et al. Vertebral osteonecrosis associated with the use of intradiscal electrothermal therapy: a case report. Spine. 2002;27(13):E325-E328.
56 Wetzel FT. Cauda equina syndrome from intradiscal electrothermal therapy. Neurology. 2001;56:1607.
57 Cohen SP, Williams S, Kurihara C, et al. Nucleoplasty with or without intradiscal electrothermal therapy (IDET) as a treatment for lumbar herniated disc. J Spinal Disord Tech. 2005;18(suppl):S119-S124.
58 Klessig HT, Showsh SA, Sekorski A. The use of intradiscal antibiotics for discography: an in vitro study of gentamicin, cefazolin, and clindamycin. Spine. 2003;28:1735-1738.
59 Esimont FJ, Wiesel SW, Brighton CT, et al. Antibiotic penetration into rabbit nucleus pulposus. Spine. 1987;12:254-256.
60 Thomas RW, Batten JJ, Want S, et al. A new in-vitro model to investigate antibiotic penetration of the intervertebral disc. J Bone Joint Surg Br. 1995;77:967-970.
61 Rhoten RL, Murphy MA, Kalfas IH, et al. Antibiotic penetration into cervical discs. Spine. 1995;37:418-421.
62 Boscardin JB, Ringus JC, Feingold DJ, et al. Human intradiscal levels with cefazolin. Spine. 1992;17(suppl):S145-S148.
63 Kleinstueck FS, Diederich CJ, Nau WH, et al. Acute biomechanical and histological effects of intradiscal electrothermal therapy on human lumbar discs. Spine. 2001;26:2198-2207.
64 Komori H, Shinomiya K, Nakai O, et al. The natural history of herniated nucleus pulposus with radiculopathy. Spine. 1996;21:225-229.
65 Takahashi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976). 1996;15(2):218-224. 21
66 Lierz P, Alò KM, Felleiter P. Percutaneous lumbar discectomy using the Dekompressor system under CT-control. Pain Pract. 2009;9(3):216-220.
67 Amoretti N, Huchot F, Flory P, et al. Percutaneous nucleotomy: preliminary communication on a decompression probe (Dekompressor) in percutaneous discectomy. Ten case reports. Clin Imaging. 2005;29:98-101.
68 Alò KM, Wright RE, Sutcliffe J, et al. Percutaneous lumbar discectomy: clinical response in an initial cohort of fifty consecutive patients with chronic radicular pain. Pain Pract. 2004;4:19-29.
69 Deer T, Kapural L. Imaging for disc decompression procedures. Tech Reg Anesth Pain Med. 2007;11(2):81-89.
70 Singh V, Piryani C, Liao K, et al. Percutaneous disc decompression using coblation (Nucleoplasty™). Pain Physician. 2002;5:250-259.
71 Sharps LS, Zacharia I. Percutaneous disc decompression using nucleoplasty. Pain Physician. 2002;5:121-126.
72 Mirzai H, Tekin I, Yaman O, et al. The results of nucleoplasty in patients with lumbar herniated disc: a prospective clinical study of 52 consecutive patients. Spine J. 2007;7:88-92.
73 Yakovlev A, Tamimi MA, Liang H, et al. Outcomes of percutaneous disc decompression utilizing nucleoplasty for the treatment of chronic discogenic pain. Pain Physician. 2007;10:319-328.
74 Calisaneller T, Ozdemir O, Karadeli E, et al. Six months post-operative clinical and 24 hour post-operative MRI examinations after nucleoplasty with radiofrequency energy. Acta Neurochir (Wien). 2007;149:495-500.
75 Gerges FJ, Lipsitz SR, Nedeljkovic SS. A systematic review on the effectiveness of the Nucleoplasty™ procedure for discogenic pain. Pain Physician. 2010;13:117-132.
76 Bhagia SM, Slipman CW, Nirschl M, et al. Side effects and complications after percutaneous disc decompression using coblation technology. Am J Phys Med Rehabil. 2006;85:6-11.
77 Domsky R, Goldberg ME, Hirsh RA, et al. Critical failure of a percutaneous discectomy probe requiring surgical removal during disc decompression. Reg Anesth Pain Med. 2006;31:177-179.