CHAPTER 34 Microtubules and Centrosomes
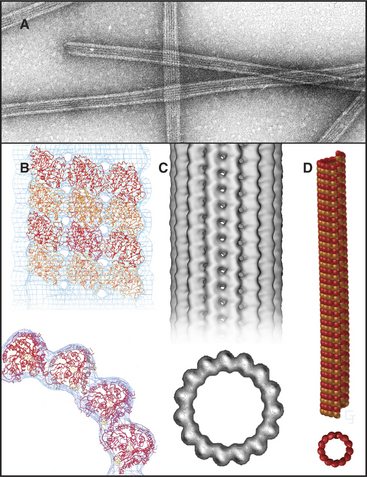
Microtubules are stiff, cylindrical polymers of α- and β-tubulin (Fig. 34-1) that provide support for a variety of cellular components and tracks for movements powered by motor proteins called kinesins and dyneins. Microtubules are 25nm in diameter and can grow longer than 20μm in cells and 3mm in vitro. The head-to-tail arrangement of dimers of α- and β-tubulin in the wall of microtubules give the polymer a molecular polarity. The “plus” (β-tubulin) end grows faster than the “minus” end.
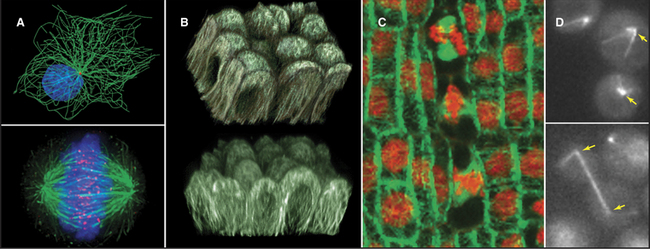
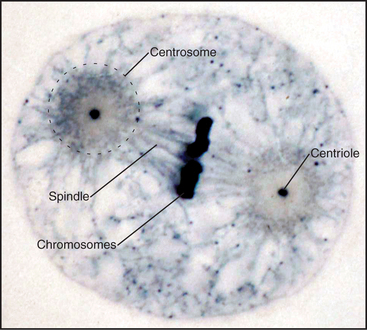
A great simplifying principle is that microtubules have a radial organization in many types of cells (Fig. 34-2A). Typically, the plus end is peripheral, and the minus end is anchored in a microtubule-organizing center. One exception to this radial organization is found in dendrites of nerve cells, where about 40% of the microtubules are oriented with the minus end away from the cell body.
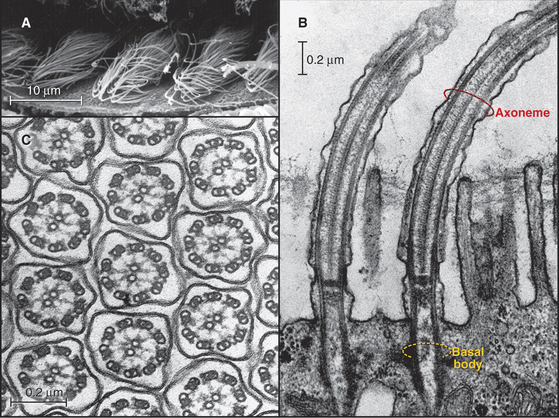
In most animal cells, the organizing center for cytoplasmic microtubules is the centrosome. Centrosomes consist of centrioles and a surrounding matrix containing the active component in microtubule nucleation—a complex of proteins including the specialized tubulin isoform γ-tubulin. The microtubule-organizing center is more diffuse in columnar epithelial cells, where microtubules originate from a broad zone containing γ-tubulin near the apex of the cell (Fig. 34-2B). In plant cells, γ-tubulin and microtubules are found throughout the cortex rather than in a discrete array, although they form a bipolar mitotic spindle during cell division (Fig. 34-2C). Microtubules in fungi grow from a spindle pole body, an organizing center containing γ-tubulin that is associated with the nuclear envelope (Fig. 34-2D). Animals and protozoa with cilia and flagella use basal bodies (Fig. 34-3B) to nucleate the assembly of microtubules for their motile structure, called an axoneme.
Microtubules vary considerably in stability. Those that form the axonemes in eukaryotic cilia and flagella are stable for days to weeks. Cytoplasmic microtubules turn over much more rapidly, within minutes in the case of the interphase array of microtubules and within tens of seconds for mitotic spindle microtubules. These dynamic microtubules randomly undergo rapid depolymerization and then regrow over a period of seconds to minutes. This “dynamic instability” helps to remodel the network of microtubules in cytoplasm and contributes to some forms of motility, including the assembly of the mitotic spindle and movements of chromosomes during mitosis (see Fig. 44-7).
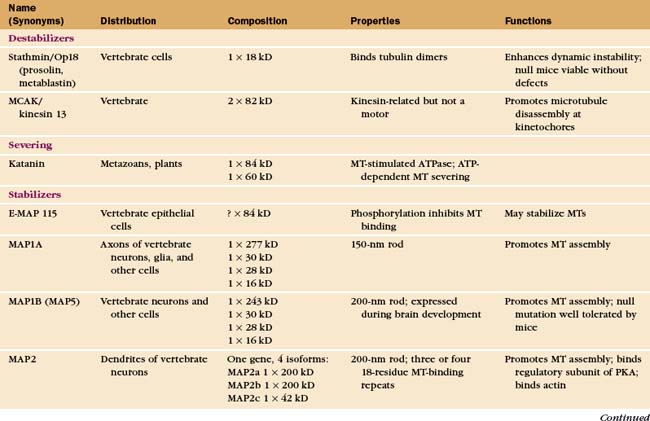
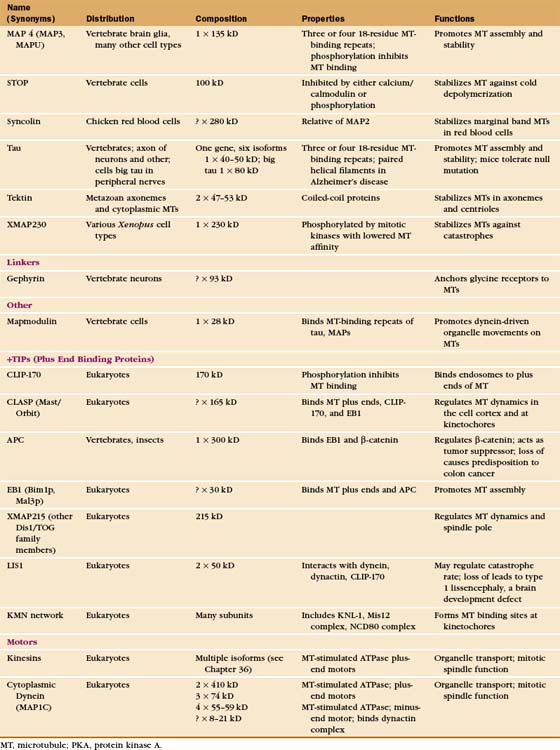
Because the same tubulin dimers can form dynamic single microtubules in cytoplasm and stable doublet microtubules in axonemes, it is believed that accessoryproteins specify both the stability and diverse structures assembled from tubulin. The various families of microtubule-associated proteins (MAPs) bind tubulin dimers, stabilize polymers, associate with microtubule ends, or sever cytoplasmic microtubules. In the axonemes of cilia and flagella, more than 100 accessory proteins organize and stabilize the regular array of nine outer doublet microtubules and two central single microtubules (see Fig. 38-16).
Microtubule motor proteins (see Figs. 36-13 and 36-14) power movements ranging from the slow movements of chromosomes on the mitotic spindle (see Fig. 44-14) to the rapid beating of cilia and flagella (see Fig. 38-14). Different motors move toward the plus and minus ends of microtubules. The main minus-end-directed motor, dynein, drives the beating of cilia and flagella. Dynein and the kinesin family of plus-end motors move membrane-bound organelles, RNA particles, viruses, and other cargo along microtubules (see Fig. 37-7). These active movements determine, to a great extent, the distribution of cellular organelles and the shape of cells.
Tubulin Structure
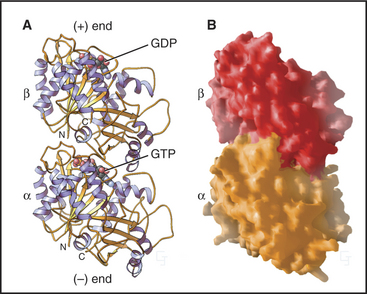
The tubulin molecule is a heterodimer of a and β subunits that share a common fold and 40% identical residues (Fig. 34-4). Dimers of α- and β-tubulin are stable and rarely dissociate at the 10- to 20-mM concentr-tions of tubulin found in cells. γ-tubulins have the same fold.
Each tubulin subunit binds a guanine nucleotide, either guanosine triphosphate (GTP) or guanosine diphosphate (GDP). The fold and the GTP-binding site of tubulin do not resemble those of other GTP-binding proteins (see Fig. 25-7). GTP on α-tubulin is buried in the dimer, so it does not exchange with solution GTP; hence, it is called the nonexchangeable N-site. GTP on β-tubulin is exposed in the dimer and exchanges slowly (Kd = 50nM), so this is known as the exchangeable site, or E-site. When incorporated into a microtubule, contacts with the adjacent a subunit bury the GTP on the β subunit and promote its hydrolysis. Neither bound guanine nucleotide can exchange when tubulin is buried in the wall of a microtubule. As is explained later in this chapter, the nature of the nucleotide on the β subunit profoundly affects microtubule assembly.
Most Archaea and Bacteria have a protein called FtsZ with the same fold as tubulin. The two proteins are most likely to have had a common ancestor, but their sequences have diverged considerably. FtsZ also forms polymers and is required for cytokinesis of prokaryotes (see Fig. 44-21). One group of Bacteria lost their FtsZ but have acquired genes for both α- and β-tubulin, most likely by lateral transfer from an eukaryote.
Tubulins are modified in a number of different ways in cells. Over time, stable microtubules accumulate two modifications—acetylation of lysine-40 and removal of the C-terminal tyrosine of α-tubulin—but neither modification is responsible for the stability. The enzyme carboxypeptidase B removes the tyrosine, leaving a glutamic acid exposed. Another enzyme, tyrosine-tubulin ligase, can replace the tyrosine. Other microtubules are modified by the addition of a polymer of up to six glutamic acid residues to the γ-carboxyl groups of glutamic acid residues of both α-tubulin and β-tubulin. Addition of one or more glycines to the γ-carboxyl of other glutamate residues stabilizes the central pair of microtubules in axonemes (see Fig. 38-16).
Structure of Microtubules
Microtubules are cylinders constructed of longitudin-ally oriented protofilaments with a 4-nm longitudinal repeat arising from the tubulin subunits (Fig. 34-5). Most cytoplasmic microtubules have 13 protofilaments, but microtubules in some cells have 11, 15, or 16 protofilaments. Microtubules assembled in vitro can have 11 to 15 protofilaments, but 13 is the favored number. For years, microtubules were thought to be true helices, but microtubules with 13 protofilaments have a longitudinal seam or discontinuity between two of the protofilaments, breaking up perfect helical packing of the subunits (Fig. 34-5D). The other protofilaments are aligned identically with respect to their neighbors.
The stiffness, length, and polarity of microtubules make them valuable both for cytoskeletal support and as tracks for microtubule-based motors. Because they resist compression, microtubules are called on more frequently than actin filaments or intermediate filaments to support asymmetrical cellular structures, including axonemes, the mitotic spindle, and elaborate surface processes of some protozoa (see Fig. 38-4). Plants provide a spectacular example of the influence of microtubules on morphology: Point mutations in tubulin influence whether climbing plants wrap in a left-handed or right-handed helix around their supports.
Microtubule Assembly from GTP Tubulin
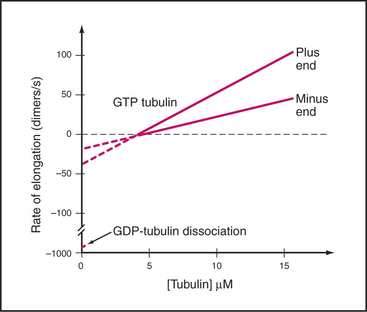
Microtubules assemble from pure GTP-tubulin subunits much like actin filaments do using subunits with bound ATP (see Figs. 5-6 and 33-8). Superficially, the assembly of microtubules is a simple bimolecular reaction of tubulin dimers with the ends of the polymer. Association and dissociation of tubulin occurs only at the ends, not from the walls of microtubules. Growth is faster at the plus end than at the minus end (Fig. 34-6), and assembly of GTP-tubulin is much more favorable than that of GDP-tubulin. The rate of elongation is proportional to the concentration of GTP-tubulin dimers above the critical concentration at each end. The rate constants measured from the slopes and intercepts in elongation experiments (Table 34-1) are similar to those for actin (see Fig. 33-8), although this similarity might be misleading, as the number of sites growing at the end of a microtubule is unknown and is most likely to be greater than one as on actin filaments.
Table 34-1 RATE CONSTANTS FOR THE ASSEMBLY OF MICROTUBULES IN VITRO AND IN CELLS
| Reaction | Plus End | Minus End |
|---|---|---|
| In Vitro Elongation of Purified Tubulin | ||
| Association of GTP tubulin | 9 μM−1s−1 | 4 M−1s−1 |
| Dissociation of GTP tubulin | 44 s−1 | 23 s−1 |
| Association of GDP tubulin | Unknown | Unknown |
| Dissociation of GDP tubulin | 733 s−1 | 915 s−1 |
| Steady-State Dynamic Instability | ||
| Frequency of catastrophe in vitro at 7 μM tubulin | 0.0045 s−1 | 0.003 s−1 |
| Frequency of rescue in vitro at 7 μM tubulin | 0.02 s−1 | 0.06 s−1 |
| Microtubule Dynamic Instability in Live Cells | ||
| Frequency of catastrophe in vivo (interphase) | 0.014 s−1 | |
| Frequency of catastrophe in vivo (mitosis) | 0.017 s−1 | |
| Frequency of rescue in vivo (interphase) | 0.046 s−1 | |
| Frequency of rescue in vivo (mitosis) | 0 | |
Data from Walker RA, O’Brien ET, Pryer NK, et al: Dynamic instability of individual microtubules. J Cell Biol 107:1437–1448, 1988.
Drugs (Box 34-1) and environmental conditions can depolymerize microtubules. Pioneering light microscopic observations of live cells established that mitotic spindle fibers (later shown to be microtubules) are sensitive to both cold and high hydrostatic pressure. This makes it possible to purify microtubules and tightly associated proteins by cycles of depolymerization in the cold and repolymerization at higher temperatures. Dissociated microtubule components in ice-cold cellular extracts polymerize when rewarmed to body temperature. Pelleting in a centrifuge separates microtubules and any associated proteins from soluble components. Resuspension in cold buffer with GTP depolymerizes the microtubules. The cycle is repeated until the desired degree of purity is achieved. Adaptations of tubulins or accessory proteins allow cold-water organisms to assemble microtubules at temperatures near freezing.
Microtubule-Organizing Centers
Instead of spontaneous nucleation, virtually all cellular microtubules arise from microtubule-organizing centers. Microtubules in cilia and flagella grow directly from basal bodies (see Fig. 38-17), but other microtubules originate in the pericentrosomal material surrounding centrioles in the centrosome (Figs. 34-2A and 34-18), from fungal spindle pole bodies (Fig. 34-19) or nuclear envelopes and less-organized material in the cortex of some epithelial cells (Fig. 34-2B) and plant cells (Fig. 34-2C). As is described in the section on centrosomes later in this chapter, these organizing centers use assemblies of γ-tubulin to initiate microtubules.
Steady-State Dynamics of Microtubules in Vitro
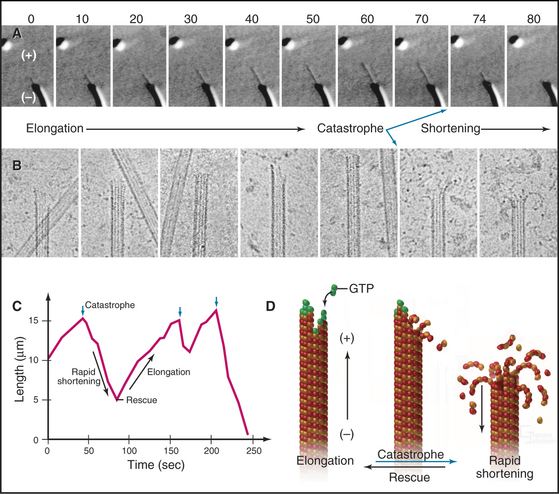
If the GTP-tubulin reactions were all that contributed to assembly, microtubules would grow until the concentration of free tubulin dimers decreased to the critical concentration, after which polymers would be relatively stable, since the critical concentrations are similar at the two ends. Under these conditions, assembly at one end would be balanced by disassembly at the other. However, this is not what happens at steady state in either test tubes or cells. In vitro, the overall microtubule polymer and monomer concentrations are stable over time, but the microtubule number declines as some microtubules disappear, and the survivors grow longer. Direct observation by light microscopy (Fig. 34-7A)shows that this behavior is attributable to random, rapid fluctuations in the length of microtubules. Amazingly, growing and shrinking microtubules coexist at steady state; some shrinking microtubules disappear while others grow. This behavior is called dynamic instability.
At steady state, individual microtubules grow slowly until they undergo a random transition to a phase of rapid shortening. This transition is called a catastrophe. As they shorten, tubulin is lost from the end at a rate of nearly 1000 dimers per second, so the polymer shrinks more than 0.5μm per second. Electron micrographs of rapidly shortening microtubules show curved segments of protofilaments peeling out from the end (Fig. 34-7B). Dimers dissociate from these curved protofilaments and sheets before or after they break free from an end. Rapid shortening can be terminated by another stochastic event called a rescue, after which the microtubule grows again at a steady rate. Rescue is more likely at the minus end than at the plus end (Table 34-1). Occasionally, a microtubule disappears completely during a shortening phase if its length reaches zero before a rescue event occurs.
Hydrolysis of GTP bound to the (exchangeable) E-site on β-tubulin drives dynamic instability. Dimeric tubulin hydrolyzes E-site GTP very slowly, but when it is incorporated into a microtubule, interactions with the adjacent a subunit stimulate hydrolysis 250-fold (k = 0.3s−1, corresponding to a half time of 2s). The g-phosphate then dissociates (k = 0.02s−1, corresponding to a half time of 35s). In contrast, GTP bound to the a subunit is not hydrolyzed because the β subunit does not provide the residues required to complete the active site. Thus, “GDP-tubulin” has GDP on the β subunit and GTP on the a subunit. Microtubules composed of GDP-tubulin are extremely unstable in comparison to microtubules assembled from dimers with slowly hydrolyzed GTP analogs bound to the β subunit.
Microtubule Dynamics in Cells
Microtubule dynamics in cells (Fig. 34-8A) are remarkably similar to those in simple buffers in vitro (Table 34-1). The centrosome nucleates and stabilizes the minus ends of most microtubules. At any moment in time, the plus ends of these microtubules are growing, as revealed by the presence of tip-binding proteins (Fig. 34-14). Despite catastrophes about once each minute (k = 0.01s−1), during which they shorten rapidly (0.28μms−1), interphase microtubules generally are long, both because the chance of rescue is high (k = 0.05s−1) and because steady growth during the elongation phase (at a rate of 0.11μms−1) restores their length before the next catastrophe. As a result of dynamic instability, thebulk of interphase microtubules have a half-life of about 10 minutes.
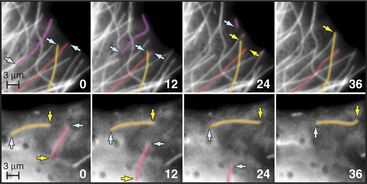
Dynamic instability allows the plus ends of microtubules to explore the entire cytoplasm as they search for targets such as kinetochores on chromosomes during mitosis (Fig. 34-2A) and to deliver tip-binding proteins, including signaling proteins, to the cell cortex. Fluctuations in microtubule length can do mechanical work such as moving cargo away from the center of the cell (see Fig. 37-6), positioning the nucleus in the center of fission cells as in yeast, or locating the mitotic spindle off center in cells that divide asymmetrically. Reciprocally, the local environment can influence the behavior of the microtubules as they explore the cytoplasm. For example, signaling pathways in the cortex involving Rho-family GTPases and kinases can stabilize microtubules locally and polarize the microtubule array.
Microtubules can also treadmill in the cytoplasm, growing at one end and shrinking at the other. This behavior is common in plants in which dynamic stability at the plus end is biased toward net growth and minus ends depolymerize steadily. Treadmilling is seen in animal cells when an occasional microtubule detaches from the centrosome (Fig. 34-8B).
Regulation by Microtubule-Associated Proteins
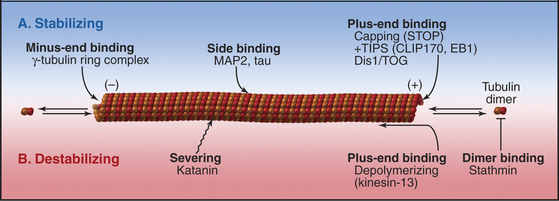
The properties of pure tubulin cannot explain all microtubule behavior in cells, which use microtubule-associated proteins (MAPs) to regulate initiation, elongation, shortening, catastrophes, and rescues (Appendix 34-1 and Fig. 34-9). MAPs stabilize some microtubules, such as those in ciliary axonemes. Tubulin purified from axonemes forms microtubules that are just as labile as cytoplasmic microtubules, but microtubules in axonemes with their associated MAPs are stable for days, even under conditions that would depolymerize cytoplasmic microtubules. Modulation of the activity of MAPs allows cells to change the dynamics of microtubules, as when dynamic instability becomes more pronounced when the cell enters mitosis (Fig. 34-2A). Developmentally programmed gene expression establishes the mix of MAPs in each cell type.
Microtubule-Stabilizing MAPs
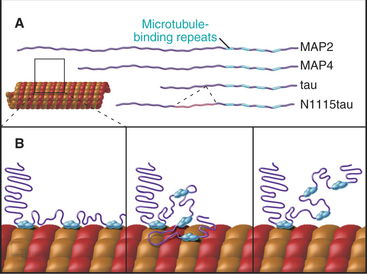
At least a dozen distinct MAPs stabilize microtubules (Appendix 34-1). Most bind along the length of microtubules, but some interact only at or near ends. Some are expressed widely, but others are restricted to specialized cells. Members of the tau family, including MAP2 and MAP4, differ in size and pattern of expression but share many common features (Fig. 34-10). These MAPs are abundant in brain, the historical tissue of choice for isolation of microtubules.
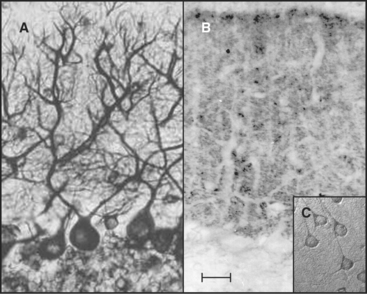
Tau, named for tubulin-associated protein, is the major MAP in the axons of neurons in vertebrate brains (Fig. 34-12B). It is also present in some neuronal cell bodies and glial cells. Alternate splicing produces seven tau isoforms from a single tau gene. Most of these isoforms have molecular weights of 40 to 50 kD, but a higher-molecular-weight tau is found in peripheral nerves. As is expected from its ability to stabilize microtubules in vitro, reduction in the tau concentration in cultured nerve cells by depletion of the mRNA reduces the numbers of microtubules. Nevertheless, mice survive the loss of their single tau gene with only minor alterations of their neurons. Compensation by other MAPs is postulated to account for the differences in the acute and chronic loss of tau.
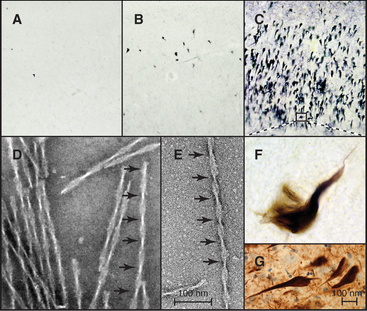
In Alzheimer’s disease, the most common dementia of older persons, as well as other neurodegenerative diseases and strokes, tau forms intracellular paired helical filaments (Fig. 34-13) that aggregate in “neurofibrillary tangles.” These tangles are a hallmark of Alzheimer’s disease, and their number judged by light microscopy correlates with the severity of the dementia. Dozens of different tau mutations are responsible for rare cases of inherited dementia, but for individuals with normal tau, excess phosphorylation can cause tau to dissociate from microtubules and leads to its fragmentation by proteolysis. Over time, short, phosphorylated tau fragments assemble into highly insoluble paired helical filaments and tangles. It is not known which molecular species along this pathway cause neuronal degeneration. Inhibition of the several kinases that initiate the conversion of tau into tangles is a possible therapeutic strategy.
MAP2 is concentrated in dendrites of neurons (Fig. 34-12A). The mechanism giving preferential distribution of MAP2 in dendrites and tau in axons of the same cell is still largely a mystery. MAP4 shares many features with MAP2 but is expressed by glial cells in the nervous system and by many other cells and tissues. High-molecular-weight MAP1A and 1B are related proteins that are distinct from the tau family, but they also form rod-shaped projections on the surface of microtubules in neurons. As in the case of tau, mice with null mutations of MAP1B are viable with minimal defects.
A variety of other proteins stabilize microtubules in distinct ways. Vertebrates have a protein called STOP that binds microtubules, making them resistant to depolymerization by cold, dilution, or drugs. Bird red blood cells express syncolin, which stabilizes the marginal band of microtubules. Tektins are fibrous proteins that stabilize microtubules in axonemes (see Fig. 38-16) and centrioles.
Microtubule-Destabilizing MAPs
Second, two classes of microtubule motor proteins, kinesin-13 and kinesin-8, promote microtubule disassembly by forming rings around the microtubules. These kinesins were discovered independently in several systems, leading to a variety of historic names. Most kinesins have a motor domain at one end of the polypeptide and use ATP hydrolysis to move cargo along the sides of microtubules (see Fig. 36-13). Kinesin-13 is not a typical motor. The ATPase domain in the middle of the polypeptide binds with high affinity to curved protofilaments that peel off the ends of microtubules. Energy from ATP hydrolysis is used to promote catastrophes and disassembly. Different isoforms of kinesin-13 are concentrated at the two ends of microtubules in the mitotic spindle—with minus ends at the poles and plus ends at kinetochores—where they regulate the lengths of the two ends (see Chapter 44).
Third, katanin, an AAA ATPase (see Box 36-1), uses ATP hydrolysis as the energy source to sever microtubules into short fragments. It disrupts the noncovalent bonds between the subunits in the microtubule wall. Tubulin dimers dissociate from the cut ends and return to the cytoplasmic pool for reassembly. Activation of severing at the onset of mitosis might contribute to remodeling the interphase microtubule network.
MAPs Associated with Growing Microtubule Plus Ends
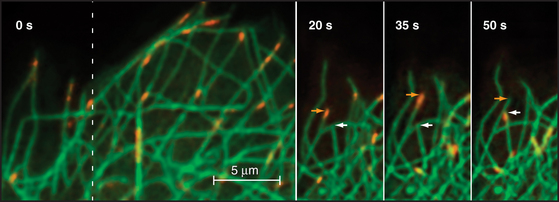
Given the dynamic way that microtubules probe the cytoplasm, the plus-end is an ideal location for proteins that regulate microtubule dynamics and connect microtubules to membranes. Indeed, several proteins concentrate at the plus ends of growing microtubules (Fig. 34-14). These plus-end tracking proteins (+TIPs) are widely distributed in eukaryotes. End-binding protein 1 (EB1 in vertebrates) is also found in plants and Giardia. CLIP-170 is found in vertebrates as well as budding and fission yeasts. Dynamic microtubules carry CLIP-170/Tip1p and an associated protein, Tea1p, to the ends of S. pombe cells (see Fig. 6-3D), where Tea1p directs the polar growth of the cell. EB1 and CLIP-170 interact with each other and a complex of other proteins. Both stabilize plus ends by reducing the frequency of catastrophes. +TIPS are involved in membrane transport along microtubules (see Fig. 37-6), but the details are still being investigated.
Three different mechanisms contribute to the association of +TIPs with plus ends. Experiments in budding and fission yeast provide evidence that some +TIPs are carried to plus ends by kinesin motors or relocated onto newly incorporated GTP subunits. Animal CLIP-170 and EB1 do not actually move along microtubules but are concentrated on plus ends because of a higher affinity for tubulin dimers than microtubules. Tubulin dimers carry CLIP-170 piggyback onto plus ends, where it dissociates quickly and recycles back to the cytoplasm. Consequently, such +TIPs are not found on the plus ends of shortening microtubules (Fig. 34-14).
Several other proteins that function at or near the plus ends of microtubules are not classical +TIPS. These include the chromosomal passenger protein INCENP (see Fig. 44-10), which targets the aurora-B kinase to the central spindle and regulates its kinase activity toward spindle components. An emerging family of MAPs that includes human CLASP appears to be required to regulate the dynamic behavior of microtubule plus ends near the cell cortex in interphase and at kinetochores during mitosis.
Linker Proteins
Protein connectors link microtubules to many other cellular structures. For example, MAP2 links to actin filaments, and plectin links to intermediate filaments. Gephyrin binds microtubules and is required for clustering glycine receptors (see Fig. 10-12) in the plasma membrane of neurons.
The Centrosome
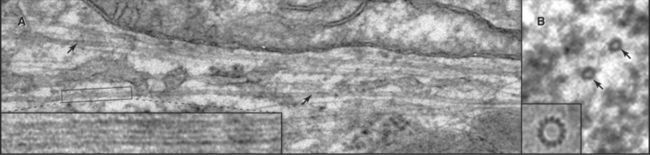
When Flemming and Van Beneden discovered the centrosome in 1875, it was thought to be one of the three main cell components, together with the nucleus and the cell body. In fact, it was correctly regarded as a key organelle that regulates mitosis and was called the “dynamic centre” of the cell by Boveri, who coined the term centrosome. The largecentrosomes of certain eggs appeared to contain one or two central granules, which Boveri called “centrioles” (Fig. 34-15). Centrioles are the “architects of centrosomes,” spatially organizing the organelle, but some cells possess bona fide centrosomes that lack centrioles.
Most metazoan cells have a centrosome containing a centriole. Multinucleated animal cells such as megakaryocytes (see Fig. 28-7) and osteoclasts (see Fig. 32-6) have multiple centrosomes. On the other hand, vertebrate muscle cells and mature oocytes lack centrosomes, as do the cells of lower plants such as ferns, which have basal bodies only in male gametes. Since diverging from animals about a billion years ago, most plants and fungi lost their centrioles but retain other structures that serve as microtubule-organizing centers.
Organization of the Centrosome
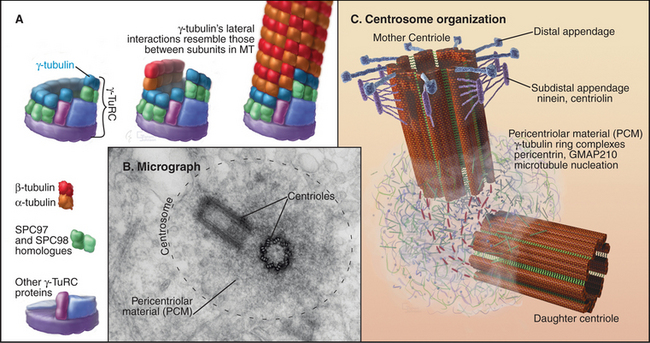
In most cells, the centrosome is organized around two cylindrical centrioles about 0.5μm long by about 0.2μm in diameter and composed of nine microtubule triplets (Fig. 34-16). Centrioles and basal bodies, located at the base of cilia and flagella (see Fig. 38-17), are equivalent structures. Both were present in ancient eukaryotes, since they are found in organisms that branched earliest from the eukaryotic lineage (see Fig. 2-4). In most vertebrate tissues, centrioles retain the capacity to grow a nonmotile primary cilium, which serves as a sensory organelle (see Fig. 38-21). Similarly, flagella grow from both poles of the meiotic spindle of divid-ing spermatocytes of species such as the silk mothBombyx mori.
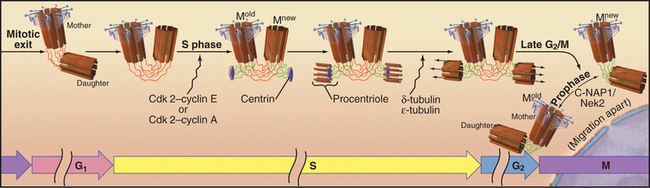
Cells that are about to enter mitosis have three types of centrioles that differ in age, structures, and activities. Two distinct mother centrioles, Mold and Mnew, are each linked to one of two identical daughter centrioles. Mold was assembled at least two cell cycles previously. Mnew was assembled in the previous cell cycle, and the daughter centrioles were assembled during the S phase (DNA synthesis phase) of the present cell cycle. Mold is characterized by the presence of distal and subdistal appendages (Fig. 34-16C), which function in microtubule anchoring. Although the pericentriolar material that surrounds both mother and daughter centrioles nucleates microtubules, the microtubules are predominantly anchored near Mold. Consequently, Mold acts as the cell center, surrounded by a radial array of microtubules (Fig. 34-2A) with their minus ends at the centrosome and plus ends at the periphery. In some G0 cells (cells that are no longer actively proliferating—see Chapter 41), the daughter centriole is motile. Centrosomes usually associate tightly with the nucleus, allowing the microtubule network to anchor it to the cell cortex during cell migration. This is important for nuclear positioning and migration in fungi, as well as during brain development in vertebrates.
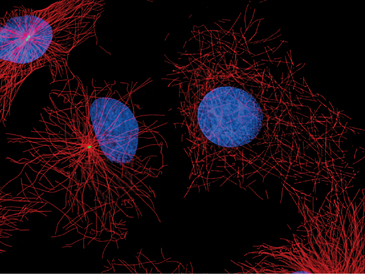
Centrosomes and their associated microtubules contribute to the shape of specialized cells. The array of polarized microtubules radiating from the centrosome in leukocytes and fibroblasts accounts for the fact that membrane bound organelles have a specific distribution within the cell. Membranes associated with minus-end-directed motors such as cytoplasmic dynein accumulate near the centrosome. The most prominent example is the Golgi apparatus. After disruption of centrosomes by RNA interference or by laser microsurgery, cytoplasmic microtubules emanating from the centrosome disappear, but other cytoplasmic microtubules persist (Fig. 34-17). In many differentiated cells, microtubules initially nucleated at centrosomes subsequently detach and become anchored elsewhere in the cytoplasm, for example at the apical membrane in epithelial cells (Fig. 34-2B). In neurons, microtubules detach from the centrosome and are transported along axons and dendrites (see Fig. 37-5).
A Few Key Proteins of the Centrosome
The most prominent protein components of centrioles are α-,β,γ. and e-tubulin (Fig. 34-16). Centriolar microtubules are more stable than most cytoplasmic microtubules, exchanging only about 10% of their tubulin per cell cycle. Like other stable microtubules, the α- and β-tubulins of centrioles are highly modified by polyglutamylation. Microinjection of cells with antibodies to glutamylated tubulin causes centrioles and centrosomes to disassemble.
Centrioles also contain multiple isoforms of centrin (see Fig. 38-6), EF-hand, Ca2+ binding proteins similar to calmodulin (see Fig. 3-12). Centrins are essential for the biogenesis of centrioles and spindle pole body biogenesis in yeasts. In Chlamydomonas, centrins form links between the centrioles and the nucleus. Despite their association with centrioles, most centrins are cytoplasmic; and in the cytoplasm, their functions are unknown.
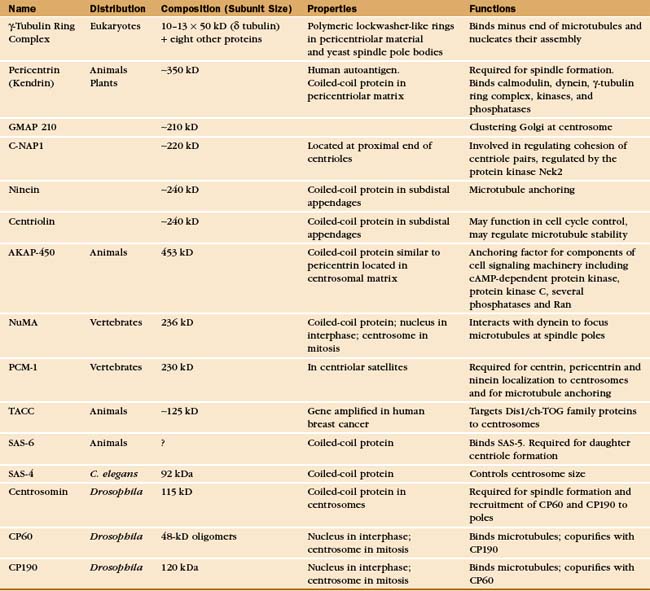
Additional proteins make up other functionally important centriolar structures (Appendix 34-2). The subdistal appendages of the mother centriole contain ninein, centriolin, and presumably a number of other proteins. Ninein is thought to play some role in anchoring the minus ends of microtubules to the centrosome. Centriolin appears to both anchor cell cycle control proteins to the centrosome and regulate microtubule stability.
The pericentriolar material contains many more proteins; up to 110 were identified in one proteomic study. The best characterized are components of the γ-tubulin ring complex (usually abbreviated gTuRC), which is primarily responsible for nucleating microtubules with 13 protofilaments in higher eukaryotes. This complex of 10 to 13 γ-tubulin molecules and 8 associated polypeptides resembles a dislocated ring or lock washer. It is now thought that the γTuRC acts as a seed from which α/β tubulin dimers polymerize in a polar manner (Fig. 34-16A). Consistent with this, the complex appears to cap the minus ends of microtubules that it initiates. Many microtubules initiated by γ-tubulin ring complexes subsequently detach, possibly as a result of being severed by katanin (Fig. 34-9). The pericentriolar material surrounding the mother centriole is then thought to recapture the slow-growing minus ends of these microtubules.
The other pericentriolar material proteins assemble a matrix around the centrioles. About 75% of these proteins are predicted to contain a-helical coiled-coils. Well-studied proteins include pericentrin/kendrin, C-NAP1 (see later), and GMAP210, among many others. Pericentrin/kendrin is a large coiled-coil protein that was identified using autoantisera from scleroderma patients (see Fig. 13-22). It binds calmodulin plus several regulatory enzymes, including protein kinases and phosphatases. Pericentrin can also bind dynein and the γ-tubulin ring complex, presumably contributing to the transport and organization of centrosomal components. Another coiled-coil protein, GMAP210 appears to be involved in, among other things, clustering of the Golgi apparatus around the centrosome.
Centrosome Duplication
The cycle of semiconservative centrosome duplication and division is closely linked to the cell cycle (Fig. 34-18; see Chapter 40 for an introduction to the cell cycle). Centrosome replication begins during S phase, when the nuclear DNA is also replicated. This connection plus the semiconservative mode of duplication initially suggested that centrioles, like mitochondria and chloroplasts, might have their own genomes. However, genetic experiments in the green algaChlamydomonas showed that nuclear genes regulate centriolar behavior, and careful morphologic analysis showed that centrioles lack their own DNA. Centriole duplication requires two cyclin dependent kinases (Cdk2–cyclin E and/or Cdk2–cyclin A; the latter is more important in mammalian cells). However, the critical phosphorylated target proteins are unknown.
The mother and daughter centrioles lose their strict orthogonal orientation during the exit from mitosis but remain linked at their proximal ends, possibly by protein fibers of the centrosomal matrix (Fig. 34-18). In G1 phase, the daughter moves slightly away from its mother. In S phase, each centriole initiates the formation of a new daughter, thus converting the previous daughter into a new mother centriole, Mnew. Newly formed daughter centrioles are oriented at right angles to what is known as the proximal end of the mother centrioles. Centriole formation is thought to begin with a disk-like structure containing centrin, which nucleates the assembly of a ring of nine single microtubules, the procentriole. The ring is converted into an array of nine triplets in a process that requires δ- and σ-tubulin. In organisms such as Drosophila andCaenorhabditis elegans, that lack these specialized tubulin isoforms, centrosomes are built of doublet and singlet microtubules. The daughter centrioles gradually lengthen during the remainder of the cell cycle, reaching their mature length just before mitosis. Then Mnew acquires distal and subdistal appendages and becomes morphologically and biochemically equivalent to Mold. The two replicated centrosome pairs separate during prophase of mitosis and in many cells then migrate apart over the surface of the nuclear envelope to set up the two poles of the mitotic spindle.
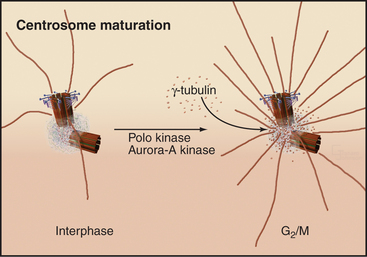
The capacity of centrosomes to nucleate microtubules fluctuates during the cell cycle. As cells enter mitosis, the microtubule-nucleating activity of centrosomes increases dramatically. This “maturation” is associated with accumulation of γ-tubulin, apparently dri-ven by the action of the Polo and Aurora-A kinases (Fig. 34-19).
Centrosomes and astral microtubules also contribute to both the initiation of cytokinesis (by helping to specify the position of the contractile ring; see Fig. 44-22) and the completion of cytokinesis. As the cleavage furrow forms during late anaphase, mother and daughter centrioles detach from each other. In some cells, the mother centriole moves transiently into the intercellular bridge between the two daughter cells just before they separate. These movements of the mother centriole might somehow influence the cleavage of the bridge.
The “Centrosome” of Yeasts
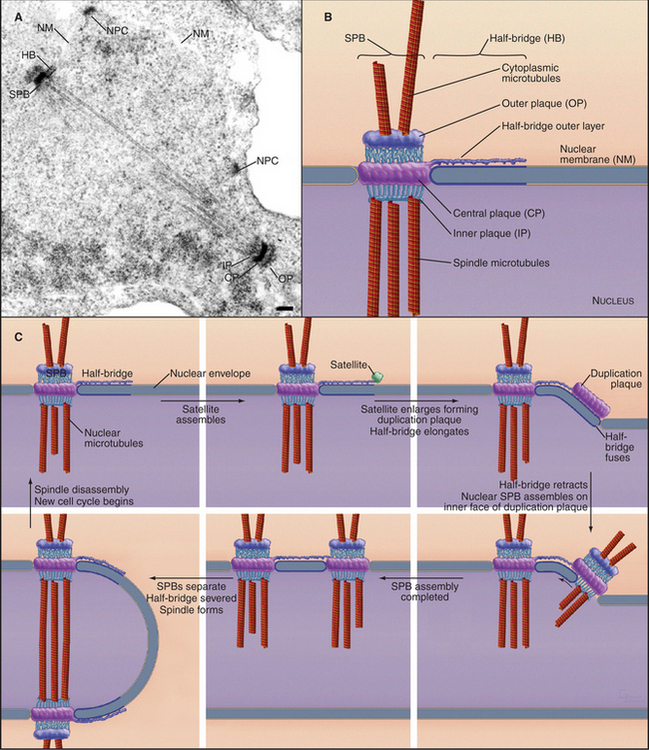
For many fungi, including budding and fission yeasts, the role of the centrosome is played by the spindle pole body (SPB), a plaque-like structure embedded in the nuclear envelope (Fig. 34-20). Like centrosomes, SPBs organize the microtubule cytoskeleton, particularly during mitosis. Centrin and γ-tubulin are two of the 45 proteins that are known to be associated with SPBs, but few of the other proteins have been found in vertebrate centrosomes. A small tetrameric complex containing two γ-tubulins and two distinct but related proteins is sufficient for yeast SPBs to nucleate microtubules. SPB duplication is analogous to centrosome duplication in that a new SPB forms adjacent to and attached to the original SPB during the S phase of the cell cycle. As with centrosomes, SPB duplication is therefore tightly linked to cell cycle progression.
Fungal SPBs regulate mitosis and cytokinesis. In fission yeast, a GTPase and a series of three kinases associate transiently with SPBs before triggering constriction of the contractile ring and formation of septa (see Fig. 44-24). The corresponding GTPase in budding yeast, Tem1p, is anchored to the SPB by a protein that resembles a portion of mammalian centriolin. The guanine nucleotide exchange factor that activates Tem1p is concentrated in the bud, far from either SPB, until the elongating mitotic spindle relocates an SPB to the bud during anaphase. Only then can the guanine nucleotide exchange factor activate the GTPase and trigger a signaling cascade that ultimately drives the cell out of mitosis.
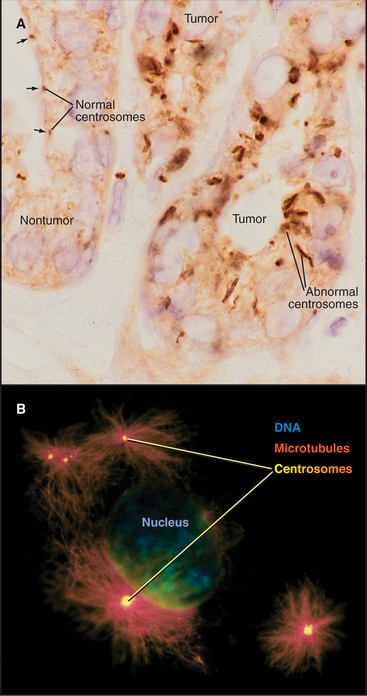
Centrosomes and Cancer
The rediscovery that centrosome abnormalities are common in cancer cells has contributed to the resurgence of interest in centrosomes. Centrosomal defects in malignant cells include enlarged size (5 to 10 times larger than normal), abnormal shape, and increased number (Fig. 34-21). In one study, 217 out of 227 high-grade tumors had centrosomal abnormalities. Overexpression of the Aurora-A protein kinase can cause centrosomal abnormalities. The Aurora-A genetic locus is amplified in certain cancers. Furthermore, the kinase can function as an oncogene when overexpressed in tissue culture cells, causing the cells to lose their normal growth regulation and to form tumors when the cells are injected into mice.
A link between centrosomes and cell cycle progression was discovered when researchers studied the results of surgically removing the centrosome. Cells that lack centrosomes complete the cell cycle, enter mitosis, and assemble apparently normal bipolar mitotic spindles (presumably by a pathway that involves the organization of spindle poles by microtubule motor proteins that bundle and remodel microtubule networks). After a lengthy delay, the cells exit mitosis, and about 40% manage to complete cytokinesis. The surprise is that after completing mitosis, these cells never rereplicate their DNA or enter a subsequent mitotic cycle. Thus, the centrosome appears to be required to pass the G1 restriction point and to enter S phase (see Fig. 41-7). Chapter 41 explains how defects in this key control point in the cell cycle are common in many types of cancer.
Centrosomal abnormalities in cancer arise when cells fail to complete cytokinesis, giving rise to progeny that have twice the normal content of DNA, number of chromosomes, and number of centrosomes. It has been suggested that normal cells have a checkpoint that is dependent on the p53 tumor suppressor protein (see Fig. 41-13) that arrests the cycle of cells that fail to complete cytokinesis. Cell lines from mice that lack p53 often have multiple centrosomes. Cancer cells with multiple centrosomes are prone to error when they attempt to segregate their chromosomes during subsequent divisions. The loss or gain of chromosomes can contribute to uncontrolled cell proliferation if the balance between growth-promoting oncogenes and growth-regulating tumor suppressor genes is upset.
Akhmanova A, Hoogenraad CC. Microtubule plus-end-tracking proteins: Mechanisms and functions. Curr Opin Cell Biol. 2005;17:47-54.
Beisson J, Wright M. Basal body/centriole assembly and continuity. Curr Opin Cell Biol. 2003;15:96-104.
Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25-34.
Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18-24.
Dogterom M, Kerssenmakers JWJ, Romet-Lemonne G, Janson ME. Force generation by dynamic microtubules. Curr Opin Cell Biol. 2005;17:67-74.
Doxsey S. Re-evaluating centrosome function. Nat Rev Mol Cell Biol. 2001;2:688-698.
Doxsey S, McCollum D, Theurkauf W. Centrosomes in cellular regulation. Annu Rev Cell Dev Biol. 2005;21:411-434.
Drewes G. MARKing tau for tangles and toxicity. Trends Biochem Sci. 2004;29:548-555.
Dutcher SK. Long-lost relatives appear: Identification of new members of the tubulin superfamily. Curr Opin Microbiol. 2003;6:634-640.
Gundersen GG, Gomes ER, Wen Y. Cortical control of microtu-bule stability and polarization. Curr Opin Cell Biol. 2004;16:106-112.
Job D, Valiron O, Oakley B. Microtubule nucleation. Curr Opin Cell Biol. 2003;15:111-117.
Kreis T, Vale R, editors. Guidebook to the Cytoskeletal and Motor Proteins, 2nd ed., New York: Oxford University Press, 1999.
Lange BM. Integration of the centrosome in cell cycle control, stress response and signal transduction pathways. Curr Opin Cell Biol. 2002;14:35-43.
Marshall WF. Centrioles take center stage. Curr Biol. 2001;11:R487-R496.
Moritz M, Agard DA. Gamma-tubulin complexes and microtubule nucleation. Curr Opin Struct Biol. 2001;11:174-181.
Nigg EA. Centrosome aberrations: Cause or consequence of cancer progression? Nat Rev Cancer. 2002;2:815-825.
Nogales E. Structural insights into microtubule function. Annu Rev Biophys Biomol Struct. 2001;30:397-420.
Ohkura H, Garcia MA, Toda T. Dis1/TOG universal microtubule adaptors: One MAP for all? J Cell Sci. 2001;114:3805-3812.
Westerman S, Weber K. Post-translational modifications regulate microtubule function. Nature Rev Molec Cell Biol. 2003;4:938-947.
Wordeman L. Microtubule depolymerizing kinesins. Curr Opin Cell Biol. 2005;17:81-88.