Chapter 81A Metastatic malignant liver tumors
Colorectal cancer
Overview
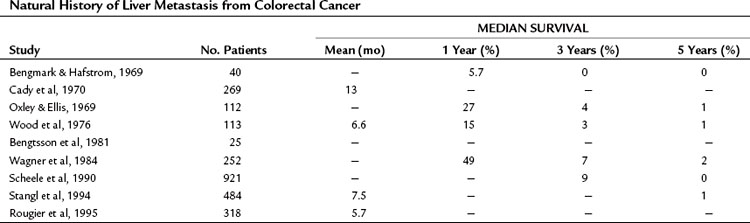
The liver is the most common site for hematogenous metastasis from colorectal cancers (CRCs). One quarter of patients with primary colorectal carcinoma present with synchronous hepatic metastasis, and nearly half of the patients resected of their colorectal primary will eventually develop metachronous liver metastasis (Bozzetti et al, 1987; Ekberg et al, 1987). In patients with isolated hepatic metastases, the extent of liver disease is the principal determinant of survival, and when left untreated, survival is measured in months (Norstein & Silen, 1997). Despite recent improvements in regional therapies, systemic chemotherapies, and biologic agents, survival is rarely over 3 years in the absence of surgical intervention (Table 81A.1).
Over the past 3 decades, surgery has been clearly demonstrated to be a safe and potentially curative modality in the treatment of colorectal metastases to the liver. The current 5-year survival following a margin-negative hepatic resection is 40%, with a 10-year survival approaching 20%. Although no prospective randomized trials comparing liver resection to systemic, regional, or other local therapies have been performed, the outcome for patients after liver resection for metastatic CRC is sufficiently favorable that surgery is now considered the primary therapy in selected patients (Pawlik & Choti, 2007; Ito et al, 2010).
Natural History of Metastases From Colorectal Cancer
Invasive CRC principally spreads through two mechanisms: cancer cells can metastasize to regional lymph nodes and then through central lymphatics into the systemic circulation, or they can spread directly to the liver via portal venous drainage (Knosel et al, 2004). This has been tested experimentally for CRC. In one study, investigators measured circulating transcripts of guanylyl cyclase C as a surrogate marker for circulating tumor cells and detected these two mechanisms at work in patients. During surgery for stage I or stage II primary CRC, circulating cancer cells were more frequently detectable in the portal blood as opposed to in the periphery, suggesting that the portal route may be more important in early stage patients; however, in some patients with documented lymph node spread, circulating cells were evident in peripheral blood samples but not in the portal blood (Tien et al, 2002).
When cancer spreads through the portal system, the liver acts effectively as a filter and minimizes or even prevents spread to distant sites (Picardo et al, 1998; Wang et al, 2004). At a cellular level, this process has been imaged through the labeling of cancer cells with green fluorescent protein, transplanting them into the ascending colon in mice. In this model, metastatic foci in the liver emerge within days after transplantation (Wang et al, 2004). The importance of portal spread is further validated by clinical data. Liver metastases were not observed in patients with cirrhosis (0 of 40 patients) but were observed in patients without cirrhosis (46 of 210, P < .001) in a Japanese study. The authors speculated that decreased portal flow to liver parenchyma in cirrhotic patients accounted for this phenomenon (Uetsuji et al, 1992).
It is unclear why some cancers spread via lymphatics and others spread via the portal circulation, although some theories based on experimental data have been suggested. One study found that in colon primaries, tumors located on the mestenteric side of the colon had a higher rate of lymph node spread than those on the antimesenteric side (207 cases, 51% vs. 30%; P = .004), probably related to the distribution and density of lymphatic vessels in the mesocolon (Boni et al, 2007). A separate group analyzed chromosomal copy number in primary and metastatic cancer foci using comparative genomic hybridization and found that certain reproducible allelic losses or gains were observed in hematogenous deposits, as compared with lymph node metastases.
The outcome of untreated metastatic CRC to the liver has been well documented in the older metastatic CRC literature and has been summarized in detail elsewhere (Norstein & Silen, 1997). The median survival of untreated CRC with synchronous liver metastases is just 5 to 10 months, and 3-year survival is very unusual. Survival at 5 years is essentially limited to patients who are seen initially with solitary liver metastases.
The extent of liver disease is an important determinant of prognosis. In a highly cited retrospective study by Wood and colleagues, 113 patients from the Glasgow Royal Infirmary with stage IV CRC were followed. The 1-year survival rate was 5.7% in the setting of widespread liver disease, 27% for those with metastases localized to a single segment or lobe of the liver, and 60% for patients with a solitary metastasis (Norstein & Silen, 1997). The patients with solitary metastases had a 3-year survival of 13% and a median survival of 17 months. A similar study by Wagner and colleagues (1984) found that 20% of patients who had an unresected solitary liver lesion lived 3 years.
The authors of these two retrospective natural history studies attempted to distinguish potentially resectable disease from unresectable disease and determined that the survival was substantially better in the former. In the study by Wood and colleagues (1976), 13 patients were believed to have had resectable disease; the 1-, 3-, and 5-year survival of these untreated patients were 77%, 23%, and 8%, respectively, compared with 15%, 0%, and 0% for the unresectable group. Similarly, Wagner and colleagues (1984) reported 3- and 5-year survivals for untreated resectable disease of 14% and 2%, respectively, compared with 4% and 0% for patients with unresectable disease. Notably, even patients with resectable, solitary liver metastases consistently have very poor long-term survival (<10%).
Results of Resection for Colorectal Liver Metastases
Long-Term Results
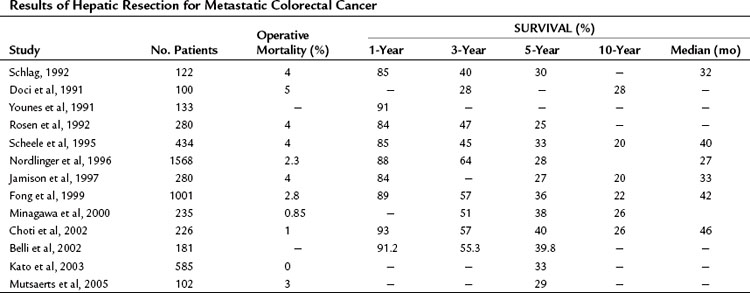
Since the studies of Wood and Wagner and colleagues, numerous large, single institutional and multicenter reports from around the world have demonstrated that liver resection is safe and results in long-term survival (Table 81A.2). In recent series, 5-year survival rates approach 40%, and median survival exceeds 40 months (Fong et al, 1999; Choti et al, 2002); this represents a dramatic improvement from the seminal study by Foster (1978) on liver resection, when the reported 5-year survival was 20%.
With newer multimodal treatments and careful patient selection, it is anticipated that 5-year survival approaching 70% can be achieved after resection (Nikfarjam et al, 2009), and comparable outcomes are likely to be reported in larger studies in the near future. Many patients with CRC at stage IV are cured by surgery. A recent review of actual 10-year survivors indicated that one third of actual 5-year survivors will eventually succumb to a cancer-related death; however, 10-year survival is tantamount to a cure in all but the rarest cases (<1%) (Tomlinson et al, 2007).
Outcome and Surgical Volume
A number of studies have correlated perioperative outcome to hospital volume for hepatectomy. Choti and colleagues (1998) and Glasgow and colleagues (1999) used the state registries in Maryland and California, respectively, and found that hepatectomy performed at a high-volume center resulted in improved outcomes. Surgery at high-volume centers was also associated with reductions in perioperative mortality, length of hospital stay, and cost. A recent study by Fong and colleagues (2005) based on a national Medicare database provides further support for superior outcomes at high-volume centers. The authors demonstrated that perioperative outcome correlated with the expertise of the institution, and the survival advantage persisted after the perioperative period. These data lend support to the concept of regionalization of hepatectomy to high-volume centers.
Medical Treatment of Metastatic Colorectal Disease to the Liver
Over the past 3 decades, the most widely used chemotherapeutic agent in the treatment of metastatic CRC has been 5-fluorouracil (5-FU), used either alone or in combination with other chemotherapies. Randomized clinical trials and meta-analyses in advanced CRC during the 1980s and 1990s established that 5-FU was best administered in combination with leucovorin (LV) and that 5-FU should be given as a continuous infusion over several days, as opposed to a series of daily bolus injections (ACCMAP, 1992; D’Angelica et al, 2002; de Gramont et al, 1997; MAGIC, 1998). Tumor response rates with the optimized 5-FU/LV dosing regimen reached 30%, with a median survival around 12 months. This response rate was a substantial improvement over what had been previously reported for 5-FU alone (10% to 15%), although the overall survival rates were similar (ACCMAP, 1992; MAGIC, 1998).
In 2000, two studies compared systemic therapy using irinotecan (CPT-11) in combination with 5-FU/LV with 5-FU/LV alone. Patients had stage IV CRC and had not received treatment for their metastatic disease (Douillard et al, 2000; Saltz et al, 2000). In the study by Saltz and colleagues, patients who recived the irinotecan-containing regimen—referred to as the IFL regimen, because it used bolus 5-FU—had a longer progression-free survival (PFS; 7.0 vs. 4.3 months), a higher response rate (39% vs. 21%), and a longer overall survival (median, 14.8 months vs. 12.6). Similar results were observed by Douillard and colleagues; in their study, irinotecan was combined with infusional 5-FU/LV (FOLFIRI). Based on these studies, combination therapy using irinotecan, 5-FU, and LV became the standard treatment for unresectable stage IV CRC. Subsequently oxaliplatin plus 5-FU/LV (FOLFOX) was accepted as second effective first-line combination regimen (de Gramont et al, 2000; Goldberg et al, 2004). In the study by de Gramont and colleagues (2000), PFS was 9.0 months versus 6.2 months with 5-FU/LV alone; the response rate was 50.7%, versus 22.3%; overall survival was 16.2 months versus 14.7 months (P = .1). In the study by Goldberg and colleagues (2004), outcomes were consistently superior with FOLFOX, including an overall survival of 19.5 months versus 15.0 months (P = .0001). This latter trial further demonstrated that FOLFOX was superior to a combination regimen consisting of irinotecan and oxaliplatin.
Two randomized controlled trials have compared FOLFOX and FOLFIRI against each other. Both trials concluded that the regimens had similar efficacy for metastatic CRC as a first-line treatment (Tournigand et al, 2004; Colucci et al, 2005). In the study by Tournigand and colleagues, patients were randomized to one of the two regimens and were crossed over to the opposite treatment arm upon progression. Overall survival was 21.5 months and 20.6 months in the FOLFIRI-first and FOLFOX-first treatment arms, respectively (P = .99). Roughly 15% of the patients in the trial became resectable during treatment and underwent metastasectomy. This was a more frequent occurrence in the FOLFIRI-first arm. In the second trial, no crossover was built into the study design, although a high proportion of patients received second-line treatment. The overall survival in the FOLFIRI and FOLFOX arms were 14 and 15 months, respectively (P = .28; Colucci et al, 2005). Secondary surgery was performed in about 5% in each group. Based on these studies, FOLFOX and FOLFIRI are widely considered to be equivelent in the metastatic setting and are generally selected according to the toxicity profile. The FOLFOX regimen is characterized by a higher rate of grade 3 and 4 neurotoxicity (30% vs. 5%) and neutropenia (44% vs. 24%). The FOLFIRI is associated with more nasuea and vomiting (20% vs. 5%), mucositis (10% vs. 0%), and alopecia (50% vs. 20%).
Falcone and colleagues (2007) explored an aggressive regimen with all three chemotherapeutic agents (FOLFOXIRI) and compared this treatment with FOLFIRI, and an increased rate of neutropenia and neurotoxicity was observed with FOLFOXIRI treatment; however, the more aggressive regimen was associated with better PFS (9.8 vs. 6.9 months), response rate (66% vs. 41%), and overall survival (22.6 vs. 16.7 months). In the FOLFOXIRI group, 15% of patients had secondary metastasectomy compared with 6% in the FOLFIRI group (P = .03). The difference was even greater when patients with liver-only disease were considered (36% vs. 12%, P = .02) (Falcone et al, 2007). In the above trials that evaluated the standard first-line combination regimens, the complete response rate was consistently around 5%.
Several small-molecule and antibody therapies have been developed that target growth factors or cell surface receptors important to CRC biology. Bevacizumab (Avastin), for instance, is a monoclonal antibody against vascular endothelial growth factor (VEGF) and has been approved in the first-line treatment of metastatic CRC in combination with 5-FU–based chemotherapy. FDA approval was a response to a study that compared bevacizumab and FOLFIRI to FOLFIRI alone (Hurwitz et al, 2004). The bevacizumab-containing regimen was associated with longer overall survival (median 20.3 vs. 15.6 months, P < .001) and a higher response rate (44.8 vs. 34.8%; P = .004). Bevacizumab was also evaluated in the context of oxaliplatin-based chemotherapy using either FOLFOX or XELOX (capecitabine and oxaliplatin; Saltz et al, 2008). Although the PFS was superior in the bevacizumab group (9.4 vs. 8.0 months; P = .0023), no improvement in overall survival was found (21.3 vs. 19.9 months; P .077). The response rates were the same in both arms (47% vs. 49%; P = .3). The pattern of increased PFS with novel biologic agents despite equivalent response rates is a common finding in trials with targeted agents. This phenomenon suggests that targeted therapies may improve PFS through stabilization of disease or perhaps by causing tumor necrosis, which is a confounding factor with conventional Response Evaluation Criteria in Solid Tumors (RECIST) (Tuma, 2006).
Cetuximab (Erbitux) and panitumumab (Vectibix) are both monoclonal antibodies against the epidermal growth factor receptor (EGFR) and are now an important part of the treatment algorithm for unresectable colorectal metastases (Cunningham et al, 2004; Jonker et al, 2007; Van Cutsem et al, 2007). Cetuximab is a chimeric monoclonal antibody approved for treatment of metastatic CRC in combination with irinotecan in patients with disease refractory to irinotecan (Cunningham et al, 2004) or as a single agent in patients who cannot tolerate irinotecan or oxaliplatin (Jonker et al, 2007).
Cunningham and colleagues (2004) established the first indication in their study, which tested the addition of cetuximab to irinotecan-based treatment in patients who were progressing and compared this strategy with cetuximab therapy alone. The response rates in the two groups were 22.9% and 10.8% (P = .007), and the times to progression were 4.1 and 1.5 months (P < .001), respectively. Overall survival was not significantly different between the two groups (8.6 vs. 6.9 months; P = .5; Cunningham et al, 2004). Jonker and colleagues demonstrated that cetuximab montherapy was superior to best supportive care in patients who were unable to take irinotecan or oxaliplatin-based therapy (Jonker et al, 2007). Overall survival was 6.1 months in the cetuximab group compared with 4.6 months in the control group (P < .001). The response rate was 8% with cetuximab, and disease stabilization occurred in 31.4% versus 10.9% in the control group (P < .05).
Panitumumab is a fully humanized monoclonal antibody and therefore appears to have a lower rate of serious infusion reactions compared with cetuximab (Van Cutsem et al, 2007). Like cetuximab, panitumumab is approved for single-agent therapy in patients who have progressed on standard chemotherapy (Van Cutsem et al, 2007). Patients on panitumumab had an 8-week PFS compared with 7.3 weeks with best supportive care (P < .0001), and it was associated with a 10% response rate; however, the overall survival was 7 months in both groups (P = .99). Panitumumab was also evaluated as part of a first-line regimen for metastatic CRC, but a benefit was not apparent in the absence of KRAS mutation profiling (Hecht et al, 2009). Hecht and colleagues (2009) compared standard FOLFOX or FOLFIRI plus bevacizumab with or without panitumumab. Interestingly, patients receiving panitumumab had significantly inferior progression-free and overall survival compared with the control arms.
Although both cetuximab and panitumumab are antibodies directed against EGFR, there is no evidence that EGFR expression influences response rate; however, there appears to be a strong treatment interaction with both medications, and KRAS is mutated in 40% of CRCs (Bos et al, 1987). A prospective trial of first-line treatment comparing FOLFIRI with and without cetuximab demonstrated prolonged PFS in patients with a wild-type KRAS in their tumors (Van Cutsem et al, 2009). Similarly, a recent trial evaluating first-line treatment using FOLFOX with and without panitumumab showed prolonged PFS in the setting of wild-type KRAS. Those patients with a KRAS mutation actually had significantly worse PFS (Douillard et al, 2010). Peeters and colleagues (2010) evaluated FOLFIRI plus panitumumab versus FOLFIRI alone in chemorefractory patients, demonstrating improved PFS with second-line chemotherapy in patients having wild-type KRAS tumors. Additionally, when administered as monotherapy as salvage treatment for metastatic CRC, EGFR inhibitors again were only found to be effective in patients with tumors with wild-type KRAS. In the two trials described earlier that compared EGFR inhibitor monotherapy with best supportive care (Jonker et al, 2007; Van Cutsem et al, 2007), cetuximab and panitumumab were associated with improvements in tumor response, PFS, and overall survival (Amado et al, 2008; Karapetis et al, 2008). Based on these studies, EGFR inhibitors are now used just in those patients with tumors that do not have a mutated KRAS gene. This paradigm, in which the genotype of a tumor dictates therapy, is likely to become even more prevalent in the future.
In summary, patients who have yet to receive treatment for metastatic CRC, even if adjuvant treatment was previously administered for the colorectal primary, have a 50% or greater chance at responding to modern systemic chemotherapy and can expect a median survival around 18 to 20 months in the absence of resection. Unresected patients, however, still seldom live more than 3 years after the diagnosis of metastatic disease. After a patient fails first-line therapy, the results are discouraging. Response rates of 20% or less are typically seen, regardless of the second-line treatment selected (Bensmaine et al, 2001; Comella et al, 2002; Cunningham et al, 1998, 2004; Falcone et al, 2007).
Prognostic Variables and Staging Systems
Characteristics of the Patient and Primary Tumor
Age has not been found to be a significant prognostic variable for long-term survival, although analyses examining the role of age are confounded by the fact that older patients are carefully selected for surgery (Cady et al, 1992; Ballantyne & Quin, 1993). The location of primary cancer has not been shown to affect outcome, as metastatic rectal and colon cancer have similar prognoses (Doci et al, 1991). On the other hand, the stage of the primary tumor is quite useful in staging metastatic disease. Patients who had positive lymph nodes associated with their primary CRC do worse, as do patients who present with synchronous liver disease. Histologic grade of the primary cancer is not a significant predictor of long-term survival in patients with metastatic disease (Doci et al, 1991; Ballantyne & Quin, 1993).
Clinical Characteristics of Liver Metastases
The most important clinical predictor of outcome related to the liver metastases themselves is the disease-free interval: the shorter the interval, the worse the prognosis (Rosen et al, 1992; Scheele et al, 1995). Other negative factors include multiple liver metastases—four lesions is a commonly used cutoff—bilateral disease, tumor size greater than 5 cm, and a markedly elevated preoperative carcinoembryonic antigen (CEA >200 ng/mL; Nordlinger et al, 1996).
The prognostic value of response to neoadjuvant chemotherapy is controversial, and opinions vary in the literature. In a study by Adam and colleagues (2004), the 5-year disease-free survival was so poor following progression during neoadjuvant chemotherapy—3% versus 20% in patients who had a response or stable disease—the authors proposed that such a finding should preclude liver resection. This phenomenon was confirmed in our own experience (Allen et al, 2003). Although other groups have not found this to be true, such studies were based on patients who received older chemotherapy regimens. Roughly half of the patients only received 5-FU, which might account for the high rate of progression (Gallagher et al, 2009; Neumann et al, 2009). With modern chemotherapy, the expected rates of progression would be less than 20% compared with 60% in the studies mentioned above that used 5-FU (Nordlinger et al, 2008).
Operative and Pathologic Characteristics of Liver Metastases
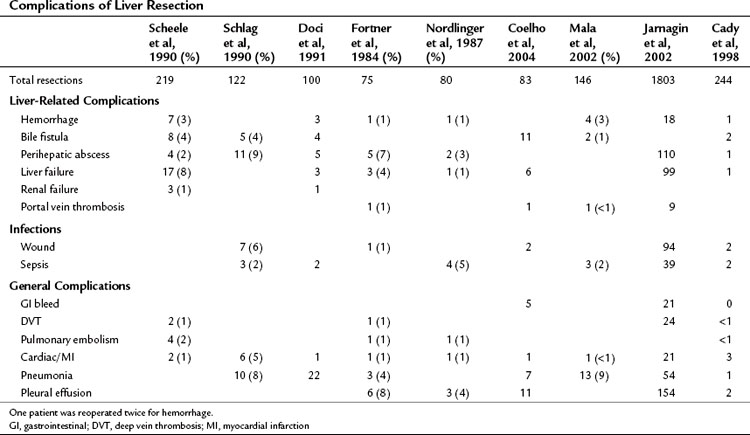
Higher operative blood loss and transfusion requirements have been found to be associated with an increased number of perioperative complications and mortality but not with a worse long-term survival (Table 81A.3; Kooby et al, 2003). Anatomic resections are believed to indirectly improve long-term outcomes, because this approach is associated with a significantly lower rate of positive margins. In a retrospective analysis from our institution, patients who had an anatomic segmental resection had a better outcome than patients subjected to wedge resections (DeMatteo et al, 2000). In an anatomic resection, planes are developed along major hepatic veins, and inadvertent disruption of the tumor is avoided. Wedge resections are usually guided by palpation and can be complicated by inadequate exposure or retraction. The attendant problems of poor visibility and impaired tactile sense at the depth of the resection will often lead to tearing of the specimen along the hard tumor–soft liver interface, thus resulting in a positive margin.
Perhaps the most informative surgical prognostic factor is the finding of extrahepatic disease (Rosen et al, 1992; Fong et al, 1999; Abdalla et al, 2006). The existence of nonpulmonary extrahepatic disease has long been considered by many surgeons to be a contraindication to hepatectomy (Fong et al, 1999; Abdalla et al, 2006); however, the approach to extrahepatic disease has been reevaluated in recent years, as some studies have demonstrated acceptable survival in selected patients with limited extrahepatic disease managed with resection, particularly in the setting of low-volume pulmonary metastases. The two largest studies on this question evaluated combination resection of liver and lung metastases and noted 5-year survival rates of 30% (Headrick et al, 2001; Miller et al, 2007).
The presence of metastatic disease to the portal lymph nodes remains a particularly poor prognostic factor. Portal metastases are found in 3% to 30% of patients with metastatic CRC to the liver (Abdalla et al, 2006), and disease recurs in virtually all patients with resected portal lymph nodes (Carpizo et al, 2009). A consensus paper on the management of extrahepatic disease concluded that no convincing data suggest that therapeutic portal lymphadenectomy for metastatic CRC improves survival (Abdalla et al, 2006). In contrast to portal lymph node metastases, there appears to be a role for hepatic resection in conjunction with metastases to other sites. The question has been examined in the form of large retrospective studies that included resections of the peritoneum or mesentery, retroperitoneal lymph nodes, and a variety of solid organs (Minagawa et al, 2000; Elias et al, 2003; Carpizo et al, 2009). The 5-year survival rates reported in these series range from 20% to 28%. Based on these studies, hepatectomy combined with surgical resection of extrahepatic disease is believed to be appropriate in selected patients.
A macroscopic positive margin, or R2 resection, is widely accepted as a poor prognostic factor. The presence of microscopic disease at the resection margin (R1 resection) or the precise thickness of the negative margin (R0 resection) is perhaps more controversial. For instance, the preponderance of data on the impact of an R1 resection suggests that a microscopically positive margin is associated with worse survival. The 5-year survivals are consistently above 30% for negative margins but are 20% or less with microscopic disease at the margin (Fong et al, 1999; Pawlik et al, 2005; Nuzzo et al, 2008); however, multivariate analyses that include R1 resection status often do not find it to be a significant predictor of outcome after adjusting for other competing risk factors, which suggests that a positive microscopic margin is merely a marker of poor biology (Pawlik et al, 2005; Nuzzo et al, 2008). As adjuvant therapy improves, patients with R1 resections will likely have outcomes that more closely approach patients with negative resection margins. Indeed, a recent report suggests that a microscopic positive resection margin obtained out of necessity does not negatively impact the 5-year survival, although the recurrence rate is increased (de Haas et al, 2008).
With regard to the extent of negative margin, a 1-cm rim of normal liver parenchyma surrounding a resected tumor was traditionally espoused in the older literature (Shirabe et al, 1997); however, recent studies suggest that subcentimeter margins are acceptable (Are et al, 2007) and are perhaps even associated with equivalent outcomes to R0 resections (Scheele et al, 1995; Pawlik et al, 2005; Hamady et al, 2006; Figueras et al, 2007). Low rates of micrometastastic disease, or satellitosis, were found in resection specimens in two Japanese studies, further supporting this idea (Yamamoto et al, 1995; Kokudo et al, 2002). Anatomic segmental resection, as opposed to wedge resections (DeMatteo et al, 2000), and neoadjuvant chemotherapy are two proposed strategies to improve R0 resection rates in patients with colorectal liver metastases (Parikh et al, 2003).
In summary, the prospect of a positive microscopic or close microscopic margin is not a contraindication to liver metastasectomy in appropriately selected patients with modern chemotherapy. In addition to a macroscopically positive resection margin, the findings of hepatic satellite lesions and intrabiliary invasion have also been shown to predict tumor recurrence (Gayowski et al, 1994; Scheele et al, 1995; Okano et al, 1999; Povoski et al, 2000; Kubo et al, 2002).
Predictive Models and Clinical Risk Scores
Four large studies have enabled robust multivariate analyses of prognostic factors with metastatic CRC and design of useful predictive models for favorable survival after metastasectomy (Nordlinger et al, 1996; Fong et al, 1999; Kattan et al, 2008; Rees et al, 2008). Nordlinger and colleagues (1996) reported on a multicenter series of more than 1500 patients. Fong and colleagues (1999) reported on a single institutional series of 1001 patients and later on a cohort of 1477 patients (Kattan et al, 2008), and Rees and colleagues (2008) evaluated long-term survival in 929 patients from a tertiary referral center in the United Kingdom.
In the series by Fong and colleagues (1999), seven parameters were found to be independent predictors of an unfavorable prognosis: 1) the presence of extrahepatic disease, 2) a positive resection margin, 3) nodal metastases associated with the primary CRC, 4) a short disease-free interval, 5) largest liver metastasis greater than 5 cm, 6) more than one liver metastasis, and 7) a serum CEA greater than 200 ng/mL. The presence of extrahepatic disease and a postive resection margin are generally determined intraoperatively. When presumed preoperatively, these two variables are often considered relative contraindications to metastasectomy. A preoperative clinical risk score (CRS) system was therefore created using the last five factors (Box 81A.1), with each positive criterion counting as one point. This CRS is a simple and facile staging system used to classify patients with liver-exclusive metastatic CRC (Fig. 81A.1).
The presence of any one of these characteristics was still associated with a 5-year survival of 24% to 41%; therefore no single criteria can be considered a contraindication to resection; rather, the total score out of 5 is highly predictive of outcome, with a score of 2 or less suggestive of a particularly favorable prognosis—the optimal candidate for liver metastasectomy. Patients with a CRS of 3 or 4 have less favorable outcomes and may be appropriate for clinical trials involving adjuvant chemotherapy. Long-term disease-free survival is rare in patients with a CRS of 5, and these patients are also typically best served in clinical adjuvant chemotherapy trials. Importantly, actual 10-year survival has been observed in patients with even the highest CRS, as patients with a CRS of 3 to 5 have a 10% disease-specific survival (DSS). This is roughly equal to the cure rate of treatment in these patients; therefore a high CRS should not be considered a contraindication to hepatic resection (Tomlinson et al, 2007).
Current Use of Clinical Risk Score
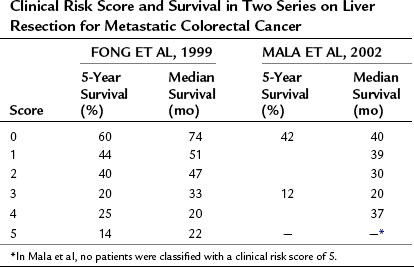
The above prognostic scoring system has been validated by an independent group from Norway (Mala et al, 2002), which demonstrated that the CRS is generalizable to populations outside of the index cohort from a single, large, tertiary American center. In addition to appropriate patient selection for surgery, the CRS has proven useful in selecting patients for neoadjuvant treatment, ablation, and stratification in clinical trials (Table 81A.4).
Table 81A.4 Clinical Risk Score and Survival in Two Series on Liver Resection for Metastatic Colorectal Cancer
Additionally, the CRS has been shown to be useful in guiding the preoperative evaluation of a patient with metastatic CRC. As the number and cost of available tests increases, there is a growing need to develop effective ways to stratify patients so as to determine which tests are necessary for a given individual. For instance, a CRS of 1 or more was associated with a 14% rate of occult metastatic disease detectable by fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging but not by conventional imaging (Schussler-Fiorenza et al, 2004). PET scanning did not have any utility in patients with a CRS of 0. Similarly, laparoscopy can be helpful for staging patients with a high CRS (>2). The finding of extrahepatic disease at laparoscopy in more than 40% of patients with elevated risk scores can prevent the added morbidity associated with an unnecessary laparotomy; however, the yield of laparoscopy is sufficiently low in the setting of a low CRS (~10% incidence of occult unresectable disease) that the added procedure is unnecessary (Jarnagin et al, 2001).
Predictive models with added sophistication have been developed recently, such as a nomogram (Kattan et al, 2008) and a multifactorial predictive index (Rees et al, 2008). Although these models are based on the same basic prognostic factors as the earlier models described above, they are more complex and difficult to use; however, as computer software and accessible handheld technology progresses, improved and more accurate predictive models will likely achieve clinical relevance in the near future.
Molecular Determinants of Outcome
Molecular profiling of CRC is an evolving area and will likely become an essential component of prediction models for cancer recurrence in the future. As stated earlier, variable response to EGFR inhibitors based on tumor KRAS status is well documented (Bos et al, 1987; Amado et al, 2008; Karapetis et al, 2008; Van Cutsem et al, 2009; Peeters et al, 2010). Similarly, response rate and the presence of microsatellite instability in the tumor are associated (Ribic et al, 2003; Nash et al, 2010). In a manner akin to the HER2 amplification and breast cancer, it is easy to imagine that these and other molecular factors will be incorporated into postoperative prognostic scales in the future.
Preoperative Investigations
Preoperative investigations prior to resection of metastatic colon cancer are directed at 1) establishing the diagnosis, 2) anatomic definition of the liver lesion for surgical planning, and 3) staging to rule out extrahepatic disease. A confirmatory biopsy of hepatic lesions is only indicated to confirm the diagnosis when the clinical picture is unclear. The differentiation between metastatic tumors and benign hepatic lesions can usually be done with imaging modalities, including ultrasound (US), magnetic resonance imaging (MRI), and PET scan as discussed below (see Chapters 13, 15, and 17). The risk of tract seeding from percutaneous fine needle aspiration appears to be quite small, with only a few case reports in the literature. The workup to determine the extent of disease includes imaging, as outlined below, and recent colonoscopy (within 6 months). Although a detailed discussion of each imaging modality is beyond the scope of this chapter, the following sections will focus on the practical aspects of imaging for preoperative workup of patients with hepatic colorectal metastases.
The Role of Preoperative Imaging
Computed Tomographic Scans
Computed tomographic (CT) scans have become indispensable in the staging of patients with metastatic CRC (see Chapter 16). A contrast-enhanced CT scan of the chest, abdomen, and pelvis are routinely obtained, although the yield from scanning the chest with regard to detecting metastatic disease is limited to just a few percent (Kronawitter et al, 1999). The most important images in the evaluation of the liver in the setting of metastatic CRC are obtained in the portal venous phase, because the lesions are not typically well vascularized. Arterial phase images are principally used to distinguish metastatic disease from benign vascular lesions, such as hemangiomas, or to better define the arterial anatomy of the liver. Such a step is necessary when planning for the placement of hepatic arterial infusion pumps. Standard CT scanning involves a triple-phase protocol through the liver that includes arterial, portal venous, and delayed phases. Slices are usually 5 to 7 mm thick through the liver, but these are reduced to 1.5-mm thick overlapping slices when high-resolution hepatic angiography is desired. Coronal and sagittal reconstructions are performed to further delineate the anatomy.
Metastatic lesions from colorectal primaries tend to push structures away, rather than invade directly, respecting the liver capsule and intersegmental planes (Baer et al, 1989; Scheele, 1989). Even when large lesions appear to involve the inferior vena cava on preoperative imaging, they often do not on surgical exploration; therefore such a finding on CT should not preclude an operation. Diaphragmatic invasion is a rare event as well (Weinbren & Blumgart, 1986).
Magnetic Resonance Imaging
MRI is most useful to evaluate indeterminate hepatic lesions and to better define the relationship of tumors to the hepatic vasculature and biliary tree using magnetic resonance cholangiopancreatography (MRCP; see Chapter 17). In particular, MRI is excellent at distinguishing between benign lesions such as hemangiomas, adenomas, and fibronodular hyperplasia. Moreover, MRI is better than CT for investigating pathology in the background of a fatty liver, such as in patients who have a history of obesity, diabetes, alcoholism, or previous chemotherapy treatment (Bipat et al, 2005; Fernandez et al, 2005). Because MRI is much more expensive than CT, this modality is not used routinely; however, it is warranted to better characterize indeterminate lesions or for the patient with an elevated CEA and a fatty liver with no evidence of disease on CT.
Ultrasonography
Ultrasonography is a relatively inexpensive test that can provide detailed information about the number, extent, and anatomic relationships of liver metastases (see Chapter 13). Duplex US can define the proximity of the tumor to the portal vessels, hepatic veins, and inferior vena cava, and US is particularly useful for characterizing cystic lesions within the liver. In expert hands, it can be very sensitive at detecting small metastatic lesions, although the test is highly operator dependent.
Hepatic Angiography and Computed Tomographic Portography
Direct hepatic angiography has largely been replaced by CT and MR angiography, which are less invasive alternatives (see Chapter 19). The main role for direct angiography in the modern era of high-resolution cross-sectional imaging is CT portography. In this technique, contrast is administered directly into the splanchnic bed by injection into the superior mesenteric artery, and a CT portal venogram of the liver is acquired after a suitable delay. This technique is highly sensitive at detecting small metastatic tumors (Sica et al, 2000); however, because the test requires arterial catheterization and is associated with a relatively high false-positive rate because of perfusion abnormalities, its primary role is in the setting of a planned portal vein embolization (PVE; see Chapter 93A, Chapter 93B ).
Positron Emission Tomography
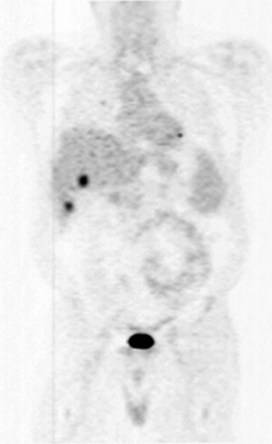
The role of whole-body positron emission tomography as an adjunct to CT in the evaluation of metastatic CRC has been growing in recent years (see Chapter 15). In the United States, the use of FDG PET for staging and diagnosis of CRC was approved by Medicare in 2001 (Kelloff et al, 2005). The imaging test utilizes an intravenously administered radioactive tracer, which in most cases is 18F-FDG. The radioactive glucose analogue cannot proceed down the glycolytic pathway and therefore accumulates within glucose-avid cancer cells. PET capitalizes on this phenomenon, as 18F-FDG reveals the distribution of metabolically active metastases overexpressing cell surface GLUT1 transporters (Fig. 81A.2). The limitations of PET include poor sensitivity for lesions less than 1 cm, that some larger lesions are not FDG avid, and that the test lacks a high degree of anatomic detail. Moreover, false positives occur in the setting of inflammation, which may exist early after surgery and radiotherapy, or in the context of an infectious process. Integration of FDG PET imaging and CT addresses some of these limitations, particularly with regard to anatomic details.
PET has emerged as a relevant test in several areas of metastatic CRC management. Reported applications include patient selection for metastasectomy, evaluation of a patient with a suspected recurrence, radiotherapy planning, response assessment, and the discovery of incidental colorectal lesions (Herbertson et al, 2007). With regard to the initial staging of patients with metastatic CRC, FDG PET imaging leads to a change in management in 2% to 36% of patients. For instance, Strasberg and colleagues (2001) examined the utility of PET in evaluating patients with hepatic colorectal metastases for possible hepatic resection. Out of 43 patients, the authors identified 6 with unresectable disease detected by PET imaging, which amounted to a 14% rate of occult disease detection by PET. Futile surgery was avoided in these patients. This improved detection rate is comparable to the experience described above by Schussler-Fiorenza and colleagues (2004) in the section on uses of the CRS. Interestingly, Strasberg and colleagues (2001) report quite favorable outcomes after metastasectomy, which they attribute to improved patient selection from the use of preoperative PET imaging: the resectability rate, 3-year disease-free survival, and 3-year overall survival were 95%, 40%, and 77% respectively. A meta-analysis on the subject found that the sensitivity and specificity of FDG PET were 91.5% and 95.4% for extrahepatic disease compared with 60.9% and 91.1% for CT (Wiering et al, 2005). The advantage of FDG PET over CT alone in the evaluation of a patient with a suspected recurrence, in the setting of a rising serum CEA, is similar to the experience described above for the patient undergoing a complete staging workup for stage IV disease (Huebner et al, 2000). FDG PET may also be particularly useful at identifying omental or peritoneal disease. Finally, the prospect of using functional imaging to assess treatment response is a promising application of PET technology. For instance, multiple studies have demonstrated that early metabolic response to chemotherapy correlates with eventual RECIST response, based on CT imaging, for both CRC and other cancer types (Findlay et al, 1996; Weber et al, 2003; Juweid & Cheson, 2006). One can imagine treatment adjustments based on FDG PET scans after just a few cycles of chemotherapy.
The Role of Laparoscopic Staging (See Chapter 21)
Laparoscopy has been shown to have a role in staging patients with metastatic CRC, particularly in patients with associated poor prognostic factors or an elevated CRS (Babineau et al, 1994; John et al, 1994; Jarnagin et al, 2000). The principal advantage of laparoscopy is that it allows the detection of occult peritoneal or periportal disease that would preclude resection, but it is not appreciated on preoperative imaging. In a study by Jarnagin and colleagues (2001), laparoscopic evaluation identified the majority of cases of unresectable metastatic disease (14 of 26), sparing a laparotomy in many instances. Moreover, the authors demonstrated that laparoscopic detection of extrahepatic or unresectable disease resulted in decreased surgical morbidity, a shorter hospital stay, decreased hospital costs, and shorter delay to systemic therapy.
Of course, a negative laparoscopy lengthens anesthetic time and increases operating costs; therefore the technique is typically reserved for patients deemed to be at high risk for having unresectable or extrahepatic disease. Such patients include those with findings on imaging that are suspicious, but not diagnostic for, extrahepatic disease and those with a CRS greater than 2 (Jarnagin et al, 2001).
Synchronous Metastases and Timing of Resection
The timing of hepatic resection in patients presenting with liver metastases during workup for the primary tumor is controversial. Certainly, studies have demonstrated that synchronous metastatic disease portends a worse prognosis compared with metachronous metastases (Scheele et al, 1995), which factors into the discussion of whether to resect the primary and metastatic disease in one operation. Several factors related to perioperative and long-term outcome should be considered when deciding between simultaneous and delayed liver resection for metastatic CRC to the liver. With regard to surgical morbidity, de Haas and colleagues (2010) demonstrated that fewer complications were observed in patients who underwent simultaneous colorectal and liver resection (11% vs. 24%), but perioperative mortality was the same. Patients in this study were included if their liver resection involved three segments or less. A similar or decreased complication rate associated with simultaneous resection has also been validated by other groups (Vogt et al, 1991; Martin et al, 2003; Chua et al, 2004; Capussotti et al, 2007).
In the above mentioned study by de Haas and colleagues (2010), the 3-year overall survivals were 74% and 70% in the two groups, respectively; however, the simultaneous resection group experienced more recurrences (85% vs. 63%; P = .002), resulting in a decreased 3-year PFS compared with the staged resection patients (8% vs. 26%, respectively). The authors concluded that although simultaneous resection is safe, there is an associated detriment in PFS. When interpreting such a study, it is important to recognize that the group of patients who underwent simultaneous resection may have included some patients who would have progressed to unresectabilty during the interval between removal of the primary and metastasectomy. This would bias the long-term results in favor of the delayed resection group. Indeed, Lambert and colleagues (2000) showed that roughly two thirds of patients can be spared unnecessary hepatic resection by using a delayed-resection approach to liver metastases. Taking the data together, it is reasonable to perform a simultaneous resection in patients with low-volume disease in the liver, when the risk of early and rapid progressive disease is relatively low. Simultaneous resection should be avoided in patients who appear frail and in certain clinical scenarios in which the liver disease is best addressed in a delayed fashion, such as with obstruction or bleeding.
Operative Management
The principles of hepatic resection are similar for colorectal metastases as for any other hepatic surgery (see Chapter 90B). The immediate preoperative preparation includes a single dose of prophylactic antibiotics and placement of sequential compression devices to prevent the development of deep vein thromboses. The choice of incision includes a subcostal, long midline, or short midline incision with a rightward extension approximately 3 cm above the umbilicus. The latter is most frequently used at our institution, as it provides excellent exposure while minimizing the length of the midline incision in the upper abdomen, potentially reducing postoperative pulmonary compromise. The maintenance of a low central venous pressure (<5 mm Hg) reduces operative blood loss by decreasing bleeding from the hepatic venous radicles during dissection (Cunningham et al, 1994; see Chapter 22). This is facilitated by performing the dissection with the patient in a 15-degree Trendelenburg position, which increases the venous return to the heart and improves cardiac output.
Intraoperative Staging and the Role of Intraoperative Ultrasound (See Chapters 21 and 95)
As with any laparotomy for cancer, the abdomen should be carefully explored for evidence of extrahepatic metastases. In particular the celiac axis and portocaval and hilar lymph nodes must be palpated, and any suspicious nodes should be removed and examined by frozen section. Most surgeons routinely use intraoperative US after mobilization of the liver. With experience this practice may detect 5% to 10% of lesions missed on noninvasive imaging with CT, MRI, and transabdominal US (Boldrini et al, 1987; Machi et al, 1987, 1991; Olsen, 1990; Stone et al, 1994). In addition, intraoperative US is useful to delineate the interior anatomy of the liver, such as the intrahepatic vessels; therefore hepatic resection can be performed more safely with an anatomic approach. Intraoperative US is also useful throughout parenchymal transection, so that the resection line can be viewed in relation to the lesion and blood vessels. Prior to the development of PET imaging, several reports demonstrated that surgical management of hepatic tumors was influenced by intraoperative US in 30% to 50% of operations (Castaing et al, 1986; Rifkin et al, 1987).
Recently, interest is mounting with regard to contrast-enhanced intraoperative liver US with contrast agents that consist of microbubbles of air or gas that create greater contrast between tumors and normal liver parenchyma. Typically, the contrast is delivered in the form of a 4-mL intravenous bolus of SonoVue, sulfur hexafluoride gas stabilized by a phospholipid shell (Bracco SpA, Milan, Italy), followed by a flush of normal saline. In a series of 60 patients from the United Kingdom with metastatic CRC, the acccuracy of preoperative MRI/CT, standard intraoperative US, and contrast-enhanced US were 74%, 79%, and 96% respectively. The median size of lesions identified on contrast-enhanced US, but not on preoperative imaging, was 0.8 cm. In 17 patients (29%), findings on intraoperative contrast-enhanced US changed the surgical plan (Leen et al, 2006). In a second study from the United Kingdom, contrast-enhanced US altered the management plan in 4 (19%) of 21 patients compared with standard intraoperative US (Shah et al, 2010).
Postoperative Management
Perioperative Mortality and Morbidity
Mortality
The mortality associated with an elective liver resection for colorectal metastases has decreased significantly over the past 3 decades, and in the last 10 years, it has been shown to be less than 10% across major series (Butler et al, 1986; Hughes et al, 1986; Pagana, 1986; Cobourn et al, 1987; Nordlinger et al, 1987; Schlag et al, 1990; Scheele et al, 1991; Younes et al, 1991; Rosen et al, 1992). Advances in the understanding of liver anatomy, resection techniques, and anesthetic care have translated into favorable survival rates even with major resections. At the highest volume centers, operative mortality rates are now 3% to 5% (see Table 81A.2). The majority of deaths occur from perioperative hemorrhage or liver failure. Further improvements in mortality rates will be challenging, despite ongoing advances in surgical technique, as surgeons have taken an increasingly aggressive approach to hepatic resections.
Morbidity
The complication rate associated with hepatectomy is attributable in large part to the metabolic and immunologic derangements associated with liver surgery. The reported complication rate in most series is over 20% (see Table 81A.3), but this is likely an underestimate and reflects the fact that these reports are retrospective. Nonspecific or nonliver morbidities include cardiac, pulmonary, and infectious complications. The incidence of cardiovascular complications is around 9%, but the overwhelming majority of these are arrhythmogenic in nature (Jarnagin et al, 2002). The low rate of severe cardiac complications reflects careful selection of patients for liver resection. Pulmonary complications occur in close to 20% of patients and are related to the large upper abdominal incision and postsurgical sympathetic pleural effusions. Specifically, symptomatic pleural effusions occur in 10% of cases, pneumonia in 3%, and pulmonary embolism in 1% (Jarnagin et al, 2002).
Liver insufficiency and liver failure remain the most dangerous liver-related complications and occur in 3% to 8% of major hepatic resections (Schlag et al, 1990; Doci et al, 1991; Scheele et al, 1991; Cunningham et al, 1994; Jarnagin et al, 2002). Other hepatobiliary complications include bile leak in 4% of patients and perihepatic abscess in 2% to 10% (Schlag et al, 1990; Scheele et al, 1991; Jarnagin et al, 2002). Significant hemorrhage is rare (1% to 3%) but can be an important cause of perioperative mortality.
It is worth emphasizing that a high complication rate does not always translate into a prolonged hospital stay. If recognized and treated promptly, most complications do not result in a poor outcome. For instance, in the experience by Jarnagin and colleagues (2002), with more than 1800 liver resections performed at a single center, the median hospital stay was 8 days, and intensive care unit (ICU) admission was required for only 112 patients (6%). On the other hand, an association between the development of complications and decreased long-term outcome, both disease-free and overall survival, has been observed for liver surgery (Ito et al, 2008; Farid et al, 2010); this is similar to the findings in a large, population-based analysis of patients undergoing surgical procedures (Khuri et al, 2005), a finding that again underscores the importance of safe liver surgery.
Follow-up After Resection
No consensus exists regarding the extent and frequency of follow-up after surgery for primary CRC, as no specific strategy has been shown to conclusively improve survival (Makela et al, 1995; Ohlsson et al, 1995; Kjeldsen et al, 1997; Schoemaker et al, 1998). Similarly, no conclusive data demonstrate improved survival with close follow-up after hepatic resection for CRC metastases (Metcalfe et al, 2004). Nevertheless, patients who have undergone hepatic resection are usually monitored closely with the goal to identify early and potentially curable resectable recurrences. Our paradigm is as follows: physical examination, serum CEA level, and CT of the abdomen and pelvis every 3 to 4 months for the first 2 years and then every 6 months thereafter, and patients undergo chest imaging annually. We continue surveillance for more than 5 years, because roughly a third of 5-year survivors will still recur (Tomlinson et al, 2007).
Role of Adjuvant Therapy
Patterns of Recurrence
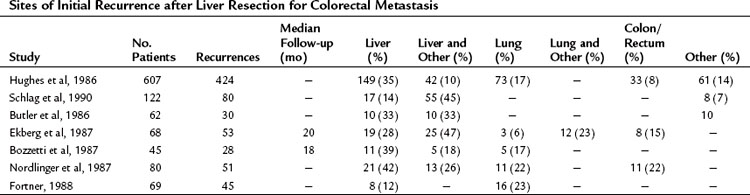
Although the cure rate for liver metastasectomy is now considered to be roughly 20% (Tomlinson et al, 2007), the majority of patients with metastatic CRC to the liver die from recurrent disease. It stands to reason that these patients harbored occult disease at surgery that ultimately progressed and led to their demise. The most common sites for failure include the liver and lung (Table 81A.5). Indeed, the recurrences occur within the liver in 60% of patients (de Jong et al, 2009) and exclusively in the liver in up to 40% (Nordlinger et al, 1987).
Adjuvant Systemic Chemotherapy
A number of nonrandomized studies have evaluated the utility of perioperative systemic chemotherapy in the setting of hepatic resection for CRC metastases with equivocal results. Most of these studies compared patients receiving 5-FU–based chemotherapy with or without irinotecan or oxaliplatin. A large retrospective cohort study from European and American centers compared 792 patients who underwent hepatic resection alone to hepatic resection with perioperative 5-FU chemotherapy. The basis for receiving chemotherapy was largely a function of where the patients were treated, thereby removing the bias toward chemotherapy in higher risk patients, which confounds other studies (Hewes et al, 2007). In this study, chemotherapy was associated with an improved 5-year overall survival (37% vs. 31%; Parks et al, 2007). Postoperative systemic 5-FU chemotherapy was evaluated in two prospective, randomized, controlled trials, the Fédération Francophone Cancerologie (FFCD) trial 9002 and the ENG trial, but both studies were closed early as a result of poor accrual. The pooled results included 278 patients randomized to surgery alone or surgery with 6 months of 5-FU. The authors demonstrated a marginal improvement in PFS (18.8 vs. 27.9 months; P = .058) without an improvement in overall survival at 3 or 5 years (Mitry et al, 2008).
More recently, Nordlinger and colleagues (2008) reported the results of the European Organization for the Research and Treatment of Cancer (EORTC) intergroup trial 4098. This prospective and randomized trial included 364 patients with resectable hepatic colorectal metastases who were treated by surgery alone or surgery with perioperative FOLFOX chemotherapy, six preoperative cycles and six postoperative cycles (Nordlinger et al, 2008). Based on an intention-to-treat analysis, an insignificant improvement was seen in PFS at 3 years of 7.3% (28.1% vs. 35.4%; P = .058); however, when only eligible patients were analyzed, the absolute difference in 3-year PFS was 8.1% and reached statistical significance (P = .041). The difference was 9.2% when just patients who underwent surgical resection were included (P = .025). This was technically a negative study, because the groups were statistically equivalent in the intent-to-treat analysis, but this is frequently quoted as a study demonstrating that perioperative chemotherapy improves PFS in patients undergoing hepatic resection for CRC metastases. Furthermore, one cannot comment on the role of neoadjuvant therapy from this study, because patients received chemotherapy before and after surgery in the adjuvant arm. No prospective and randomized study to date has examined the role of newer, biologic agents in the adjuvant setting in patients with resectable metastatic CRC.
Adjuvant Hepatic Arterial Infusion Chemotherapy (See Chapter 86)
Although regional liver-directed chemotherapy is not universally used, some liver surgeons and gastrointestinal oncologists support a role for this strategy in the setting of metastatic CRC to the liver. The rationale for this approach is that the liver is the most common site for tumor recurrence after liver resection, and it is often the only site of recurrence. Moreover, most metastatic liver tumors preferentially derive their blood supply from the hepatic artery, as opposed to normal hepatic tissue, which relies on the portal venous blood supply. In addition, the hepatic parenchyma can extract and metabolize chemotherapy drugs to less toxic byproducts, which allows the administration of very high doses to tumor cells with minimal systemic toxicity. For a more detailed discussion of the technical aspects of regional chemotherapy, refer to Chapter 86.
The most extensively studied agent used in hepatic artery infusional therapy is 5-FU-2-deoxyuridine (FUDR). This agent is a 5-FU analogue, which is concentrated 100 to 400 times in the liver as a result of its 95% hepatic extraction ratio (Ensminger et al, 1978). Most of the data on regional hepatic chemotherapy focuses on unresectable metastatic CRC. Randomized trials have clearly demonstrated that the technique is safe and is associated with a higher response rate than systemic chemotherapy alone; responses are 48% to 62% versus 0% to 21%, respectively (Chang et al, 1987; Kemeny et al, 1987; Hohn et al, 1989; Martin et al, 1990; Rougier et al, 1992); however, improved treatment response has translated into an improved survival in just a single study (Chang et al, 1987). Four relatively large randomized trials of adjuvant regional chemotherapy have been completed, and three were positive trials in favor of regional chemotherapy (Kemeny et al, 1999; Lygidakis et al, 2001; Kemeny et al, 2002). The fourth trial by Lorenz and colleagues (1998) found no difference in outcome for patients treated with resection alone versus resection plus regional 5-FU chemotherapy in an intention-to-treat analysis; however, the patients randomized to regional therapy had a very high operative mortality (8%), and 5-FU was used instead of FUDR, which has a much higher hepatic extraction rate and therefore less systemic toxicity. In addition, implanted ports were used as opposed to pump devices. Technical complications prevented chemotherapy administration in many of the patients randomized to regional therapy, so that just 74% and 30% of patients in the experimental arm initiated and completed treatment, respectively. These findings highlight the importance of technical expertise with regional liver chemotherapy (Lorenz et al, 1998).
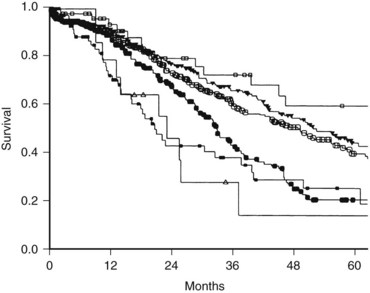
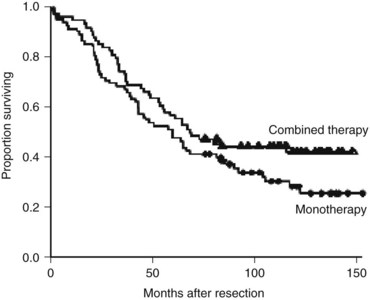
Two of the positive adjuvant trials were large American studies. The Eastern Oncology Cooperative Group/Southwest Oncology Group (ECOG/SWOG) study consisted of 109 patients randomized to surgery alone or surgery plus hepatic arterial infusion (HAI) with FUDR. The 4-year hepatic disease-free survival was significantly better in the HAI group (67% vs. 43%; P = .03) (Kemeny et al, 2002). No difference in overall survival was found, although the study was insufficiently powered to test this end point. In a larger single-institution study from Memorial Sloan-Kettering Cancer Center (MSKCC), 156 patients were randomized to resection plus systemic 5-FU or resection plus combined systemic 5-FU and HAI with FUDR. The patients who were treated with regional therapy had a significantly higher 2-year survival (86% vs. 72%; P = .03) and markedly improved liver disease control, and 10-year follow-up demonstrated significantly improved PFS associated with regional therapy (31.3 vs. 17.2 months; Fig. 81A.3). Survival rates at 10 years were 41.1% and 27.2% in the two groups, although the difference was not statistically significant (P = .1) (Kemeny & Gonen, 2005).
Future prospective trials should explore regimens combining HAI chemotherapy with FUDR and modern systemic chemotherapy with agents such as irinotecan (CPT-11), oxaliplatin, bevacizumab, and cetuximab. Phase I and II studies combining systemic CPT-11 and HAI-FUDR or systemic oxaliplatin with HAI-FUDR are presently ongoing. As second-line therapy in the palliative setting for unresectable hepatic colorectal metastases, systemic CPT-11 and/or oxaliplatin combined with HAI-FUDR was associated with a response rate of over 70% (Kemeny et al, 2001; Kemeny & Gonen, 2005). This compares quite favorably to second-line systemic chemotherapy, which has a response rate of only 20%.
Recent Phase I and II studies of HAI with floxuridine and dexamethasone combined with systemic irinotecan (Kemeny et al, 2003) or oxaliplatin (Kemeny et al, 2009) demonstrated safety and feasibility in the adjuvant setting. Active investigation is now directed at determining the efficacy of this strategy. While awaiting the results of larger scale trials, regional FUDR should be considered an option in the adjuvant setting in patients at high risk for liver recurrence, particularly after failure of first-line systemic therapy.
Neoadjuvant Chemotherapy for Downstaging of Unresectable Disease
Several practical and conceptual advantages attend neoadjuvant chemotherapy for metastatic CRC involving the liver. Improved efficacy of modern chemotherapy has the potential to convert unresectable metastatic CRC into resectable disease, or it may reduce the extent of resection required to remove the hepatic disease (see Chapters 65 and 87). In addition, neoadjuvant chemotherapy can treat micrometastatic disease early as opposed to addressing it in a delayed fashion. Finally, this strategy may function as an in vivo test of chemoresponsiveness to a particular agent. Bismuth and colleagues (1996) were the first to publish this observation in a study of 330 consecutive patients with unresectable disease. They found that a combination of 5-FU/LV and oxaliplatin converted 53 patients to resectability (16%). Furthermore, the long-term outcome of these individuals after resection was similar to patients who were initially resectable. Since then, the potential to downstage unresectable into resectable disease with neoadjuvant chemotherapy has been validated in a number of studies and ranges between 3% and 41% of patients (Giacchetti et al, 1999; Adam et al, 2001; Wein et al, 2001; Clavien et al, 2002; Adam et al, 2004; Pozzo et al, 2004; Vauthey et al, 2006; Adam et al, 2009). Patients who undergo complete resection have a median survival between 30 and 60 months. Of course, patient selection plays a vital role when estimating the potential benefit of neoadjuvant treatment. In the largest prospective study to date, 138 of 1104 unresectable patients (12%) became resectable after a course of neoadjuvant chemotherapy using either FOLFIRI or FOLFOX. Factors associated with a poor long-term outcome in these patients included large tumors, an increased number of tumors (≥3), increased preoperative CEA, and periportal or celiac lymph node metastases (Adam et al, 2004).
Regional chemotherapy alone or in conjunction with systemic chemotherapy may also convert unresectable disease into resectable disease. For example, 6 (26%) of 23 patients became resectable after HAI-FUDR treatment (Clavien et al, 2002). The 3-year actual survival rate was 84% for the patients who responded to HAI treatment compared with 40% for nonresponders. As part of a Phase I trial conducted at MSKCC, 21 patients with unresectable metastastic CRC received systemic oxaliplatin and CPT-11 in conjunction with HAI-FUDR. Seven of these patients (33%) became resectable after treatment (Kemeny et al, 2005).
Chemotherapy-Induced Liver Damage (See Chapters 65 and 87)
Neoadjuvant chemotherapy has been shown to cause liver injury in some patients. Specifically, oxaliplatin is associated with hepatic sinusoidal obstruction (Rubbia-Brandt et al, 2004), and CPT-11 is associated with hepatic steatosis (Kooby et al, 2003; Parikh et al, 2003); these agents can even cause severe steatohepatitis (Fernandez et al, 2005). Obese patients are at increased risk, and a preoperative liver biopsy should be considered to evaluate the liver parenchyma in such patients. When a major hepatic resection is planned in a patient suspected of having impaired liver function after exposure to systemic chemotherapy, preoperative portal vein embolization may be warranted to induce hypertrophy in the future liver remnant. In some instances, concern about the function of the future liver remnant should push the liver surgeon to proceed straight to surgery, and plan for chemotherapy postoperatively, after the liver has regenerated. In addition to steatohepatitis, the treated liver may become fibrotic and may develop perivascular adhesions, which can complicate a planned resection.
Despite well-documented histologic changes in the liver that can occur with chemotherapy, the actual clinical impact is not entirely clear. Indeed some studies have demonstrated a negative impact of oxaliplatin or irinotecan chemotherapy on postoperative morbidity (Karoui et al, 2006; Vauthey et al, 2006; Nakano et al, 2008) and mortality (Vauthey et al, 2006), whereas other studies have not confirmed these findings (Hewes et al, 2007; Mehta et al, 2008).
Disappearing Hepatic Metastases
A radiologic complete response, defined as the disappearance of a lesion on cross-sectional imaging, occurs in approximately 6% to 9% of lesions treated with neoadjuvant chemotherapy (Benoist et al, 2006; Elias et al, 2007; Auer et al, 2010). A lesion that disappears during chemotherapy may represent a pathologic complete response or a reduction in the sensitivity of cross-sectional imaging secondary to chemotherapy-induced steatosis. When a hepatic metastasis is present in a segment of the liver that cannot be removed, disappearance of this lesion during chemotherapy may preclude wedge resection or ablation and thereby jeopardize a complete resection. Moreover, in some patients, resection is only a consideration because previously apparent liver disease had disappeared during chemotherapy. When approaching such a patient, surgeons must understand that removal of visible disease is likely to be ineffective if occult and viable tumor remains in the foci that correspond to the “vanished” lesions.
Three institutions have published retrospective studies on the significance of liver metastases that disappear with chemotherapy (Benoist et al, 2006; Elias et al, 2007; Auer et al, 2010), and although all three studies agree that a complete radiologic response does not often equate to a true clinicopathologic response, the strength of this association varied across the studies. Benoist and colleagues (2006) found the lowest rate of true clinicopathologic response. The authors identified 38 patients with at least one or more disappearing liver metastases, a total of 66 complete radiologic responses, by CT scan (Benoist et al, 2006). All of these patients eventually underwent surgical exploration after a lesion disappeared. In 20 of 66 instances, macroscopic disease was appreciated at the site of disappearance. In 15 cases, the area of disappearance was resected, even though no residual disease was observed at surgery, and viable tumor cells were seen on histologic examination in 12 of these specimens. The remaining 31 sites were left in situ, and 23 recurred within 1 year of surgery. In summary, a true and durable response was only appreciated in 17% of the disappearing lesions.
Elias and colleagues reviewed data from 16 patients who had complete disappearance for at least 3 months during HAI or systemic chemotherapy in at least one liver metastasis in the future liver remnant on cross-sectional imaging. The disappearing lesion reappeared in 6 patients (38%) but represented a complete response in 10 patients (62%) based on a minimum of 2 years of follow-up. Interestingly, none of the lesions that disappeared with HAI therapy reappeared in follow-up. In the most recent study by Auer and colleagues, 38 patients had 118 disappearing liver metastases; a total of 68 disappearing lesions were resected, and 50 were followed clinically (Auer et al, 2010). In the resected liver that harbored the 68 vanished lesions, a complete pathologic response was observed in 44 patients (65%). Similarly, there were 31 recurrences (62%) in the 50 disappearing lesions that were followed clinically for a median of 41 months. Factors associated with a true complete response included HAI chemotherapy, inability to visualize the disappearing lesion on MRI, and normalization of serum CEA with treatment.
Re-Resection for Recurrence after Resection
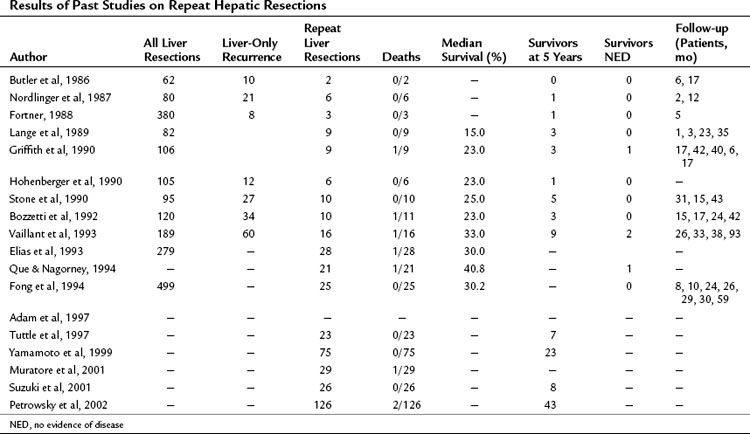
As stated previously, the most common site of recurrence after hepatic resection for colorectal metastases is the liver, and the liver is the sole site of recurrence in 15% to 40% of cases (see Table 81A.5). In the absence of extrahepatic disease, and in the patient with a good performance status and adequate hepatic reserve, a repeat hepatectomy may be considered. In approximately one third of liver recurrences, such an operation is feasible (Table 81A.6). The presence of adhesions and the altered anatomy of the liver, particularly the position of the vasculature and biliary system, make these operations very challenging from a technical standpoint. Series of repeat liver resections report an operative mortality rate less than 3% and a perioperative complication rate that is similar to initial resections (Lange et al, 1989; Bozzetti et al, 1992). The most common complications include biliary fistula, hepatic duct stenosis, hemorrhage, hepatic failure, and subphrenic abscess. Reported 5-year overall survival was 30% to 40%, and the median survivals were often more than 30 months after repeat resection.
The largest published series of repeat liver resection for metastatic CRC is a combined series of 126 patients from MSKCC and the University of Frankfurt Medical Center (Petrowsky et al, 2002). The operative procedures included 90 minor resections and 36 major hepatic resections. The 1-, 3-, and 5-year survivals were 86%, 51%, and 34%, respectively, and there were 19 actual 5-year survivors (15%). On multivariate regression analysis (proportional hazard model), the number of lesions greater than 1 (P = .01) and tumor size greater than 5 cm (P = .04) were independent prognostic indicators of reduced survival. Contrary to previous smaller studies (Bozzetti et al, 1992; Adam et al, 1997), the interval between the first and second liver resection was not associated with long-term survival.
Abdalla EK, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13(10):1271-1280.
Adam R, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225(1):51-60. discussion 60-52
Adam R, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8(4):347-353.
Adam R, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644-657. discussion 657-648
Adam R, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240(6):1052-1061. discussion 1061-1054
Adam R, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27(11):1829-1835.
Advanced Colorectal Cancer Meta-Analysis Project (ACCMAP). Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol. 1992;10(6):896-903.
Allen PJ, et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7(1):109-115. discussion 116-107
Amado RG, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626-1634.
Are C, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246(2):295-300.
Auer RC, et al. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer. 2010;116(6):1502-1509.
Babineau TJ, et al. Role of staging laparoscopy in the treatment of hepatic malignancy. Am J Surg. 1994;167(1):151-154. discussion 154-155
Baer HU, et al. Resectability of large focal liver lesions. Br J Surg. 1989;76(10):1042-1044.
Ballantyne GH, Quin J. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer. 1993;71(12 Suppl):4252-4266.
Belli G, et al. Liver resection for hepatic metastases: 15 years of experience. J Hepatobiliary Pancreat Surg. 2002;9(5):607-613.
Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969;23(1):198-202.
Bengtsson G, et al. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg. 1981;141(5):586-589.
Benoist S, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24(24):3939-3945.
Bensmaine MA, et al. Factors predicting efficacy of oxaliplatin in combination with 5-fluorouracil (5-FU) +/− folinic acid in a compassionate-use cohort of 481 5-FU–resistant advanced colorectal cancer patients. Br J Cancer. 2001;85(4):509-517.
Bipat S, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis: meta-analysis. Radiology. 2005;237(1):123-131.
Bismuth H, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224(4):509-520. discussion 520-502
Boldrini G, et al. The systematic use of operative ultrasound for detection of liver metastases during colorectal surgery. World J Surg. 1987;11(5):622-627.
Boni L, et al. The mesenteric and antimesenteric site of the tumor as possible prognostic factor in colorectal cancer: 5-year survival analysis. Surg Oncol. 2007;16(Suppl 1):S79-S82.
Bos JL, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327(6120):293-297.
Bozzetti F, et al. Patterns of failure following surgical resection of colorectal cancer liver metastases: rationale for a multimodal approach. Ann Surg. 1987;205(3):264-270.
Bozzetti F, et al. Repeated hepatic resection for recurrent metastases from colorectal cancer. Br J Surg. 1992;79(2):146-148.
Butler J, et al. Hepatic resection for metastases of the colon and rectum. Surg Gynecol Obstet. 1986;162(2):109-113.
Cady B, et al. Survival of patients after colonic resection for carcinoma with simultaneous liver metastases. Surg Gynecol Obstet. 1970;131(4):697-700.
Cady B, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992;127(5):561-568. discussion 568-569
Cady B, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227(4):566-571.
Capussotti L, et al. Major liver resections synchronous with colorectal surgery. Ann Surg Oncol. 2007;14(1):195-201.
Carpizo DR, et al. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16(8):2138-2146.
Castaing D, et al. Utility of operative ultrasound in the surgical management of liver tumors. Ann Surg. 1986;204(5):600-605.
Chang AE, et al. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987;206(6):685-693.
Choti MA, et al. Should hepatic resections be performed at high-volume referral centers? J Gastrointest Surg. 1998;2(1):11-20.
Choti MA, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759-766.
Chua HK, et al. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis Colon Rectum. 2004;47(8):1310-1316.
Clavien PA, et al. Downstaging of hepatocellular carcinoma and liver metastases from colorectal cancer by selective intra-arterial chemotherapy. Surgery. 2002;131(4):433-442.
Cobourn CS, et al. Examination of patient selection and outcome for hepatic resection for metastatic disease. Surg Gynecol Obstet. 1987;165(3):239-246.
Coelho JC, et al. Liver resection: 10-year experience from a single institution. Arq Gastroenterol. 2004;41(4):229-233.
Colucci G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23(22):4866-4875.
Comella P, et al. Oxaliplatin plus raltitrexed and leucovorin–modulated 5-fluorouracil i.v. bolus: a salvage regimen for colorectal cancer patients. Br J Cancer. 2002;86(12):1871-1875.
Cunningham D, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352(9138):1413-1418.
Cunningham D, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337-345.
Cunningham JD, et al. One hundred consecutive hepatic resections: blood loss, transfusion, and operative technique. Arch Surg. 1994;129(10):1050-1056.
D’Angelica MI, et al. Randomized clinical trials in advanced and metastatic colorectal carcinoma. Surg Oncol Clin N Am. 2002;11(1):173-191. ix
de Gramont A, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15(2):808-815.
de Gramont A, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938-2947.
de Haas RJ, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248(4):626-637.
de Haas RJ, et al. Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. Br J Surg. 2010;97(8):1279-1289.
de Jong MC, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440-448.
DeMatteo RP, et al. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4(2):178-184.
Doci R, et al. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg. 1991;78(7):797-801.
Douillard JY, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041-1047.
Douillard JY, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697-4705.
Ekberg H, et al. Pattern of recurrence in liver resection for colorectal secondaries. World J Surg. 1987;11(4):541-547.
Elias D, et al. Repeat hepatectomy for cancer. Br J Surg. 1993;80(12):1557-1562.
Elias D, et al. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003;90(5):567-574.
Elias D, et al. Outcome of posthepatectomy-missing colorectal liver metastases after complete response to chemotherapy: impact of adjuvant intra-arterial hepatic oxaliplatin. Ann Surg Oncol. 2007;14(11):3188-3194.
Ensminger WD, et al. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2′-deoxyuridine and 5-fluorouracil. Cancer Res. 1978;38(11 Pt 1):3784-3792.
Falcone A, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670-1676.
Farid SG, et al. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251(1):91-100.
Fernandez FG, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200(6):845-853.
Figueras J, et al. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases: evidences from 663 liver resections. Ann Oncol. 2007;18(7):1190-1195.
Findlay M, et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol. 1996;14(3):700-708.
Fong Y, et al. Repeat hepatic resections for metastatic colorectal cancer. Ann Surg. 1994;220(5):657-662.
Fong Y, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309-318. discussion 318-321
Fong Y, et al. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242(4):540-544. discussion 544-547
Fortner JG. Recurrence of colorectal cancer after hepatic resection. Am J Surg. 1988;155(3):378-382.
Fortner JG, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg. 1984;199(3):306-316.
Foster JH. Survival after liver resection for secondary tumors. Am J Surg. 1978;135(3):389-394.
Gallagher DJ, et al. Response to neoadjuvant chemotherapy does not predict overall survival for patients with synchronous colorectal hepatic metastases. Ann Surg Oncol. 2009;16(7):1844-1851.
Gayowski TJ, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116(4):703-710. discussion 710-701
Giacchetti S, et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol. 1999;10(6):663-669.
Glasgow RE, et al. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg. 1999;134(1):30-35.
Goldberg RM, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23-30.
Griffith KD, et al. Repeat hepatic resections for colorectal metastases. Surgery. 1990;107(1):101-104.
Hamady ZZ, et al. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1 cm rule. Eur J Surg Oncol. 2006;32(5):557-563.
Headrick JR, et al. Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg. 2001;71(3):975-979. discussion 979-980
Hecht JR, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672-680.
Herbertson RA, et al. The expanding role of PET technology in the management of patients with colorectal cancer. Ann Oncol. 2007;18(11):1774-1781.
Hewes JC, et al. Preoperative chemotherapy and the outcome of liver resection for colorectal metastases. World J Surg. 2007;31(2):353-364. discussion 365-356
Hohenberger P, et al. Tumor recurrence and options for further treatment after resection of liver metastases in patients with colorectal cancer. J Surg Oncol. 1990;44(4):245-251.
Hohn DC, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989;7(11):1646-1654.
Huebner RH, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41(7):1177-1189.
Hughes KS, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100(2):278-284.
Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335-2342.
Ito H, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247(6):994-1002.
Ito K, et al. Surgical treatment of hepatic colorectal metastasis: evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010;16(2):103-110.
Jamison RL, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;132(5):505-510. discussion 511
Jarnagin WR, et al. A prospective analysis of staging laparoscopy in patients with primary and secondary hepatobiliary malignancies. J Gastrointest Surg. 2000;4(1):34-43.
Jarnagin WR, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001;91(6):1121-1128.
Jarnagin WR, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397-406. discussion 406-397
John TG, et al. Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg. 1994;220(6):711-719.
Jonker DJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040-2048.
Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354(5):496-507.
Karapetis CS, et al. Kras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757-1765.
Karoui M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243(1):1-7.
Kato T, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46(10 Suppl):S22-S31.
Kattan MW, et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247(2):282-287.
Kelloff GJ, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11(8):2785-2808.
Kemeny MM, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy—an intergroup study. J Clin Oncol. 2002;20(6):1499-1505.
Kemeny N, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma: a randomized trial. Ann Intern Med. 1987;107(4):459-465.
Kemeny N, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341(27):2039-2048.
Kemeny N, et al. Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol. 2001;19(10):2687-2695.
Kemeny N, et al. Phase I/II study of hepatic arterial therapy with floxuridine and dexamethasone in combination with intravenous irinotecan as adjuvant treatment after resection of hepatic metastases from colorectal cancer. J Clin Oncol. 2003;21(17):3303-3309.
Kemeny N, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23(22):4888-4896.
Kemeny N, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol. 2009;20(7):1236-1241.
Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352(7):734-735.
Khuri SF, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326-341. discussion 341-323
Kjeldsen BJ, et al. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84(5):666-669.
Knosel T, et al. Chromosomal alterations during lymphatic and liver metastasis formation of colorectal cancer. Neoplasia. 2004;6(1):23-28.
Kokudo N, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137(7):833-840.
Kooby DA, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237(6):860-869. discussion 869-870
Kronawitter U, et al. Evaluation of chest computed tomography in the staging of patients with potentially resectable liver metastases from colorectal carcinoma. Cancer. 1999;86(2):229-235.
Kubo M, et al. Less aggressive features of colorectal cancer with liver metastases showing macroscopic intrabiliary extension. Pathol Int. 2002;52(8):514-518.
Lambert LA, et al. Interval hepatic resection of colorectal metastases improves patient selection. Arch Surg. 2000;135(4):473-479. discussion 479-480
Lange JF, et al. Repeat hepatectomy for recurrent malignant tumors of the liver. Surg Gynecol Obstet. 1989;169(2):119-126.
Leen E, et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg. 2006;243(2):236-240.
Lorenz M, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg. 1998;228(6):756-762.
Lygidakis NJ, et al. Metastatic liver disease of colorectal origin: the value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection: results of a prospective randomized study. Hepatogastroenterology. 2001;48(42):1685-1691.
Machi J, et al. Intraoperative ultrasonography in screening for liver metastases from colorectal cancer: comparative accuracy with traditional procedures. Surgery. 1987;101(6):678-684.
Machi J, et al. Accuracy of intraoperative ultrasonography in diagnosing liver metastasis from colorectal cancer: evaluation with postoperative follow-up results. World J Surg. 1991;15(4):551-556. discussion 557
Makela JT, et al. Five-year follow-up after radical surgery for colorectal cancer: results of a prospective randomized trial. Arch Surg. 1995;130(10):1062-1067.
Mala T, et al. Hepatic resection for colorectal metastases: can preoperative scoring predict patient outcome? World J Surg. 2002;26(11):1348-1353.
Martin JKJr, et al. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer: a randomized trial. Arch Surg. 1990;125(8):1022-1027.
Martin R, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197(2):233-241. discussion 241-232
Mehta NN, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34(7):782-786.
Meta-analysis Group In Cancer (MAGIC). Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16(1):301-308.
Metcalfe MS, et al. Choice of surveillance after hepatectomy for colorectal metastases. Arch Surg. 2004;139(7):749-754.
Miller G, et al. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg. 2007;205(2):231-238.
Minagawa M, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231(4):487-499.
Mitry E, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26(30):4906-4911.
Muratore A, et al. Repeat hepatectomy for colorectal liver metastases: a worthwhile operation? J Surg Oncol. 2001;76(2):127-132.
Mutsaerts EL, et al. Prognostic factors and evaluation of surgical management of hepatic metastases from colorectal origin: a 10-year single-institute experience. J Gastrointest Surg. 2005;9(2):178-186.
Nakano H, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247(1):118-124.
Nash GM, et al. KRAS mutation and microsatellite instability: two genetic markers of early tumor development that influence the prognosis of colorectal cancer. Ann Surg Oncol. 2010;17(2):416-424. Epub Oct 8 2009
Neumann UP, et al. Nonresponse to pre-operative chemotherapy does not preclude long-term survival after liver resection in patients with colorectal liver metastases. Surgery. 2009;146(1):52-59.
Nikfarjam M, et al. Survival outcomes of patients with colorectal liver metastases following hepatic resection or ablation in the era of effective chemotherapy. Ann Surg Oncol. 2009;16(7):1860-1867.
Nordlinger B, et al. Hepatic resection for colorectal liver metastases: influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg. 1987;205(3):256-263.
Nordlinger B, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77(7):1254-1262.
Nordlinger B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007-1016.
Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg. 1997;1(5):398-407.
Nuzzo G, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008;143(3):384-393.
Ohlsson B, et al. Follow-up after curative surgery for colorectal carcinoma: randomized comparison with no follow-up. Dis Colon Rectum. 1995;38(6):619-626.
Okano K, et al. Macroscopic intrabiliary growth of liver metastases from colorectal cancer. Surgery. 1999;126(5):829-834.
Olsen AK. Intraoperative ultrasonography and the detection of liver metastases in patients with colorectal cancer. Br J Surg. 1990;77(9):998-999.
Oxley EM, Ellis H. Prognosis of carcinoma of the large bowel in the presence of liver metastases. Br J Surg. 1969;56(2):149-152.
Pagana TJ. A new technique for hepatic infusional chemotherapy. Semin Surg Oncol. 1986;2(2):99-102.
Parikh AA, et al. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7(8):1082-1088.
Parks R, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204(5):753-761. discussion 761-753
Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11(8):1057-1077.
Pawlik TM, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715-722. discussion 722-714
Peeters M, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706-4713.
Petrowsky H, et al. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg. 2002;235(6):863-871.
Picardo A, et al. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery. 1998;124(1):57-64.
Povoski SP, et al. Recognition of intrabiliary hepatic metastases from colorectal adenocarcinoma. HPB Surg. 2000;11(6):383-390. discussion 390-381
Pozzo C, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15(6):933-939.
Que FG, Nagorney DM. Resection of ‘recurrent’ colorectal metastases to the liver. Br J Surg. 1994;81(2):255-258.
Rees M, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929. patients. Ann Surg. 2008;247(1):125-135.
Ribic CM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247-257.
Rifkin MD, et al. Intraoperative ultrasound of the liver: an important adjunctive tool for decision making in the operating room. Ann Surg. 1987;205(5):466-472.
Rosen CB, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216(4):493-504. discussion 504-495
Rougier P, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol. 1992;10(7):1112-1118.
Rougier P, et al. Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer. Fondation Française de Cancerologie Digestive. Br J Surg. 1995;82(10):1397-1400.
Rubbia-Brandt L, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15(3):460-466.
Saltz LB, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905-914.
Saltz LB, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013-2019.
Scheele J. [Segment-oriented liver resection: principles—technic—status]. Chirurg. 1989;60(4):251-265.
Scheele J, et al. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77(11):1241-1246.
Scheele J, et al. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110(1):13-29.
Scheele J, et al. Resection of colorectal liver metastases. World J Surg. 1995;19(1):59-71.
Schlag P, et al. Resection of liver metastases in colorectal cancer: competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol. 1990;16(4):360-365.
Schoemaker D, et al. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114(1):7-14.
Schussler-Fiorenza CM, et al. Clinical risk score correlates with yield of PET scan in patients with colorectal hepatic metastases. J Gastrointest Surg. 2004;8(2):150-157. discussion 157-158
Shah AJ, et al. Contrast-enhanced intraoperative ultrasound improves detection of liver metastases during surgery for primary colorectal cancer. HPB (Oxford). 2010;12(3):181-187.
Shirabe K, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84(8):1077-1080.
Sica GT, et al. CT and MR imaging of hepatic metastases. AJR Am J Roentgenol. 2000;174(3):691-698.
Stangl R, et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343(8910):1405-1410.
Stone MD, et al. Surgical therapy for recurrent liver metastases from colorectal cancer. Arch Surg. 1990;125(6):718-721. discussion 722
Stone MD, et al. Intraoperative ultrasound imaging of the liver at the time of colorectal cancer resection. Arch Surg. 1994;129(4):431-435. discussion 435-436
Strasberg SM, et al. Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. Ann Surg. 2001;233(3):293-299.
Suzuki S, et al. Impact of repeat hepatectomy on recurrent colorectal liver metastases. Surgery. 2001;129(4):421-428.
Tien YW, et al. Simultaneous detection of colonic epithelial cells in portal venous and peripheral blood during colorectal cancer surgery. Dis Colon Rectum. 2002;45(1):23-29.
Tomlinson JS, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575-4580.
Tournigand C, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229-237.
Tuma RS. Sometimes size doesn’t matter: reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst. 2006;98(18):1272-1274.
Tuttle TM, et al. Repeat hepatic resection as effective treatment of recurrent colorectal liver metastases. Ann Surg Oncol. 1997;4(2):125-130.
Uetsuji S, et al. Absence of colorectal cancer metastasis to the cirrhotic liver. Am J Surg. 1992;164(2):176-177.
Vaillant JC, et al. Repeat liver resection for recurrent colorectal metastases. Br J Surg. 1993;80(3):340-344.
Van Cutsem E, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658-1664.
Van Cutsem E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408-1417.
Vauthey JN, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24(13):2065-2072.
Vogt P, et al. Resection of synchronous liver metastases from colorectal cancer. World J Surg. 1991;15(1):62-67.
Wagner JS, et al. The natural history of hepatic metastases from colorectal cancer: a comparison with resective treatment. Ann Surg. 1984;199(5):502-508.
Wang J, et al. Visualizing portal vein metastatic trafficking to the liver with green fluorescent protein-expressing tumor cells. Anticancer Res. 2004;24(6):3699-3702.
Weber WA, et al. Positron emission tomography in non–small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21(14):2651-2657.
Wein A, et al. Impact of surgery on survival in palliative patients with metastatic colorectal cancer after first line treatment with weekly 24-hour infusion of high-dose 5-fluorouracil and folinic acid. Ann Oncol. 2001;12(12):1721-1727.
Weinbren K, Blumgart LH. Pathological changes in the liver and computed tomography. Recent Results Cancer Res. 1986;100:58-67.
Wiering B, et al. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer. 2005;104(12):2658-2670.
Wood CB, et al. A retrospective study of the natural history of patients with liver metastases from colorectal cancer. Clin Oncol. 1976;2(3):285-288.
Yamamoto J, et al. Repeat liver resection for recurrent colorectal liver metastases. Am J Surg. 1999;178(4):275-281.
Yamamoto J, et al. Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann Surg. 1995;221(1):74-78.
Younes RN, et al. The influence of intraoperative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg. 1991;214(2):107-113.