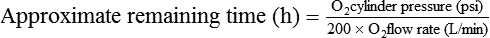Medical gas supply
Medical gases most common to anesthesia include oxygen (O2), nitrous oxide (N2O), and air. Historically the less frequently used medical gases include helium (He), nitrogen (N2), and carbon dioxide (CO2), but there has been a recent surge in the use of CO2 secondary to the advancement of laparoscopic and robotic procedures. Several governing bodies regulate medical gases, but the containment and delivery of these gases via a medical gas cylinder system is controlled via standards set by the United States Department of Transportation. Medical gas cylinders are the foundation for central pipeline supply of gases to the operating room (OR) and hospital. Additionally, a cylinder system (typically the smaller E cylinders) exists in the OR as a backup for unanticipated failure of the central pipeline supply (Figures 1-1 to 1-3).
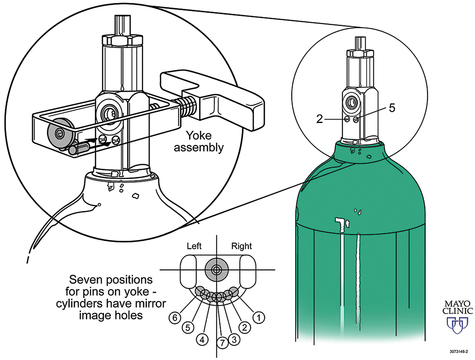
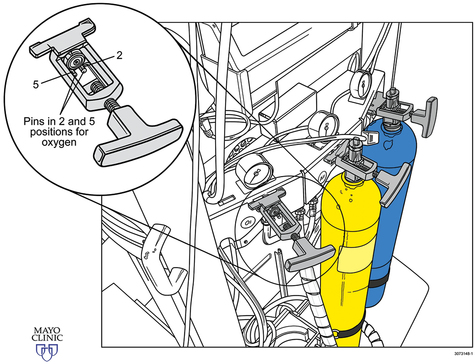
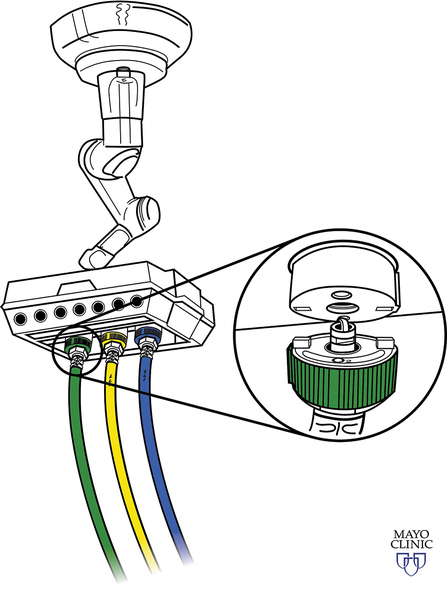
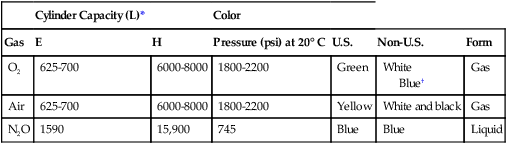
Medical gas cylinders store compressed gas. Cylinder sizes vary and are designated by letters, with A being the smallest and H being the largest. H cylinders are large-capacity storage containers that typically provide the central pipeline supply of medical gas that is piped into the OR. E cylinders are smaller and are the most commonly encountered cylinders in the OR. A typical anesthesia machine will have an attachment for two (O2 and N2O) or three (two O2 and one N2O) E cylinders. E cylinders are also commonly used to supply O2 to patients during transport. Cylinders are color coded according to the gas they contain. Unfortunately, there is no global agreement, and the colors in the United States are not the same as those accepted internationally. Table 1-1 lists the common medical gases, the cylinder capacity, the color of the cylinders, and the form under which medical gases are stored.
Table 1-1
| Cylinder Capacity (L)* | Color | |||||
| Gas | E | H | Pressure (psi) at 20° C | U.S. | Non-U.S. | Form |
| O2 | 625-700 | 6000-8000 | 1800-2200 | Green | White Blue† |
Gas |
| Air | 625-700 | 6000-8000 | 1800-2200 | Yellow | White and black | Gas |
| N2O | 1590 | 15,900 | 745 | Blue | Blue | Liquid |
*Cylinder sizes are designated by letters, with A being the smallest and H the largest.