12 Medical Complications in the Management of Brain Tumors
Symptomatic Management
SYMPTOMS
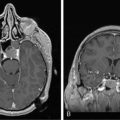
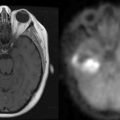
Headaches are present in 50% of patients at hospital presentation, although less than 10% of all patients have the “classical” headache of raised intracranial pressure.1 Patients with headaches are more likely to have larger tumors.2 The brain parenchyma does not contain pain fibers. Blood vessels, dura, and choroid plexus do have sensory nerve endings, and pain from stretching of these structures causes vascular or referred pain. In general, patients with supratentorial tumors have headaches referred frontally (cranial nerve V), and those with tumors involving the posterior fossa have headaches referred to the occipitocervical region (cranial nerves IX and X).
Sadly, the headaches associated with tumors have very few good discriminating features. Papilledema is found at some stage in more than 50% of patients with headache; however, less than 15% are identified as having papilledema at first presentation.1,3 Some patients with midline shift do not have any headache.2 The headache of tonsillar herniation is maximal in the neck and occiput and may be associated with nuchal rigidity and painful extensor spasms of the spine and limbs. These may mimic a generalized tonic seizure, but are not associated with loss of consciousness. Seizures are the first symptom of an intracerebral tumor in 21% of patients.Their frequency increases to 26.5% by hospital presentation, and approximately 50% of patients seize at some stage during the illness.4
By the time of hospital presentation, 81% of patients have some neurological signs on examination. The most common signs are unilateral focal motor or sensory signs involving the limbs; dysphasia; or impairment of memory and/or cognition. Neurological impairments are more common with increasing age, and older patients are more likely to have multiple and more severe impairments.5 Some authors have identified subtle cognitive problems in as many as 91% of patients with brain tumors before surgery.6 Anxiety is present in 17% to 30% prior to surgical operation; this may be more common in patients with right hemisphere tumors.7–9 Clinical depression reaching the DSM-IV criteria for major depressive disorder occurs in 19% to 38% of patients with a brain tumor and has been found to be the single most important independent predictor of quality of life.10 Depression is most common in women and those with a past or family history of psychiatric disorder.7,11 Patients with left hemisphere tumors reported significantly more memory problems and depressive symptoms.12 If the depression is severe, suicide is a real risk.
MANAGEMENT OF SYMPTOMS
Steroids have been shown to improve symptoms and neurological impairments and to reduce cerebral edema seen on imaging. In many patients with primary CNS lymphoma, and occasional patients with malignant glioma, the tumor can disappear on imaging. Steroids are not indicated in patients without significant symptoms or without cerebral edema seen on imaging, since the side effects of steroids are likely to outweigh any benefit.13 Patients with focal neurological deficits, and without symptoms or signs of raised intracranial pressure, respond well to dexamethasone 4 mg/day. This dose is as effective as 8 mg/day or 16 mg/day and has fewer side effects.14 If patients have symptomatic raised intracranial pressure or a significant shift seen on imaging studies, or in cases where herniation is suspected, an initial dose of 12 to 16 mg intravenously may be followed by 16 mg orally each day, until the tumor has been decompressed surgically or until a maximum benefit has been achieved. The dose should then be titrated downwards to the minimum effective dose. Steroids should be avoided after 6 pm because of the common side effect of insomnia. A clinical response to steroids was found in 37% of patients with a primary brain tumor at the time of diagnosis, 40% of patients with symptoms during radiotherapy, and 6% of patients after completion of radiotherapy.13 The frequency of improvement in symptoms was similar in patients with brain metastases.
There is an uncommon phenomenon sometimes found in patients with large mass lesions, in which paroxysmal neurological symptoms are triggered by standing. The attacks may occur despite dexamethasone therapy, but the addition of acetazolomide will often promptly stop symptoms.15 Patients with dysphasia, dysarthria and dysphagia and dysphonia should be assessed by a speech therapist and a swallowing assessment should be performed where indicated. Patients with hemiparesis or gait problems should be assessed by a physiotherapist and given advice regarding gait, mobility, and prevention of deep vein thrombosis. Resective surgery will lead to further improvements in many patients, often after an early postoperative worsening. Postoperative neurological deterioration occurs in 30% of patients undergoing resection and 36% who were biopsied.5 The postoperative nonneurological complication rate is highest for complete (9.7%) or partial resection (8%), and lowest for stereotactic biopsy (3.8%).
Where imaging has revealed an intracranial tumor and the patient presents with seizures, the patient should receive the advice of the neuro-oncology multidisciplinary team about seizure control and treatment of the tumor. The seizure threshold will be lowered by poor sleep, irregular diet, anxiety, noncompliance with medications, and the use of certain medications (e.g., antipsychotics, antidepressants, or alcohol). It is essential to discuss risk-avoidance strategies with the patient, both for work (i.e., dangerous machinery, heights) and leisure (i.e., swimming or bathing unsupervised). Issues such as: driving, oral contraceptives, pregnancy, education, and management of tonic-clonic seizures must be discussed. Relatives must be informed about first aid measures. The legislation regarding work and driving will vary from country to country and in the US, from state to state. UK regulations can be found on www.dvla.gov.uk.16
Tonic-clonic seizures are most likely to become completely controlled with medication, whereas focal seizures are only completely controlled by a single anticonvulsant in 30% to 40%, with an additional 30% to 40% achieving a 50% reduction in seizure frequency. Any of several anticonvulsants can be used to control seizures. Anticonvulsants have not been compared head to head in tumor-associated epilepsy. The anticonvulsant used will depend on the urgency for treatment (e.g., status epilepticus), possible interactions with other drugs likely to be used in management of the tumor and cost-effectiveness issues. Carbamazepine, phenytoin, and phenobarbital are enzyme-inducing agents whereas valproate, lamotrigine, levetiracetam, and topiramate are not. The newer anticonvulsants may be better tolerated, have fewer drug interactions, and may have fewer cognitive side effects, but they are more expensive.17,18 Guidelines are available on the efficacy and tolerability of the newer antiepileptic drugs.19 These recommend initial use of the older agents unless there is a satisfactory reason for not using them. Certain antiepileptic agents may alter the bioavailability of chemotherapy (see later). Whether this has any significant effect on survival or outcome is still uncertain. Acute seizure management is crucial, because prolonged seizures cause neurochemical changes occur that lead to neuronal damage. Evidence from a trial comparing four treatment regimes for the management of status epilepticus has demonstrated that intravenous injection of lorazepam 0.1 mg/kg, at a rate of less than 2 mg/min, is effective in the initial treatment in 65% of patients. Phenobarbital (15 mg/kg) was effective in 58%, diazepam (0.15 mg/kg) followed by phenytoin 18 mg/kg was effective in 56%, and phenytoin alone in 44% of cases. The American Academy of Neurology Guidelines for the management ofstatus epilepticus have been summarized in Table 12-1.
Surgical resection of some low-grade neoplasms (gangliogliomas, pilocytic astrocytomas, dysembryoplastic neuroepithelial tumors) can cure epilepsy or reduce seizure frequency, particularly if the epileptogenic area, as determined by noninvasive recordings, is resected in addition to the tumor.20 Fractionated radiotherapy and radiosurgery can reduce seizure frequency to Engel classification I or II in 54% of patients with brain tumors and intractable epilepsy.21 As with tumor-associated epilepsy surgery, temporal lobe tumors had a better success rate than extratemporal tumors. Temozolomide may reduce the seizure frequency in intractable epilepsy.22
There does not seem to be a difference in how patients with brain tumors cope with their illness as compared to patients with other brain diseases such as stroke and Parkinson disease.23 Anxiety or depression may occur due to uncertainty about symptoms, likelihood of control of symptoms, and worries about the immediate future, including death, or family and financial matters. All of these affect quality of life. Psychological morbidity is related to physical and neuropsychological functioning. Psychological morbidity is associated with high levels of physical disability and also with cognitive dysfunction, but is not related to the grade of tumor or the extent to which the patient was aware of the nature of his or her disease. In one prospective study, 5% had clinically significant levels of anxiety, and six (15%) had clinically significant levels of depression, yet 92% felt they had full or intermediate knowledge about their prognosis.
A variety of psychological approaches have been used, but cognitive-behavior therapy (CBT) has been most researched in people with cancer. Treatment with antidepressants has been shown to be more effective than either placebo or no treatment in patients with physical illness.24 However, there are no randomized controlled trials of the use of antidepressants in patients with brain tumors. Management guidelines for the treatment of depression in those who are also physically ill have been published.25 Short-term, highly focused forms of psychotherapy such as cognitive-behavioral, supportive, and group therapies are helpful but time-consuming. They may be the only forms of treatment available in patients disinclined to accept antidepressants or who are intolerant of them. CBT is as effective as antidepressants. By 3 to 8 months, 50% to 60% of patients are in remission, compared with only 27% treated with placebo.26,27
Depression should be and can be successfully treated.28 The importance of this is supported by the observation that the presence of depression impacts negatively on both psychological and physical quality-of-life outcomes in patients with brain tumors.29 Neuropsychiatrists may be able to help identify whether sedation, confusion, and possible lowered seizure threshold are related to the disease or to antidepressant or antipsychotic medication.30 There are no randomized controlled trials of different treatment approaches in the neuropsychiatric management of patients with brain or CNS tumors. The advice, therefore, is at the level of recommendations for best practice from a consensus group of experts. In the “Glioma Outcome” study (2004) that included 598 patients diagnosed with a glioma,31 concordance between physician recognition of depression and treatment of depression was initially low (33%), but increased at 3 and 6 months (51%and 60%, respectively). Antidepressants may have an adverse effect on seizure control, although this is rarely a serious complication in my experience, and it has not been found in recent prospective studies.32 Antidepressants should be prescribed when necessary, as depression is a major cause of poor quality of life in patients with epilepsy.33 Selective serotonin-reuptake inhibitors have largely replaced tricyclic antidepressants in the management of somatic psychiatric conditions, but good comparative treatment studies in patients with brain tumors are not available.
Prophylactic Perioperative Care
PROPHYLACTIC ANTICONVULSANTS
Although it has been common practice in the USA and parts of Europe to prescribe prophylactic anticonvulsants prior to neurosurgery for malignant brain tumors, there is now evidence from a meta-analysis of randomized controlled trials showing that anticonvulsant prophylaxis does not reduce the seizure incidence (OR 1.09; 95% CI, 0.63 to 1.89; p=0.8), seizure-free survival (OR 1.03; 95% CI, 0.74 to 1.44; p=0.9), or overall survival (OR 0.93; 95% CI, 0.65 to 1.32; p=0.7) in patients with brain tumors who have not had an epileptic seizure.34 More recent studies, in which patients were randomized either to no treatment or to treatment with carbamazepine or phenytoin for 6 or 24 months, showed no significant differences in any group. A high incidence of drug-related side effects was found in this and other studies.35–37 Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors is therefore not justified.
PROPHYLACTIC ANTICOAGULATION
Cancer, immobility, hemiplegia, and surgery are all risk factors for deep vein thrombosis and pulmonary embolus. Risk of DVT and/or pulmonary embolus is age-related, with an annual incidence of less than 1:3000 in patients under the age of 40 and 1:500 in those over the age of 80.38 The frequency of DVT in malignant glioma is between 19% and 28%, and the risk that a patient with a brain tumor will display a DVT in follow-up studies is between 22% and 45%.39 The frequency of DVT is highest in meningioma (72%), followed by glioma (60%) and metastasis (20%).40,41 Despite the very high likelihood of thrombotic complications in patients with malignant glioma, there is no consensus about DVT prophylaxis.42 The risk of DVT is reduced by use of compression stockings, pneumatic intermittent compression boots, early mobilization, low-dose heparin or fractionated heparin. A meta-analysis of four randomized controlled trials of heparin prophylaxis in neurosurgical patients has shown that with placebo, 29% of people develop DVT, compared with 16% of those receiving low molecular weight heparin or unfractionated heparin.43 A critical appraisal of the literature on prophylactic anticoagulation therapy in neurosurgery concluded that treatment with heparin resulted in a 45% relative risk reduction of venous thromboembolism (OR 0.48; 95% CI 0.35-0.66; p<0.001).44 Major bleeding complications were infrequent, but heparin (both UFH and LMWH) resulted in a 71% increased relative risk of major bleeding events (OR 1.71; 95% CI 0.69-4.27; p=0.24). A randomized controlled trial of heparin 5000 U subcutaneously, starting 2 hours before surgery and continuing every 12 hours thereafter until full ambulation or for 7 days, in 103 patients undergoing surgery for removal of a supratentorial tumor, showed no increase in bleeding tendency in any of the parameters examined compared with the placebo arm.45 There is no evidence to suggest that enoxaparin 40 mg/day is superior to unfractionated heparin. In the randomized controlled trial comparing these agents as prophylaxis, none of the 150 participants in the study suffered symptomatic DVT and less than 10% had asymptomatic DVT (mostly calf) using the combination of graduated compression stockings, intermittent pneumatic compression, and either enoxaparin or subcutaneous heparin 5000 U twice daily.46 A multimodality approach to prophylaxis, using compression stockings, pneumatic intermittent compression boots, and prophylactic perioperative minidose heparin during surgery is safe and is recommended.
Complications Indirectly Related to the Tumor and Its Effects
DEEP VEIN THROMBOSIS AND PULMONARY EMBOLUS
Deep vein thrombosis (DVT) may be difficult to diagnose. Although DVT may cause unilateral calf or thigh swelling, pain and tenderness along the line of the deep veins, low-grade pyrexia, distension of superficial veins, or color change, many cases are asymptomatic. Homan sign is poorly predictive. There is a 10% rate of symptomatic pulmonary embolus in untreated proximal DVT, and 18% to 30% mortality if this is left untreated.47 Pulmonary embolus is rare if the DVT is treated by anticoagulation therapy.48 DVT can be complicated by critical limb ischemia (less than 5%), recurrent DVT (20% in 5 years), or postthrombotic syndrome (50% to 75%). If clinically suspected, intravenous heparin should be started until the diagnosis is excluded by diagnostic imaging.49 as the benefits of anticoagulation outweigh the risks of tumor hemorrhage. Compression ultrasound will reliably identify proximal DVT, but contrast venography is the gold standard if ultrasound is negative.
Unfractionated heparin is the initial treatment of choice followed by oral anticoagulation.49,50 Low molecular weight heparin is an effective alternative to unfractionated heparin and may reduce both the occurrence of major bleeding during initial treatment and overall mortality.51 Low molecular weight heparin is more costly, but it can be given once per day by subcutaneous injection and there is no need to monitor blood tests. If DVT is confirmed, heparin should be continued for 4 to 6 days while oral anticoagulation is being introduced. An international normalized ratio (INR) of greater than 2.0 on 2 consecutive days should be obtained before heparin is stopped. The optimal INR after a first DVT is 2.5. Anticoagulation should be continued for at least 3 months, although 6 months is commonly recommended. Aspirin and nonsteroidal antiinflammatory drugs should be avoided when on heparin or warfarin. Hypersensitivity (drug-induced thrombocytopenia), local injection site bruising, and bleeding are common complications of heparin. Graduated elastic compression stockings worn on the affected leg for at least 2 years after a DVT reduces the incidence of postthrombotic syndrome from 23% to 11%.52,53
Effort dyspnea, syncope, and tiredness may herald small pulmonary emboli, while medium-sized emboli usually cause pleuritic chest pain, cough, and hemoptysis. Large pulmonary emboli cause central chest pain, collapse, shock, tachypnea, and tachycardia. A spiral computed tomogram of the chest usually demonstrates emboli well, but occasionally a pulmonary angiogram is required. Alternatively, a ventilation-perfusion scan using technecium-99m may show mismatch. Blood gases, electrocardiogram, chest x-ray, erythrocyte sedimentation rate, and full blood count may be supportive where pulmonary angiogram or spiral CT are not readily available. Medical management of pulmonary embolism consists of 100% oxygen, intravenous fluids to increase the right ventricular filling pressure, heparin, and oral anticoagulation. Venous thrombectomy and pulmonary embolectomy may be indicated for massive pulmonary emboli. If there is a clear contraindication to anticoagulation (e.g., known hypersensitivity to heparin or pork products, major active bleeding, thrombocytopenia <50,000 platelets/μl, or a history of heparin-induced thrombocytopenia), inferior vena caval filters can be used. Filters often do not prevent pulmonary emboli, and some studies suggest a 40% recurrence rate and a high complication rate (62%).54,55
Complications from medical treatment
STEROIDS
Cautions for usage include advanced age, past history of tuberculosis, heart, liver or renal failure, diabetes, hypertension, glaucoma, or a past history of severe psychosis. With long-term usage, elevation in blood sugar occur in 47% to 72%, peripheral edema in 11%, anxiety or psychiatric disorders in 10%, oropharyngeal candidiasis in 6% to 8%, Cushing syndrome in 15%, and muscular weakness in 60%. Rarely, an acute myopathy can come on within a week of starting high-dose corticosteroids and can involve respiratory muscles. Myopathy is more common with 9-alpha-fluorinated corticosteroids, such as dexamethasone; classical steroid myopathy is painless, with a slow onset, and affects proximal lower limb muscles and occasionally proximal upper limbs.56 The mechanism of this steroid myopathy is felt to be due to inhibition of messenger RNA synthesis of muscle-specific proteins. Withdrawal of dexamethasone after usage for some months must be done fairly slowly, and may be associated with changes in mood, myalgia, arthropathy, headaches, or loss of appetite. Dexamethasone-withdrawal headache is nonspecific and may lead to the reinstatement of higher doses due to concerns of raised intracranial pressure. Evidence of reduced tumor mass effect and reassurance that withdrawal can cause headache may aid eventual withdrawal and limit psychological dependence.
ANTIEPILEPTIC DRUGS (AEDS)
AEDs should be prescribed after discussions with the patient about the benefits and side effect profiles. Ideally, epilepsy should be treated with a single anticonvulsant agent at the lowest effective dose. Combination therapy may be necessary, but is commonly associated with complex drug interactions and increased frequency of side effects. A careful watch must be kept in the early stages of drug introduction, especially for early hematological, hypersensitivity, or central nervous system side effects. Early allergic responses necessitate withdrawal. AED hypersensitivity syndrome is characterized by multisystem involvement, fever, lymphadenopathy, mucocutaneous rash, hypertransaminasemia, and peripheral eosinophilia. This potentially lethal complication is a feature of aromatic enzyme-inducing antiepileptics (phenytoin, phenobarbital, carbamazepine) through the hepatic cytochrome P450 (CYP) isoenzyme mechanism.57 There is cross-reactivity between these antiepileptics. The risk of allergic rash or blood dyscrasias is approximately 5% to 10% with carbamazepine, phenytoin, and lamotrigine. This generally occurs within the first 2 months of starting therapy. Stevens-Johnson syndrome, toxic epidermal necrolysis, and hepatic complications are rare but serious complications. In patients with drug-induced skin rash, valproate, gabapentin, topiramate, tiagabine, and levetiracetam are moderately safe choices.
The enzyme-inducing AEDs will commonly increase the gamma GT by up to three times the upper limit, but this usually only requires monitoring rather than drug alteration. However, all drugs (especially valproate) can cause serious hepatic toxicity and liver failure; therefore, although these complications are rare, it is advisable to monitor liver function during the introduction and the following 6 months. The incidence of hematological toxicity from valproate is low and mainly consists of dose-dependent thrombocytopenia. Interactions are usually due to hepatic enzyme induction or inhibition, and are variable and often unpredictable. AED interactions are summarized in Table 12-2.
TABLE 12-2 Antiepileptic Drug Addiction and the Effect on Existing Drugs
| Carbamazepine | Often lowers concentration | Clonazepam, clobazam, lamotrigine, phenytoin, valproate, topirimate, oxcarbazepine, tiagabine |
| May increase concentration | Phenytoin, phenobarbital | |
| Gabapentin | No interactions | |
| Lamotrigine | May increase | Active metabolite carbamazepine |
| Levetiracetam | No interactions | |
| Oxcarbazepine | May lower concentration | Carbamazepine |
| May raise concentration | Carbamazepine | |
| Phenobarbital | Often lowers concentration | Clonazepam, carbamazepine |
| Lamotrigine, phenytoin, valproate, topirimate, oxcarbazepine,tiagabine | ||
| May increase concentration | Phenytoin | |
| Phenytoin | Often lowers concentration | Clonazepam, carbamazepine |
| Lamotrigine, valproate, topirimate, oxcarbazepine, tiagabine | ||
| Often increases concentration | Phenobarbital | |
| Topiramate | May raises concentration | Phenytoin |
| Valproate | May lower concentration | Active metabolite oxcarbazepine |
| Often raises concentration | Active metabolite carbamazepine | |
| Lamotrigine, Phenobarbital, Phenytoin |
Metabolism of phenytoin may be altered by drugs influencing CYP2C9 or CYP2C19, such as diazepam, leading to phenytoin toxicity. Tricyclic antidepressants inhibit CYP2C19. P450 enzyme-inducing anticonvulsants reduce serum levels of chemotherapeutic agents metabolized by CYP3A4 and CYP2A6. This could have a beneficial effect on reducing the toxicity of chemotherapeutic agents, but may also reduce the effectiveness of these agents. Whether this has any significant effect on survival or outcome is still uncertain. When survival was studied in chemotherapy trials of patients with glioblastoma who were or were not on enzyme-inducing AEDs, enzyme-inducing AEDs were associated with better overall survival and progression-free survival (HR=0.75, p=0.0029 and HR 0.81, p=0.024, respectively).58 Enzyme-inhibiting AEDs (e.g., valproic acid) can increase the concentration of chemotherapy. The newer generation of AEDs (e.g., lamotrigine, gabapentin, and levetiracetam) are not metabolized by CYP, and may be as effective in managing seizures.59 Procarbazine oxidation is enhanced by enzyme-inducing AEDs and may increase the likelihood of hypersensitivity reactions to procarbazine.60 The risk of thrombocytopenia with valproate may be worsened if patients are receiving fotomustine-cisplatinum chemotherapy, but it responds to valproate dose reduction.61
In the context of management of patients with brain tumors, AEDs may cause central nervous system side effects that may be difficult to distinguish from tumor progression or may influence management in other ways. These are summarized in Table 12-3. Carbamazepine can cause a mild neutropenia and hyponatremia that may influence the later use of chemotherapy. Valproate is often associated with weight gain that can be a problem if the patient also requires steroids. Valproate can also inhibit platelet aggregation and the coagulation cascade which may lead to a higher tendency to hemorrhage when used with heparins, warfarin, or nonsteroidal antiinflammatory drugs. Valproate can cause a fine tremor, which is often more noticeable in the hemiparetic limb in patients with an existing hemiparesis. Toxicity, with AEDs inducing dysarthria, ataxia, lethargy, and weakness, can be easily mistaken for tumor progression, although the presence of nystagmus and intermittent diplopia and the absence of papilledema or focal neurological deficit are helpful pointers to the likely diagnosis of AED toxicity. Many AEDs are associated with headache, cognitive, speech or memory problems, or psychiatric symptoms that may be mistaken for tumor progression. Some suggest that patients with cognitive problems may be best suited to treatment with newer agents such as lamotrigine, gabapentin, tiagabine, levetiracetam or oxcarbazepine, although this is only by extrapolation from studies in patients with nontumor-associated epilepsy. Obese patients may benefit from topiramate or zonisamide, as these have a tendancy to produce weight loss. Acute angle closure glaucoma has been associated with topiramate and reverses when the drug is withdrawn. Vigabatrin is not commonly used now because of its association with irreversible visual field loss and the requirement for visual fields to be checked regularly.62
TABLE 12-3 Important, Common, or CNS Side Effects of Anticonvulsants
| Carbamazepine | Cautions: | Hepatic, renal, cardiac disease. Glaucoma. |
| Beware of early severe blood and skin disorders | ||
| Oxcarbazepine | Toxicity: | Diplopia, dizziness; confusion, ataxia, tremor |
| Side effects: | Rash, agitation; leukopenia and other blood disorders, jaundice, renal failure, hypersensitivity reaction, depression, psychosis, alopecia, hyponatremia, edema, osteomalacia | |
| Gabapentin | Cautions: | Psychotic illness, renal impairment, diabetes |
| Toxicity: | Tiredness, diplopia, dizziness, ataxia, tremor | |
| Side effects: | Rash, leukopenia and other blood disorders, myalgia, headache, memory problems, hypersensitivity reaction, cough, paresthesia | |
| Lamotrigine | Cautions: | Hepatic, renal. Beware of early severe blood and skin disorders and aplastic anemia |
| Toxicity: | Diplopia, dizziness; confusion and ataxia | |
| Side effects: | Rash, hypersensitivity reaction, flu-like illness, worsening seizures, agitation; leukopenia and other blood disorders, dizziness, drowsiness, insomnia, headache, agitation | |
| Levetiracetam | Cautions: | Hepatic, renal disease |
| Toxicity: | Drowsiness, fatigue, and dizziness | |
| Side effects: | Drowsiness, fatigue, and dizziness. Rarely amnesia, psychiatric symptoms, insomnia, headache, rash | |
| Phenobarbital | Cautions: | Hepatic, renal disease; careful in elderly |
| Toxicity: | Drowsiness, fatigue, and dizziness | |
| Side effects: | Drowsiness, fatigue, and dizziness, psychiatric symptoms(depression or agitation), insomnia, megaloblastic anemia (folate deficiency) | |
| Phenytoin | Cautions: | Hepatic disease. Beware of early severe blood and skin disorders. |
| Toxicity: | Diplopia, dizziness; confusion, ataxia, tremor | |
| Side effects: | Rash, agitation; leukopenia and other blood disorders, jaundice, SLE, hypersensitivity reaction, depression, psychosis, gum hypertrophy, peripheral neuropathy, megaloblastic anemia, osteomalacia | |
| Topiramate | Cautions: | Hepatic disease and renal disease. May cause secondary acute angle closure glaucoma in myopes in first month. |
| Toxicity: | Diplopia, dizziness; confusion, ataxia, tremor | |
| Side effects: | Rash, agitation; leukopenia and other blood disorders, jaundice, weight loss, paresthesia, memory problems, fatigue, speech problems, depression, psychosis | |
| Valproate | Cautions: | Hepatic disease, clotting disorders. Pancreatitis |
| Toxicity: | Tremor. Diplopia, dizziness; confusion, ataxia | |
| Side effects: | Leukopenia and other blood disorders, alopecia, weight gain, gastrointestinal side effects, memory problems, dementia, gynecomastia |
Neurological and Psychiatric Postoperative Complications
NEUROLOGICAL IMPAIRMENTS
In a prospective database, 788 patients who underwent their first or second craniotomy for malignant glioma were analyzed for perioperative complications.63 Perioperative complications occurred in 24% of patients after a first operation and 33% of patients after a second (p = 0.1). Most patients were the same or better neurologically after surgery, but 8% displayed significant worsening after the first operation and 18% after the second (p = 0.007). Regional complications occurred at similar rates in both groups, but systemic infections occurred more frequently in the second surgery group (4.4% compared with 0%; p < 0.0001), as did depression (20% compared with 11%; p = 0.02). The perioperative mortality rate was 1.5% following a first operation and 2.2% after a second. Nevertheless, most selected patients are neurologically stable or improved after either their first or second craniotomy.64 Studies using intraoperative image guidance and functional mapping in patients with low-grade glioma identified a high percentage of postoperative cognitive deficits, but these often resolve within 3 months.65,66 In one study of neurocognitive dysfunction in patients with insular tumors undergoing surgery, neurosurgeons presurgically identified altered mental status in 13%; however, neuropsychological testing demonstrated cognitive problems in 73%. Postsurgical decline (median 16 days postoperatively) was identified in all patients in at least one cognitive domain.67 See Table 12-4.
ACUTE CONFUSIONAL STATES/ACUTE PANIC ATTACKS
In patients who are confused or demented or who have receptive dysphasia, all forms of treatment should be discussed with the next of kin. In patients with postsurgical acute confusional states, it is essential to consider infection, hypoxia, endocrine or metabolic derangement, drug-related side effects, and underlying psychiatric conditions. Psychiatrists are skilled in assessments of mental capacity and consent, and if the psychiatric disturbance is severe, the patient may require to be detained and treated under section. Patients with acute confusional states in the early postsurgical phase are almost always being treated with steroids. The management of these patients generally follows the usual principles of management of an acute delirium in a medically sick patient. Some advice concerning psychiatric complications in cancer patients has been published in the literature.68 A study from Memorial Sloan-Kettering Cancer Center found that 41% of inpatient psychiatric referrals were diagnosed with organic mental disorders.30
Acute panic attacks are uncommon and must be differentiated from epileptic panic attacks. They usually occur in patients with a past history of anxiety or depression and are more common in younger patients (under 50 years of age). Early identification of patients with anxiety and appropriate counseling (information clearly given, reassurance, message of hope, advice about side effects of medication, a contact telephone number to call if anxious, e.g., neuro-oncology nurse), may be highly effective in reducing the likelihood of panic attacks. Epileptic panic attacks are usually short-lived, stereotyped, and nonsituational; also, the patient is well between attacks. An electroencephalogram may show a discharging seizure focus. The treatment is appropriate antiepileptic drugs. The recognized treatments for severe acute (nonepileptic) panic attacks include cognitive behavior therapy and, particularly in the USA, alprazolam treatment.28,30 In general, short-acting drugs with a good side-effect profile (less potent anticholinergic effects) should be prescribed at low doses.
1. R. Grant. Overview: Brain tumour diagnosis and management/Royal College of Physician Guidelines. J Neurol Neurosurg Psychiat. 2004;75(Suppl. 2):18-23.
2. P.A. Forsyth, J.B. Posner. Headaches in patients with brain tumors. Neurology. 1993;43:1678-1683.
3. C.M. Fisher. Brain herniation: a revision of classical concepts. Can J Neurol Sci. 1995;22(2):83-91.
4. B. Schaller, S.J. Ruegg. Brain tumor and seizures: pathophysiology and its implications for treatment revisited. Epilepsia. 2003;44(9):1223-1232.
5. Z. Clyde, J. Chataway, D. Signorini, A. Gregor, R. Grant. Significant change in tests of neurological impairment in patients with brain tumours. J Neurooncol. 1998;39:81-90.
6. O. Tucha, C. Smely, M. Preier, K.W. Lange. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47:324-333.
7. A. Pringle, R. Taylor, I.R. Whittle. Anxiety and depression in patients with intracranial neoplasm before and after tumour surgery. Br J Neurosurg. 1999;13(1):46-51.
8. A. Mainio, H. Hakko, A. Niemela, T. Tuurinkoski, J. Koivukangas, P. Rasanen. The effect of brain tumour laterality on anxiety levels among neurosurgical patients. J Neurol Neurosurg Psychiat. 2003;74:1278-1282.
9. R.K. Gupta, R. Kumar. Benign brain tumours and psychiatric morbidity: a 5 years retrospective data analysis. Austral New Zealand J Psychiat. 2004;38:316-319.
10. G. Pelletier, M. Verhoef, N. Khatri, N. Hagen. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress and existential issues. J Neurooncol. 2002;57(1):41-49.
11. D. Wellisch, T. Kaleita, D. Freeman, T. Cloughesy, J. Goldman. Predicting major depression in brain tumor patients. Psychooncol. 2002;11(3):230-238.
12. C.A. Hahn, R.H. Dunn, P.E. Logue, J.H. King, C.L. Edwards, E.C. Halperin. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55(4):992-999.
13. C. Hemper, E. Weiss, C.F. Hess. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side effects? Sup Care Cancer. 2002;10:322-328.
14. C.J. Vecht, A. Hovestadt, H.B. Verbiest, J.J. van Vliet, C.J. van Puten. Dose effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16mg per day. Neurology. 1994;44(4):675-680.
15. Watling, J.G. Cairncross. Acetazolamide therapy for symptomatic plateau waves in patients with brain tumors. Report of three cases. J Neurosurg. 2002;97(1):224-226.
17. G.K. Bergey. Initial treatment of epilepsy: special issues in treating the elderly. Neurology. 2004;63(10 Suppl. 4):S40-S48.
18. G.L. Wagner, E.B. Wilms, C.A. van Donselaar, Ch.J. Vecht. Levetiracetam: preliminary experience in patients with primary tumours. Seizure. 2003;12(8):585-586.
19. E. Beghi. efficacy and tolerability of the newer anti-epileptic drugs: comparison of two recent guidelines. Lancet Neurol. 2004;3(10):618-621.
20. J. Zenter, A. Hufnagel, H.K. Wolf, B. Ostertun, E. Behrens, M. Campos, et al. Surgical treatment of neoplasms associated with medically intractable epilepsy. Neurosurgery. 1997;41(2):378-386.
21. O. Schrottner, H.G. Eder, F. Unger, K. Feichtinger, G. Pendl. Radiosurgery in lesional epilepsy: brain tumors. Stereotact Funct Neurosurg. 1998;70(Suppl. 1):50-56.
22. K. Hoang-Xuan, L. Capelle, M. Kujas, et al. Temozolomide as initial treatment for adults with low-grade oligodendroglioma or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22(15):3133-3138.
23. M. Herrmann, N. Curio, T. Petz, H. Synowitz, S. Wagner, C. Bartels, et al. Coping with illness after brain diseases—a comparison between patients with malignant brain tumors, stroke, Parkinson’s disease and traumatic brain injury. Disabil Rehab. 2000 Aug 15;22(12):539-546.
24. D. Gill, S. Hatcher. A systematic review of the treatment of depression with antidepressant drugs in patients who also have physical illness. J Psychosomat Res. 1999;47(2):131-143.
25. R. Voellinger, A. Berney, P. Bauman, J-M Annoni, C. Bryois, T. Buclin, et al. Major depressive disorder in the general hospital: adaptation of the practice guidelines. Gen Hosp Psychiat. 2003;25(3):185-193.
26. L. Mynors-Wallis, et al. Problem-solving treatment: evidence for effectiveness and feasibility in primary care. Int J Psychiat Med. 1996;26:249-262.
27. H.C. Schulberg, M.R. Block, M.J. Madonia, C.P. Scott, E. Rodriguez, S.D. Imber, et al. Treating major depression in primary care practice: eight month clinical outcomes. Arch Gen Psychiat. 1996;58:112-118.
28. S. Wein. Cancer. In: R. Robinson, W. Yates, editors. Psychiatric Treatment of the Mentally Ill. New York: Marcel Dekker, Inc; 1999:229-251.
29. M.E. Huang, J. Wartella, J. Kreutzer, W. Broaddus, L. Lyckholm. Functional outcomes and quality of life in patients with brain tumours: a review of the literature. Brain Inj. 2001;15:843-856.
30. S. Passik, P. Ricketts. Central Nervous System Tumors. In: Psycho-oncology. JC Holland. Oxford: Oxford University Press; 1998:303-313.
31. N.S. Litofsky, E. Farace, F. AndersonJr, C.A. Meyers, W. Huang, E.R. LawsJr. Glioma Outcomes Project Investigators. Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery. 2004;54(2):358-366. Feb (discussion 366–7)
32. K.U. Kuhn, B.B. Quednow, M. Thiel, P. Falkai, W. Maier, C.E. Elger. Anti-depressive treatment in patients with temporal lobe epilepsy and major depression: a prospective study with three different antidepressants. Epilepsy Behav. 2003;4(6):674-679.
33. L.S. Boylan, L.A. Flint, D.L. Labovitz, S.C. Jackson, K. Starner, O. Devinsky. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology. 2004;62(2):258-261.
34. M.J. Glantz, B.F. Cole, P.A. Forsyth, L.D. Recht, P.Y. Wen, M.C. Chamberlain, et al. Practice Parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumours. Report of the Quality Standards Subcommittee of the American Academy of Neurology. 2000. http://www.aan.com/professionals/practice/index.
35. P.M. Foy, D.W. Chadwick, N. Rajgopalan, A.L. Johnson, M.D. Shaw. Do prophylactic anticonvulsant drugs alter the pattern of seizures after craniotomy? J Neurol Neurosurg Psychiat. 1992 Sep;55(9):753-757.
36. A. De Santis, R. Villani, M. Sinisi, N. Stocchetti, E. Perucca. Add-on phenytoin fails to prevent early seizures after surgery for supratentorial brain tumors: a randomized controlled study. Epilepsia. 2002;43(2):175-182.
37. A. Pace, L. Bove, P. Innocenti, et al. Epilepsy in gliomas: incidence and treatment in 119 patients. J Exp Clin Cancer Res. 1998;17(4):479-482.
38. DH. Advice on travel related deep vein thrombosis. London Department of Health. 2002. www.doh.gov.uk/dvt/index.htm.
39. R.E. Sawaya, B.L. Ligon. Thromboembolic complications associated with brain tumors. J Neurooncol. 1994;22:173-181.
40. R. Sawaya, M. Zuccarello, M. Elkalliny, H. Nishiyama. Post-operative venous thromboembolism and brain tumors: part 1. Clinical profile. J Neurooncol. 1992;14:119-125.
41. A.D. Levi, M.C. Wallace, M. Bernstein, B.C. Walters. Venous thromboembolism after brain tumor surgery: a retrospective review. Neurosurgery. 1991;28:859-863.
42. S.F. Danish, M.G. Burnett, S.C. Stein. Prophylaxis for deep venous thrombosis in patients with craniotomies: a review. Neurosurg Focus. 2004;17:1-8.
43. A. Iorio, G. Agnelli. Low molecular weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery—a meta-analysis. Arch Int Med. 2000;160(15):2327-2332.
44. O. Abdulwadud. Anticoagulation therapy as prophylaxis for prevention of DVT or pulmonary embolism in neurosurgery. 2002. [Online]. Available from http://www.med.monash.edu.au/healthservices/cce/
45. S. Constantini, A. Kanner, A. Friedman, Y. Shoshan, Z. Israel, E. Ashkenazi, et al. Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double blind study. J Neurosurg. 2001;94(6):918-921.
46. S.Z. Goldhaber, K. Dunn, M. Gerhard-Herman, J.K. Park, PMcL. Black. Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest. 2002;122:1933-1937.
47. R.D. Hull, G.F. Pineo. Prophylaxis of deep venous thrombosis and pulmonary embolism. Current recommendations. Med Clin North Am. 1998;82(3):477-493.
48. B.A. Hutten, M.H. Prins. Duration of oral anticoagulant treatment for symptomatic venous thromboembolism (Cochrane Review). The Cochrane Library. (4):2002. Oxford. Update Software
49. SIGN Anti-thrombotic therapy. Scottish Intercollegiate Guidelines Network. 1999. www.sign.ac.uk. Report Number 36
50. D.P. Brandjes, H. Heijboer, H.R. Butler, M. de Rijk, H. Jagt, J.W. ten Cate. Acenocoumerol and heparin compared with acenocoumerol alone in the initial treatment of proximal vein thrombosis. N Engl J Med. 1992;327(21):1485-1489.
51. A.G.M. van den Belt, M.H. Prins, A.W. Lensing, et al. Fixed dose subcutaneous low molecular weight heparin for the long term treatment of symptomatic venous thromboembolism. (Cochrane Review). The Cochrane Library. (4):2002. Oxford. Update software
52. A.A. Brandes, E. Scelzi, G. Salmistraro, M. Ermani, C. Carollo, F. Berti, et al. Incidence of risk of thromboembolism during treatment of high grade gliomas: a prospective study. Eur J Cancer. 1997;33:1592-1596.
53. C. McCollum. Avoiding the consequences of deep vein thrombosis. Elevation and compression are important and too often forgotten. Brit Med J. 1998;317:696.
54. J.M. Levin, D. Schiff, J.S. Loeffler, H.A. Fine, P.M. Black, P.Y. Wen. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology. 1993;43:1111-1114.
55. D. Schiff, L. DeAngelis. Therapy for venous thromboembolism in patients with brain metastases. Cancer. 1994;73:493-498.
56. T.T. Batchelor, L.P. Taylor, H.T. Thaler, J.B. Posner, L.M. DeAngelis. Steroid myopathy in cancer patients. Neurology. 1997;48:1234-1238.
57. R.N. Maldonado, S.J. Tello, R.E. Garcia-Baquero, H.A. Castano. Anticonvulsant hypersensitivity syndrome with fatal outcome. Eur J Dermatol. 2002;12(5):503-505.
58. K.A. Jaekle, K. Ballman, J. Uhm, J. O’Fallon, P. Schomberg, C. Scheithauer, et al. Comparison of survival endpoints in glioblastoma patients receiving or not receiving enzyme-inducing anticonvulsants in NCCTG Trails. 113s ASCO 2004. Abstract No 1525. J Clini Oncol. 2004;22(Suppl. 14):113s.
59. C.J. Vecht, G.L. Wagner, E.B. Wilms. Treating seizures in patients with brain tumors: Drug interactions between antiepileptic and chemotherapeutic agents. Semin Oncol. 2003;30(6 Suppl. 19):49-52.
60. D.F. Lehmann, T.E. Hurteau, N. Newman, T.E. Coyle. Anticonvulsant usage is associated with an increased risk of procarbazine hypersensitivity reactions in patients with brain tumors. Clin Pharmacol Ther. 1997;62(2):225-229.
61. V. Bourg, C. Lebrun, R.M. Chichmanian, P. Thomas, M. Frenay. Nitroso-urea-cisplatin-based chemotherapy associated with valproate: increase of haematological toxicity. Ann Oncol. 2001;12(2):217-219.
62. J.J. Asconape. Some common issues in the use of antiepileptic drugs. Semin Neurol. 2002;22(1):27-39.
63. S.M. Chang, I.F. Parney, M. McDermott, F.G. Barker2nd, M.H. Schmidt, W. Huang, et al. Glioma Outcomes Investigators. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98(6):1175-1181. Jun
64. R. Grant, J. Slattery, A. Gregor, I.R. Whittle. Recording neurological impairment in clinical trials of glioma. J Neuro-Oncol. 1994;19:37-49.
65. H. Duffau, L. Capelle, D. Denvil, et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiat. 2003;74:901-907.
66. H. Duffau, L. Capelle, D. Denvil, et al. Usefulness of intra-operative electrical subcortical mapping during surgery for low grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98:764-785.
67. J.S. Wefel, F.F. Lang, R. Nadar, C.A. Meyers. Neurocognitive sequelae of surgical resection for previously untreated intrinsic insular region tumor. Neurooncol. 2003:3350.
68. S. Olofsson, M. Weitzner, A. Valentine, W. Baile, C. Meyers. A retrospective study of the psychiatric management of delirium in the cancer patient. Support Care Cancer. 1996;4(5):351-357.