Chapter 76D Mesocaval shunt
History of the Mesocaval Shunt
Eck first described decompression of the portal system via the portacaval shunt in 1877. The first mesocaval shunt was developed primarily for children with portal vein thrombosis by Marion in 1953 (Marion et al, 1965) and Clatworthy in 1955. In the Marion-Clatworthy J-shunt, the inferior vena cava (IVC) is divided just proximal to the confluence of the iliac veins, and the proximal end is anastomosed to the lateral aspect of the superior mesenteric vein (Clatworthy, 1955). In 1954, Valdoni also described this procedure in a discussion regarding treatment options for portal hypertension. Because of the complications of venous stasis, including intractable lower extremity edema, this shunt never gained popularity in adults.
In 1970, Read and colleagues described the use of homologous vein graft as a conduit for the mesocaval shunt. In 1972, Drapanas reported good success with prosthetic mesocaval shunts by showing efficacy in 25 patients with acute exsanguinating variceal hemorrhage and popularized the procedure. Throughout the 1970s, several authors reported success with autologous, homologous, heterologous, and synthetic mesocaval shunts for the treatment of bleeding varices.
Indications for Mesocaval Shunts
With remarkable advances in endoscopic therapy, interventional radiology, and liver transplantation, the role of surgical shunts in the treatment of complications related to portal hypertension has been revised (see Chapter 76A). Although alternatives exist, surgical shunts remain an important intervention in some patients. The use of transjugular intrahepatic portosystemic shunts (TIPS; see Chapter 76E) has decreased the need for surgical shunts dramatically. TIPS provides only short-term treatment, however; it requires constant monitoring and frequent intervention, and such shunts are appropriate only in patients with advanced liver disease, as a “bridge” to transplantation, or in severely ill patients with a short life expectancy. In contrast, surgical shunt are best performed in patients who have bleeding varices refractory to endoscopic intervention with good hepatic reserve (i.e., Child-Turcotte-Pugh [CTP] classes A and B). Indications for surgical shunt procedures include variceal hemorrhage with good hepatic reserve, failed TIPS, portal vein thrombosis, Budd-Chiari syndrome, refractory ascites, portal hypertensive biliopathy, and small-for-size syndrome in liver transplantation; although more recently, portal decompression has emerged for the treatment of small-for-size syndrome in liver transplantation.
The most common indication for surgical shunt is bleeding varices refractory to medical and endoscopic therapy in patients who have good hepatic reserve and do not need liver transplantation in the near future. Henderson and colleagues (2000) showed excellent outcomes in CTP class A and B cirrhotic patients with refractory bleeding who received surgical shunts. In their series, the overall survival and rebleeding rates were 86% and 6.3%, respectively, with a median follow-up of 36 months. The choice between TIPS and surgical shunts depends on the patient’s hepatic reserve and timing to possible transplantation. If transplantation is expected within 1 to 2 years, TIPS is likely to bridge the patient to transplantation without the need for surgical intervention. However, if transplantation is unlikely to occur within 1 to 2 years, surgical shunting is warranted; more than 50% of TIPS patients require intervention within this time period.
Orloff and coworkers (2002) reported the use of portosystemic shunts in the treatment of 200 children and adults with extrahepatic portal hypertension secondary to portal vein thrombosis. In contrast to patients with intrahepatic portal hypertension secondary to cirrhosis, this patient population is often younger and healthier and often has preserved liver function, making them excellent surgical candidates. In this large series by Orloff and colleagues (2002), 100% of patients had normal liver biopsies with normal liver function, and 66 patients (33%) underwent Marion-Clatworthy–type cavomesenteric shunts with no immediate postoperative mortality. Of note, six adult patients experienced postoperative lower extremity edema. The 15-year actuarial survival was noted to be 95% for all shunt types, with shunt patency at 97.5%. Liver function remained normal for 99% of these patients at 5 years. The authors concluded that portosystemic shunting is the most effective treatment for these patients and that these procedures should be performed early in the course of variceal bleeding episodes.
Although Budd-Chiari syndrome (see Chapter 77) with obstruction of the hepatic venous outflow was traditionally treated with portosystemic shunt procedures, other treatment options—including hepatic vein angioplasty, catheter-directed thrombolysis, TIPS, and liver transplantation—have been successfully introduced. After reviewing 54 Budd-Chiari syndrome patients treated at Johns Hopkins Hospital over 20 years, Slakey and colleagues (2001) concluded that shunting and transplantation result in 5-year survival rates of at least 75% in these patients. Fisher and associates (1999) advocated the use of hepatic vein angioplasty for Budd-Chiari syndrome patients with short-length hepatic vein stenosis or occlusion and surgical shunting for patients with diffuse hepatic vein occlusion or failed angioplasty; others recommend TIPS. However, recanalization of the hepatic veins is not always successful and may require a shunt procedure.
A study using the porcine transplant model suggested that mesocaval shunts may provide protective effects for small-for-size liver grafts by protecting them from portal flow–related injuries (Smyrniotis et al, 2003). Case reports are emerging that demonstrate successful use of this technique to alleviate portal hypertension associated with small-for-size syndrome after living-donor liver transplantation (Boillot et al, 2002; Kokai et al, 2008). A porcine model has demonstrated retention of liver regenerative capabilities despite reduction of venous inflow after left hepatectomy with mesocaval shunt (Pouyet et al, 2007). Given the critical organ shortage and increasing use of segmental grafts, this approach may prove clinically useful in the future.
Because subsequent transplantation often is anticipated in patients receiving portosystemic shunts, the shunt procedure should minimize as much as possible any potential compromise of the technical aspects of the transplant operation. In contrast to portacaval shunts, the distal splenorenal and mesocaval interposition shunts do not alter the porta hepatis anatomy. In a case control study comparing decompressive procedures involving the hepatic hilum with both TIPS and other surgical shunts, Dell’Era and colleagues (2005) noted significantly higher operative times, higher transfusion requirements, increased hospital and intensive care unit (ICU) stays, and higher perioperative mortality in patients with procedures that affected the hepatic hilum. These findings have been reproduced by other authors as well (Menegaux et al, 1994; Mazzaferro et al, 1990; Brems et al, 1989), however, other studies have not found this association (Gonzalez et al, 2005; Abou Jaoude & Almawi, 2001; Boillot et al, 1991). The mesocaval interposition shunt is technically less challenging than the distal splenorenal shunt and requires minimal retroperitoneal dissection. It effectively relieves portal hypertension, and in contrast to the distal splenorenal shunt, it also relieves or prevents secondary ascites.
Shunt Materials and Mesocaval Shunt Size
Multiple variations of the mesocaval shunt procedure have been described in the literature, and significant debate has ensued regarding the ideal graft material and diameter for the mesocaval shunt. In 1970, Read and colleagues presented a series of eight mesocaval shunts with homologous vena cava. Also in 1970, Lord and associates published a report on seven mesocaval H-grafts with polytetrafluoroethylene (PTFE) prostheses, of which six remained patent for 20 months. Despite Lord’s early success, many clinicians remained fearful of using prosthetic grafts in the venous system because of experimental failures in long-term patency. In 1972, Drapanas published data on 25 more mesocaval shunt procedures that used 19- to 22-mm Dacron interposition grafts and showed excellent patency rates. Drapanas cited a superiority of Dacron over PTFE for better incorporation into host tissues and continued to use Dacron in his studies.
Throughout the 1970s, several other groups reported success with large-diameter prosthetic grafts. In 1977, Filtzer and coworkers reviewed their institution’s experience with 20 patients who underwent mesocaval shunt procedures using 18- to 22-mm Dacron grafts for bleeding esophageal varices. The long-term patency rate approached 90%. In 1975, Drapanas and associates published a follow-up study on the hemodynamics of 80 mesocaval shunts using large-diameter (18 to 22 mm) Dacron grafts. In this series, a decrease in mean portacaval pressure by 50% was noted, and 44% maintained hepatopetal portal flow. Only three graft thromboses occurred, and overall patency rate was 95%. One patient developed graft infection, however, with subsequent sepsis and death.
Although recurrent esophageal varices and hemorrhage were rare after these procedures, the large-diameter portacaval and mesocaval grafts (16 to 22 mm) often resulted in total portal shunting and loss of prograde portal flow, which many investigators thought led to progressive liver dysfunction and high rates of encephalopathy. Sarfeh and colleagues (1986) investigated the importance of maintaining hepatic portal perfusion for prevention of encephalopathy by systemically reducing the portacaval H-graft diameters. After reviewing 88 patients treated with portacaval shunting for bleeding esophageal varices, they reported maintenance of hepatopetal flow in 82% of patients with 8-mm PTFE H-grafts, 46% with 10 mm PTFE H-grafts, and only 3% with 14- to 20-mm Dacron grafts. Preserved prograde portal flow correlated with decreased rates of encephalopathy, which was observed in only 9% of patients with 8-mm PTFE H-grafts compared with 39% of those with large-diameter Dacron grafts. Although early graft thrombosis was more common in the small-diameter grafts (16%), the cumulative shunt patency rate was 97% for those grafts. One patient experienced graft infection with subsequent sepsis and death. In another study, Isaksson and colleagues (2005) noted that patients with a 14-mm mesocaval shunt had a steady, worsening performance in postoperative follow-up on several psychometric tests, and the rate of hepatofugal portal flow was 90% at 12 months in these patients.
Throughout the 1980s, most reports of small-diameter grafts focused on their use in the portacaval position as opposed to a mesocaval shunt. All studies showed a slightly increased rate of perioperative shunt thrombosis over the large-diameter shunts; this was mostly managed via angiographic revision (Collins et al, 1994; Rypins et al, 1988), and all small-diameter portacaval shunt procedures were coupled with aggressive portal vein collateral ablation.
More recent studies have shown the efficacy of small-diameter mesocaval shunts. Mercado and associates (2000) reviewed a 10-year experience with 10-mm PTFE mesocaval shunts for the treatment of bleeding esophageal varices in mostly low-risk patients with preserved hepatic function. Thirty-three patients underwent the procedure; 81% maintained long-term patency evaluated by angiography, but 15% developed episodes of rebleeding. Only 11% had encephalopathy, and 12-month survival was 81%. Mercado and colleagues (1996) also compared the distal splenorenal shunt with the 10-mm PTFE mesocaval shunt and showed decreased encephalopathy, decreased shunt thrombosis, and fewer changes in portal flow with the distal splenorenal shunt but no differences in mortality or rebleeding rates. They concluded that the mesocaval shunt continues to have a role in the surgical treatment of portal hypertension, when a selective shunt cannot be performed.
Reynolds and Southwick in 1951 first described the use of autologous vein graft for interposition between the portal vein and vena cava. Stipa and colleagues (1978) compared their experience with the side-to-side portacaval shunt versus autologous internal jugular vein interposition mesocaval shunt in 79 patients with variceal hemorrhage and portal hypertension. Although no differences in survival were reported between the two groups, encephalopathy was greater in the portacaval shunt patients (54%) compared with the mesocaval shunt patients (35%), and 95% of the patients with mesocaval shunts maintained hepatopetal portal flow. Gonzalez and associates (1977) also reported the use of autologous vein graft in 26 patients; no graft infections were reported for patients undergoing mesocaval shunts with either homologous or autologous vein graft. These shunts require more time to harvest vein, however, and they may not be appropriate in an emergency or urgent setting.
The literature also shows favorable patency rates with mesocaval shunting. Ilkgul and coworkers (2005) reported an 89% overall patency rate over a median follow-up of 42 months in patients with autologous internal jugular vein grafts. Sigalet and colleagues (2001) found a 100% shunt patency at 4.3 years in 20 pediatric patients using autologous vein graft. It is important to note that all of the mesocaval shunts discussed in this review provided adequate portal decompression with low perioperative mortality and infrequent recurrence of variceal hemorrhage.
Preoperative Evaluation and Management
Technique
Exposure
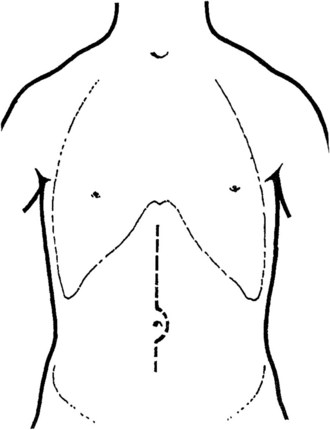
A transverse mid-abominal, right low subcostal, or midline incision may be used (Fig. 76D.1). Because of portal hypertension, a few large collateral vessels may be encountered in the subcutaneous tissue; these should be doubly ligated and divided instead of divided with electrocautery. When the peritoneum is opened, ascites is removed, and the abdomen is explored; a mechanical retractor is placed to provide maximal exposure. A liver biopsy often is performed and always should be done for any areas suspicious for hepatoma.
Exposure of the Superior Mesenteric Vein
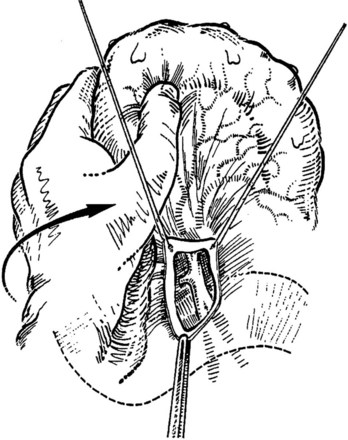
The transverse mesocolon is retracted cephalad, and the small intestine is retracted caudad and to the left to expose the root of the mesentery and the ligament of Treitz. A transverse incision is made in the posterior parietal peritoneum to initiate dissection of the SMV, which usually overlies the right lateral margin of the vertebral bodies. The superior mesenteric artery usually is located anterior and to the left of the SMV (Fig. 76D.2); however, these relationships are inconstant; Doppler may help identify the vascular structures.
Exposure of the Inferior Vena Cava
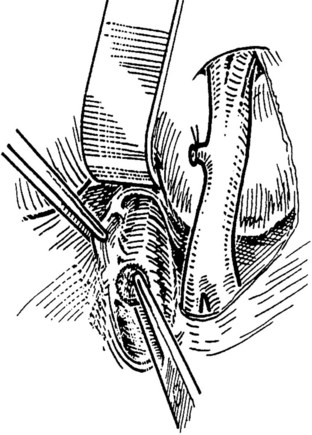
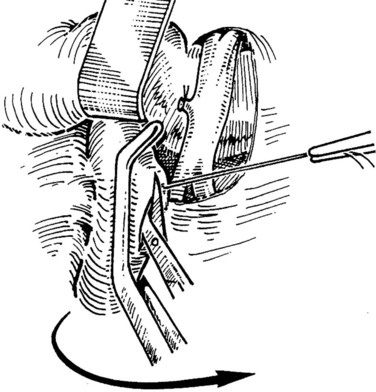
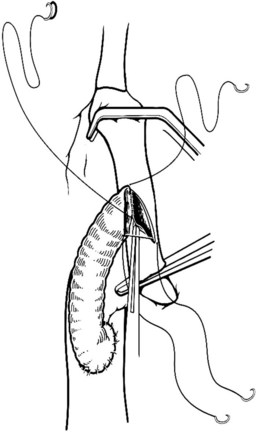
When an adequate length of the SMV is circumferentially mobilized, the IVC is identified by a direct approach through a window in the right colonic mesentery. Alternatively, the right colon and mesentery may be reflected to the midline, allowing access to the right retroperitoneum. The distal second portion and third portion of the duodenum are mobilized with a reverse Kocher maneuver. It is important to ligate and divide the large lymphatic channels in the retroperitoneum to avoid a significant lymphatic leak. A 6- to 7-cm length of IVC is dissected along the anterior and lateral borders (Fig. 76D.3). It is not necessary to mobilize the cava circumferentially but only enough to allow placement of a large Satinsky clamp for partial occlusion.
Preparation of the Vena Cava and Venotomy
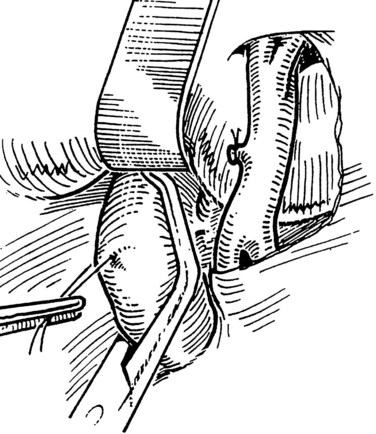
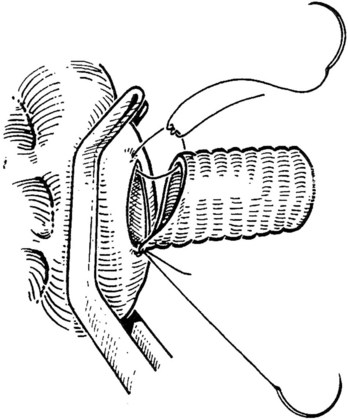
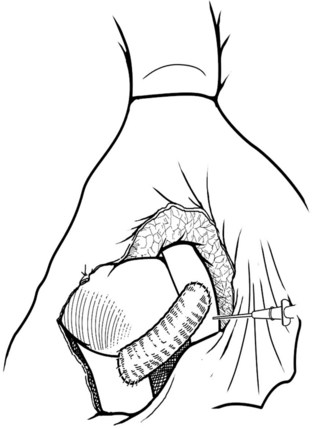
The IVC-graft anastomosis is performed first, which eliminates the problems of diminished exposure for the caval anastomosis and prolonged SMV occlusion. A vascular suture can be placed in the anterior wall of the vena cava and used to tent it upward, while a large Satinsky clamp is placed to occlude the cava partially (Fig. 76D.4). It is important that the clamp occludes the superior and inferior ends of the vena cava segment to prevent bleeding during the anastomosis. The suture used to tent the cava is excised with an ellipse of anterior caval wall to form the venotomy (Fig. 76D.5). Using angled Pott scissors, the venotomy is enlarged to the same diameter, usually 8 to 12 mm, as the chosen ringed Gore-Tex or internal jugular vein graft. If the opening is too large, the graft does not assume a circular shape at the anastomosis.
Inferior Vena Cava–Graft Anastomosis
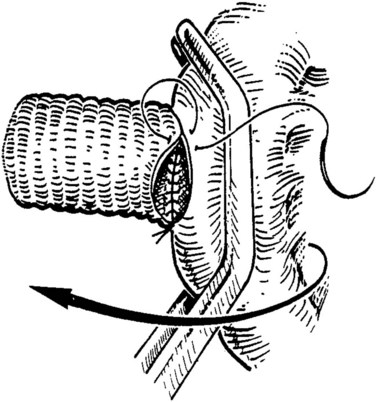
The caval anastomosis is located in the deepest and most dependent area of the wound, and good exposure is essential. The duodenum is retracted superiorly, and the rest of the viscera are retracted inferiorly. Two corner sutures are placed using 5-0 synthetic, nonabsorbable suture material at each end of the venotomy, and these are tied to position the graft over the venotomy (Fig. 76D.6). A stay suture also is placed midway along the medial caval venotomy lip for gentle traction to distract it away from the lateral suture line and to prevent incorporation into the opposite wall. The Satinsky clamp is rotated to the patient’s left, and the right side of the anastomosis is completed first. It is started at the inferior margin using one end of the corner stitch and working upward, placing the continuous sutures outside-in on the graft and inside-out on the vein (Fig. 76D.7). At the superior margin, the suture is tied to one end of the superior corner stitch.
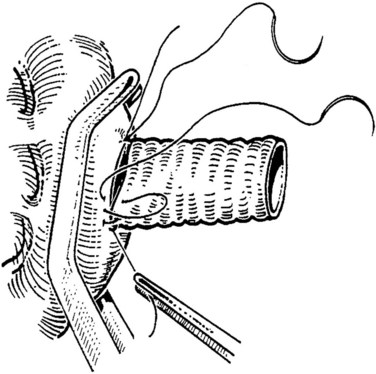
FIGURE 76D.7 The cava and graft are rotated medially, and the right lateral suture line is performed first.
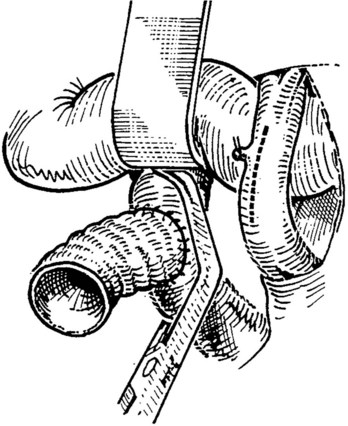
After completion of the right-side anastomosis, the Satinsky clamp is rotated to the patient’s right, and the other end of the superior corner suture is run inferiorly to complete the left side of the anastomosis (Fig. 76D.8). Figure 76D.9 shows the completed anastomosis. A nerve hook may be used to ensure proper positioning of the cava or graft before each needle bite.
Superior Mesenteric Vein–Graft Anastomosis
A small ellipse is excised from the anterior-lateral aspect of the SMV, and the venotomy is extended proximally and distally using angled Pott scissors to match the length of the obliquely cut graft. For H-shaped shunts, the venotomy is more right posterior-lateral on the vein. Sutures are placed at both ends of the venotomy and graft using 5-0 or 6-0 synthetic nonabsorbable suture material tied to approximate the vein and graft. The right lateral suture line is performed first in a continuous fashion from within the graft and vein, and is carried from the superior suture to the inferior suture (Fig. 76D.10). The left lateral suture line is created in a similar fashion by rotating the vein to the patient’s right for better exposure (see Fig. 76D.10). The left lateral suture line also is started at the superior suture and carried down and tied to the inferior stay suture.
Evaluating the Anastomosis
The pressures in the portal system are measured by positioning an 18 gauge needle connected to a water manometer into the SMV near the SMV-graft anastomosis (Fig. 76D.11). With the graft patent and mesenteric systemic flow intact, the pressure is measured as the decompressed portal pressure. The graft is clamped, and the pressure is rechecked to confirm that the system is indeed decompressed. A reduction in the pressure gradient by 12- to 18-cm H2O should be expected. Also, a thrill may be palpated in the graft. When it is established that no technical problems exist with the graft, such as kinking or compression by surrounding tissues, the abdomen is irrigated, hemostasis is ensured, and the abdomen is closed.
Postoperative Evaluation and Management
Immediately after the procedure, shunt patency should be confirmed by duplex ultrasound, MRI, or CT angiography (Fig. 76D.12). If it is not possible to determine shunt patency via noninvasive techniques, an angiogram must be performed.
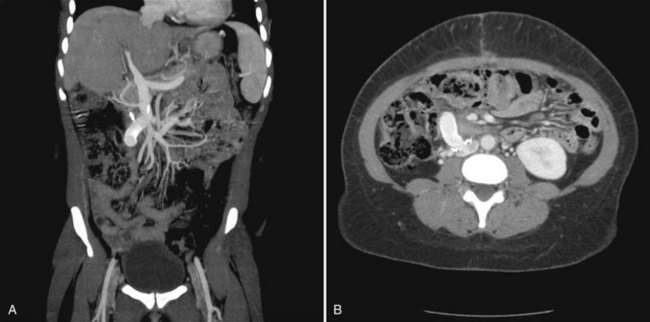
FIGURE 76D.12 Computed tomographic angiography of a patent interposition mesocaval shunt. A, Coronal image. B, Transverse image.
(Image courtesy Dr. Ravi Chari.)
If gastrointestinal bleeding recurs, the patient should undergo shunt interrogation to determine shunt patency. Several studies also recommend routine shunt evaluation by either duplex ultrasound or angiography (Mercado et al, 1996; Orloff et al, 2002; Rypins et al, 1988). We perform follow-up duplex ultrasound every 3 to 6 months after the procedure for the lifetime of the shunt. Shunt stenosis is managed routinely with percutaneous transfemoral dilation and, rarely, stenting of the stenosis; operative revision is done only with failure of the less invasive interventional procedures. For early and late shunt thrombosis, most studies advocate shunt thrombectomy. After balloon catheter placement and dilation across any stenosis, an angiocatheter is placed at the SMV-graft anastomosis, and streptokinase is infused at a rate of 5000 U/hr for 24 hours (Rypins et al, 1988; Sarfeh et al, 1986).
Abou Jaoude MM, Almawi WY. Liver transplantation in patients with previous portosystemic shunt. Transplant Proc. 2001;33:2723-2725.
Boillot O, et al. Liver transplantation in patients with a surgical portosystemic shunt. Gastroenterol Clin Biol. 1991;15:876.
Boillot O, et al. Small-for-size partial liver graft in an adult recipient: a new transplant technique. Lancet. 2002;359:406-407.
Brems JJ, et al. Effect of a prior portosystemic shunt on subsequent liver transplantation. Ann Surg. 1989;209:51-56.
Clatworthy WH. A new type of portal to systemic shunt for portal decompression. Arch Surg. 1955;1:588.
Collins JC, et al. Narrow-diameter portacaval shunts for management of variceal bleeding. World J Surg. 1994;18:211-215.
Dell’Era A, et al. Impact of prior portosystemic shunt procedures on outcome of liver transplantation. Surgery. 2005;137:620-625.
Drapanas T. Interposition mesocaval shunt for treatment of portal hypertension. Ann Surg. 1972;176:435-448.
Drapanas T, et al. Hemodynamics of the interposition mesocaval shunt. Ann Surg. 1975;181:523-533.
Eck NV. Concerning ligation of the vena porta: preliminary notification [translation]. Voen Med Zh. 1877;130:1-2.
Filtzer HS, et al. Experience with interposition mesocaval shunt for management of variceal bleeding. Arch Surg. 1977;112:593-595.
Fisher NC, et al. Managing Budd-Chiari syndrome: a retrospective review of percutaneous hepatic vein angioplasty and surgical shunting. Gut. 1999;44:568-574.
Gonzalez EE, et al. Results of liver transplantation in patients with previous portosystemic shunts. Transplant Proc. 2005;37:1491-1492.
Gonzalez EM, et al. Transduodenopancreatic interposition mesocaval shunt using internal jugular vein autograft. Surg Gynecol Obstet. 1977;145:565-569.
Henderson JM, et al. Surgical shunts and TIPS for variceal decompression in the 1990s. Surgery. 2000;128:540-547.
Ilkgul O, et al. Experience with mesocaval shunt with autologous jugular vein interposition in patients with Budd-Chiari syndrome. Hepatogastroenterology. 2005;52:662-665.
Isaksson B, et al. Hepatic encephalopathy verified by psychometric testing and EEG in cirrhotic patients: effects of mesocaval interposition shunt or sclerotherapy. HPB. 2005;7:65-72.
Kokai H, et al. Successful super-small-for-size graft liver transplantation by decompression of portal hypertension via splenectomy and construction of a mesocaval shunt: a case report. Transplant Proc. 2008;40:2825-2827.
Lord JW, et al. Mesocaval shunt modified by the use of a Teflon prosthesis. Surg Gynecol Obstet. 1970;130:525-526.
Marion P, et al. Mesenterico-caval anastomosis [in French]. Lyon Chir. 1965;61:448-449.
Mazzaferro V, et al. Liver transplantation in patients with previous portosystemic shunt. Am J Surg. 1990;160:111-116.
Menegaux F, et al. Comparison of transjugular and surgical portosystemic shunts on the outcome of liver transplantation. Arch Surg. 1994;129:1018-1024.
Mercado MA, et al. Distal splenorenal shunt versus 10-mm low-diameter mesocaval shunt for variceal hemorrhage. Am J Surg. 1996;171:591-595.
Mercado MA, et al. Small-diameter mesocaval shunts: a 10-year evaluation. J Gastrointest Surg. 2000;4:453-457.
Orloff MJ, et al. Bleeding esophagogastric varices from extrahepatic portal hypertension: 40 years’ experience with portal-systemic shunt. J Am Coll Surg. 2002;194:717-728.
Pouyet M, et al. Liver regeneration and hemodynamics in pigs with mesocaval shunt. J Surg Res. 2007;138:128-134.
Read RC, et al. Mesocaval H venous homografts. Arch Surg. 1970;101:785-791.
Reynolds JT, Southwick HW. Portal hypertension: use of venous grafts when side to side anastomosis is impossible. Arch Surg. 1951;62:789-800.
Rypins EB, et al. Advantages and disadvantages of polytetrafluoroethylene (PTFE) grafts for portacaval shunting. Vasc Surg. 1988;2:88-92.
Sarfeh IJ, et al. A systemic appraisal of portacaval H-graft diameters: clinical and hemodynamic perspectives. Ann Surg. 1986;204:356-363.
Sigalet DL, et al. Portal venous decompression with H-type mesocaval shunt using autologous vein graft: a North American experience. J Pediatr Surg. 2001;36:91-96.
Slakey DP, et al. Budd-Chiari syndrome: current management options. Ann Surg. 2001;233:522-527.
Smyrniotis VE, et al. Effect of mesocaval shunt on survival of small-for-size liver grafts: experimental study in pigs. Transplantation. 2003;75:1737-1740.
Stipa S, et al. Mesentericocaval shunt with the internal jugular vein. Surg Gynecol Obstet. 1978;146:391-399.
Valdoni P. Portal hypertension: I. orientation and therapeutic directions in portal hypertension [in Italian]. Clin Nuova Rass Prog Med Internazionale. 1954;19:349-350.