Chapter 13 Medial Branch Blocks
Cervical, Thoracic, and Lumbar
 Findings on radiographs, computed tomography, and magnetic resonance imaging are often inconclusive.
Findings on radiographs, computed tomography, and magnetic resonance imaging are often inconclusive. Diagnostic local anesthetic nerve blocks of the medial branches are the best way to diagnose facet pain.
Diagnostic local anesthetic nerve blocks of the medial branches are the best way to diagnose facet pain.Establishing a Diagnosis
History and Physical Examination
History and physical examination are important for exclusion of other causes of spinal pain. On reviewing the medical history, several conditions can affect the facet joints. These include osteoarthritis, inflammatory arthritides (rheumatoid arthritis, ankylosing spondylitis, and reactive arthritis), synovial impingement, meniscoid entrapment, chondromalacia facetae, pseudogout, synovial inflammation, and villonodular synovitis,1–4 Signs on clinical examination that may point to facet pain include tenderness over the facet joints on palpation; pain that is characterized as deep, dull, and aching; pain that is difficult to localize; and stiffness, especially in the morning. Sudden onset of pain noted with twisting, bending, or rotary movements and pain aggravated on extension and lateral bending more than flexion are also consistent with facet joint–mediated pain, although the latter finding is unreliable. Additional physical examination findings may include muscle spasm and either hypalgesia or hyperalgesia over the area of pain. Flexion, extension, and rotation may or may not be decreased. The straight-leg raise results are usually negative. Given all of the above, the clinical criteria for facet joint–mediated pain are generally nonspecific and unreliable.5–7 Most maneuvers used in physical examinations are likely to stress several structures simultaneously, especially the discs, muscles, and facet joints, thus failing to provide any reasonable diagnostic criteria based on physical examination.
As stated previously, history and physical examination, along with radiologic imaging, cannot reliably be used to diagnose painful zygapophyseal joints. Therefore, medical branch blocks are needed to diagnose facetogenic pain.8 Numerous studies have tried to predict who will respond to facet joint injections. Revel et al9 identified seven variables associated with a positive response to facet joint injections: age older than 65 years, pain that was not exacerbated by coughing, pain not worsened by hyperextension, pain not worsened by forward flexion, pain not worsened when rising from flexion, pain not worsened by extension-rotation, and pain well relieved by recumbency. A later study by Revel et al10 found the presence of five among seven variables distinguished 92% of the patients responding to lidocaine injection and 80% of those not responding to lidocaine. However, further investigations of Revel et al’s variables by other investigators have been proven wrong.5
Injections in Facilitating the Diagnosis
Facet joint–mediated pain can be diagnosed with placebo-controlled or controlled local anesthetic diagnostic blocks using either facet joint blocks or medial branch blocks. The choice of technique used should be based on evidence of effectiveness of diagnosis and ease of technique. Evidence for diagnostic medial branch blocks using 80% pain relief with controlled diagnosed blocks are noted to be Level I or II-1 based on U.S. Preventive Services Task Force criteria.11,12 Medial branch blocks are relatively easier to perform and safer than intraarticular blocks because medial branch blocks can be performed even with osteophyte formation, which prevents entry into the facet joint. Additionally, the target area of the medial branch prevents overpenetration of the needle into the spinal canal during placement, therefore decreasing the possibility that the needle will pass through the target and into the spinal canal and cord, which can occur during intraarticular injections. Medial branch blocks also have therapeutic validity, but the therapeutic validity of intraarticular injection is lacking.11
False-positive rates of single local anesthetic blocks are high, ranging from 27% to 63% for cervical, 42% to 58% for thoracic, and 21% to 33% for lumbar medial branch blocks.12–14
Because of high false-positive rate of single local anesthetic blocks, numerous experts advocate performing diagnostic double blocks with either saline control or two different local anesthetics. Control blocks using normal saline under double-blind conditions is the most rigorous form of control blocks, requiring three blocks of the same joint. The first block would have to be with the local anesthetic to establish prima facie that the joint is symptomatic. To maintain the controlling effect of change and blinding, the second block would have to be either normal saline or inactive agent, and the third block would need to be the reciprocal agent.8 Despite this evidence, in a systematic study of the cost-effectiveness of using controlled facet blocks, Bogduk and Holmes15 determined that the use of placebo-controlled injections cannot be justified in the United States based on financial considerations.
In clinical practice, controlled diagnostic blocks with two local anesthetics with different durations are used. A true-positive response is one in which the patient reports complete relief of pain for a shorter duration when short-acting local anesthetic is used and a longer duration when a long-acting agent is used.16 Lidocaine 1% to 2% commonly used for the first block with a positive response is followed by a later block with bupivacaine 0.25% to 0.5% 2 to 4 weeks later.11,17 Response patterns of medial branch blocks using bupivacaine and lidocaine were defined by Barnsley et al.16
A concordant response confirms that the joint is the source of pain with confidence of 85%.18
Anatomy
The Facet Joint
The basic anatomical unit of the spine is referred to as a three-joint complex that consists of paired zygapophyseal joints and an intervertebral disc (Fig. 13-1).19 This articular triad functions to stabilize and support the spine along with limiting motion of the spine.
The term zygapophyseal joint (or facet joint) originated from the Greek root zygos, meaning yoke or bridge, and physis, meaning outgrowth. Therefore, the facet joints bridge the vertebrae behind the vertebral foramina.20 Facet joints are paired diarthrodial synovial joints formed by inferior articular process of one vertebra and superior articular process of the subjacent vertebra. Facet joints share the same general characteristics of synovial joints. As true synovial joints, each facet joint contains distinct joint space capable of accommodating 1 to 1.5 mL of fluid, a synovial membrane, hyaline cartilage surfaces, and fibrous capsule. The fibrous capsule of the facet joint is composed mostly of collagenous tissue arranged more or less in transverse fashion to provide maximum resistance to flexion. The thick fibrous joint capsule is covered by synovial membrane superiorly, posteriorly, and inferiorly. Anteriorly, the synovial membrane lacks a true fibrous capsule and instead is in direct contact with the ligamentum flavum.19
Facet joints are richly innervated with three types of nerve endings: encapsulated (Ruffini-type endings, pacinian corpuscles), unencapsulated, and free nerve endings.19 The presence of low-threshold, rapidly adapting mechanosensitive neurons lining the facet capsule suggests that in addition to transmitting nociceptive information, the facet capsule also serves proprioceptive function.21,22
Facet capsule nerve endings contain substance P, calcitonin gene-related peptide, and neuropeptide Y.23,24 Inflammatory mediators such as prostaglandins, inflammatory cytokinase, interleukin 1B, interleukin 7, and tumor necrosis factor have also been found in facet joint cartilage and synovial tissue.25,26
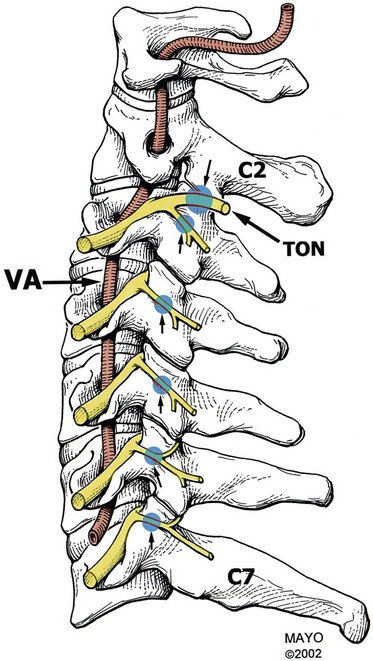
The orientation of the facet joints varies at the cervical, thoracic, and lumbar regions. Facet joints oriented parallel to the sagittal plane provide substantial resistance to axial rotation but minimal resistance to shearing forces (backward and forward sliding), and facet joints oriented more in a coronal plane tend to protect against flexion and shearing forces but provide minimal protection against rotation. The cervical facets are orientated in a coronal plane, which allows for flexion, extension, and lateral bending. The C2-C3 to C5-C6 facets are angled 35 degrees from the coronal plane, and C7-T1 are angled 22 degrees from the coronal plane. Orientation of the C5-C6 facet joint is between 22 and 35 degrees. All cervical facet joints from C2-C3 to C7-T1 are angled 110 degrees from the midline posterior sagittal plane. In the cervical spine, it is important to remember that the vertebral artery passes through the foramen of the transverse process of C1-C6.27
Facet joints in the thoracic spine differ from that of the cervical and lumbar spines in that their orientation is in a more coronal direction, which allows thoracic facet joints to play an important role in stabilization of thoracic spine during flexion loading. The T1-T2 facet joint is angled 66 degrees from a transverse plane, with the cephalad end more anterior than the caudad end. The T3-4 to T11-T12 facet joints are angled 75 degrees from the transverse plane. The T1-T2 to the T11-T12 facet joints are uniformly angled 110 degrees from the midline posterior sagittal plane. The T12-L1 facet joint is 25 degrees oblique to the sagittal plane from the midline posteriorly, which is similar to the lumbar orientation.28,29
The upper lumbar facet joints (T12-L2) are oriented closer to the midsagittal plane of the vertebral body, which allows limited rotational movements and favors flexion and extension. The lower facet joints tend to be oriented in a more coronal angle, which allows for greater rotational movements. The lumbar facet joints’ cephalad ends are farther anterior than the caudad ends because the facet joints are tipped approximately 10 degrees. In the upper lumbar spine, approximately 80% of the facet joints are curved, and 20% are flat; in the lower lumbar spine, these numbers are reversed.30,31 The lumbar facet joints transition from a coronal orientation to a sagittal positioning with age. Some studies have shown a positive association between degenerative spondylolisthesis and more sagitally oriented lower lumbar facet joints.32
Innervation
Cervical
The facet joints are innervated by the dual nerve supply from the medial branch of the dorsal ramus. Cervical facet joints from C3-C4 to C7-T1 are supplied by medial branches from the same level and the level above. Medial branch nerves arise from the posterior primary rami located in the cervical intertransverse space and then passes dorsally and medially to wrap around the waist of the articular pillar. The location of this nerve on the articular pillar is essentially the same as those of nerves C4-C8. The medial branches are bound to the periosteum by an investing fascia and are held against the articular pillars by tendons of the semispinalis capitis. The C7 medial branch crosses the root of the C7 transverse process and therefore lies higher on the lateral projection of the C7 articular pillar.33
The C2-C3 facet joint differs in innervation in that their innervation comes from the third occipital nerve along with C2 and C3 medial branches. Two medial branches usually arise separately from the C3 dorsal ramus. The superior and larger branch is the third occipital nerve (also known as the superficial medial branch), and the inferior branch is the deep medial branch. The third occipital nerve innervates C2-C3 joint. It curves dorsally and medially around the superior articular process of the C3 vertebra and crosses the C2-C3 facet joint either just below or across the joint margin. It also provides cutaneous neural supply for the suboccipital region. The C3 dorsal ramus is the only cervical dorsal ramus below C2 that has a cutaneous distribution.34,35
The C8 medial branch runs a course similar to the upper thoracic nerves. It arises from the dorsal ramus within lateral margin of the intervertebral foramen of C7-T1. Because of the C8 medial branch and seven cervical vertebrae, numbering of medial branches is different in the cervical region compared with in the thoracic and lumbar spine. The facet joint is innervated by a cervical medial branch at the same level and level above for C4-C7. For example, the C5-C6 facet joint is innervated by the medial branches of C5 and C6. This numbering applies to the C4 through C7 levels.34,36
Thoracic Innervation
The course of the medial branches of T11 and T12 also vary because of different osseous anatomy at these levels. The T12 transverse process is shorter compared with other levels. Therefore, the T11 branch runs across the lateral surface of the root of the relatively short T12 transverse process. At the T12 level, the medial branch assumes a course analogous to those of the lumbar medial branches.28,37,38
Lumbar Innervation
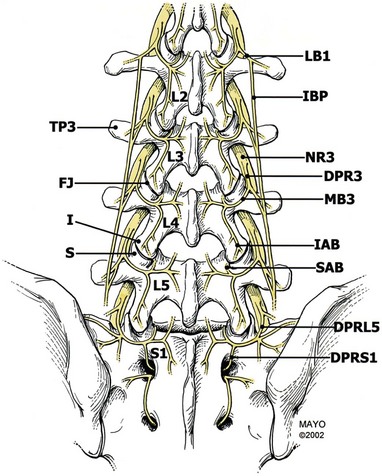
The primary dorsal rami divide into three nerves as they approach their respective transverse processes: the medial branch, intermediate branch, and lateral branch. The medial branch is the largest of the dorsal branch nerves. The medial branch has three branches as well. The proximal branch hooks around the articular process to supply the facet above. The medial descending branch passes medially and downward to innervate the superior and medial portions of the facet capsule below, the multifidus and interspinales muscles and ligament, and the periosteum of the neural arch. The ascending branch supplies the facet above. The other two main branches of the dorsal ramus are the intermediate branch and the lateral branch. The intermediate branch sends fibers into the longissimus muscles. The lateral branch innervates the iliocostalis muscle, the thoracolumbar fascia, the skin of the lower back and buttock, and the sacroiliac joint, but does not innervate the facets.22,39
The medial branches of L1-L4 rami have a predictable course. These medial branches come off the dorsal ramus and exit from the intervertebral foramen, piercing the intertransverse ligament and crossing the superior border of the transverse process. The branches then travel along the junction of the transverse process and superior articular process. This junction is often referred to as a “groove.” The medial branch nerve runs in the groove along the lateral aspect of the neck of the superior articular process, traveling caudally and posteriorly where it is in direct contact with the base of the superior surface of the transverse process, passing under the mamilloaccessory ligament. At L3, L4, and L5 levels, this ligament can become calcified. The medial branch nerve then proceeds inferiorly and posteriorly, where it sends fibers cephalad to innervate the caudad capsular margin of the adjacent superior joint capsule. Then it sends fibers to the next lower level at its cephalad capsular margin.20,40
The course of the L5 medial branch is somewhat modified because the transverse process is replaced by the ala of the sacrum. The L5 medial branch is not the medial branch of L5 but rather the dorsal primary ramus of L5, which is longer. The dorsal ramus pierces the intertransverse ligament and runs caudally and posteriorly along the groove formed by the junction of the superior articular process and sacral ala. The L5 dorsal ramus divides into two branches, a medial and lateral branch. The L5 medial branch curves medially around the base of the L5-S1 joint, sending an articular branch to this joint and then supplying the multifidus muscles. There is no mamilloaccessory ligament at this level. However, fibrous tissue fixes the position of the nerve at the base of the superior articular process. The lateral branch of the L5 dorsal ramus runs caudad to communicate with the S1 dorsal ramus lateral branch.41,42
Basic Science
Pain Referral Patterns
The distribution of referred pain appears to be related to the innervation of the medial and lateral branches of the dorsal rami. Studies of mapping out referral patterns have used asymptomatic volunteers and pain patients. Referral patterns from cervical zygapophyseal joints were studied by Dwyer et al.43 In their first study, the facet injections were done in normal volunteers, which showed certain referral patterns. The accuracy of referral patterns were later studied on symptomatic patients. Referral patterns obtained were C2-C3 as pain located in the upper cervical region and extending at least onto the occiput. The C3-C4 pattern is located over the posterolateral cervical region without substantial extension into the occiput and extending caudally over the posterolateral aspect of the neck without entering the region of the shoulder girdle. The C3-C4 pattern basically covers the area that is coextensive with the underlying levator scapulae muscle. Whereas the C4-C5 pattern is concentrated over the angle formed by the top of the shoulder and side of the neck, the C5-C6 pattern spreads laterally toward the shoulder with the main area draping over the top, front, and back of the shoulder girdle, with a base coinciding with the spine of the scapula. The C6-C7 pattern extends below the spine of the scapula. The typical C7-T1 pain pattern extends into the paravertebral area with coverage over the scapulae.44
The thoracic facets may produce middle back pain that is paraspinous with neuralgic characteristics. Thoracic referral patterns were noted by joint distention in normal volunteers. Some overlap was noted in the thoracic area. The pain was usually one to two segments inferior and lateral to the involved joint and unilateral. The T1-T2 joint pain was noted to be below the inferior angle of the scapulae. Despite this, there is a wide overlap in thoracic referral patterns that makes them unreliable.44–46
Referred pain from the lumbar zygapophyseal joints and medial branches has been examined in numerous studies showing overlap of referred pain between each level.47,48 Pain from L1-L3 has been shown to extend into the flank, hip, and upper lateral thighs. Pain from L3-S1 has been shown to extend deeper into the thigh, usually laterally and posteriorly and also into the groin area. Referred pain from the L4-5 and L5-S1 facet joints can also cause pain extending into the lower lateral leg, below the knee, and even to the foot at times.20,47,49 Referred pain may also assume a pseudoradicular pattern, making the underlying diagnosis difficult to confirm based on pain patterns.50
Imaging
Radiologic imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) have shown to be nonspecific in the diagnosis of facetogenic-mediated pain. Studies in asymptomatic volunteers showed a prevalence of facet degeneration of 8% to 14%. Findings on imaging do not correlate well with symptoms.51,52 Stojanovic et al53 studied the correlation between MRI pathology and response to diagnostic medial branch blocks and radiofrequency denervation. Their study showed a possible correlation between lumbar facetogenic pain and MRI abnormalities.53 Schwarzer et al54 showed that the degree of degenerative changes seen on CT did not correlate well with facet joint–mediated spinal pain. Single-photon emission computed tomography (SPECT) has been shown to identify probable joint inflammation, but its effectiveness in predicting successful medial branch blocks is not well known.55 The potential of fluorodeoxyglucose positron emission tomography (FDG-PET) has limited value for the diagnosis of facet joint arthropathy and is expensive.56 In conclusion, accurately diagnosing facet joint–mediated pain via correlation with radiological findings is unreliable.
Indications and Contraindications
Indications
The purpose of medial branch blocks is to test if the patient’s pain is relieved by anesthetizing the target nerves. Patients who should be included for diagnostic medial branch blocks may have pain that is somatic or nonradicular pain in the neck, upper back, mid back, lower back, upper or lower extremity, or chest wall, or headaches. Pain should be intermittent or continuous with a visual analog score an average of 6 or more on a scale of 0 to 10 that causes functional disability. Pain should last for at least 3 months or longer. Patients should have spinal pain of unknown origin and lack neurological deficits. Patients should have tried and failed conservative management, which includes oral medications such as nonsteroidal antiinflammatory drugs (NSAIDs), physical therapy, exercise, and bed rest.11,13
Techniques
Target Identification
Cervical
Needle Placement
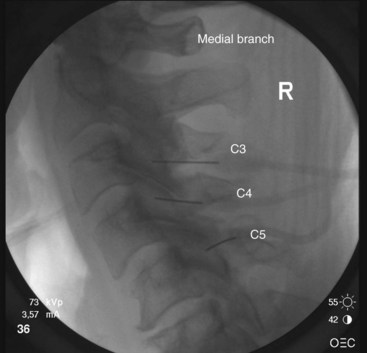
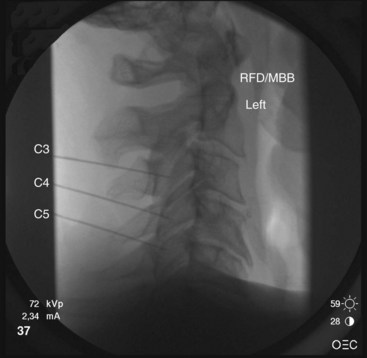
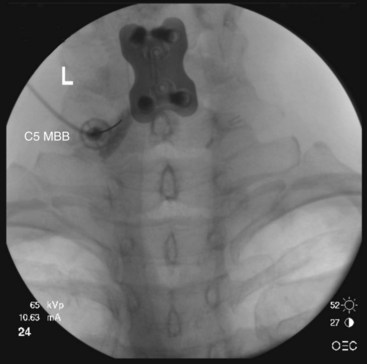
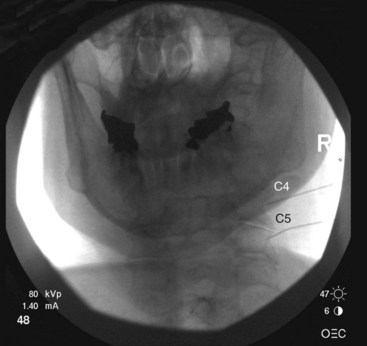
The target points for cervical medial branch blocks from the third to the sixth medial branches is the “centroid” of the articular pillars with the same segmental number as the target nerve (Fig. 13-2). The centroid is found at the intersection of the two diagonals of the diamond-shaped pillar. This target point can be seen with true lateral fluoroscopic imaging in which the silhouettes of the articular pillars on each side at a given segment are superimposed. To confirm superimposition, the x-ray beam should be tilted around the long axis of the patient for splitting of the superimposed articular pillar silhouettes to occur. Therefore, the ipsilateral articular processes are noted. It is important to confirm a true lateral view because it is essential to eliminate any risk of aiming the needle toward the contralateral side of the neck. It is important to have the target point in the center of the x-ray beam so it appears on the center screen when using fluoroscopy. Keeping the target point in the center avoids parallax errors.
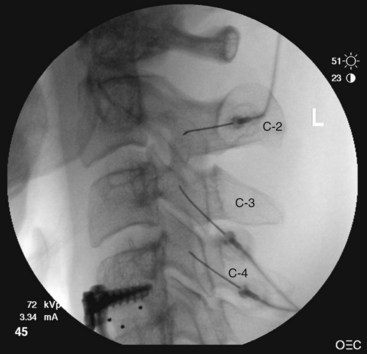
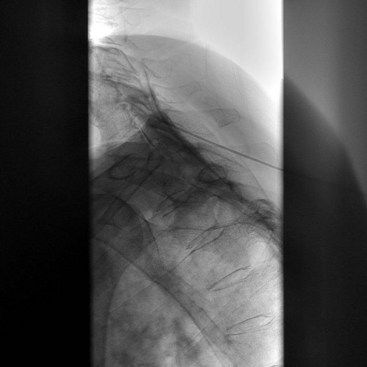
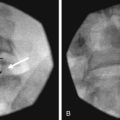
The third occipital nerve is thicker than the medial branches of the typical cervical dorsal rami; therefore, multiple target points are needed for blocking the nerves involving the C2-C3 facet joint. The target points are located along a vertical line that bisects the articular pillar of C3. There are three target points; the highest target point is immediately above the subchondral plate of the C2 inferior articular process. The lowest point is immediately below the subchondral plate of the C3 superior articular process. The middle point lies midway between these points, usually on the subchondral plate of the superior articular process of C3. Needle advancement is toward the middle of the three target points lateral to the C2-C3 facet joint capsule at the level of the joint. When the needle rests on bone and is in position, the needle is withdrawn slightly (≈3 mm) to ensure the needle has not penetrated the joint capsule. After injection of contrast to rule out venous uptake, injection with 0.3 mL of local anesthetic is done. The needle tip is then readjusted either to the cranial target point (anteroinferior margin of C2 inferior articular facet) or caudad target point (anterosuperior margin of C3 superior articular facet), where 0.3 mL of local anesthetic will also be injected at each spot. If contrast is used, distribution when one has achieved correct needle placement will show spread across the surface of the joint, where the target nerve is held against the joint by the overlying splenius capitis muscle (Figs. 13-3 to 13-7).
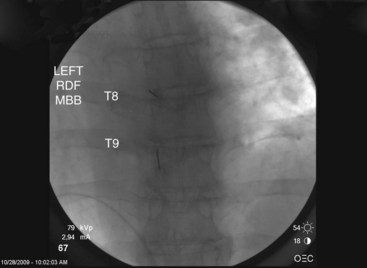
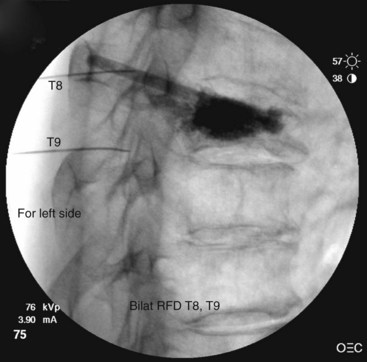
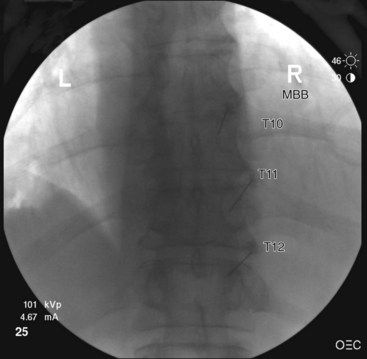
Thoracic
Needle Placement
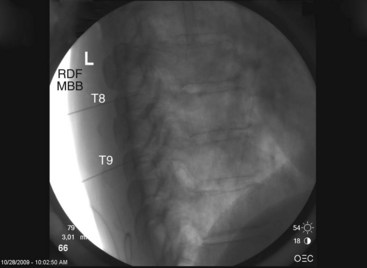
Contrast is injected to confirm adequate spread covering the target area for the nerve and exclude intravascular uptake. At the midthoracic level (T5-T8), there is no validated technique for blocking the medial branches because of the unusual and variable anatomy of the medial branches at this level. The test dose of contrast under live fluoroscopy should show cephalad spread in an elongated ovoid shape covering the area where the target nerve lies (Figs. 13-8 to 13-11).
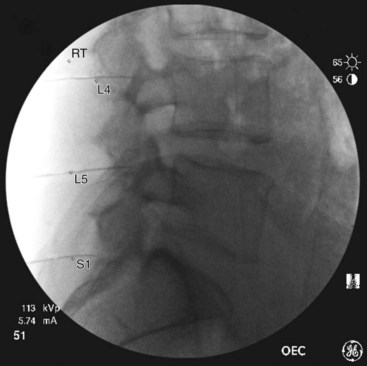
Lumbar
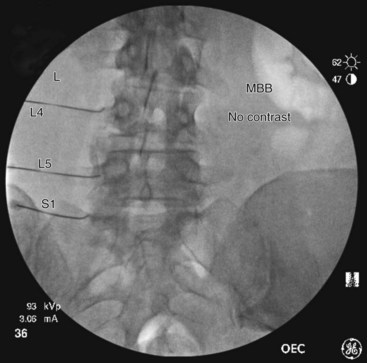
Needle Placement
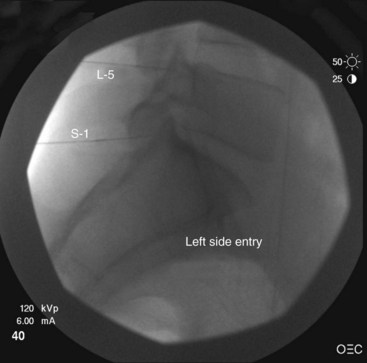
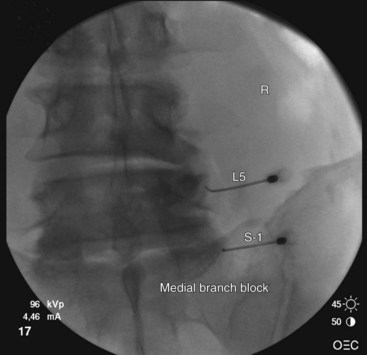
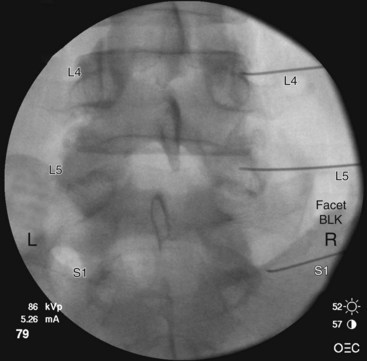
At the L5 level, is it important to remember the target nerve is not the medial branch of L5. The L5 medial branch is actually the L5 dorsal primary ramus, which is located against the bone along the superomedial aspect of the groove formed by the sacral ala and superior articulating process of S1. The target is along the superior articulating process sacral ala junction just below the upper margin of the sacral ala. The target is found using an oblique view. Less oblique tilt is needed when compared with the L1-L4 levels. Usually 0 to 10 degrees is sufficient for the L5 dorsal primary ramus. Too much oblique causes obstruction of the target point with the “Scotty dog” view because of the iliac crest being in the path of the needle. The skin puncture site should be slightly lateral to the target point but medial to the adjacent iliac crest. Needle advancement should be monitored under fluoroscopic guidance, making sure the needle tip is always below the upper margin of the sacrum with its course being in a ventral and medial direction toward the target point. When the needle contacts the bone forming the superior edge of the sacral ala, the needle is advanced carefully over the bone 2 to 3 mm. PA and oblique views are taken to confirm correct needle position. On the oblique view, the needle tip should be seen resting on the ala of sacrum, at the base of the S1 superior articular process. On the AP view, the needle tip should be against and under the lateral margin of the superior articular process of the sacrum. The needle bevel should be pointed medially to decrease risk of inadvertent spread of injectate into the S1 foramen or to the L5-S1 intervertebral foramen. Contrast should be injected to rule out intravascular spread and to confirm correct placement of the needle (Figs. 13-12 to 13-16).
Patient Management and Evaluation
When evaluating diagnostic medial branch blocks, the patients should have two comparative blocks with short- and long-acting local anesthetics to rule out false-positive results. Patients should have shorter relief with lidocaine than when using bupivacaine. The pain relief should last at least 2 hours with lidocaine and at least 3 hours or longer than the duration of relief with lidocaine when bupivacaine is used. The patient should not take oral opioid medications before the procedure. Immediately after the procedure, the patient should repeat maneuvers that normally reproduce or aggravate his or her pain. Although it is subject to debate, pain relief should be at least 50% to 80% or greater. In the past, there has been a lack of consensus on a definition of successful diagnostic block.57 Schwarzer et al58 defined positive diagnostic block as 50% or greater reduction in pain. Guidelines by the American Society of Interventional Pain Physicians (ASIPP) use positive diagnostic block response as 80% pain relief with movements that normally cause pain.11 An observational study by Manchikanti et al59 examined using 50% or greater versus 80% or greater as a cut-off for positive diagnostic blocks. The authors found that of the patients who received greater than 80% relief from diagnostic medial branch blocks, 89.5% of them continued to have facet joint–mediated pain at 2-year follow-up. In contrast, in the 50% relief arm of the study, only 51% of these patients had continued lumbar-mediated facet pain at 2-year follow-up. They concluded that it is optimal to use 80% pain relief and being able to perform previously painful movements as a positive diagnostic response. This is to improve patient outcomes and avoid performing unnecessary procedures.59
For patients having positive controlled diagnostic blocks, they can undergo therapeutic medial branch blocks or radiofrequency ablation. Therapeutic medial branch evidence is level II-1 or II-2. These blocks should provide at least 50% pain relief or greater for a minimum of 8 weeks. Blocks should preferably not be performed at intervals sooner than 2 to 3 months apart.11
Outcomes and Evidence
In 2005, it was noted that evidence-based evaluations showed level III for short- and long-term relief with lumbar and cervical medial branch blocks in managing chronic low back or neck pain.60 A repeat review of the same authors in 2007 showed that the evidence is moderate for short- and long-term pain relief with repeat interventions for cervical, thoracic, and lumbar medial branch nerve blocks with local anesthetics.50 In a review study by Falco et al12 in 2009, cervical medial branch blocks were concluded as level II-1 with strong recommendation of 1B or 1C.12 The ASIPP guidelines show both moderate recommendation for short- and long-term relief of cervical, thoracic, and lumbar medial branch blocks.11
The ASIPP guidelines state that for the diagnostic phase, the two medial branch procedures should be at intervals of no sooner than 1 week or preferably 2 weeks. For the therapeutic phase, 2 to 3 months or longer are needed between injections (≥50% relief for at least 3 months). If the therapeutic procedures are planned for different regions, the practitioner should wait at least 1 to 2 weeks to treat the different areas.11
The evidence for cervical, thoracic, and lumbar medial branch nerve blocks with local anesthetics (with or without steroids) is moderate for short- and long-term pain relief with repeat interventions.50 Atluir et al14 noted that the evidence for thoracic MBB is level 1 or II-1 with strong recommendations of 1A or 1B.
A systematic review by Falco et al12 noted that the recommendation is a strong 1B or 1C for the use of therapeutic facet joint medial branch blocks in providing both short-term and long-term relief in the treatment of chronic cervical facet joint neck pain. For cervical medial branch blocks, there is level II-1 evidence with strong recommendation of 1B or 1C. For cervical radiofrequency neurotomy, there is level II-1 to level II-2 evidence with strong recommendation of 1B or 1C. There is no evidence available for cervical intraarticular facet joint injections.
Risks and Complications
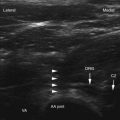
A strict sterile technique should be used to decrease infection. Patients taking coagulopathic medications should have appropriate time off these medications and appropriate laboratory test results checked. Correct needle placement is important to decrease the chance of epidural, subarachnoid spread, or pneumothorax. For thoracic medial branch blocks, needle placement must always be dorsal to target bone to prevent the risk of pneumothorax. A study by Dreyfuss et al41 showed that needle placement for lumbar medial branch blocks at the middle of the transverse process at its junction with the superior articular process decreased the risk of aberrant spread. For the L5 dorsal ramus, the needle bevel should point medially, and the target point is halfway between the upper end and middle of the ala of the sacrum to reduce aberrant spread. Contrast should be used to rule out intravascular injection. With regards to radiation exposure, new techniques using ultrasound technique and a single-needle technique may decrease or completely eliminate radiation exposure. More studies are needed to confirm the application and feasibility of these techniques.61,62
Intravascular injections can cause burning pain and anaphylactic reactions. They can also cause false-negative responses. Contrast injection with fluoroscopy should therefore be used. Dreyfuss et al41 found 8% intravascular injection when studying lumbar medial branch blocks in 15 subjects and 120 injections. Kaplan et al63 found that inadvertent venous uptake occurred in 33% of nerve blocks with initial venous uptake carrying 50% false-negative results despite subsequent lack of venous uptake with needle repositioning. Lee et al64 showed that the overall incidence of intravascular injection in lumbar medial branch blocks was 6% and the prediction of intravascular injection by aspiration test sensitivity was 34%. Spot radiographs demonstrated intravascular uptake in 40% of those documented by real-time ultrasonography. Real-time fluoroscopy with contrast enhancement should be performed during the blocks to increase diagnostic and therapeutic values and to avoid complications because both aspiration and spot radiography frequently missed intravascular uptake.
1 Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30:213-222.
2 de Vlam K, Mielants H, Verstaete KI, et al. The zygapophyseal joint determines morphology of the enthesophyte. J Rheumatol. 2000;27:1732-1739.
3 Fujishiro T, Nabeshima Y, Yasui S, et al. Pseudogout attack of the lumbar facet joint: a case report. Spine. 2002;27:E396-E398.
4 Campbell AJ, Wells IP. Pigmented villonodular synovitis of a lumbar vertebral facet joint. J Bone Joint Surg Am. 1982;64:145-146.
5 Manchikanti L, Pampati V, Fellows B, et al. The Inability of the clinical picture to characterize pain from facet joints. Pain Physician. 2000;3:158-166.
6 Mironer YE, Somerville JJ. Protocol for diagnosis and treatment of facet joint. Pain Digest. 1999;9:188-190.
7 Sehgal N, Dunbar E, Shah RV, et al. Systematic review of diagnostic utility of facet/zygapophysial joint injections in chronic spinal pain: an update. Pain Physician. 2007;10:213-228.
8 Bogduk N. International Spinal Injection Society guidelines for the performance of spinal injection procedures. Part I: zygapophysial joint blocks. Clin J Pain. 1997;13:285-302.
9 Revel ME, Listrat VM, Chevalier XJ, et al. Facet joint block for low back pain. Identifying predictors of a good response. Arch Phys Med Rehabil. 1992;73:824-828.
10 Revel M, Poiraudeau S, Auleley GR, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia: proposed criteria to identify patients with painful facet joints. Spine. 1998;23:1972-1976.
11 Manchikanti L, Boswell MV, Singh V, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12:699-802.
12 Falco FJE, Erhart S, Wargo BW, et al. Systematic review of diagnostic utility and therapeutic effectiveness of cervical facet joint interventions. Pain Physician. 2009;12:323-344.
13 Manchikanti L, Boswell MV, Singh V, et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskeletal Disorders. 2004;5:15-21.
14 Atluir S, Datta S, Falco FJE, et al. Systematic review of diagnostic utility and therapeutic effectiveness of thoracic facet joint interventions. Pain Physician. 2008;11:611-629.
15 Bogduk N, Holmes S. Controlled zygapophysial joint blocks: the travesty of cost-effectiveness. Pain Med. 2000;1:24-34.
16 Barnsley L, Lord S, Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain. 1993;55:99-106.
17 Manchikanti L, Singh V, Pampati V. Are diagnostic lumbar medial blocks valid? Results of a 2-year follow-up. Pain Physician. 2003;6:147-153.
18 Lord SM, Barnsley L, Bogduk N. The utility of comparative local anaesthetic blocks versus placebo-controlled blocks for diagnosis of cervical zygapophysial joint pain. Clin J Pain. 1995;11:208-213.
19 Hirsch C, Ingelmark BE, Miller M. The anatomical basis for low back pain: Studies on the presence of sensory nerve endings in ligamentous, capsular, and intervertebral disc structures in the human lumbar spine. Acta Orthop Scand. 1963;33:1-17.
20 Manchikanti L, Singh V. Review of chronic low back pain of facet joint origin. Pain Physician. 2002;5:83-101.
21 Cavanaugh JM, Lu Y, Chen C, et al. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88:63-67.
22 Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007;106:591-614.
23 Ashton IK, Ashton BA, Gibson SJ, et al. Morphological basis for back pain: the demonstration of nerve fibers and neuropeptides in the lumbar facet joint capsule but not in ligamentum flavum. J Orthop Res. 1992;10:72-78.
24 El-Bohy AA, Cavanaugh JM, Getchell ML, et al. Localization of substance P and neurofilament immunoreactive fibers in the lumbar facet joint capsule and supraspinous ligament of the rabbit. Brain Res. 1988;460:379-382.
25 Beaman DN, Graziano GP, Glover RA, et al. Substance P innovation of lumbar spine facet joints. Spine. 1993;18:1044-1049.
26 Kallakuri S, Singh A, Chen C, et al. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine. 2004;29:1182-1186.
27 Panjabi MM, Oxland T, Takata K, et al. Articular facets of the human spine: quantitative three-dimensional anatomy. Spine. 1993;18:1298-1310.
28 Ebraheim NA, Xu R, Ahmad M, et al. The quantitative anatomy of the thoracic facet and the posterior projection of its inferior facet. Spine. 1997;22:1811-1817.
29 Panjabi MM, Takata K, Geol V, et al. Thoracic human vertebrae: quantitative three-dimensional anatomy. Spine. 1991;16:888-901.
30 Masharawi Y, Rothschild B, Dar G, et al. Facet orientation in the thoracolumbar spine: three-dimensional anatomic and biomechanical analysis. Spine. 2004;29:1755-1763.
31 Tulsi RS, Hermanis GM. A study of the angle of inclination and facet curvature of superior lumbar zygapophyseal facets. Spine. 1993;18:1311-1317.
32 Boden SD, Riew KD, Yamaguchi K, et al. Orientation of the lumbar facet joints: association with degenerative disc disease. J Bone Joint Surg Am. 1996;78:403-411.
33 Bogduk N, Marsland A. The cervical zygapophyseal joints as a source of neck pain. Spine (Phila Pa 1976). 1988;13(6):610-617.
34 Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7:319-330.
35 Lord S, Barnsley L, Wallis B, et al. Third occipital nerve headache: a prevalence study. J Neurol Neurosurg Psychiatry. 1994;57:1187-1190.
36 Zhang J, Tsuzuki N, Hirabayashi S, et al. Surgical anatomy of the nerves and muscles in the posterior cervical spine: a guide for avoiding inadvertent nerve injuries during the posterior approach. Spine. 2003;28:1379-1384.
37 Chua WH: Clinical anatomy of the thoracic dorsal rami. BMedSci Thesis, University of Newcastle, Australia, 1994.
38 Chua WH, Bogduk N. The surgical anatomy of thoracic facet denervation. Acta Neurochir (Wien). 1995;136:140-144.
39 Selby DK, Paris SV. Anatomy of facet joints and its clinical correlation with low back pain. Contemp Orthop. 1981;12:1097-1103.
40 Suseki K, Takahashi Y. Innervation of lumbar facet joints: origins and functions. Spine. 1997;22:477-485.
41 Dreyfuss P, Schwarzer AC, Lau P, et al. Specificity of lumbar medial branch and L5 dorsal ramus blocks: a computed tomography study. Spine. 1997;22:895-902.
42 Bogduk N. Low back pain. In Clinical anatomy of lumbar spine and sacrum, ed 4, New York: Churchill Livingstone; 2005:183-216.
43 Dwyer A, Aprill N, Bogduk N. Cervical zygapophysial joint pain patterns I: a study in normal volunteers. Spine. 1990;15:453-457.
44 Aprill C, Dwyer A, Bogduk N. Cervical zygapophyseal joint pain patterns II: a clinical evaluation. Spine. 1990;15:458-461.
45 Dreyfuss P, Tibiletti C, Dreyer S. Thoracic zygapophyseal joint pain patterns. Spine. 1994;19:807-811.
46 Fukie A, Ohseto K, Shiotan M. Patterns of pain induced by distending the thoracic zygapophyseal joints. Reg Anesth. 1997;22:332-336.
47 McCall IW, Park WM, O’Brien JP. Induced pain referral from posterior elements in normal subjects. Spine. 1979;4:441-446.
48 Marks R. Distribution of pain provoked from lumbar facet joints and related structures during diagnostic spinal infiltration. Pain. 1989;39:37-40.
49 Fukie S, Ohseto K, Shiotani M, et al. Distribution of referred pain from the lumbar zygapophyseal joints and dossal rami. Clin J Pain. 1997;13:303-307.
50 Boswell MV, Colson JD, Seghal N, et al. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007;10:229-253.
51 Weisel SW, Tsourmas N, Feffer HL, et al. A study of computer assisted tomography: 1. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:548-555.
52 Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;333:69-73.
53 Stojanovic MP, Sethee J, Mohiuddin M, et al. MRI analysis of the lumbar spine: can it predict response to diagnostic and therapeutic facet procedures? Clin J Pain. 2010;26:110-115.
54 Schwarzer AC, Wang SC, O’Driscoll D, et al. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine. 1995;20:907-912.
55 Ackerman WE, Ahmad M. Pain relief with intraarticular or medial branch nerve blocks in patients with positive lumbar facet joint SPECT imaging: a 12-week outcome study. South Med J. 2008;101:931-934.
56 Houseni M, Chamroonrate W, Zhuang H, et al. Facet joint arthropathy demonstrated on FDG-PET. Clin Nucl Med. 2006;31:418-419.
57 Binder DS, Nampiaparampil DE. The provocative lumbar facet joint. Curr Rev Musculoskeletal Med. 2009;2:15-24.
58 Schwarzer AC, Aprill CN, Derby R, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195-200.
59 Manchikanti L, Pampati S, Cash KA. Making sense of the accuracy of diagnostic lumbar facet joint nerve blocks: an assessment of the implications of 50% relief, 80% relief, single block, or controlled blocks. Pain Physician. 2010;13:133-143.
60 Boswell MV, Colson JD, Spillane WF. Therapeutic facet joint interventions in chronic spinal Pain: a systematic review of effectiveness and complications. Pain Physician. 2005;8:101-104.
61 Shin JK, Moon JC, Yoon KB, et al. Ultrasound-guided lumbar medial-branch block: a clinical study with fluoroscopy control. Reg Anesth Pain Med. 2006;31:451-454.
62 Stojanovic MP, Zhou Y, Hord D, et al. Single needle approach for multiple medial branch blocks: a new technique. Clin J Pain. 2003;19:134-137.
63 Kaplan M, Dreyfuss P, Halbrook B, et al. The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint. A physiologic challenge. Spine. 1998;23:1847-1852.
64 Lee CJ, Kim YC, Shin JH, et al. Intravascular injection of lumbar medial branch block: a prospective evaluation of 1433 injections. Anesth Analg. 2008;106:1274-1278.