CHAPTER 388 Microsurgical Management of Arteriovenous Malformations
This chapter is intended to address aspects that are found to be most relevant when attempting to treat cerebral arteriovenous malformations (AVMs) microsurgically. Although other chapters in this volume are devoted to nonmicrosurgical AVM treatment modalities, in this chapter we provide a brief discussion of the aspects that we have found most important in the treatment of AVMs by embolization and radiosurgery. We know that different treatment modalities for AVMs are frequently presented as “alternatives” to the patient, but we try to make the point that for each patient there is only one “best” alternative, although that alternative may include more than one modality (e.g., embolization followed by microsurgery). The different modalities of treatment and treatment paradigms should not be presented as interchangeable.
In this chapter we concentrate on decision making, as well as on the operative strategies and technical considerations that the senior author (R.C.H.) has found to be most useful.
General Considerations
Although much information on etiology, pathophysiology, natural history, and clinical findings is covered in other chapters, a brief review of this topic, from our perspective, seems necessary before directly discussing microsurgical treatment.
In principle, AVMs are arteriovenous shunts composed of feeding arteries that connect into an abnormal vascular nest (nidus), which in turn is drained by abnormal veins. Consequently, there is no or only an abnormal capillary bed. The so-called nidus is the network of channels that is interposed between the feeding arteries and draining veins. A compact nidus is characterized by a well-circumscribed vascular network, whereas a diffuse nidus refers to an abnormal vascular network that is more widespread within normal brain parenchyma.1 In both cases the abnormal vessel conglomerate is intermingled with gliotic brain tissue. This pathology needs to be differentiated from a dural AVM, or fistula, which has no nidus but a more direct communication between enlarged arteries and veins. AVMs are thought to be congenital in nature, but they frequently grow during childhood, adolescence, and young adulthood. It is rare for them to grow significantly in more mature adults. Therefore, it is important to stress that these lesions are not neoplastic and thus the term “angioma” should be avoided because it is inappropriate. Yet it is becoming more and more evident that these lesions also exhibit dynamic features that make them change over time, a fact that could influence even “successfully treated” AVMs.
It is obvious that a good understanding of the natural history of a disease is essential for the process of decision making if one is to weigh the risks and potential benefits of different treatment modalities against the risk of doing nothing. Fortunately, we already have several very good studies in the literature that give us robust data about the natural history of both AVMs that are initially manifested as hemorrhage and those that have never bled.
Table 388-E1 ![]() isa compilation of the natural history studies that we consider most important.2–7 In summary, patients with cerebral AVMs bleed at a rate of approximately 2% to 4% per year. For decision making and timing of potential procedures, it is crucial to understand that this rate of bleeding is similar in most studies regardless of whether the patient has ever bled, although patients initially seen with hemorrhage have about twice the risk for rebleeding during the first year. It appears clear, then, that unruptured cerebral AVMs have a much greater annual rate of bleeding than unruptured aneurysms do, although of course the consequences of hemorrhage from an aneurysm are worse than those of hemorrhage from an AVM; AVM hemorrhages result in significant morbidity in about 25% to 30% of patients and death in approximately 10%.8
isa compilation of the natural history studies that we consider most important.2–7 In summary, patients with cerebral AVMs bleed at a rate of approximately 2% to 4% per year. For decision making and timing of potential procedures, it is crucial to understand that this rate of bleeding is similar in most studies regardless of whether the patient has ever bled, although patients initially seen with hemorrhage have about twice the risk for rebleeding during the first year. It appears clear, then, that unruptured cerebral AVMs have a much greater annual rate of bleeding than unruptured aneurysms do, although of course the consequences of hemorrhage from an aneurysm are worse than those of hemorrhage from an AVM; AVM hemorrhages result in significant morbidity in about 25% to 30% of patients and death in approximately 10%.8
Spontaneous obliteration of an AVM is rare, but does occur, and has been reported in single cases.9–13 Several factors appear to be associated with spontaneous occlusion: a single draining vein, a solitary arterial feeder, and nidus size smaller than 3 cm.14,15
In the process of decision making, it is important to try to understand what factors may influence the rate of bleeding, which undoubtedly is not identical for every patient with an AVM. Several anatomic and hemodynamic factors seem to increase the risk for bleeding. Apart from associated aneurysms on the feeding artery (see later), the significance of nidus size has engendered controversy, with some suggesting that small AVMs bleed more frequently.16,17 Deep location has been suggested to increase the risk for hemorrhage (basal ganglia, periventricular or intraventricular space, posterior fossa),18,19 as has deep venous drainage,20 feeding by perforators, and location in the vertebrobasilar system.21 We believe that an intraventricular or periventricular location has a higher risk for hemorrhage, although we cannot substantiate this opinion with numbers. The role of feeding artery and draining vein pressure in this regard has been investigated. Norbash and coworkers saw no correlation in a group of 32 patients.22 In a large series of 340 reviewed patients, Duong and colleagues identified “high arterial input pressure” and “venous outflow restriction (exclusively deep venous drainage)” as the most powerful risk predictors for a hemorrhagic AVM manifestation.23 We also believe that venous outlet obstruction potentiates the risk for hemorrhage. Spetzler and colleagues associated AVM size smaller than 3 cm with higher feeding artery pressure and larger hemorrhage.16 Graf and collaborators calculated a 5-year risk for hemorrhage of 10% for AVMs larger than 3 cm and 52% for AVMs smaller than 3 cm.2 Whether smaller size really results in a higher risk for bleeding is still subject to controversy.
Pregnancy does not seem to increase the likelihood of hemorrhage.24,25 Horton and colleagues reviewed the cases of 451 women with AVMs who had 540 pregnancies, 17 of which were complicated by hemorrhage. None of the hemorrhages occurred during labor, vaginal delivery, or cesarean section. The authors concluded that the method of delivery should be based on obstetric consideration alone and the decision to operate after intracranial hemorrhage “should be based on neurosurgical principles.”25
Because of their size, giant AVMs differ somewhat in their typical findings from smaller lesions. Very large malformations are frequently manifested not only as hemorrhage or seizures (or both) but also as transient and progressive neurological dysfunction because of steal phenomena; the large shunt volume can initiate relative hypoperfusion in the surrounding functional parenchyma that sometimes results in ischemia.
Microsurgical Treatment
Indications for Microsurgical Treatment
Patient-Related Factors
When evaluating a patient for treatment of an AVM, we must consider factors such as age, general health, neurological status, history of recent or past hemorrhage, symptoms from the AVM, occupation and hobbies, and psychological makeup. The patient’s age is most important in determining the cumulative risk for AVM rupture during the remainder of the patient’s life expectancy. Assuming an annual hemorrhage rate of 2% to 4% and an average life expectancy of 70 years, the cumulative risk (in percentage) for AVM rupture may be estimated by the following formula: 105 minus the patient’s age in years.26,27 Younger age may thus justify a more aggressive approach because the cumulative risk is so high. At the same time, of course, it is easier for the young to tolerate a prolonged operation, and the chances for satisfactory recovery from any neurological deficit that might occur are better. Likewise, the general health of the patient needs to be taken into account. The current overall and neurological condition of a patient may dictate the timing, and severe comorbid conditions may preclude surgery. The occupation and lifestyle of a patient are also important aspects to consider. For a schoolteacher or lawyer it may be intolerable to have a speech deficit, but a mild visual field deficit might not hinder them from practicing their profession, whereas the latter would be intolerable for a pilot or truck driver. Some patients simply cannot live with the thought of harboring a brain lesion that has a relatively high potential to bleed at some point, whereas others live perfectly well with this knowledge and might prefer to decline treatment.
Arteriovenous Malformation–Related Factors
Location, size, configuration (compact versus diffuse), location and pattern of arterial supply, presence of deep perforators, location and pattern of venous drainage, evidence of outflow obstruction, associated aneurysms, blood flow to the AVM, evidence of “steal,” and fresh or old hematoma from the AVM are all factors that must be considered in determining the feasibility of surgery and the involved surgical risk.28 To help in this process, several classifications have been developed,29 including the one that is used most frequently today—the Spetzler-Martin grading scheme.30 Although such a classification is very helpful, especially in terms of reporting and comparing results, no classification could possibly take all the necessary variables into account that an experienced surgeon should consider when estimating surgical risk. Such factors as the presence of deep perforator arterial supply,31 location of the venous drainage, nidus configuration, and others would be difficult to account for in a classification that is still of practical use.32 Other classifications that have attempted to specifically address the risk for hemorrhage from an AVM (in contrast to surgical risk) never gained popularity in daily use (e.g., that of Nataf and coworkers20).
Surgeon-Related Factors
Finally, experience, availability of embolization and radiosurgery, familiarity with the results and morbidity of each treatment modality, supporting facilities, appropriate intensive care unit, anesthesia, intraoperative angiography, and the availability of experienced surgical assistance must be considered.
As will be emphasized, a good understanding by the surgeon of how to select the most appropriate treatment for the patient seems to be as important as mere technical skills at surgery. Besides considerable experience with all the surgical aspects, good understanding of the indications, efficacy, and complications of other treatment modalities is essential. We have emphasized repeatedly that it is the ethical responsibility of the concerned surgeon to give an answer-seeking patient a clear opinion of what in his or her view would be the best treatment of the patient’s AVM. This means that after weighing the pros and cons of the different modalities and their combination, the surgeon should supply the patient with one straightforward and understandable treatment recommendation. If not possible, it is prudent to refer the patient to a more experienced colleague. Of course, it is always the patient’s prerogative to accept or reject the recommendation, but an expert recommendation should never have the form of a potpourri of treatment modalities from which to choose. To this effect, the microsurgeon must have a thorough understanding of other treatment modalities that may be used in preference to or in combination with microsurgery. These other treatment modalities (embolization and radiosurgery) are covered in detail in other chapters but are discussed here from the perspective of microsurgery.
Embolization
Embolization is used with curative intention, as a palliative maneuver, or before surgical excision33,34 or radiosurgery. Partial embolization has its indications for palliation and presurgical treatment, but even in experienced hands it carries substantial risk. As can be seen from the review in![]() Table 388-E2,35–53 embolization in all its forms has morbidity and mortality rates that are not negligible, with morbidity ranging from 2% to 27% and mortality from 0% to 8%. Preoperative embolization has made it possible to operate on cerebral AVMs that before this technique became available could not undergo surgery without risking substantial morbidity. Frequent use has also been made of pre-radiosurgical embolization. The rationale to “downsize” large inoperable AVMs in an attempt to reach a so-called critical size for effective Gamma Knife surgery is very weak in our opinion. In many of these cases radiosurgery fails to completely obliterate the nidus, and in fact it has been shown to reduce the obliteration rate of linear accelerator radiosurgical treatment.54 Previous embolization had been identified as a negative predictor of successful AVM radiosurgery.55 After it became evident that particle embolization before radiosurgery resulted in a 15% to 20% recanalization rate, it was generally abandoned and liquid embolic agents are now preferred.55–57 However, with these agents the problem is inadvertent embolization of the feeding artery, thus resulting in proximal occlusion with only minimal or no nidal penetration.33 Inadvertent, premature closure of draining veins is a specific significant risk with all liquid agents. With a partially “open” nidus, creation of new arteriovenous shunts can be anticipated. In addition, there is no evidence that pre-radiosurgical embolization reduces the risk for hemorrhage during the critical period after radiosurgery until the induced devascularization and fibrosis lead to complete obliteration of the nidus.33 This is why in general we see only rare indications for preradiosurgical embolization.
Table 388-E2,35–53 embolization in all its forms has morbidity and mortality rates that are not negligible, with morbidity ranging from 2% to 27% and mortality from 0% to 8%. Preoperative embolization has made it possible to operate on cerebral AVMs that before this technique became available could not undergo surgery without risking substantial morbidity. Frequent use has also been made of pre-radiosurgical embolization. The rationale to “downsize” large inoperable AVMs in an attempt to reach a so-called critical size for effective Gamma Knife surgery is very weak in our opinion. In many of these cases radiosurgery fails to completely obliterate the nidus, and in fact it has been shown to reduce the obliteration rate of linear accelerator radiosurgical treatment.54 Previous embolization had been identified as a negative predictor of successful AVM radiosurgery.55 After it became evident that particle embolization before radiosurgery resulted in a 15% to 20% recanalization rate, it was generally abandoned and liquid embolic agents are now preferred.55–57 However, with these agents the problem is inadvertent embolization of the feeding artery, thus resulting in proximal occlusion with only minimal or no nidal penetration.33 Inadvertent, premature closure of draining veins is a specific significant risk with all liquid agents. With a partially “open” nidus, creation of new arteriovenous shunts can be anticipated. In addition, there is no evidence that pre-radiosurgical embolization reduces the risk for hemorrhage during the critical period after radiosurgery until the induced devascularization and fibrosis lead to complete obliteration of the nidus.33 This is why in general we see only rare indications for preradiosurgical embolization.
Complete cure of AVMs with embolization alone is usually very difficult to achieve, although in very experienced hands success rates have reached 40% ![]() (Table388-E2). Complete cure in this context should be defined as disappearance of the nidus and early venous drainage. Generally, only relatively “simple” AVMs with readily accessible feeders are potential candidates for a curative endovascular approach. However, in many instances these AVMs are also the ones that are easy to remove surgically with minimal morbidity but with a higher certainty of complete resection. Therefore, we would recommend attempts at endovascular curative obliteration only for lesions that in addition to accessible feeders have a deep, critical location, where the risk associated with surgery would exceed the risk related to embolization, or in patients with advanced age or significant comorbid conditions.
(Table388-E2). Complete cure in this context should be defined as disappearance of the nidus and early venous drainage. Generally, only relatively “simple” AVMs with readily accessible feeders are potential candidates for a curative endovascular approach. However, in many instances these AVMs are also the ones that are easy to remove surgically with minimal morbidity but with a higher certainty of complete resection. Therefore, we would recommend attempts at endovascular curative obliteration only for lesions that in addition to accessible feeders have a deep, critical location, where the risk associated with surgery would exceed the risk related to embolization, or in patients with advanced age or significant comorbid conditions.
In general, we believe that there are very limited indications for palliative embolization because the procedure changes flow dynamics and the actual bleeding risk can be increased with this form of partial treatment. The best indications for palliative embolization are “steal” symptoms (progressive neurological deficits) and chronic venous hypertension as a result of high-flow lesions with limited drainage capacity.42,58,59 Other indications can be associated aneurysms and large inoperable AVMs with inherent angiographic “risk factors” that could be addressed endovascularly. Patients with “inoperable” AVMs sometimes undergo palliative embolization after they have already experienced multiple hemorrhages to reduce the flow in an attempt to lower the risk for further rupture. Thus far, however, there is no evidence to support this empirical method. Other indications are intractable headaches and seizures.
Probably the most important role for embolization is preoperative reduction of arterial inflow, occlusion of deep feeders (Fig. 388-1), and management of prenidal aneurysms. The goal is to enable or facilitate surgery. In our opinion, preoperative embolization should only be performed when in the estimate of an experienced neurosurgeon and endovascular surgeon it will reduce the overall risk—in other words, when it can be estimated that the risk of preoperative embolization added to the risk of surgical excision after embolization would be less than the risk of surgical excision without embolization. Additionally, preoperative embolization should be guided by the surgeon who intends to excise the AVM and knows which feeding pedicles are easy to access at surgery and therefore need not be embolized and which are the deep inaccessible feeders that would be helpful to have occluded by embolization before surgery.
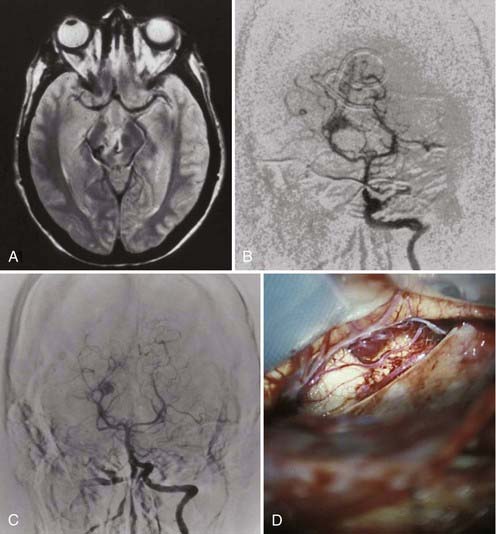
FIGURE 388-1 Small inferolateral thalamic arteriovenous malformation. A, Axial magnetic resonance image. B, Anteroposterior (AP) vertebral injection showing a large thalamic perforator. C, AP vertebral injection after successful embolization of the thalamic perforator. D, Intraoperative photograph of the subtemporal approach to the malformation, which was successfully resected after embolization of the large single thalamic perforator.
Radiosurgery
Radiosurgery was developed and to date is mostly applied by neurosurgeons trained in this treatment modality. Its impact on the treatment of AVMs has been huge. In all its different techniques it is still most effective for smaller AVMs that are 3 cm or less in diameter. Obviously, the great advantages of radiosurgery are its minimal invasiveness and its potential to treat lesions that would otherwise not be amenable to surgical excision because of deep or critical location, or both.60–64 Other indications are patients who as a result of age and general condition are not considered good candidates for surgery. Achievable obliteration rates are related to size and configuration (diffuse versus compact) and range between 60% and 80% over a 2- to 3-year period (Table 388-E3)![]() .44,55,65–73 Obliteration rates are clearly higher for smaller AVMs.70
.44,55,65–73 Obliteration rates are clearly higher for smaller AVMs.70
TABLE 388-E3 Outcomes of Arteriovenous Malformations after Radiosurgery
| SERIES | OBLITERATION RATES | MORBIDITY/MORTALITY RATES |
|---|---|---|
| Steinberg et al.,65 1991 | 100% (<4 cc); 70% (>3.7 cm in diameter) by angiography | 0.9% permanent morbidity; 11% hemorrhage |
| Friedman,66 1997* | 79% (<10 cc); 47% (>10 cc) | N/A |
| Pollock et al.,55 1998 | 61% overall; 83% (<4 cc) by angiography | N/A |
| Nozaki et al.,44 2000 | N/A | 36% morbidity from rehemorrhage; 0% mortality |
| Stieg et al.,67 2000* | 76% at 3 yr | N/A |
| Inoue and Ohye,68 2002 | 81.3% by angiography | N/A |
| Pollock et al.,69 2003 | 78.0% by angiography | 11.9% morbidity; 4.2% mortality |
| Shin et al.,70 2004 | 87.1% by angiography | 1.9% annual hemorrhage rate; 1.5% permanent morbidity |
| Pollock et al.,71 2004† | 75.4% by angiography; 22.8% by MRI | 12% morbidity from hemorrhage; 17.6% morbidity from radiation; 9% mortality |
| Maruyama et al.,72 2004 | 66% by angiography | 1.7% latency-interval hemorrhage rate per year for 1st 3 yr, then 0% |
| Fractionated Radiation Therapy for Large Arteriovenous Malformations | ||
| Karlsson et al.,73 2005 | 8% obliterated | 6% annual hemorrhage rate after radiation; 18% died of hemorrhage; overall 25% mortality (7 deaths, 2 of unknown cause) |
N/A, not available.
* It is not clear that the obliteration rates in these two articles were confirmed by angiography or magnetic resonance imaging and angiography.
† A series of grade IIIB and IV arteriovenous malformations (AVMs) in the basal ganglia, thalamus, and brainstem. Grade IIIB is defined as small Spetzler-Martin grade III AVMs located in areas where surgical resection is either too difficult or prohibitive.20
The main disadvantage of radiosurgery is its uncertainty of cure in terms of complete AVM obliteration and the fact that this process takes between 1 and 3 and sometimes 4 years to occur.61,74–76 It has been shown that during this “latent period” of incomplete obliteration the risk for hemorrhage is similar to the risk with untreated AVMs (3% to 4% per year),77–81 although there are also reports claiming lower rates72,80,82 and some series suggest that it may be higher (5.3% to 10%).83,84 In addition, there is a small but significant risk for neurological injury and other types of complications from radiation damage (3% to 10%, depending on location).84–87 There are a few reports of late hemorrhage after angiographically proven complete obliteration, and some reports describe histologically proven patency of some of the components of the AVM after angiographic obliteration. However, in view of the large number of AVMs treated by radiosurgery within the past 20 years, it can be concluded that the risk for future hemorrhage is extremely low and practically negligible once angiographic obliteration has been demonstrated. Clinically significant complications that can be directly attributed to radiosurgery, such as symptomatic radiation necrosis, appear in 3% to 6% of treated patients ![]() (Table388-E3). The morbidity of treating AVMs in very critical regions such as the brainstem and internal capsule is most likely substantially higher.71
(Table388-E3). The morbidity of treating AVMs in very critical regions such as the brainstem and internal capsule is most likely substantially higher.71
Coexisting Intracranial Aneurysms
Thompson, Deruty, and their associates found coexisting intracranial aneurysms in 45 of 600 patients with AVMs (7.5%),88,89 and Cunha e Sa and colleagues’ analysis of 400 AVMs identified aneurysms in 39 patients (10%).90 They can be proximal to the lesion (prenidal on a feeding artery), within the lesion (intranidal), or in a remote location. The risk of these patients having an intracranial hemorrhage is higher than the risk in patients without associated aneurysms and is estimated at 7% per year91; another study found a higher rebleeding rate in AVMs associated with intranidal aneurysms.92 Patients with coexisting aneurysms tend to be older and are more frequently initially evaluated for epilepsy and neurological events.93 Half of these patients have multiple aneurysms. The majority (85%) of aneurysms are prenidal on a feeding artery or on a major arterial trunk that is participating in the supply. If the aneurysms are postnidal and remote from the AVM, their risk for rupture seems to be lower. In consideration of these numbers, we think that aneurysms need to be addressed during the initial evaluation and early in care if the location of the hemorrhage suggests aneurysmal rupture. Some authors also recommend initial treatment of the aneurysm, even when the AVM has bled, or in cases in which the source is not clear.
It has been proposed that hemodynamic stress from high flow or high arteriovenous shunting in feeding arteries is an important factor for the development of coexisting aneurysms; however, they can also develop in low-flow shunt situations. In high-flow situations, coexisting prenidal aneurysms may diminish in size and disappear when the shunt is obliterated.93
Timing of Surgery
In general, AVM resection should be elective surgery. Patients who sustain neurological deficits from AVM hemorrhage tend to improve over time, especially young patients. The occasional patient with a substantial intracerebral hemorrhage that requires evacuation as a lifesaving measure is an exception. Even in these cases, however, we recommend that the hematoma be evacuated in a very conservative manner in an effort to avoid having to deal with the AVM if it bleeds. The AVM in such a case should be treated at a later time under more favorable conditions. A few weeks after the hemorrhage, a repeat angiogram not infrequently reveals different angioarchitecture. The exception, of course, would be a very superficial, easily identifiable, and safely resectable AVM.
General Principles of Microsurgical Treatment
Positioning and Craniotomy
Positioning should help enable a rather perpendicular approach to convexity lesions and at the same time use gravity to minimize brain retraction. For this reason, the surface of convexity lesions is usually placed parallel to the floor—in other words, with the convexity representation of the lesion uppermost in the field.
For deep-seated AVMs, frameless stereotactic guidance can be a very helpful adjunct to allow a relatively small craniotomy and to tailor an optimized approach trajectory through noncritical areas of the brain—again, with the point of cortical entry uppermost in the field. Large superficial lesions, however, are approached through larger than necessary craniotomies to be able to map the vascular surface anatomy. Feeding arteries can be identified easier on the surface before they plunge deeply into a sulcus as they approach the AVM. One of the most important reasons for a larger craniotomy is to allow thorough mapping of the anatomy of the superficial draining veins. In the event of intraoperative hemorrhage, a craniotomy well beyond the confines of the lesion is highly appreciated when the operative field suddenly needs to be extended to remove the hematoma.
For intraoperative angiography, a radiolucent head frame can be used; however, carbon frames do not usually offer the same degree of positional freedom. With modern portable angiographic units, we have found the standard Mayfield clamp to work adequately. It is important to emphasize that in AVM surgery there are no “routine positions”; rather, they need to be tailored to the lesion and patient and are therefore highly individual.
Identification of the Malformation
After opening the dura carefully in an attempt to not injure potentially attached veins, the superficial anatomy needs to be very well defined, understood, and correlated with the angiographic image to identify superficial feeding arteries and at the same time distinguish them from arterialized draining veins. It is essential to correlate the preoperative angiographic findings with the surgical surface anatomy.
Elimination of Superficial Feeding Arteries
With convexity AVMs, the next step is to open all the sulci around the AVM under microscopic magnification in a search for superficial feeders, which are then coagulated and divided as they enter the AVM. Every effort has to be made to take feeders right at the point where they enter the nidus. It can be difficult at times to differentiate an arterialized vein from a feeding artery. If inspection under microscopic magnification is not sufficient for this process, use of a temporary clip greatly helps in differentiation: after clip application, an arterialized vein will tumesce between the clip and nidus but collapse or become softer and bluer distal to the clip; a feeding artery will collapse between the clip and nidus and continue to pulsate proximal to the clip.
In critical areas of the brain, it is of utmost importance to ensure that a particular artery actually ends in the AVM rather than being an “en passage” vessel that supplies brain tissue distal to the AVM. These en passage arteries feed the AVM via small lateral branches and are a particular problem with AVMs in the sylvian fissure and with pericallosal AVMs. Such arteries need to be very carefully dissected to be able to just take the small AVM-feeding side branches and preserve the main branch.
If larger feeding arteries cannot be coagulated completely because of the high flow, temporary aneurysm clips can be placed before transection. Usually, more proximal coagulation is then possible, and the clip can be taken off at a later time or left. Sometimes the flow before transection is so high that the closing pressure of a temporary clip is not high enough, in which case it needs to be exchanged for a permanent clip of similar size or a hemoclip. Generally, we prefer to use a permanent clip proximally (usually a hemoclip) in all feeders larger than about 1 mm.
An attempt is made to preserve all arterialized veins, but occasionally one or more superficial veins are sacrificed to facilitate dissection, provided that the major venous drainage is left intact and undisturbed.
Circumferential Dissection of the Nidus
In AVMs with a surface representation, the nidus is “developed” by circumferential corticectomy around the AVM after all superficial feeders have been identified and disconnected by opening the adjacent sulci as a first step. It is very helpful to carry this perpendicular dissection and corticectomy around the nidus to a depth of 2.5 to 3 cm before proceeding with deeper dissection. We have found empirically that a perpendicular corticectomy to such a depth will have disconnected all the superficial arterial supply. Once this has been accomplished, the dissection is continued in a more vertical plane around the AVM, usually in a “spiral” fashion until its deepest aspect is reached. During this deeper, spiraling dissection phase it is very important to not mistake loops and eccentric lobules of the AVM that project into normal brain for feeding arteries or draining veins. Coagulation and interruption of such loops can lead to significant and hard-to-control bleeding from the AVM. Great care should be taken to avoid coagulation of the AVM itself during the early phase, when internal AVM pressure and flow are still very high, because any decrease in nidus volume without a decrease in inflow will increase internal pressure and thus the risk for rupture. After the major arterial feeding pedicles have been taken, the turgor of the nidus will substantially diminish. Only then can coagulation be used to stroke some of the AVM loops in an effort to shrink them away from eloquent brain areas. It is not advisable at all to use such a maneuver in the early portion of the procedure when the AVM is still under high inflow pressure and thus more prone to rupture with manipulation.94 As the dissection approaches deeper areas, it usually becomes more tedious because more persistent bleeding will frequently be encountered as a result of the smaller and more fragile arterial supply from deep perforators or subependymal choroidal branches as a ventricle is approached. Once inadvertently lacerated or cut, bleeding from these deep vessels is extremely difficult to control because the vessel diameter is small and there is an element of retraction into parenchyma once cut. The late Dr. Thoralf Sundt designed microclips specifically for placement on these tiny fragile vessels. Sundt microclips are a far better option than creating an ever-deeper tunnel into normal brain in an attempt to reach the retracted and still patent bleeder with bipolar coagulation. Bleeding from the AVM itself can usually be stopped by placing a cottonoid pad, gently retracting on the bleeding point, and moving to a different plane of dissection. If one uses such a maneuver, it is important to remember to never pack when bleeding in a direction away from the AVM because this will risk significant parenchymal or intraventricular hemorrhage. In the case of AVMs that reach the ventricle, oftentimes the bleeding will not stop until the ependyma of the ventricle has been reached and small ependymal feeders to the AVM are controlled.
Transection of Deep Venous Drainage and Removal of the Lesion
If an important draining vein prematurely bleeds, it is usually advisable to attempt hemostasis with hemostatic agents and gentle pressure instead of coagulation and sacrifice. When the major arterial pedicles have been disconnected, the color of the large draining veins should become darker and finally change from red to blue because the drained blood will be progressively less arterialized. After inflow to the nidus has been completely eliminated, the nidus should deflate. The draining veins can be taken at that point unless they still have an arterialized element, which is frequently the case with the last large draining vein. Quite often there is one hidden arterial feeder left, in close proximity or underneath the vein, that needs to be identified and disconnected. After this final step the nidus can be removed. Complete resection of the malformation should be confirmed by intraoperative angiography.95,96 Promising new techniques are intraoperative ICG angiography (near-infrared indocyanine green fluorescence angiography),97–101 intraoperative computed tomographic (CT) angiography,102 and three-dimensional intraoperative ultrasound.103,104 It can be foreseen that the intraoperative application of magnetic resonance imaging (MRI) will not remain limited to tumor surgery.105,106
Hemostasis
The mainstay of AVM dissection is proper use of bipolar coagulation. Novices will instantly notice that because of the high flow it is a lot more difficult to coagulate an AVM feeder than a normal artery of the same diameter. We prefer intermittent coagulation for 1 or 2 seconds under constant irrigation. It is essential that the bipolar forceps tips not be brought together to avoid “sticking” of the tips. For these dissection steps the surgeon has to be very diligent in general and specifically with regard to instruments, and the bipolar tips need to be maintained absolutely clean at all times. This necessitates very frequent cleaning by the operating room nurse, and it is therefore more efficient to have an identical set of bipolar forceps available that can be alternated. If one of the aforementioned deep fragile AVM vessels “sticks” to the bipolar tip, avulsion of this vessel can lead to serious problems at any point in the operation but particularly during the end phase of AVM resection. Frequently, a refractory source of bleeding in the depth of the nidus resection cavity is a small satellite AVM lobule that is still hidden under a thin layer of parenchyma after having mistaken the AVM to have a single feeder.
Once the lesion has been removed, the resection cavity needs to be checked meticulously for potential points of breakthrough hemorrhage. After thorough irrigation and inspection, systolic blood pressure is raised by 15 to 20 mm Hg above normal mean arterial pressure for that patient for 10 to 15 minutes. If bleeding can be provoked, usually a residual portion of the AVM is left. By rubbing gently with a small cottonoid against the resection wall, suspicious areas can be tested. If no bleeding can be provoked, it is time to line the cavity with the usual hemostatic agent. From then on the patient’s blood pressure is kept at or slightly below the normal mean for 24 hours.
Specific Surgical Considerations for Arteriovenous Malformations in Different Locations
Convexity Arteriovenous Malformations
Resection of convexity AVMs follows the principles outlined earlier. In patients with additional arterial supply from external carotid branches, enhanced alertness is necessary when the craniotomy flap is turned to avoid serious bleeding in the very early proceedings of the surgery. Multiple bur holes and very careful stripping and opening of the dura can help avoid injury to meningeal vascular pedicles. Elimination of all or most of the external supply by preoperative embolization is most helpful in these cases. Generally, we have noted exuberant external supply only in AVMs that have bled or have been embolized.
Deep Parasagittal Arteriovenous Malformations
Anterior frontal parasagittal lesions are approached through a unilateral frontal craniotomy that crosses the midline. The position is dependent on the location of the AVM and the dominant arterial supply. If the lesion does not come to the surface and the main supply is from the anterior cerebralartery (ACA), we prefer the lateral position with the ipsilateral side down to allow the brain to fall away from the falx by gravity. This position is also preferred for parietal and occipital parasagittal malformations. Essentially, the lateral position should be reserved for parasagittal AVMs that do not reach the convexity because only then can the dura be opened with a very narrow, sinus-based flap that allows the brain to fall under the dura. If a wide dural flap were used in this position, the brain would potentially fall against the dural edge, which can result in substantial damage. Another option is a contralateral transfalcine approach. With larger lesions that come to the surface of the convexity, we position the patient so that the convexity portion of the AVM is uppermost in the field to be able to perform a larger craniotomy and wider dural flap to control any middle cerebral artery (MCA) feeders. Posterior frontal and parietal lesions frequently take their arterial supply from more than one major arterial territory. Their drainage is through sizable arterialized veins into the superior and inferior sagittal sinus, a fact that limits access to the interhemispheric fissure. To circumvent tight corridors between superficial bridging veins, the bone flap should be broad based to have ample and safe choices for the interhemispheric route between these veins. Preoperative embolization of the ACA-pericallosal supply can be a very important adjunct that can obviate the need for early parasagittal retraction. Larger frontoparietal lesions involve the motor strip and therefore present a serious surgical concern, and hence a more conservative approach may be favored sometimes. A major concern with these large parasagittal lesions is premature injury to the arterialized draining veins, which can occur easily as a result of retraction. Under unfortunate conditions the entire AVM might be stuck to the falx because of many small veins draining to the falx and the sinus. In such circumstances it is advisable to make full use of a very broad-based bone flap so that the lesion is approachable from a more anterior or posterior direction alternatively.
Occipital parasagittal lesions are preferably approached with a craniotomy that extends to the sagittal and transverse sinuses. For this approach, the patient is positioned either prone or in a lateral position with the ipsilateral side down when the arterial supply is only from the posterior cerebral artery.
Hypothalamic and Inferior Frontal Arteriovenous Malformations
Malformations in this location are usually rather small and involve the septal, anterior hypothalamic, and medial subfrontal regions, with feeding vessels from the anterior communicating complex. A pterional subfrontal approach provides direct access to the feeding arteries.
Anterior Corpus Callosum Arteriovenous Malformations
Frequently, the ACA supply of these malformations is by en passage arteries, which makes embolization risky and control of small side branches to the AVM tedious. Oftentimes, the head of the caudate nucleus is involved, with extension to the anterior limb of the internal capsule. Such a situation in turn invariably implies that there is additional supply by deep perforators from the medial aspects of the MCA and from the recurrent artery of Heubner. If these lesions are large, they can involve the basal-medial aspect of the frontal lobe. Such circumstances may require the sequential use of a basal pterional and then a superior parasagittal approach through a large combined craniotomy and a change in position of the head (Fig. 388-2).
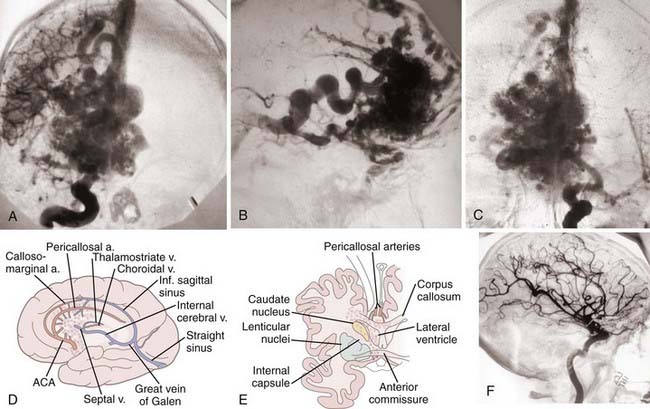
FIGURE 388-2 Large anterior callosal arteriovenous malformation (AVM) involving the caudate nucleus and anterior hypothalamus. A, Anteroposterior (AP) right carotid injection. B, Lateral right carotid injection showing a large septal draining vein. C, AP left carotid injection. D and E, Sketches of the arterial supply and venous drainage, as well as the major brain structures involved by the AVM. F, Postoperative lateral carotid arteriogram showing complete removal of the malformation with preservation of both pericallosal arteries. ACA, anterior cerebralartery.
(D and E, From Heros R. Surgery for arteriovenous malformations of the brain. In: Ojemann R, Ogilvey CS, Crowell R, et al., eds. Surgical Management of Neurovascular Disease, 3rd ed. Baltimore: Williams & Wilkins; 1995:420-475.)
Posterior Corpus Callosum (Splenial) and Posterior Third Ventricle Arteriovenous Malformations
There is an important difference in the vascular supply of AVMs in the splenial–posterior third ventricle region and trigonal AVMs. Trigonal AVMs are fed from the anterior choroidal and posterolateral choroidal arteries. Because of their more midline position, splenial AVMs have additional pedicles from posteromedial choroidal branches, direct branches of the posterior cerebral artery, and pericallosal branches from the anterior and posterior cerebral artery complexes (Fig. 388-3). The parasagittal approach allows control over the medial blood supply. A good way to gain control over the posteromedial choroidal branches with a parasagittal approach is by placing the ipsilateral side down in the lateral position; the prone position is also satisfactory. In the past we have used the semisitting position but have discarded it because of problems with outward herniation of the occipital lobe during surgery. Prior placement of a lumbar drain is important to achieve brain relaxation and gain space without needing to use forcible retraction. The surgical challenge arises toward the end of the procedure, when the AVM needs to be followed laterally in a tangential direction toward the trigone. The trigonal region is difficult to reach from that approach yet will contribute significantly to the AVM’s blood supply. AVMs in the area of the velum interpositum at the roof of the third ventricle can be reached with the same parasagittal approach. To reach the velum, a small callosal incision is made just anterior to the splenium.
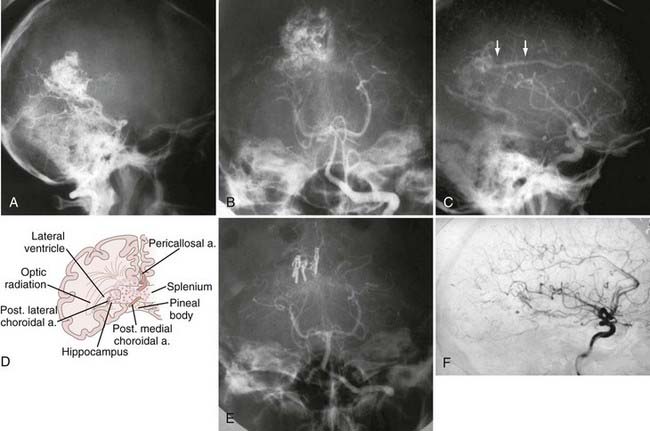
FIGURE 388-3 Splenial arteriovenous malformation (AVM). A, Lateral vertebral injection. B, Anteroposterior (AP) vertebral injection. C, Lateral carotid injection. Arrows show a pericallosal feeder. D, Sketch showing the arterial supply to a typical splenial AVM. E, Postoperative AP vertebral injection showing complete removal of the malformation. F, Postoperative lateral carotid injection.
(D, From Heros R. Surgery for arteriovenous malformations of the brain. In: Ojemann R, Ogilvey CS, Crowell R, et al., eds. Surgical Management of Neurovascular Disease, 3rd ed. Baltimore: Williams & Wilkins; 1995:420-475.)
Trigonal Arteriovenous Malformations
With regard to the optimal surgical approach, we like to differentiate two groups of trigonal AVMs: first, those that are more inferiorly located and involve the floor and the lateral wall of the trigone, and second, the ones that relate to the medial aspect of the trigone, which has been called the parasplenial region,107 as well as the roof of the trigone and sometimes the dorsal surface of the pulvinar of the thalamus.
For the first, lateral group, we use a transtemporal approach through the inferior temporal gyrus on the dominant side to avoid speech problems and either through the inferior or the middle temporal gyrus on the nondominant side. Superior quadrantanopia is a common consequence of this approach, but frequently these patients have already had a field cut, and in general this deficit is not very disabling.
For the second, more medial group, we prefer to use a superior parieto-occipital approach108 over a parasagittal one (Fig. 388-4). For the latter, considerable retraction is required because the trigone is about 3.5 cm lateral to the midline. In addition, the line of vision toward the trigone is more tangential. Yasargil, however, advocates this parasagittal, posterior interhemispheric-transprecuneus approach for medial trigonal AVMs.109 We have found this approach extremely difficult for anything but very small AVMs. The superior parieto-occipital approach is a transcerebral approach that traverses between the parietal sensory association fibers and the occipital visual association fibers. This approach has been applied routinely for various pathologies in the same location, such as tumors involving the trigone, without resulting in significant sensory or visual deficits. With larger AVMs, some visual field loss cannot be prevented because the visual radiations along the tapetum are injured. Frequently, it is possible to use a sulcus, which then shortens the distance to the target by 1.5 to 2 cm. This approach can be greatly helped by using frameless stereotactic guidance to ensure the correct trajectory or by using ultrasound. Several positions are possible for this approach (semisitting, prone, lateral). Lately, we have most often used a lateral position with the lesion side up, the head elevated, and the neck flexed so that the point of entry is in the cortex uppermost in the field. The vertical coordinate of the entry point for the cortical incision is approximately 7 cm up from the occipital tip, which corresponds to about 9 cm above the inion as an external landmark. The horizontal entry point coordinate is 3.5 to 4 cm lateral to the superior sagittal sinus. The trajectory from the entry point is then directed toward the trigonum. If frameless stereotaxis or ultrasound is not available, the surgeon can aim at the ipsilateral pupil.
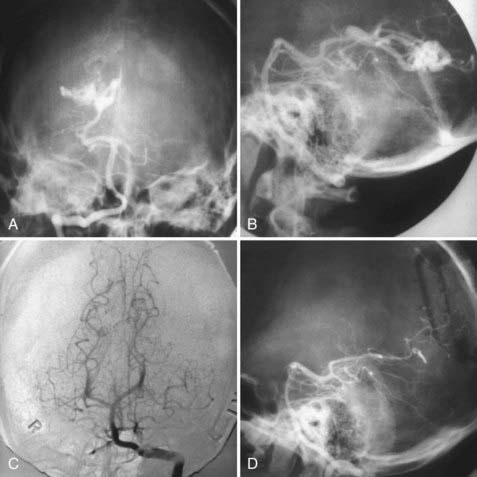
FIGURE 388-4 Trigonal arteriovenous malformation (AVM). A, Anteroposterior (AP) vertebral injection. B, Lateral vertebral injection. C, Postoperative AP vertebral injection. D, Postoperative lateral vertebral injection showing complete removal of the malformation through a posterior parietal bone flap.
Sylvian and Insular Arteriovenous Malformations
These lesions are located within the sylvian fissure or on the cortical surface of the insula, and their venous drainage is into the sylvian veins. The peculiarity of these AVMs is their propensity to have supply by en passage branches of the MCA. The branches of the MCA pass adjacent to or right through the malformation on their course toward supplying normal brain and supply the AVM by short perpendicular side branches. MRI has made it possible to accurately estimate when most of the AVM is within the sylvian fissure itself, and in these cases even large AVMs can be resected with safety (Fig. 388-5).
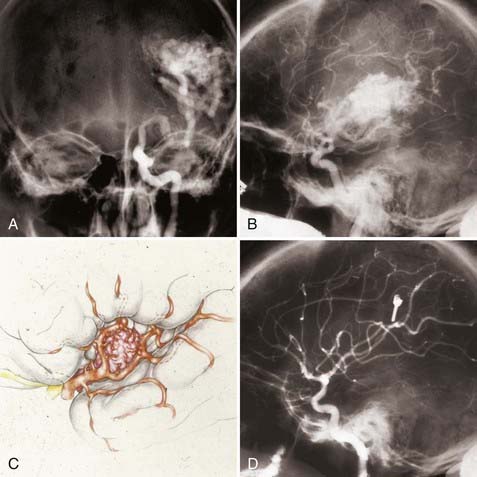
FIGURE 388-5 Sylvian arteriovenous malformation (AVM). A, Anteroposterior left carotid injection. B, Lateral left carotid injection. C, Sketch of a typical sylvian AVM demonstrating arteries en passage feeding the AVM through small side branches. D, Postoperative left carotid injection showing complete removal of the AVM.
Insular AVMs, which are approached via the sylvian fissure, require skeletonization of the MCA sylvian branches as they pass lateral to the AVM. The aim is to control the medially directed feeders. Unfortunately, there is frequently a lenticulostriate perforator supply, a fact that renders surgery very problematic (Fig. 388-6). If such a supply were predominant, one should consider radiosurgery if the size would allow this alternative. A medial lenticulostriate perforator supply implies that the AVM extends too medially and involves the deep basal ganglia and possibly the internal capsule, which generally makes it inoperable. Some insular AVMs occupy an extensive region involving the temporal lobe stem, the medial temporal lobe, and parts of the atrium (Fig. 388-6).
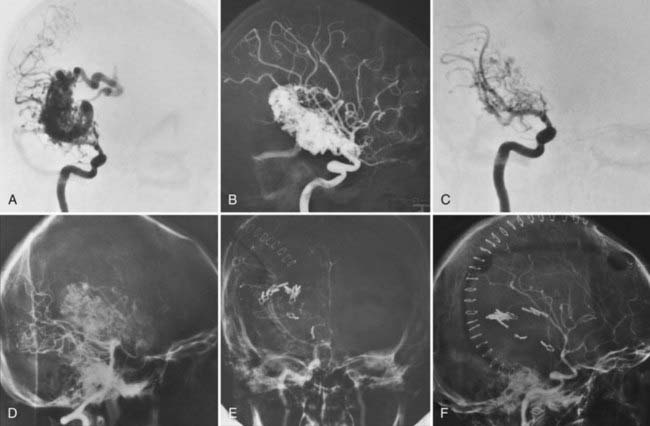
FIGURE 388-6 Large insular/medial temporal arteriovenous malformation (AVM). A, Right anteroposterior (AP) carotid injection. B, Right lateral carotid injection. C, Early AP carotid injection demonstrating lenticulostriate feeders. D, Lateral vertebral injection demonstrating posterior cerebral feeders. E, Postoperative AP carotid injection demonstrating complete removal of the AVM. F, Postoperative lateral carotid injection.
Medial Temporal Arteriovenous Malformations
Medial temporal AVMs that are anteriorly located in the vicinity of the uncus, amygdala, and anterior hippocampal complex are approached through the medial aspect of the sylvian fissure via a pterional craniotomy (Fig. 388-7). When the sylvian fissure is opened and the temporal lobe is retracted laterally, the feeders of the AVM come into view and are easy to identify. From superficial to deep, the usual sequence of branches is anterior temporal branches of the MCA, anterior choroidal artery and branches thereof, posterior communicating artery branches, and some early temporal branches of the posterior cerebral artery. All feeding pedicles must be controlled and disconnected while at the same time preserving the draining veins, which drain both anteriorly into the sphenoparietal sinus and medially into the basal vein. Once the arterial inflow has been disconnected, the lesion can be removed without difficulty.
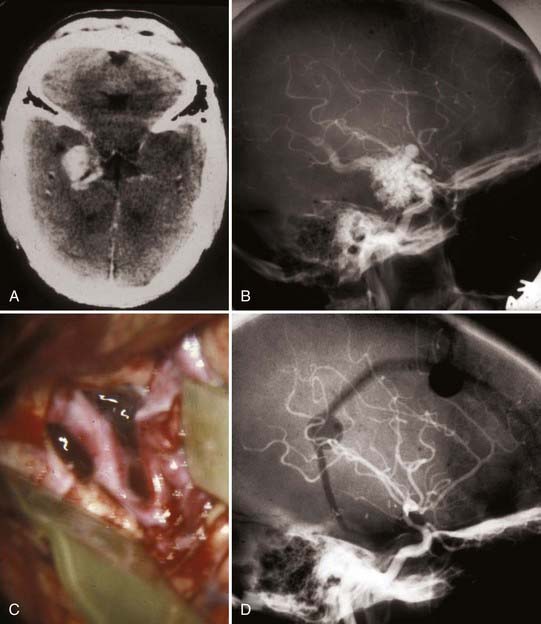
FIGURE 388-7 Anteromedial temporal arteriovenous malformation (AVM). A, Computed tomography scan. B, Lateral right carotid injection. C, Intraoperative transsylvian exposure demonstrating the internal carotid artery and feeders to the medial aspect of the AVM by a very enlarged anterior temporal branch. D, Postoperative right carotid arteriogram demonstrating complete removal of the AVM.
Posteriorly located malformations of the medial temporal lobe typically involve the region of the hippocampus and parahippocampal gyrus, as well as the fusiform gyrus, and can extend back to the trigone. Such AVMs are approached via a temporal craniotomy that offers choices for a subtemporal corridor or via a transcortical route through the inferior temporal gyrus.110 With the transcortical route it is easier to spare the vein of Labbé, which might be arterialized and is far more endangered with the subtemporal approach, which necessitates retraction of the temporal lobe (Fig. 388-8). With either approach, the trajectory is toward the temporal horn. Once identified, it serves as a good anatomic landmark for orientation. In the most anterior aspect of the temporal horn, at the choroidal fissure, the anterior choroidal artery can be identified. It invariably supplies these lesions and can be controlled through the choroidal fissure on the medial aspect of the temporal horn. Either of these approaches risks superior quadrantanopia, and the patient needs to be informed about this probable complication beforehand. Posterior cerebral artery feeders can be controlled either subtemporally or directly transtemporally.
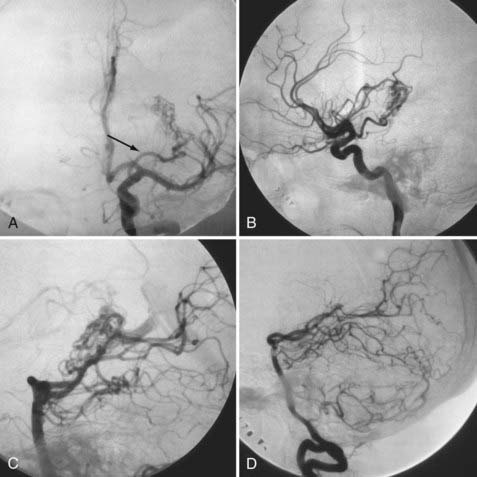
FIGURE 388-8 Left posteromedial temporal arteriovenous malformation (AVM). A, Left carotid injection demonstrating a large anterior choroidal feeder (arrow). B, Lateral left carotid injection. C, Lateral vertebral injection. D, Postoperative vertebral injection demonstrating complete removal of the AVM.
Intraventricular Arteriovenous Malformations
Purely intraventricular AVMs are less frequently encountered. It is the experience of the senior author that these AVMs have a greater tendency to bleed than those completely embedded in brain and, accordingly, have a bad natural history with repeated hemorrhages. Our inclination, therefore, is to treat them aggressively whenever possible. The feasibility of surgery is highly dependent on the prevailing arterial supply in these cases. Lesions with exclusive or predominant choroidal arterial supply are usually considered operable because these pedicles can be controlled at the ependymal surfaces. This even applies when the malformation extends along the entire roof of the third ventricle (Fig. 388-9). The transcallosal approach provides an excellent avenue to reach these AVMs. For the reasons outlined earlier, we prefer to use this approach in conjunction with a lateral position and the side of the parasagittal approach (usually the right side) down. Extreme care must be taken to not compromise the internal cerebral veins, which are frequently intimately related to the lesion.
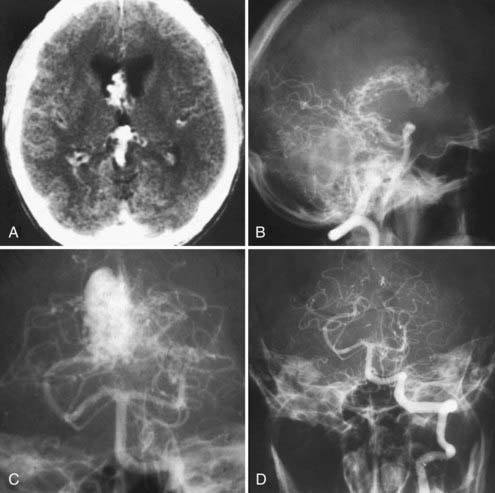
FIGURE 388-9 Intraventricular arteriovenous malformation (AVM) involving the tela choroidea of the third ventricle. A, Computed tomography scan showing an intraventricular AVM. B, Lateral vertebral injection demonstrating the posteromedial choroidal supply to the AVM. C, Anteroposterior (AP) vertebral injection. D, Postoperative AP vertebral injection demonstrating complete removal of the AVM (transcallosal, transchoroidal approach).
Lesions located at the head of the caudate nucleus are safer to excise. They are supplied by choroidal branches and from the depth by the recurrent artery of Heubner and by medial lenticulostriate arteries. Choices for approaching the AVM are an anterior transcallosal route or, if ventriculomegaly is present, a transfrontal route. Of course, in this location bleeding from perforators poses a problem, but parenchymal damage anteromedial to the internal capsule can be well tolerated. A predominant perforator supply across the basal ganglia and the thalamus makes attempts at surgical resection dangerous. These lesions are mostly deemed inoperable, but if small enough, radiosurgery is a very good alternative.
Striatocapsulothalamic Region Arteriovenous Malformations
Malformations in this region have a predominant perforator supply, which in our opinion precludes surgery. When small enough, these lesions should be treated by radiosurgery. The situation is somewhat different if the lesion is located lateral to the internal capsule and involves the lateral basal ganglia and insula—many of these lesions can be excised with acceptable morbidity (see Fig. 388-6). Small lesions that involve the posterolateral inferior aspect of the thalamus can oftentimes be removed with reasonable safety if the predominant supply is by lateral circumferential arteries, which can be controlled in the ambient cistern or the anteromedial ventricular surface of the thalamus (choroidal supply, controllable in the ventricle). Malformations of the posterolateral inferior aspect of the thalamus involve the lateral geniculate body and should therefore not be removed unless the patient already has complete hemianopia; if the visual pathway is functionally intact, radiosurgery should be considered. Other neurosurgeons have been more aggressive with thalamic and basal ganglia parenchymal lesions and frequently report good results.111–115
Cerebellar Vermis Lesions
Superior vermian AVMs are invariably supplied by branches of the superior cerebellar arteries (SCAs). Most often the supply is bilateral. To reach these branches, we prefer an infratentorial supracerebellar approach. For this, our preference is to use a sitting position to allow the cerebellum to fall down after drainage of cerebrospinal fluid from the cisterna magna. With the cerebellum falling down, one has to be particularly careful to not tear any arterialized veins that drain from the cerebellum to the tentorium. As described earlier, the surgeon has to be aware to not take any of these arterialized veins before the bulk of the arterial supply is controlled. At times this is more difficult to achieve than with other exposures because the space is more confined and one has to work around these veins without exerting too much force on the cerebellum with downward retraction.
Posterior and inferior vermian AVMs are approached with the patient in the prone or three-quarter “park bench” position. The predominant supply of these malformations is by posterior inferior cerebellar artery (PICA) branches, but frequently there is additional deep supply from SCA branches. If these lesions reach the fourth ventricle, there is an additional transependymal supply, which is difficult to control.
Cerebellar Hemisphere Lesions
Hemispheric AVMs can be quite large and, in contrast to vermian malformations, are mostly located more lateral and have a unilateral blood supply. This characteristic helps differentiate the rare medially located hemispheric AVM that abuts the vermis from a true vermian lesion, which generally has a bilateral supply. Large AVMs of the cerebellar hemisphere usually involve all three cerebellar arterial territories: the SCA, anterior inferior cerebellar artery (AICA), and PICA. Venous drainage is typically into the petrosal, the galenic, or both systems. If the lesion is located laterally, it can be approached via a retromastoid craniotomy, which can be extended to the midline and be supplemented with a far-lateral suboccipital approach if more anteromedial exposure is necessary (Fig. 388-10). In addition, this expands the operative field in a caudal direction, and thus the potential arterial supply from the PICA can be controlled from an inferolateral direction, the AICA supply at the cerebellopontine angle, and the SCA supply from a superolateral direction above the cerebellum.
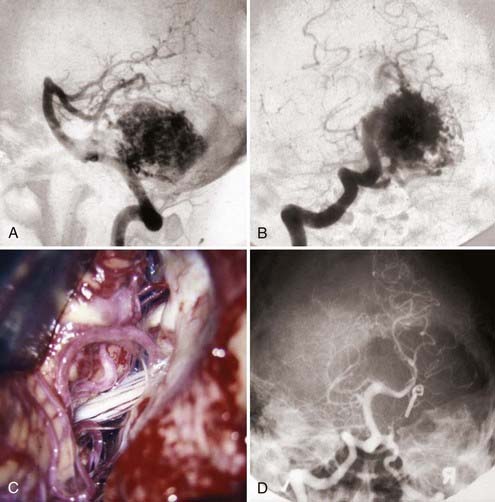
FIGURE 388-10 Lateral cerebellar hemispheric arteriovenous malformation (AVM). A, Lateral vertebral injection. B, Anteroposterior vertebral injection. C, Operative exposure through a far-lateral suboccipital approach demonstrating feeders from the anterior inferior and posterior inferior cerebellar arteries. D, Postoperative vertebral injection demonstrating complete removal of the AVM.
Brainstem Arteriovenous Malformations
We believe that most AVMs located within the parenchyma of the brainstem should not be operated on. Fortunately, these lesions are not common.116–119 Nozaki and colleagues found a considerably higher annual risk for hemorrhage (15.1%) and rehemorrhage (14.2%) in a review of 25 lesions treated.120 These lesions are fed by perforating arterial branches, which cannot be controlled early in the course of the dissection. This is one reason why the surgical morbidity is forbidding. Another option for treatment would be radiosurgery. However, there is high morbidity if radionecrosis occurs in this location.121 For these reasons we generally recommend a conservative approach to these patients unless they have bled more than once or have progressive symptomatology. The few lesions that were operated on by the senior author were mainly pial, superficial lesions with a primarily circumferential arterial supply that could be controlled at one of the surgical surfaces of the brainstem. This is more or less in line with the recommendation of other authors.120 The resectable lesions are usually located at the cerebellopontine angle, at the anterolateral aspect of the brainstem. Their arterial supply is typically from dilated AICA or PICA branches. However, when located on the ventral brainstem surface, there can be supply from basilar artery perforators. Venous drainage is generally to the lateral pontine vein into the petrosal or galenic system. Some tectal lesions are also amenable to surgery, even more so when the patient already has Parinaud’s syndrome (Fig. 388-11).
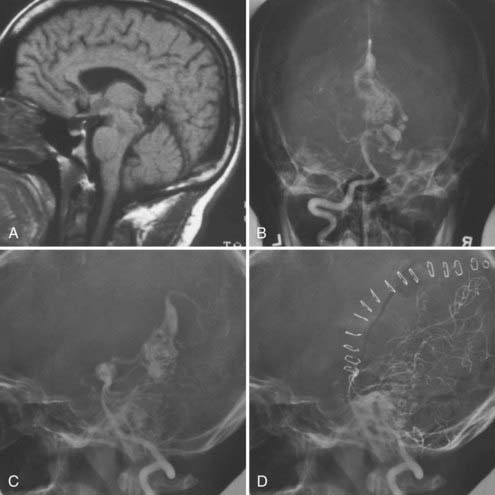
FIGURE 388-11 Tectal arteriovenous malformation (AVM). A, Sagittal magnetic resonance image. B, Anteroposterior vertebral injection. C, Lateral vertebral injection. D, Postoperative lateral vertebral injection showing complete removal of the AVM through a posterior temporal occipital transtentorial approach.
“Pial” cerebellopontine angle AVMs are approached laterally. The transverse and sigmoid sinus, as well as the foramen magnum region, need to be extensively exposed, which is why a far-lateral approach variant that also takes the posterior arch of C1 down is generally used. This allows wide exposure of the cisterns from the petrosal vein above to the vertebral artery below. Various degrees of condyle drilling allow more or less anterior pontine and brainstem exposure.
Complications and Their Avoidance
The complexity and challenge of treatment of AVMs find expression in the abundant potential to produce untoward effects. The most careful of preoperative and intraoperative judgment is required, which extends well into the postoperative period. Our own complications have been categorized and discussed before.8,122–124 The major share of significant complications can be attributed to faulty surgical judgment, that is, patients who underwent surgery and in retrospect should not have been operated on. Such misjudgment mostly had to do with an incorrect conception of the exact topographic localization and vicinity to eloquent areas, and this in turn was more common before the days of conventional and functional MRI.125
Modern high-resolution MRI sequences provide very precise resolution of anatomic details, although it is still difficult to conceptualize the three-dimensional relationships of the lesion to critical areas. Still, MRI offers a distinct advantage over the days when the surgeon had to strictly rely on an angiography and a relatively crude anatomic CT image to define three-dimensional relationships. Many of our complications with high-grade AVMs date back to this era. Undoubtedly, there will be an important role for future navigational applications with improved multimodal image fusion capabilities that incorporate angiographic, anatomic, hemodynamic, and cerebral functional data in a practical way.
Intraoperative Complications
Hemorrhage in most instances is a consequence of AVM bleeding during dissection close to the nidus or a secondary consequence of premature occlusion of significant draining veins.
Parenchymal damage can also occur when the circumnidal margin of resection is too generous. It is crucial to be as close as possible to the nidus without entering it. Dissection that is more than a few millimeters away from the nidal margin might already have resulted in a functional deficit in eloquent brain. Functional deficit can also be induced by indirect parenchymal damage, when feeders are inadvertently cut too far away from the nidus. The difficulties of deep perforator disconnection with regard to hemostasis, the usefulness of Sundt clips, and the danger of packing when bleeding away from the AVM are discussed earlier.
The problems resulting from excessive retraction have been alluded to as well. When retraction is necessary, it should be kept to the minimum sufficient to create corridors that are wide enough to securely manipulate the instruments. Retraction can be minimized by intelligent use of position, wide enough craniotomies, skull base approaches, and release of cerebrospinal fluid. Sometimes it is advisable to use a short transcortical route through noncritical brain areas to avoid excessive retraction with the potential for bridging vein and parenchymal damage (e.g., subtemporal versus inferior temporal gyrus approach).110,126,127 With AVMs in the sylvian fissure and callosal region, it is essential to identify all arteries en passage because they are most frequent in these locations as discussed previously. Bridging vein injury contributes to parenchymal damage when veins draining normal brain are torn or thrombose because of excessive stretching. With the parasagittal and subtemporal approaches, the risk for such injury clearly is higher.
Visual radiation injury is a concern with lesions of the temporal and occipital lobes.128 The spatial course of the geniculocalcarine fibers and Meyer’s loop around the temporal horn of the lateral ventricles should be understood and avoided whenever possible when planning the approach. For example, a transcortical inferior temporal gyrus approach for resection of AVMs in the mid and posterior temporal lobe allows traversal underneath the optic radiation and at the same time can avoid retraction injury.
Postoperative Complications
Hemorrhage
Intraparenchymal hemorrhage is the most serious concern in the immediate postoperative period. It can be the result of incomplete hemostasis or, more commonly, residual AVM fragments left hidden under a thin layer of parenchyma. This problem of residual AVM has mostly been resolved since intraoperative angiography has become routinely available, as discussed earlier. To secure robust hemostasis, several maneuvers have been mentioned before: surgery under normotension, “cottonoid rub” of suspicious areas, and hypertensive challenge before closing.
De Novo Seizures
New onset of seizures was encountered in 15% of our patients. In those who had seizures initially, seizure frequency improved in 55%, remained unchanged in 35%, and worsened in 12%.129 This is why it seems reasonable to treat all patients with anticonvulsant prophylaxis after they have undergone surgical excision of a supratentorial parenchymal AVM.130 We recommend at least a 6-month treatment interval.
Other Postoperative Problems
The term normal perfusion pressure breakthrough (NPPB) refers to a theory of hemodynamic changes that are induced once an AVM has been excised and that lead to postoperative edema or hemorrhage (or both).131 Although this theory remains controversial, we are convinced of its validity. Fortunately, this problem can now be avoided before surgery with the routine use of preoperative embolization for large, high-flow AVMs. The angiographic features of patients with potential for NPPB include a combination of long, high-flow, large-caliber feeding arteries and diminished perfusion of adjacent brain because of a steal phenomenon (angiographic steal).
Retrograde venous thrombosis can occur postoperatively as a result of stasis in large draining veins after AVM resection.132 The senior author had a dramatic example of this complication (Fig. 388-12).
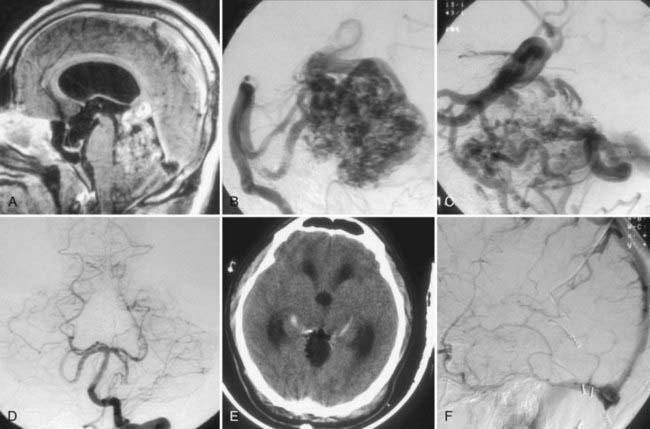
FIGURE 388-12 Superior vermian cerebellar arteriovenous malformation (AVM). A, Sagittal magnetic resonance image. B, Lateral vertebral injection, early arterial phase. C, Lateral vertebral injection, late arterial phase, demonstrating a very enlarged vein of Galen and internal cerebral veins draining the malformation. D, Postoperative anteroposterior vertebral injection demonstrating complete removal of the AVM. E, Postoperative computed tomography scan demonstrating absence of the AVM and fresh thrombi in the basal veins bilaterally. F, Postoperative vertebral injection, venous phase, demonstrating absence of filling of the deep venous system.
Vasospasm after AVM excision must be rare because the senior author has not encountered such a problem in his personal series. However, in his series of 414 patients, Yasargil described 2 patients with this complication after extensive dissection of the A1 and M1 segments.109
Retrograde feeding artery thrombosis can occur as a result of stasis in long dilated AVM feeders. Stasis is a common angiographic finding after AVM resection that can last for about a month.133 Although stasis is a common finding, actual thrombosis of these former feeding arteries is relatively rare. Miyasaka and coauthors reported five cases in a series of 76 patients in an earlier work (6.6%)134 and pointed out in a later report that the risk is much higher in the elderly.135
Outcome
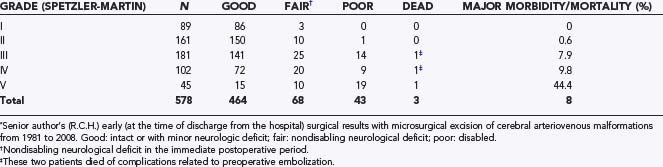
The senior author’s personal surgical results with open microsurgical resection of cerebral AVMs from 1981 until 2008 are summarized in Table 388-4. These are the results at the time of discharge from the hospital after surgery. As we have reported before, many of the patients with mild or moderate neurological deficits improve with time.129 A review of these results indicates that grade I and II AVMs were operated on with minimal morbidity and no mortality. This is in line with the results of almost all large AVM series![]() (Tables 388-E5 and 388-E6).30,44,62,129,136–148 The excellent surgical results with grade I and II and with the majority of grade III lesions in most series support the strong bias toward surgical excision of these lesions. Admittedly, all the large surgical series are biased by careful selection of cases. For example, the senior author has not recommended surgical excision to many asymptomatic patients who because of age or surgical comorbid conditions were not thought to be good surgical candidates. Likewise, some patients with grade III AVMs involving critical regions of the brain were not offered surgical excision. Some of these patients who were not offered surgical excision were treated conservatively, others with radiosurgery, and a few exceptional ones with curative (complete obliteration) embolization. In sharp contrast to the results for grade I and II and most grade III lesions, the morbidity with grade IV and even more so with grade V lesions has been unacceptably high, and this is mirrored in most of the large series. Patients with grade V lesions in the senior author’s personal series were almost exclusively operated on before 1990, when a review of his early experience was reported.129 Similarly, this also applied to many patients with grade IV lesions. Our reported permanent serious morbidity rate for grade IV and V AVMs after a mean follow-up time of 3 years and a minimum of 6 months was 17.7%, with a mortality rate of 3.2%.129 Currently, the senior author has a far more conservative attitude with these high-grade lesions, and this view is shared by the majority of experienced neurovascular surgeons.149
(Tables 388-E5 and 388-E6).30,44,62,129,136–148 The excellent surgical results with grade I and II and with the majority of grade III lesions in most series support the strong bias toward surgical excision of these lesions. Admittedly, all the large surgical series are biased by careful selection of cases. For example, the senior author has not recommended surgical excision to many asymptomatic patients who because of age or surgical comorbid conditions were not thought to be good surgical candidates. Likewise, some patients with grade III AVMs involving critical regions of the brain were not offered surgical excision. Some of these patients who were not offered surgical excision were treated conservatively, others with radiosurgery, and a few exceptional ones with curative (complete obliteration) embolization. In sharp contrast to the results for grade I and II and most grade III lesions, the morbidity with grade IV and even more so with grade V lesions has been unacceptably high, and this is mirrored in most of the large series. Patients with grade V lesions in the senior author’s personal series were almost exclusively operated on before 1990, when a review of his early experience was reported.129 Similarly, this also applied to many patients with grade IV lesions. Our reported permanent serious morbidity rate for grade IV and V AVMs after a mean follow-up time of 3 years and a minimum of 6 months was 17.7%, with a mortality rate of 3.2%.129 Currently, the senior author has a far more conservative attitude with these high-grade lesions, and this view is shared by the majority of experienced neurovascular surgeons.149
TABLE 388-E5 Microsurgery: Postoperative Outcomes for Spetzler-Martin Grade I-III Arteriovenous Malformations
| SERIES | NO. OF PATIENTS | MORBIDITY/MORTALITY RATES |
|---|---|---|
| Spetzler and Martin,30 1986 | 100 | 5% early morbidity |
| Heros et al.,129 1990 | 91 | 1.1% late morbidity; 1.1% late mortality |
| Sisti et al.,136 1993 | 67 (small AVMs) | 1.5% combined morbidity and mortality |
| Pikus et al.,62 1998 | 19 | 0% |
| Tokunaga et al.,137 2000 | 12 | 0% for grades I and II; 75% early morbidity, 50% late morbidity, and 0% mortality for grade III |
| Irie et al.,138 2000 | 27 | 0% |
| Hongo et al.,139 2000 | 20 | 4% mortality |
| Pik and Morgan,140 2000 | 110 | 10.9% early morbidity; 2.7% late morbidity |
| Hartmann et al.,141 2000 | 95 | 5.3% morbidity; 0% mortality |
| Russell et al.,142 2002 | 35 | 8.6% morbidity; 0% mortality |
| Lawton,143 2003 | 76 (grade III) | 3.9% morbidity; 3.9% mortality |
| Morgan et al.,144 2004 | 220 (grades I and II) | 0.9% morbidity; 0.5% mortality |
AVM, arteriovenous malformation.
TABLE 388-E6 Microsurgery: Postoperative Outcomes for Grade IV and V Arteriovenous Malformations
| SERIES | NO. OF PATIENTS | MORBIDITY/MORTALITY RATES |
|---|---|---|
| Heros et al.,129 1990 | 62 | 17.7% late morbidity; 3.2% late mortality |
| Hamilton and Spetzler,145 1994 | 44 | 21.9% combined M&M for grade IV; 16.7% combined M&M for grade V |
| Hassler and Hejazi,146 1998 | 62 | 20.5% combined M&M for grade IV; 30.4% combined M&M for grade V |
| Hartmann et al.,141 2000 | 29 | 6.9% morbidity; 0% mortality |
| Nozaki et al.,44 2000 | 32 | 9% morbidity; 0% mortality |
| Jizong et al.,147 2000 | 50 | 26% early morbidity; 12% late morbidity; 0% mortality |
| Tokunaga et al.,137 2000 | 4 | 25% morbidity; 0% mortality |
| Irie et al.,138 2000 | 4 | 25% morbidity; 0% mortality |
| Hashimoto et al.,148 2000 | 3 | 75% morbidity; 0% mortality |
| Russell et al.,142 2002 | 9 | 22.2% morbidity; 11.1% mortality |
M&M, morbidity and mortality.
In four of our patients included in Table 388-4, reoperation was necessary because of residual AVM. Intraoperative or postoperative angiography was performed in all patients, except a handful who harbored a very small AVM in a noneloquent area of the brain and the surgeon felt absolutely sure about complete excision. With the exception of patients who needed to be reoperated on for residual AVM demonstrated by postoperative angiography, all others had complete excision confirmed with intraoperative or postoperative angiography. With one very notable exception of a 15-year-old boy who had complete AVM resection demonstrated on postoperative angiography and died 2 years later of an intraventricular hemorrhage after rupture of a recurrent AVM, no other patient, to our knowledge, has had hemorrhage from recurrent AVMs.
Conclusion
Brain AVMs are complex lesions that require well-founded decision making by a neurosurgeon with significant experience in the treatment of these lesions. Robust natural history data indicate a 2% to 4% annual risk for hemorrhage regardless of whether the AVM has bled in the past. This risk is only somewhat higher (6%) during the first year after an AVM has ruptured. Hemorrhage from AVM carries an approximate average of 30% morbidity and 10% mortality rates with both previously ruptured and unruptured AVMs.
The senior author feels strongly that patients with AVMs seeking expert opinion should be informed of the significance of harboring an AVM and, in general, of the different treatment modalities used for their treatment, but ultimately, expert surgeons should tell patients unambiguously, if they know, what in their opinion is the best treatment or combination of treatments for patients with that particular AVM. Unequal options should not be presented as equivalent alternative forms of treatment. Granted, in some instances even the most experienced surgeon may not be sure of what the best option is and, in such cases, should clearly convey this to the patient and, if appropriate, refer the patient for a second opinion.
We believe that grade I and II AVMs in relatively young, healthy patients should be treated by surgical excision occasionally preceded by embolization for some of the grade II lesions. Radiosurgery is a good treatment alternative for older patients or those with significant surgical comorbid conditions. Decision making is more difficult with grade III AVMs, although most of them can still be treated safely with surgical excision frequently preceded by preoperative embolization. Most grade V AVMs should be treated conservatively because surgical excision carries unacceptably high risk and it is unlikely that these lesions will be obliterated completely, even with multimodality treatment. Some grade IV lesions can be excised safely after preoperative embolization, but many of these lesions should also be treated conservatively. Our experience, as well as a thorough review of the literature, has convinced us that partial (incomplete) treatment of AVMs by embolization does not improve and may worsen the natural history; however, there are specific indications for palliative embolization, such as symptoms of cerebral steal or venous hypertension, intractable seizures, repeated hemorrhages, and specific “high-risk” angiographic findings. Radiosurgery is a wonderful treatment modality for relatively small AVMs that because of their location cannot be surgically resected with safety.
The surgical approach to cerebral AVMs has to be very carefully individualized for each patient because practically no AVM is identical to another. Except for surgical excision of small superficial AVMs in noncritical areas, AVM surgery requires considerable experience and careful planning. The position of the patient and design of the craniotomy to optimize exposure, minimize brain retraction, and provide early control of major arterial feeders are most important. Well-known surgical principles include identification and control of arterial feeders only when they are entering the AVM and preservation of as much of the venous drainage as possible until all the arterial input has been eliminated. Intraoperative angiography or early postoperative angiography when the former is not available is mandatory for all but the simplest small superficial lesions. Once complete surgical excision has been demonstrated angiographically, the risk for hemorrhage in the future, at least in adults, seems to approximate zero.
Baskaya MK, Heros RC. Indications for and complications of embolization of cerebral arteriovenous malformations. J Neurosurg. 2006;104:183-186.
Brown RDJr, Wiebers DO, Forbes GS. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859-863.
Drake CG. Surgical removal at arteriovenous malformations from the brain stem and cerebellopontine angle. J Neurosurg. 1975;43:661-670.
Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98:3-7.
Hashimoto N, Nozaki K, Takagi Y, et al. Surgery of cerebral arteriovenous malformations. Neurosurgery. 2007;61(suppl 1):375-387.
Heros RC. Embolization of arteriovenous malformations. J Neurosurg. 2004;100:807-809.
Heros RC. Brain resection for exposure of deep extracerebral and paraventricular lesions. Surg Neurol. 1990;34:188-195.
Heros R. Arteriovenous malformations of the brain. In: Ojemann R, Heros R, Crowell R, editors. Surgical Management of Cerebrovascular Disease. Baltimore: Williams & Wilkins; 1988:347-413.
Heros RC. Arteriovenous malformations of the medial temporal lobe. Surgical approach and neuroradiological characterization. J Neurosurg. 1982;56:44-52.
Heros R, Korosue K. Parenchymal cerebral arteriovenous malformations. In: Appuzzo M, editor. Brain Surgery: Complication Avoidance Management. New York: Churchill Livingstone; 1993:1175-1192.
Heros RC, Korosue K. Radiation treatment of cerebral arteriovenous malformations. N Engl J Med. 1990;323:127-129.
Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations: late results. Neurosurgery. 1990;26:570-577.
Kondziolka D, McLaughlin MR, Kestle JR. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995;37:851-855.
Korosue K, Heros R. Complications of complete surgical resection of AVMs of the brain. In: Barrow D, editor. Intracranial Vascular Malformations. Neurosurgical Topics. Park Ridge, IL: AANS Publications; 1990:157.
Pikus HJ, Beach ML, Harbaugh RE. Microsurgical treatment of arteriovenous malformations: analysis and comparison with stereotactic radiosurgery. J Neurosurg. 1998;88:641-646.
Pollock BE, Flickinger JC, Lunsford LD, et al. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998;42:1239-1244.
Schaller C, Schramm J, Haun D. Significance of factors contributing to surgical complications and to late outcome after elective surgery of cerebral arteriovenous malformations. J Neurol Neurosurg Psychiatry. 1998;65:547-554.
Sisti MB, Stein BM. Arteriovenous malformations of the brain stem. Neurosurg Clin N Am. 1993;4:497-505.
Solomon RA, Stein BM. Management of arteriovenous malformations of the brain stem. J Neurosurg. 1986;64:857-864.
Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651-672.
Tew J, Lewis A. Honored guest presentation: Management strategies for the treatment of intracranial arteriovenous malformations. Clin Neurosurg. 2000;46:267-284.
Yasargil M. AVM of the brain, clinical considerations, general and special operative techniques, surgical results, nonoperated cases, cavernous and venous angiomas, neuroanesthesia. In: Microneurosurgery. Stuttgart: Thieme; 1988:479.
Yasargil M. AVM of the brain, history, embryology, pathological considerations, hemodynamics, diagnostic studies, microsurgical anatomy. In: Microneurosurgery. Stuttgart: Thieme; 1987:49-211.
1 Chin LS, Raffel C, Gonzalez-Gomez I, et al. Diffuse arteriovenous malformations: a clinical, radiological, and pathological description. Neurosurgery. 1992;31:863-868.
2 Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
3 Crawford PM, West CR, Chadwick DW, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
4 Brown RDJr, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
5 Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
6 Mast H, Young WL, Koennecke HC, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065-1068.
7 Halim AX, Johnston SC, Singh V, et al. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697-1702.
8 Baskaya MK, Jea A, Heros RC, et al. Cerebral arteriovenous malformations. Clin Neurosurg. 2006;53:114-144.
9 Chen JW, Kerber C, U HS. Spontaneous regression of large bilateral basal ganglia arteriovenous malformations. AJNR Am J Neuroradiol. 1991;12:835-837.
10 Panciani PP, Fontanella M, Carlino C, et al. Progressive spontaneous occlusion of a cerebellar AVM: pathogenetic hypothesis and review of literature. Clin Neurol Neurosurg. 2008;110:502-510.
11 Peretta P, Carlino C, Gennari F, et al. Spontaneous occlusion of brainstem arteriovenous malformation following ligature of a hepatic patent ductus venosus. Case report and review of the literature. J Neurosurg. 2007;106(2 suppl):147-152.
12 Sawlani V, Handique A, Phadke RV. Spontaneous regression of cerebral AVM due to thrombosis of draining vein—angiographic and MRI demonstration. J Neurol Sci. 2004;223:195-198.
13 Pascual-Castroviejo I, Pascual-Pascual JI, Blazquez MG, et al. Spontaneous occlusion of an intracranial arteriovenous malformation. Childs Brain. 1977;3:169-179.
14 Krapf H, Siekmann R, Freudenstein D, et al. Spontaneous occlusion of a cerebral arteriovenous malformation: angiography and MR imaging follow-up and review of the literature. AJNR Am J Neuroradiol. 2001;22:1556-1560.
15 Abdulrauf SI, Malik GM, Awad IA. Spontaneous angiographic obliteration of cerebral arteriovenous malformations. Neurosurgery. 1999;44:280-287.
16 Spetzler RF, Hargraves RW, McCormick PW, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:918-923.
17 Mansmann U, Meisel J, Brock M, et al. Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery. 2000;46:272-279.
18 Marks MP, Lane B, Steinberg GK, et al. Hemorrhage in intracerebral arteriovenous malformations: angiographic determinants. Radiology. 1990;176:807-813.
19 Willinsky R, Lasjaunias P, Terbrugge K, et al. Brain arteriovenous malformations: analysis of the angio-architecture in relationship to hemorrhage (based on 152 patients explored and/or treated at the Hôpital de Bicêtre between 1981 and 1986). J Neuroradiol. 1988;15:225-237.
20 Nataf F, Meder JF, Merienne L, et al. [Therapeutic strategy for cerebral arteriovenous malformations. Proposal for classification of individual hemorrhagic risk.]. Neurochirurgie. 1998;44:83-93.
21 Turjman F, Massoud TF, Viñuela F, et al. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery. 1995;37:856-860.
22 Norbash AM, Marks MP, Lane B. Correlation of pressure measurements with angiographic characteristics predisposing to hemorrhage and steal in cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 1994;15:809-813.
23 Duong DH, Young WL, Vang MC, et al. Feeding artery pressure and venous drainage pattern are primary determinants of hemorrhage from cerebral arteriovenous malformations. Stroke. 1998;29:1167-1176.
24 Finnerty JJ, Chisholm CA, Chapple H, et al. Cerebral arteriovenous malformation in pregnancy: presentation and neurologic, obstetric, and ethical significance. Am J Obstet Gynecol. 1999;181:296-303.
25 Horton JC, Chambers WA, Lyons SL, et al. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1990;27:867-871.
26 Kondziolka D, McLaughlin MR, Kestle JR. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995;37:851-855.
27 Brown RDJr. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 2000;46:1024.
28 Schaller C, Schramm J, Haun D. Significance of factors contributing to surgical complications and to late outcome after elective surgery of cerebral arteriovenous malformations. J Neurol Neurosurg Psychiatry. 1998;65:547-554.
29 Luessenhop AJ, Gennarelli TA. Anatomical grading of supratentorial arteriovenous malformations for determining operability. Neurosurgery. 1977;1:30-35.
30 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
31 Du R, Keyoung HM, Dowd CF, et al. The effects of diffuseness and deep perforating artery supply on outcomes after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2007;60:638-646.
32 Spears J, Terbrugge KG, Moosavian M, et al. A discriminative prediction model of neurological outcome for patients undergoing surgery of brain arteriovenous malformations. Stroke. 2006;37:1457-1464.
33 Baskaya MK, Heros RC. Indications for and complications of embolization of cerebral arteriovenous malformations. J Neurosurg. 2006;104:183-186.
34 Heros RC. Embolization of arteriovenous malformations. J Neurosurg. 2004;100:807-809.
35 Hurst RW, Berenstein A, Kupersmith MJ, et al. Deep central arteriovenous malformations of the brain: the role of endovascular treatment. J Neurosurg. 1995;82:190-195.
36 Vinuela F, Duckwiler G, Gugliemi G. Intravascular embolization of brain arteriovenous malformations. In: Macinunas R, editor. Endovascular Neurological Intervention. Park Ridge, IL: AANS Publications; 1995:189-199.
37 Frizzel RT, Fisher WS3rd. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery. 1995;37:1031-1039.
38 Deruty R, Pelissou-Guyotat I, Amat D, et al. Complications after multidisciplinary treatment of cerebral arteriovenous malformations. Acta Neurochir (Wien). 1996;138:119-131.
39 Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
40 Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations: part I—technique, morphology, and complications. Neurosurgery. 1996;39:448-457.
41 Debrun GM, Aletich V, Ausman JI, et al. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery. 1997;40:112-120.
42 Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131-214.
43 Kwon OK, Han DH, Han MH, et al. Palliatively treated cerebral arteriovenous malformations: follow-up results. J Clin Neurosci. 2000;7(suppl 1):69-72.
44 Nozaki K, Hashimoto N, Miyamoto S, et al. Resectability of Spetzler-Martin grade IV and V cerebral arteriovenous malformations. J Clin Neurosci. 2000;7(suppl 1):78-81.
45 Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48:984-995.
46 Hartmann A, Pile-Spellman J, Stapf C, et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33:1816-1820.
47 Dowzenko A, Jaworski M. [Endovascular embolization of cerebral arteriovenous malformation (AVM).]. Neurol Neurochir Pol. 2003;37:861-870.
48 Yu SC, Chan MS, Lam JM, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. AJNR Am J Neuroradiol. 2004;25:1139-1143.
49 Taylor CL, Dutton K, Rappard G, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810-812.
50 Haw CS, terBrugge K, Willinsky R, et al. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104:226-232.
51 Kim LJ, Albuquerque FC, Spetzler RF, et al. Postembolization neurological deficits in cerebral arteriovenous malformations: stratification by arteriovenous malformation grade. Neurosurgery. 2006;59:53-59.
52 Ledezma CJ, Hoh BL, Carter BS, et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58:602-611.
53 Jayaraman MV, Marcellus ML, Hamilton S, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol. 2008;29:242-246.
54 Andrade-Souza YM, Ramani M, Scora D, et al. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery. 2007;60:443-451.
55 Pollock BE, Flickinger JC, Lunsford LD, et al. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998;42:1239-1244.
56 Mathis JA, Barr JD, Horton JA, et al. The efficacy of particulate embolization combined with stereotactic radiosurgery for treatment of large arteriovenous malformations of the brain. AJNR Am J Neuroradiol. 1995;16:299-306.
57 Pollock BE, Flickinger JC, Lunsford LD, et al. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery. 1996;38:652-659.
58 Rosenkranz M, Regelsberger J, Zeumer H, et al. Management of cerebral arteriovenous malformations associated with symptomatic congestive intracranial hypertension. Eur Neurol. 2008;59:62-66.
59 Berthelsen B, Lofgren J, Svendsen P. Embolization of cerebral arteriovenous malformations with bucrylate. Experience in a first series of 29 patients. Acta Radiol. 1990;31:13-21.
60 Heros RC, Korosue K. Radiation treatment of cerebral arteriovenous malformations. N Engl J Med. 1990;323:127-129.
61 Heros RC. Treatment of arteriovenous malformations: gamma knife surgery. J Neurosurg. 2002;97:753-754.
62 Pikus HJ, Beach ML, Harbaugh RE. Microsurgical treatment of arteriovenous malformations: analysis and comparison with stereotactic radiosurgery. J Neurosurg. 1998;88:641-646.
63 Sasaki T, Kurita H, Saito I, et al. Arteriovenous malformations in the basal ganglia and thalamus: management and results in 101 cases. J Neurosurg. 1998;88:285-292.
64 Tew J, Lewis A. Honored guest presentation: Management strategies for the treatment of intracranial arteriovenous malformations. Clin Neurosurg. 2000;46:267-284.
65 Steinberg GK, Fabrikant JI, Marks MP, et al. Stereotactic helium ion Bragg peak radiosurgery for intracranial arteriovenous malformations. Detailed clinical and neuroradiologic outcome. Stereotact Funct Neurosurg. 1991;57:36-49.
66 Friedman W. Radiosurgery versus surgery for arteriovenous malformations: the case for radiosurgery. Clin Neurosurg. 1997;45:18-20.
67 Stieg PE, Friedlander RM, Loeffler JS, et al. Arteriovenous malformations: indications for stereotactic radiosurgery. Clin Neurosurg. 2000;47:242-248.
68 Inoue HK, Ohye C. Hemorrhage risks and obliteration rates of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 2002;97(5 suppl):474-476.
69 Pollock BE, Gorman DA, Coffey RJ. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery. 2003;52:1291-1296.
70 Shin M, Maruyama K, Kurita H, et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: the University of Tokyo experience. J Neurosurg. 2004;101:18-24.
71 Pollock BE, Gorman DA, Brown PD. Radiosurgery for arteriovenous malformations of the basal ganglia, thalamus, and brainstem. J Neurosurg. 2004;100:210-214.
72 Maruyama K, Kondziolka D, Niranjan A, et al. Stereotactic radiosurgery for brainstem arteriovenous malformations: factors affecting outcome. J Neurosurg. 2004;100:407-413.
73 Karlsson B, Lindqvist M, Blomgren H, et al. Long-term results after fractionated radiation therapy for large brain arteriovenous malformations. Neurosurgery. 2005;57:42-49.
74 Liscak R, Vladyka V, Simonová G, et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005-1014.
75 Kwon Y, Jeon SR, Kim JH, et al. Analysis of the causes of treatment failure in gamma knife radiosurgery for intracranial arteriovenous malformations. J Neurosurg. 2000;93(suppl 3):104-106.
76 Schaller C, Pavlidis C, Schramm J. [Differential therapy of cerebral arteriovenous malformations. An analysis with reference to personal microsurgery experiences.]. Nervenarzt. 1996;67:860-869.
77 Jones J, Jang S, Getch CC, et al. Advances in the radiosurgical treatment of large inoperable arteriovenous malformations. Neurosurg Focus. 2007;23(6):E7.
78 Prat R, Galeano I, Conde R, et al. Surgical removal after first bleeding of an arteriovenous malformation previously obliterated with radiosurgery: case report. Surg Neurol. 2009;71:211-214.
79 Kiran NA, Kale SS, Vaishya S, et al. Gamma Knife surgery for intracranial arteriovenous malformations in children: a retrospective study in 103 patients. J Neurosurg. 2007;107(6 suppl):479-484.
80 Karlsson B, Lindquist C, Steiner L. Effect of Gamma Knife surgery on the risk of rupture prior to AVM obliteration. Minim Invasive Neurosurg. 1996;39:21-27.
81 Coffey RJ, Nichols DA, Shaw EG. Stereotactic radiosurgical treatment of cerebral arteriovenous malformations. Gamma Unit Radiosurgery Study Group. Mayo Clin Proc. 1995;70:214-222.
82 Karlsson B, Lax I, Soderman M. Risk for hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001;49:1045-1051.
83 Andrade-Souza YM, Ramani M, Scora D, et al. Radiosurgical treatment for rolandic arteriovenous malformations. J Neurosurg. 2006;105:689-697.
84 Friedman WA, Bova FJ, Bollampally S, et al. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296-307.
85 Levegrun S, Hof H, Essig M, et al. Radiation-induced changes of brain tissue after radiosurgery in patients with arteriovenous malformations: dose/volume-response relations. Strahlenther Onkol. 2004;180:758-767.
86 Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359:863-873.
87 Chapman PH, Ogilvy CS, Loeffler JS. The relationship between occlusive hyperemia and complications associated with the radiosurgical treatment of arteriovenous malformations: report of two cases. Neurosurgery. 2004;55:228-233.
88 Thompson RC, Steinberg GK, Levy RP, et al. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery. 1998;43:202-211.
89 Deruty R, Mottolese C, Soustiel JF, et al. Association of cerebral arteriovenous malformation and cerebral aneurysm. Diagnosis and management. Acta Neurochir (Wien). 1990;107:133-139.
90 Cunha e Sa MJ, Stein BM, Solomon RA, et al. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1992;77:853-859.
91 Brown RDJr, Wiebers DO, Forbes GS. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859-863.
92 Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46:793-800.
93 Lasjaunias P, Piske R, Terbrugge K, et al. Cerebral arteriovenous malformations (C. AVM) and associated arterial aneurysms (AA). Analysis of 101 C. AVM cases, with 37 AA in 23 patients. Acta Neurochir (Wien). 1988;91:29-36.
94 Hashimoto N, Nozaki K, Takagi Y, et al. Surgery of cerebral arteriovenous malformations. Neurosurgery. 2007;61(suppl):375-389.
95 Anegawa S, Hayashi T, Torigoe R, et al. Intraoperative angiography in the resection of arteriovenous malformations. J Neurosurg. 1994;80:73-78.
96 Hernesniemi J, Dashti R, Mateo O, et al. Historical landmarks in vascular neurosurgery “On July 10th 2006, at the 70th Anniversary of the Department of Neurosurgery of Zurich Medical School. ”. Acta Neurochir Suppl. 2008;103;:131-137.
97 Raabe A, Beck J, Gerlach R, et al. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132-139.
98 Raabe A, Beck J, Seifert V. Technique and image quality of intraoperative indocyanine green angiography during aneurysm surgery using surgical microscope integrated near-infrared video technology. Zentralbl Neurochir. 2005;66:1-6.
99 Woitzik J, Horn P, Vajkoczy P, et al. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005;102:692-698.
100 Raabe A, Nakaji P, Beck J, et al. Prospective evaluation of surgical microscope–integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005;103:982-989.
101 Unno N, Suzuki M, Yamamoto N, et al. Indocyanine green fluorescence angiography for intraoperative assessment of blood flow: a feasibility study. Eur J Vasc Endovasc Surg. 2008;35:205-207.
102 Chibbaro S, Tacconi L. Image-guided microneurosurgical management of vascular lesions using navigated computed tomography angiography. An advanced IGS technology application. Int J Med Robot. 2006;2:161-167.
103 Tanaka Y, Imai H, Terada T, et al. [Intraoperative ultrasonography in AVM surgery.]. No Shinkei Geka. 2006;34:37-43.
104 Woydt M, Perez J, Meixensberger J, et al. Intra-operative colour-duplex-sonography in the surgical management of cerebral AV-malformations. Acta Neurochir (Wien). 1998;140:689-698.
105 Schmitz B, Nimsky C, Wendel G, et al. Anesthesia during high-field intraoperative magnetic resonance imaging experience with 80 consecutive cases. J Neurosurg Anesthesiol. 2003;15:255-262.
106 Nimsky C, Ganslandt O, von Keller B, et al. Intraoperative high-field MRI: anatomical and functional imaging. Acta Neurochir Suppl. 2006;98:87-95.
107 Yasargil M. AVM of the brain, history, embryology, pathological considerations, hemodynamics, diagnostic studies, microsurgical anatomy. In: Microneurosurgery. Stuttgart: Thieme; 1987:49-211.
108 Heros R. Arteriovenous malformations of the brain. In: Ojemann R, Heros R, Crowell R, editors. Surgical Management of Cerebrovascular Disease. Baltimore: Williams & Wilkins; 1988:347-413.
109 Yasargil M. AVM of the brain, clinical considerations, general and special operative techniques, surgical results, nonoperated cases, cavernous and venous angiomas, neuroanesthesia. In: Microneurosurgery. Stuttgart: Thieme; 1988:479.
110 Heros RC. Arteriovenous malformations of the medial temporal lobe. Surgical approach and neuroradiological characterization. J Neurosurg. 1982;56:44-52.
111 U HS. Microsurgical excision of paraventricular arteriovenous malformations. Neurosurgery. 1985;16:293-303.
112 Shi YQ, Chen XC. Surgical treatment of arteriovenous malformations of the striatothalamocapsular region. J Neurosurg. 1987;66:352-356.
113 Malik GM, Umansky F, Patel S, et al. Microsurgical removal of arteriovenous malformations of the basal ganglia. Neurosurgery. 1988;23:209-217.
114 Solomon RA, Stein BM. Interhemispheric approach for the surgical removal of thalamocaudate arteriovenous malformations. J Neurosurg. 1987;66:345-351.
115 Waga S, Shimosaka S, Kojima T. Arteriovenous malformations of the lateral ventricle. J Neurosurg. 1985;63:185-192.
116 Solomon RA, Stein BM. Management of arteriovenous malformations of the brain stem. J Neurosurg. 1986;64:857-864.
117 Drake CG. Surgical removal at arteriovenous malformations from the brain stem and cerebellopontine angle. J Neurosurg. 1975;43:661-670.
118 Chyatte D. Vascular malformations of the brain stem. J Neurosurg. 1989;70:847-852.
119 Sisti MB, Stein BM. Arteriovenous malformations of the brain stem. Neurosurg Clin N Am. 1993;4:497-505.
120 Nozaki K, Hashimoto N, Kikuta K, et al. Surgical applications to arteriovenous malformations involving the brainstem. Neurosurgery. 58(4 suppl 2), 2006. ONS-270-278
121 Izawa M, Hayashi M, Chernov M, et al. Long-term complications after gamma knife surgery for arteriovenous malformations. J Neurosurg. 2005;102(suppl):34-37.
122 Heros R, Korosue K. Parenchymal cerebral arteriovenous malformations. In: Appuzzo M, editor. Brain Surgery: Complication Avoidance Management. New York: Churchill Livingstone; 1993:1175-1192.
123 Heros R. Surgery for arteriovenous malformations of the brain. In: Ojemann R, Ogilvey CS, Crowell R, et al, editors. Surgical Management of Neurovascular Disease. 3rd ed. Baltimore: Williams & Wilkins; 1995:420-475.
124 Heros R. Prevention and management of therapeutic complications. In: Jafar J, Awad I, Rosenwasser R, editors. Vascular Malformations of the Central Nervous System. Philadelphia: Williams & Wilkins; 1999:363.
125 Latchaw RE, Hu X, Ugurbil K, et al. Functional magnetic resonance imaging as a management tool for cerebral arteriovenous malformations. Neurosurgery. 1995;37:619-625.
126 Heros RC, Ojemann RG, Crowell RM. Superior temporal gyrus approach to middle cerebral artery aneurysms: technique and results. Neurosurgery. 1982;10:308-313.
127 Heros RC. Brain resection for exposure of deep extracerebral and paraventricular lesions. Surg Neurol. 1990;34:188-195.
128 Korosue K, Heros R. Complications of complete surgical resection of AVMs of the brain. In: Barrow D, editor. Intracranial Vascular Malformations. Neurosurgical Topics. Park Ridge, IL: AANS Publications; 1990:157.
129 Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations: late results. Neurosurgery. 1990;26:570-577.
130 Thorpe ML, Cordato DJ, Morgan MK, et al. Postoperative seizure outcome in a series of 114 patients with supratentorial arteriovenous malformations. J Clin Neurosci. 2000;7:107-111.
131 Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651-672.
132 Miyasaka Y, Yada K, Ohwada T, et al. Hemorrhagic venous infarction after excision of an arteriovenous malformation: case report. Neurosurgery. 1991;29:265-268.
133 Meyer B, Urbach H, Schaller C, et al. Is stagnating flow in former feeding arteries an indication of cerebral hypoperfusion after resection of arteriovenous malformations? J Neurosurg. 2001;95:36-43.
134 Miyasaka Y, Yada K, Ohwada T, et al. Retrograde thrombosis of feeding arteries after removal of arteriovenous malformations. J Neurosurg. 1990;72:540-545.
135 Miyasaka Y, Kurata A, Tanaka R, et al. The significance of retrograde thrombosis following removal of arteriovenous malformations in elderly patients. Surg Neurol. 1998;49:399-405.
136 Sisti MB, Kader A, Stein BM. Microsurgery for 67 intracranial arteriovenous malformations less than 3 cm in diameter. J Neurosurg. 1993;79:653-660.
137 Tokunaga K, Kinugasa K, Meguro T, et al. Curative treatment of cerebral arteriovenous malformations by embolisation using cellulose acetate polymer followed by surgical resection. J Clin Neurosci. 2000;7(suppl 1):1-5.
138 Irie K, Nagao S, Honma Y, et al. Treatment of arteriovenous malformation of the brain—preliminary experience. J Clin Neurosci. 2000;7(suppl 1):24-29.
139 Hongo K, Koike G, Isobe M, et al. Surgical resection of cerebral arteriovenous malformation combined with pre-operative embolisation. J Clin Neurosci. 2000;7(suppl 1):88-91.
140 Pik JH, Morgan MK. Microsurgery for small arteriovenous malformations of the brain: results in 110 consecutive patients. Neurosurgery. 2000;47:571-575.
141 Hartmann A, Stapf C, Hofmeister C, et al. Determinants of neurological outcome after surgery for brain arteriovenous malformation. Stroke. 2000;31:2361-2364.
142 Russell SM, Woo HH, Joseffer SS, et al. Role of frameless stereotaxy in the surgical treatment of cerebral arteriovenous malformations: technique and outcomes in a controlled study of 44 consecutive patients. Neurosurgery. 2002;51:1108-1116.
143 Lawton MT. Spetzler-Martin Grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery. 2003;52:740-748.
144 Morgan MK, Rochford AM, Tsahtsarlis A, et al. Surgical risks associated with the management of grade I and II brain arteriovenous malformations. Neurosurgery. 2004;54:832-837.
145 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-6.
146 Hassler W, Hejazi N. Complications of angioma surgery: personal experience in 191 patients with cerebral angiomas. Neurol Med Chir (Tokyo). 1998;38:238-244.
147 Jizong Z, Shuo W, Jingsheng L, et al. Combination of intraoperative embolisation with surgical resection for treatment of giant cerebral arteriovenous malformations. J Clin Neurosci. 2000;7(suppl 1):54-59.
148 Hashimoto H, lida J, Hironaka Y, et al. Surgical management of cerebral arteriovenous malformations with intraoperative digital subtraction angiography. J Clin Neurosci. 2000;7(suppl 1):33-35.
149 Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98:3-7.