Chapter 81B Metastatic malignant liver tumors
Neuroendocrine
Overview
The liver is second only to regional lymph nodes as the dominant site of metastases from all gastrointestinal (GI) tract malignancies. Because progression of hepatic metastases is the predominant cause of death in patients with GI cancers, hepatic metastases have been the focus of a multimodal treatment approach. Importantly, the accumulated experience documenting the survival potential of hepatic resection for selected patients with colorectal metastases (see Chapter 81A) has prompted evaluation of this approach for other malignancies metastatic to the liver. Whether similar approaches will prove effective for neuroendocrine metastases remains unclear.
Evidence supporting hepatic resection for metastatic GI neuroendocrine tumors (NETs) continues to emerge. Hepatic resection of metastatic NET to the liver is appealing because gastrointestinal NET has a similar route of metastatic dispersal through the portal venous system, as do colorectal cancers. Moreover, their natural history is typically prolonged compared with other GI tract cancers and, in fact, with other solid tumors. Initial experience with hepatic resection for metastatic NET (Foster & Berman, 1977) suggested that patients might benefit in terms of survival and symptom relief from clinical endocrinopathies when antihormonal and antineoplastic therapies were ineffective. Consequently, interest in hepatic resection for metastatic NET has grown. In addition to the prolonged natural history of NET and the clinically significant endocrinopathies, several other observations have supported further assessment of hepatic resection: 1) the prolonged presence of intrahepatic disease before evidence of extrahepatic progression, 2) the impression that the severity of clinical endocrinopathy correlates with the intrahepatic volume of metastatic disease, 3) the resectability of the primary and regional NET despite metastatic disease, and 4) the presence of normal, nonmetastatic liver. This chapter details the clinical data supporting hepatic resection for metastatic NET; presents the current outcomes for hepatic resection, hepatic transplantation, and ablative therapy for metastatic NET; and presents an algorithm for long-term management of patients with metastatic NET in the liver.
Classification of Gastroenteropancreatic Neuroendocrine Tumors
Most neuroendocrine metastases to the liver are of GI or pancreatic origin, so-called gastroenteropancreatic (GEP) tumors (Rindi et al, 1998). GEP neuroendocrine tumors have historically been divided into two broad types, carcinoid and noncarcinoid, either of which may or may not be associated with hormone production that causes a clinical endocrinopathy (functional or nonfunctional, respectively). Traditionally, GI carcinoids (GICs) have been classified by their site of origin—foregut (lung, thymus, stomach, duodenum, pancreas, bile duct, gallbladder, and liver), midgut (small intestine, appendix, and proximal colon), and hindgut (distal colon and rectum)—because biologic and biochemical features within these groups vary. In contrast, pancreatic neuroendocrine tumors have been classified simply by whether they are functional (see Chapter 61). Regardless of origin, neuroendocrine tumors are similar histopathologically. Many histologic and morphologic features are shared by both benign and malignant tumors. Importantly, only the confirmed presence of metastases confers an unequivocal diagnosis of malignancy.
Clinical behavior for NET has ranged from an indolent to an aggressive clinical course with rapid cancer progression and death. For GEP neuroendocrine tumors, two schemes have been employed (Capella et al, 1995; Solcia et al, 2000). Broadly these classifications stratify patients with malignant GEP into low-grade (well-differentiated) or high-grade (poorly differentiated) NET (World Health Organization [WHO] classification) and subtype each as either functioning or nonfunctioning. In general, only patients with liver metastases from well-differentiated (low-grade) but not poorly differentiated (high-grade) NET are approached surgically. The TNM (tumor-node-metastases) staging has also been proposed (Rindi et al, 2006, 2007) and correlated with survival (Fischer et al, 2008; Pape et al, 2008a, 2008b; Skov et al, 2008). Some classifications tend to limit patients based on the number and extent of hepatic metastases on the radiologic imaging: a single metastasis (type I), isolated metastatic bulk accompanied by smaller deposits (type II), and disseminated metastatic spread throughout the liver (type III). These three groups differed significantly in regard to tumor-related characteristics, but this classification correlated with therapeutic approach and long-term survival (Frilling et al, 2009). The recently published guidelines by the North American Neuroendocrine Tumors Society (NANETs) highlight the importance of a uniform approach to pathologic reporting. This is especially relevant regarding differentiation and grade of individual tumors, which have a major impact on prognostication and choice of therapy (Klimstra et al, 2010).
GIC cancers produce a variety of proteins and peptide hormones (Onaitis et al, 2000; Schnirer et al, 2003). Almost all NETs are positive for neuroendocrine markers chromogranin A and neuron-specific enolase, but serum levels have correlated poorly with prognosis (Tomassetti et al, 2001); however, they are useful for treatment follow-up (Jensen et al, 2007; Nikou et al, 2008). Pancreatic NETs produce a wide variety of one or more peptides: gastrin, insulin, glucagon, and vasoinhibitory peptide, among others (Gumbs et al, 2002; Mansour & Chen, 2004). Nonfunctional pancreatic NET implies either the production of an inactive peptide, subclinical hormone production, or no peptide production. Reviews for the specific endocrinopathies from pancreatic neuroendocrine tumors are cited for reference to recognize their implications in clinical management (Gumbs et al, 2002; Mansour & Chen, 2004).
Epidemiology
A brief overview of the most commonly encountered primary GEP NETs is presented because combined resection of the primary and regional extent of disease and hepatic metastases is frequently undertaken. GICs comprise nearly 75% of all carcinoids with the remainder primarily of bronchopulmonary origin. The distribution of GIC is composed of the small intestine (35%), rectum (23%), appendix (19%), colon (12%), stomach (6%), duodenum (4%), and hepatobiliary-pancreatic region (2%) (Modlin et al, 2003). The relative incidence of gastric and rectal carcinoids has increased over the last 3 decades but has decreased in the appendix. GICs are frequently associated with noncarcinoid tumors in the small intestine (29%), stomach (21%), and appendix (18%). Overall, localized disease characterizes the presentation of rectal (80%) and gastric (70%) carcinoids, and nonlocalized disease is represented in colonic (80%) and small intestinal (67%) carcinoids. Although GICs are often considered to behave benignly, 13% of patients have metastatic disease at presentation, and overall 5-year survival for GIC is only 67% (Kulke & Mayer, 1999).
Nonfunctional NETs of the pancreas have an equal sex distribution (Hochwald et al, 2001). NET of the pancreas occurs in 0.5 to 1 person per 100,000 population. Nonfunctional NET accounts for more than 50% of pancreatic NET in most series, and wide variations in incidence between types of NET are likely related to the definition of “nonfunctional.” Multicentricity is not infrequent (15%). The epidemiology of functional pancreatic neuroendocrine tumors varies widely, with or without the multiple endocrine neoplasia (MEN) 1 syndrome, and is beyond the scope of this chapter; however, references are provided.
Small Intestinal Carcinoids
Small intestinal carcinoids are the most common GIC (Kulke & Mayer, 1999; Modlin et al, 2003). Patients usually present with abdominal pain, diarrhea, GI obstruction, and bleeding. Small intestinal carcinoids account for 90% of patients with the carcinoid syndrome. Approximately 20% of patients with the syndrome will have clinically evident carcinoid heart disease, and an even larger proportion will have occult heart disease detectable by echocardiography (Bernheim et al, 2007). Small intestinal carcinoids are often associated with dense mesenteric fibrosis, intestinal obstruction, hemorrhage, and intestinal ischemia secondary to vessel compression by metastatic regional lymph nodes and local release of vasoactive hormones (mesenteric angiopathy) (Eckhauser et al, 1981). Nearly 20% to 30% of patients have multiple GI carcinoids, and more than a third of patients have concurrent noncarcinoid cancers. Besides regional lymph node metastases, these carcinoids frequently metastasize to the liver, peritoneum, retroperitoneal nodes, and ovaries.
Resection is the only potentially curative treatment for small intestinal carcinoids (Hellman et al, 2002a; Loftus & van Heerden, 1995; Soreide et al, 1992; Woodside et al, 2004). Because of the frequency of the associated syndrome, careful preoperative evaluation and preparation are necessary. When suspected clinically, baseline urinary 5-hydroxyindole acetic acid (5-HIAA) levels should be obtained. The extent and site of the primary small intestinal carcinoid can be defined by barium contrast GI series, enteric computed tomographic (CT) scan, enteric magnetic resonance imaging (MRI), or by small bowel enteroclysis. Further imaging of the abdomen and chest for metastatic disease is best defined by contrast-enhanced CT. Often CT will identify mesenteric adenopathy and stranding characteristic of regionally advanced small bowel carcinoids even without identification of the site of the primary carcinoid on small bowel contrast studies.
Assessment of the relationship of the regional lymphatic metastases to the vessels of the small bowel is important in planning resection of the primary and regional extent of disease (Mullen et al, 2005). Despite the typical desmoplastic reaction adjacent to the involved regional lymph nodes, resection is feasible, except with extensive invasion of the third portion of the duodenum and the uncinate process of the pancreas, circumferential arterial and venous encasement without patent proximal arterial collaterals, or distal mesenteric arteries not large enough for reconstruction (Ohrvall et al, 2000; Schindl et al, 2002).
Various imaging methods may be helpful. Although not anatomically useful in operative planning, somatostatin receptor scintigraphy and octreoscanning are useful is evaluating the overall disease extent and the site of the primary, when not recognized by other studies; these are also helpful in modifying treatment strategies (Slooter et al, 2001). The role of MRI is increasing, primarily in assessing the extent of hepatic metastases. MRI is particularly sensitive in detecting small metastases within the liver and often will detect disease not seen by other imaging techniques inclusive of CT; however, MRI has limited applicability for defining the site of the primary carcinoid.
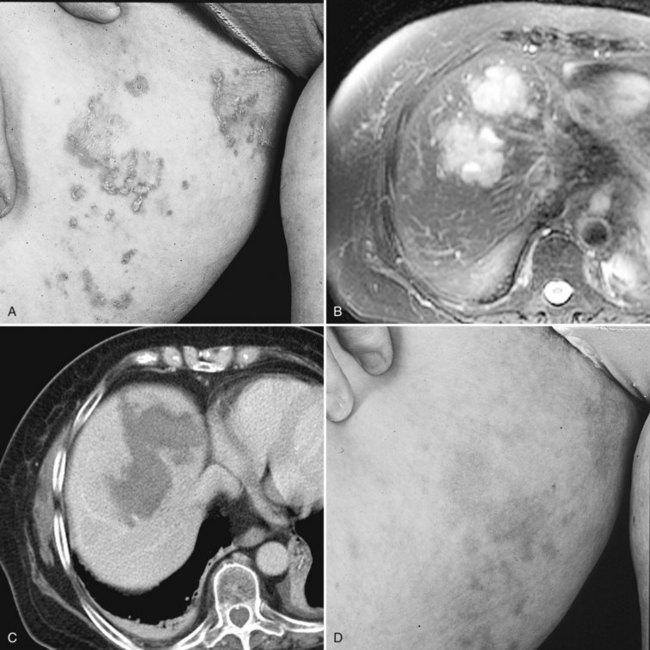
The recognition of carcinoid heart disease demands a thorough cardiac evaluation (Fox & Khattar, 2004). The major implication of carcinoid heart disease is the presence of right heart failure and the consequently elevated systemic venous pressures that can cause a pulsatile liver (implying hepatic vein pressures >25 mm Hg), which precludes hepatic resection. The presence of clinical significant carcinoid heart disease dictates medical treatment, and occasionally even valve replacement, prior to resection of the primary small intestinal carcinoid and the hepatic metastases (Moller et al, 2005). Survival after surgical repair of carcinoid heart disease is improved compared with medical treatment, even without surgical treatment of hepatic metastases (Connolly et al, 1995). Some patients may be candidates for hepatic resection after repair of carcinoid heart disease, depending upon objective decreases in systemic venous hypertension and the degree of functional cardiac improvement (Connolly et al, 2002; McDonald et al, 1999). Conversely, hepatic resection has been shown to be associated with decreased cardiac progression of the carcinoid heart disease and improved prognosis (Bernheim et al, 2008).
Any patient with the carcinoid syndrome requires preoperative and intraoperative somatostatin analogue therapy to prevent a carcinoid crisis (Oberg et al, 2004), a clinical syndrome of life-threatening intraoperative hypotension or hypertension and severe flushing with or without concurrent bronchospasm or arrhythmias. To date, the frequency and factors predictive of this perianesthetic complication remain unknown. Prevention is essential, and appropriate treatment should be prescribed in all patients undergoing intervention for metastatic carcinoid tumors (Kinny et al, 2001). Short-acting analogues are preferred, even if the patient has received the long-acting analogue within 30 days. Management should consist of subcutaneous short-acting somatostatin analogue on call to operation and intravenous infusion of the analogue throughout the operation and in recovery. Additional intraoperative increases in infusion rates are appropriate for unexplained intraoperative hemodynamic instability.
Resection of small intestinal carcinoids should encompass the primary tumor, any multicentric tumors, and the regional lymph nodes. When intestinal ischemia is present, resection of the thickened, chronically ischemic bowel is indicated; resection of the regional lymph nodes is often possible by keeping the dissection immediately adjacent to the nodes and retracting the visceral vessels away from the involved nodes (Hellman et al, 2002a). Preservation of the blood supply to the GI tract is essential to prevent short-bowel syndrome. As mentioned previously, preoperative CT with intravenous contrast will define those patients with resectable nodal disease, which is very difficult to assess intraoperatively. Resection of hepatic metastases is generally indicated only if complete resection of the primary and regional disease is feasible. Resection of hepatic metastases should be performed concurrently, if all gross metastases can be excised. If nearly all of the hepatic disease can be resected (or ablated), cytoreductive resection should be considered, because both survival and symptom-free quality of life may be improved (Knox et al, 2004; Sarmiento et al, 2003; Chambers et al, 2008). Staged resections can be performed with similar expectations in outcomes. Overall 5-year survival for small intestinal carcinoid ranges from 50% to 67%.
Pancreatic Neuroendocrine Tumors (See Chapter 61)
An aggressive operative approach generally is warranted for NET of the pancreas (Fendrich et al, 2006; Hochwald et al, 2001; Matthews et al, 2002; Phan et al, 1998; Schurr et al, 2007; Solorzano et al, 2001). Resection remains the treatment of choice for patients with localized NET of the pancreas and for selected patients with hepatic metastases (Oberg & Jelic, 2008). Resection of the primary NET and the regional lymph nodes is generally possible despite their often large size. The extent and type of pancreatectomy is dictated by the site of the primary NET; pancreatoduodenectomy is used for NET of the head of the pancreas, and distal pancreatectomy/splenectomy is best for body or tail NET (see Chapter 62A, Chapter 62B ). In some cases, enucleation may be an alternative approach. For patients with locally invasive NET of adjacent structures or the GI tract, resection should be en bloc. Laparoscopic pancreatectomy is currently applicable for resection of most pancreatic NET (Fernandez-Cruz et al, 2002, 2008). Whether concurrent laparoscopic resection of the primary pancreatic NET and hepatic metastases is feasible has not been proven. Concurrent resection of primary pancreatic NET and hepatic metastases can be performed safely, although staged resections may be preferable, particularly in patients with involvement of the head of the pancreas (Sarmiento et al, 2002). Overall actuarial 5-year survival rates for NET of the pancreas range from 45% to 63% with a median survival of about 4 years.
Natural History
Regardless of whether NETs are classified as carcinoid or noncarcinoid, the natural history of patients with unresected or unresectable hepatic metastases has been similar. Overall, patients with unresected hepatic metastases from NETs have a 5-year survival of approximately 30% (Proye, 2001) and a median survival of 17 months (House et al, 2006).
Several factors affect the natural history of both carcinoid and noncarcinoid cancers (Durante et al, 2009). Clearly, liver metastases are the most significant factor adversely affecting outcome (Moertel, 1987; Pape et al, 2008a). With or without liver metastases from NET, 5-year survival is approximately 30% to 40% and 90% to 100%, respectively. Poorly differentiated NET and progressive neuroendocrine metastases in the liver (>25% volume increase on two CT scans within 3 months) further adversely affects survival among patients with hepatic metastasis (Madeira et al, 1998). Progression in size or number of hepatic neuroendocrine metastases can be expected in 90% after a median follow-up of 11.5 months (Skinazi et al, 1996). Carcinoid heart disease occurs only in the presence of metastatic carcinoid tumor to the liver independently and predicts poor survival. The survival of patients with clinically severe carcinoid heart disease is approximately 1.6 years; the survival rate is only 31% at 3 years, unless cardiac surgery is undertaken successfully (Connolly et al, 1995; Fox & Khattar, 2004). Patients with carcinoid heart disease should be considered for hepatic surgery, as stated before, because cardiac disease progression is reduced, and prognosis is improved (Bernheim et al, 2008).
Although uncontrolled functioning endocrinopathies from metastatic neuroendocrine tumors can be life threatening—for example, severe hypoglycemia from insulinoma and GI perforation from gastrinoma—the impact of such events on the natural history of these patients is unknown. Conversely, it is likely that clinical control of endocrinopathies can affect natural history of NET; however, specific studies to document clinical control of endocrinopathies by antihormonal therapy with objective tumor responses have not been well documented, except for gastrinoma before and after the use of H2 blockers or proton pump inhibitors (Norton et al, 2003a).
Similar to carcinoid tumors, the 5-year survival of patients with pancreatic NET ranges from 30% to 40% with a median survival of approximately 40 months (Chen et al, 1998; Thompson et al, 1985). Whether the presence or absence of clinical endocrinopathies affects the natural history of patients with metastatic pancreatic NET is unclear; however, the specific NET likely affects overall natural history. Up to 75% of patients who present with midgut or hindgut tumors are likely to have liver metastases, in particular the nonfunctioning group, and those with a pancreatic primary (likely to be high grade) have the highest rate of liver involvement (Steinmuller et al, 2008). In a series of 35 patients with hepatic metastases, 60% of which were from gastrinomas, the 5-year survival was approximately 70%. The prognosis also is affected by the extent of metastatic NET in the liver (Chamberlain et al, 2000; Phan et al, 1998); the 5-year survival was only 24% with more than 75% tumor replacement of the liver, whereas 5-year survival approached 80% for less than 50% tumor replacement.
Surgical and Ablative Treatment of Hepatic Neuroendocrine Metastases
Hepatic Resection for Neuroendocrine Metastases
The current mainstay of treatment for metastatic GI neuroendocrine tumors to the liver is resection (see Chapter 90E); however, no randomized controlled data support improved survival from hepatic resection of metastatic NET. Although hepatic resection of malignancy is employed most often with curative intent, the tumor biology of these typically indolent and slow-growing cancers renders them less responsive to more commonly employed palliative therapies, such as chemotherapy and radiation; thus, resection with palliative intent (cytoreduction) may afford patients a real and significant advantage in palliation and may prolong survival. Importantly, the focus on palliation and extension of life, rather than on complete remission, is conceptually a paradigm shift, because most hepatic resections are undertaken for cure in patients with metastatic solid cancers.
The treatment of hepatic metastases from NET is aimed at reduction of the mass of malignant tissue (cytoreduction) chiefly for two reasons: first and foremost, metastatic gastrointestinal NET is usually indolent and slow growing, with a well-differentiated histopathologic grade (WHO classification) (Solcia et al, 2000); thus chemotherapy and radiotherapy regimens targeted toward rapidly dividing cells are relatively ineffective; second, symptoms secondary to expression and secretion of biologically active peptides by these tumors is directly related to overall mass of tumor, although production of peptides may be heterogeneous among individual metastases. Similarly, pain and decreased performance status may negatively impact on quality of life for patients with nonfunctional NET metastatic to the liver. In patients who do respond to medical treatment, debulking surgery—cytoreduction of more than 90% of the liver tumors—is a valid and direct method of providing relief from symptoms. These reasons, coupled with improved safety for hepatic resection, have prompted some to advocate hepatic resection as a primary therapeutic option for patients with both functional and nonfunctional metastatic NET (Chambers et al, 2008). Based on these premises, hepatic cytoreduction, whether by hepatic resection or transplantation, should address clinical endocrinopathies and improve survival; as a corollary, the duration of response should be in proportion to the extent of the debulking or cytoreduction of the metastatic NET and the growth rate of the residual NET (House et al, 2006).
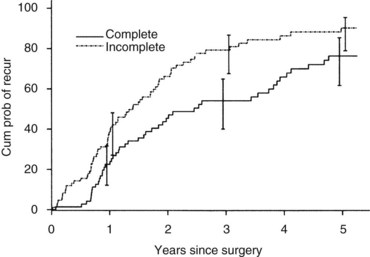
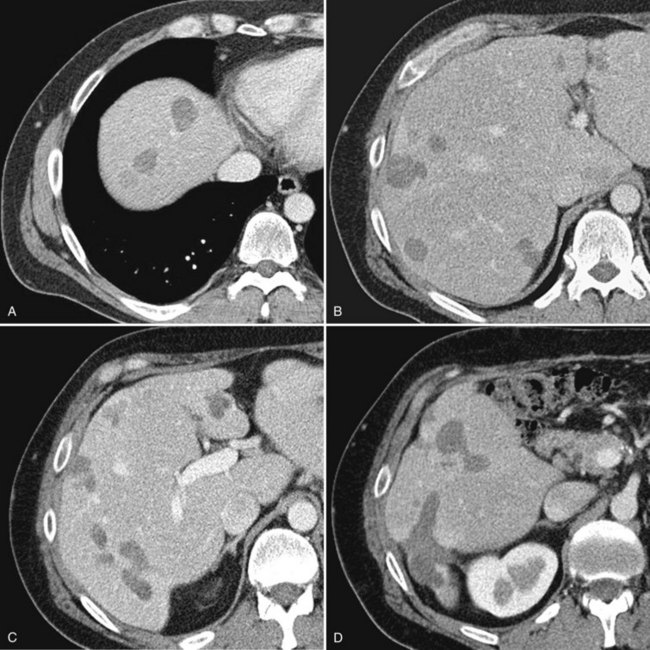
Currently, hepatic resection of metastatic NET is recommended if the primary tumor and regional disease are resectable or resected, and a minimum of 90% of the volume of hepatic metastases is resectable or ablatable (Steinmuller et al, 2008). Our initial results showed that debulking hepatic resection could be performed safely, and overall survival was about 75% at 4 years (Que et al, 1995). No significant difference in survival was found between patients undergoing complete (R0) or incomplete (R1 to R2) resections. Mean duration of symptom response was nearly 2 years. Subsequently, our findings in 170 patients undergoing hepatic resections showed that symptoms resolve in 98% of patients. Median time to symptom recurrence was 45 months, and 59% of patients experienced recurrent symptoms at 5 years. Overall survival was 61% and 35% at 5 and 10 years, respectively, and perioperative mortality rate was 1.2%. Recurrence, however, was 84% at 5 years and 94% at 10 years (Fig. 81B.1; Sarmiento et al, 2003). Examples of results obtained from cytoreductive hepatic resection for neuroendocrine tumors are shown in Figure 81B.2.
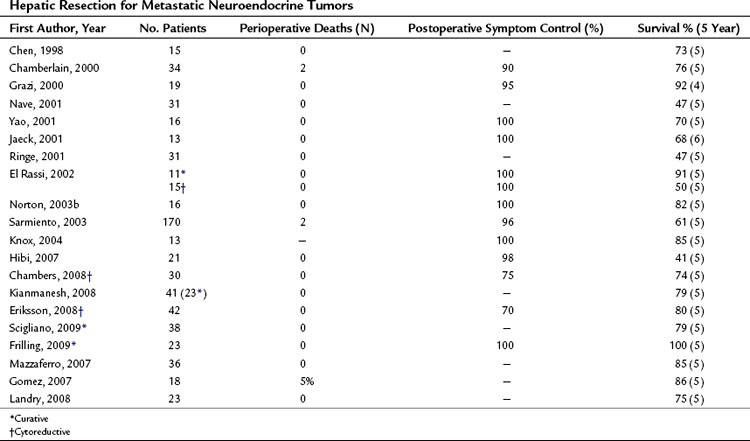
A review of the current literature on hepatic resection for metastatic NET is summarized in Table 81B.1. The cumulative findings from these reports support an aggressive operative approach for metastatic NET. Overall 5-year survival has ranged from 41% to 82% with a median 5-year survival of up to 100%. Only four perioperative deaths have been reported among the tabulated patients (n = 556). When addressed, resolution of endocrine symptoms exceeded 90% in most series, although duration of response was not routinely reported.
Although the majority of patients reported to date have had metastatic carcinoid cancer to the liver, survival has not differed appreciably among patients with carcinoid and noncarcinoid (primarily islet cell) cancers. Current data do not show that survival is affected by the presence or absence of clinical endocrinopathy. Interestingly, although complete macroscopic resection of hepatic metastases (R0/R1) has been undertaken more frequently, survival has not significantly differed among patients with complete or incomplete hepatic resection of metastatic NET, provided cytoreduction exceeds 90% of the estimated hepatic cancer volume (Que et al, 1995; Sartori et al, 2005). Significant improvement in quality of life with prolonged survival also has been confirmed for patients undergoing adequate (>90%) cytoreduction (Knox et al, 2004). These data suggest that hepatic resection of metastatic NET is safe and clinically effective, and overall operative survival is nearly double that of patients with unresected metastases. Few other factors related to the primary NET, metastatic disease, or patients appear to correlate with survival.
A major challenge is providing treatment for bilobar synchronous hepatic metastases, which are relatively common. Although one-step surgery may be feasible, it might require right portal vein embolization (see Chapter 93A, Chapter 93B ) to enhance the size of the future liver remnant. Compared with two-step surgical procedures that include resection of the primary and clearance of left liver, followed by right portal vein occlusion and a second step consisting of right or extended right hepatectomy, it seems that with or without preoperative portal vein embolization, one-step surgery is associated with a higher rate of R2 resection and more postoperative complications (Elias et al, 2003; Kianmanesh et al, 2008; Steinmuller et al, 2008). This might in part be due to preoperative intensive selection of patients in two-step procedures and dissociation of major liver and abdominal surgery; however, compared with liver transplantation, the two-step procedures represent a valid alternative by pushing the limit of respectability in type III patients, especially those with bilobar, synchronous liver metastases (Kianmanesh et al, 2008; Steinmuller et al, 2008).
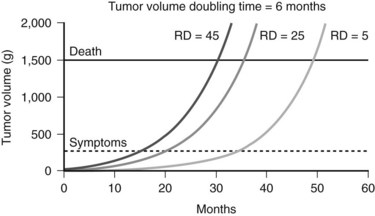
Complete resection remains the goal of cytoreductive hepatic resection, because both progression rate and median time to clinical recurrence are significantly reduced. The theoretic impact of minimizing residual metastatic NET in the liver on outcome is depicted in Figure 81B.3. Resection of more than 90% of the volume of hepatic metastases can result in significantly different residual cancer volumes based on variations of the incident metastatic volume. Figure 81B.3 shows the projected outcomes of three different residual tumor volumes on symptom recurrence and survival, assuming a greater than 90% resection and a cancer doubling time of 6 months. Both time to recurrence of symptoms and survival are related to residual metastatic cancer volume. The clinical impact of cytoreductive hepatic resection is not more than 90% resection of metastatic NET in the liver but the minimization of residual metastatic volume, that is, not resected volume but rather residual liver volume.
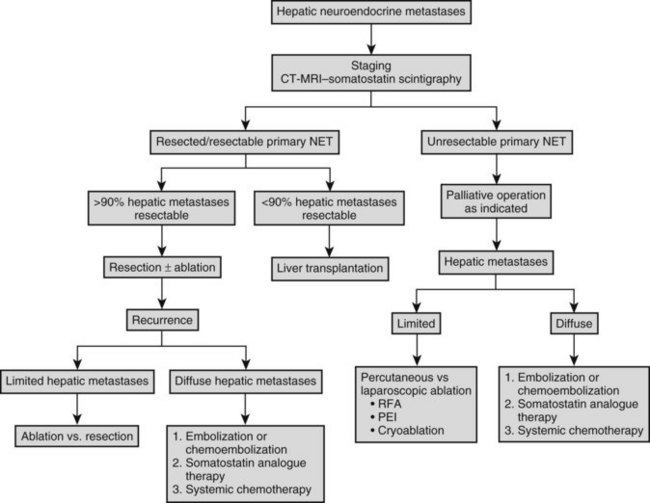
The overall frequency of recurrence or progression after hepatic resection is approximately 80% at 5 years. Intrahepatic progression of residual metastases after R1 or R2 resection or recurrence after R0 resection dictates subsequent liver-directed therapy, unless the risk of liver failure or extrahepatic disease spread override this issue. Although cure of patients with metastatic NET to the liver is infrequent, prolonged palliation is possible. Patterns of intrahepatic recurrence have not been well studied, but intrahepatic recurrence is typically multicentric in our experience. The extent and distribution of hepatic recurrence dictates the choice of therapy. For solitary recurrences, either resection or ablation is appropriate. Given that serial imaging usually identifies small recurrences after hepatic resection, percutaneous ablative approaches are preferable, because morbidity associated with percutaneous RFA is less than that after repeat resection, which is advised for lesions in sites that preclude safe RFA. Sequential ablation or resection is undertaken as recurrence is recognized, until precluded by extent of recurrence within the liver. Metastatic NET that precludes ablation or resection is treated by embolization or chemoembolization, with or without systemic chemotherapy in the absence of extrahepatic disease and with chemotherapy in the presence of extrahepatic disease. A schema for management of neuroendocrine metastases to the liver is outlined in Figure 81B.4.
Hepatic Transplantation for Neuroendocrine Metastases (See Chapters 97A and 97E)
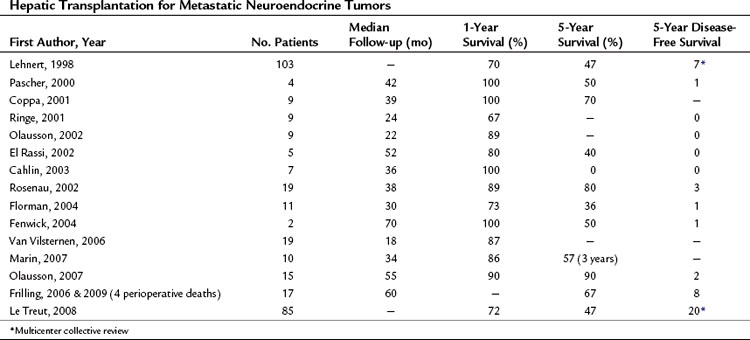
Orthotopic liver transplantation (OLT) has been frequently employed for the treatment of metastatic NET (Table 81B.2). The rationale for OLT is similar to that for resection, with the caveat that adequate cytoreduction by resection is precluded by extensive intrahepatic disease and the absence of extrahepatic metastases. OLT during the time period of intrahepatic disease may be curative or at least significantly palliative. Unlike resection, OLT addresses all hepatic metastases and should provide similar, if not greater, duration of palliation of symptoms and survival. The attractiveness of R0 resection afforded by OLT and the high recurrence rate of NET associated with resection has fostered ongoing evaluation of OLT for metastatic NET (Mazzaferro et al, 2007).
Several studies have attempted to correlate clinicopathologic factors to survival. Age, location of the primary NET, extent of the primary operation, extent of hepatic metastases, Ki67 index, E-cadherin expression, genetic instability (TP53), and histologic features have been assessed. Age less than 50 years, limited hepatic metastases, low Ki67 index, regular E-cadherin staining, and R0 resection of the primary NET with no evidence of locoregional progression for 1 or more years has correlated with prolonged survival (Fernandez et al, 2003). Current data preclude routine use of these factors as selection criteria for OLT until further confirmed.
Current data do not support OLT as a primary treatment modality for metastatic NET (Clark et al, 2006; Fernandez et al, 2003; Mazzaferro et al, 2007; Steinmuller et al, 2008), but it should be considered an investigative treatment alternative in specialty centers. OLT should probably be considered only in patients with resected primary NET, after a period of disease stability, with exclusion of any extrahepatic disease, and only for selected NET. Whether other biologic or genetic markers will improve the reliability for selection remains unclear.
Radiofrequency Ablation of Neuroendocrine Hepatic Metastases
Multiple minimally invasive ablative modalities exist for the treatment of liver tumors, including cryotherapy, percutaneous ethanol injection (PEI), and thermal techniques such as radiofrequency ablation (RFA), microwave ablation, and interstitial laser thermotherapy (ILT) (see Chapter 83, Chapter 84A, Chapter 84B, Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D ). Currently, RFA is the most widely used ablative technique for hepatic tumors in general because of its versatility, ease of use, and relatively low risk of complications (Bleicher et al, 2003; Giovannini & Seitz, 1994; Livraghi et al, 2003; Pearson et al, 1999; Wong et al, 2001); however, its utility for NET metastases will be limited by disease extent in most patients.
The application of RFA for metastatic NET arose after its efficacy was established in the treatment of hepatocellular carcinoma and metastatic colorectal cancer (Curley et al, 1999; Pawlik et al, 2003; Solbiati et al, 2001a; Wood et al, 2000). Local tumor control was achieved in 82% to 98% of treated metastases, although duration of follow-up varied (Bleicher et al, 2003; Bowles et al, 2001; Curley et al, 2000; de Baere et al, 2000; Pawlik et al, 2003, Pearson et al, 1999; Rhim & Dodd, 1999; Solbiati et al, 2001a). Local recurrence rates were higher in patients with colorectal metastases and lower in those with neuroendocrine hepatic metastases (Bleicher et al, 2003; Solbiati et al, 2001b), which is likely related to differences in disease biology and length of follow-up. Successful ablation typically occurred in the treatment of metastases smaller than 3 cm (de Baere et al, 2000; Kettenbach et al, 2003; Solbiati et al, 2001b; Wood et al, 2000); only 50% of tumors greater than 5 cm were ablated completely, even with repeated ablation (Goldberg & Ahmed, 2002).
Published experience with RFA for neuroendocrine hepatic metastases has been limited. RFA has been employed both primarily and as an adjunct to resection. Objective tumor destruction has been well documented and has been shown highly effective in local tumor control, with local recurrence in 3% to 5% of treated metastases (Berber et al, 2002; Hellman et al, 2002b). Improved survival with RFA of metastatic NET has been suggested for selected patients (Elias et al, 2003, 2009).
Duration of follow-up is insufficient to determine actual durability of local control given the protracted natural history of neuroendocrine cancers. Progression of hepatic metastases occurs in about 30% of patients within 2 years (Berber et al, 2002; Hellman et al, 2002b). Specific factors of the hepatic metastases—such as site, degree of necrosis or fibrosis, and hormonal markers—have not yet been correlated to RFA response.
RFA effectively relieves clinical endocrinopathies related to neuroendocrine hepatic metastases; nearly 90% of patients report some degree of symptom relief following ablation (Berber et al, 2002; Hellman et al, 2002b; Henn et al, 2003; Siperstein et al, 1997; Siperstein & Berber, 2001), and duration of relief from symptoms usually is 10 months or longer (Berber et al, 2002; Henn et al, 2003). These findings approach those reported after hepatic resection of metastatic NET (Chamberlain et al, 2000; Sarmiento et al, 2003). For limited hepatic metastases, RFA can achieve similar results percutaneously; however, it must be emphasized that the proportion of patients with disease that is amenable to percutaneous ablation is small. In the setting of extrahepatic disease, isolated hepatic ablation will have a limited effect on symptoms (Henn et al, 2003); however, clinical efficacy will depend upon the volume and site of extrahepatic tumor and the specific hormone expression.
Serum and urine neuroendocrine tumor markers are generally proportional to tumor burden (Jensen et al, 2007; Moertel, 1987). The response of such markers to RFA has been variable. In a study of 34 patients treated with intraoperative RFA, only 65% had a decrease in serum tumor markers afterward (Berber et al, 2002). Persistently elevated tumor markers after RFA likely reflect patient selection, completeness of ablation, and occult or overt neuroendocrine cancer within the abdomen or elsewhere. A favorable response in reduction of serum tumor markers following ablation may predict the durability of symptomatic response and may be associated with improved survival and decreased incidence of disease progression (Berber et al, 2002).
Ethanol Ablation of Hepatic Neuroendocrine Metastases (See Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D )
Ethanol ablation has also been incorporated into the treatment of primary and secondary hepatic neoplasms, including metastases from neuroendocrine malignancies in selected patients (Castells et al, 1993; Giovannini, 2002; Giovannini & Seitz, 1994; Lencioni et al, 1995; Livraghi et al, 1995, 1999; Shiina et al, 1993). Several studies have documented a complete intrahepatic response in patients with hepatic neuroendocrine metastases treated with percutaneous ethanol injection (PEI) (Livraghi et al, 1991), which permits ablation of metastases adjacent to structures at risk of damage by RFA.
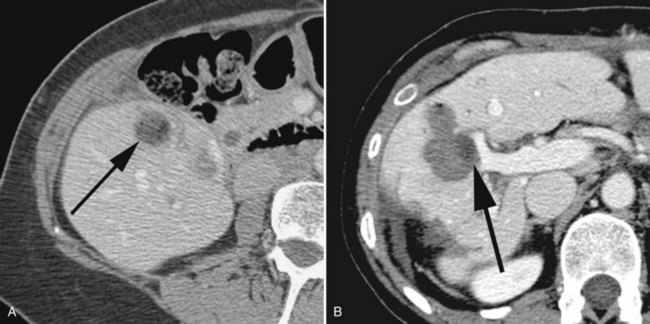
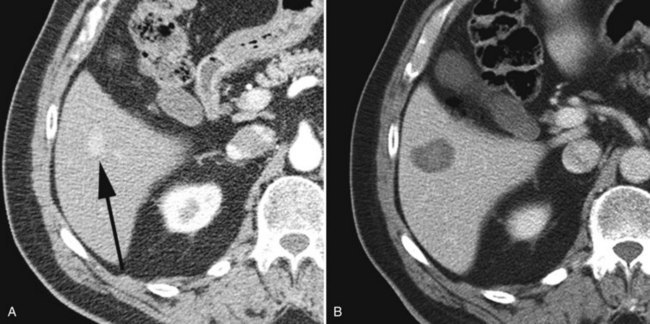
Highly selective ethanol ablation can be performed on metastases adjacent to vital structures, such as the hepatic flexure of the colon; those adjacent to large vessels vulnerable to the heat-sink effect; and those adjacent to central bile ducts, where subsequent biliary stricture may occur (Fig. 81B.5). Moreover, metastases of very small size can also be ablated successfully with ethanol, with limited collateral injury to adjacent liver.
Cryoablation of Hepatic Neuroendocrine Metastases (See Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D )
Intraoperative cryoablation is effective in the treatment of primary and secondary tumors of the liver (Finlay et al, 2000; Mahvi & Lee, 1999; Neeleman et al, 2001; Seifert et al, 1998b; Sheen et al, 2002; Zhou & Tang, 1998). Based on a collective review of 1990s literature, local recurrence develops in about 30% of cryoablated tumors (Seifert et al, 1998b). In a direct comparison of cryoablation and RFA in the treatment of both primary and secondary hepatic tumors, RFA was shown to be more effective in local control (2% vs. 14% local recurrence) with fewer complications (3% vs. 41% complication rate) (Chung et al, 2001; Pearson et al, 1999).
Current experience with cryoablation for hepatic neuroendocrine metastases reflects ablations performed intraoperatively (Bilchik et al, 1997; Cozzi et al, 1995; Goering et al, 2002; Seifert et al, 1998a). Most often, the intraoperative cryoablation is performed in conjunction with resection of the primary tumor and hepatic metastases (Cozzi et al, 1995; Seifert et al, 1998a). Local control of neuroendocrine hepatic metastases has occurred in 95% of treated tumors (Chung et al, 2001; Cozzi et al, 1995; Seifert et al, 1998a), which is similar to other ablative techniques. Some relief of symptoms has been shown in all patients, even with recognized subtotal ablative debulking (Bilchik et al, 1997; Cozzi et al, 1995; Seifert et al, 1998b), and postoperative ablative adjuvant therapy with a long-acting somatostatin analogue can prolong symptom-free survival (Chung et al, 2001).
Guidelines for Ablation
Resection is precluded in many patients with neuroendocrine metastases to the liver because of the extent of tumor burden, comorbid disease, or prior hepatic resection. In such patients, percutaneous ablation allows for less invasive tumor debulking and may have some advantage over chemotherapy alone, as shown by Ruers and colleagues (2007).
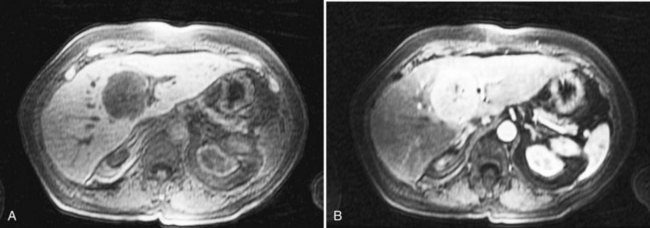
In contrast to nonneuroendocrine metastases, surgical experience justifies subtotal debulking of significant hepatic tumor by the ablation of multiple metastases (Fig. 81B.6). Generally speaking, if the metastasis is visible with imaging, it can usually be treated in some manner with percutaneous ablation, using either RFA or ethanol, provided sufficient liver parenchyma has been preserved to prevent hepatic decompensation.
This large-volume ablation contrasts with the ablation of limited disease. Such a patient might have one or two very small hepatic metastases such that surgical resection might be considered overly aggressive given the effectiveness of percutaneous RFA in treating such tumors (Fig. 81B.7). Percutaneous treatment is typically performed on an outpatient basis and obviates the longer hospitalization after resection. Moreover, given the invariable recurrence of metastases following surgical resection (Sarmiento et al, 2003), percutaneous ablation is well suited for the patient who has undergone prior liver surgery. Unlike surgical resection, ablation can easily be performed on multiple occasions based on occurrence of new metastases.
RFA of neuroendocrine hepatic metastases allows a relatively noninvasive mechanism to treat patient symptoms, similar to the role of hepatic resection. In our experience, less than 5% of patients are treated solely for tumor-related symptoms. The focus of such ablative treatment is usually twofold, with both debulking of the patient’s hepatic tumor and secondary symptom management (Fig. 81B.8).
Microwave (MW) ablation therapy is a local treatment by which tumors are destroyed by coagulation from the passage of MWs into tissue. MW therapy is emerging as a reliable technique under a variety of clinical situations. Two main zones are described after ablative therapy: central and transitional. No viable cells were demonstrated in 93% of lesions after treatment, even up to 6 cm in diameter (Gravante et al, 2008). Although data comparing RFA with MW are awaited, the results reported in observational studies indicate that MW may have a role when larger ablation zones are required (Boutros et al, 2010).
Medical Treatment of Hepatic Neuroendocrine Metastases
The several approaches for the medical treatment of metastatic NET include somatostatin analogues, chemotherapy, immunotherapy, embolization or chemoembolization, and internal irradiation with iridium 131 conjugates (de Vries et al, 2002; Van Essen et al, 2009).
Somatostatin Analogues
Octreotide is a synthetic somatostatin analogue with a significantly longer half-life and duration of action than native somatostatin (Lamberts et al, 1996). It is primarily cytostatic but can also be cytotoxic. The effect of somatostatin analogues is mediated through type 2 and type 5 somatostatin receptors, inhibiting the cellular release of hormone. Response may correlate to somatostatin receptor scintigraphy (Janson et al, 1994); the analogues also can affect cell-cycle arrest in G1 phase, induce apoptosis, and inhibit angiogenesis. Lantreotide is currently the longest acting or slowest release analogue. Octreotide dose ranges from 100 to 500 µg three times daily, and lantreotide is given 60 to 120 mg every 4 weeks.
Somatostatin analogues have been associated with a biochemical response in about 70% of patients, and symptomatic relief is seen in 60% to 90% (Oberg et al, 2004). Objective reduction in tumor size, or more than 50% of the largest diameter, has occurred in less than 10% of patients. Stabilization of NET has been observed in 36% to 70% of patients for a median duration of 12 months (Oberg et al, 2004). As expected, symptomatic response has been correlated with improved quality of life, although response to somatostatin analogue therapy varies by type of NET. As noted previously, short-acting somatostatin analogue therapy is used to prevent or to treat the carcinoid crisis periprocedurally for any intervention such as resection, transplantation, ablation, or embolization. In general, somatostatin analogue treatment is well tolerated. Steatorrhea, diarrhea, abdominal discomfort, and biliary sludge or gallstones can develop but rarely preclude continued use (Kaltsas et al, 2004; Kvols et al, 1987; Trendle et al, 1997). More recently, the long-acting analogues of somatostatin, such as lanreotide and long-acting octreotide, have become available and are the mainstay of symptomatic treatment (Modlin et al, 2008).
Chemotherapy
Systemic chemotherapy is generally reserved for patients with advanced or progressive disease, where other treatment efforts have failed (Brentijens & Saltz, 2001; Kaltsas et al, 2001; Rivera & Ajani, 1998). Recent results of randomized trials have established the role of sunitinib in the management of pancreatic NETs. It has been shown to increase progression-free and overall survival (Raymond et al, 2011). Data from previous trials are also presented. Patients with pancreatic NET have been more responsive to chemotherapy than those with carcinoid tumors (Rivera & Ajani, 1998), and streptozocin-based combinations with 5-fluorouracil (5-FU) and doxorubicin have resulted in objective responses in 45% to 69% of patients (Oberg, 2001; Moertel et al, 1992); however, a more recent study reported disappointing results using a 5-FU and streptozocin combination (Maire et al, 2009). Median duration of response for high-grade NET is about 8 to 9 months. Carcinoid tumors may be less sensitive to cytotoxic agents because of the preponderance of low-grade malignant (well-differentiated) histology and low proliferation index (Bajetta et al, 2002). Dacarbazine, 5-FU, and epirubicin combination therapy has achieved objective tumor response in about 30% of patients with carcinoid tumors (Bajetta et al, 2002; Oberg et al, 2004), and median duration of response for carcinoid tumors is about 6 months. Some success has been achieved with hepatic artery infusional cisplatin in pancreatic NET metastases (Igarashi et al, 2007).
α-Interferon
Systemic α-interferon (α-IFN) may be used to treat advanced NET. The mechanism of α-IFN is mediated through direct inhibition of the cell cycle (G1/S phase), protein and hormone production, and antiangiogenesis; it is mediated indirectly by increased immune stimulation (Oberg et al, 2004). Although an objective tumor response is seen in only 10% to 15%, both symptomatic and biochemical responses have been observed in about 40% to 60% of patients (Biesma et al, 1992; Fjällskog et al, 2002; Jacobsen et al, 1995; Moertel et al, 1989; Oberg et al, 1986, 2004). Disease stabilization has occurred in 40% to 60% of patients. Adverse reactions to α-IFN are common and may dictate its discontinuation; chronic fatigue and hematologic cytopenias are the most common side effects.
Embolization or Chemoembolization of Hepatic Neuroendocrine Metastases (See Chapter 83)
Neuroendocrine metastases are intensely hypervascular. Embolization of the hepatic arteries results in ischemia of the metastases and causes variable degrees of tumor necrosis, which can alleviate symptoms or endocrinopathies (Perry et al, 1994). Embolization alone or in combination with intraarterial chemotherapy (chemoembolization) has been employed for relief of symptoms. Repeated embolization is possible depending upon the interventional vascular technique used, selective or nonselective. Objective tumor responses to embolization alone have ranged from 30% to 70% with similar symptomatic response rates (Brown et al, 1999; Oberg et al, 2004). Intermittent or temporary dearterialization with the use of an implantable hepatic artery occluder provides similar symptomatic relief for 6 to 12 months (Bengmark et al, 1982). Duration of response has ranged from 15 to 30 months, and chemotherapy following embolization has prolonged duration of response (Moertel et al, 1994). These findings, coupled with the theoretical advantages of high intrahepatic concentration afforded by arterial infusion of chemotherapeutic agents, have prompted evaluation of chemoembolization. Although chemoembolization has been used for metastatic NET, range and duration of response has been similar to embolization alone (Fiorentini et al, 2004). To date, no randomized trial comparing embolization with and without chemotherapy have confirmed a significant difference in outcomes or response, and observational data have shown no statistical difference between the two groups (Pitt et al, 2008; Ruutiainen et al, 2007). A combination of chemoembolization with hepatic artery chemoinfusion for patients with unresectable hepatic disease has achieved better than 3-year survival for the majority of patients (Christante et al, 2008).
New Drugs and Targets
Recent randomized trials assessing the utility of sunitinib maleate notwithstanding (Raymond et al, 2011) the crucial issue with rigorous assessment of medical treatment is that most studies are retrospective, assess heterogeneous tumors, commonly lack standardized entry criteria, reflect single-center experience, and are underpowered. As previously discussed, the tyrosine kinase inhibitor sunitinib malate has shown proven efficacy in the management of metastatic pancreatic NETs (Raymond et al, 2011). It has recently been approved by the FDA and has been adopted by several European countries.
Traditional DNA-damaging cytotoxic drugs are of limited efficacy in GEP NETs for reasons outlined above. Several proangiogenic molecules are overexpressed in NETs, such as vascular endothelial growth factor (VEGF) and its receptors and related signaling-pathway components: epithelial growth factor receptor, insulin-like growth factor-1 receptor, phosphoinositide-3-kinase, RAC-α serine/threonine-protein kinase (AKT), and mammalian target of rapamycin (mTOR). New drugs that target some of these molecules are under assessment in early clinical trials, such as bevacizumab (monoclonal antibody against VEGF). Angiogenesis and mTOR inhibitors (temsirolimus, everolimus) may have potential, but less than 20% of patients show a radiologic response (Konings et al, 2009; Yao et al, 2008). Although strategies that use these biologic agents might advance the management of GEP NETs, they were first developed for other tumor types. The development of more effective drugs for GEP NETs will need improved understanding of GEP NET biology and perhaps the discovery of a molecular target specific to all or some subtypes of GEP NETs. Use of somatostatin receptors to target so-called passenger drugs—that is, active cytotoxic drugs that are physically linked to agents that bind to somatostatin receptors—might hold promise.
Recently, the combination of two oral cytotoxic agents, capecitabine and temozolomide, has been shown to have significant activity in patients with advanced pancreatic endocrine tumors. A response rate of 70%, combined with a progression-free survival of 18 months, was recently reported (Strosberg et al, 2010).
Peptide Receptor Radionuclide Therapy
GEP NETs overexpress peptide receptors, mainly subtype 2, which become internalized after ligand binding; therefore they are targets for cytotoxic drugs coupled to somatostatin, such as radiolabeled somatostatin analogues. Using indium 111 diagnostic scintigraphy for these receptors can identify tumors that express somatostatin receptors (Nasir et al, 2008; de Jong et al, 2009). This new treatment is proving to be safe and effective and might become an important treatment strategy for lesions that express adequate densities of somatostatin receptors (Kwekkeboom et al, 2008).
Primary Hepatic Neuroendocrine Tumors
Albeit rare, neuroendocrine tumors may arise primarily within the liver. Hepatobiliary neuroendocrine tumors accounted for only 2% of all GI carcinoids in the Surveillance Epidemiology and End Results (SEER) database over a 30-year period, and primary hepatic carcinoids accounted for less than half of these tumors (Modlin et al, 2003). Most reports of primary hepatic carcinoids are single-case experiences, and case series are usually limited to less than 6 patients (Knox et al, 2003). Primary hepatic carcinoids have similar histopathologic and immunohistochemical findings as extrahepatic carcinoids. Most primary hepatic carcinoids are nonfunctional and are rarely associated with the carcinoid syndrome; however, because they can be functional and may be associated with a specific endocrinopathy, and not limited only to the carcinoid syndrome, they should probably be termed primary hepatic neuroendocrine tumors and not hepatic carcinoids.
The diagnosis presumes a thorough search and exclusion of an extrahepatic neuroendocrine tumor. The cell of origin is unknown. Pancreatic heterotopia has been postulated as a source of these tumors. Some may arise from intrahepatic biliary tract radicles, because carcinoids of the extrahepatic biliary tract are more common. Moreover, most primary hepatic neuroendocrine tumors arise centrally or in the perihilar area within the liver (Hwang et al, 2008). Finally, some primary hepatic neuroendocrine tumors may in fact be metastases from an occult primary NET or a primary NET that had spontaneously regressed. A history of prior GEP resections warrants careful pathologic review of the resected specimen to exclude a missed primary NET. Conversely the absence of an identifiable extrahepatic site of NET after resection is posited as evidence supporting the liver as a primary site.
NET shows a female predominance (65%), and mean age at presentation is 50 years. Typical symptoms and signs at presentation include abdominal pain, jaundice, weight loss, and rarely a specific endocrinopathy. The imaging features are similar to other neuroendocrine tumors (Fig. 81B.9). The criteria for resectability of primary hepatic NET are similar for other hepatic malignancies. Because of the central location within the liver, extended lobar hepatic resections are frequently required for resection of these tumors. Hepatic transplantation has also been employed.
A review of the literature reveals an overall 5- and 10-year survival of 78% and 59%, respectively (Knox et al, 2003). Among the 44 patients reported to have had hepatic resections for treatment, 5- and 10-year survival were 80% and 68% respectively (Knox et al, 2003). Predictors of long-term survival have been few, and analysis of such factors is limited by the low prevalence of primary hepatic NET. In fact, hepatic multicentricity and bilaterality, extrahepatic metastases at the time of resection, advanced age, and gender have not correlated adversely with survival. Recurrence within the liver has not been uncommon, and repeat resections have been reported and are associated with further prolongation of survival. Hepatic transplantation has also proven effective for primary hepatic NET deemed unresectable otherwise by standard techniques (Fenwick et al, 2004). The overall and disease-free survival after hepatic transplantation are similar to those of standard resection, albeit limited in duration of follow-up and number of patients reported. Current data preclude assessment of antihormonal therapy and chemotherapy, although anecdotal reports suggest responses are similar to those in patients with extrahepatic NET.
Bajetta E, et al. Efficacy of a chemotherapy combination for the treatment of metastatic neuroendocrine tumours. Ann Oncol. 2002;13:614-621.
Bengmark S, et al. Temporary liver dearterialization in patients with metastatic carcinoid disease. World J Surg. 1982;6:46-53.
Berber E, Flesher N, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg. 2002;26:985-990.
Bernheim AM, Connolly HM, Pellikka PA. Carcinoid heart disease. Curr Treat Options Cardiovasc Med. 2007;9:482-489.
Bernheim AM, et al. Role of hepatic resection for patients with carcinoid heart disease. Mayo Clin Proc. 2008;83:143-150.
Biesma B, et al. Recombinant interferon alpha-2b in patients with metastatic apudomas: effect on tumours and tumour markers. Br J Cancer. 1992;66:850-855.
Bilchik AJ, et al. Cryosurgical palliation of metastatic neuroendocrine tumors resistant to conventional therapy. Surgery. 1997;122:1040-1047.
Bleicher RJ, et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10:52-58.
Boutros C, et al. Microwave coagulation therapy for hepatic tumors: review of the literature and critical analysis. Surg Oncol. 2010;19:e22-e32.
Bowles BJ, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864-869.
Brentijens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist’s perspective. Surg Clin North Am. 2001;81:527-542.
Brown KT, et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Intervent Radiol. 1999;10:397-403.
Cahlin C, et al. Liver transplantation for metastatic neuroendocrine tumor disease. Transplant Proc. 2003;35:809-810.
Capella C, et al. Revised classification of neuroendocrine tumours of the lung, pancreas, and gut. Virch Arch. 1995;425:547-560.
Castells A, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18:1121-1126.
Chamberlain RS, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432-445.
Chambers AJ, et al. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645-651.
Chen H, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88-93.
Christante D, et al. Hepatic artery chemoinfusion with chemoembolization for neuroendocrine cancer with progressive hepatic metastases despite octreotide therapy. Surgery. 2008;144:885-893.
Chung MH, et al. Hepatic cytoreduction followed by a novel long-acting somatostatin analog: a paradigm for intractable neuroendocrine tumors metastatic to the liver. Surgery. 2001;130:954-962.
Clark OH, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2006;4:102-138.
Connolly HM, et al. Outcome of cardiac surgery of carcinoid heart disease. J Am Coll Cardiol. 1995;25:410-416.
Connolly HM, et al. Carcinoid heart disease: impact of pulmonary valve replacement on right ventricular function and remodeling. Circulation. 2002;106:151-156.
Coppa J, et al. Resection versus transplantation for liver metastases from neuroendocrine tumors. Transplant Proc. 2001;33:1537-1539.
Cozzi PJ, Englund R, Morris D. Cryotherapy treatment of patients with hepatic metastases from neuroendocrine tumors. Cancer. 1995;76:501-509.
Curley SA, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8.
Curley SA, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381-391.
de Baere T, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619-1625.
de Jong M, et al. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc Chem Res. 2009;42:873-880.
de Vries H, et al. Diagnostic, surgical, and medical aspect of the midgut carcinoid. Cancer Great Rev. 2002;28:11-25.
Durante C, et al. Prognostic factors influencing survival from metastatic (stage IV) gastroenteropancreatic well-differentiated endocrine carcinoma. Endocr Relat Cancer. 2009;16:585-597.
Eckhauser FE, et al. Mesenteric angiopathy, intestinal gangrene, and midgut carcinoids. Surgery. 1981;90:720-728.
Elias D, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery. 2003;133:375-382.
Elias D, et al. Combined liver surgery and RFA for patients with gastroenteropancreatic endocrine tumors presenting with more than 15 metastases to the liver. Eur J Surg Oncol. 2009;35:1092.
El Rassi ZS, et al. Primary and secondary liver endocrine tumors: clinical presentation, surgical approach and outcome. Hepatogastroenterology. 2002;49:1340-1346.
Eriksson J, et al. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg. 2008;32:930-938.
Fendrich V, et al. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg. 2006;244:845-851.
Fenwick SW, et al. Hepatic resection and transplantation for primary carcinoid tumors of the liver. Ann Surg. 2004;239:210-219.
Fernandez JA, et al. Role of liver transplantation in the management of metastatic neuroendocrine tumors. Transplant Proc. 2003;35:1832-1833.
Fernandez-Cruz L, et al. Outcome of laparoscopic pancreatic surgery: endocrine and nonendocrine tumors. World J Surg. 2002;26:1057-1065.
Fernandez-Cruz L, et al. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumors? World J Surg. 2008;32:904-917.
Finlay IG, et al. Resection with cryotherapy of colorectal hepatic metastases has the same survival as hepatic resection alone. Eur J Surg Oncol. 2000;26:199-202.
Fiorentini G, et al. Intra-arterial hepatic chemoembolization in liver metastases from neuroendocrine tumors: a phase II study. J Chemother. 2004;16:293-297.
Fischer L, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95:627-635.
Fjällskog ML, et al. Treatment of malignant endocrine pancreatic tumors with a combination of alpha-interferon and somatostatin analogs. Med Oncol. 2002;19:35-42.
Florman S, et al. Liver transplantation for neuroendocrine tumors. J Gastrointest Surg. 2004;8:208-212.
Foster, JH, Berman, MM. Solid Liver Tumors. Philadelphia: WB Saunders, 1977.
Fox DJ, Khattar RS. Carcinoid heart disease: presentation, diagnosis, and management. Heart. 2004;90:1224-1228.
Frilling A, et al. Liver transplantation for patients with metastatic endocrine tumors: single-center experience with 15 patients. Liver Transpl. 2006;12:1089-1096.
Frilling A, et al. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg. 2009;96:175-184.
Giovannini M. Percutaneous alcohol ablation for liver metastasis. Semin Oncol. 2002;29:192-195.
Giovannini M, Seitz JF. Ultrasound-guided percutaneous alcohol injection of small liver metastases: results in 40 patients. Cancer. 1994;73:294-297.
Goering JD, et al. Cryoablation and liver resection for noncolorectal liver metastases. Am J Surg. 2002;183:384-389.
Goldberg SN, Ahmed M. Minimally invasive image-guided therapies for hepatocellular carcinoma. J Clin Gastroenterol. 2002;35:S115-S129.
Gomez D, et al. Hepatic resection for metastatic gastrointestinal and pancreatic neuroendocrine tumours: outcome and prognostic predictors. HPB (Oxford). 2007;9:345-351.
Gravante G, et al. Hepatic microwave ablation: a review of the histological changes following thermal damage. Liver Int. 2008;28:911-921.
Grazi GL, et al. Highly aggressive policy of hepatic resections for neuroendocrine liver metastases. Hepatogastroenterology. 2000;47:481-486.
Gumbs AA, et al. Review of the clinical, histological, and molecular aspects of pancreatic endocrine neoplasms. J Surg Oncol. 2002;81:45-53.
Hellman P, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg. 2002;26:991-997.
Hellman P, et al. Radiofrequency tissue ablation using cooled tip for liver metastasis of neuroendocrine tumors. World J Surg. 2002;26:1052-1056.
Henn AR, et al. Percutaneous radiofrequency ablation of hepatic metastases for symptomatic relief of neuroendocrine syndromes. AJR Am J Roentgenol. 2003;181:1005-1010.
Hibi T, et al. Surgery for hepatic neuroendocrine tumors: a single institutional experience in Japan. Jpn J Clin Oncol. 2007;37:102-107.
Hochwald SN, Conlon KC, Brennan MR. Nonfunctional pancreatic islet cell tumors. In: Doherty, G, Skogseid, B. Surgical Endocrinology. Philadelphia. Lippincott: Williams & Wilkins; 2001:361-373.
House MG, et al. Differences in survival for patients with resectable versus unresectable metastases from pancreatic islet cell cancer. J Gastrointest Surg. 2006;10:138-145.
Hwang S, et al. Surgical treatment of primary neuroendocrine tumors of the liver. J Gastrointest Surg. 2008;12:725-730.
Igarashi H, et al. Successful management of multiple liver metastases from pancreatic neuroendocrine tumor by hepatic arterial administration of cisplatin powder. Pancreas. 2007;35:288-290.
Jacobsen MB, et al. Interferon-alpha 2b, with or without prior hepatic artery embolization: clinical response and survival in mid-gut carcinoid patients: the Norwegian Carcinoid Study. Scand J Gastroenterol. 1995;30:789-796.
Jaeck D, et al. Hepatic metastases of gastroenteropancreatic neuroendocrine tumors: safe hepatic surgery. World J Surg. 2001;25:689-692.
Janson ET, et al. [111In-DTPA-D-Phe1] octreotide scintigraphy in patients with carcinoid tumours: the predictive value for somatostatin analogue treatment. Eur J Endocrinol. 1994;131:577-581.
Jensen EH, et al. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol. 2007;14:780-785.
Kaltsas G, et al. The role of chemotherapy in the nonsurgical management of malignant neuroendocrine tumours. Clin Endocrinol (Oxf). 2001;55:575-587.
Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458-511.
Kettenbach J, et al. Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. AJR Am J Roentgenol. 2003;180:1537-1545.
Kianmanesh R, et al. Two-step surgery for synchronous bilobar liver metastases from digestive endocrine tumors: a safe approach for radical resection. Ann Surg. 2008;247:659-665.
Kinny MAO, et al. Perianaesthetic risks and outcomes of abdominal surgery for metastatic carcinoid tumours. Br J Anaesth. 2001;87:447-452.
Klimstra SD, et al. The pathological classification of neuroendocrine tumors. A review of nomenclature, grading and staging systems. Pancreas. 2010;39:707-712.
Knox CD, et al. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171-1175.
Knox CD, et al. Survival and functional quality of life after resection of hepatic carcinoid metastasis. J Gastrointest Surg. 2004;8:653-659.
Konings IRHM, et al. The applicability of mTOR inhibition in solid tumors. Curr Cancer Drug Targets. 2009;9:439-450.
Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999;340:858-868.
Kvols LK, et al. Treatment of metastatic islet cell carcinoma with a somatostatin analogue (SMS 201-995). Ann Intern Med. 1987;107:162-168.
Kwekkeboom DJ, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3] octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124-2130.
Lamberts SW, et al. Octreotide. N Engl J Med. 1996;334(4):246-254.
Landry CS, et al. Proposed staging system for gastrointestinal carcinoid tumors. Am Surg. 2008;74:418-422.
Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma. Transplantation. 1998;66:1307-1312.
Lencioni R, et al. Treatment of small hepatocellular carcinoma with percutaneous ethanol injection: analysis of prognostic factors in 105 Western patients. Cancer. 1995;76:1737-1746.
Le Treut Y, et al. Predictors of long-term survival after liver transplantation for metastatic endocrine tumors: an 85-case French multicentric report. Am J Transplant. 2008;8:1205-1213.
Livraghi T, Vettori C, Lazzaroni S. Liver metastases: results of percutaneous ethanol injection in 14 patients. Radiology. 1991;179:709-712.
Livraghi T, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101-108.
Livraghi T, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661.
Livraghi T, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451.
Loftus JP, van Heerden JA. Surgical management of gastrointestinal carcinoid tumors. Adv Surg. 1995;28:317-336.
Madeira I, et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 1998;43:422-427.
Mahvi DM, Lee FTJr. Radiofrequency ablation of hepatic malignancies: is heat better than cold? Ann Surg. 1999;230:9-11.
Maire F, et al. Is adjuvant therapy with streptozotocin and 5-fluorouracil useful after resection of liver metastases from digestive endocrine tumors? Surgery. 2009;145:69-75.
Mansour JC, Chen H. Pancreatic endocrine tumors. J Surg Res. 2004;120:139-161.
Marin C, et al. Role of liver transplantation in the management of unresectable neuroendocrine liver metastases. Transplant Proc. 2007;39:2302-2303.
Matthews BD, et al. Surgical experience with functioning pancreatic neuroendocrine tumors. Am Surg. 2002;68:660-665.
Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47:460-466.
McDonald ML, et al. Carcinoid heart disease and carcinoid syndrome: successful surgical treatment. Ann Thorac Surg. 1999;67:537-539.
Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
Modlin IM, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72.
Moertel CG. Karnofsky memorial lecture: an odyssey in the land of small tumors. J Clin Oncol. 1987;5:1502-1522.
Moertel CG, Rubin J, Kvols LK. Therapy of metastatic carcinoid tumor and the malignant carcinoid syndrome with recombinant leukocyte A interferon. J Clin Oncol. 1989;7:865-868.
Moertel CG, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519-523.
Moertel CG, et al. The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Int Med. 1994;120:302-309.
Moller JE, et al. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2005;348:1005-1015.
Mullen JT, et al. Carcinoid tumors of the duodenum. Surgery. 2005;138:971-977.
Nasir A, et al. Multimodality management of a polyfunctional pancreatic endocrine carcinoma with markedly elevated serum vasoactive intestinal polypeptide and calcitonin levels. Pancreas. 2008;36:309-313.
Nave H, et al. Surgery as primary treatment in patients with liver metastases from carcinoid tumors: a retrospective, unicentric study over 13 years. Surgery. 2001;129:170-175.
Neeleman N, et al. Cryosurgery as treatment modality for colorectal liver metastases. Hepatogastroenterology. 2001;48:325-329.
Nikou GC, et al. Chromogranin a levels in diagnosis, treatment and follow-up of 42 patients with non-functioning pancreatic endocrine tumours. Pancreatology. 2008;8:510-519.
Norton JA, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057-1065.
Norton JA, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859-866.
Oberg K. Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S111-S114.
Oberg K, Jelic S. Neuroendocrine gastroenteropancreatic tumors: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(Suppl 2):ii104-ii105.
Oberg K, et al. Treatment of malignant carcinoid tumors with human leukocyte interferon: long-term results. Cancer Treat Rep. 1986;70:1297-1304.
Oberg K, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Acta Oncol. 2004;43:617-625.
Ohrvall U, et al. Method for dissection of mesenteric metastases in mid-gut carcinoid tumors. World J Surg. 2000;24:1402-1408.
Olausson M, et al. Indication and results of liver transplantation in patients with neuroendocrine tumours. World J Surg. 2002;26:998-1004.
Olausson M, et al. Orthotopic liver or multivisceral transplantation as treatment of metastatic neuroendocrine tumors. Liver Transpl. 2007;13:327-333.
Onaitis MW, et al. Gastrointestinal carcinoids: characterization by site of origin and hormone production. Ann Surg. 2000;232:549-556.
Pape UF, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083-1097.
Pape UF, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256-265.
Pascher A, et al. Primary and secondary hepatic manifestation of neuroendocrine tumors. Lang Arch Surg. 2000;385:265-270.
Pawlik TM, et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059-1069.
Pearson AS, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592-599.
Perry LJ, et al. Hepatic arterial embolization for metastatic neuroendocrine tumors. Surgery. 1994;116:1111-1116.
Phan GQ, et al. Surgical experience with pancreatic and peripancreatic neuroendocrine tumors: review of 125 patients. J Gastrointest Surg. 1998;2:472-482.
Pitt SC, et al. Hepatic neuroendocrine metastases: chemo- or bland embolization? J Gastrointest Surg. 2008;12:1951-1960.
Proye C. Natural history of liver metastases of gastroenteropancreatic neuroendocrine tumors: place for chemoembolization. World J Surg. 2001;25:685-688.
Que FG, et al. Hepatic resection for neuroendocrine carcinomas. Am J Surg. 1995;169:36-42.
Raymond E, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513.
Rhim H, Dodd GD3rd. Radiofrequency thermal ablation of liver tumors. J Clin Ultrasound. 1999;27:221-229.
Rindi G, Capella C, Solcia E. Cell biology, clinicopathological profile, and classification of gastro-enteropancreatic endocrine tumors. J Mol Med. 1998;76:413-420.
Rindi G, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401.
Rindi G, et al. TNM staging of midgut and hindgut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757-762.
Ringe B, et al. Treatment of hepatic metastases from gastroenteropancreatic neuroendocrine tumors: role of liver transplantation. World J Surg. 2001;25:697-699.
Rivera E, Ajani JA. Doxorubicin, streptozocin, and 5-fluorouracil chemotherapy for patients with metastatic islet-cell carcinoma. Am J Clin Oncol. 1998;21:36-38.
Rosenau J, et al. Ki67, E-cadherin, and p53 as prognostic indicators of long-term outcome after liver transplantation for metastatic neuroendocrine tumors. Transplantation. 2002;73:386-394.
Ruers TJ, et al. Comparison between local ablative therapy and chemotherapy for non-resectable colorectal liver metastases: a prospective study. Ann Surg Oncol. 2007;14:1161-1169.
Ruutiainen AT, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18:847-855.
Sarmiento JM, et al. Concurrent resections of pancreatic islet cell cancers with synchronous hepatic metastases: outcomes of an aggressive approach. Surgery. 2002;132:976-983.
Sarmiento JM, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29-37.
Sartori P, et al. Palliative management strategies of advanced gastrointestinal carcinoid neoplasms. Langenbecks Arch Surg. 2005;390:391-396.
Schindl M, et al. Treatment of small intestinal neuroendocrine tumors: is an extended multimodal approach justified? World J Surg. 2002;26:976-984.
Schnirer II, Yao JC, Ajani JA. Carcinoid: comprehensive review. Acta Oncol. 2003;42:672-692.
Schurr PG, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273-281.
Scigliano S, et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15 year monocentric study. Endocr Relat Cancer. 2009;16:977-990.
Seifert JK, Cozzi PJ, Morris DL. Cryotherapy for neuroendocrine liver metastases. Semin Surg Oncol. 1998;14:175-183.
Seifert JK, Junginger T, Morris DL. A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb. 1998;43:141-154.
Sheen AJ, Poston GJ, Sherlock DJ. Cryotherapeutic ablation of liver tumours. Br J Surg. 2002;89:1396-1401.
Shiina S, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023-1028.
Siperstein AE, Berber E. Cryoablation, percutaneous alcohol injection, and radiofrequency ablation for treatment of neuroendocrine liver metastases. World J Surg. 2001;25:693-696.
Siperstein AE, et al. Laparoscopic thermal ablation of hepatic neuroendocrine tumor metastases. Surgery. 1997;122:1147-1154.
Skinazi F, et al. Liver metastases of digestive endocrine tumours: natural history and response to medical treatment. Eur J Gastroenterol Hepatol. 1996;8:673-678.
Skov BG, et al. Reclassification of neuroendocrine tumors improves the separation of carcinoids and the prediction of survival. J Thorac Oncol. 2008;3:1410-1415.
Slooter GD, et al. Somatostatin receptor imaging, therapy and new strategies in patients with neuroendocrine tumours. Br J Surg. 2001;88:31-40.
Solbiati L, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166.
Solbiati L, et al. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound. 2001;13:149-158.
Solcia E, Kloppel G, Sobin L. Histological Typing of Neuroendocrine Tumors. World Health Organization Classification of Tumors. New York: Springer; 2000. pp 38-74
Solorzano CC, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078-1085.
Soreide O, et al. Surgical treatment is a principle in patients with advanced abdominal carcinoid tumors. Surgery. 1992;111:48-54.
Steinmuller T, et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87:47-62.
Strosberg JR, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2010;117:268-275. Epub Sep 7 2010
Thompson GB, et al. Carcinoid tumors of gastrointestinal tract: presentation, management and prognosis. Surgery. 1985;98:1054.
Tomassetti P, et al. Diagnostic value of plasma chromogranin in neuroendocrine tumours. Eur J Gastroenterol Hepatol. 2001;13:55-58.
Trendle MC, Moertel CG, Kvols LK. Incidence and morbidity of cholelithiasis in patients receiving chronic octreotide for metastatic carcinoid and malignant islet cell tumors. Cancer. 1997;79:830-834.
van Essen M, et al. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev. Endocrinol. 2009;5:382-393.
van Vilsteren FG, et al. Liver transplantation for gastroenteropancreatic neuroendocrine cancers: defining selection criteria to improve survival. Liver Transpl. 2006;12:448-456.
Wong SL, et al. Radiofrequency ablation for unresectable hepatic tumors. Am J Surg. 2001;182:552-557.
Wood TF, et al. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593-600.
Woodside KJ, Townsend CMJr, Evers RM. Current management of gastrointestinal carcinoid tumors. J Gastrointest Surg. 2004;8:742-756.
Yao JC, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316-1323.
Yao KA, et al. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery. 2001;130:677-685.
Zhou XD, Tang ZY. Cryotherapy for primary liver cancer. Semin Surg Oncol. 1998;14:171-174.