Chapter 24 Menopause
INTRODUCTION
Natural menopause occurs between ages 45 and 55, with a median age of 51 for women in industrialized countries.1 Available evidence suggests that in less-developed countries with lower socioeconomic and nutritional level this event takes place earlier.2,3 These differences in onset of menopause support the hypothesis that menopause might not only be genetic, but may also be a biological marker echoing society’s longevity.
The significant medical and technologic gains of the past century in medicine and to some extent, the constant betterment of living standards, have yielded an improvement in life expectancy. In some countries increases of more than 40 years of life expectancy have been observed during the past 100 years alone.4 The life expectancy for women at birth in the United States is age 79.9.5
As a consequence of this demographic evolution, a large proportion of the female population will spend more than one third of their lives after the menopause. In 1950 there were 220 million women in the world older than age 50. More than half of these women lived in the so-called developed world (112 million). In 1990, there were 467 million women older than age 50 around the globe, and by 2030 this number is expected to approach 1.2 billion. Roughly three out of four of these menopausal women will be in the developing world (total of 912 million).6 At a national level, the U.S. Census Bureau calculated a total of 35.5 million women older than age 50 during the 1990 census, and by 1997 the menopausal population in the United States represented almost 30% of the total female population. By 2030, the menopausal population in the United States is expected to reach 66.5 million, representing 38% of the female population and 20 % of the entire population.7
MENOPAUSE PHYSIOLOGY AND PATHOPHYSIOLOGY
The supply of primordial follicles in the female gonad is predetermined before birth and diminishes with age until the gonad is unable to provide enough mature follicles to sustain menstrual cyclicity.8 The peak number of germ cell count is found at 20 weeks’ gestation, with decreased numbers at birth and puberty. Based on the number of follicles at three successive stages of development, which were obtained by counting follicles in histologic sections of ovaries from 52 normal women, a mathematical model was developed to describe the rates of growth and death of ovarian follicles in human ovaries between ages 19 and 50.9 While the number of oocytes dwindles throughout a woman’s life,10 there seems to be a transition at age 38 when the rate of follicle disappearance is augmented considerably with age. As a consequence, an estimated total of 1500 follicles remain at age 50 from the 300,000 present at age 19. This high death rate of small follicles appears to be responsible for advancing the timing of ovarian failure, and therefore of menopause, to midlife in our species.
Age of Menopause
The mean age of menopause in normal women in the United States ranges between age 50 and 52.11 This number was based on a cross-sectional study, which is associated with recall bias. However, these findings have been confirmed in a large prospective cohort study of middle-aged women: the Massachusetts Women’s Health Study. The cohort of 2570 women that was followed confirmed that the median age of natural menopause was 51.3 years with a mean difference of 1.8 years between current smokers and nonsmokers. This study also showed that smokers not only show an earlier menopause, but also a shorter perimenopause.12
In other parts of the world, population-based surveys have shown earlier onset of menopause. For example, the median age of onset of menopause was 48 in a survey of 742 United Arab Emirates women.13 In certain regions of India, such as the state of Himachal Pradesh, the mean age of onset of menopause can be as low as 43.5 years, according to data from 500 postmenopausal women.14
Factors Affecting the Onset of Menopause
Table 24-1 includes several factors that have been linked with an earlier onset of menopause. Among these factors, smoking has been the most often linked environmental agent to early age of onset of menopause. However, these studies have been inconclusive with regard to duration and intensity of smoking. Midgette and Baron concluded after a review of 14 studies that the risk of being menopausal was approximately doubled for current smokers compared with nonsmokers among women age 44 to 55.15 However, in 2004 van Asselt and colleagues, using data from a Dutch population-based cohort of 5544 women, assessed the effect of smoking duration and intensity on age at menopause, correcting for the chronologic age-dependency of the variables concerned. After their modeling they concluded like previous researchers that smoking lowers the menopausal age. However, the reduction in the menopausal age appears not to be dependent on smoking duration, and it appears that smoking cigarettes could have an effect only around the time of menopause itself.16 In other words, the number of cigarettes smoked during perimenopause is apparently more significant than smoking history as the culprit of earlier age of onset of menopause among smoking women.
Table 24-1 Factors Associated with Earlier Onset of Menopause
Time Course of Oocyte Pool Depletion
It is estimated that the entire process from initiation of follicle growth until complete maturation and finally ovulation takes months.17,18 The majority of follicles will experience atresia by apoptosis at some point in their developmental process.19 Fifty percent of apoptosis occurs at the small antral follicle stage of 2.1 to 5mm. On the other hand, atresia of the resting follicles in the human fetus seems to be set off by a process of necrosis rather than apoptosis.20 It seems clear now that the age-related decline in ovarian function in women is the result of the decline in both quantity and quality of the resting ovarian follicle pool. Recently a total of 182 resting follicles from a young cadre of women (age 25–32) were compared with 81 resting follicles from an older group (age 38–45) for signs of age-related changes by transmission-electron microscopy. De Bruin and colleagues concluded that, in resting follicles, the morphologic changes with age are different from the changes seen in quality decline by atresia.20 The morphologic changes with age specifically comprise the mitochondria, the dilated smooth endoplasmic reticulum, and the Golgi complex.
Genetic Contribution
The age of natural menopause is determined by the interplay of genetic and environmental factors.21 There are cross-sectional22 and case-control23 population studies suggesting the existence of genetic variability in the age of menopause to be as high as 70%. However, a study conducted in the Netherlands in 164 mother-daughter pairs with a natural menopausal age estimated a hereditability of 44% (95% confidence interval [CI] 36%–50%). The authors conclude that these estimates are more accurate than those of previous studies that were done in twins and siblings because siblings shared many environmental factors.24
STAGES OF REPRODUCTIVE AGING
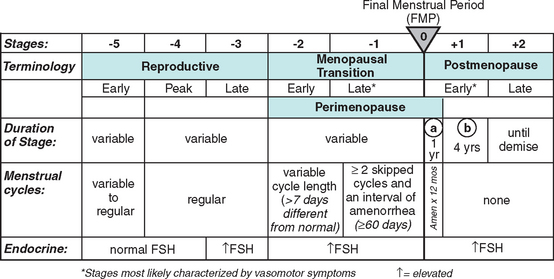
Until recently there was an absence of a relevant organized nomenclature system for the different stages of female reproductive aging. With this in mind the Stages of Reproductive Aging Workshop (STRAW) was held in Park City, Utah on July 23 and 24, 2001 (Fig. 24-1).
As noted in Figure 24-1 the anchor for the staging system is the final menstrual period (FMP). Five stages occur before and two after this anchor point. Stages −5 to −3 cover the reproductive period; stages −2 and −1 are the menopausal transition; and stages +1 and +2 are the postmenopause.
Among the most concrete achievements of STRAW was the development of clear and specific nomenclature, which was previously vague and confusing in the literature. The authors of STRAW recognize that this is a draft and these concepts might evolve as knowledge advances.25–27
CLINICAL SIGNS AND SYMPTOMS OF MENOPAUSE
The clinical diagnosis of menopause is established retrospectively once a patient has had more than 12 months of amenorrhea in conjunction with vasomotor symptoms such as hot flashes and headaches. At this point the patient has made the transition in the STRAW classification from −1 to +1.The range of symptoms due to immediate estrogen deficiency in women during STRAW stages −1 to +1 includes hot flashes and urogenital changes (see Table 24-1).25–27
Hot Flashes
The vasomotor flush is the most characteristic trait of estrogen deficiency. It is experienced at least once in 75% of women during menopause. It is also one of the most puzzling symptoms of menopause, because the etiology and physiology remain incompletely understood.28 It is thought to be the result of a hypothalamic dysregulation from estrogen withdrawal that culminates in peripheral vasodilation and increase in blood flow. This results in heat loss and a decrease in core body temperature. The hypothalamic dysfunction is also manifested by simultaneous pulse of LH and presumably gonadotropin-releasing hormone (GnRH) that is coincident with the hot flash. Hot flash, hot flush, night sweats, and vasomotor symptoms are words frequently used to express the same experience. Hot flashes are defined subjectively as the recurrent transient sensation of heat that can be accompanied by palpitations, perspiration, chills, shivering, and feeling of anxiety. It is then a heat dissipation response that habitually begins in the face, neck, and chest and often becomes generalized.29
In general we can divide the potential causes of hot flashes into seven categories: systemic diseases, neurologic, alcohol– medication interaction, drugs, food additives, eating, and miscellaneous30 (Fig. 24-2). However, is important to emphasize that these other causes are much less common than those associated with hypoestrogenemia.
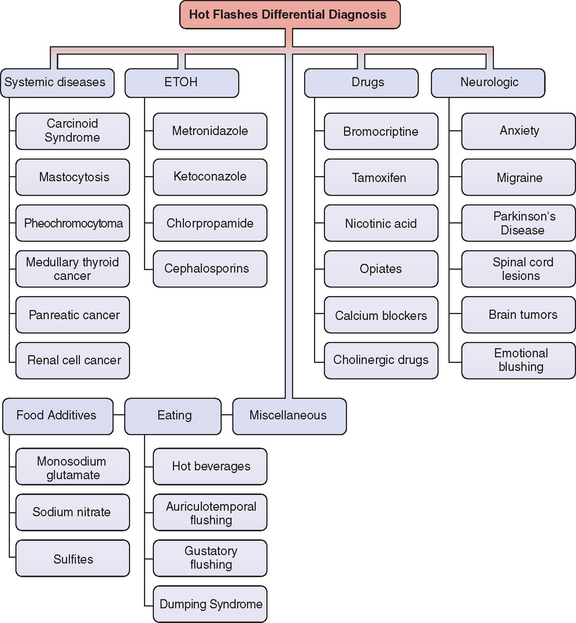
Figure 24-2 Differential diagnosis of flushing.
(Adapted from Mohyi D: Differential diagnosis of hot flashes. Maturitas 27:203–214, 1997.)
Systemic Diseases
Carcinoid Syndrome
These are mainly neuroendocrine tumors of the bowel. They may also be found in the bronchus, pancreatic islets, retroperitoneum, liver,31 and even in the ovary.32 They probably arise from gastrointestinal or bronchopulmonary pluripotential stem cells.33 The carcinoid syndrome clinically has a classic triad of diarrhea, flushing, and valvular heart lesions. Skin flushing is the most common sign and is present in more than 90% of patients. The mechanism of flushing is at least partially due to serotonin release, but other substances, such as kinin, substance P, neurotensin, and prostaglandin, may play a role.33
Mastocytosis
Mast cell proliferations can be limited to the skin (cutaneous) or can be spread to extracutaneous tissues (systematic). Vasomotor-like symptoms may be present in these patients because the mast cell granules contain a number of acid hydrolases, leukotrienes, histamine, heparin, and slow-reacting substance.34
Pheochromocytoma
These tumors often arise from the adrenal medulla. Most of their clinical characteristics are due to the production, storage, and secretion of catecholamines. The hallmark sign in these patients is hypertension, which is present in 60% of patients. A significant number of them suffer hot flashes. Documenting increased urinary catecholamines makes the diagnosis.35
Medullary Carcinoma of the Thyroid
Medullary carcinoma of the thyroid is a malignant tumor that originates from the parafollicular or thyroid C cells. These tumor cells typically produce an early biochemical signal (hypersecretion of calcitonin).36 This cancer can occur sporadically, but many times is inherited in an autosomal dominant way as a part of the syndrome multiple endocrine neoplasia type 2.37 Other bioactive substances that can be secreted by medullary carcinomas and may be responsible for the vasomotor symptoms include corticotropin, corticotropin-releasing hormone, and prostaglandins.30
Alcohol–Medication Interaction
Alcohol and numerous drugs are associated with vasomotor symptoms. In some cases the drug is not the real vasoactive agent, but rather a metabolite or another mediator triggered by the drug ingested. Some drugs will only create vasomotor symptoms when combined with alcohol.38 Others, such as calcium channel blockers, have a direct impact on the vessels.39 On the other hand, tamoxifen or bromocriptine produce hot flashes by triggering different mediators.
Food Additives and Eating Habits
The main limitation in our ability to assess the value of a treatment for hot flashes is the lack of an objective measure. This complex task is due to the current inability to reliably identify when a hot flash has taken place. The main objective method used today, sternal skin conductance monitoring, has some limitations, but the main weakness is the failure of sternal skin conductance to provide any information on duration, intensity, and interference with patient activities. Therefore, all data are derived from imperfect methods.28
Morbidity Associated with Hot Flashes
Sleep Disturbances
The relationship between hot flashes and interference with sleep is controversial. Multiple epidemiologic studies have shown an association between awakening and arousal from sleep and hot flashes in menopausal women.40–42 This has led to the commonly held conception that hot flashes and night sweats cause awakening, which subsequently creates fatigue, and possibly decreases performance and quality of life.43 The flaw in these studies is that these hypotheses have not been properly tested in controlled laboratory investigations. Also, neither of these investigations screened out patients with apnea and other sleep disturbances that are also prevalent in menopause and may represent a confounder factor.
Recently, Freedman and Roehrs studied 31 patients between ages 46 and 51 who were classified into three groups: premenopausal asymptomatic (cycling), postmenopausal asymptomatic (asymptomatic), and postmenopausal symptomatic (symptomatic). They then assessed several outcome measures: sleep electroencephalogram recordings, sternal skin conductance to record hot flashes, multiple sleep latency tests to assess sleepiness, simple and divided attention performance tests, and sleep and fatigue questionnaires. There were no significant differences among the three groups on any sleep variable. Of the awakenings taking place within 2 minutes of a hot flash, 55.2% happened before the hot flash, 40% after the hot flash, and 5% simultaneously. Of arousals taking place within 2 minutes of a hot flash, 46.7% occurred before, 46.7% after, and 5.6% simultaneously. There were no significant group differences on any self-report measure or on any performance measure. They concluded that there is no evidence that hot flashes produce sleep disturbances in symptomatic postmenopausal women.44
Migraines
There is sufficient observational data to suggest a link between hormones and migraines.45,46 However, the relationship between menopause and migraine is still being debated. Observational studies suggest that migraine worsens just before menopause and improves after cessation of menses in approximately two thirds of cases.
Neri, in a sample of 556 postmenopausal patients, studied the prevalence and characteristics of headaches in this cohort and found that many of them had migraine with aura. Interestingly, women with prior migraine generally improved with the onset of spontaneous menopause. In contrast, women with bilateral oophorectomy usually experienced worsening of their migraines.47 More recently, a cross-sectional, community-based study of 1436 women using the 1988 International Headache Society Criteria showed the highest prevalence of migraines in the perimenopausal group (31%) and the lowest (7%) in the postmenopausal group.48
Urogenital Changes
The lack of estrogen has been associated with a decrease in the moisture of the genital tissues. The persistent dryness of the vaginal mucosal surfaces may lead to symptoms of vaginitis, pruritus, dyspareunia, and even stenosis. Other symptoms that may be related to estrogen deprivation in the urogenital tissues are dysuria, urgency incontinence, and urinary frequency. It is unclear whether all these symptoms are related to the lack of estrogen or are part of the degenerative process of aging. It is postulated that changes in estrogen levels change the quality of the collagen content and the connective tissues in the urogenital area.49
More controversial are data suggesting that lack of estrogen increases the likelihood of menopausal women to experience recurrent urinary tract infections (UTIs). A randomized, placebo-controlled trial of vaginal estrogen in 93 postmenopausal women demonstrated that patients being treated could reduce their number of UTIs per year. This study observed 0.5 episodes of UTI in the treatment groups versus 5.9 episodes in the placebo group.50 On the other hand data from the Heart and Estrogen/Progestin Replacement Study (HERS) showed that urinary tract infection frequency was higher in the group randomized to hormone treatment, although the difference was not statistically significant (odds ratio, 1.16; 95% CI, 0.99–1.37).51
However conflicting these results are, from the clinician’s point of view it seems prudent to attempt a trial of vaginal estrogen therapy to address several of these postmenopausal urogenital symptoms. It should be considered in the presence of a vaginal pH greater than 4.5. Like FSH, elevated vaginal pH appears to be a good predictor of estrogen status.52,53
LONG-TERM MORBIDITY ASSOCIATED WITH POSTMENOPAUSAL STATUS
The two major long-term risks associated with menopause are osteoporosis and cardiovascular disease.
Osteoporosis
Chapter 25 reviews osteoporosis in detail. In this section we discuss its relationship as a long-term risk factor in menopausal women. The demographic changes and longevity increase described at the beginning of this chapter, coupled with the fact that osteoporosis rises dramatically with age, makes osteoporosis a serious economic burden for healthcare systems in our society.54 The estimated total direct expenditures (hospitals and nursing homes) for osteoporotic and associated fractures was $17 billion in 2001 ($47 million each day).55 The National Osteoporosis Foundation estimates that 50% of white women will suffer at least one osteoporosis-related fracture in their remaining lifetime. At least 90% of hip and spine fractures among elderly women can be attributed to osteoporosis.56 These statistics are the result of an accelerated decline in bone mass after menopause. However, this decline begins at approximately age 35 when a disparity between bone formation and bone resorption begins to occur. After menopause there are two periods of net bone loss: an accelerated stage that begins with the onset of menopause (1 to 3 years) and continues for 5 to 8 years57 (STRAW 0, 1, and 2) and a prolonged, slower stage of bone loss that remains throughout STRAW 2. The initial accelerated phase may account for bone loss of up to 30%.58
The three most common osteoporotic related fractures are hip, vertebral, and wrist. The most common are vertebral fractures, accounting for 700,000 cases a year in the United States.55 These should be suspected in postmenopausal women with back pain, loss of height, and kyphosis. In one observational study of 7223 postmenopausal women over age 65, patients with radiographically detected vertebral fractures were found to have significantly more limited-activity days, whether they were symptomatic or not.59 These data should raise awareness for the clinician of the decreased quality of life that menopausal patients may experience even with asymptomatic fractures.
The second most common fracture is hip fracture, accounting for 300,000 cases a year in the United States.55 These are without doubt the more serious consequence of osteoporosis in postmenopausal women. One in 5 women will die within 1 year after fracture, and 1 in 2 will have permanent loss of function.60 Lastly, distal forearm fractures occur in 250,000 patients a year in the United States.55 Only half of the patients who suffer these fractures recover full function of the arm in 6 months.61
Cardiovascular Disease
The American Heart Association has designated cardiovascular disease a “silent epidemic.” Despite the overall decline in the mortality rate due to cardiovascular disease in the United States, the absolute number of deaths due to cardiovascular disease is actually increasing.62 This is in part due to the demographic changes in our society described in the introduction of this chapter. Cardiovascular disease, which encompasses heart attacks and strokes, is responsible each year for more deaths than all other causes combined in postmenopausal women.62,63 The burden and threat of this disease during menopause is in part due to the discrepancy among the reality of the problem and the perception of its magnitude by both physicians and patients. Nothing exemplifies this better than a 1995 Gallup survey, which revealed that 4 out of 5 women ages 45 to 75 were unaware that cardiovascular disease was the first cause of death for their age group. Instead most of the women quoted cancer, specifically breast cancer, as their most probable cause of death. In reality this represents only 4% of the causes of death in this age group. The primary care physicians questioned did not do very well either; 32% of them did not know that heart disease was the main cause of death in this age group of women.64
The incidence of cardiovascular disease and particularly myocardial infarction dramatically increases after menopause and approximates the mortality of this entity in men.65,66 Furthermore, bilateral oophorectomy or premature ovarian failure increases the risk of cardiovascular disease beyond that of natural menopause.67 Despite this seemingly logical association between estrogen cardioprotection and other coherent data from observational studies, the Women’s Health Initiative (WHI) group of trials and the HERS have found no role for estrogen as a primary or secondary prevention for cardiovascular disease in postmenopausal women.
MEDICAL TREATMENT OF MENOPAUSE
The results of the Women’s Health Initiative study (WHI) have altered the principles of medical practice in menopausal women. We have changed from the concept of prevention of chronic diseases encompassed in the term hormone replacement therapy to the concept of hormone therapy. Thus, the U.S. Food and Drug Administration (FDA) and professional organizations such as the American College of Obstetricians and Gynecologists recommend the use of estrogen-containing medications to be restricted to the treatment of vasomotor and vaginal symptoms. They also affirm that the lowest effective dose be prescribed for the shortest duration of time.68–70
Principles of Hormone Therapy
Key Findings from the Women’s Health Initiative
The WHI was a group of clinical trials designed to examine the impact of hormone therapy on cardiovascular disease and breast cancer, the effect of low-fat diet on breast and colon cancer, and the impact of vitamin D in calcium supplementation on fractures and colon cancer.71
In May 2002 the clinical trial that aimed to assess the cardiovascular effects of estrogen and progestin therapy in postmenopausal women with intact uterus was halted. The Data and Safety Monitoring Board reported that the estrogen/progestin treatment group had an increased risk of cardiovascular disease, thromboembolism, and breast cancer after 5.2 years of follow-up.72 In 2004, after 6.8 years of follow-up, the estrogen-only trial was halted.73 In this clinical trial, estrogen-only treatment demonstrated an increase risk of strokes similar to the one found in the estrogen/progestin clinical trial previously halted. They also reported a lack of benefit on cardiovascular disease incidents and a probable increase in dementia. The risks and benefits findings of the WHI are summarized in Table 24-2.74
Table 24-2 Women’s Health Initiative Findings: Outcomes Associated with Use of Combined Estrogen and Progestin and Estrogen Alone in Healthy Postmenopausal Women, Aged 50 to 79 Years
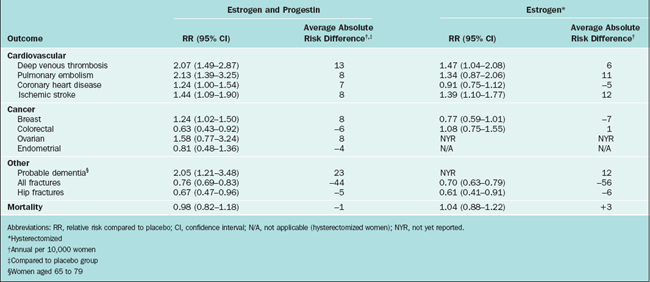
It should be emphasized that the WHI trial did not intend to evaluate the effects of estrogen or estrogen/progestin on vasomotor symptoms; therefore, these results must be translated into the specific needs of our patients when they request relief for hot flashes or other postmenopausal symptoms. It is also critical to point out that the rate of serious adverse events in patients treated with estrogen therapy is low, calculated to be 2 out of 1000 women treated per year.75 Healthcare providers and patients must balance the benefits of estrogen treatment versus the risk for adverse events. Today, more than ever, the concept of individualized menopausal care should be applied in the clinical setting.
Key Findings of the Heart and Estrogen/Progestin Replacement Study
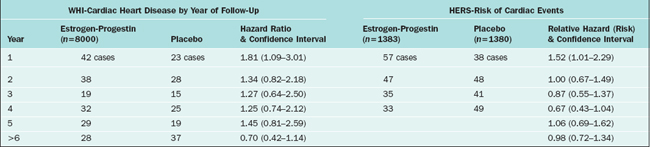
Whereas the WHI aimed to test the hypothesis that hormone therapy prevented cardiovascular disease in healthy postmenopausal women (primary prevention), the Heart and Estrogen/Progestin Replacement Study (HERS) intended to evaluate whether hormone therapy decreased the risk of coronary heart disease in postmenopausal women with established coronary disease. In this randomized trial, all the 2763 postmenopausal women had uterus and were allocated to either placebo (n = 1383) or 0.625 mg of conjugated equine estrogens plus 2.5 mg of medroxyprogesterone acetate daily (n = 1380).76,77 The primary outcome measures were nonfatal myocardial infarction and death from coronary heart disease.
The results of the HERS trial have been reported in two publications: HERS and HERS II. The HERS report is the result of randomized, blinded, placebo-controlled trial for 4.1 years; the HERS II reflects the unblinded follow-up for 2.7 more years.76,77 Both of the studies demonstrated that in patients with established heart disease, the use of estrogen and progestin does not prevent additional cardiovascular events. There were no differences in the primary or secondary outcomes of patients in the placebo or the treatment group.
The conclusion of HERS and HERS II is that postmenopausal hormone therapy should not be recommended for the purpose of reducing the risk of cardiovascular events. Table 24-3 shows a comparison between the WHI and HERS trials.
Key Findings of the Million Women Study
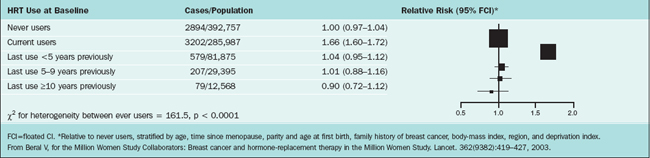
The Million Women Study is a prospective cohort study, which included 1,084,110 British women age 50 to 64 recruited between 1996 and 2001. This study was undertaken by the UK National Health Service Breast Screening Programme, targeting women age 50 to 64 undergoing routine screening once every 3 years. Because only approximately half of these patients had ever taken estrogen after menopause, the aim of the study was to investigate the relation between various combinations of hormone therapy and two main outcomes: breast cancer and mortality.78 All patients filled out a questionnaire and were monitored in this manner. This questionnaire is Available at: http://www.millionwomenstudy.org. A major strength of this study was the unparalleled database size, which was sufficiently powered to quantify absolute and relative risks and enabled researchers to discern the effects among different preparations of hormones in use among postmenopausal women. One weakness of the study was that hormone use versus nonuse was determined on admission to the study and was not modified during follow-up even though there were potential multiple crossover treatments in some of the subjects. The authors reached these conclusions:
The relative risks for invasive breast cancer in relation to current of use of hormone therapy and type of hormone preparations are illustrated in Tables 24-4 and 24-5.
Candidates for Hormone Therapy during Menopause
The medical management of menopausal symptoms has evolved to a personalized approach. Personal choice is important, and some will never take hormone therapy under any circumstances. The physician must consider many variables before offering hormone therapy, such as personal, cardiovascular, osteoporosis, breast cancer risk, the number and severity of estrogen-deficient symptoms.
Systemic Estrogen Therapy
Oral Estrogen
Several oral preparations of estrogen are available, the most commonly used being Premarin (Table 24-6). This compound of conjugated equine estrogens derived from pregnant mare’s urine consists mostly of estrone sulfate, equilin sulfate, dihydroequilin sulfate, and many other minor estrogens. There are also conjugated synthetic estrogens such as Cenestin that are derived from plant sources. Esterified estrogens, such as Estratab or Menest, are derived from plant sources as well. Ethinyl estradiol is found in oral contraceptives but also in one product for hormone therapy called FemHRT. Estrace is a micronized form of estradiol. Although there are different dosages and potencies of estrogens, there is no difference in their efficacy.79
Table 24-6 Estrogen Products, Routes of Administration, and Available Doses
| Product | Available Doses Oral |
|---|---|
| Synthetic conjugated estrogens (Cenestin, Enjuvia) | 0.3 mg, 0.45 mg, 0.625 mg, 1.25 mg |
| Equine conjugated estrogens (Premarin) | 0.3 mg, 0.45 mg, 0.625 mg, 0.9 mg, 1.25 mg, 2.5 mg |
| Micronized estradiol (Estrace, Gynodiol) | 0.5 mg, 1.0 mg, 2 mg |
| Esterified estrogens (Menest) | 0.3 mg, 0.625 mg, 1.25 mg, 2.5 mg |
| Estropipate (Ogen, Ortho-Est) | 0.625 mg, 1.25 mg, 2.5 mg |
| Transdermal Systems | |
| 17-β estradiol (Estraderm, Vivelle, Alora, Climara, Esclim, Menostar) | 0.025 mg, 0.0375 mg, 0.05 mg, 0.075 mg–0.1 mg/day 14 g/day |
| 17-β estradiol plus norethindrone acetate or levonorgestrel (Combipatch, Climara Pro) | |
Due to the concerns about cardiovascular disease and thromboembolism with the use of estrogen therapy, new low-dose regimens have been approved by the FDA for treatment of vasomotor symptoms and osteoporosis: Prempro in dosage strengths of 0.45mg conjugated estrogen/1.5mg medroxyprogesterone acetate and 0.3mg conjugated estrogen/1.5mg medroxyprogesterone acetate. The HOPE trial showed no evidence of endometrial hyperplasia in women treated with conjugated equine estrogens/medroxyprogesterone acetate regardless of the dose.80 These clinical trials have also demonstrated that low doses of estrogen are adequate for the prevention of bone loss.
Transdermal Estrogen
All transdermal systems currently available contain the same estrogen, 17β-estradiol, in different dosages. A dose of 50μg/day in a transdermal delivery system is bioequivalent to an oral dose of 0.625μg of conjugated equine estrogens.81 The lowest-dose transdermal delivery system approved for prevention of osteoporosis (Menostar) has only 0.014mg/day of estradiol.82
Topical Estrogen
The products available are Estrogel and Estrasorb. A randomized, controlled trial of 225 menopausal women demonstrated that a topical gel containing 1.25 or 2.5g was much more effective than placebo in alleviating the frequency and intensity of moderate and severe hot flashes in symptomatic women.83
Selective Estrogen Receptor Modulators
The usefulness of alternative medications to treat or prevent chronic diseases such as osteoporosis has been increasing. The mixed estrogen agonist-antagonist action that selective estrogen receptor modulators (SERMs) demonstrated in specific target tissues such as bone and their binding with estrogen receptors makes them a good option for some patients.84 There are many reasons why SERMs produce their tissue-selective effects, such as differences in receptor binding affinities and interaction with different coactivators and corepressors.85
Tamoxifen
Tamoxifen is a SERM that may act as an estrogen receptor agonist (i.e., uterus) or as an antagonist (i.e., breast).86 As a result tamoxifen can have beneficial effects in osteoporosis, breast cancer, and cardiovascular disease. Tamoxifen also stimulates endometrial proliferation and increases vasomotor symptoms.
In the largest randomized clinical trial aimed to test the hypothesis that tamoxifen reduces the incidence of breast cancer in patients at high risk for the disease, fracture risk was determined as a secondary endpoint. In The National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 Trial, 13,338 women were randomized to placebo or 20mg of tamoxifen a day for 5 years. The trial demonstrated a significant decrease in the incidence of invasive and noninvasive breast cancer, and a decrease in the incidence of vertebral, wrist (Colles’), and hip fractures in the treatment group when compared with the placebo. In this same study, patients in the tamoxifen group did not experience a higher rate of ischemic heart disease. However, the risk for endometrial cancer, especially in women over age 50, was increased (RR, 2.53; 95% CI, 1.35–4.97), with all of the endometrial cancers detected in early stage (I).87
Raloxifene
Raloxifene was discovered over 20 years ago in an effort to develop an antiestrogenic drug to treat and/or prevent breast cancer and was previously known as keoxifene.88,89 Raloxifene, like other SERMs, seems to compete with endogenous estrogens for attaching to the cytoplasmic receptor and may either turn on or turn off estrogen-mediated transcription. The complete molecular mechanism by which estrogens and SERMs exercise their biologic actions on various tissues has not been entirely understood and still is an area of intensive investigation. Raloxifene reduced the risk of osteoporosis in postmenopausal women without increasing their risk for uterine cancer or endometrial hyperplasia.90,91 Raloxifene at 60mg/day is currently approved for the prevention and treatment of osteoporosis.
Some clinicians prefer to use biphosphonates as a first option because of their more potent antiresorptive activity compared to raloxifene.92 A comparative trial compared the two classes of drugs. In the EFFECT trial (EFficacy of Fosamax versus Evista Comparison Trial), patients in the alendronate group were found to have substantially greater increases in bone mineral density (BMD) than those in the raloxifene group at both lumbar spine and hip sites at 12 months. Lumbar spine BMD increased 4.8% with alendronate versus 2.2% with raloxifene (P < 0.001). The increase in total hip BMD was 2.3% with alendronate versus 0.8% with raloxifene (P < 0.001). Decreased bone turnover was more significant with alendronate than raloxifene. Overall tolerability was comparable. However, the number of patients reporting vasomotor symptoms was considerably higher with raloxifene (9.5%) than with alendronate (3.7%, P = 0.010); an equal number of patients reported adverse gastrointestinal side effects.91
Besides the positive impact in intermediate outcome measures such as BMD and bone markers, raloxifene has been proven to reduce the risk of osteoporotic vertebral fractures. In the MORE trial (Multiple Outcomes of Raloxifene Evaluation) a multicenter, international, double-blind, placebo-controlled trial, 7705 women age 31 to 80 years in 25 countries who had been postmenopausal for at least 2 years were randomized to one of three groups: 60mg/day of raloxifene, 120 mg/day of raloxifene, or placebo. After 36 months of follow-up the risk of vertebral fracture was reduced in both study groups receiving raloxifene (for 60-mg/d group: relative risk [RR], 0.7; 95% CI, 0.5–0.8; for 120-mg/d group: RR, 0.5; 95% CI, 0.4–0.7).93 This study also demonstrated that breast cancer was less common in the treatment arms than in the placebo arm. Thirteen cases of breast cancer were confirmed among the women assigned to raloxifene versus 27 among the women assigned to placebo (RR, 0.24; 95% CI, 0.13–0.44; P<.001).94 A follow-up STAR trial (Study of Tamoxifen And Raloxifene) will compare raloxifene and tamoxifen for the prevention of breast cancer.
Raloxifene studies have also shown improvement in some intermediate cardiovascular outcome measures, such as serum low-density lipoprotein, lipoprotein (a), homocysteine, and plasma fibrinogen.95,96 To test the hypothesis that raloxifene reduces risk of coronary events (coronary death, nonfatal myocardial infarction, or hospitalized acute coronary syndromes other than myocardial infarction) the Raloxifene Use for The Heart (RUTH) trial was begun in 1998.97
Tibolone
Tibolone is a synthetic steroid that has been shown to be effective in alleviating menopausal symptoms and preventing bone loss. It has been widely used in Europe and the rest of the world since 1988. Clinical trials are under way in the United States. Tibolone has estrogenic actions in the brain, vagina, and bone tissues, but lacks estrogenic activity in the endometrium and in breast tissue. Its multifaceted hormone properties may stem from the fact that tibolone is quickly biotransformed into three metabolites: 3α- and 3β-hydroxy-tibolone, each with estrogenic effects, and the Δ4-isomer, with progestogenic and androgenic effects. The tissue-selective actions of tibolone are the product of metabolism, enzyme control, and receptor activation that vary in responsive target tissues. This biotransformation takes places principally at the liver and intestine. These pharmacologic characteristics make tibolone unique and different from the typical SERMs.98
More recently, an even lower dose of tibolone was evaluated in a randomized clinical trial. This study included 90 postmenopausal women who were followed for 2 years and were allocated to tibolone 2.5mg (n = 30), tibolone 1.25mg (n = 30), and a control group (n = 30). All subjects received 1000mg of calcium per day. Gambacciani and colleagues demonstrated tibolone to be effective at lower doses, not only at preventing bone loss as measured by BMD, but also at alleviating vasomotor symptoms.99
Another interesting potential benefit of tibolone in postmenopausal women is the positive effect shown on libido in some clinical trials. In a small randomized trial, patients with postmenopausal changes in sexual desire were allocated to 2.5mg/day of tibolone (n = 14) or to 500mg/day of calcium. This trial showed that the patients treated with tibolone experienced an improvement in sexual desire after the third month of treatment, which was maintained until the end of treatment (12 months).100 Similar beneficial findings in sexual desire were shown in a small study of 50 postmenopausal patients who were treated with either tibolone or conjugated estrogens and medroxyprogesterone.101
In the large cohort Million Women Study, there was increased relative risk of breast cancer in current users of tibolone (RR, 1.45; 95% CI, 1.25–1.68).78 These results were not anticipated, because tibolone does not increase breast density and has been shown to have much less estrogenic activity in breast tissues than other agents in postmenopausal clinical trials.102,103
Nonhormonal Therapies
Clonidine
Clonidine is an antihypertensive medication that acts centrally as an α2-adrenergic agonist and can be administered orally or via a transdermal delivery system. Clonidine has been used to treat hot flashes in postmenopausal women and in postorchiectomized men.104 The evidence to support its use is limited. One controlled trial randomized 15 patients to placebo and 14 to transdermal clonidine. Subjects were followed for 8 weeks; 86% of the patients in the treatment group had a significant decrease in the frequency of vasomotor episodes, 73% had a decrease in the severity of flushes, and 67% saw a decrease in the duration of hot flushes. In the placebo groups these benefits were present in 36%, 29%, and 21%, respectively.105 One characteristic consistent throughout the retrospective and prospective clinical trials of clonidine was the high percentage of patients with side effects. Dry mouth was experienced in up to 40% of patients, drowsiness and dizziness in up to 35%, and skin rash and irritation in 15% of oral and up to 50% in the transdermal system.
Selective Serotonin Reuptake Inhibitors
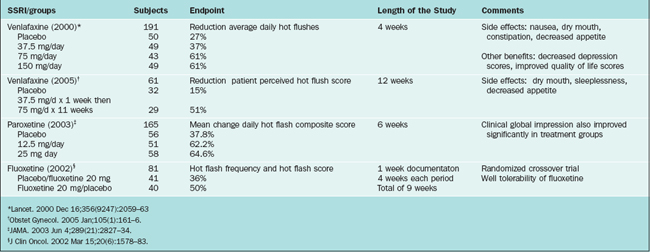
Th SSRIs (Table 24-7) seem to effectively decrease vasomotor instability in menopausal women by increasing the availability of serotonin in the central nervous system. These compounds should be considered a possible option to control hot flashes in women not willing to take estrogen or in whom estrogen is contraindicated. There are several open-label trials and enough randomized, controlled trials to currently recommend them as a reasonable option. There are studies evaluating citaprolam, paroxetine, and sertraline. Perhaps the most widely studied and used agent is venlafaxine, which is not strictly an SSRI because it inhibits both serotonin and norepinephrine reuptake.
Gabapentin
Gabapentin is a γ-aminobutyric acid analogue approved in 1994 for the treatment of seizures and has been used by neurologists to treat neuropathic pain, essential tremor, and migraines.106 In a randomized, double-blind, controlled trial that included 59 menopausal women with 7 or more severe hot flashes a day, a dose of 900mg/day of gabapentin was given for 12 weeks. Gabapentin was associated with a 45% reduction in hot flash frequency and a 54% reduction in hot flash composite score (frequency and severity combined into one score) from baseline, compared with 29% (P = 0.02) and 31% (P = 0.01) reductions, respectively, for placebo.107 One out of five patients taking gabapentin will experience dizziness and somnolence. Thus, this very expensive compound has minimal efficacy and is not an effective option for treatment of vasomotor flushes.
Veralipride
Veralipride is a neuroleptic antidopaminergic with antigonadotropic activity that is extensively used in Latin America and Europe for the treatment of vasomotor symptoms. This medication is currently not available in the United States. In a short 20-day, double-blind, randomized trial that included 43 symptomatic postmenopausal women, 21 subjects received 100mg/day veralipride and 22 received 1.25mg/day of conjugated estrogens. Individuals taking veralipride had a significant improvement comparable to estrogen. Tolerance was adequate in both groups.108 These findings have been confirmed in another pilot trial.109 Veralipride has also shown to be useful in treating the hot flashes caused by raloxifene. In fact a study demonstrated that giving raloxifene 60mg/day continuously and veralipride 100mg every other day eliminated the complaints of vasomotor symptoms in patients taking this SERM.110
DIETARY AND LIFESTYLE RECOMMENDATIONS DURING MENOPAUSE
Calcium Recommendations
One of the key elements to preventing osteoporosis is maintaining a positive calcium balance. This task becomes challenging during menopause because calcium absorption declines with age. Furthermore, the lower levels of estrogen during menopause decrease the levels of 1,25-dihydroxyvitamin D, with the subsequent consequence of even less calcium absorption.111 Nordin and coworkers measured radiocalcium absorption in 262 postmenopausal women age 40 to 87 and demonstrated a late age-related decrease (only after age 75) in calcium absorption in postmenopausal women in addition to the decline that occurs at menopause. They concluded that this decrease could be due to a decline in either the active calcium transport or the diffusion component of the calcium absorption system.112
According to the National Institutes of Health Consensus Development Conference on Optimal Calcium Intake held on 6–8 June 1994, the following are the calcium intake recommendations in women:113
The preferred method of reaching optimal calcium intake is through dietary sources. Among the foods with higher content of calcium are dairy products, green vegetables (e.g., broccoli, kale, turnip greens, Chinese cabbage), calcium-set tofu, some legumes, canned fish, seeds, nuts, and certain fortified food products, like bread and cereal. A good rule of thumb to calculate the daily intake of calcium is to multiply the number of milk servings (8 oz = 240mL = 1 cup) by 300mg.115 There are tables available that can be used to estimate the daily intake of calcium in a patient’s diet.114
Due to the challenges of achieving adequate calcium intake via food sources, many physicians advocate the use of calcium supplements. There are two main types of calcium compounds available in the market: calcium carbonate and calcium citrate. Some authors advocate the use of calcium citrate (Citracal) over calcium carbonate (Os-Cal), arguing that calcium citrate is better absorbed, especially on an empty stomach.115,116 On the other hand, calcium carbonate is usually less expensive and according to at least one study of absorbability, bioavailability, and cost-effectiveness the carbonate formulation is a better supplement choice in a population at risk for both low BMD and hip fracture.117
Vitamin D
Vitamin D is not usually found in food. It is produced by the skin in response to light exposure. There is controversy regarding the right amount of oral intake of vitamin D that is required. The Recommended Dietary Allowance (RDA) in women age 51 or older is 400 IU (10μg/day) and is based on the dose needed to prevent rickets.118,119 A dose of 800 IU should be considered for those living in Northern climates.
Exercise
Many health-related exercise recommendations are based on studies done in men. However, a recent systematic review of all the randomized, controlled exercise trials in postmenopausal women delineates some benefits in intermediate outcome measures. In a systematic review, Asikainen and colleagues assessed 28 randomized, controlled trials with 2646 subjects. Based on this review of studies on postmenopausal women, they describe specific guidelines for health professionals to use when counseling their patient to adequately develop an exercise program.120
The training described probably will preserve normal body weight. When combined with a weight-reducing diet, exercise will likely reduce bone loss and increase muscle strength. Based on limited evidence, such exercise might also improve flexibility, balance, and coordination; decrease hypertension; and improve dyslipidemia.120
Tobacco and Alcohol
Cigarette smoking and excessive alcohol intake are related to increased risk of bone loss and cardiovascular disease. In women, more than two drinks per day is associated with an elevated risk of developing hypertension. On the other hand, mild consumption of alcohol (less than 7 units/week) in women may be associated with a cardioprotective effect. In a 12-year prospective study which included 85,709 healthy women age 35 to 59, Fuchs and colleagues concluded that mild to moderate consumption of alcohol could be associated with a decreased mortality rate for women, but mainly for those at high risk for coronary heart disease.121 Nevertheless, we should to be cautions when counseling for mild alcohol intake because it is also associated with increased incidence of breast cancer.122 This issue raises difficulties in the decision making for alcohol consumption for women.
ALTERNATIVE MEDICINE DURING MENOPAUSE
Botanical Dietary Supplements
In recent years more natural products have gained popularity for treatment of vasomotor symptoms. Among these products phytoestrogens are perhaps the ones with more widespread use. Phytoestrogens are plant-derived substances structurally related to estrogens that have weak affinity to estrogen receptors.123 Phytoestrogen-containing dietary supplements commonly employed to relieve vasomotor symptoms include flaxseed, red clover extract, evening primrose oil, and soy compounds. The difficulty of consuming these botanical dietary supplements is that their purity, potency, and effectiveness are not well established; however, they are popularly believed to be safe and effective for treatment of menopausal symptoms.124 These products are used by as many as 46% to 79% of menopausal patients in some surveys.125,126
A review of randomized, controlled trials of complementary and alternative medicine for the treatment of menopausal symptoms by Kronenberg and colleagues evaluated a total of 29 randomized, controlled trials of complementary and alternative therapies for hot flashes and other menopausal symptoms; of these, 12 dealt with soy or soy extracts, 10 with herbs, and 7 with other therapies. They concluded that although soy products seem to have modest benefit for hot flashes, studies are not definitive. Isoflavone preparations seem to be even less effective than soy products. Black cohosh may be effective for menopausal symptoms, especially hot flashes, but the lack of adequate long-term safety data (mainly on estrogenic stimulation of the breast or endometrium) precludes recommending long-term use.127
A more recent systematic review of 25 randomized, controlled trials from the Cochrane Library and MEDLINE from 1966 to March 2004 involving a total of 2348 patients concluded that the available evidence suggests that phytoestrogens available as soy foods, soy extracts, and red clover extracts do not improve hot flashes or other menopausal symptoms.128
For mild hot flashes, lifestyle-related strategies such as keeping the core body temperature cool, participating in regular exercise, and using paced respiration have shown some efficacy without adverse effects. Among nonprescription remedies, clinical trial results are insufficient to either support or refute efficacy for soy foods and isoflavone supplements (from either soy or red clover), black cohosh, or vitamin E; however, no serious side effects have been associated with short-term use of these therapies.129
Progesterone Cream from Yam Root
Progesterone can be synthesized commercially from the wild yam. Its use for alleviating menopausal symptoms is growing in the United States. The scientific evidence is controversial at best. A 12-month randomized, controlled trial of 102 healthy postmenopausal women given either a quarter teaspoon of cream (containing 20 mg progesterone) or placebo to the skin daily showed that although there was a significant improvement in the relief of vasomotor symptoms (83% in the treatment group vs. 19% in the placebo group) there was no difference in the BMD.130
In contrast a randomized, double-blind, placebo-controlled, crossover trial performed in 23 postmenopausal women with vasomotor symptoms using a topical cream containing wild yam extract (Dioscorea villosa), vitamin E, and other oils found no detrimental side effects nor any improvement in menopausal symptoms as documented in weekly diaries for 3 months.131 Similar results were found in a parallel, double-blind, randomized, placebo-controlled trial in 80 symptomatic postmenopausal women comparing the effect of a transdermal cream containing a progesterone (32mg/daily) with a placebo cream. This study showed no changes in mood characteristics or sexual feelings, nor was there any change in blood lipid levels or in bone metabolic markers, despite a slight elevation of blood progesterone levels.132
Acupuncture
In a small prospective open trial, 11 menopausal symptomatic patients who underwent acupuncture for 5 weeks experienced a significant improvement in menopausal vasomotor symptoms without any changes in reproductive hormones or psychosocial or sexual symptoms, as measured by the Menopause Specific Quality of life Questionnaire.133
A pilot study evaluated the effectiveness of acupuncture for the treatment of menopausal symptoms in 15 patients with breast cancer treated with tamoxifen 20mg/day. With the exception of libido, all of the other dimensions of the Green Menopause Index showed significant improvement (P<0.001) with acupuncture.134
Wyon and colleagues randomized 45 postmenopausal women with complaints of vasomotor symptoms to three study groups: electro-acupuncture, superficial needle insertion, or oral estradiol treatments for 12 weeks with a 6-month follow up. The patients in the electro-acupuncture group had a decrease in the mean number of hot flashes from 7.3 to 3.5, whereas superficial needle insertion patients decreased the mean number of hot flashes from 8.1 to 3.8. In the estrogen group, the number of hot flashes decreased from 8.4 to 0.8. The Kupperman index and the general climacteric symptoms score decreased and remained unchanged 24 weeks after treatment in all groups. Superficial needle insertion and electro-acupuncture were similar in efficacy to acupuncture treatment of vasomotor symptoms, although not as effective as estrogen.135
In Sweden a randomized, single-blind, controlled design was used to evaluate the effects of electro-acupuncture on general psychological distress related to climacteric symptoms in 30 postmenopausal women. In this study one group of patients received electro-acupuncture and another extremely superficial needle insertion, functioning as a near-placebo control. Patients were treated for 12 weeks and general psychological well-being, mood, and experience of climacteric symptoms were used as outcome measures. This study showed enhancement in the mood scale in the acupuncture group, but climacteric symptoms and psychological well-being improved in both placebo and treatment groups, suggesting that electro-acupuncture is not any better than superficial needle insertion for amelioration of climacteric symptoms or improvement of well-being.136
1 McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med. 1985;103:350-356.
2 McKinlay SM. The normal menopause transition: An overview. Maturitas. 1996;23:137-145.
3 Weg RB. Demography. In: Mishell DRJr, editor. Menopause: Physiology and Pharmacology. Chicago: Year Book Medical Publishers; 1987:23-40.
4 World Health Organization. The World Health Report 1999: Making a difference. Geneva: World Health Organization, 1999.
5 Kochanek KD, Smith BL. Deaths: Preliminary data for 2002. Natl Vital Stat Rep. 2004;52:1-47.
6 World Health Organization. Research on the Menopause in the 1990s. WHO Technical Report Series No. 866. Geneva: World Health Organization, 1996.
7 U.S. Bureau of the Census: Statistical Abstract of the United States: 1998. (118th ed.) Washington, DC, 1998.
8 Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11:1484-1486.
9 Faddy MJ, Gosden RG. A mathematical model of follicle dynamics in the human ovary. Hum Reprod. 1995;10:770-775.
10 Faddy MJ, Gosden RG, Gougeon A, et al. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum Reprod. 1992;7:1342-1346.
11 McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med. 1985;103:350-356.
12 McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103-115.
13 Rizk DE, Bener A, Ezimokhai M, et al. The age and symptomatology of natural menopause among United Arab Emirates women. Maturitas. 1998;29:197-202.
14 Randhawa I, Premi HK, Gupta T. The age at menopause in the women of Himachal Pradesh, and the factors affecting the menopause. Ind J Public Health. 1987;31:40-44.
15 Midgette AS, Baron JA. Cigarette smoking and the risk of natural menopause. Epidemiology. 1990;1:474-480.
16 van Asselt KM, Kok HS, van Der Schouw YT, et al. Current smoking at menopause rather than duration determines the onset of natural menopause. Epidemiology. 2004;15:634-639.
17 Gougeon A. Some aspects of the dynamics of ovarian follicular growth in the human. Acta Eur Fertil. 1989;20:185-192.
18 Gougeon A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr Rev. 1996;17:121-155.
19 Yuan W, Giudice LC. Programmed cell death in human ovary is a function of follicle and corpus luteum status. J Clin Endocrinol Metab. 1997;82:3148-3155.
20 de Bruin JP, Dorland M, Spek ER, et al. Ultrastructure of the resting ovarian follicle pool in healthy young women. Biol Reprod. 2002;66:1151-1160.
21 de Bruin JP, Bovenhuis H, van Noord PAH, et al. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16:2014-2018.
22 Torgerson DJ, Thomas RE, Reid DM. Mothers and daughters menopausal ages: Is there a link? Eur J Obstet Gynecol Reprod Biol. 1997;74:63-66.
23 Cramer DW, Xu H, Harlow BL. Family history as a predictor of early menopause. Fertil Steril. 1995;64:740-745.
24 van Asselt KM, Kok HS, Pearson PL, et al. Heritability of menopausal age in mothers and daughters. Fertil Steril. 2004;82:1348-1351.
25 Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric. 2001;4:267-272.
26 Soules MR, Sherman S, Parrott E, et al. Stages of Reproductive Aging Workshop (STRAW). J Womens Health Gend Based Med. 2001;10:843-848.
27 Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril. 2001;76:874-878.
28 Miller HG, Li RM. Measuring hot flashes: Summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79:777-781.
29 Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13:453-464.
30 Mohyi D, Tabassi K, Simon J. Differential diagnosis of hot flashes. Maturitas. 1997;27:203-214.
31 Sonnet S, Wiesner W. Flush symptoms caused by a mesenteric carcinoid without liver metastases. JBR-BTR. 2002;85:254-256.
32 Torvik A. Carcinoid syndrome in a primary tumour of the ovary. Acta Pathol Microbiol Scand. 1960;48:81-88.
33 Lips CJ, Lentjes EG, Hoppener JW. The spectrum of carcinoid tumours and carcinoid syndromes. Ann Clin Biochem. 2003;40:612-627.
34 Valent P, Sperr WR, Schwartz LB, Horny HP. Diagnosis and classification of mast cell proliferative disorders: Delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114:3-11.
35 Manger WM, Eisenhofer G. Pheochromocytoma: Diagnosis and management update. Curr Hypertens Rep. 2004;6:477-484.
36 Moley JF. Medullary thyroid cancer. Surg Clin North Am. 1995;75:405-420.
37 Gertner ME, Kebebew E. Multiple endocrine neoplasia type 2. Curr Treat Options Oncol. 2004;5:315-325.
38 Wilkin JK. Quantitative assessment of alcohol-provoked flushing. Arch Dermatol. 1986;122:63-65.
39 Wilkin JK. Effect of nadolol on flushing reactions in rosacea. J Am Acad Dermatol. 1989;20:202-205.
40 Baker A, Simpson S, Dawson D. Sleep disruption and mood changes associated with menopause. J Psychosom Res. 1997;43:359-369.
41 Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30:41-50.
42 Erlik Y, Tataryn IV, Meldrum DR, et al. Association of waking episodes with menopausal hot flushes. JAMA. 1981;245:1741-1744.
43 Stone AB, Pearlstein TB. Evaluation and treatment of changes in mood, sleep, and sexual functioning associated with menopause. Obstet Gynecol Clin N Am. 1994;21:391-403.
44 Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82:138-144.
45 MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354-361.
46 Silberstein SD. Headache and female hormones: What you need to know. Curr Opin Neurol. 2001;14:323-333.
47 Bono G, Neri I, Granella F, et al. Characteristics of headache at menopause: A clinico-epidemiologic study. Maturitas. 1993;17:31-37.
48 Wang SJ, Fuh JL, Lu SR, et al. Migraine prevalence during menopausal transition. Headache. 2003;43:470-478.
49 Falconer C, Ekman-Ordeberg G, Ulmsten U, et al. Changes in paraurethral connective tissue at menopause are counteracted by estrogen. Maturitas. 1996;24:197-204.
50 Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. NEJM. 1993;329:753-756.
51 Brown JS, Vittinghoff E, Kanaya AM, et alfor the Heart and Estrogen/Progestin Replacement Study Research Group. Urinary tract infections in postmenopausal women: Effect of hormone therapy and risk factors. Obstet Gynecol. 2001;98:1045-1052.
52 Caillouette JC, Sharp CFJr, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270-1277.
53 Roy S, Caillouette JC, Roy T, Faden JS. Vaginal pH is similar to follicle-stimulating hormone for menopause diagnosis. Am J Obstet Gynecol. 2004;190:1272-1277.
54 Melton LJIII, Chrischilles EA, Cooper C, et al. Perspective: How many women have osteoporosis? J Bone Miner Res. 1992;7:1005-1010.
55 National Osteoporosis Foundation. Osteoporosis: Disease statistics: “Fast facts.”. Available at http://www.nof.org/osteoporosis/stats.htm. Accessed 6 December 2004.
56 Melton LJ3rd, Thamer M, Ray NF, et al. Fractures attributable to osteoporosis: Report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:16-23.
57 Ahlborg HG, Johnell O, Nilsson BE, et al. Bone loss in relation to menopause: A prospective study during 16 years. Bone. 2001;28:327-331.
58 Riggs BL, Melton LJ3rd. Involutional osteoporosis. NEJM. 1986;314:1676-1686.
59 Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: A prospective study. Ann Intern Med. 1998;128:793-800.
60 Forsen L, Sogaard AJ, Meyer HE, et al. Survival after hip fracture: Short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73-78.
61 Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull WHO. 2003;81:646-656.
62 American Heart Association. 1997 Heart and Stroke Facts: Statistical Update. Dallas: American Heart Association, 1996.
63 Eaker ED, Chesebro JH, Sacks FM, et al. Cardiovascular disease in women. Circulation. 1993;88:1999-2009.
64 Gallup Poll. Coronary Heart Disease: Women’s Heart Health Initiative. Princeton, N.J.: American Medical Women’s Association, 1995.
65 National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation. 1994;89:1333.
66 Gorodeski GI. Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women. Exp Gerontol. 1994;29:357-375.
67 Colditz GA, Willett WC, Stampfer MJ, et al. Menopause and the risk of coronary heart disease in women. NEJM. 1987;316:1105-1110.
68 American College of Obstetricians and Gynecologists. Hormone therapy executive summary. Obstet Gynecol. 2004;104:S1-S4.
69 North American Menopause Society. Report. Menopause. 2003;10:6-12.
70 This guidance was developed by the Division of Reproductive and Urologic Drug Products (DRUDP) in the Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA). Available at http://www.fda.gov/cder/guidance/5412dft.doc. Accessed 9 December 2004.
71 Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18-S77.
72 Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
73 Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee: Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
74 Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy: Scientific review. JAMA. 2002;288:872-881.
75 Grady D. Postmenopausal hormones—therapy for symptoms only. NEJM. 2003;348:1835-1837.
76 Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
77 Grady D, Herrington D, Bittner V, et alfor the HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57.
78 Beral V, et alfor the Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
79 Mashchak CA, Lobo RA, Dozono-Takano R, et al. Comparison of pharmacodynamic properties of various estrogen formulations. Am J Obstet Gynecol. 1982;144:511-518.
80 Pickar JH, Yeh IT, Wheeler JE, et al. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: Two-year substudy results. Fertil Steril. 2003;80:1234-1240.
81 Baker VL. Alternatives to oral estrogen replacement. Transdermal patches, percutaneous gels, vaginal creams and rings, implants, other methods of delivery. Obstet Gynecol Clin North Am. 1994;21:271-297.
82 Menostar—a low-dose estrogen patch for osteoporosis. Med Lett Drugs Ther. 2004;46:69-70.
83 Archer DF, for the EstroGel Study Group. Percutaneous 17β-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
84 Fontana A, Delmas PD. Selective estrogen receptors modulators in the prevention and treatment of postmenopausal osteoporosis. Endocrinol Metab Clin North Am. 2003;32:219-232.
85 Khovidhunkit W, Shoback DM. Clinical effects of raloxifene hydrochloride in women. Ann Intern Med. 1999;130:431-439.
86 Jensen EV, Khan SA. A two-site model for antiestrogen action. Mech Ageing Dev. 2004;125:679-682.
87 Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
88 Black LJ, Jones CD, Falcone JF. Antagonism of estrogen action with a new benzothiophene derived antiestrogen. Life Sci. 1983;32:1031-1036.
89 Black LJ, Jones CD, Clark JH, Clemens JA. A unique antiestrogen displaying high affinity for estrogen receptors, negligible estrogenic activity and near-total estrogen antagonism in vivo. Breast Cancer Res Treat. 1982;2:279. 279
90 Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. NEJM. 1997;337:1641-1647.
91 Sambrook PN, Geusens P, Ribot C, et al. Alendronate produces greater effects than raloxifene on bone density and bone turnover in postmenopausal women with low bone density: Results of EFFECT (Efficacy of Fosamax versus Evista Comparison Trial) International. J Intern Med. 2004;255:503-511.
92 Johnell O, Scheele WH, Lu Y, et al. Additive effects of raloxifene and alendronate on bone density and biochemical markers of bone remodeling in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87:985-992.
93 Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637-645.
94 Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
95 Walsh BW, Kuller LH, Wild RA, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451.
96 Mijatovic V, van der Mooren MJ, Kenemans P, et al. Raloxifene lowers serum lipoprotein (a) in healthy postmenopausal women: A randomized, double-blind, placebo-controlled comparison with conjugated equine estrogens. Menopause. 1999;6:134-137.
97 Mosca L, Barrett-Connor E, Wenger NK, et al. Design and methods of the Raloxifene Use for The Heart (RUTH) study. Am J Cardiol. 2001;88:392-395.
98 Kloosterboer HJ. Tissue-selectivity: The mechanism of action of tibolone. Maturitas. 2004;48(Suppl 1):S30-S40.
99 Gambacciani M, Ciaponi M, Cappagli B, et al. A longitudinal evaluation of the effect of two doses of tibolone on bone density and metabolism in early postmenopausal women. Gynecol Endocrinol. 2004;18:9-16.
100 Palacios S, Menendez C, Jurado AR, et al. Changes in sex behaviour after menopause: Effects of tibolone. Maturitas. 1995;22:155-161.
101 Kokcu A, Cetinkaya MB, Yanik F, et al. The comparison of effects of tibolone and conjugated estrogen-medroxyprogesterone acetate therapy on sexual performance in postmenopausal women. Maturitas. 2000;36:75-80.
102 Pantidou A, Kaplanis K, Chrissogonidis I, Destouni C. Mammographic changes during postmenopausal hormonal replacement therapy with tibolone. Eur J Gynaecol Oncol. 2004;25:493-494.
103 Kutlu T, Ficicioglu C, Basaran T, et al. Mammographic breast density changes after 1 year of tibolone use. Maturitas. 2004;48:133-136.
104 Parra RO, Gregory JG. Treatment of post-orchiectomy hot flashes with transdermal administration of clonidine. J Urol. 1990;143:753-754.
105 Nagamani M, Kelver ME, Smith ER. Treatment of menopausal hot flashes with transdermal administration of clonidine. Am J Obstet Gynecol. 1987;156:561-565.
106 Magnus L. Nonepileptic uses of gabapentin. Epilepsia. 1999;40(Suppl 6):S66-S72.
107 Guttuso TJr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2003;101:337-345.
108 Wesel S, Bourguignon RP, Bosuma WB. Veralipride versus conjugated oestrogens: A double-blind study in the management of menopausal hot flushes. Curr Med Res Opin. 1984;8:696-700.
109 Verbeke K, Dhont M, Vandekerckhove D. Clinical and hormonal effects of long-term veralipride treatment in post-menopausal women. Maturitas. 1988;10:225-230.
110 Morgante G, Farina M, Cianci A, et al. Veralipride administered in combination with raloxifene decreases hot flushes and improves bone density in early postmenopausal women. Gynecol Endocrinol. 2004;18:194-198.
111 Heaney RP, Recker RR, Stegman MR, Moy AJ. Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr. 2004;80:998-1002.
112 Nordin BE, Need AG, Morris HA, et al. Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr. 2004;80:998-1002.
113 NIH Consensus Development Panel on Optimal Calcium Intake. Optimal calcium intake. JAMA. 1994;272:1942-1948.
114 Weaver CM, Proulx WR, Heaney R. Choices for achieving adequate dietary calcium with a vegetarian diet. Am J Clin Nutr. 1999;70(3 Suppl):543S-548S.
115 Heller HJ, Greer LG, Haynes SD, et al. Pharmacokinetic and pharmacodynamic comparison of two calcium supplements in postmenopausal women. J Clin Pharmacol. 2000;40:1237-1244.
116 Heller HJ, Stewart A, Haynes S, Pak CY. Pharmacokinetics of calcium absorption from two commercial calcium supplements. J Clin Pharmacol. 1999;39:1151-1154.
117 Heaney RP, Dowell SD, Bierman J, et al. Absorbability and cost effectiveness in calcium supplementation. J Am Coll Nutr. 2001;20:239-246.
118 National Academy of Sciences. Recommended Dietary Allowances, 10th ed. Washington, D.C.: National Academy Press, 1989.
119 Available at http://www.nal.usda.gov/fnic/dga/rda.pdf (government website for RDA). Accessed on 23 December 2004.
120 Asikainen TM, Kukkonen-Harjula K, Miilunpalo S. Exercise for health for early postmenopausal women: A systematic review of randomised controlled trials. Sports Med. 2004;34:753-778.
121 Fuchs CS, Stampfer MJ, Colditz GA, et al. Alcohol consumption and mortality among women. NEJM. 1995;332:1245-1250.
122 Singletary KW, Gapstur SM. Alcohol and breast cancer: Review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143-2151.
123 Kam IW, Dennehy CE, Tsourounis C. Dietary supplement use among menopausal women attending a San Francisco health conference. Menopause. 2002;9:72-78.
124 Adams C, Cannell S. Women’s beliefs about “natural” hormones and natural hormone replacement therapy. Menopause. 2001;8:433-440.
125 Gokhale L, Sturdee DW, Parsons AD. The use of food supplements among women attending menopause clinics in the West Midlands. J Br Menopause Soc. 2003;9:32-35.
126 Mahady GB, Parrot J, Lee C, et al. Botanical dietary supplement use in peri- and postmenopausal women. Menopause. 2003;10:65-72.
127 Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: A review of randomized, controlled trials. Ann Intern Med. 2002;137:805-813.
128 Krebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: A systematic review. Obstet Gynecol. 2004;104:824-836.
129 North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: Position statement of The North American Menopause Society. Menopause. 2004;11:11-33.
130 Leonetti HB, Longo S, Anasti JN. Transdermal progesterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol. 1999;94:225-228.
131 Komesaroff PA, Black CV, Cable V, Sudhir K. Effects of wild yam extract on menopausal symptoms, lipids and sex hormones in healthy menopausal women. Climacteric. 2001;4:144-150.
132 Wren BG, Champion SM, Willetts K, et al. Transdermal progesterone and its effect on vasomotor symptoms, blood lipid levels, bone metabolic markers, moods, and quality of life for postmenopausal women. Menopause. 2003;10:13-18.
133 Dong H, Ludicke F, Comte I, et al. An exploratory pilot study of acupuncture on the quality of life and reproductive hormone secretion in menopausal women. J Altern Complement Med. 2001;7:651-658.
134 Porzio G, Trapasso T, Martelli S, et al. Acupuncture in the treatment of menopause-related symptoms in women taking tamoxifen. Tumori. 2002;88:128-130.
135 Wyon Y, Wijma K, Nedstrand E, Hammar M. A comparison of acupuncture and oral estradiol treatment of vasomotor symptoms in postmenopausal women. Climacteric. 2004;7:153-164.
136 Sandberg M, Wijma K, Wyon Y, et al. Effects of electro-acupuncture on psychological distress in postmenopausal women. Complement Ther Med. 2002;10:161-169.