Chapter 110 Metabolic Bone Disease
Beyond immediate surgical concerns, medical evaluation and treatment of the underlying metabolic bone disease is crucial to a successful long-term outcome. For many patients, the presenting symptom of previously unrecognized and untreated osteoporosis is a painful vertebral fragility fracture. Unfortunately, the majority of patients who have experienced a fracture do not receive appropriate treatment for osteoporosis. Approximately 50% of female patients who have sustained a compression fracture do not receive osteoporosis treatment.1 The rate of treatment after fracture is even lower for men. A recent retrospective study of 1171 men aged 65 or older demonstrated that only 7.1% of osteoporotic subjects and 16.1% of those with a hip or vertebral fracture received medication for osteoporosis.2 This represents a significant missed opportunity to reduce the risk of future fractures, particularly since the likelihood of refracture approaches 10% to 20% within 1 year of the initial fracture.
Osteoporosis
Definition
Osteoporosis is defined as “systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture.”3 Although the diagnosis of osteoporosis in asymptomatic individuals is typically based on bone density measurement alone, this definition emphasizes the important role of unmeasured ultrastructural abnormalities that contribute to the clinical end point of fracture. In fact, the incidence of hip fracture increases between the ages of 50 to 90 years seven times more than predicted on the basis of decline in bone density alone.4
Prevalence and Costs
The prevalence of osteoporosis increases with age in both men and women. It is estimated that currently about 10 million Americans older than 50 years of age have osteoporosis.5 This number is expected to increase to more than 14 million people in 2020.6 Though osteoporosis is commonly conceived of as a disease of women, more than 30% of people with osteoporosis are men. The clinical consequence of osteoporosis is fracture. The 2004 U.S. Surgeon General’s report on Bone Health and Osteoporosis concluded that osteoporosis results in approximately 1.5 million fragility fractures annually. Vertebral compression fractures are the most common, accounting for about 700,000 fractures per year. More than 50% of women and 30% of men will experience a vertebral compression in their lifetime. As many as 20% of people who suffer from a vertebral fragility fracture will experience another within 1 year.5
Beyond the personal suffering and functional impact, the economic burden to society of osteoporosis is considerable. The estimated cost of caring for the greater than 2 million osteoporotic fractures in 2005 was estimated to be $17 billion.6,7 These figures are expect to increase by 50% by 2025, when the annual fracture incidence will surpass 3 million and costs will exceed $25 billion. A significant portion of this anticipated increase is a result of the growing problem of osteoporosis in the Hispanic population.6
Pathophysiology
Peak bone mass is achieved by about age 30 years in both sexes. Differences in peak bone mass account for some of the variation in osteoporosis risk between men and women as well as between racial and ethnic groups. For example, African American women have higher bone densities than non-Hispanic women at all ages and are at lower risk for fractures of the spine and hip.8 Since achievement of genetically determined peak bone mass occurs primarily before the age of 20 years, osteoporosis in later life may, in part, be regarded as a pediatric disease with geriatric consequences.9 Juvenile calcium intake is positively associated with bone mass in the fourth decade of life.10
Age-related bone loss begins in the fourth decade and continues throughout life in men and women. By the eighth or ninth decade of life, women have lost approximately 35% of their cortical bone mass and 50% of their trabecular bone mass.11 Men lose about 60% as much during their lifetimes. Menopause in women is associated with a period of accelerated loss of trabecular bone that persists for about 10 years. Thereafter, bone loss from trabecular and cortical sites continues at a slower rate, similar to that of men. Skeletal sites that are predominantly trabecular in composition, including vertebral bodies and distal forearm bones, are therefore at greatest risk for earlier osteoporosis. Rates of Colles and vertebral fractures in women rise sharply after menopause.12
The major cause of primary age-related osteoporosis in both men and women is loss of gonadal function. In young adults, skeletal remodeling is an ongoing process with closely coupled bone resorption and formation. In estrogen-deficient women, bone resorption as assessed by biochemical markers increases by 90%, while bone formation markers increase only 45%, reflecting an imbalance between bone formation and resorption with net bone loss.12 Estrogen has multiple effects on both osteoclast and osteoblast function. Estrogen suppresses osteoclast development by suppressing RANKL production as well as regulating production of osteolytic cytokines including interleukin-1, interleukin-6, tumor necrosis factor alpha, and prostaglandins.12,13 Estrogen plays a role in bone formation by stimulating production of growth factors by osteoblasts.14 The principal risk factors for primary osteoporosis in women are related to estrogen deficiency: postmenopausal status; nulliparity; late menarche; early menopause (before age 45), either natural or surgical; and secondary amenorrhea related to exercise or eating disorders. As in women, estrogen plays a critical and dominant role in maintaining bone density in men. While androgens are important determinants of muscle mass in males, serum estradiol levels are more predictive of bone density. Peripheral aromatization of androgens to estrogen is important in maintaining estradiol levels above the threshold required to maintain skeletal homeostasis.2
In older men and women, other factors contribute to age-related bone loss, including physiologic hyperparathyroidism, vitamin D deficiency, and secretion of various bone-resorbing cytokines.7,12 Recent studies suggest a remarkably high level of vitamin D deficiency among older adults. In a recent study, more than 50% of older North American women currently treated for osteoporosis were found to have suboptimal vitamin D levels.15 In the patients with fractures or falls, the prevalence of vitamin D deficiency exceeds 90%.16 In most studies of older adults, the prevalence of low serum vitamin D levels is unrelated to gender, race, latitude, or global location.17
Risk Assessment
In addition to the changes in bone density related to decline in gonadal function in both men and women, multiple lifestyle factors, medical disorders, and drugs may exacerbate or accelerate “age-related” bone loss (Box 110-1).18 Approximately 50% of men with osteoporosis have underlying “secondary” causes, and as many as one third of women with osteoporosis are found to have other conditions beyond estrogen deficiency.2,19,20 In men with osteoporosis, the most common secondary causes are hypogonadism, glucocorticoid use, and alcoholism. In women, secondary causes are more common in perimenopausal women and include glucocorticoid use, thyroid hormone excess, hypoestrogenemia, and anticonvulsant treatment.18
Measurements of bone mineral density alone are insensitive as predictors of risk of clinical fragility fractures. More than 90% of these fractures occur in individuals who do not have osteoporosis as defined by bone density measurement criteria.21 Incorporating assessment of clinical risk factors for bone loss and fracture risk into predictive models for fragility fracture, with or without bone mineral density measurement, greatly enhances the assessment of fracture risk in both men and women.22 The World Health Organization (WHO) has developed such a fracture risk assessment tool (FRAX), which incorporates multiple risk factors including body mass index, personal history of previous fracture, history of parental hip fracture, current smoking, history of long-term glucocorticoid use, rheumatoid arthritis, and daily alcohol consumption of three or more units.23 The tool also includes the presence or absence of other secondary causes of osteoporosis, including hypogonadism, inflammatory bowel disease, prolonged immobility, organ transplantation, type 1 diabetes, and thyroid disorders. FRAX is available to clinicians online (www.shef.ac.uk/FRAX) and provides estimates of the 10-year probability of hip fracture and major osteoporotic fracture.
Morbidity and Mortality
Osteoporosis is similar to hypertension as a disorder with a long asymptomatic interval before resulting in clinical manifestation. If unrecognized and untreated in its preclinical phase, osteoporosis may result in significant morbidity and mortality. Fragility fractures are the single most morbid and clinically significant consequence of osteoporosis, occurring most frequently in the vertebral body, proximal femur, and distal radius.5
The presence of a vertebral fracture, even if asymptomatic, increases the risk of future vertebral fracture fivefold and doubles the risk of hip fracture.1 In addition, these patients may experience chronic low back pain, loss of height, and kyphosis, leading to symptomatic biomechanical changes in the spine.7 Pulmonary compromise may become evident as a result of the kyphosis and compression fractures manifested by restrictive lung disease with decreased vital capacity.24 On average, each thoracic vertebral fracture reduces pulmonary vital capacity by 9%.24,25 In addition, shortening of the thoracic spine may result in compression of the abdominal contents, resulting in symptoms of early satiety and bloating. This may result in anorexia and weight loss, which is a great concern in a population of individuals who are already frail.
Patients with compression fractures also experience lower levels of functional performance compared with controls, including difficulty with performance of activities of daily living. These patients may become more sedentary as a result, with progressive deconditioning and further bone loss. The constellation of the preceding symptoms, as well as low self-esteem due to body image changes, may result in depression in up to 40% of individuals with osteoporosis. Patients who are at greater likelihood of developing depression are those with more than one compression fracture, those who are older, and those who are more socially isolated.24
Patients with a compression fracture were found to have a 23% higher age-adjusted mortality rate in a recent prospective cohort study of almost 10,000 women age 65 years or older.26 The mortality rate is even greater in patients with hip fractures, reaching 20% more than age-matched controls in the first year after hip fracture.7 Increased mortality after hip fracture is usually due to coexisting illness or deep venous thrombosis with pulmonary embolism from the relative immobilization associated with the fracture.27 Patients who survive the hip fracture often have limited ability to perform activities of daily living and require prolonged institutional care. In individuals over 75 years of age, hip fracture mortality is greater in men than in women: 20.7% and 7.5%, respectively.2,6
Diagnosis of Osteoporosis
A painful fracture is the most obvious clinical consequence of osteoporosis, but as many as 70% of osteoporotic spine fractures are asymptomatic.28 While a clinical diagnosis of osteoporosis may be made in the presence of a fragility fracture in the absence of bone mineral density measurement, low bone mass is recognized as a more sensitive diagnostic parameter in the absence of symptoms.29 Low bone mass is also a strong predictor of future fracture risk. The WHO has defined osteoporosis and osteopenia on the basis of bone mineral density (BMD) (Table 110-1). A T score is defined as the number of standard deviations above or below the average BMD for healthy young white females. The Z score is defined as the number of standard deviations above or below the average BMD for age- and sex-matched controls. Osteoporosis is present when the T score is at least −2.5. Severe osteoporosis is defined as a T score of at least −2.5 in the presence of one or more fragility fractures. Z scores are used preferentially to assess bone loss in premenopausal women and males younger than 50 years of age. A low Z score (<−2.0) represents bone loss in excess of age-expected loss and suggests that secondary causes of bone loss may be present.18,30 The WHO thresholds were chosen on the basis of fracture risk in postmenopausal Caucasian women. Similar diagnostic threshold values for men are less well defined. However, several studies have demonstrated that the age-adjusted fracture risk for any given BMD is similar in men and women.31 The International Society for Clinical Densitometry (ISCD) advises that the WHO criteria be used in postmenopausal women and in men age 50 and older but not in premenopausal women or men less than 50 years old, as the fracture risk is not the same in younger men and women.
TABLE 110-1 Osteoporosis and Osteopenia: World Health Organization Criteria
| Classification | Criteria |
|---|---|
| Normal | BMD up to ±1 SD of the main of the young adult reference range |
| Osteopenia | BMD between 1 and 2.5 SD below the main of the young adult reference range |
| Osteoporosis | BMD greater than 2.5 SD below the mean of the young adult reference range |
| Severe osteoporosis | BMD greater than 2.5 SD below the mean of the young adult reference range in the presence of one or more insufficiency fractures |
BMD, bone mineral density; SD, standard deviation.
Several technologies are available to measure bone mass, including forearm single-photon absorptiometry, spine and hip dual-photon absorptiometry, and quantitative ultrasound of the calacaneus. While multiple studies have shown that these various measurement techniques may predict osteoporotic risk,32–37 the gold standard remains dual-energy x-ray absorptiometry (DEXA) of the hip and spine for the diagnosis of osteoporosis.8,18,30 DEXA is considered the gold standard because it has been shown to be precise (1%–2%) and to have acceptable accuracy and good reproducibility. It is also the most extensively validated test for fracture outcomes.29,30 Other advantages of DEXA include relatively low radiation exposure, wide availability, and the capacity to measure bone density at multiple skeletal sites. The ISCD recommends obtaining BMD measurements of the spine and hip. Numerous studies have shown that BMD measured at the femur (neck or total hip) is the best for predicting hip fracture risk.3,30,31,38 Spinal BMD is the optimum for monitoring response to treatment. The risk of hip fracture is increased 2.6 times for each standard deviation decrease at the femoral neck.31,39 Serial BMD measurements should be performed on the same machine for the same patient, owing to variability of BMD assessment between machines of different manufacturers.
Most clinical guidelines recommend screening healthy women for osteoporosis at age 65 and testing higher-risk women earlier. The ISCD recommends screening men without risk factors for osteoporosis at age 70 and testing higher-risk men earlier. Risk factors are as noted previously. Another indication for screening is radiographic evidence of osteopenia or vertebral fracture.30 In addition, screening is recommended for people who have diseases associated with bone loss, such as rheumatoid arthritis, as well as people initiating long-term corticosteroid therapy. Current evidence does not support routine screening of all perimenopausal women, as its value in directing preventive therapy against future fractures has not been established8 (Box 110-2).
BOX 110-2 Osteoporosis Screening Guidelines
• Women age 65 and older, men age 70 and older (regardless of clinical risk factors)
• Younger postmenopausal women and men ages 50 to 69 for whom there may be concern based on their clinical profile
• Women in menopausal transition who may have a risk factor for increased fracture (low body weight, high-risk medication, or prior low trauma fracture)
• Adults who sustain a fracture after age 50
• Adults taking glucocorticoids in a daily dose of ≥5 mg or equivalent for 3 months or more
• Any person being considered for pharmacologic therapy for osteoporosis
• Anyone being treated with osteoporosis to monitor effect (generally every 2 years)
• Anyone not receiving therapy in whom evidence of bone loss would lead to treatment
Bone turnover markers reflecting bone formation and resorptive activity are not recommended for routine diagnostic purposes, as they have not been found to predict bone mass or fracture risk. Indices of bone formation include alkaline phosphatase and osteocalcin. Markers for resorption include serum and urine levels of type I collagen C- and N-telopeptides. While not reliable diagnostically, they have been found helpful in clinical trials in understanding the mechanism of bone loss. Bone markers may also be helpful for monitoring response to therapy and compliance.8
Evaluation of patients with osteoporosis should include a thorough history and physical examination, as most of the secondary causes of osteoporosis can be excluded with a careful history and physical examination. A minimum screening laboratory profile should be considered for all patients who have been diagnosed with osteoporosis. This is particularly important in men, as 30% to 60% will have an identifiable secondary cause. Approximately 50% of perimenopausal women with osteoporosis also have a secondary cause, including hypoestrogenemia, glucocorticoid usage, thyroid hormone excess, and anticonvulsant therapy.18 As was mentioned previously, patients with an abnormal Z score should also be studied more aggressively for secondary causes. Initial general screening should include a complete blood count, erythrocyte sedimentation rate, serum calcium, serum 25 hydroxyvitamin D, phosphorus, alkaline phosphatase, creatinine, aspartate aminotransferase, thyroid-stimulating hormone (TSH), and serum protein electrophoresis. Tannenbaum et al. looked at the yield of laboratory testing to identify secondary causes of osteoporosis in otherwise healthy women. Their findings suggest that a basic screen of serum calcium, serum parathyroid hormone (PTH), and 24-hour urinary calcium excretion in all patients, and a serum TSH in patients on thyroid replacement, provides a high diagnostic yield (86% in their study) at a low cost (mean cost of $75/patient).40 In male patients, serum testosterone should be obtained. Additional studies such as 24-hour urinary calcium, PTH, and serum immunoelectrophoresis should be obtained selectively on the basis of risk factors and preliminary studies (Table 110-2).
TABLE 110-2 Screening Laboratory Tests
| Test | Purpose |
|---|---|
| CBC | Evaluate for bone marrow malignancy, infiltrative process, or malabsorption |
| Serum calcium | Decreased in those with malabsorption or vitamin D deficiency, increased in hyperparathyroidism |
| Liver function | Evaluate for intrinsic liver abnormality |
| Alkaline phosphatase | Increased in acute fractures, prolonged immobilization, and Paget disease of the bone |
| TSH | Screen for hyperthyroidism |
| ESR | May indicate an inflammatory process or monoclonal gammopathy (associated with bone loss) |
| Serum 25-hydroxyvitamin D | Evaluate for vitamin D deficiency |
| Serum calcium | Decreased in those with malabsorption or vitamin D deficiency, increased in hyperparathyroidism |
| Serum phosphorus | Decreased in patients with osteomalacia |
| PTH | Screening for hyperparathyroidism |
| Creatinine | Renal failure is associated with secondary hyperparathyroidism |
| Serum testosterone | In all men to screen for hypogonadism |
| Serum estradiol | Screening for hypogonadism in premenopausal or perimenopausal women |
| Urinary calcium excretion | 24-hour urinary excretion on a high-calcium-intake diet screens for malabsorption and hypercalciuria |
| SPEP/UPEP | If monoclonal gammopathy is suspected |
CBC, complete blood count; ESR, erythrocyte sedimentation rate; PTH, parathyroid hormone; SPEP/UPEP, serum protein electrophoresis/urine protein electrophoresis; TSH, thyroid-stimulating hormone.
Prevention of Osteoporosis
Because currently available treatments for established osteoporosis reduce fracture rates by 50% to 60% at best and restore only a small portion of skeletal bone, prevention of osteoporosis remains the ideal objective in maintaining skeletal health. Optimizing peak adult bone mass is crucial, as low peak adult bone mass is a major risk factor for subsequent development of osteoporosis. Although as much as 75% of peak adult bone mass is genetically determined, nutrition and physical activity play important roles in optimizing bone mass from infancy to adulthood.41
Hormone Replacement Therapy
Until the Women’s Health Initiative studies were reported in 2002 through 2004, perimenopausal women were typically considered for hormone replacement therapy to preserve bone and prevent the steep escalation in bone loss in the early postmenopausal years.42–44 Although this trial demonstrated a 34% reduction in hip and vertebral fractures in postmenopausal women treated with conjugated estrogens or estrogens plus progestin, other health risks, including coronary artery disease, stroke, and venous thromboembolism, exceeded benefits. Because of the unfavorable risk-to-benefit ratio and the availability of other effective nonhormonal drugs, hormone replacement therapy is not recommended for prevention of osteoporosis in women without vasomotor or other menopausal symptoms requiring treatment.
Nutrition
Lifelong adequate intake of calcium and vitamin D is essential to achieving peak bone mass and prevention of osteoporosis. Calcium supplementation has been shown to have a positive effect on accrual of bone mass throughout childhood and adolescence.45 Calcium supplementation has been shown to retard bone loss in postmenopausal women.46 For children ages 3 to 8 years, 800 mg of calcium per day is recommended.8 After age 8 and through adult life, most guidelines recommend a daily intake of 1200 to 1500 mg of calcium from both dietary and supplemental sources.8,47 Unfortunately, more than 50% of adolescents and young adults do not ingest sufficient dairy products to achieve dietary calcium requirements.8
Vitamin D plays an important role in optimal calcium absorption. Vitamin D adequacy has been defined as the level of vitamin D necessary to achieve maximal suppression of PTH, while avoiding the negative skeletal effects of secondary hyperparathyroidism.45 In adolescents, vitamin D levels correlate with bone mineral content, and the vitamin probably plays a crucial role in achieving peak bone mass.48 In older adults, a dose-response relationship between vitamin D and fracture reduction has been demonstrated, with a 20% reduction in hip fracture risk in individuals taking higher doses.49 As with calcium, the decrease in dairy product consumption, particularly of vitamin D enriched or fortified milk products, contributes to inadequate consumption of this vitamin. For adults, the National Osteoporosis Foundation recommends an intake of 800 to 1000 international units (IU) of vitamin D per day. The best measure of vitamin D status is the serum level of 25(OH)D. A 25(OH)Dlevel of greater than 32 ng/mL is the target that is considered optimal to achieve full suppression of PTH for osteoporosis prevention. The requirement for other micronutrients that are important in skeletal health, including magnesium, fluoride, vitamin C, vitamin K, and potassium, are easily met by a healthy diet that includes five servings daily of fruits and vegetables.45
Exercise
In prepubertal and peripubertal children, active weight-bearing exercise has been demonstrated to increase bone mineral density as measured by DEXA or calcaneal ultrasound.50,51 In premenopausal women, total weight-bearing physical activity correlates with bone density, the strongest association with physical activity being during early age periods.52 These studies suggest that early active, weight-bearing exercise is important in achieving peak adult bone mass. There is little evidence that exercise in midlife significantly increases BMD, however.8 In older patients, weight-bearing exercise slows bone loss but has not been shown to decrease fracture risk.53 However, regular exercise in older patients has been shown to reduce the risk of falls by about 25%, potentially reducing the risk of osteoporotic fracture.54 The National Osteoporosis Foundation strongly endorses lifelong physical activity at all ages, including weight-bearing exercise and muscle-strengthening exercise.
Who Should Be Treated?
Because osteoporosis, like hypertension, is a silent disease until it manifests clinically as fracture, screening for asymptomatic disease in high-risk individuals represents an initial step before treatment. BMD measurement by DEXA represents the screening tool of choice. As was noted earlier, the WHO has defined osteopenia and osteoporosis on the basis of this measurement. However, though fracture rates are highest in individuals with osteoporosis as defined by these criteria, more than 80% of postmenopausal women with fractures have T scores better than −2.5.55 Most fractures occur in patients with BMD in the range defined as osteopenia. However, pharmacologic treatment of women with osteopenia defined by BMD in the absence of other risk factors is not cost effective.56 Fracture risk depends not only on BMD values but also on other independent variables, including body mass index, age, history of prior fracture, parental history of hip fracture, glucocorticoid use, tendency to fall, poor mobility, and other secondary factors.57,58 The FRAX tool described previously represents one attempt to predict fracture risk by using a model that incorporates BMD and clinical risk factors.31 Although biochemical markers of bone remodeling have been shown to identify perimenopausal women who are at increased risk for rapid bone loss, the role of markers in predicting fracture risk and determining who should be treated is uncertain.59
Current guidelines recommend that pharmacologic treatment should be offered to men and women who have known osteoporosis and to those who have experienced fragility fractures.47,60 Both the American College of Physicians and the National Osteoporosis Foundation recommend that clinicians consider pharmacologic treatment for men and women who are at increased risk for developing osteoporosis based on a analysis of risk factors. The National Osteoporosis Foundation specifically defines this population with a 10-year probability of a hip fracture greater than 3% or a 10-year risk of a major osteoporotic fracture greater than 20% based on the FRAX calculator.
Pharmacologic Therapy
Pharmacologic therapy for the treatment of osteoporosis can be classified as antiresorptive or anabolic (Table 110-3).
Antiresorptive agents work by inhibiting osteoclast activity, therefore reducing bone resorption. The current available antiresorptives include bisphosphonates, selective estrogen-receptor modulators, and calcitonin.
The bisphosphonates alendronate and risedronate are approved for both the treatment and prevention of osteoporosis. Both alendronate and risedronate have been shown to reduce vertebral and nonvertebral fragility fractures by 50%.47,61–64 Both drugs have also been shown to be effective in the treatment of glucocorticoid-induced osteoporosis.65,66 Another bisphosphonate, ibandronate, which has been approved for the treatment of osteoporosis, has also been shown to reduce the incidence of vertebral fracture by about 50%, but reduction in hip fracture risk remains unproven. An intravenous bisphosphonate, zoledronic acid, has been demonstrated to decrease the incidence of vertebral fractures by 70%, hip fracture by 41%, and nonvertebral fractures by 25% over 3 years in a recent double-blind, placebo-controlled trial of 3889 postmenopausal women with osteoporosis.67 Zoledronic acid is also indicated for the prevention of new clinical fractures in patients who have recently sustained a hip fracture, as it has been shown in a recent study to decrease new clinical fracture and death.68,47
The most common adverse event of bisphosphonates is esophagitis. A higher risk of developing atrial fibrillation with IV zoledronic acid was noted when compared to placebo (1.3% vs. 0.5%); the atrial fibrillation occurred more than 30 days after infusion in most patients.67 The incidence of atrial fibrillation with the treatment of the other bisphosphonates is unclear, and no definitive association has been demonstrated. 47 Rarely, osteonecrosis of the jaw has been reported, mainly in cancer patients receiving high-dose intravenous bisphosphonates. In a recent review article, osteonecrosis of the jaw was rare in osteoporosis patients treated with bisphosphonates, with an estimated incidence of less than 1 case per 100,000; on the basis of the current data, there was insufficient evidence to confirm an association of osteonecrosis of the jaw and low-dose bisphosphonate usage in the treatment of osteoporosis.69 There is currently no consensus on how long to continue bisphosphonate therapy. However, stopping therapy after 5 years for some women may be reasonable, because there appears to be residual benefit on BMD and fractures for 5 years.70
Calcitonin is an antiresorptive hormone for the treatment of osteoporosis in women who are at least 5 years postmenopausal. Calcitonin is administered nasally (200 IU) or subcutaneously. In a 5-year study of postmenopausal women, calcitonin reduced the vertebral fracture risk by 33% to 36% compared to placebo.71 Calcitonin did not decrease the risk of nonvertebral fractures compared to placebo. Unlike other antiresorptives, some patient may experience an analgesic effect from calcitonin that may be of benefit in the treatment of symptomatic vertebral compression fractures.72 This effect may be due to modulation of beta-endorphin levels.73
Raloxifene is an estrogen agonist/antagonist that acts as an estrogen agonist at the bone but an antagonist in uterine and breast tissue in postmenopausal women.47,74 It is approved for both the treatment and prevention of osteoporosis and to reduce the risk of invasive breast cancer in postmenopausal women with osteoporosis.47 Raloxifene has been shown to decrease vertebral fractures by 30% to 50% in postmenopausal women with osteoporosis. It has also been show to increase both spinal and hip BMD.47,75,76 Reduction of hip fracture risk has not been shown. Raloxifene is associated with increased risk of venous thromboembolism and does not decrease the risk of coronary heart disease. Hot flashes are also increased (6% over placebo).47
Teriparatide (recombinant human parathyroid hormone 1-34) is the only currently available anabolic agent that stimulates bone formation by stimulating osteoblasts more than osteoclasts. Administered subcutaneously daily, teriparatide has been shown to increase bone mass by 10% and to decrease vertebral and nonvertebral fracture risk by more than 50%.77 The recommended duration of treatment is a maximum of 2 years, and observational studies suggest benefit for at least 18 months after discontinuation.78 It is common practice to follow teriparatide treatment with an antiresorptive agent, usually a bisphosphonate, to maintain or further increase BMD.79 Several studies have also compared teriparatide with bisphosphonates, and McClung et al. found that 20 μg/day of teriparatide resulted in significantly greater increases in lumbar spine BMD (10.3%) compared to 10 mg/day of alendronate (5.5%).80 Common side effects seen in clinical trials include hypercalcemia, leg cramps, nausea, and dizziness.79 The most serious concern is the risk of osteogenic sarcoma, as rat studies found a dose-dependent increase in teriparatide-treated animals.81 The risk in humans is felt to be small. Patients with an increased risk of osteosarcoma (i.e., Paget disease of the bone); a prior history of radiation therapy of the skeleton, bony metastasis, or hypercalcemia; or a history of a skeletal malignancy should not be treated with teriparatide. This agent should be reserved for patients who are at high risk for fracture or who are unresponsive to or intolerant of antiresorptive drugs.
There is no consensus on the optimal approach for monitoring therapy. The National Osteoporosis Foundation recommends that repeat BMD assessments be performed every 2 years, which is in accordance with Medicare guidelines but recognizes that testing more frequently may be warranted in certain clinical situations.47 Poor compliance and persistence with long-term treatment are major barriers in the management of osteoporosis. Measurement of bone markers has been shown to help overcome such barriers. Suppression of biochemical markers of bone turnover after 3 to 6 months of antiresorptive therapies has been demonstrated.
Osteomalacia
Osteomalacia is a metabolic bone disorder characterized by deficient mineralization of newly formed matrix or osteoid. Overall, there may be decreased, normal, or even increased bone mass but with decreased mechanical strength. The diminished deposition of mineral is the result of disorders that lead to a low calcium-phosphate product. Because osteomalacia is commonly asymptomatic and often coexists with osteoporosis in the elderly, its prevalence is difficult to measure. Estimates range from 1% based on unselected autopsy cases to as high as 18% in elderly nursing home patients.82 Osteomalacia in growing children before fusion of the epiphyseal growth plates is referred to as rickets.
Pathophysiology
The most common cause of osteomalacia in the United States is vitamin D deficiency. Vitamin D plays a central role in calcium and phosphate homeostasis. The two primary sources of vitamin D are dietary and endogenous skin synthesis with exposure to ultraviolet light.83 If serum calcium falls, parathyroid hormone levels rise and stimulate renal synthesis of 1,25(OH)2D, the most active form of the vitamin. Vitamin D increases intestinal calcium and phosphate absorption, calcium and phosphate release from the skeleton, and renal calcium and phosphate reabsorption. A negative feedback loop exists as 1,25(OH)2D also stimulates osteocyte production of fibroblast growth factor 23, which acts on the kidney to increase phosphate excretion and decrease production of 1,25(OH)2D.84 This integrated metabolic system functions to maintain serum ionized calcium and phosphate at optimal levels for bone mineralization. In contrast, when vitamin D levels are decreased, intestinal calcium absorption is reduced by as much as 45% to 65% of normal. Absorption of dietary phosphate is also impaired. This results in a reduced calcium-phosphate product at the bone mineralization front, leading to osteomalacia.
Diagnosis
When symptomatic, osteomalacia presents with diffuse bone pain and tenderness as well as proximal muscle weakness.82 The lumbar spine, pelvis, and lower extremities are the most common sites of involvement, which is usually bilateral. Pain is typically worse with weight bearing and diminished by rest. The bone pain is due, at least in part, to insufficiency fractures in these areas. Looser’s zones are thin radiolucent lines oriented at right angles to the long axis of the bone and are typically located above the lesser trochanters, pubic rami, ribs, and axillary border of the scapula and along the shaft of long bones. Looser’s zones represent insufficiency fractures in inadequately mineralized bone. A technetium-99 bone scan demonstrates increased uptake in Looser’s zones. The most common radiographic abnormality in patients with osteomalacia is osteopenia. Since bone mineralization is deficient in osteomalacia, bone mineral density measurements such as DEXA are reduced, risking misdiagnosis of osteomalacia as osteoporosis.85 Laboratory findings in osteomalacia vary by etiology (Table 110-4). In vitamin D–deficient states, for example, alkaline phosphatase is elevated and serum calcium is decreased. The best serologic marker to assess a patient’s vitamin D status is the 25-hydroxyvitamin D metabolite. The lower end of the normal range of this metabolite is 25 ng/dL. In osteomalacia, serum 25(OH)D levels are often less than 10 ng/dL.82
In rare, atypical cases, particularly when osteomalacia is suspected in patients with coexisting osteoporosis, a transiliac tetracycline-labeled bone biopsy may be indicated. Bone biopsy may also be required when a patient fails to respond as expected to appropriate treatment. In osteomalacia, the ratio of mineral to organic matrix is low. Bone histology provides the only certain method of proving the diagnosis of osteomalacia.86
Treatment
The objectives of treatment include relieving pain, strengthening bone by promoting mineralization, and correcting secondary hyperparathyroidism. Vitamin D–deficient patients may require initial supplementation with 50,000 IU of vitamin D2 until levels are normal, with continued maintenance of 800 to 1000 IU daily.82 Higher doses of vitamin D may be required in patients with osteomalacia and chronic renal failure or defective 25 hydroxylation of vitamin D due to anticonvulsant therapy.
Paget Disease of the Bone
Definition, Epidemiology, and Pathophysiology
Paget disease of the bone (PDB), also known as osteitis deformans, was first described in 1876 by Sir James Paget. It is a chronic skeletal disorder that is characterized by focal areas of excessive osteoclastic resorption along with increased and disorganized osteoblastic activity resulting in abnormal bone formation, which results in a “mosaic” pattern of lamellar bone. The osteoclasts are increased in size and number and have more nuclei than is normal.87 The newly laid bone is associated with hypertrophy, sclerosis, and increased vascularity.88,89 The bone is architecturally abnormal, mechanically weaker, and more susceptible to various stresses than normal bone,89 resulting in skeletal deformity and an increased tendency for pathologic fracture.90 PDB may involve a single bone (monostotic) or multiple bones (polyostotic). The axial skeleton is preferentially affected, and common sites of involvement include the pelvis (70%), femur (55%), lumbar spine (53%), skull (42%), and tibia (32%).87
The incidence of PDB has been estimated at 3% in people older than 50 years in North America and Europe.91 The disease seldom appears before the age of 40. The prevalence increases with age and has been reported to double with each decade beyond 50, so more than 10% of people over the age of 90 have the disease.92 The disease is slightly more prevalent in men than in women and is more common in white than nonwhite individuals.93,94 The marked geographic variance in the prevalence of PDB of the bone suggests genetic or environmental factors in causation. Fifteen percent of patients report a family history,95 and children or siblings of affected patients have a sevenfold higher chance of developing PDB.87,96 Recent epidemiologic data suggest declining rates of severity and prevalence of the condition in the last 30 years, suggesting that this disorder has an environmental component.87,92,93
The etiology of PDB is unknown. Environmental and genetic factors may play a role. Four genetic mutations have been identified in selected patients with PDB. The most important appears to be sequestosome 1 (SQSTM1).87 Inclusions that resemble virus particles have been detected in the osteoclasts of pagetic bone; further studies have shown that these inclusions cross-react with antibodies against the measles virus and respiratory syncytial virus. These intranuclear particles are not specific for pagetic osteoclasts and have also been found in osteoclasts of osteoporotic bone.87,92
History and Clinical Presentation
The majority of cases of PDB are asymptomatic. The symptomatic cases tend to present with bone pain, and about 30% of the patients will note deep, aching bone pain that is persistent throughout the day and worse at night.92,97 Pain is often associated with bone deformities such as bending of the long bones and enlargement of the skull and a warm sensation of the affected bone due to increased blood flow. The gait abnormalities result in increases in spine and joint pain. Fractures are common and may be incomplete.87 Most fractures heal rapidly, although malunion is quite common.87,92 Secondary osteoarthritis is also common in PDB, and epidemiologic studies have shown hip and knee replacements to be more common in patients with PBD.87 Spine problems are common, with spinal stenosis and radiculopathies reported. Spinal cord lesions may result in paraparesis with loss of sphincter control and spasticity of the lower extremities.98
Hearing loss is also common, with deafness occurring in 50% of patients owing to temporal bone distortion or fixation of the stapes.98 Invagination of the skull may also result in vertebrobasilar insufficiency, hydrocephalus, cerebellar herniation, ataxia, or lower cranial nerve lesions. High-output cardiac failure can arise as a complication of active disease, owing to increased blood flow through the affected bone. This is proportional to the extent of the disease.87,89 Hypercalcemia can also occur, especially in patients who are immobilized.
Sarcomatous degeneration of a pagetic area is a rare complication of the disease, occurring in 0.2% of the cases, although the overall risk of sarcoma is 30 times greater in pagetic patients than in the general population.92 The 5-year survival rate is between 5% and 8%.99 Malignant transformation is suspected in any long-standing disease with constant and worsening bone pain or with involvement of a new skeletal segment.92 Sarcoma can affect any site but is most commonly found at the femur and pelvis and is rarely multifocal.85 In a recent study that analyzed 119 patients presenting with PBD over 35 years, 18 cases degenerated into sarcoma. Malignant transformation was not found in patients who were treated with bisphosphonates, suggesting a possible preventative effect.99
Diagnosis
Radiographic Findings
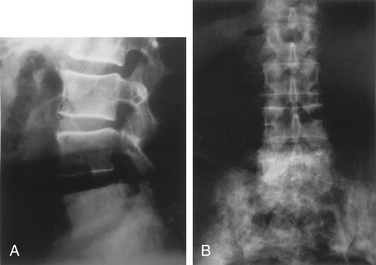
Radiographic changes of PBD have been described in three phases. The first, or osteolytic, phase is most common in the long bones. Osteolysis can also affect any portion of the bone, but when it affects the diaphysis, it presents as a wedge-shaped radiolucent area that is clearly demarcated from the other bone. This has been described as a “blade of grass” or “flame.” Osteolysis is followed by deposition of poorly mineralized osteoid with fibrosis. This finding is typical in the skull, where it is termed osteoporosis circumscripta. During the second or mixed phase, the skull develops a so-called cotton-wool appearance. In the late phase, increased bone density with trabecular and cortical thickening has been described92,98 (Fig. 110-1). The skull may become flattened and mottled. Characteristically, the bones in PDB demonstrate local enlargement and thickening. The increase in bone size is deceptive, as pagetic bone does not have the compressive strength and deforms with weight-bearing stress.
Radionuclide bone scanning is more sensitive than radiography for the detection of metabolically active lesions, which present as “hot spots.”85 Literature regarding the usage of MRI and CT in the evaluation of PDB is scarce. There is evidence that MRI may be helpful to better distinguish pagetic bone from sarcoma compared to radiography and CT.85
Laboratory Manifestations
Serum osteocalcin levels may be elevated but are inconsistent and less reliable. Other indices of bone resorption, including urinary excretion of hydroxyproline and pyridinium cross-links, may correlate with disease activity.89,92
Treatment
The majority of treatment agents are directed at reducing osteoclastic activity. Calcitonin was the first therapeutic agent available for PDB, but it is no longer considered a first-line agent. The bisphosphonates are considered first in the treatment of PDB, as they strongly inhibit bone resorption and result in longer remission when compared to calcitonin.92 Bisphosphonates are synthetic analogues of inorganic pyrophosphate that bind the surface of hydroxyapatite and are localized at the site of active bone formation. They decrease bone resorption and formation by decreasing osteoclastic activity and number.95,96,100 Randomized placebo-controlled trials have shown that bisphosphonate therapy reduces bone turnover, improves joint pain, promotes healing of osteolytic lesions, and restores normal bone histology in Paget disease with replacement of woven bone with lamellar bone. On the basis of these findings, it is postulated that bisphosphonate therapy may help decrease bone complications; however, adequately powered studies have not been undertaken to establish this. Bisphosphonates have also anecdotally been reported to be effective in improving paraplegia in isolated patients with Paget disease of the spine and improving spinal stenosis. In addition, second-generation bisphosphonates such as pamidronate, risedronate, and zoledronic acid are preferable to the older bisphosphonates such as etidronate for treatment of PDB, as they are more effective at reducing bone turnover.87 Alendronate (40 mg/day for 6 months) and risedronate (30 mg/day for 2 months) have shown normalization of total alkaline phosphatase in about 70% of patients with moderate to severe PDB after a course of treatment. Pamidronate is also useful in the treatment of active PDB, leading to normalization of total alkaline phosphatase about 50% of the time.92 In a recent randomized double-blind controlled 6-month trial, a single infusion of zoledronic acid (5 mg infused over 15 minutes) was compared to daily oral risedronate (30 mg/day for 60 days). Zoledronic acid was found to produce more rapid, more complete, and more sustained response in PDB than risedronate. Another study showed that a single intravenous dose of zoledronic acid led to favorable clinical, biochemical, and scintigraphic responses in patients with PDB starting as early as 3 months after treatment and lasting no less than 12 months.88
Patients receiving bisphosphonate therapy should receive 1000 to 1500 mg of calcium and 400 to 800 IU of vitamin Dby diet or supplementation as new bone formation occurs throughout the process of repair in pagetic bone.89 Focal osteomalacia has been reported in patients treated with etidronate and pamidronate despite supplementation with vitamin D and calcium.87
Measurements of alkaline phosphatase are used to assess the activity of PDB and monitor treatment to the bisphosphonates. Total alkaline phosphatase should be measured every 3 months for the first 6 months of therapy and every 6 months thereafter. Retreatment is indicated when there are persistent symptoms or biochemical relapse. Continuation of pain may be an indication for retreatment. In patients who are asymptomatic, retreatment should be based on biochemical markers of bone turnover.89
Surgical Considerations in Metabolic Bone Disease
Osteoporosis
Surgical Treatment of Osteoporotic Fractures
Most osteoporotic fractures will heal spontaneously and do not require surgical intervention.101 A portion of osteoporotic compression fractures are persistently painful and interfere with function.102 In recent years, many patients with such fractures have been treated with vertebral augmentation procedures such as vertebroplasty or kyphoplasty. The indications, techniques, and outcomes of these procedures are dealt with in Chapter 128.
A very small subset of osteoporotic fractures will result in a neurologic deficit, and surgery may be required to treat these fractures. The treatment goals for these fractures are to decompress the neural elements, to restore alignment, and to stabilize the fracture generally with rigid internal fixation. Ventral decompression via corpectomy and strut graft with fusion has been performed but has a relatively high failure rate of this construct.103,104 Anterior column reconstruction followed by dorsal instrumented fusion is an option, but this procedure is extensive and has a high rate of morbidity.105 Long-segment dorsal reconstruction with a dorsolateral corpectomy through a costotransversectomy or lateral extacavitary approaches are also options. This allows adequate decompression and reconstruction of the anterior column through a single incision. The anterior column is then reconstructed with autograft, allograft, or intervertebral body cages.106 The addition of expandable intervertebral body cages has made the anterior column reconstruction somewhat easier.107 These procedures are also associated with a relatively high morbidity rate. A third option is an all-dorsal procedure with the resection of the dorsal elements and a spinal column shortening through a variation of the pedicle subtraction osteotomy. This procedure corrects the kyphosis and restores anterior column weight-bearing capability, but it requires a strut graft or cage.108–110
Some surgeons have advocated a hybrid approach utilizing decompression and short-segment reconstruction combined with vertebral augmentation through kyphoplasty or vertebroplasty. Relatively good results have been reported with relatively low morbidity.111–113 Long-term results of these procedures are not well known. No randomized controlled trials exist comparing these alternative options to the treatment of the neurologically compromised vertebral compression fractures.
Surgery for Degenerative Disease in the Presence of Osteoporosis
Osteoporosis does not directly cause symptoms that would lead to surgery in the absence of a fracture. However, many patients who present with typical symptoms of degenerative disease and require surgery will have associated osteoporosis.114 The indications for surgery do not change, but the osteoporosis may affect the types of procedures that are chosen. Although osteoporosis is common in older patients with degenerative conditions, most surgeons do not routinely screen for or treat the osteoporosis prior to surgery.115 Consideration should be given to doing so, as it may improve outcomes. Screening for osteoporosis with a DEXA scan and assessing the patient’s preoperative serum calcium and vitamin D levels are likely to be warranted in older patients undergoing fusion surgery.116
The use of perioperative bisphosphonates in patients undergoing fusion surgery is somewhat controversial. Some studies have suggested a higher fusion failure rate; others have not.117–120 Anabolic agents such as teriparatide may be of benefit both preoperatively and perioperatively in improving bone strength and enhancing fusion.121–123
Use of Instrumentation in Osteoporosis
Spine instrumentation is used in patients undergoing spine fusion. Osteoporosis weakens the holding power of the implants in bone.124 This leads to increased rates of instrumentation failure in elective surgery in patients with osteoporosis. This in turn leads to poorer outcome and higher complication rates in osteoporotic patients undergoing spine reconstructive surgery.
Numerous strategies have been proposed for improving the outcomes of spine surgery in patients with osteoporosis. Two review articles by Ponnusamy et al.125 and Hu126 summarize a number of options for increasing fixation and improving outcome in osteoporotic patients. Box 110-3 summarizes some of the available options.
Uninstrumented Fusion
An obvious way of avoiding hardware failure is to avoid the use of hardware in the first place. Although hardware is commonly used in lumbar spine fusion, particularly in the presence of degenerative spondylolisthesis, studies have not shown a clear, consistent benefit of the use of instrumentation.127–129 It is reasonable in patients with osteoporosis and stable spines to fuse without hardware. Fusion rates will be lower, but clinical results in the near term are equivalent.
Increased Number of Fixation Points
Increasing the number of fixation points decreases the loads on each individual component of the construct. Thus, the loads are distributed across more fixation points, overall construct stiffness increases, and the chance of failure decreases.130,131
Cross-Fixators and Triangulation
Cross-fixators have their effect by further distributing forces across the implant. They are particularly useful in rotation.132,133 By placing the screws in a convergent fashion and adding a cross-link, further improvement in strength can be obtained through triangulation.134 One caveat is that cross-links have been suggested to increase pseudarthrosis rates in scoliosis constructs.135
Use of Interbody Fusion and Osteotomies to Obtain Correction
Placement of large interbody implants across the disc space or eccentrically placed implants on the concavity can correct scoliosis. This allows the dorsal hardware to be placed as a neutralization device, and with lower loads on the screws, there should be less failure.136–140
Use of Hooks and Wires Instead of Screws
In osteoporosis, relatively more strength is lost in cancellous bone than in cortical bone. The lamina is more cortical than cancellous and loses proportionally less strength.141 As a result, laminar hooks or wires may be an option. In addition, hooks and wires have superior holding power in pull-out and should be considered at the ends of a kyphosis construct in which pull-out is a common motor failure.142–144
Cement Augmentation of Pedicle Screws
Numerous studies have shown that cement augmentation improves the holding power of screws.145–147 To date, no studies of clinical effectiveness have been published, though the technique is widely used. In our practice in long constructs, cement augmentation is used only at one or two levels at the top or bottom of a construct. If a constructs fails, the failure is usually at the end segments, and should revision be required, it is easier to do if not all segments have been cement augmented. Another interesting idea is to augment adjacent segments to lessen the risk of proximal junctional kyphosis.148
Use of Implants Designed for Osteoporotic Bone
A number of modifications of pedicle screws have been proposed for use in osteoporotic bone. These include hollow screws for insertion of cement,149,150 expandable screws,151–153 and variable pitch or thread depth screws.153 These modifications all lead to increased pull-out strength of the implants in biomechanical testing. To date, there have been no good clinical studies showing improved outcomes with these implants.
Meticulous Attention to Spinal Balance
In studies examining outcomes of adult deformity surgery, there is a clear correlation between outcome and restoration of sagittal balance.154 Late complications, such as implant failure or junctional kyphosis, are correlated with failure to achieve balance.155–157 As a result, it is vitally important that surgeons performing surgery for osteoporosis pay meticulous attention to overall spinal balance, particularly the sagittal balance.
Surgical Considerations in Paget Disease of the Spine
As was mentioned previously and in this chapter, PDB can lead to spinal stenosis and neurologic compromise. Medical management will usually be successful in treating this.158,159 In rare cases, stenosis from underlying degenerative disease may combine with PDB to create symptoms. Treatment goals and options are the same as those in degenerative disease alone. In patients with deficit due to pathologic fracture through a pagetic vertebra, surgery may be indicated.160 Bleeding risk is higher in patients with PDB, and bisphosphonate or calcitonin treatment may lessen this risk if surgery is not urgent.161
A rare complication of PDB is sarcomatous degeneration. Spinal osteosarcoma in PDB is not treated differently from primary osteosarcomas.162,163
Black D., Schwartz A., Ensrud K., et al. Effects of continuing or stopping alendronate after 5 years of Treatment. The Fracture Intervention Trial Long-Term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
Cho S.K., Bridwell K.H., Lenke L.G., et al. Major complications in revision adult deformity surgery: Risk factors and clinical outcomes with two- to seven-year follow-up. Spine (Phila Pa 1976). 2011. [Epub ahead of print]
Kanis J.A., Johnell O., Oden A., et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385-397.
Khosla S., Riggs B.L. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005;34:1015-1030.
Kleerekoper M., Gold D.T. Osteoporosis prevention and management. An evidence-based review. Clin Obstet Gynecol. 2008;51:556-563.
Lyritis G.P., Mayasis B., Tsakalakos N., et al. The natural history of the osteoporotic vertebral fracture. Clin Rheumatol. 1989;8(Suppl 2):66-69.
National Osteoporosis Foundation. Clinician’s guideline to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation; 2008.
Ponnusamy K.E., Iyer S., Gupta G., Khanna A.J. Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J. 2011;11:54-63.
1. Schafer A., Sellmeyer D. Interventions to improve treatment of osteoporosis following fracture. J Clin Outcomes Manag. 2008;15:587-592.
2. Gennari L., Bilezikian J. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
3. Consensus Development Conference. Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646-650.
4. Kanis J.A., Johnell O., Oden A., Johansson H., McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385-397.
5. Freedman B., Potter B., Nesti L., et al. Osteoporosis and vertebral compression fractures-continued missed opportunities. Spine J. 2008;8:756-762.
6. Burge R., Dawson-Hughes B., Solomon D., et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465-471.
7. Clarke B. Case study and commentary. Diagnosis and management of postmenopausal osteoporosis. J Clin Outcomes Manag. 2002;9:397-404.
8. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
9. Raisz L.G. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;45:1353-1358.
10. Nieves J.W., Golden A.L., Siris E., et al. Teenage and current calcium intake are related to bone mineral density of the hip and forearm in women aged 30-39 years. Am J Epidemiol. 1995;141:341-351.
11. Hunter D.J., Sambrook P.N. Bone loss. Epidemiology of bone loss. Arthritis Res. 2000;2:441-445.
12. Khosla S., Riggs B.L. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005;34:1015-1030.
13. Eghbali-Fatourechi G., Khosla S., Sanyal A., et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221-1230.
14. Oursler M.J., Cortese C., Keeting P.E., et al. Modulation of transforming growth factor beta production in normal human osteoblast-like cells by 17-beta estradiol and parathyroid hormone. Endocrinology. 1991;129:3313-3320.
15. Holick M.F., Siris E.S., Binkley N., et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215-3224.
16. Simonelli C., Morancey J.A., Swanson L., et al. A high prevalence of vitamin D insufficiency/deficiency in a minimal trauma fracture population. J Bone Miner Res. 2004;19:S433.
17. Reginster J.Y. The high prevalence of inadequate serum vitamin D levels and implications for bone health. Curr Med Res Opin. 2005;21:579-585.
18. Mauck K.F., Clarke B.F. Diagnosis, screening, prevention, and treatment of osteoporosis. Mayo Clin Proc. 2006;81:662-672.
19. Johnson B.E., Lucasey B., Robinson R.G., Lukert B.P. Contributing diagnoses in osteoporosis. The value of a complete medical evaluation. Arch Intern Med. 1989;149:1069-1072.
20. Clark J., Tamenbaum C., Postnett K., et al. Laboratory testing in healthy, osteoporotic women. J Bone Miner Res. 1997;12:S141.
21. Kanis J.A., Johnell O., Oden A., et al. Ten-year risk of osteoporotic fracture and the effect of risk factors on screening strategies. Bone. 2002;30:251-258.
22. Kanis J.A., Johnell O., Oden A., et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385-397.
23. Kanis J.A., Oden A., Johnell O., et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033-1046.
24. Mazanec D., Mompoint A., Podichetty V., Potnis A. Vertebral compression fractures: manage aggressively to prevent sequelae. Cleve Clin J Med. 2003;70:147-154.
25. Leech J.A., Dulberg C., Kellie S., et al. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141:68-71.
26. Licata A. Quality of life of osteoporotic patients. The impact of vertebral compression fractures. Adv Osteoporotic Fracture Manag. 2001;1:2-6.
27. Cummings S.R., Black D.M., Rubin S.M. Lifetime risks of hip, Colles’, or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med. 1989;149:2445-2448.
28. Majumdar S., Kim N., Colman I., et al. Incidental vertebral fractures discovered with chest radiography in the emergency department. Arch Intern Med. 2005;165:905-909.
29. Nelson H., Helfand M., Woolf S., Allan J. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:529-541.
30. El Maghraoui A., Roux C. DXA scanning in clinical practice. Q J Med. 2008;101:605-617.
31. Kanis J., McCloskey E., Johansson H., et al. A reference standard for the description of osteoporosis. Bone. 2008;42:467-475.
32. Bauer D.C., Gluer C.C., Cauley J.A., et al. Broadband ultrasonic attenuation predicts fractures strongly and independently of densitometry in older women. Arch Intern Med. 1997;157:629-634.
33. Gardsell P., Johnell O., Nilsson E. Predicting fractures in women by using forearm bone densitometry. Calcif Tissue Int. 1989;44:235-242.
34. Grampp S., Genant H.K., Mathur A., et al. Comparisons of non-invasive bone mineral measurements in assessing age-related loss, fracture discrimination, and diagnostic classification. J Bone Miner Res. 12, 1997. 697–711
35. Hans D., Dargent-Molina P., Schott A.M., et al. Ultrasonicgraphic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet. 1996;348:511-514.
36. Melton L.J., Atkinson E.J., O’Fallon W.M., et al. Long-term fracture prediction by bone mineral assessed at different sites. J Bone Miner Res. 1993;10:1227-1233.
37. Bauer D.C., Weing S.K., Cauley J.A., et al. Quantitative ultrasound predicts hip and non-spine fracture in men: the MrOS study. Osteoporos Int. 2007;18:771-777.
38. Cummings S.R., Cawthon P.M., Ensrud K.E., et al. BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res. 2006;21:1550-1556.
39. Marshal D., Johnell O., Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254-1259.
40. Tannenbaum C., Clark J., Schwartzman K., et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87:4431-4437.
41. Kleerekoper M., Gold D.T. Osteoporosis prevention and management. An evidence-based review. Clin Obstet Gynecol. 2008;51:556-563.
42. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
43. Cauley J.A., Robbins J., Chen Z., et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density. The Women’s Health Initiative Randomized Trial. JAMA. 2003;290:1729-1738.
44. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701-1712.
45. Nieves J.W. Osteoporosis: the role of micronutrients. Am J Clin Nutr. 2005;81(Suppl):S1232-S1239.
46. Shea B., Wells G., Cranney A., et al. Osteoporosis Methodology Group and the Osteoporosis Research Advisory Group: Meta-analysis of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002;23:522-529.
47. National Osteoporosis Foundation. Clinician’s guideline to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation; 2008.
48. Valimaki V.V., Alfthan H., Lehmuskalllio E., et al. Vitamin D status as determinant of peak bone mass in young Finnish men. J Clin Endocrinol Metab. 2004;89:76-80.
49. Bischoff-Ferrari H.A., Willett W.C., Wong J.B., et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency. Arch Intern Med. 2009;169:551-561.
50. Linden C., Alwis G., Ahlborg H., et al. Exercise, bone mass and bone size in prepubertal boys: one-year data from the pediatric osteoporosis prevention study. Scand J Med Sci Sports. 2007;17:340-347.
51. Daly R.M., Rich P.A., Klein R., et al. Effects of high-impact exercise on ultrasonic and biochemical indices of skeletal status: a prospective study in young male gymnasts. J Bone Miner Res. 1999;14:1222-1230.
52. Ulrich C.M., Georgiou C.C., Gillis D.E., Snow C.M. Lifetime physical activity is associated with bone mineral density in premenopausal women. J Womens Health. 1999;8:365-375.
53. Bonaiuti D., Shea B., Oivine R., et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 3, 2002. CD000333
54. Taaffe D.R., Duret C., Wheeler S., Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47:1208-1214.
55. Siris E.S., Chen Y., Abbott T.A., et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
56. Schousboe J.T., Nyman J.A., Kane R.L., Ensrud K.E. Cost-effectiveness of alendronate therapy for estrogenic postmenopausal women. Ann Intern Med. 2005;142:34-41.
57. Miller P.D., Barlas S., Brenneman S.K., et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113-1120.
58. McClung M.R. Osteopenia: to treat or not to treat [editorial]. Ann Intern Med. 2005;142:796-797.
59. Garnero P., Sornay-Rendu E., Duboeuf F., et al. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res. 1999;14:1614-1621.
60. Qaseem A., Snow V., Shekelle P., et al. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404-415.
61. Black D.M., Cummings S.R., Karpf D.B., et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541.
62. Cummings S.R., Black D.M., Thompson D.E., et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077-2082.
63. Harris S.T., Watts N.B., Ganant H.K., et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial: Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352.
64. Reginster J., Minne H.W., Sorenson O.H., et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91.
65. Cohen S., Levy R.M., Keller M., et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double blind placebo-controlled, parallel group study. Arthritis Rheum. 1999;42:2309-2318.
66. Saag K.G., Emkey R., Schnitzer T.J., et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Inducted Osteoporosis Intervention Study Group. N Engl J Med. 1998;339:292-299.
67. Black D.M., Delmas P.D., Eastell R., et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
68. Lyles K.W., Colon-Emeric C.S., Magaziner J.S., et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
69. Khan A., Sandor G., Dore E., et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36:478-490.
70. Black D., Schwartz A., Ensrud K., et al. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-Term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
71. Chestnut C.H., Silverman S., Andriano K., et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fracture study. Am J Med. 2000;109:267-276.
72. Pontiroli A.E., Pajetta E., Scaglia L., et al. Analgesic effect of intranasal and intramuscular salmon calcitonin in postmenopausal osteoporosis: a double-blind, double placebo study. Aging Clin Exp Res. 1994;6:459-463.
73. Gennari C., Agnusdei D., Camporeale A. Use of calcitonin in the treatment of bone pain associated with osteoporosis. Calcif Tissue Int. 1991;49(Suppl 2):S9-S13.
74. Khovidhunkit W., Shoback D.M. Clinical effects of raloxifene hydrochloride in women. Ann Intern Med. 1999;130:431-439.
75. Ettinger B., Black D.M., Mitlak B.H., et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized trial. JAMA. 1999;282:637-645.
76. Maricic M., Adachi J.D., Sarkar S., et al. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med. 2002;162:1140-1143.
77. Neer R.M., Arnaud C.D., Zanchetta J.R., et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434-1441.
78. Lindsay R., Scheele L.R., Clancy W.H. Incident vertebral fractures during an 18-month observation period following discontinuation of LY333334 (recombinant human parathyroid hormone [1-34], rhPTH [1-34]) use in postmenopausal women with osteoporosis [abstract]. J Bone Miner Res. 2001;16(Suppl 1):1105.
79. Black D.M., Bilezikiari J.P., Ensrud K.E., et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555-565.
80. McClung M.R., San Martin J., Miller P.D., et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762-1768.
81. Vahle J.L., Sato M., Long G.G., et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human data. Toxicol Pathol. 2002;30:312-321.
82. Maricic M. Osteomalacia. Curr Osteoporos Rep. 2008;6:130-133.
83. Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(Suppl 2):S153-S164.
84. Shoback D. Update in osteoporosis and metabolic bone disorders. J Clin Endocrinol Metab. 2007;92:747-753.
85. Haugeberg G. Imaging of metabolic bone diseases. Best Pract Res Clin Rheumatol. 2008;22:1127-1139.
86. Boyce B.F. Uses and limitations of bone biopsy in management of metabolic bone disease. Baillieres Clin Endocrinol Metab. 1988;2:31-57.
87. Ralston S.H., Langston A.L., Reid I.R. Pathogenesis and management of Paget’s disease of bone. Lancet. 2008;372:155-163.
88. Avramidis A., Polyzos S., Moralidis E., et al. Scintigraphic, biochemical, and clinical response to zoledronic acid treatment in patients with Paget’s disease of bone. J Bone Miner Metab. 2008;26:635-641.
89. Silverman S.L. Paget disease of bone: therapeutic options. J Clin Rheumatol. 2008;14:299-305.
90. Siris E.S. Extensive personal experience: Paget’s disease of bone. J Clin Endocrinol Metab. 1995;80:335-338.
91. Delmas P.D., Meunier P.J. The management of Paget’s disease of bone. N Engl J Med. 1997;336:558-566.
92. Colina M., LaCorte R., DeLeonardis F., Trotta F. Paget’s disease of bone: a review. Rheumatol Int. 2008;28:1069-1075.
93. Cooper C., Harvey N., Dennison E., et al. Update on the epidemiology of Paget’s disease of bone. J Bone Miner Res. 2007;21:3-8.
94. Barker D.J. The epidemiology of Paget’s disease. Metab Bone Dis Rel Res. 1981;3(4–5):231-233.
95. Alden J.C. Osteoporosis: a review. Clin Ther. 1989;11:3-14.
96. Siris E.S., Ottman R., Flaster E., Kelsey J.L. Familial aggregation of Paget’s disease. J Bone Miner Res. 1991;6:495-500.
97. Singer F.R., Krane S.M. Paget’s disease of bone. In: Avioli L.V., Krane S.M., editors. Metabolic bone disease and clinically related disorders. Philadelphia: WB Saunders; 1990:546.
98. Douglas D.L., Bickerstaff D.R. Metabolic bone disease. Part 2. Surgery. 1990;78:1882.
99. Zati A., Bilotta T. Degeneration of Paget’s disease into sarcoma: clinical and therapeutic influencing factors. Chir Organi Mov. 2008;92:33-37.
100. Krane S.M. Etidronate disodium in the treatment of Paget’s disease of the bone. Ann Intern Med. 1982;96:619-625.
101. Lyritis G.P., Mayasis B., Tsakalakos N., et al. The natural history of the osteoporotic vertebral fracture. Clin Rheumatol. 1989;8(Suppl 2):66-69.
102. Klazen C.A., Verhaar H.J., Lohle P.N., et al. Clinical course of pain in acute osteoporotic vertebral compression fractures. J Vasc Interv Radiol. 2010;21:1405-1409.
103. Uchida K., Kobayashi S., Matsuzaki M., et al. Anterior versus posterior surgery for osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine. Eur Spine J. 2006;15:1759-1767.
104. Kanayama M., Ishida T., Hashimoto T., et al. Role of major spine surgery using Kaneda anterior instrumentation for osteoporotic vertebral collapse. J Spinal Disord Tech. 2010;23:53-56.
105. Nakashima H., Yukawa Y., Ito K., et al. Combined posteroanterior surgery for osteoporotic delayed vertebral fracture and neural deficit in patients with Parkinson’s disease. Orthopedics. 32, 2009. ii
106. Kim K.T., Suk K.S., Kim J.M., et al. Delayed vertebral collapse with neurological deficits secondary to osteoporosis. Int Orthop. 2003;27:65-69.
107. Uchida K., Kobayashi S., Nakajima H., et al. Anterior expandable strut cage replacement for osteoporotic thoracolumbar vertebral collapse. J Neurosurg Spine. 2006;4:454-462.
108. Suk S.I., Kim J.H., Lee S.M., et al. Anterior-posterior surgery versus posterior closing wedge osteotomy in posttraumatic kyphosis with neurologic compromised osteoporotic fracture. Spine (Phila Pa 1976). 2003;28:2170-2175.
109. Chang K.W., Chen Y.Y., Lin C.C., et al. Apical lordosating osteotomy and minimal segment fixation for the treatment of thoracic or thoracolumbar osteoporotic kyphosis. Spine (Phila Pa 1976). 2005;30:1674-1681.
110. Saita K., Hoshino Y., Higashi T., Yamamuro K. Posterior spinal shortening for paraparesis following vertebral collapse due to osteoporosis. Spinal Cord. 2008;46:16-20.
111. Blondel B., Fuentes S., Metellus P., et al. Severe thoracolumbar osteoporotic burst fractures: treatment combining open kyphoplasty and short-segment fixation. Orthop Traumatol Surg Res. 2009;95:359-364.
112. Sudo H., Ito M., Abumi K., et al. One-stage posterior instrumentation surgery for the treatment of osteoporotic vertebral collapse with neurological deficits. Eur Spine J. 2010;19:907-915.
113. Uchida K., Nakajima H., Yayama T., et al. Vertebroplasty-augmented short-segment posterior fixation of osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine: comparisons with posterior surgery without vertebroplasty and anterior surgery. J Neurosurg Spine. 2010;13:612-621.
114. Andersson G.B., Bostrom M.P., Eyre D.R., et al. Consensus summary on the diagnosis and treatment of osteoporosis. Spine (Phila Pa 1976). 1997;22:S63-S65.
115. Dipaola C.P., Bible J.E., Biswas D., et al. Survey of spine surgeons on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. Spine J. 2009;9:537-544.
116. Chin D.K., Park J.Y., Yoon Y.S., et al. Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int. 2007;18:1219-1224.
117. Lehman R.A.Jr., Kuklo T.R., Freedman B.A., et al. The effect of alendronate sodium on spinal fusion: a rabbit model. Spine J. 2004;4:36-43.
118. Babat L.B., McLain R.F., Milks R., et al. The effects of the antiresorptive agents calcitonin and pamidronate on spine fusion in a rabbit model. Spine J. 2005;5:542-547.
119. Xue Q.Y., Ji Q., Li H.S., et al. Alendronate treatment does not inhibit bone formation within biphasic calcium phosphate ceramics in posterolateral spinal fusion: an experimental study in porcine model. Chin Med J. 2009;122:2770-2774.
120. Nagahama K., Kanayama M., Togawa D., et al. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011;14:500-507.
121. Arita S., Ikeda S., Sakai A., et al. Human parathyroid hormone (1-34) increases mass and structure of the cortical shell, with resultant increase in lumbar bone strength, in ovariectomized rats. J Bone Miner Metab. 2004;22:530-540.
122. Chen P., Jerome C.P., Burr D.B., et al. Interrelationships between bone microarchitecture and strength in ovariectomized monkeys treated with teriparatide. J Bone Miner Res. 2007;22:841-848.
123. Lehman R.A.Jr., Dmitriev A.E., Cardoso M.J., et al. Effect of teriparatide [rhPTH(1,34)] and calcitonin on intertransverse process fusion in a rabbit model. Spine (Phila Pa 1976). 2010;35:146-152.
124. Yamagata M., Kitahara H., Minami S., et al. Mechanical stability of the pedicle screw fixation systems for the lumbar spine. Spine (Phila Pa 1976). 1992;17:S51-S54.
125. Ponnusamy K.E., Iyer S., Gupta G., Khanna A.J. Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J. 2011;11:54-63.
126. Hu S.S. Internal fixation in the osteoporotic spine. Spine (Phila Pa 1976). 1997;22:S43-S48.
127. Fischgrund J.S., Mackay M., Herkowitz H.N., et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22:2807-2812.
128. Kuntz K.M., Snider R.K., Weinstein J.N., et al. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine (Phila Pa 1976). 2000;25:1132-1139.
129. Watters W.C.3rd, Bono C.M., Gilbert T.J., et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009;9:609-614.
130. Kostuik J.P., Munting E., Valdevit A. Biomechanical analysis of screw load sharing in pedicle fixation of the lumbar spine. J Spinal Disord. 1994;7:394-401.
131. Brodke D.S., Bachus K.N., Mohr R.A., Nguyen B.K. Segmental pedicle screw fixation or cross-links in multilevel lumbar constructs: a biomechanical analysis. Spine J. 2001;1:373-379.
132. Dick J.C., Zdeblick T.A., Bartel B.D., Kunz D.N. Mechanical evaluation of cross-link designs in rigid pedicle screw systems. Spine (Phila Pa 1976). 1997;22:370-375.
133. Carson W.L., Duffield R.C., Arendt M., et al. Internal forces and moments in transpedicular spine instrumentation. The effect of pedicle screw angle and transfixation—the 4R-4bar linkage concept. Spine (Phila Pa 1976). 1990;15:893-901.
134. Ruland C.M., McAfee P.C., Warden K.E., Cunningham B.W. Triangulation of pedicular instrumentation. A biomechanical analysis. Spine (Phila Pa 1976). 1991;16:S270-S276.
135. Kim Y.J., Bridwell K.H., Lenke L.G., et al. Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine (Phila Pa 1976). 2005;30:468-474.
136. Lippman C.R., Spence C.A., Youssef A.S., Cahill D.W. Correction of adult scoliosis via a posterior-only approach. Neurosurg Focus. 2003;14:E5.
137. Crandall D.G., Revella J. Transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion as an adjunct to posterior instrumented correction of degenerative lumbar scoliosis: three year clinical and radiographic outcomes. Spine (Phila Pa 1976). 2009;34:2126-2133.
138. Heary R.F., Karimi R.J. Correction of lumbar coronal plane deformity using unilateral cage placement. Neurosurg Focus. 2010;28:E10.
139. Wang M.Y., Mummaneni P.V. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus. 2010;28:E9.
140. Acosta F.L., Liu J., Slimack N., et al. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study. J Neurosurg Spine. 2011;15(1):92-96.
141. Eckstein F., Fischbeck M., Kuhn V., et al. Determinants and heterogeneity of mechanical competence throughout the thoracolumbar spine of elderly women and men. Bone. 2004;35:364-374.
142. Halvorson T.L., Kelley L.A., Thomas K.A., et al. Effects of bone mineral density on pedicle screw fixation. Spine (Phila Pa 1976). 1994;19:2415-2420.
143. Margulies J.Y., Casar R.S., Caruso S.A., et al. The mechanical role of laminar hook protection of pedicle screws at the caudal end vertebra. Eur Spine J. 1997;6:245-248.
144. Tan J.S., Kwon B.K., Dvorak M.F., et al. Pedicle screw motion in the osteoporotic spine after augmentation with laminar hooks, sublaminar wires, or calcium phosphate cement: a comparative analysis. Spine (Phila Pa 1976). 2004;29:1723-1730.
145. Cook S.D., Salkeld S.L., Stanley T., et al. Biomechanical study of pedicle screw fixation in severely osteoporotic bone. Spine J. 2004;4:402-408.
146. Burval D.J., McLain R.F., Milks R., Inceoglu S. Primary pedicle screw augmentation in osteoporotic lumbar vertebrae: biomechanical analysis of pedicle fixation strength. Spine (Phila Pa 1976). 2007;32:1077-1083.
147. Frankel B.M., D’Agostino S., Wang C. A biomechanical cadaveric analysis of polymethylmethacrylate-augmented pedicle screw fixation. J Neurosurg Spine. 2007;7:47-53.
148. Aydogan M., Ozturk C., Karatoprak O., et al. The pedicle screw fixation with vertebroplasty augmentation in the surgical treatment of the severe osteoporotic spines. J Spinal Disord Tech. 2009;22:444-447.
149. Chen L.H., Tai C.L., Lai P.L., et al. Pullout strength for cannulated pedicle screws with bone cement augmentation in severely osteoporotic bone: influences of radial hole and pilot hole tapping. Clin Biomech (Bristol, Avon). 2009;24:613-618.
150. Chen L.H., Tai C.L., Lee D.M., et al. Pullout strength of pedicle screws with cement augmentation in severe osteoporosis: a comparative study between cannulated screws with cement injection and solid screws with cement pre-filling. BMC Musculoskelet Disord. 2011;12:33.
151. Ngu B.B., Belkoff S.M., Gelb D.E., Ludwig S.C. A biomechanical comparison of sacral pedicle screw salvage techniques. Spine (Phila Pa 1976). 2006;31:E166-E168.
152. Wu Z.X., Cui G., Lei W., et al. Application of an expandable pedicle screw in the severe osteoporotic spine: a preliminary study. Clin Invest Med. 2010;33:E368-E374.
153. Krenn M.H., Piotrowski W.P., Penzkofer R., et al. Influence of thread design on pedicle screw fixation. Laboratory investigation. J Neurosurg Spine. 2008;9:90-95.
154. Yadla S., Maltenfort M.G., Ratliff J.K., Harrop J.S. Adult scoliosis surgery outcomes: a systematic review. Neurosurg Focus. 2010;28:E3.
155. Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925-948.
156. Rose P.S., Bridwell K.H., Lenke L.G., et al. Role of pelvic incidence, thoracic kyphosis, and patient factors on sagittal plane correction following pedicle subtraction osteotomy. Spine (Phila Pa 1976). 2009;34:785-791.
157. Cho S.K., Bridwell K.H., Lenke L.G., et al. Major complications in revision adult deformity surgery: risk factors and clinical outcomes with two- to seven-year follow-up. Spine (Phila Pa 1976). 2011;May 14,. [Epub ahead of print]
158. Walpin L.A., Singer F.R. Paget’s disease. Reversal of severe paraparesis using calcitonin. Spine (Phila Pa 1976). 1979;4:213-219.
159. Hadjipavlou A.G., Katonis P.G., Tzermiadianos M.N., et al. Principles of management of osteometabolic disorders affecting the aging spine. Eur Spine J. 2003;12(Suppl 2):S113-S131.
160. Hadjipavlou A.G., Gaitanis L.N., Katonis P.G., Lander P. Paget’s disease of the spine and its management. Eur Spine J. 2001;10:370-384.
161. Parvizi J., Klein G.R., Sim F.H. Surgical management of Paget’s disease of bone. J Bone Miner Res. 2006;21(Suppl 2):P75-P82.
162. Huang T.L., Cohen N.J., Sahgal S., Tseng C.H. Osteosarcoma complicating Paget’s disease of the spine with neurologic complications. Clin Orthop Relat Res. 1979;141:260-265.
163. Tigani D., Pignatti G., Picci P., et al. Vertebral osteosarcoma. Ital J Orthop Traumatol. 1988;14:5-13.