CHAPTER 44 Meningitis and Encephalitis
Bacterial Meningitis
Pathophysiology
Pyogenic infections of the meninges originate either by hematogenous spread of bacteria or infected thrombi or by direct extension from bacterially colonized cranial structures adjacent to the meninges. Typical sources of direct extension include surgical or traumatic breaches of the paranasal sinuses or mastoid air cells, osteomyelitic foci within the skull, or congenital sinus tracts.1 Iatrogenic infection via an infected shunt, intraoperative contamination, or even a lumbar puncture needle is also possible. In animal models of bacteremia, placement of a needle into the subarachnoid space causes clustering of bacteria at the site of injury. It is tempting to extend this by analogy to humans, although there is no proof that this occurs in systemic human bacterial infection.
Cerebral tissue itself is relatively resistant to infection, and direct injection of virulent bacteria into the brains of animals seldom yields abscess formation. Some coincident infarction of brain tissue either by venous or arterial occlusion or by direct injury is the usual (and possibly requisite) antecedent event. Bacteria in the CSF, however, attract a robust inflammatory response and require no such additional injury. Parameningeal foci of infection, whether lodged in meningeal or superficial cerebral vessels or in adjacent sinus cavities or bone, simply require a physical breach in the arachnoid membrane to initiate bacterial colonization of CSF and result in frank meningitis. When septic material embolizes from an infected lung or congenital heart lesion or when it extends directly from the ears or sinuses, more than one type of bacterium can be found in the CSF. By contrast, hematogenous infections usually permit only one bacterial type to gain entry into the subarachnoid space. Bacterial pathogens differ by age of the patient (Table 44-1), and therapy initiated before identification of the pathogen is generally chosen to cover the typical pathogens found within the age group of the patient. Once bacteria enter the CSF space, they initiate a cascade of events within that space that extends throughout its reach. Thus, meningitis starting in the spine can easily cause cranial nerve dysfunction, and the converse is equally true. CSF within the ventricles is not spared because bacteria may enter it (and cause ventriculitis) either directly from infective emboli to the choroid plexus or by reflux of bacteria through the foramina of Magendie and Luschka.
| AGE | TYPICAL PATHOGENS |
|---|---|
| Neonates (0 to 4 wk) | Escherichia coli |
| Listeria monocytogenes | |
| Streptococcus (group B) | |
| Infants (4-12 wk) | Escherichia coli |
| Haemophilus influenzae | |
| Listeria monocytogenes | |
| Streptococcus (group B) | |
| Streptococcus pneumoniae | |
| Children (3 mo to 18 yr) | Haemophilus influenzae |
| Neisseria meningitidis | |
| Streptococcus pneumoniae | |
| Adults (18-60 yr) | Neisseria meningitidis |
| Streptococcus pneumoniae | |
| Elderly adults (>60 yr) | Gram-negative bacilli |
| Haemophilus influenzae | |
| Listeria monocytogenes | |
| Streptococcus pneumoniae |
Infection does not generally spread outward from the subarachnoid space to the subdural space. Occasionally, however, a subdural effusion is produced, more often in infants than adults. Because they tend to resolve spontaneously as the infection fades with medical therapy, these effusions are tapped only if they produce a significant mass effect on adjacent brain tissue.2
Types of Bacterial Meningitis
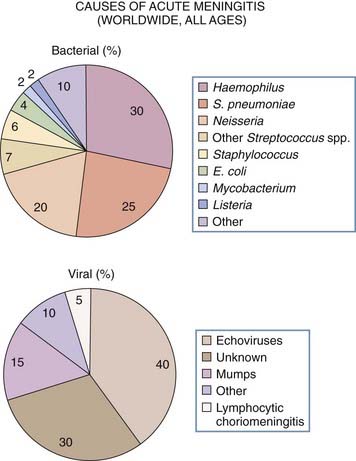
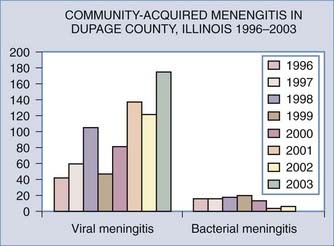
The three most common bacteria causing meningitis are Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae, which together account for 75% of cases (Fig. 44-1). Less frequently seen are Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus group A, which usually occur after head trauma or neurosurgical procedures or with a brain or epidural abscess; Streptococcus group B, which is seen in newborns; and the Enterobacteriaceae (Klebsiella, Proteus, and Pseudomonas spp.), which occur after lumbar puncture or shunt placement.
Pneumococcal, Haemophilus, and meningococcal meningitides have a seasonal pattern, with the majority of cases occurring in fall, winter, and spring and with a male preponderance. Because of the overuse of antibiotics prevalent in medicine today, drug-resistant strains occur increasingly, and it is important to know the patterns of drug resistance in general and the resistance of the bacterium in question in particular to form an effective therapeutic plan. Meningococcal meningitis occurs most often in children and adolescents but can be seen in adulthood until the age of 50, after which it sharply declines in incidence.3,4 Pneumococcal meningitis and Haemophilus meningitis both predominate in the very young and in elderly adults.
Clinical Features
Adults and Older Children
Early manifestations of bacterial meningitis include fever, headache (typically severe), generalized seizures, and impaired sensorium. Neck stiffness on flexion is common but may be abrogated in a patient already taking steroids. The older Kernig sign (inability to extend the legs completely) and Brudzinski sign (hip and knee flexion in response to neck flexion) are sometimes seen but are less reliable. Some patients even have abdominal pain because of initial onset of infection in the spine with effect on the thoracolumbar spinal roots. These symptoms are seen in any type of bacterial meningitis, but certain features suggest one or another type.5 During epidemics of meningitis, meningococcus should be suspected if evolution is rapid, if a petechial rash or ecchymosis accompanies the onset of symptoms, or when circulatory collapse occurs. The rash in particular should drive immediate therapy for N. meningitidis because in such cases time is critical and death can ensue within 2 days. In patients with preexisting infection of the lungs, sinuses, or ears or in those who have disorders of the heart valves, pneumococcus should be suspected. It is also more common in alcoholics and in patients who have dermal sinus tracts, sickle cell anemia, basal skull fracture, or previous splenectomy. The most common scenario for Haemophilus meningitis is after an upper respiratory or ear infection in a child.
Clinical Testing
Lumbar puncture is the key test in diagnosing meningitis.6 This test should be done before instituting antibiotic therapy, and a positive diagnostic yield is increased by multiple taps. Those with bleeding disorders should, if possible, have hematologic correction of their coagulopathy before lumbar puncture. Those with increased intracranial pressure should undergo computed tomography (CT) or magnetic resonance imaging (MRI) of the head before lumbar puncture. Some will have hydrocephalus sufficient to require a ventriculostomy, and CSF can be acquired by that route rather than by a redundant lumbar puncture. In general, the fear of herniation when a spinal tap is performed in the presence of an intracranial mass lesion is much overstated. It is actually very unusual for transtonsillar herniation to occur when small amounts of CSF are withdrawn from the lumbar cistern. However, it is wise to use a small (25-gauge) needle in preference to a large-bore needle when intracranial pressure is elevated because this will permit more gradual removal of CSF and may further lessen the chance of such herniation.
The CSF findings in the various types of meningitis are shown in Table 44-2. Spinal fluid pressure is typically elevated, and a normal pressure in a patient strongly suggests that meningitis is not present. Pleocytosis is the most diagnostic finding, with the typical white blood cell count in CSF ranging from 1000 to 10,000 cells/mm3. A very high count (>50,000) should raise the possibility of a bacterial abscess that has ruptured into the ventricles. Such patients will be very ill and may require intraventricular as well as intravenous antibiotics. In bacterial (as opposed to viral or fungal) meningitis, a neutrophilic predominance is seen (85% to 95% of the total cell population), but later in the course of the illness the percentage of mononuclear cells rises.
Protein levels are high in 90% of patients, in the range of 100 to 500 mg/dL. Glucose levels fall to a level of less than 40 mg/dL. In hyperglycemic patients, the diagnostic finding is a drop in glucose to less than 40% of the blood glucose level measured simultaneously. Gram stain of spinal fluid sediment may show the bacterium in patients who have not previously been treated with antibiotics. It is invariably negative in those who have been so treated. CSF samples for culture are always best obtained before treatment because they too will be affected by the presence of previously administered circulating antibiotic. In untreated cases, cultures should be expected to be positive in 20% to 90% of patients. In culture-negative patients, counterimmunoelectrophoresis may help detect bacterial antigens that linger after the bacteria themselves have disappeared. Other adjunctive methods include radioimmunoassay and enzyme-linked immunosorbent assay (ELISA), also done to detect bacterial antigens, and polymerase chain reaction (PCR) amplification of nucleic acid sequences specific for bacterial species.7
Postcraniotomy Meningitis
The risk of CSF contamination leading to meningitis begins during surgery, but seeding of bacteria can occur after surgery in patients with either controlled CSF drainage or uncontrolled CSF leakage. The most common organisms cultured in patients after craniotomy have been S. aureus and S. epidermidis, with gram-negative organisms also being common.8,9 Patients have fever, neck stiffness, and altered levels of consciousness, as do those with standard meningitis. However, those in whom meningitis develops after surgery have an increased risk for stroke secondary to venous infarction, an event precipitated by septic thrombophlebitis and encouraged by the dehydration that is often present. A lumbar puncture can be done safely in most postoperative patients, particularly those from whom a mass lesion has been resected and in whom the brain has been decompressed. CSF should be evaluated in the usual fashion for cell count, protein, and glucose. One study of meningitis in the postoperative period defined it (somewhat arbitrarily) as 100 white cells/mm3 with a minimum of 50% polymorphonuclear cells or 400 white cells/mm3 regardless of the polymorphonuclear percentage.10 However, normal lumbar puncture parameters have never been defined for specific times after craniotomy and would additionally depend on the nature of the disease for which the craniotomy was performed. It is likely that the measurements are changed by the operation itself, by anesthesia, by the use of steroids, and by the disruption of cerebral tissue that occurs. In the absence of precise information for each type of craniotomy, interpretation of the CSF results should not rely on borderline values or on one parameter alone but must be a synthesis of all relevant measurements. Gram stain of CSF can help guide selection of antibiotics because culture may take 2 or more days to yield bacterial identity and sensitivity to treatment. In patients in whom the clinical symptoms and signs point to meningitis, treatment is instituted after a spinal tap is performed. If subsequent cultures show the meningitis to be aseptic, antibiotic administration may be stopped. However, it is common to continue them because perioperative administration of antibiotics may interfere with CSF cultures done in the first few days after craniotomy.
The role of prophylactic antibiotics in preventing meningitis after craniotomy remains controversial. A study by Barker, a meta-analysis of six trials involving 1729 patients, showed that meningitis accounted for 32% of the 102 infections reported after craniotomy.11 The incidence of meningitis in antibiotic-treated patients was 1.1% versus 2.7% in untreated controls. Because statistical analysis suggested no heterogeneity among the different trials, the author concluded that antibiotics conferred significant benefit in preventing meningitis after surgery. A second study by Korinek and associates drew a different conclusion.12 In a series of 6243 consecutive craniotomies they showed an overall 1.5% incidence of meningitis and identified the presence of CSF leakage, concomitant infection of the surgical incision, and surgical duration to be independent risk factors. Antibiotic prophylaxis reduced incisional infection by half but did not change the incidence of meningitis. Such conflicting results leave the question of prophylaxis for meningitis unsettled, but it is widely accepted that prophylaxis should be used to prevent postcraniotomy infection in a more general sense. The main issue would seem to be the choice of antibiotics to allow suppression of the broadest possible range of types of postoperative infection.
Recurrent Meningitis
Meningitis will recur if the source of the bacteria remains active after treatment and suppression of the meningitis itself. Such recidivism should raise suspicion of an ongoing CSF leak through a previous basilar skull fracture or surgical procedure affecting the frontal, sphenoid, or ethmoid sinuses or the cribriform plate (Fig. 44-2). In the absence of previous trauma, a congenital fistula between the nasal sinuses and subarachnoid space may be suspected. In either case, CT with thin cuts through the skull base on bone windows is very helpful. Difficult cases are those in which the CSF leak is intermittent or very slow. We have found the best method of detecting small leaks to be injection of radioactive tracer (typically 99Tc or 111In) into the lumbar subarachnoid space with placement of nasal pledgets.13 The patient is then imaged over the next 24 to 48 hours to observe gradual movement of the tracer to the head (and if leak is present, into the nose and stomach). The pledgets are removed after the first day and also scanned; if a very slow leak is present, they may be the only source of positive detection. Finally, the kinetics of disappearance in CSF have been determined in normal patients, and an overly quick decay of tracer activity beyond the normal range suggests that a leak is present even if anatomic localization is lacking.14 Ultimately, if a fistula is found that is causing repeated bouts of meningitis, it should be closed surgically.15
Treatment
Treatment of specific pathogens is presented in Table 44-3. The antibiotics suggested are only starting points because ongoing evolution of bacterial resistance to antibiotics has changed the patterns of antibiotic use significantly over the past 20 years.16 In all cases the best antibiotics are those with good penetration into CSF, a penetration that in some instances is enhanced by the presence of meningeal irritation. Selection of an antibacterial agent should take into consideration the need for bactericidal activity and for adequate penetration into CSF. The antibacterial concentration should be at least 10 to 20 times the minimal bactericidal concentration of the agent in question. Penetration of CSF by antibiotics is enhanced by a low molecular weight and simple chemical structure of the drug, low degree of ionization at physiologic pH, high lipid solubility, and low degree of protein binding. Initial therapy is empirical according to the most likely organism suspected for the age of the patient.17 The duration of therapy should be 10 to 14 days for those with bacterial meningitis in general and 21 days for patients with gram-negative bacteria and Listeria. If a shunt is present, it will typically require externalization or removal, or both, with reinsertion done only when the meningitis has been completely treated. Thus, prolonged ventriculostomy drainage will be needed in patients in whom meningitis develops but who are heavily shunt dependent. The ventricular drains should be changed at least weekly because their rate of infection (thereby perpetuating the meningitis) goes up after that time point.18 When a persistent parameningeal focus of infection is present, this too must be cleared (medically or surgically) before the patient’s meningitis can be declared cured. Treatment is invariably by intravenous administration, and in refractory cases or in those with profound ventriculitis, intraventricular therapy may additionally be needed.19 It is not necessary to repeat lumbar puncture at intervals during therapy as long as progressive clinical improvement suggests that the disease is clearing. CSF glucose in particular remains low after other signs of infection have disappeared and must be interpreted in the context of the patient’s clinical status.
TABLE 44-3 Treatment of Specific Pathogens Causing Meningitis
| ORGANISM | DRUG OR DRUGS COMMONLY USED |
|---|---|
| Standard Bacterial Species | |
| Streptococcus pneumoniae | |
| MIC < 0.06 µg/mL | Penicillin G or ampicillin |
| MIC 0.1 to 1.0 µg/mL | Third-generation cephalosporin |
| MIC ≥ 2 µg/mL | Vancomycin |
| Neisseria meningitidis | Penicillin G or ampicillin |
| Haemophilus influenzae | |
| β-Lactamase negative | Ampicillin with chloramphenicol |
| β-Lactamase positive | Ceftriaxone or cefotaxime |
| Listeria monocytogenes | Penicillin G or ampicillin |
| Streptococcus group B | Penicillin G or ampicillin |
| Pseudomonas aeruginosa | Ceftazidime (if sensitive), consider adding an aminoglycoside |
| Enterobacteriaceae | Third-generation cephalosporin |
| Staphylococcus | |
| Methicillin sensitive | Nafcillin or oxacillin |
| Methicillin resistant | Vancomycin |
| Other Microbial Species | |
| Mycobacterium tuberculosis | 3-4 antituberculosis drugs (isoniazid, rifampin, ethambutol, pyrazinamide) |
| Fungi | Amphotericin B or voriconazole |
| Naegleria fowleri | Amphotericin B plus rifampin |
| Spirochete | |
| Borrelia burgdorferi | Ceftriaxone or penicillin G |
| Treponema pallidum | Penicillin G |
MIC, minimal inhibitory concentration.
The use of dexamethasone for meningitis has been controversial, with most data acquired from children.20 It diminishes the inflammatory response in the subarachnoid space and decreases the production of tumor necrosis factor and interleukin-1. It also reduces cerebral edema and production of CSF without attenuating bacterial killing. In adults, studies have shown a decreased incidence of sensorineural hearing loss and a decrease in mortality.21 We recommend using dexamethasone in patients of any age at a dose of 0.15 mg/kg every 6 hours during the first 4 days of antibiotic therapy. Anticonvulsants should be given when seizures are present but need not be given prophylactically. Serum sodium levels should be checked frequently because they may fall and require the institution of fluid restriction. Ventricular enlargement seen on scans may indicate postmeningitic hydrocephalus but need not be treated unless the ventriculomegaly worsens. Clearly, if a shunt is needed because of the presence of transependymal fluid flow or symptoms of increased intracranial pressure in a patient with active meningitis, a ventriculostomy drain is used rather than an indwelling shunt.
Prevention
Strategies for prevention include active immunization, passive immunization with immunoglobulins, and chemoprophylaxis. The most effective vaccine is that against Haemophilus, which decreases Haemophilus meningitis in children and is commonly given to them; consequently, many cases now occur in unvaccinated adults. The Haemophilus vaccine is recommended for high-risk individuals with complement deficiency, which predisposes them to such meningitis. Vaccines for meningococcus have also been developed and are being used with increasing frequency.22 Meningococcal meningitis is associated with a high risk of secondary infection in household members, who have 500 times the risk for infection that the general population does. Thus, rifampin (10 to 20 mg/kg per day orally for 4 days to a maximal dose of 600 mg daily) should be given to those who have had close contact with the patient in the 2 weeks before the diagnosis of meningitis. Ciprofloxacin has been shown to be effective in eradicating the meningococcal carrier state in adults, but chemoprophylaxis for other types of meningitis is not recommended. The exception is patients with posttraumatic CSF fistulas. Although a number of studies individually have not shown a significant difference in the incidence of meningitis with antibiotic prophylaxis in such patients, a meta-analysis that grouped six studies for analysis did show a 2.5% incidence of meningitis in those who received antibiotics versus 10% in those who did not, a difference that supports antibiotic use in that group.23
Prognosis
Bacterial meningitis is commonly fatal when untreated. Currently, the mortality rate for treated Haemophilus and meningococcal meningitis is 5% to 15% and has remained so for many years. Pneumococcal meningitis has a somewhat higher mortality rate of 15% to 30%. Fulminant meningococcemia is possible with or without meningitis and has a very high mortality rate from vasomotor collapse associated with adrenocortical hemorrhage (Waterhouse-Friderichsen syndrome).24 In neonates the mortality rate from this phenomenon is 40% to 75%, and at least half who recover have serious neurological injury. The prognosis for bacterial meningitis is worse in those who have proven bacteremia, coma, seizures, and such concomitant disease as alcoholism and diabetes mellitus. Death occurs for a variety of reasons, including vascular collapse from septic shock, cerebral edema leading to transtonsillar herniation, respiratory failure secondary to aspiration pneumonia in an obtunded patient, and uncontrolled seizures. Those who recover from meningococcal meningitis usually show no residual deficits, but neurological injury occurs in 25% or more of children with Haemophilus meningitis and up to 30% of those with pneumococcal meningitis. In one study that monitored children after meningitis, 31% of those with pneumococcal meningitis had persistent sensorineural hearing loss, whereas the figures for meningococcal and Haemophilus meningitis were 10% and 6%, respectively.25,26 Haemophilus meningitis in infants leaves only 50% entirely normal, with the remainder having either behavioral problems or significant ongoing neurological deficits.
Tuberculous Meningitis
The incidence of tuberculous meningitis is very low because only 1% of patients with tuberculosis contract this extrapulmonary form of the disease.27 It accounts for about 2500 cases per year in the United States. However, in developing countries, tuberculosis remains a very significant medical problem, and the lowering of barriers to immigration together with the increasing incidence of multidrug-resistant mycobacterial strains suggests that the case numbers are likely to rise in North America.
Pathogenesis
Tuberculous meningitis is caused by Mycobacterium tuberculosis in most patients and very occasionally by Mycobacterium bovis. Typically, it begins with bacterial seeding of the brain with the formation of tubercles that subsequently rupture and seed mycobacteria into the adjacent subarachnoid space. Such meningitis can be the terminal event in patients with miliary tuberculosis or occur less dangerously in the context of generalized tuberculosis with a single area of brain involvement.28
Clinical Features
Treatment of tuberculous meningitis always requires a combination of drugs, three at a minimum, with capacity to penetrate the CNS.29 Administration is prolonged and continues for 18 to 24 months. The single most effective drug is isoniazid, but it has side effects (neuropathy and hepatitis) that can be disabling in alcoholics who may have these conditions from the outset. Neuropathy can be prevented by giving pyridoxine concomitantly, but hepatitis is not treatable except by discontinuing use of the drug. In patients taking ethambutol, optic neuropathy occasionally develops, so those receiving long-term ethambutol treatment should be screened at intervals for visual acuity and color discrimination. Corticosteroids are not generally used. At times, an intracerebral tuberculoma requires resection if there is a mass effect or if it fails to shrink with drug therapy. Mortality is lower when treatment is started early, but it reaches 50% in patients in whom the condition is diagnosed while in coma. Neurological impairment is found in 20% to 30% of survivors.
Despite the basal cistern loss caused by tuberculous meningitis, the associated hydrocephalus can be treated successfully in 70% of cases by endoscopic third ventriculostomy.30
Treponemal (Syphilitic) Meningitis
Treatment still involves administration of penicillin as the drug of choice for all varieties of neurosyphilis, regardless of whether symptoms are present.31 Ideally, crystalline penicillin G is given at high doses intravenously for 2 weeks. If at 6 months the patient is symptom free and the CSF abnormalities have normalized, no further treatment is necessary, but a second lumbar puncture at 1 year is advisable. If, however, at 6 months pleocytosis or elevated protein is still present in CSF, another course of penicillin should be given.
Meningitis from Lyme Disease
Lyme disease, named after the town in Connecticut where it was first recognized, is a tick-borne multisystem disease associated with relapsing fever and caused by spirochetes of the genus Borrelia. As in syphilis, the Borrelia spirochete invades the CNS early and causes asymptomatic meningitis, with later manifestation of more profound neurological abnormality in a smaller proportion of patients.32 Also as in syphilis, the neurological complications mainly derive from the effects of chronic meningitis, but in Lyme disease a peripheral neuropathy may likewise produce neurological symptoms and make explication of the symptoms more complex. The disease starts as a tick bite with associated rash. Weeks to months later, neurological symptoms appear in 15% of patients. The neurological involvement takes the form of a fluctuating meningoencephalitis or peripheral neuritis, or both. The meningitis is manifested as headache, stiff neck, nausea and vomiting, malaise, and chronic fatigue and fluctuates over a period of weeks to months. In CSF a lymphocytosis is seen with counts of up to 3000 cells/mL and protein levels of up to 300 mg/dL. The glucose level is usually normal. Cognitive and behavioral changes sometimes occur, as do seizures, ataxia, and choreiform movements, and cranial neuropathy is seen in half of patients, most frequently a facial palsy. CSF testing with ELISA is positive in 50% of those in the early stage and 100% of those in the later stage. Recommended treatment is high-dose penicillin (20 million units intravenously daily for 10 days) or ceftriaxone.
Fungal Meningitis
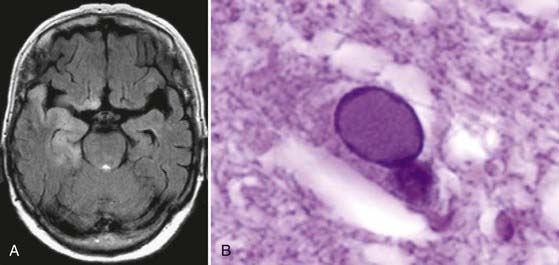
Meningitis of fungal origin is far less common than bacterial meningitis, but it causes similar pathophysiologic and clinical effects. Although many fungi can involve the nervous system, only a few do so with regularity and are listed in this section. They enter the CNS either by a hematogenous route or through infected paranasal sinuses.33 Although fungal meningitis may have no apparent predisposing cause, it usually occurs in the context of immunosuppression from another disease, such as diabetes mellitus, hematopoietic malignancy, prolonged immunosuppression in transplant patients, or chronic steroid therapy (Fig. 44-3). Patients with leukopenia are particularly susceptible. The course of onset and the symptom profile of fungal meningitis are much like that of tuberculous meningitis, and the onset is insidious. As in other chronic meningitides, these patients are often afebrile and may have cranial nerve involvement, arteritis with thrombosis and infarction, and hydrocephalus. The spinal fluid alterations also mirror those of tuberculous meningitis. CSF pressure is elevated with a moderate pleocytosis of lymphocytic predominance. CSF protein is elevated and glucose is low. The diagnosis is made from KOH preparation of CSF smears, from culture of CSF, and by antigen recognition tests or PCR amplification. Specific subtypes are summarized in the following text.
Cryptococcosis is one of the more frequent fungal infections. Cryptococcus is a fungus of the soil commonly carried by birds and transmitted to humans through the lungs or skin. The meningitis is granulomatous in nature, with granulomas containing fibroblasts, giant cells, areas of necrosis, and the organisms themselves. Its evolution is subacute, much like that of tuberculosis, but it may be fatal within a few weeks if left untreated.34 Headache, fever, and a stiff neck are usually but not always seen, and patients often have symptoms of gradually increasing intracranial pressure, ataxia, or a confusional state. Because this is not a basal meningitis, cranial nerve palsies are infrequent. Meningovascular involvement may lead to stroke. In some patients the course may be chronic, with the disease persisting for years in a waxing and waning course. To diagnose cryptococcal meningitis, the organism must be found in CSF, large volumes of which are needed to find it. The classic test is the India ink preparation, which is positive in only 60% of proven cases. Fungal cultures will often yield the organism, but in some the only evidence of infection is Cryptococcus serology by a latex agglutination test. Treatment of this and indeed most fungal meningitides is amphotericin B given intravenously. Nephrotoxicity is the main side effect of therapy, but even with treatment the mortality is 40%. In some cases, voriconazole has also proved effective.35
Candidiasis is the most frequent opportunistic fungal infection, but it causes meningitis only rarely. When candidiasis involves the CNS, it generally takes the form of parenchymal abscesses and noncaseating granulomas, with meningitis being less frequent. The diagnosis is often made post mortem. The mortality rate, with or without treatment, is very high, but occasional successes do occur.36
Coccidioidomycosis progresses from the typical influenza-like illness with pulmonary infiltration to the disseminated form of the disease in very few individuals. Meningitis is sometimes part of that dissemination (Fig. 44-4). The organism is difficult to recover from CSF and is more easily cultured from lymph nodes or skin lesions. The meningitis is again clinically much like that associated with tuberculous meningitis.37
Uncommon Meningitides Rarely Encountered
Amebas of the genus Naegleria, typically found in the southeastern states, cause a meningoencephalitis that patients acquire by swimming in ponds colonized by the organism. After a rapid onset characterized by severe headache, fever, nausea and vomiting, and a stiff neck, the disease progresses quickly to coma with a fatal outcome in most patients. CSF shows findings similar to those of acute bacterial meningitis, but it may also yield viable trophozoites in a wet preparation of unspun fluid. Culture is unhelpful. Treatment with antiprotozoal agents does not work, but with amphotericin B instituted quickly a few patients will recover.38
Toxoplasmosis, caused by the obligate intracellular parasite Toxoplasma gondii, typically affects the CNS in immunosuppressed patients. The most common scenario occurs in patients with HIV infection. The neurological manifestations of toxoplasmosis may be attributed to the background disease causing the immunosuppression, with the diagnosis being delayed and having a negative impact on outcome. The overall clinical picture includes rash, myocarditis, and polymyositis, with neurological symptoms being quite variable.39 Most often a meningoencephalitis occurs with seizures, confusion, coma, and in the CSF, increased protein and lymphocytic pleocytosis.40 Lesions are scattered throughout the brain, and presumably those adjacent to the subarachnoid space cause the meningitis component of the meningoencephalitis. The diagnosis can be made by finding organisms in CSF or in stereotactic biopsy specimens of brain lesions.41 However, treatment with sulfadiazine and pyrimethamine (with leucovorin given as well to abrogate the antifolate effect of the latter drug) is now given to any immunocompromised patient with brain lesions consistent with Toxoplasma. A positive response to medical treatment is considered diagnostic. When the immune system compromise is expected to persist, long-term treatment is advisable.
Aseptic Meningitis
Aseptic meningitis is fairly common, with the majority of cases being due to viral infection, most commonly from the enterovirus family (Fig. 44-5; also see Fig. 44-1). Such organisms include echovirus, coxsackievirus, and nonparalytic poliovirus, which together cover 80% of cases in which a specific virus is identified. The next most common cause is mumps virus, followed by herpes simplex type 2, lymphocytic choriomeningitis virus, and adenovirus.42 These viruses, together with leptospirosis, cause 95% of cases of aseptic meningitis. Very occasional cases are caused by Epstein-Barr virus or sometimes occur in conjunction with Mycoplasma pneumoniae. Patients with HIV infection can also have acute, self-limited aseptic meningitis.
The enteroviruses are by far the most common cause of viral meningitis.43 They occur most often in children, and outbreaks in families are typical because of spread by the fecal-oral route. The peak incidence is in the months of August and September. Meningitis from mumps can occur at any time during the year, but most often in the late winter and spring. Males are afflicted 3 times more often than females, and other manifestations of mumps infection are not always present. Because a previous attack of mumps confers lifelong immunity, a past history of the disease excludes this cause. Lymphocytic choriomeningitis virus infection occurs through contact with infected hamsters or food contaminated by mouse feces. Respiratory symptoms precede the onset of meningitis, and the infection is most common in late fall and winter when mice tend to congregate indoors. A previous sore throat with generalized lymphadenopathy and a transient rash may suggest infectious mononucleosis caused by Epstein-Barr virus, which occasionally causes aseptic meningitis.
Acute Encephalitis/Meningoencephalitis
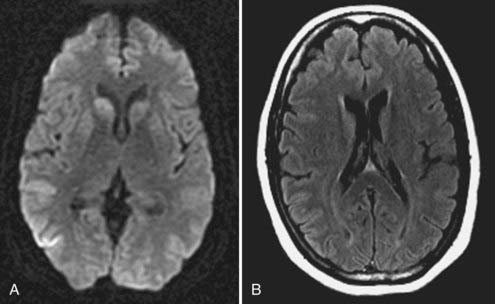
Symptoms of herpes simplex encephalitis evolve over a period of several days and resemble those of the other acute encephalitides. Occasional premonitory symptoms, including temporal lobe seizures, personality changes, anosmia, or olfactory or gustatory hallucinations, match the tendency of this disease to involve the inferomedial portions of the frontal and temporal lobes (Fig. 44-6). Changes in memory are usually identified only during the convalescent phase. In severe cases, temporal lobe edema and status epilepticus can cause uncal herniation and subsequent coma and death.44
Making the diagnosis can be difficult even with CSF analysis and characteristic MRI findings showing an appropriate geographic location of the hemorrhagic lesions. Electroencephalographic findings suggest the disease but are not specific for it and include periodic high-voltage sharp waves in the temporal area. As the disease progresses, a rising antibody titer can be demonstrated, but this does not generally become apparent early enough to affect therapy. The most certain way of diagnosing herpes simplex encephalitis is by viral culture of brain tissue obtained by brain biopsy or by immunohistochemical staining for viral antigens on such tissue. PCR analysis can also be performed, particularly if the specimen is obtained stereotactically and thus is small in volume. Biopsy can be performed either by stereotactic methods or, to increase diagnostic yield, by a small open craniotomy in which the cortical surface is exposed and a volume of tissue measuring 1 cm3 is removed from the inferior temporal region.45 Because the temporal lobe may be particularly hemorrhagic, meticulous hemostasis is required with such biopsies, and care should be taken to maintain the integrity of the specimen during its removal to avoid a crush artifact, which hinders histologic analysis.
The treatment of choice is acyclovir, which in the body is converted to acyclovir triphosphate, a potent inhibitor of the herpes simplex DNA polymerase needed for viral replication.46 The drug is given intravenously and has relatively few serious side effects, but its dose should be decreased in patients with renal dysfunction. It can cause phlebitis and gastrointestinal disturbance and is considered safe for use during pregnancy. Because most relapses occur within 3 months of completing the initial course of intravenous acyclovir, prolonged therapy with an oral antiviral agent (such as valacyclovir) has been suggested. The role of steroids in treating this disease remains unclear. Although steroids help limit the cerebral edema associated with the infectious process, they may also suppress the immune responses necessary to inhibit viral replication. Animal studies have suggested benefit without evidence of increased viral dissemination,47 and a nonrandomized retrospective study in humans has shown an improved outcome at 3 months in the steroid-treated group.48 Treatment should be given for 2 weeks to minimize relapse. Outcomes are better in patients younger than 30 years, in whom a mortality of 25% is noted, and only 10% of survivors escape neurological damage if treatment begins after the onset of coma.
In some patients with meningoencephalitis and severe cerebral edema, decompressive craniectomy can yield good results. In one report of three patients so treated by either bifrontal craniectomy or hemicraniectomy, two survived without residual focal deficit, and others have reported similar success with this procedure.49,50 In addition, temporal lobectomy has been reported with good outcome in a child with refractory brain edema from herpes encephalitis.51
Special Circumstances
Acquired Immunodeficiency Syndrome
HIV infection has a number of neurological consequences. Its specific link to meningitis and encephalitis comes through opportunistic infection by such entities as cerebral toxoplasmosis, which does not infect immunocompetent individuals. Primary CNS lymphoma develops in about 5% of patients infected with HIV and can be difficult to distinguish from toxoplasmosis clinically or radiologically. In such cases of diagnostic confusion, when CSF cytology is negative, a brain biopsy (typically done stereotactically) may be needed. Because the response to radiotherapy and corticosteroids is short-lived, the prognosis of such patients is poor. In HIV-positive patients, varicella-zoster infection is generally more severe. In addition, mycobacterial infection is more frequent in patients with HIV than in the unaffected population.52 Such infection can be caused by either M. tuberculosis or Mycobacterium avium-intracellulare. Here too the response to treatment is less than ideal, and grossly destructive cerebral lesions are more likely to be present. Both syphilitic meningitis and meningovascular syphilis have an increased incidence in patients with acquired immunodeficiency syndrome (AIDS), as do opportunistic infections caused by cytomegalovirus and Cryptococcus.53 At autopsy a third of patients with AIDS are found to be infected with cytomegalovirus, but the clinical relevance of this finding is uncertain. Cryptococcal infections may take the form of meningitis or a solitary cryptococcoma and are the most frequent fungal agents complicating HIV disease. Clear symptoms of meningitis or encephalitis may be absent, however, with little CSF abnormality. With the current widespread use of retroviral therapy and multidrug cocktails providing much better long-term suppression of HIV, such secondary afflictions are somewhat less common but remain part of the overall burden of a patient infected with HIV.54
Chemical Meningitis
Chemical meningitis refers to meningeal inflammation caused by a noninfectious agent. This typically takes the form of release of blood or cyst contents into CSF from a lesion adjacent to the subarachnoid space. Such lesions include craniopharyngiomas, Rathke’s cleft cysts, epidermoid and dermoid cysts, and cholesteatomas.55 Release of the irritative element often occurs repeatedly, and the result is a chronic headache syndrome relieved by resection of the offending lesion. This is a form of aseptic meningitis, although one without a viral etiology. In Mollaret’s meningitis, a syndrome consisting of recurrent bouts of fever and the signs and symptoms of aseptic meningitis, recovery is rapid and spontaneous. Although no cause has been definitively identified, it has been ascribed to an occult reservoir of herpes simplex virus type 1, but this association is not conclusive.
Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492.
Davis LE, Beckham JD, Tyler KL. North American encephalitic arboviruses. Neurol Clin. 2008;26:272-757.
DeGaudio M, Chiappini E, Galli L, et al. Therapeutic management of bacterial meningitis in children: a systematic review and comparison of published guidelines from a European perspective. J Chemother. 2010;22:226-237.
Hall WA, McCutcheon IE, editors. Infections in Neurosurgery. Park Ridge, Illinois: American Association of Neurological Surgeons, 1999.
Kastrup O, Wanke I, Maschke M. Neuroimaging of infections of the central nervous system. Semin Neurol. 2008;28:511-522.
Logan SA, MacMahon E. Viral meningitis. BMJ. 2008;336:36-40.
1 Sare GM, Varma A, Green K, et al. Pneumococcal meningitis secondary to intra-sphenoidal encephalocele. Acta Neurochir (Wien). 2009;151:103-104.
2 Vinchon M, Joriot S, Jissendi-Tchofo P, et al. Postmeningitis subdural fluid collection in infants: changing pattern and indications for surgery. J Neurosurg. 2006;104:383-387.
3 Heckenberg SG, de Gans J, Brouwer MC, et al. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Medicine (Baltimore). 2008;87:185-192.
4 Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcus, and Neisseria meningitides. Lancet. 2007;369:2196-2210.
5 Van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-1859.
6 Straus SE, Thorpe KE, Halroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006;296:2012-2022.
7 Chiba N, Murayama SY, Morozumi M, et al. Rapid detection of eight causative pathogens for the diagnosis of bacterial meningitis by real-time PCR. J Infect Chemother. 2009;15:92-98.
8 Blomstedt GC. Craniotomy infections. Neurosurg Clin N Am. 1992;3:275-285.
9 Kaufman BA, Tunkel AR, Pryor JC, et al. Meningitis in the neurosurgical patient. Infect Dis Clin North Am. 1990;4:677-701.
10 Blomstedt GC. Post-operative aseptic meningitis. Acta Neurochir (Wien). 1987;89:112-116.
11 Barker FGII. Efficacy of prophylactic antibiotics against meningitis after craniotomy: a meta-analysis. Neurosurgery. 2008;60:887-894.
12 Korinek AM, Baugnon T, Golmard JL, et al. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2007;60:887-894.
13 Bai J, Yokoyama K, Kinuya S, et al. Radionuclide cisternography in intracranial hypotension syndrome. Ann Nucl Med. 2002;16:75-78.
14 Moriyama E, Ogawa T, Nishida A, et al. Quantitative analysis of radioisotope cisternography in the diagnosis of intracranial hypotension. J Neurosurg. 2004;101:421-426.
15 Daudia A, Biswas D, Jones NS. Risk of meningitis with cerebrospinal fluid rhinorrhea. Ann Otol Rhinol Laryngol. 2007;116:902-905.
16 Prawad K, Karlupia N, Kumar A. Treatment of bacterial meningitis: an overview of Cochrane systematic reviews. Respir Med. 2009;103:945-950.
17 Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2009;39:1267-1284.
18 Hoefnagel D, Dammers R, Ter Laak-Poort MP, et al. Risk factors for infections related to external ventricular drainage. Acta Neurochir (Wien). 2008;150:209-214.
19 Ziai WC, Lewin JJ3rd. Improving the role of intraventricular antimicrobial agents in the management of meningitis. Curr Opin Neurol. 2009;22:277-282.
20 Assiri AM, Alasmari FA, Zimmerman VA, et al. Corticosteroid administration and outcome of adolescents and adults with acute bacterial meningitis: a meta-analysis. Mayo Clin Proc. 2009;84:403-409.
21 Van de Beek D, de Gans J, McIntyre P, et al. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis. 2004;4:139-143.
22 Zimmer SM, Stephens DS. Meningococcal conjugate vaccines. Curr Opin Pharmcother. 2004;5:855-863.
23 Brodie HA. Prophylactic antibiotics for posttraumatic cerebrospinal fluid fistulae: a meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123:749-752.
24 Rosa D, Pasqualotto A, de Quadros M, et al. Deficiency of the eighth component of complement associated with recurrent meningococcal meningitis—case report and literature review. Braz J Infect Dis. 2004;8:328-330.
25 Dodge PR, Davis H, Feigin RD, et al. Prospective evaluation of hearing impairment as a sequela of acute bacterial meningitis. N Engl J Med. 1984;311:869-874.
26 Pomeroy SL, Holmes SJ, Dodge PR, et al. Seizures and other neurologic sequelae of bacterial meningitis in children. N Engl J Med. 1990;323:1651-1657.
27 Peto HM, Pratt RH, Harrington TA, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49:1350-1357.
28 Be NA, Kim KS, Bishai WR, et al. Pathogenesis of central nervous system tuberculosis. Curr Mol Med. 2009;9:94-99.
29 Thwaites G, Fisher M, Hemingway C, et al. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59:167-187.
30 Chugh A, Husain M, Gupta RK, et al. Surgical outcome of tuberculous meningitis hydrocephalus treated by endoscopic third ventriculostomy: prognostic factors and postoperative neuroimaging for functional assessment of ventriculostomy. J Neurosurg Pediatr. 2009;3:371-377.
31 Jay CA. Treatment of neurosyphilis. Curr Treat Options Neurol. 2006;8:185-192.
32 Cadavid D, Barbour AG. Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin Infect Dis. 1998;26:151-164.
33 Epstein VA, Kern RC. Invasive fungal sinusitis and complications of rhinosinusitis. Otolaryngol Clin North Am. 2008;41:497-524.
34 Davis JA, Horn DL, Marr KA, et al. Central nervous system involvement in cryptococcal infection in individuals after solid organ transplantation or with AIDS. Transpl Infect Dis. 2009;11:432-437.
35 Bandettini R, Castagnola E, Calvillo M, et al. Voriconazole for cryptococcal meningitis in children with leukemia or receiving allogeneic hemopoietic stem cell transplant. J Chemother. 2009;21:108-109.
36 Liu KH, Wu CJ, Chou CH, et al. Refractory candidal meningitis in an immunocompromised patient cured by caspofungin. J Clin Microbiol. 2004;42:5950-5953.
37 Blair JE. Coccidioidal meningitis: update on epidemiology, clinical features, diagnosis, and management. Curr Infect Dis Rep. 2009;11:289-295.
38 Vargas-Zepeda J, Gómez-Alcalá AV, Vásquez-Morales JA, et al. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch Med Res. 2005;36:83-86.
39 Pradhan S, Yadav R, Mishra VN. Toxoplasma meningoencephalitis in HIV-seronegative patients: clinical patterns, imaging features and treatment outcome. Trans R Soc Trop Med Hyg. 2007;101:25-33.
40 Bach MC, Skarulis GJ. Acute toxoplasmic meningitis in a patient with AIDS. Clin Infect Dis. 1997;25:1482-1483.
41 Chappell ET, Guthrie BL, Orenstein J. The role of stereotactic biopsy in the management of HIV-related focal brain lesions. Neurosurgery. 1992;30:825-829.
42 Landry ML, Greenwold J, Vikram HR. Herpes simplex type-2 meningitis: presentation and lack of standardized therapy. Am J Med. 2008;122:688-691.
43 Ihekwaba UK, Kudesia G, McKendrick MW. Clinical features of viral meningitis in adults: significant differences in cerebrospinal fluid findings among herpes simplex virus, varicella zoster virus, and enterovirus infections. Clin Infect Dis. 2008;47:783-789.
44 Kumar G, Kalita J, Misra UK. Raised intracranial pressure in acute viral encephalitis. Clin Neurol Neurosurg. 2009;111:399-406.
45 Schlitt MJ, Morawetz RB, Bonnin JM, et al. Brain biopsy for encephalitis. Clin Neurosurg. 1988;33:591-602.
46 James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009;83:207-213.
47 Thompson KA, Blessing WW, Wesselingh SL. Herpes simplex replication and dissemination is not increased by corticosteroid treatment in a rat model of focal herpes encephalitis. J Neurovirol. 2000;6:25-32.
48 Kamei S, Sekizawa T, Shiota H, et al. Evaluation of combination therapy using acyclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry. 2005;76:1544-1549.
49 Adamo MA, Deshaies EM. Emergency decompressive craniectomy for fulminating infectious encephalitis. J Neurosurg. 2008;108:174-176.
50 Di Rienzo A, Iacoangeli M, Rychlycki F, et al. Decompressive craniectomy for medically refractory intracranial hypertension due to meningoencephalitis: report of three patients. Acta Neurochir (Wien). 2008;150:1057-1065.
51 Sánchez-Carpintero R, Aguilera S, Idoate M, et al. Temporal lobectomy in acute complicated herpes simplex encephalitis: technical case report. Neurosurgery. 2008;62:E1174-E1175.
52 Vinnard C, Macgregor RR. Tuberculous meningitis in HIV-infected individuals. Curr HIV/AIDS Rep. 2009;6:139-145.
53 Carmo RA, Mouras AS, Christo PP, et al. Syphilitic meningitis in HIV patients with meningeal syndrome: report of two cases and review. Braz J Infect Dis. 2001;5:280-287.
54 Maaschke M, Kastrup O, Esser S, et al. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry. 2000;69:376-380.
55 Hadden D, Allen A. Chemical meningitis due to rupture of a craniopharyngioma cyst. J R Soc Med. 2004;97:565-566.