Chapter 12 Metastatic Evaluation
INTRODUCTION
The incidence of metastatic spinal disease continues to rise, for two reasons: an increased capacity to detect metastatic lesions and improved survival of cancer patients.1 The optimal treatment of this disease requires an efficient yet thorough evaluation. Despite the ongoing evolution of surgical therapy, there is still controversy regarding the merits of surgery relative to non-surgical management with chemotherapy and radiation. The goal of the spine surgeon is to quickly and efficiently gather the information necessary to determine whether conservative non-operative management, preoperative adjuvant treatment, or initial surgical intervention is to be countenanced in the care of the patient with spinal metastases.
DIFFERENTIAL DIAGNOSIS
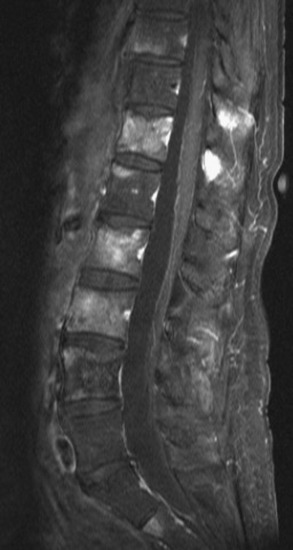
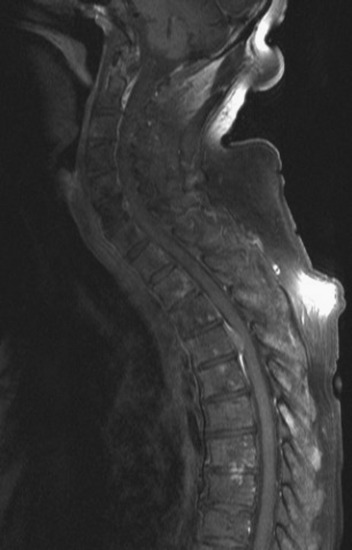
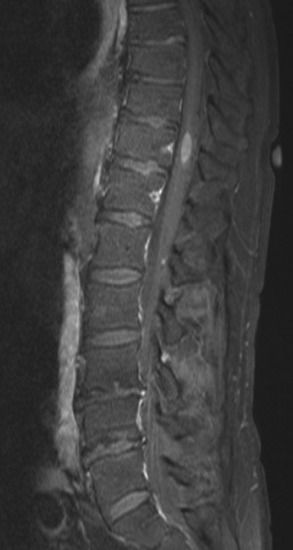
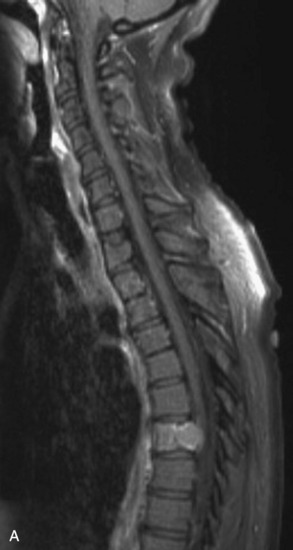
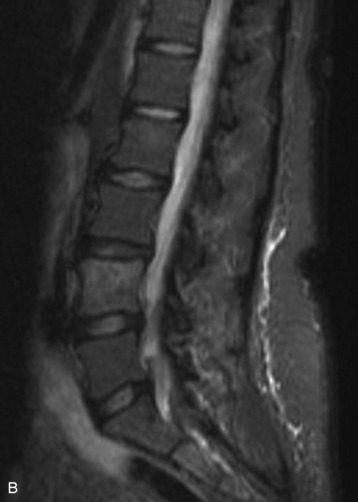
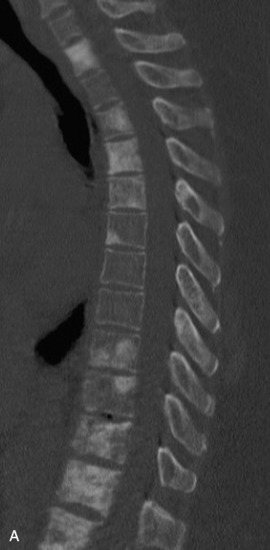
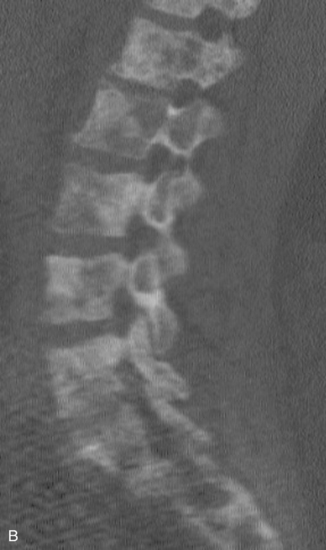
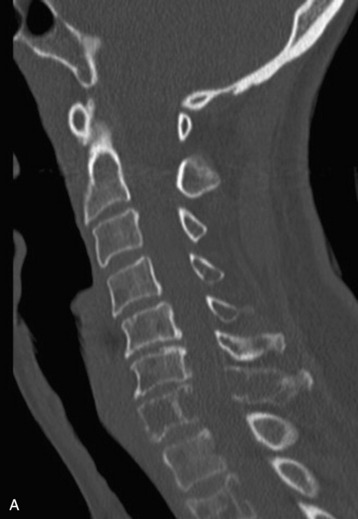
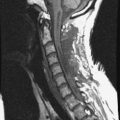
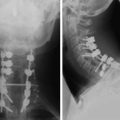
Spinal metastatic lesions are quite common. Nearly 18,000 new cases2 with demonstrated secondary tumor spread represent almost 70% of all cancer patients each year.3,4 There are five possible pathways of metastasis to the spine: arterial, venous, direct extension, lymphatic, and leptomeningeal via cerebrospinal fluid (CSF). In anatomical distribution, the vast majority of metastatic spinal lesions are extradural, initially disseminating to the vertebral bodies (Figs. 12-1 and 12-2) and expanding subsequently in destructive fashion to eventually lie adjacent to the dura.5–7 Intradural metastases typically are found in patients with concomitant intracranial metastases. Drop metastases and leptomeningeal carcinomatosis are typically intradural extramedullary, originate via the CSF from an intracranial source, and exist as multiple discrete lesions or coat the dura and enmesh within nerve roots.7,8 Intramedullary metastases from hematological seeding of the spinal cord are exceedingly rare (Fig. 12-3).
METASTASES FROM PRIMARY CENTRAL NERVOUS SYSTEM TUMORS
Medulloblastomas,9–14 ependymomas,15,16 and gliomas14,17–19 are the primary tumors of the brain known to subsequently seed other central nervous system (CNS) locations via leptomeningeal dissemination.20,21 The location of secondary metastases from primary CNS tumors is usually intradural and extramedullary. Glioblastoma multiforme (GBM) is an exception, with sporadic reports describing metastases consistent with both intradural CSF seeding as well as hematogenous spread to the vertebral bodies and other extraneural locations.17–19 The more common leptomeningeal CSF seeding occurs primarily in children with tumors of the posterior fossa, affecting the entire neuraxis and thus requiring comprehensive evaluation of the brain and spinal cord with gadolinium-enhanced magnetic resonance imaging (MRI) studies.22 Treatment of leptomeningeal metastasis is typically palliative, with an expected patient survival of 6 months, and often halts further neurological deterioration until the patient succumbs to progression of systemic or intracranial disease. Treatment is a combination of intralumbar or intraventricular drug therapy with craniospinal radiotherapy and local radiation boost to solid portions of metastatic disease. Multiple, less common primary brain tumors have been reported to spread via CSF dissemination, including pineal germinoma23 and pineoblastoma, choroid plexus papilloma and carcinoma, hemangiopericytoma,24,25 ependymoblastoma,26 and retinoblastoma.27
METASTASES FROM EXTRANEURAL TUMORS
In adults, secondary metastases typically originate from lung, breast, prostate, and hemopoietic tumors (multiple myeloma, lymphoma); renal cancer; melanoma; and sarcoma.3,4,28–31 In children, most spine metastases are caused by Ewing’s sarcoma and neuroblastoma; less common causes are sarcomas, Hodgkin’s lymphoma, and germ cell tumors.32 Extraneural tumors that subsequently spread to the spinal axis most commonly disseminate hematogenously to the bone as secondary metastases and extend to the epidural space via aggressive enlargement and bone lysis. Spread to the intradural space typically occurs as tertiary leptomeningeal metastases as a result of seeding via CSF pathways from secondary intracranial involvement (Fig. 12-3). Location in the intramedullary compartment is rare and believed to occur hematogenously.
Amongst all bony secondary metastases in the body, the spinal column is the most common site of spread,33 and it is also the initial site of spread in 12–20% of patients who present with spinal symptoms.7,34 Most metastatic spinal lesions originate from arterial hematological spread, but some, including prostate and renal metastases, also occur through the venous system in retrograde fashion via the valveless venous plexus described by Batson.35–37 Post-mortem studies also show that 15–40% of patients dying from disseminated cancer have vertebral epidural metastases38 and that, overall, extradural vertebral metastases increase in the caudal direction along the vertebral column. This is a volume-dependent relationship, corresponding to the greater volume of bone marrow within the larger vertebral bodies of the lumbar spine, followed by thoracic and then cervical vertebrae in marrow volume.28,30 Similarly, these lesions are found in the vertebral bodies 20 times more often than in the posterior elements because of the relative distribution of marrow volume.39 As a result, metastatic compression most often originates anterior to the thecal sac (Fig. 12-4). Symptomatic lesions, however, occur more often in the thoracic rather than the lumbar spine. A number of factors predispose the thoracic spine to increased risk of neurological deficit from tumor compression. The thoracic canal is small in dimension relative to the spinal cord, so there is less room for a tumor to expand before deficit onset.40 The spinal cord extends throughout the thoracic spine but only down to the second vertebra of the lumbar spine, and the spinal cord is more susceptible to neurological symptoms of compression than the roots of the cauda equina. The thoracic cord has a more tenuous blood supply. Finally, the thoracic spine with its natural kyphosis may be more prone to angular deformity with a pathological fracture, which would exacerbate the compression and mass effect from an expansile tumor anterior to the spinal cord (see Fig. 12-4).
Intradural extramedullary spinal metastases from extraneural sources most commonly occur as tertiary drop metastases from intracranial intradural secondary lesions, such as carcinomas of the lung, breast, melanoma, and hemopoietic tumors such as lymphoma and leukemia.41 These tumors begin in the brain and then subsequently seed the subarachnoid spaces and spread to other CNS locations, including the spine.20,21 The location of these tertiary CNS metastases is usually intradural and extramedullary (see Fig. 12-3), much like primary CNS tumors that spread to the spine via CSF dissemination. Once tumor cells enter the CSF, the entire neuraxis becomes affected, and comprehensive evaluation of the brain and spinal cord with gadolinium-enhanced MRI studies and CSF cytopathology is necessary.22 Treatment of leptomeningeal metastatic disease to the spine is palliative and requires a combination of intralumbar or intraventricular drug therapy and craniospinal radiotherapy with local radiation boost to solid portions. These lesions are often found on imaging and intraoperatively to be entangled within the nerve roots of the cauda equina as a diffuse meningeal covering, sometimes termed “sugar-coating,” with discrete areas of solid tumor interspersed.
Because of the small volume of the spinal cord in comparison to the mass of the spinal axis, hematogenous spread to the spinal cord is rare. Secondary intramedullary spinal cord metastases comprise approximately 0.5% of spinal axis metastases.6,7 The most common intramedullary metastases are lung and breast carcinomas, followed by hemopoietic tumors and melanoma, but all lesions that are metastatic to the brain have been reported in the spine.42–44 Treatment is via local radiotherapy, rather than surgical resection, in conjunction with systemic chemotherapy.
HISTORY AND PHYSICAL EXAMINATION
Severe axial spine pain is so commonly a manifestation of disseminated cancer that in the cancer patient, medical practitioners should treat it as a symptom of spinal metastasis until proven otherwise. Pain is by far the most common initial symptom, present in more than 90% of patients with metastatic spinal disease. The pain is usually severe, can be burning and dysesthetic, worsens at night, and is unremitting.40 This local pain may be caused by irritation of the periosteum, ligaments, or adjacent viscera. If the pain is more severe with movement and remits at rest, spinal stability must be evaluated because severe mechanical pain may be caused by extensive bone invasion and destruction.45 Radicular symptoms may be unilateral or bilateral. Paresthesias in the upper extremities may be reproduced with evaluation for Lhermitte sign, especially when cord compression is present. Nerve root irritation in the thoracic spine can be aggravated by sharp movements of the trunk, such as coughing or sneezing. When present, radicular symptoms may localize the lesion to a span of two to three vertebral levels before imaging.40 Leg pain may be present with compression of the cervical or thoracic spinal cord and is thought to arise from irritation of the anterolateral spinothalamic tract. Although the initial presentation is pain, as the lesion progresses, impingement on neurological structures eventually results in neurological deficits. Such deficits may begin as numbness and paresthesias but inevitably progress to weakness, sensory loss, bowel and bladder sphincter dysfunction, and ultimately paraplegia without appropriate intervention. Cervical and lumbar metastases are often symptomatic up to 6 months before the onset of neurological deficit, whereas thoracic lesions typically present with neurological compromise in conjunction with initial symptoms.46 Patients presenting with neurological deficit require urgent evaluation, and if the deficits are rapidly progressive, urgent surgical intervention may be indicated. Rapid progression and severe deficits indicate a poor prognosis.47
A general physical examination, followed by a detailed musculoskeletal examination, should be performed for all patients with possible metastatic spinal disease. With detailed musculoskeletal and sensory examinations performed to clearly assess the patient’s neurological status at the time of presentation, further neurological decline can be properly evaluated in the context of both ideal patient care and medicolegal documentation. Spine tenderness is often present with palpation on the spinous processes overlying the metastatic lesions.48 In addition, the general examination should emphasize the anatomical locations of primary tumors that commonly metastasize to bone and spine. These would include cervical, axillary and inguinal lymph nodes, breasts, chest, abdomen, and prostate. Lesions that compress the cervical and thoracic spinal cord produce the myelopathic findings of upper motor neuron deficits, such as weakness and spasticity. Limbs feel heavy, objects are dropped by the hands, and legs buckle with standing or ambulation. Wasting of intrinsic hand muscles may occur with lesions that affect the anterior horn neuronal cell bodies. Spasticity findings include increased deep tendon reflexes, clonus, extensor plantar responses, and Beevor sign with upward deviation of the umbilicus on abdominal contraction. Lesions above the conus also can result in bowel constipation, priapism, reflex ejaculations, spastic sphincters, and initially urinary retention followed by overflow incontinence and then intermittent emptying. In contrast, conus and cauda equina lesions produce lower motor neuron deficits without myelopathy. Conus lesions result in lower extremity weakness, absent extensor plantar responses, hypotonia, saddle anesthesia, and eventually loss of sphincter tone and incontinence. Cauda equina compression similarly produces decreased tone in the lower extremities, either unilateral or bilateral lower extremity weakness, and like conus lesions can result in flaccid incontinence of bowel and bladder and impotence from sacral root dysfunction.
Other more specific findings may be found depending on the location of the lesion and should be rigorously documented before initiation of treatment to prevent a lack of clarity with regard to deficit onset. Dorsal column dysfunction from posterior compression may result in deficits of position sense, vibration, and fine touch. Because of the anatomical layering of sensory fibers, extramedullary lesions often produce greater loss peripherally than proximally, with the reverse being true for intramedullary lesions. Intramedullary lesions also may produce a segmental pattern of sensory loss with sacral sparing. Tumors that compress the cord from a lateral to medial direction can produce Brown-Séquard’s syndrome, which is classically an ipsilateral decrement in corticospinal motor function, dorsal column sensory function, and contralateral spinothalamic pain and temperature loss. Lesions in the upper cervical spine may cause respiratory dysfunction from intercostal muscle weakness. Lesions of low cervical and high thoracic segments may produce Horner’s syndrome, with ipsilateral ptosis, miosis, anhidrosis, and enophthalmos. Eruption of herpes zoster may occur from posterior root irritation at the level of metastatic disease.48 If the history and physical examination suggest an acutely worsening neurological condition, emergent surgical intervention is warranted.
GENERAL INVESTIGATIONS
Routine laboratory studies should be performed for all patients with suspected metastatic disease. A chemistry panel to assess for electrolyte imbalances is important in this patient population undergoing multimodal therapy for cancer. Potential metabolic derangements from tumor lysis; metastatic organ involvement, including kidney and liver; and bony invasion with osteolysis and bone resorption make the knowledge of serum sodium, potassium, blood urea nitrogen, creatinine, calcium, phosphate, magnesium, liver enzymes, and coagulation times important for patient management and for any contemplation of surgical intervention.49,50 A complete blood count, erythrocyte sedimentation rate or C-reactive protein, albumin, and transthyretin are important to evaluate the nutritional and immunological status for cancer patients subject to chemotherapy and radiation treatment. Electrocardiograms are necessary if the patient is older or if electrolyte abnormalities, such as hypercalcemia, exist.
Routine studies for evaluation of new spinal metastases include chest radiographs for lung cancer and ultrasonography of the abdomen and pelvis, or CT of the chest, abdomen, and pelvis to find the primary lesion and stage the disease. Additional imaging studies for a metastatic work-up often include either a bone or positron emission tomography (PET) scan (Fig. 12-5) of the body.51 Routine laboratory tests include serum electrophoresis and urine for Bence-Jones protein in multiple myeloma, and prostate-specific antigen levels.52 Carcinoembryonic antigen (CEA) is a non-specific marker for cancer of the colon, breast, lung, pancreas, and ovaries. For patients with concomitant intracranial lesions, CSF should be sent for cytopathology.
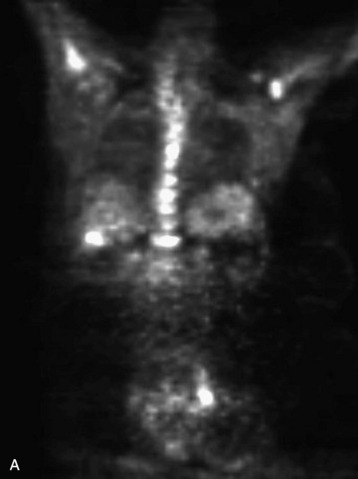
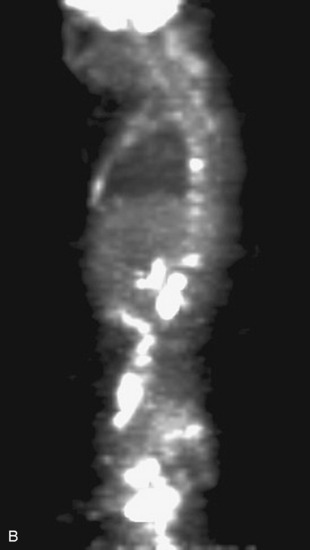
Fig. 12-5 This patient is a 42-year-old woman with ER weakly positive, progesterone receptor (PR) positive, HER-2 negative breast cancer metastatic to bone, liver, and brain. PET scan shows increased 2-deoxy-2-[18F]fluoro-D-glucose (18FDG or FDG) uptake throughout the osseous structures, with pronounced uptake in the T5, T9, T11, and L1 vertebral bodies, all suggestive of malignancy. Other osseous structures demonstrating hypermetabolism include the sternum, the left ilium adjacent to the sacroiliac joint, the left infratrochanteric region, and the proximal femora bilaterally. Foci also are in the liver and inguinal lymph nodes.
DIAGNOSTIC NEUROIMAGING
SPINE RADIOGRAPHY
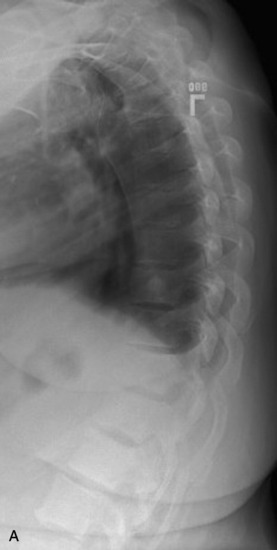
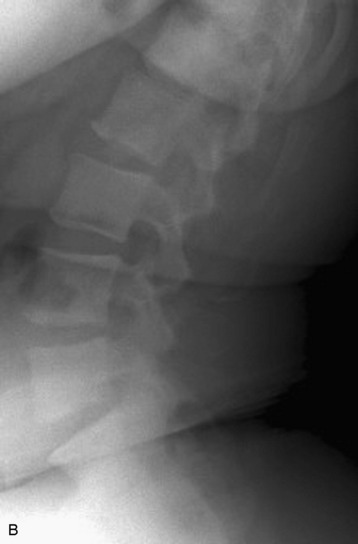
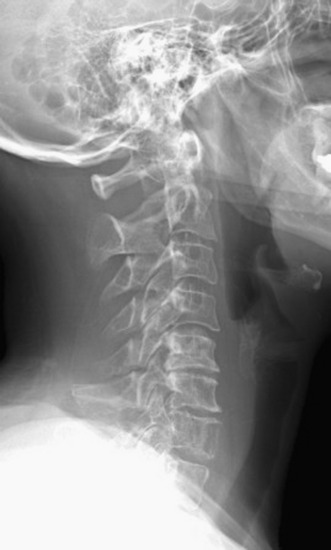
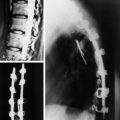
Plain radiographs are readily available and yield valuable information to assist in the diagnosis and treatment of spinal metastases. Up to 90% of patients with symptomatic metastatic spinal disease have findings on plain radiographs.53,54 Plain radiography, however, is not good for the evaluation of small metastases because 30–50% of bony destruction is usually necessary before the lesions become visible.55 Plain radiographs are therefore not sensitive indicators of the presence or extent of metastatic disease. Patients who clinically present with a high suspicion for metastatic spinal disease require further work-up in the absence of plain radiography findings. Plain radiographs, however, are invaluable in the evaluation of spinal stability. Radiographs alone are excellent for the evaluation of spinal alignment. Patients can be placed in different positions, including an upright posture to subject the spine to axial weight-bearing while the radiograph is taken, without the limitations on positioning with CT and MRI scanning. Positional flexibility also permits radiographs with the patient in flexion, neutral position, and extension to evaluate whether spinal alignment is maintained. A better evaluation of bony integrity is available with spinal CT. It should be obtained if there is significant bone destruction or a question of bone integrity evidenced on plain film or MRI and if a surgical intervention is planned that will require detailed knowledge of bone anatomy.
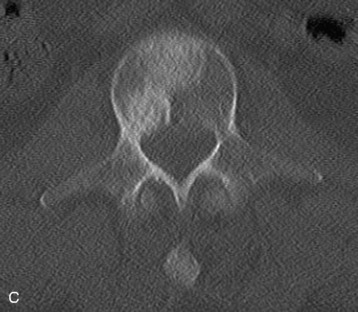
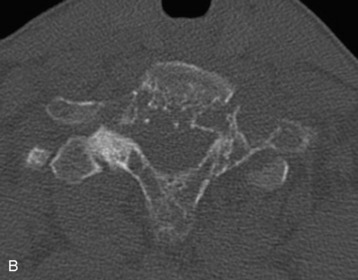
The most common finding on plain radiography is the “winking owl” sign, which provides evidence of pedicle destruction on anteroposterior radiography of the spine.56 Most metastases are osteolytic, and the significant demineralization can often be visualized as lucencies (Figs. 12-6 and 12-7). Some tumors, such as breast and prostate cancer, create osteoblastic sclerotic margins. These lucencies and sclerotic areas are typically seen in the vertebral bodies and are often multifocal. In addition, large masses that invade paraspinal structures are often seen to produce soft tissue shadows. Other features of epidural spinal metastases include an indistinct posterior vertebral margin and pathological compression fractures.57 These and other changes in the contours of the vertebral bodies suggest metastatic disease, especially in the absence of osteoporosis.55 Multiple myeloma may produce osteopenia that results in the bowing and “fishmouthing” of multiple endplates. In patients with mechanical pain, radiographs may demonstrate instability, with vertebral body collapse and kyphosis or scoliosis in conjunction with lucent areas devoid of bone. Dynamic radiography may demonstrate increasing anterolisthesis, scoliotic deformity, or kyphosis caused by ligament and bone destruction. Presence of such findings requires bed rest, immobilization, and consideration for urgent surgical stabilization to prevent neurological devastation for the unfortunate patient.
MYELOGRAPHY
Historically, before the advent of MRI, myelography was the gold standard for the evaluation of spinal stenosis and cord compression caused by various diseases, including spinal metastases. Because of dye location in the CSF subarachnoid spaces surrounding the spinal cord and the roots within the thecal sac, with myelography it is often possible to determine whether a lesion is epidural, intradural and extramedullary, or intramedullary. It also is possible to determine the spinal level of the tumor and the anatomical relationship of the lesion in relationship to the dura, spinal cord, and spinal roots. A common variation of myelography often performed is CT myelography, in which a CT scan of the spine is obtained immediately after infusion of dye into the thecal sac. CT myelography increases the resolution of the study and adds axial slices for improved evaluation of the metastatic lesion and its anatomic relationship to neural and bony elements. Access to CSF for cytopathology is an additional benefit of the procedure. As a result, some authors have recommended aggressive use of myelography for the early diagnosis of spinal metastatic disease.53
In the modern era, MRI of the spine has largely supplanted myelography in the evaluation of non-bony spinal pathology. Myelography is still useful in certain clinical situations for patients who cannot have an MRI study because of inability to tolerate MRI scanning or presence of incompatible metallic implants, or if MRI is unavailable. Limitations to myelography include the risks associated with an invasive procedure in a patient with a spinal tumor. Myelography in the presence of a complete spinal block as a result of the mass effect of the tumor carries the risk for spinal cord herniation or “coning” at the level of the block.38 In one series, 7 of 50 patients with complete spinal block who underwent lumbar puncture developed an acute neurological deterioration.58 Complete spinal block also would result in the inability to visualize anatomical elements proximal to the site of blockage, since myelography is performed by injecting contrast distally into the lumbar thecal sac. A proximal injection of contrast dye into the thecal sac would then be required, typically at C1–2, which would carry its own set of risks. The presence of two separate spinal blocks would make it impossible, even with proximal and distal dye injections, to evaluate the intervening spinal segments.
SPINAL COMPUTED TOMOGRAPHY
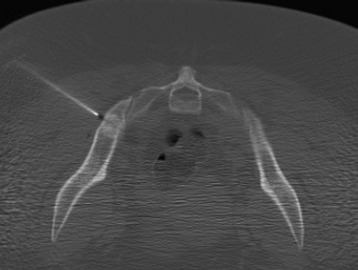
Metastatic lesions cause lytic destruction of bone and result in alteration of the bony architecture of the spinal elements that may or may not affect spinal stability. Plain radiographs may yield valuable information in the visualization of lucencies and sclerotic margins of large metastatic lesions, but they are not good for the evaluation of small metastases because 30–50% of bone destruction is required before the lesions become visible (compare Figs. 12-6 and 12-8). A full determination of spinal stability requires both plain radiographs and CT of the spine. Patients with invasive metastatic spinal disease on plain radiographs or MRI, with a large tumor load invading the vertebral body, facets, or posterior elements, require spinal CT to fully evaluate the bony architecture and the integrity of bony spinal elements (Fig. 12-9). An important component in the evaluation of spinal stability is the assessment of spinal alignment, which is limited with CT and MRI because only positions that do not permit weight-bearing are usually allowed. This is unlike plain radiography, during which patients can be placed in multiple positions, including upright, to maximally subject the spine to axial weight-bearing. Positional flexibility also permits radiographs with the patient in flexion, neutral, and extension positions to evaluate spinal alignment with movement.
SPINE MAGNETIC RESONANCE IMAGING
For the spine surgeon, evaluation of neural compression is critical in defining the role of surgical intervention. Tumor expansion and invasion into bone with the potential for compression of neural elements can be evaluated only with good visualization of the spinal column, cord, roots, and metastases so that an understanding of the anatomical interrelationships of these structures is obtained. CT imaging may be inadequate for this purpose, whereas MRI of soft tissues, including spinal metastases, is superior. In comparison to myelography, MRI is better at delineating soft tissue and more clearly distinguishes between anterior vs. posterior compression. Because MRI is not invasive, it avoids the risks of myelography. MRI is thus the gold standard in the evaluation of spinal neoplasm, with sensitivity and specificity well exceeding 90%.59 This sensitivity is even better than that of bone scan56 and has facilitated the earlier diagnosis of spinal metastasis in comparison with other modalities.60 The ability to evaluate the entire spine in multiple orthogonal views has greatly increased the capacity to adequately detect small metastatic lesions over multiple spinal levels.61,62 This leads to improved treatment with modalities such as radiation therapy, providing maximal therapeutic benefit for the patient with metastatic disease of the spine. The main disadvantage of MRI is increased acquisition time, which leads to greater expense, patient claustrophobia, and motion artifact in the thoracic spine caused by respiratory chest wall movement and cardiac motion.
MRI evaluation of patients with metastases of the spine reveals four distinct patterns of disease. The most commonly seen pattern is lytic and multifocal.38 With this pattern, tumors are bright on T2 and dark on T1. The second pattern is multifocal and sclerotic, and these lesions are dark on both T1 and T2. Diffuse lesions tend to be bright on T2 and dark on T1, like lytic lesions, but lack focality. These lesions may be either homogeneous or heterogeneous in signal intensity.63 With the application of gadolinium, there is inconsistency in the enhancement properties of spinal metastases. Some lesions enhance whereas others may not, and even lesions in the same patient may enhance dissimilarly. Some lesions become iso-intense with enhancement, so comparison with precontrast images and evaluation of other sequences, such as fat suppression, is useful.38 On MRI, pathological metastatic fractures can be distinguished from osteoporotic compression fractures, with the former brighter on T2 and darker on T1 and the latter iso-intense with normal vertebral marrow.64
BONE SCAN
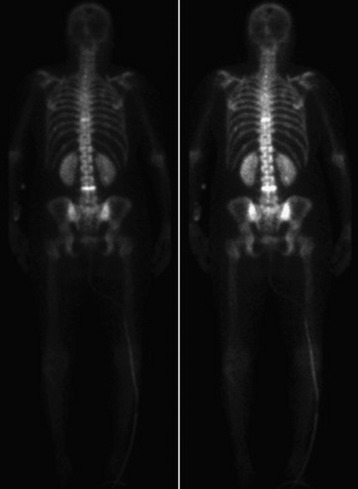
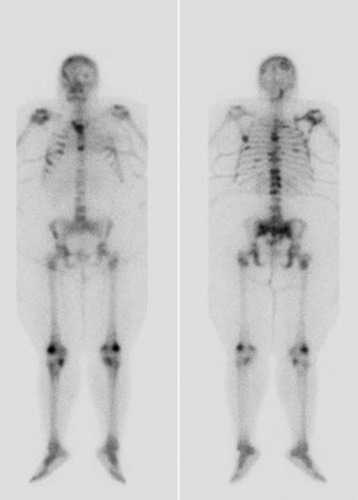
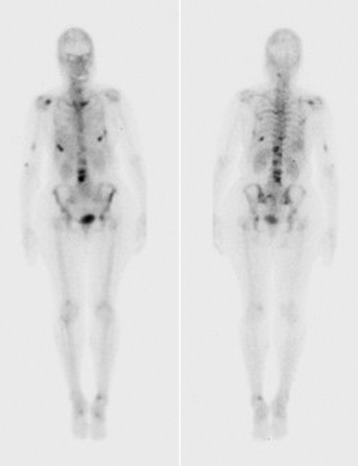
Technetium-99 bone scan is performed frequently to either detect or follow the course of metastatic disease. Radionuclide build-up in metastases occurs by two mechanisms. Immature osteoid formation occurs as the reparative host response to weakening of bone caused by the destructive metastatic process (Fig. 12-10), similar to the formation of callus around a fracture.65 In addition, intramembranous ossification occurs in tumors with large fibrous stroma.66 Unfortunately, detecting osteoblastic activity is non-specific. The reaction of the body to a destructive process may be caused by metastatic disease, but it also may be caused by trauma, infection, and degenerative disease.38 Bone scans also are poor in resolution, with no ability to evaluate bony architecture or spinal canal compromise.
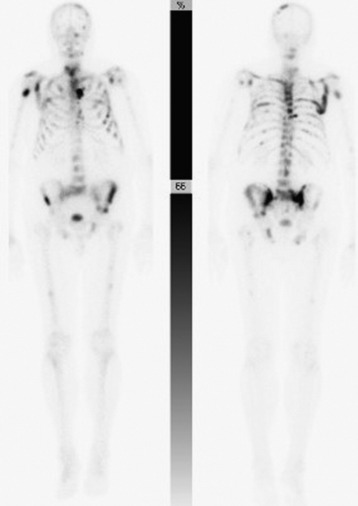
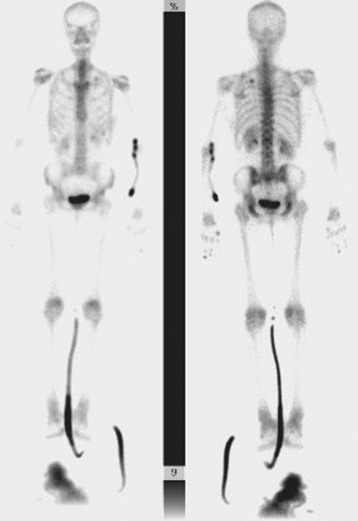
The main benefit of bone scanning is sensitivity. Bone scans are extremely sensitive to increases in local metabolic activity, and visualization of lesions may occur more than 1 year before detection with plain radiographs. In comparison with the 30–50% loss of mineralization required for radiographic detection, only a 5–10% change in lesion area is necessary for detection with bone scan.56 Bone scans also are useful in the detection of multiple lesions, especially when they are small (Figs. 12-11 to 12-13). Detection with bone scan depends, however, on the ability of the host to mount a reaction. This is difficult in very aggressive metastatic lesions, such as multiple myeloma (Fig. 12-14), renal and lung tumors, and certain sarcomas, and these lesions can thus be missed.56
SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY SCAN
Single photon emission computed tomographic (SPECT) images can be helpful in the determination between benign primary bone processes and malignant metastatic tumor.67 Lesions with uptake solely in the body are usually benign, whereas lesions with uptake in both the body and the pedicle usually are metastatic. Lesions with uptake in both the vertebral body and the posterior elements, without uptake in the intervening pedicle, also usually are benign.
SPINE ANGIOGRAPHY
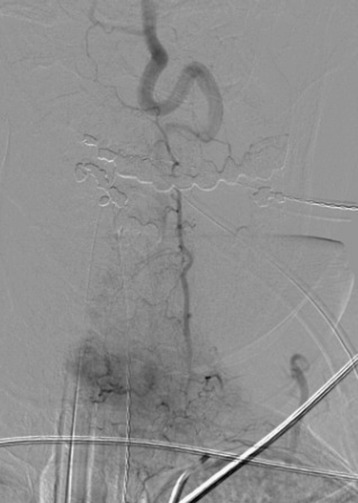
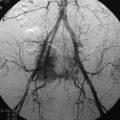
For tumors that are vascular, such as renal cell or thyroid carcinomas, operative intervention may be accompanied by substantial blood loss.68 When there is high suspicion that a metastatic spine mass is highly vascular, then angiography for visualization of the lesion’s blood supply is indicated to assist with surgical planning (Fig. 12-15). With angiography, embolization is often performed to decrease blood supply and facilitate lesion resection.69,70
DIAGNOSTIC BIOPSY
Diagnostic biopsies are performed to evaluate a suspicious lesion in the absence of a previous primary lesion or to obtain tissue to confirm a metastatic diagnosis before the initiation of treatment in a patient with a known primary tumor. A tentative diagnosis established with the discovery of a primary lesion through a metastatic work-up requires confirmation by biopsy. One or more metastatic lesions may be more accessible than the primary lesion and thus provide a less morbid means to obtain tissue when surgical decompression is not necessary (Fig. 12-16). Percutaneous biopsy was first described by Coley in 1931. Since that time, new CT-guided techniques now facilitate needle placement. Percutaneous biopsy is efficient and effective in establishing or excluding a metastatic diagnosis. Success rates in obtaining a diagnosis vary from different reports, with some approaching 95% in the diagnosis of metastatic lesions.71
Biopsy material should routinely be sent for culture, and a frozen section should be sent to ensure obtaining diagnostic tissue. The biopsy of osteoblastic lesions is more challenging because of low success in obtaining diagnostic tissue, so larger needles, more samples, and biopsy of soft tissue near the lesion are recommended. Extra samples also should be sent when the primary is unknown so that enough tissue is available for special studies and stains. Blood from the biopsy bed should be sent as clot, which is then smeared for tumor tissue that is not subject to the crush artifact of a trocar.72 When tissue cannot be obtained successfully, open biopsy may be required. Open biopsies should be undertaken with the potential for a more definitive surgical intervention in mind. The posterior vertebral elements may be sampled easily via a straight posterior approach. The vertebral body can be accessed by a transpedicular approach. The greater morbidity of an anterior thoracic, abdominal, or retroperitoneal approach precludes their use for the purpose of a biopsy alone. Needle or open biopsy allows oncologists to institute definitive treatment for the specific metastatic disease after pathological diagnosis and imaging for staging.
TREATMENT STRATIFICATION
Treatment of metastatic spinal disease depends on the degree of neurological compromise, the amount of bone destruction, when the diagnosis is determined, the patient’s prognosis, and the efficacy of radiation and chemotherapy. Patients with rapidly progressive neurological deficit who can retain a reasonable amount of neurological function with intervention may require acute surgical decompression. Even in the absence of acute neurological decompensation, evidence of spinal instability may require urgent, if not emergent, surgical stabilization. Other indications for surgery are severe pain attributable to mechanical causes, or reduction of tumor load to increase the efficacy of radiation or chemotherapy. Various authors have proposed stratification schemes to help guide the treatment of patients with metastatic spinal disease.73–75 One scheme divides patients into five categories based on the extent of neurological compromise and degree of bone destruction. Surgery is recommended for patients with severe neurological compromise or instability, with non-surgical therapy recommended for all others. Other schemes stratify patients based on general health and the extent and grade of their metastatic disease, and recommend aggressive surgical therapy for patients with less advanced metastatic disease and non-surgical palliation for those with higher-grade malignancies, widespread metastases, and greater tumor load.
1 Bailar JC3rd, Gornik HL. Cancer undefeated. N Engl J Med. 1997;336:1569-1574.
3 Fornasier VL, Horne JG. Metastases to the vertebral column. Cancer. 1975;36:590-594.
27 Lipper MH, Kishore PR. Intraspinal metastases from retinoblastoma. Radiology. 1979;131:161-163.
29 Hammerberg KW. Surgical treatment of metastatic spine disease. Spine. 1992;17:1148-1153.
38 Kamholtz R, Sze G. Current imaging in spinal metastatic disease. Semin Oncol. 1991;18:158-169.
48 O’Connor MI, Currier BL. Metastatic disease of the spine. Orthopedics. 1992;15:611-620.
52 Henneberry MO, Engel G, Grayhack JT. Acid phosphatase. Urol Clin North Am. 1979;6:629-641.
73 Harrington KD. Metastatic disease of the spine. J Bone Joint Surg Am. 1986;68:1110-1115.