Chapter 19 Mechanisms of Normal Retinal Adhesion
Independent of its clinical relevance, the study of retinal adhesion is also of considerable physiologic interest. No anatomic junctions bridge the mammalian subretinal space,1 yet this primordial dural cavity remains collapsed and, indeed, tightly closed throughout a lifetime of ocular movement and tugging from the vitreous. Our current knowledge of this system is still incomplete, but evidence suggests that adhesion depends on a mixture of anatomic, physical, and metabolic factors.2 This chapter reviews these factors and considers clinical and therapeutic implications.
Models for measuring retinal adhesion
In vitro methods
Adhesive force falls rapidly after enucleation or death,3,4 as is discussed later, and this puts a constraint of time on techniques for measuring adhesiveness in vitro.5,6 One approach is to peel the retina within a fluid bath while recording the required force with a transducer.3 A faster method is to measure the amount of RPE pigment that remains attached to the retina after separation as the index of adhesiveness.7 An eye can be enucleated and small strips of the eyecup prepared within 30 seconds, after which the retina is gently peeled by hand from the RPE. The stronger the adhesive force, the more pigment adheres to the peeled retina.
In vivo methods
Kita et al.8 developed the most direct technique for quantifying adhesiveness within the living eye. A small detachment is made in the eye by injecting fluid into the subretinal space through a micropipette, and a second micropipette is inserted simultaneously into the detachment cavity to measure fluid pressure. The pressure that is required to expand the detachment can be converted mathematically, by Laplace’s law, into a value for adhesive force at the margin of the detachment (where the separation is taking place). This technique allows the evaluation in living animals of the effects of drugs and other agents that modify retinal adhesion.9,10
Adhesive force and environmental factors
Magnitude of adhesive force
In vitro measurements, 5–20 minutes after enucleation, have shown that about 25 mg of force is required to peel a 5-mm strip of rabbit retina from the RPE.3,11,12 Pressure measurements in living eyes have shown an adhesive force in the rabbit of 100–180 dyn/cm.8,9 Adhesion is stronger in cats and monkeys, which show mean values for adhesive force that are 180% (for cats) and 140% (for monkeys) of that in the rabbits.9
Sensitivity to temperature and ionic environment
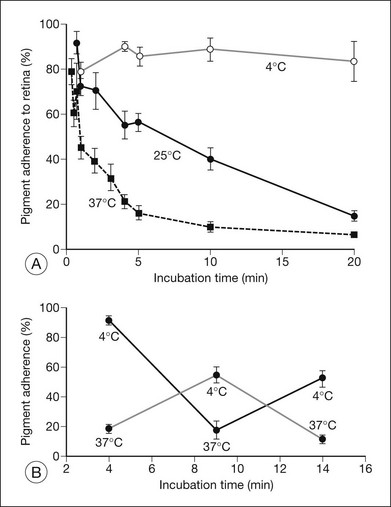
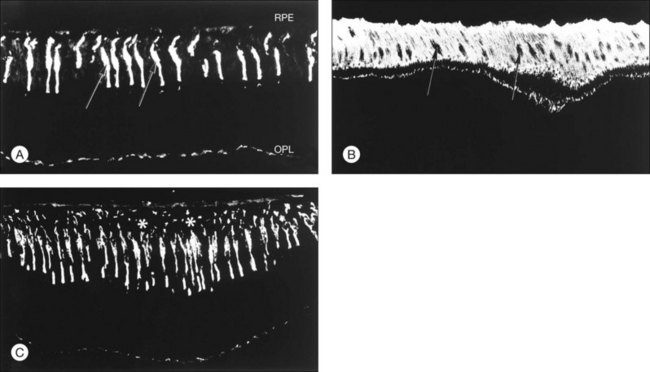
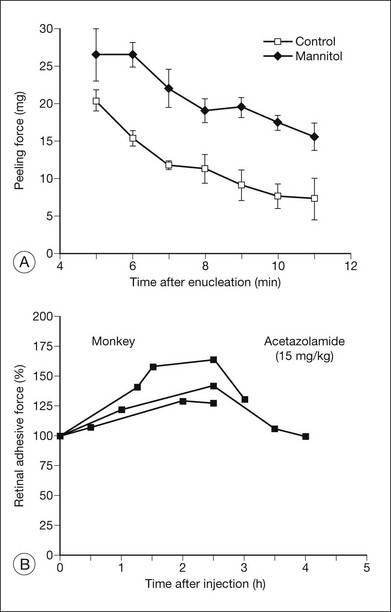
Retinal adhesiveness drops rapidly postmortem at 37 °C, but remains near control levels for hours at 4 °C7,13–15 (Fig. 19.1A). These effects of temperature are reversible. Figure 19.1B shows that changing the temperature from 37 °C to 4 °C or vice versa causes retinal adherence in the rabbit16 to rise or fall, respectively, and repeatedly. This reversibility has also been documented in primate and human tissue.14,15,17 The mechanism of these reversible temperature effects is still obscure. Temperature could act directly on physicochemical components of adhesion or modulate metabolic systems. Some of the adhesion-enhancing effect of cold temperature may result from sodium pump inhibition and secondary tissue swelling, which would make the interdigitated outer segments and RPE microvilli harder to separate.3,13 Because cellular swelling is a pathologic event, the actions of cold temperature may not be entirely relevant to normal adhesive processes.
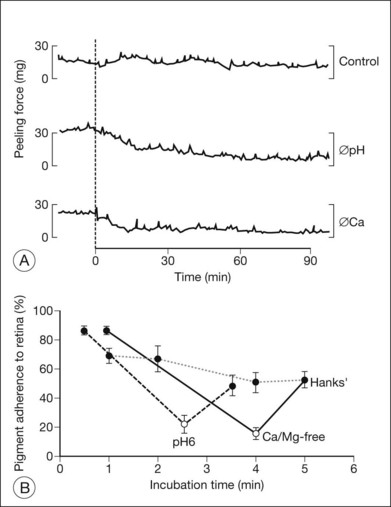
The ionic environment appears to be critical to adhesive strength. In in vitro experiments using tissue from rabbits, humans, and other primates, lowering the pH from 7.4 to 5.5, or removing calcium and magnesium ions from the bathing solution, weakened adhesive force and accelerated the rate at which adhesion falls postmortem11,13,15,17 (Fig. 19.2A). These changes can be rapidly reversible16 (Fig. 19.2B), although cold temperature will block or mask the effects of pH and calcium ions. In vivo, the removal of calcium ions from the subretinal space weakens retinal adhesion in rabbits to about 30% of normal.18 In other words, calcium appears to be a necessary element for the maintenance of normal adhesiveness in the living eye.
Fluid pressure: hydrostatic and osmotic
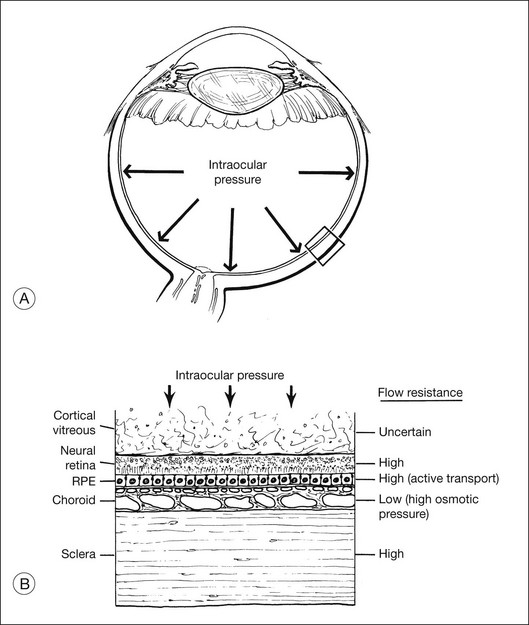
Fluid is driven passively from vitreous to choroid by both intraocular pressure and the osmotic pressure of the extracellular fluid in the choroid (estimated to be about 12 mmHg in the rabbit,19 but possibly lower in humans20). However, under normal conditions there appears to be relatively little posterior flow, and most of the fluid that enters the eye as aqueous leaves the eye through anterior drainage channels.19,21 The posterior route is limited because the retina and RPE22,23 provide a substantial resistance to water movement, and little fluid can cross these layers under the available heads of pressure. A side-effect of this retinal flow resistance is that the outward movement of fluid acts to push the retina against the RPE (Fig. 19.3). This is one mechanism contributing to normal retinal attachment. The actual pressure difference across retina or RPE is probably small because intraocular pressure is ultimately contained by the sclera, and tissue pressures will therefore equalize to a large degree between the vitreous and choroid. However, Fatt and Shantinath22 have calculated that even a very small pressure difference (only 0.52 × 10−3 mmHg) across the retina would generate a force sufficient to keep the retina firmly fixed against the wall of the eye. This low value may explain the fact that no one14,19 has been able to measure a pressure difference between the vitreous cavity and subretinal space.
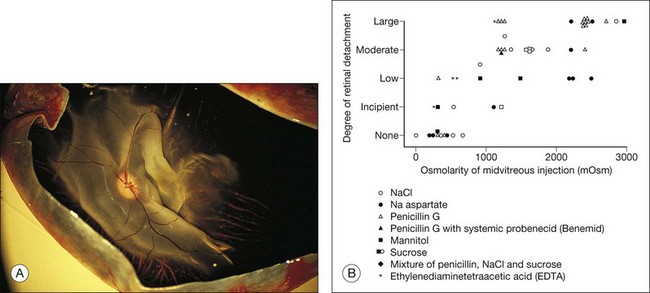
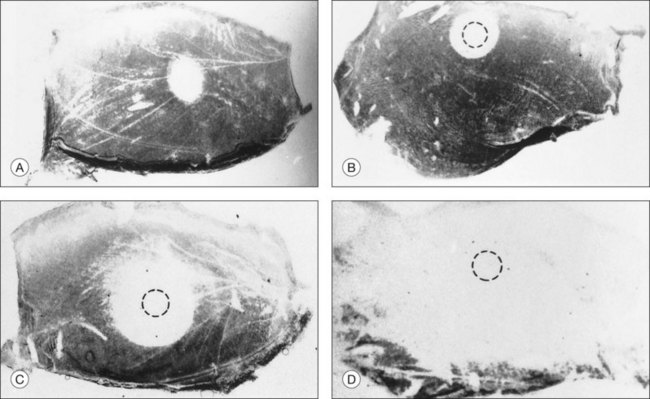
These physical forces can also work in the other direction and cause retinal detachment. For example, if hyperosmotic fluid is introduced into the vitreous cavity, fluid moving from the choroid into the vitreous will elevate the retina24,25 (Fig. 19.4). This is an important concern in the evaluation or use of intravitreal drugs that may damage the retina osmotically independently of pharmacologic effects.
It may seem puzzling that, although most intraocular fluid leaves the eye anteriorly rather than posteriorly, ample animal data26–30 indicated that the RPE can pump fluid from the subretinal space to choroid at a very high rate (about 0.3 µL/h/mm2 of RPE) comparable with that of aqueous secretion. How can the RPE have such enormous power for fluid removal, yet very little fluid leaves the normal eye by a posterior route? The answer probably lies in the flow resistance of the retina. Under normal conditions, given the flow resistance of the tissue and the tiny pressure differential across it,22,23 water will cross the retina only at a very slow rate. In other words, the tissue resistance is rate-limiting, and most aqueous fluid must leave by the anterior route. Under pathologic conditions of retinal detachment (whether rhegmatogenous or nonrhegmatogenous), fluid is present within the subretinal space and is thus available for transport at a maximal rate. Measurements in humans for the resorption of detachments showed a rate of 0.11 µL/h/mm2 of RPE, and the rate may well be higher in eyes without pathology.31 This translates into roughly 3.5 mL of fluid per day, which explains why a rhegmatogenous detachment can settle within 24 hours, and emphasizes the power of RPE fluid transport.
The clinical importance of fluid pressure as a factor in preventing retinal detachment is uncertain. It undoubtedly helps to keep the retina in place when the retina is intact, but raising intraocular pressure in rabbits to 38 mmHg or lowering it to 0 mmHg had only a modest effect on the rate of subretinal fluid absorption.32 Furthermore, clinical retinal (as opposed to choroidal) detachment is uncommon in hypotonus eyes, and even a small hole in the retina would theoretically allow fluid to reach the subretinal space and circumvent fluid pressure mechanisms of adhesion. Retinal holes are, in fact, found in about 10% of autopsy eyes without detachment,33 but many of these holes may not be functionally open because of surrounding pigmentation or because of “tamponade” by the cortical vitreous gel.34
Once the retina has detached and adhesion mechanisms that depend on retina–RPE contact can no longer work, fluid dynamics become much more important, even in the presence of a hole. Hammer35 has calculated that fluid entering a retinal hole over a scleral buckle will, by its flow pattern, create a suction force that pulls the retina backward against the buckle.
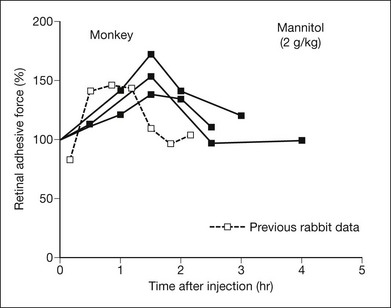
Osmotic pressure can be manipulated more easily and may turn out to have therapeutic applications. Both in vitro and in vivo experiments, using rabbits and primates,9,36,37 have shown that an intravenous injection of mannitol will increase retinal adhesiveness by roughly 50% within 1–2 hours of injection (Fig. 19.5). The magnitude of the effect correlates rather closely with blood osmolality. Mannitol is known to enhance the absorption of fluid out of the subretinal space.38 Some of the adhesive effect may come from fluid absorption “pulling” the retina against the RPE; however, most of the effect probably comes from dehydration of the subretinal space (which enhances the binding properties of the interphotoreceptor matrix (IPM)), because the mannitol effect is still evident in excised tissue where there is no flow toward the choriocapillaris.
Vitreous support and other physical aspects of adhesion
The role of the vitreous in creating retinal detachments through traction and contraction is well known. The role of the vitreous in maintaining retinal attachment is less clear. Vitreous gel has a physical structure that may help to keep the retina in place,34,39 although retinas do not just come off when vitreous detachment or syneresis occurs. A thin cortical layer of vitreous might remain after vitreous detachment or syneresis and serve as a seal or tamponade for retinal holes,34 thus aiding the action of fluid pressure in keeping the retina apposed (Fig. 19.3).
There is other, indirect, evidence for a vitreous role in adhesion. It is very difficult to produce and maintain an experimental rhegmatogenous detachment if the vitreous body remains intact.24,40 Vitreous removal (either mechanical or with hyaluronidase) presumably weakens the tamponade and the structural qualities of the gel, but it also allows liquid vitreous to reach the subretinal space. The status of the gel may help account for the vastly different incidence of retinal detachment among young individuals, in whom the gel is largely intact, and among older individuals, in whom syneresis and vitreous detachment have occurred. A retinal hole in a young eye is blocked by gel pressure and can seal uneventfully; a hole in an old eye is more likely to allow fluid to enter the subretinal space and cause detachment. On the other hand, the integrity of the vitreous gel seems of little consequence to the formation or persistence of experimental nonrhegmatogenous detachments32 or to the retinal adhesive force measured by peeling,11 so the role of the vitreous gel in attachment may be more one of preventing pathologic fluid access to the subretinal space than one of providing a direct adhesive support or force.
The weight of retina is potentially a factor in attachment and detachment, as influenced by gravity and ocular movement. Once a detachment has occurred, the influence of gravity is well known, because appropriate positioning of the patient can be enormously effective in inducing retina to settle. However, it is hard to envision retinal weight as a major factor in retinal attachment under normal conditions,27,32 because our upright posture and eye movements would dictate that benefits and adverse effects be simultaneously present in different parts of the eye.
Mechanical forces inside the subretinal space
Anatomic bridges do not exist between retina and RPE,1 but it is reasonable to ask to what extent matrix material between the layers serves as “glue” and to what extent the physical interdigitation of photoreceptor outer segments and RPE microvilli serves to hold the tissues together.
Mechanical interdigitation
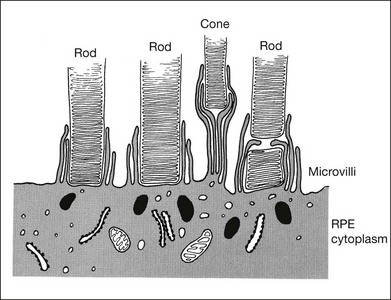
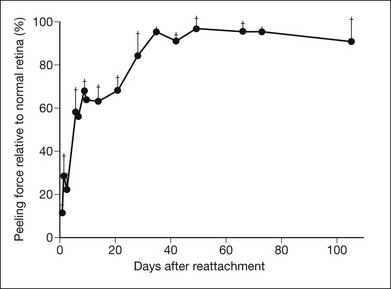
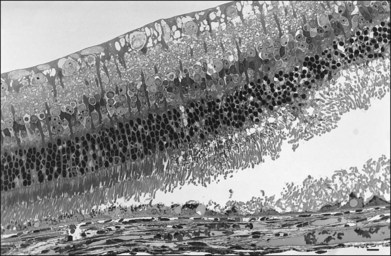
RPE microvilli wrap closely around the tips of the outer segments (Fig. 19.6), and this connection is strong enough to allow for daily phagocytosis of outer-segment fragments as the photoreceptors renew their disc material.1,41,42 However, as a factor in adhesion, interdigitation seems of variable importance. The RPE microvilli are subject to continuous cellular remodeling during the outer-segment renewal cycle. After surgical repair of a retinal detachment, reattachment takes place rather promptly, before the outer segments have regenerated and microvillous connections have been re-established.43,44 Interdigitation begins to develop within 3 days of reattachment, but retinal adhesiveness does not return to normal until 5–6 weeks after reapposition of experimentally detached retina in the rabbit45 (Fig. 19.7).
The mechanisms by which interdigitation could produce adhesion are not known. During outer-segment phagocytosis, the microvilli indent the outer segments and must physically impede attempts to withdraw them. Close ensheathment might also provide a frictional resistance to withdrawal, just as a finger is hard to pull from a narrow tube. There may be electrostatic forces that oppose separation of the membranes.46 The magnitude of all of these effects will also depend on other factors, such as the composition of the intervening matrix and capability of the RPE microvilli for motility and remodeling.
Interphotoreceptor matrix properties
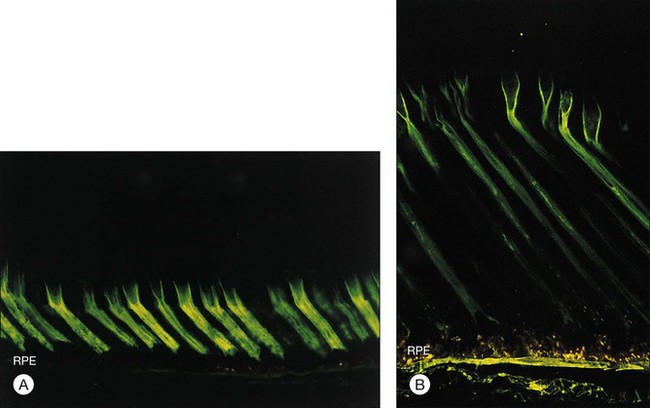
The IPM is a viscous material composed largely of proteins, glycoproteins, and proteoglycans47,48 but containing a substantial concentration of glycosaminoglycans.49 The idea that IPM might serve as a viscous glue was proposed many years ago by Berman,50 but current evidence indicates the IPM is not just a layer of glue but has component structures that play a role in the adhesive process. For example, the cones are surrounded by a specialized sheath of matrix51,52 that can be recognized histochemically by the binding of peanut agglutinin (PNA) (Fig. 19.8A). These cone matrix sheaths remain attached to both RPE and photoreceptor cells when the neural retina is peeled from the RPE.48,51,53 If fresh primate or human retina is peeled from the RPE, the matrix material will stretch markedly before breaking (Fig. 19.8B), suggesting a rather strong bonding at both the outer-segment and RPE interfaces.17,54 The evidence for a bonding function of the IPM is strongest with respect to the cone matrix sheaths, but the structural material that surrounds the rods may play a similar role. It can be recognized histochemically by staining with wheatgerm agglutinin (WGA) or other lectins,55,56 and stretching of WGA-staining material may be observed on peeling freshly enucleated eyecup material.17,54,57 The role of newly recognized matrix components such as galectins and IPM proteoglycans remains to be determined.58,59
The ability of cone or rod matrix sheaths to serve as a structural bond between retina and RPE may well depend on the presence of specific receptors that bind IPM components to the cell membranes. (“Cell adhesion molecules” represent a group of substances that mediate adhesion between individual cells or between cells and substrates.60) Experiments have shown that extracted IPM material does not automatically stick cells to one another,61 but adhesion is unlikely unless specific receptors are present to bind the macromolecular substances. Several receptor systems, including sites for fibronectin, integrins, and mannose, have been proposed and disputed as being involved with retinal adhesion.48,62–65 There is functional evidence that inhibition of chondroitin sulfate proteoglycan synthesis with xylosides causes spontaneous detachments in primates.48,66
Other types of evidence support the concept that IPM contributes significantly to retinal adhesion. Physical factors that affect adhesiveness, such as temperature, pH, and calcium concentration, are known to be modulators of the physicochemical properties of IPM macromolecules and may also affect the bonding of large-molecular species at receptoral sites.67,68 There is also a marked loss of retinal adhesiveness when the subretinal space is exposed to enzymes that degrade matrix components,15,69 such as chondroitinase ABC (which degrades chondroitin sulfate70) or neuraminidase (which breaks sialic acid bonds71). These enzymes, given intravitreally or into the subretinal space itself, weaken adhesiveness markedly in correlation with cytochemical evidence of damage to the IPM (Figs 19.9 and 19.10). It is unknown whether the effects of these enzymes on adhesiveness are a result of changes in viscosity or damage to the structural elements of the IPM. However, adhesiveness returns to normal roughly 3 weeks after exposure of an eye to neuraminidase or chondroitinase ABC, and the recovery of adhesiveness correlates closely with the recovery of normal PNA-binding properties in the IPM.72
Experiments have shown that the chemical nature of the IPM may change with light and dark adaptation.73 To the extent that photic effects might modify the viscosity or bonding properties of the IPM, light could be a modulating factor of retinal adhesion. However, experimental data on adhesion in the light and dark have been contradictory and of uncertain significance.11,74,75
Subcellular components and mobility
Subcellular components of the RPE, such as microtubules and microfilaments, will be relevant to retinal adhesion to the extent that they influence remodeling and movement of the RPE microvilli.76 Actin filaments control melanin movement in the amphibian RPE11 and are present in mammalian RPE,77,78 but a movement of melanin granules has never been observed in mammals. A few attempts have been made to alter adhesion by inhibiting subcellular components of the RPE. Cytochalasin B, which blocks microfilaments, was found to have no effect on adhesion when the drug was presented to excised tissue briefly after enucleation.12 However, injecting cytochalasin into the vitreous 4–72 hours before enucleation caused a 70–90% reduction in pigment adherence as a measure of adhesiveness.79
When retina detaches, the surface morphology of the RPE changes literally within minutes.23 The evolution of these changes can be altered by both colchicine (which dissembles microtubules) and cytochalasin,80 suggesting that microtubules and microfilaments are important to apical RPE morphology. Actin filament contraction and subcellular motility are known to require calcium,81 the absence of which is severely detrimental to retinal adhesion. However, we do not know whether the action of calcium in weakening adhesiveness relates to this mechanism or to effects that calcium may have on transport systems and matrix-binding properties.
Critical dependence on oxygen
It was noted previously that adhesiveness in living rabbit eyes falls severely within a few minutes of death5 (Fig. 19.1A). Furthermore, just 1 minute of ocular ischemia in living rabbits weakens adhesion dramatically, whereas restoration of circulation restores adhesiveness.82,83 The postmortem adhesive loss appears to be slower in primates and humans, and under similar in vitro conditions, at least a moderate adhesive force was measurable for 30 minutes or more after enucleation of primate and human tissue15,17,83 (Fig. 19.11A).
One possible explanation for the postenucleation loss of adhesion is that the rapid release of lysosomal enzymes by injured RPE alters the IPM.84 Enzyme release can occur quickly after injury,84,85 and lysosomal enzyme activity is generally enhanced by warm temperature, low pH, and low calcium levels, all of which reduce retinal adhesiveness. However, the loss of adhesion under warm temperature, low pH, and low calcium conditions is rapidly reversible by restoring normal environmental conditions,16 which would seem unlikely if irreversible degradation of the IPM had taken place. We have also been unable to find any cytochemical or morphologic changes in the IPM that correlate with the postmortem period loss of adhesion.
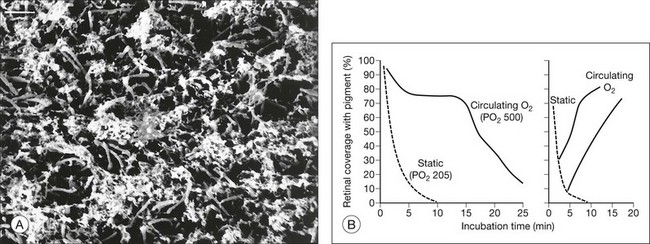
The postmortem failure can be retarded or even reversed simply by improving or restoring oxygenation.82 With enhanced oxygenation, retinal adhesion in excised rabbit tissue can be maintained for 15–30 minutes (instead of 1–2 minutes) without postmortem failure (Fig. 19.11B); adhesive failure that occurred after a few minutes without oxygen was rapidly reversed by oxygenating the bath. In monkeys, adhesiveness may persist in oxygenated Ames solution for 1 hour or more.83
Metabolic inhibitors and other agents
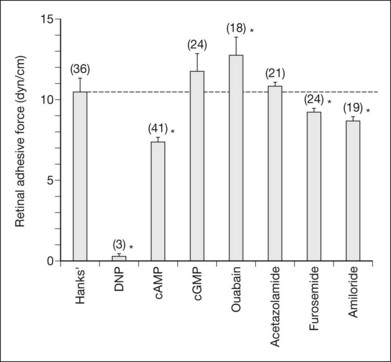
A number of experiments have shown that general metabolic inhibitors have a deleterious effect on the process of retinal adhesion. For example, the introduction of cyanide into the experimental bath reduces the measured adhesive force and hastens the postmortem failure of adhesion.3 In vivo experiments with dinitrophenol placed in the subretinal space showed that retinal adhesiveness was rapidly reduced to an unmeasurable level10 (Fig. 19.12). Sodium iodate and hemicholinium-3 also cause retinal adhesiveness to fall rapidly to a low level,86–89 but these effects may involve a variety of mechanisms because these agents affect not only metabolic functions of the RPE (and, more slowly, the photoreceptors) but also the physical integrity of the RPE cells and blood–retinal barrier.87,90
Specific transport inhibitors, in contrast, may act very selectively on different aspects of the adhesive process. For example, ouabain, which blocks the electrogenic sodium pump, will ultimately kill photoreceptor and RPE cells. However, in the short term its effect is to strengthen adhesiveness3,10 (Fig. 19.12) because sodium entry causes cellular swelling that tightens (presumably) the interdigitation between RPE microvilli and outer segments.13 This is a pathologic effect, of course, and is probably of little relevance to normal mechanisms of adhesion. Acetazolamide, which inhibits carbonic anhydrase, causes an increase in retinal adhesiveness, measured in vitro in rabbits3,88 and in vivo in rabbit and monkey7 (Fig. 19.13). The drug is effective only when given systemically and does not enhance adhesion if placed in the subretinal space8 or in an experimental bath.3,91 Acetazolamide enhances the apical-to-basal transport of fluid across the RPE,3,89 and its effects on adhesion may well be a result of fluid movement, since the enhancement of outward fluid movement by other means such as mannitol36 also increases adhesiveness.36,37
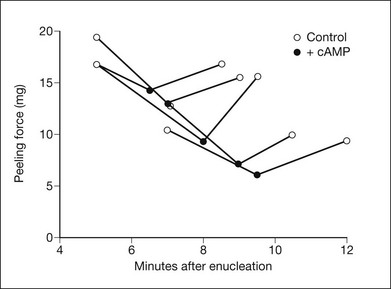
Cyclic adenosine monophosphate (cAMP), which has been shown by several groups to inhibit the outward transport of fluid from the subretinal space,92–94 reversibly weakens retinal adhesive force in vitro12 (Fig. 19.14). cAMP is present in the RPE, but it is not known whether it actively modulates adhesive force in the living eye. In rabbits with experimental detachments, cyclic guanosine monophosphate (cGMP) has the opposite effect of cAMP on RPE fluid transport (i.e., it facilitates outward movement),93,95 but it has not been shown to have an effect on retinal adhesiveness.
Retinal adhesiveness in vivo in the rabbit was also decreased, relative to normal, to 86% with furosemide and 81% with amiloride10 (Fig. 19.12). Both these agents reduce apical–basal fluid transport across epithelial cells.96,97
Relationship of adhesion to subretinal fluid transport and subretinal protein
The RPE actively transports water from the subretinal space to the choroid23,92,98 and experiments on the control of subretinal fluid transport3,27,28,32,91,93,95,99 show that many of the factors affecting adhesion also influence transport in a similar direction. This concurrence may, in part, be fortuitous, since both physiologic processes involve the RPE; thus, conditions that nonspecifically stimulate or inhibit the RPE will have a similar effect on adhesion and fluid absorption. However, there may also be a more direct connection insofar as conditions that dehydrate the subretinal space should serve to keep the retina closely apposed, the matrix viscous, and the matrix molecules tightly interactive at ionic or receptor-binding sites. Conversely, conditions that block subretinal fluid transport and allow the subretinal space to become hydrated would seem intuitively to weaken adhesiveness. Thus, part of the metabolic requirement for retinal adhesion is almost certainly a direct result of the fact that subretinal fluid transport under normal conditions takes place primarily by active metabolic transport across the RPE. Adhesiveness is also increased by raising systemic osmolality with mannitol,9,36,37 which passively enhances the absorption of subretinal fluid.38
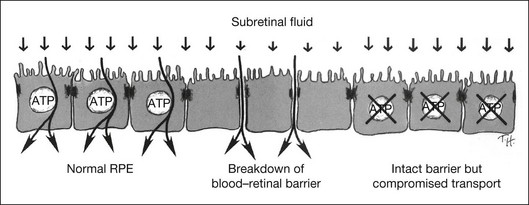
In certain conditions the efficacy of subretinal fluid transport is clearly a critical factor in maintaining adhesion. For example, under conditions where defects occur in the RPE barrier and choroidal pressure is relatively elevated (e.g., through venous obstruction, ocular hypotony, systemic hypertension, or other causes), there will be a risk of fluid following the pressure gradient to enter the subretinal space and cause a nonrhegmatogenous (serous) detachment. Passive adhesive systems are important to minimize the spread of fluid laterally, but the critical determinant of whether a detachment forms and how large it grows will be the ability of the RPE surrounding the “leak” to remove fluid as quickly as it enters the subretinal space. This concept of serous detachment2 is discussed later.
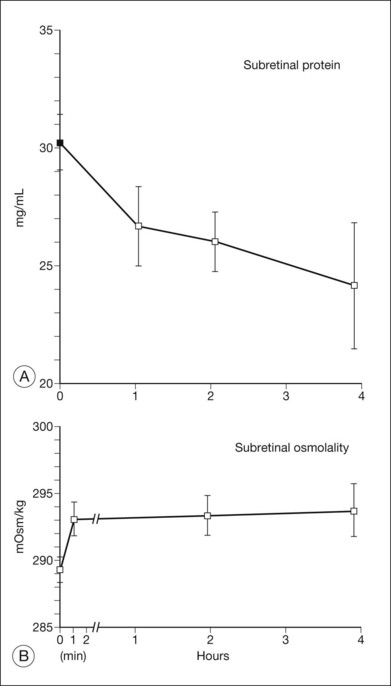
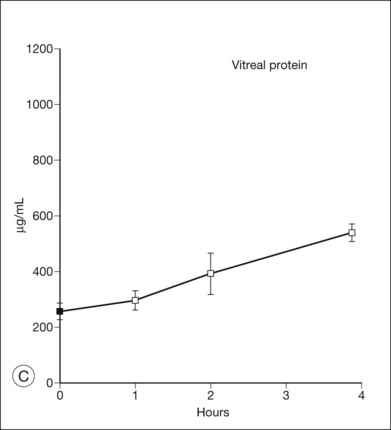
Since the subretinal fluid of both serous and rhegmatogenous detachments has a high concentration of protein, the question may be asked whether oncotic pressure from this protein contributes to the formation or persistence of detachments by drawing in fluid. However, in experiments that introduced albumin (serum) or larger molecules into the subretinal space of rabbits (Fig. 19.15), fluid was absorbed within a few hours regardless of whether protein or other larger molecules remained in the subretinal space.100–102 Furthermore, retina is sufficiently permeable to ions and water that the osmotic pressure of the subretinal space is continuously equilibrating with that of the vitreous to neutralize gradients102,103 (Fig. 19.15B). In fact, albumin can also diffuse slowly across the retina. When serum is injected into the subretinal space, the subretinal fluid loses roughly 5% of its protein concentration per hour into the vitreous (Fig. 19.15A and C). Thus, the high protein content of subretinal fluid is not self-maintaining and is not the cause of detachment; rather it is the result of continued protein influx from the RPE or vitreous. Faulty RPE transport may also contribute to the retention of fluid within the retina, i.e., macular edema.104
Pharmacologic modification of adhesion
Pharmacologic agents that modify retinal adhesive force could be useful clinically in several ways. Agents that enhance adhesion could help reduce the risk of retinal detachment in high-risk eyes. They might minimize the spread of an existing detachment while awaiting surgical repair. They could improve or hasten the healing process after surgical repair of a detachment. To the extent that they enhance the absorption of subretinal fluid, they would speed the recovery of nonrhegmatogenous, as well as rhegmatogenous, detachments and minimize the risk of redetachment. If drugs can compensate in part for metabolic factors that fail in older eyes (which are more susceptible to detachment than younger ones) or in specific diseases that are predisposed to detachment, the risk of detachment might be decreased in these selected populations. Agents that reduce adhesion could facilitate an atraumatic separation of the retina in surgical procedures such as the removal of subretinal lesions, macular translocation, RPE transplantation, and injections of therapeutic agents such as a viral vector for gene transfer or stem cells for retinal regeneration.105,106
Mannitol
Mannitol is an accepted therapeutic agent in humans for glaucoma and cerebral edema. In systemic doses that are used clinically, it causes a 50% increase in retinal adhesiveness, measured both in excised tissue in vitro37 and in the living eyes of primates and rabbits9,36 (Fig. 19.5). The magnitude and duration (2–3 hours) of the mannitol effect are directly related to the increase in blood osmolality. However, the effect is not simply a result of fluid movement “pulling” the retina against the RPE, because it is measurable in vitro (where there is no transretinal flow). More likely, it depends on dehydration of the IPM, which strengthens bonding characteristics.
When hyperosmotic solutions are injected into the vitreous cavity,25 the gradient is in the opposite direction and retinal detachment can be induced (Fig. 19.4). This is a risk with certain drugs, but could in theory be used to produce or facilitate retinal separation in conjunction with procedures that require surgical elevation of the macula. Limitations are the minutes required for detachment to develop and the associated cellular damage if the osmotic load is too great.
Acetazolamide
The acetazolamide effect is slightly weaker than mannitol in the clinical doses we have used, causing a 30–45% increase in retinal adhesiveness in primates and rabbits9,17 (Fig. 19.13). The acetazolamide effect lasts for 3–4 hours after a single injection, but acetazolamide can be given safely over long periods (although currently it is unknown whether the positive effects on adhesion would persist). Acetazolamide increases subretinal fluid transport91 and thus could theoretically help to absorb fluid postoperatively or in cases of serous detachment. However, judging by anecdotal reports, researchers have not found acetazolamide to be dramatically effective in the management of central serous retinopathy. The problem may be that the effect of acetazolamide on subretinal fluid transport is not very strong and can only occur when the RPE is basically healthy and receptive to metabolic stimulation.107 In a disorder such as central serous retinopathy, where RPE transport is probably compromised as a part of the disease, there may not be a normal substrate of RPE on which the acetazolamide can work.
Although acetazolamide and related carbonic anhydrase inhibitors have not been clinically useful for detachment disorders, they have proved quite useful for the removal of foveal cystic fluid in a number of disorders. They have not been of great use in postcataract cystoid edema and diabetic macular edema, where fluid continuously enters from vascular sources, but they can clear intraretinal cysts dramatically where the fluid is relatively static in disorders such as retinitis pigmentosa, X-linked juvenile retinoschisis, enhanced S-cone syndrome and sometimes macular epiretinal membrane formation.104,107–111 The effectiveness probably depends on reasonable preservation of RPE metabolic function under the fovea, so that the drug can augment transport. One limitation to its use for retinal edema is the systemic side-effects, which may relate in part to its action on intracellular carbonic anhydrase throughout the body. Agents like benzolamide, which affect only the membrane-bound enzymes that are relevant to RPE transport, may have fewer side-effects.112 Recent work has shown that enough drug may enter from topical carbonic anhydrase drops to produce a clinical effect, although the onset may take months instead of days.113,114 The action of both oral and topical carbonic anhydrase inhibitors seems subject to tachyphylaxis (rebound of cystic edema), and cysts may return after roughly a year of usage; however, the effects will usually return after several months off treatment.115,116
Cold temperature and ouabain
Both cold temperature and ouabain increase retinal adhesiveness,3,7 but their action depends on pathologic changes (cellular swelling) rather than a true enhancement of the mechanisms of adhesion.13 Neither cold nor ouabain therefore can be used for any long-term clinical applications, but they may be valuable in special situations. For example, cooling the irrigating solution during vitrectomy might reduce the risk of retinal separation during the manipulation of surgery.
Ionic changes
The removal of local calcium and magnesium ions, or lowering the pH, causes a dramatic fall in retinal adhesive force. Thus transient irrigation of the retina with calcium-free solution, or a solution of low pH, might facilitate the production of atraumatic surgical separation in association with submacular surgery. Alternatively a small amount of such solution might be injected into the subretinal space to weaken adhesion and make enlargement of the surgical detachment easier. Preliminary studies on this approach have been reported,75,105 but RPE or retinal toxicity is a risk, and guidelines for safe administration still need to be established.
Implications for vitreoretinal surgery
Conversely, conditions that reduce adhesiveness, such as intravitreal hyperosmolarity, metabolic inhibition, low Ca2+, and low pH, might prove useful in subretinal surgery where retinal separation needs to be produced transiently and with little cellular damage. Low-Ca2+, low-Mg2+ solutions have already been shown to weaken adhesion and facilitate experimental detachment in rabbits.75,105,117 Metabolic inhibitors, such as dinitrophenol, should in theory also be effective if their effects could be made transient and nontoxic. Another agent that might reduce adhesiveness is cAMP. Some of these approaches are likely to reach clinical practice within the next few years.
Recovery of adhesiveness without retinopexy
Recovery after retinal detachment depends on a variety of factors, including the length of detachment (which may affect the survival of cells) and effectiveness with which adhesive mechanisms can be re-established. We have measured in rabbits the retinal peeling force in vitro45 after making small experimental detachments that spontaneously settled within hours to a few days. The results show that restoration of normal adhesive strength requires 4–6 weeks (Fig. 19.7). Keep in mind that these experiments involved the injection of a small amount of balanced salt solution into the subretinal space; in clinical detachments, where the fluid is serous and the retina is separated for longer periods, there could well be more trauma to the RPE or outer segments and thus an even more prolonged recovery period. It remains to be shown which adhesive factors are rate-limiting for the recovery process, considering that full recovery involves the restoration of anatomic interdigitation, RPE synthetic capabilities, IPM structure and binding, and subretinal fluid transport. These data show how clinical recovery after retinal detachment is possible without retinopexy,118–120 although it is slow and would only be advisable when mechanical (vitreous) traction forces have been fully relieved.
Effects of retinopexy
Retinal surgeons frequently use retinopexy (i.e., scarification of the retina and RPE with laser photocoagulation, diathermy, or cryotherapy) as a means of creating an extra-strong adhesion between retina and RPE that will strengthen attachment and prevent the lateral movement of subretinal fluid. Experiments on the in vitro peeling force45 and the in vivo adhesive force120 show that laser photocoagulation produces a bond that approaches normal adhesive strength within 24 hours, possibly from local effects such as fibrin formation (Fig. 19.16). The adhesive strength continues to increase gradually and reaches levels roughly twice normal by 2–3 weeks after the photocoagulation.45,121,122 Diathermy effects, measured in vivo, have a similar time course. Cryotherapy, however, weakens adhesion for the first week, after which the adhesive force rises to the same levels as with other forms of retinopexy. The initial weakening after cryotherapy probably relates to local inflammation or edema. Thus, all forms of retinopexy appear to be effective in the long run, presumably by inducing scar formation; however, if a rapid bond is required, laser photocoagulation or diathermy is preferable.
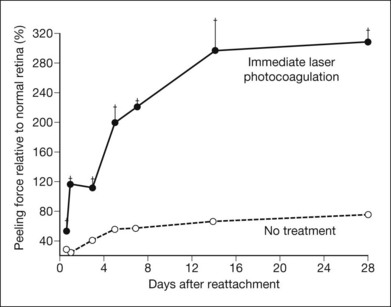
Fig. 19.16 Laser photocoagulation of freshly reattached retina can rapidly enhance the strength of retinal adhesion. Two experimental detachments were made in each eye (Fig. 19.7), and after the subretinal fluid had spontaneously absorbed, the base of one detachment was covered with laser photocoagulation. The force required to peel retina from the reattached areas, with or without photocoagulation, was compared to the force required in the intervening normal areas. The adhesive force in the photocoagulated regions exceeded normal within hours and reached an apparent maximum after about 2 weeks (although at these levels of force the retina frequently tore rather than separated, and the “true” adhesive strength may be even greater).
(Reproduced from Yoon YH, Marmor MF. Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology 1988;95:1385–8. Copyright 1988, with permission from the American Academy of Ophthalmology.)
Effects of vitreous in the subretinal space
One of the factors contributing to many rhegmatogenous detachments is vitreous syneresis and liquefaction, which allow fluid to percolate through the retinal tear and expand or maintain the elevation of retina. Although there is some evidence that hyaluronic acid may be toxic in high concentrations to RPE,123 clinical data indicate that liquid vitreous is tolerated insofar as a reasonable recovery of retinal function generally occurs if detachments are repaired within a few days or weeks. The presence of liquefied vitreous in the subretinal space has no immediate effect on the rate of fluid transport across the RPE.124 The concentration of protein in normal vitreous is only about 1% of that in serum, but when vitreous enters the subretinal space the concentration of subretinal protein rises slowly.124 This occurs because water is pumped out faster than the protein can be removed, whereas the continued entry of liquid vitreous brings in more protein. This mechanism may contribute to the high protein concentration of subretinal fluid in chronic rhegmatogenous detachments. Serum and protein may also leak in from the choroid to the extent that the RPE has been damaged under the conditions of detachment.
Pathophysiology of serous detachment
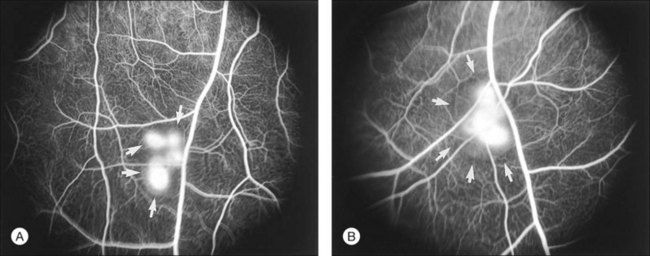
For detachment to occur there must be forces that cause the retina to separate and also conditions that allow subretinal fluid to persist. The clinical condition of serous detachment provides a case in point. In central serous chorioretinopathy (CSC), fluorescein angiography demonstrates apparent “leaks” through the RPE, defects through which fluid enters the subretinal space from the choroid. However, experimental work has clearly shown that subretinal fluid overlying an intact RPE is removed very actively by active metabolic transport and that damage to the RPE barrier in general makes the absorption of fluid faster rather than slower (Fig. 19.17). For example, damaging the RPE focally by mechanical micropipette injury or laser burns or diffusely by the systemic administration of sodium iodate hastens the absorption of subretinal fluid.125,126 This occurs because intraocular pressure and the osmotic absorption of the choroid both move fluid in an outward direction, and their effectiveness is increased when the RPE flow barrier is removed. Even serous fluid is absorbed at least as fast over damaged as over intact RPE.101,103 Furthermore, to cause detachment the entering fluid must overcome the retinal adhesive forces at the margin of the leak. Thus the mere presence of a focal defect in the RPE does not “cause” serous detachment.127
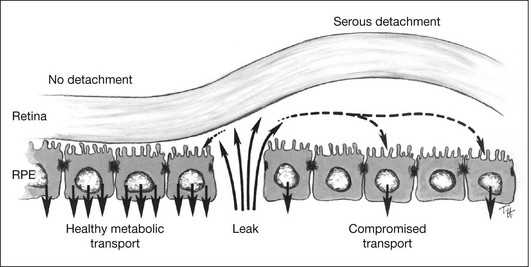
How then do serous detachments form?2,128,129 The question is not whether the leaks are the source of fluid – they must be – but why the fluid accumulates when entry is slow and one would expect subretinal fluid to be cleared rapidly by active transport (across a normal RPE) or by passive mechanisms (across damaged RPE). On reflection, the one condition that would allow fluid to accumulate is when the RPE barrier function remains intact around the leak, whereas active fluid transport is impaired (Fig. 19.18). In this situation fluid cannot leave passively or actively. I suspect that many serous detachments form because there is widespread functional damage to the process of transport across the RPE (either from RPE disease directly or from disease of the underlying choroid and choriocapillaris). For example, the absorption of subretinal fluid in rabbits is slowed by the ligation of vortex veins.130 Furthermore the choroid is typically thickened in CSC, which may signify increased hydrostatic pressure that would foster serous detachment both directly and through effects upon RPE transport.106 Under conditions that compromise fluid absorption and possibly adhesiveness but do not destroy the blood–retinal barrier, fluid that enters from a focal leak may persist and accumulate within the subretinal space (Fig. 19.19). One may ask whether serous detachment can be accounted for simply by oncotic pressure when a protein-rich fluid leaks into the subretinal space. However, as noted earlier, protein in the subretinal space does not prevent fluid absorption; it is osmotically neutralized by the diffusion of water and ions from the vitreous, and it will not persist unless there is a continual influx of new proteinaceous fluid.
Three conditions appear to be necessary for serous fluid to accumulate129,130: (1) a defect in the RPE barrier that allows access to the subretinal space; (2) a source of fluid pressure, to move fluid in; and (3) an impairment of outward fluid transport (or a broad area of leakage), so fluid spreads and persists in the subretinal space. Weakness of retinal adhesion may be an additional facilitating factor. Unless all three basic conditions are present, detachment will not occur. For example, attempts to induce serous detachment in rabbits by damaging the RPE focally while altering intraocular pressure (conditions 1 and 2 only) have been largely unsuccessful.118 However, diffuse injury to the RPE and underlying choriocapillaris (by photosensitization with rose Bengal dye131 or by toxic effects of N-ethylmaleimide132) leads to the formation of bullae (Fig. 19.20), since the damaged capillaries not only serve as a source of fluid, but also compromise RPE transport and barrier functions. In clinical practice, choroidal vascular damage is probably the most frequent primary cause of serous detachments and may underlie even the focal RPE defects of CSC.106,128
If CSC is more a disease of diffuse transport dysfunction, involving RPE or choriocapillaris, than a result of the focal “leak” seen on angiography,2 why does laser photocoagulation of the leak help to clear the fluid? Because laser therapy may effectively seal the visible leak, it removes the immediate source of fluid and allows the existing fluid to absorb slowly. However, recurrences will be common as long as the underlying transport dysfunction persists, and this is indeed the clinical course of the disease. In further support of the concept of diffuse dysfunction, serous retinopathy is often associated with systemic disease of the vascular system (especially choroidal ischemic disorders) or with emotional stress,133–136 and the serous fluid accumulations are often bilateral and multifocal. Serous detachments may occur in patients taking high levels of corticosteroids,137,138 and the chronic administration of epinephrine can produce serous detachments in experimental animals.139 The most effective treatment for CSC may ultimately be pharmacologic intervention that blocks the adverse adrenergic and corticosteroid effects.128 Treatment with acetazolamide or other carbonic anyhdrase inhibitors that enhance RPE transport has not shown great value, presumably because the RPE transport mechanisms are compromised by the disease. However, as noted earlier, these agents can be very effective in clearing cystic intraretinal fluid, especially in retinal dystrophies including retinitis pigmentosa, X-linked retinoschisis, and enhanced S-cone syndrome.
1 Steinberg RH, Wood I. Pigment epithelial cell ensheathment of cone outer segments in the retina of the domestic cat. Proc R Soc Lond B. 1974;187:461–478.
2 Marmor MF. New hypothesis on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1988;226:548–552.
3 Marmor MF, Abdul-Rahim AS, Cohen DS. The effect of metabolic inhibitors on retinal adhesion and subretinal fluid resorption. Invest Ophthalmol Vis Sci. 1980;19:893–903.
4 Zauberman H, DeGuillebon H. Retinal traction in vivo and postmortem. Arch Ophthalmol. 1972;87:549–554.
5 De Guillebon H, De la Tribonniere MM, Pomerantzeff O. Adhesion between retina and pigment epithelium. Arch Ophthalmol. 1971;86:679–684.
6 De Guillebon H, Zauberman H. Experimental retinal detachment: biophysical aspects of retinal peeling and stretching. Arch Ophthalmol. 1972;87:545–548.
7 Endo EG, Yao XY, Marmor MF. Pigment adherence as a measure of retinal adhesion: dependence on temperature. Invest Ophthalmol Vis Sci. 1988;29:1390–1396.
8 Kita M, Negi A, Kawano SI, et al. Measurement of retinal adhesive force in the in vivo rabbit eye. Invest Ophthalmol Vis Sci. 1990;31:624–628.
9 Kita M, Marmor MF. Retinal adhesive force in living rabbit, cat, and monkey eyes: normative data and enhancement by mannitol and acetazolamide. Invest Ophthalmol Vis Sci. 1992;33:1879–1882.
10 Kita M, Marmor MF. Effects on retinal adhesive force in vivo of metabolically active agents in the subretinal space. Invest Ophthalmol Vis Sci. 1992;33:1883–1887.
11 Marmor MF, Maack T. Local environmental factors and retinal adhesion in the rabbit. Exp Eye Res. 1982;34:727–733.
12 Yoon YH, Marmor MF. Effects on retinal adhesion of temperature, cyclic AMP, cytochalasin B, and enzymes. Invest Ophthalmol Vis Sci. 1988;29:910–914.
13 Marmor MF, Yao XY. The enhancement of retinal adhesiveness by ouabain appears to involve cellular edema. Invest Ophthalmol Vis Sci. 1989;30:1511–1514.
14 Maurice DM, Salmon J, Zauberman H. Subretinal pressure and retinal adhesion. Exp Eye Res. 1971;12:212–217.
15 Yao XY, Hageman GS, Marmor MF. Retinal adhesiveness in the monkey. Invest Ophthalmol Vis Sci. 1994;35:744–748.
16 Yao XY, Endo EG, Marmor MF. Reversibility of retinal adhesion in the rabbit. Invest Ophthalmol Vis Sci. 1989;30:220–224.
17 Marmor MF, Yao XY, Hageman GS. Retinal adhesiveness in surgically enucleated human eyes. Retina. 1994;14:181–186.
18 Kita M, Negi A, Marmor MF. Lowering the calcium concentration in the subretinal space in vivo loosens retinal adhesion. Invest Ophthalmol Vis Sci. 1992;33:23–29.
19 Bill A. Some aspects of tissue fluid dynamics in the eye. In: Cant JS, ed. Vision and circulation. St Louis: Mosby, 1976.
20 Toris CB, Pederson JE, Tsuboi S, et al. Extravascular albumin concentration of the uvea. Invest Ophthalmol Vis Sci. 1990;31:43–53.
21 Maurice DM. Flow of water between aqueous and vitreous compartments in the rabbit eye. Am J Physiol. 1987;252:104–108.
22 Fatt I, Shantinath K. Flow conductivity of retina and its role in retinal adhesion. Exp Eye Res. 1971;12:218–226.
23 Tsuboi S. Measurement of the volume flow and hydraulic conductivity across the isolated dog retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1987;28:1776–1782.
24 Marmor MF. Retinal detachment from hyperosmotic intravitreal injection. Invest Ophthalmol Vis Sci. 1979;18:1237–1244.
25 Marmor MF, Martin LJ, Tharpe S. Osmotically induced retinal detachment in the rabbit and primate: electron microscopy of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1980;19:1016–1029.
26 Cantrill HL, Pederson JE. Experimental retinal detachment. VI. The permeability of the blood–retinal barrier. Arch Ophthalmol. 1984;102:747–751.
27 Frambach DA, Marmor MF. The rate and route of fluid resorption from the subretinal space of the rabbit. Invest Ophthalmol Vis Sci. 1982;22:292–302.
28 Negi A, Marmor MF. Quantitative estimation of metabolic transport of subretinal fluid. Invest Ophthalmol Vis Sci. 1986;27:1564–1568.
29 Pederson JE, Cantrill HL. Experimental retinal detachment. V. Fluid movement through the retinal hole. Arch Ophthalmol. 1984;102:136–139.
30 Hamann S, Kiilgaard JF, la Cour M, et al. Cotransport of H+, lactate, and H2O in porcine retinal pigment epithelial cells. Exp Eye Res. 2003;76:493–504.
31 Chihara E, Nao IN. Resorption of subretinal fluid by transepithelial flow of the retinal pigment epithelium. Graefes. Arch Ophthalmol. 1985;223:202–204.
32 Negi A, Kawano S, Marmor MF. Effects of intraocular pressure and other factors on subretinal fluid resorption. Invest Ophthalmol Vis Sci. 1987;28:2099–2102.
33 Rutnin U, Schepens CL. Fundus appearance in normal eyes. IV. Retinal breaks and other findings. Am J Ophthalmol. 1978;64:1063–1078.
34 Foulds WS. The vitreous in retinal detachment. Trans Ophthalmol Soc UK. 1975;95:412–416.
35 Hammer ME. Retinal re-attachment forces created by absorption of subretinal fluid. Doc Ophthalmol. 1981;25:61–75.
36 Kita M, Marmor MF. Systemic mannitol increases the retinal adhesive force in vivo. Arch Ophthalmol. 1991;109:1449–1450.
37 Yao XY, Moore KT, Marmor MF. Systemic mannitol increases retinal adhesiveness measured in vitro. Arch Ophthalmol. 1991;109:275–277.
38 Negi A, Marmor MF. Effects of subretinal and systemic osmolality on the rate of subretinal fluid resorption. Invest Ophthalmol Vis Sci. 1984;25:616–620.
39 Osterlin S. On the molecular biology of the vitreous in the aphakic eye. Acta Ophthalmol. 1977;55:353–361.
40 Foulds WS. Experimental retinal detachment. Trans Ophthalmol Soc UK. 1963;83:153–170.
41 Anderson DH, Fisher SK. The relationship of primate foveal cones to the pigment epithelium. J Ultrastruct Res. 1986;67:23–32.
42 Young RW. Visual cells and the concept of renewal. Invest Ophthalmol. 1976;15:700–725.
43 Anderson DH, Guerin CJ, Erickson PA, et al. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;227:168–183.
44 Kroll AJ, Machemer R. Experimental retinal detachment in the owl monkey. V. Electron microscopy of the reattached retina. Am J Ophthalmol. 1969;67:117–130.
45 Yoon YH, Marmor MF. Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology. 1988;95:1385–1388.
46 Gingell D, Fornes JA. Demonstration of intermolecular forces in cell adhesion using a new electrochemical technique. Nature. 1975;256:210–211.
47 Adler AJ, Klucznik KM. Proteins and glycoproteins of the bovine interphotoreceptor matrix: composition and fractionation. Exp Eye Res. 1982;34:423–434.
48 Hageman GS, Kirchoff MA, Anderson DH. Biochemical characterization and distribution of retinal interphotoreceptor matrix glycoconjugates. Glycoconj J. 1990;7:512.
49 Porello K, LaVail MM. Histochemical demonstration of spatial heterogeneity in the interphotoreceptor matrix of the rat retina. Invest Ophthalmol Vis Sci. 1986;27:1577–1586.
50 Berman ER. Mucopolysaccharides (glycosaminoglycans) of the retina: identification, distribution, and possible biological role. Mod Probl Ophthalmol. 1969;8:5–31.
51 Hollyfield JG, Varner H, Rayborn ME, et al. Retinal attachment to the pigment epithelium. Retina. 1989;9:59–68.
52 Johnson LV, Hageman GS, Blanks MC. Interphotoreceptor matrix domains ensheath vertebrate cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1986;27:129–135.
53 Hageman GS, Marmor MF, Yao XY, et al. The interphotoreceptor matrix mediates primate retinal adhesion. Arch Ophthalmol. 1995;113:655–660.
54 Hageman GS, Johnson LV. Structure, composition, and function of the retinal interphotoreceptor matrix. In: Osborne N, Chader J. Progress in retinal research. Oxford: Pergamon Press, 1991.
55 Hageman GS, Johnson LV. The “cone matrix sheath”: structural, compositional, and functional analyses. Invest Ophthalmol Vis Sci. 1988;29(suppl):108.
56 Kirchoff MA, Anderson K, Johnson LF, et al. Composition and distribution of insoluble interphotoreceptor matrix components. Invest Ophthalmol Vis Sci. 1990;31(suppl):153.
57 Marmor MF. Mechanisms of retinal adhesion. In: Osborne NN, Chader GJ. Progress in retinal research. Oxford: Pergamon Press, 1993.
58 Kuehn MH, Wietecki DT, Hageman GS. Molecular characterization of the murine orthologue of the human retinal proteoglycan IPM 150. Mol Vis. 2000;6:148–156.
59 Uebara F, Ohba N, Ozawa M. Isolation and characterization of galectins in the mammalian retina. Invest Ophthalmol Vis Sci. 2001;42:2164–2172.
60 Edelman GM. Cell adhesion molecules. Science. 1983;219:450–457.
61 Adler AJ, Klucznik KM. Interaction of bovine pigment epithelium cells, photoreceptor outer segments, and interphotoreceptor matrix: a model for retinal adhesion. Curr Eye Res. 1982;1:579–589.
62 Kohno T, Sorgente N, Ishibashi T, et al. Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci. 1987;28:506–514.
63 Philp NJ, Nachmias VT. Polarized distribution of integrins and fibronectin in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1987;28:1275–1280.
64 Shirakawa H, Ishiguro SI, Itoh Y, et al. Are sugars involved in the binding of rhodopsin-membranes by the retinal pigment epithelium? Invest Ophthalmol Vis Sci. 1987;28:628–632.
65 Opas M, Kalnins VI. Distribution of spectrin and lectin-binding materials in surface lamina of RPE cells. Invest Ophthalmol Vis Sci. 1985;26:621–627.
66 Lazarus HS, Hageman GS. Xyloside-induced disruption of interphotoreceptor matrix proteoglycans results in retinal detachment. Invest Ophthalmol Vis Sci. 1992;33:364–376.
67 Ishikawa M, Johnson LV, DeWing MD, et al. pH-Dependent changes in interphotoreceptor matrix domains surrounding cone photoreceptors. Ophthalmol Res. 1996;28:117–124.
68 Ishikawa M, Fujiwara T, Yoshitomi T. Temperature-dependent ultrastructural changes in the cone interphotoreceptor matrix. Jpn J Ophthalmol. 2009;53:536–540.
69 Yao XY, Hageman GS, Marmor MF. Retinal adhesiveness is weakened by enzymatic modification of the interphotoreceptor matrix in vivo. Invest Ophthalmol Vis Sci. 1990;31:2051–2058.
70 Yamada K. The effect of digestion with chondroitinases upon certain histochemical reactions of mucosaccharide-containing tissues. J Histochem Cytochem. 1974;22:266.
71 Uehara F, Muramatsu T, Sameshima M, et al. Effects of neuraminidase on lectin binding sites in photoreceptor cells of monkey retina. Jpn J Ophthalmol. 1985;29:54.
72 Yao XY, Hageman GS, Marmor MF. Recovery of retinal adhesion after enzymatic perturbation of the interphotoreceptor matrix. Invest Ophthalmol Vis Sci. 1992;33:498–503.
73 Uehara F, Yasumura D, LaVail MM. Rod- and cone-associated interphotoreceptor matrix in the rat retina. Invest Ophthalmol Vis Sci. 1991;32:285–292.
74 Owczarek FR, Marak GE, Pilkerton AR. Retinal adhesion in light- and dark-adapted rabbits. Invest Ophthalmol. 1975;14:353–358.
75 Faude F, Wendt S, Biedermann B, et al. Facilitation of artificial retinal detachment for macular translocation surgery tested in rabbits. Invest Ophthalmol Vis Sci. 2001;42:1328–1337.
76 Burnside MB. Possible roles of microtubules and actin filaments in retinal pigmented epithelium. Exp Eye Res. 1976;23:257–275.
77 Burnside B, Laties AM. Actin filaments in apical projections of the primate pigmented epithelial cell. Invest Ophthalmol. 1976;15:570–575.
78 Burnside B, Adler R, O’Connor P. Retinomotor pigment migration in the teleost retinal pigment epithelium. I. Roles for actin and microtubules in pigment granule transport and cone movement. Invest Ophthalmol Vis Sci. 1983;24:1–15.
79 Moore KT, Yao XY, Marmor MF. Intravitreal cytochalasin D decreases retinal adhesion. Invest Ophthalmol Vis Sci. 1991;32(suppl):772.
80 Immel J, Negi A, Marmor MF. Acute changes in RPE apical morphology after retinal detachment in rabbit: a SEM study. Invest Ophthalmol Vis Sci. 1986;27:1770–1776.
81 Dearry A, Burnside B. Effects of extracellular Ca++, K+, and Na+ on cone and retinal pigment epithelium retinomotor movements in isolated teleost retinas. J Gen Physiol. 1984;83:589–611.
82 Kim RY, Yao XY, Marmor MF. Oxygen dependency of retinal adhesion. Invest Ophthalmol Vis Sci. 1993;34:2074–2078.
83 Marmor MF, Yao XY. The metabolic dependency of retinal adhesion in rabbit and primate. Arch Ophthalmol. 1995;113:232–238.
84 Kain HL, Libondi T. Experimentelle Netzhautabldaung-Untersuchungen zum Pathomechanismus. Fortschr Ophthalmol. 1986;83:590–596.
85 Adler AJ, Martin KJ. Lysosomal enzymes in the interphotoreceptor matrix: acid protease. Curr Eye Res. 1983;2:359–366.
86 Ashburn FS, Jr., Pilkerton A, Rao NA, et al. The effects of iodate and iodoacetate on the retinal adhesion. Invest Ophthalmol Vis Sci. 1980;19:1427–1432.
87 Negi A, White MP, Marmor MF. Effects of hemicholinium-3, a photoreceptor and pigment epithelial toxin, on retinal adhesiveness and subretinal fluid absorption. Doc Ophthalmol. 1993;83:331–336.
88 Takeuchi A, Negi A, Yamamoto F, et al. Effects of sodium iodate on retinal adhesive force in vivo. Invest Ophthalmol Vis Sci. 1991;32(suppl):667.
89 Yoon YH, Marmor MF. Effects of hemicholinium-3 and sodium iodate on RPE and retinal adhesiveness. Invest Ophthalmol Vis Sci. 1991;32(suppl):667.
90 Nilsson SEG, Knave B, Persson HE. Changes in ultrastructure and function of the sheep pigment epithelium and retina induced by sodium iodate. II. Early effects. Acta Ophthalmol. 1977;55:1007–1026.
91 Marmor MF, Maack T. Enhancement of retinal adhesion and subretinal fluid resorption by acetazolamide. Invest Ophthalmol Vis Sci. 1982;23:121–124.
92 Hughes BA, Miller SS, Machen TE. Effects of cyclic AMP on fluid absorption and ion transport across frog retinal pigment epithelium: measurements in the open-circuit state. J Gen Physiol. 1984;83:875–899.
93 Marmor MF, Negi A. Pharmacologic modification of subretinal fluid absorption in the rabbit eye. Arch Ophthalmol. 1986;104:1674–1677.
94 Miller S, Farber D. Cyclic AMP modulation of ion transport across frog retinal pigment epithelium: measurements in the short-circuit state. J Gen Physiol. 1984;83:853–874.
95 Kawano SI, Marmor MF. Metabolic influences on the absorption of serous subretinal fluid. Invest Ophthalmol Vis Sci. 1988;29:1255–1257.
96 Frambach DA, Misfeldt DS. Furosemide-sensitive Cl transport in embryonic chicken retinal pigment epithelium. Am J Physiol. 1983;244:679–685.
97 Tsuboi S, Pederson JE. Experimental retinal detachment. XI. Furosemide-inhibitable fluid absorption across retinal pigment epithelium in vivo. Arch Ophthalmol. 1986;104:602–603.
98 Frambach DA, Weiter JJ, Adler AJ. A photogrammetric method to measure fluid movement across isolated frog retinal pigment epithelium. Biophys J. 1985;47:547–552.
99 Negi A, Marmor MF. Mechanisms of subretinal fluid resorption in the cat eye. Invest Ophthalmol
100 Marmor MF, Negi A, Maurice DM. Kinetics of macromolecules injected into the subretinal space. Exp Eye Res. 1985;40:687–696.
101 Negi A, Marmor MF. Healing of photocoagulation lesions affects the rate of subretinal fluid resorption. Ophthalmology. 1984;91:1678–1683.
102 Takeuchi A, Kricorian G, Marmor MF. Albumin movement out of the subretinal space after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:1298–1305.
103 Takeuchi A, Kricorian G, Yao XY, et al. The rate and source of albumin entry into saline-filled experimental retinal detachments. Invest Ophthalmol Vis Sci. 1994;35:3792–3798.
104 Wolfensberger TJ, Gregor ZJ. Macular edema – rationale for therapy. Macular edema. Coscas G, ed. Macular edema. Basel: Karger; 2010;vol 47:49–58.
105 Szurman P, Roters S, Grisanti S, et al. Ultrastructural changes after artificial retinal detachment with modified retinal adhesion. Invest Ophthalmol Vis Sci. 2006;47:4983–4989.
106 Imamura Y, Fujiwara T, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–1473.
107 Marmor MF. Hypothesis concerning carbonic anhydrase treatment of CME: example with epiretinal membrane. Arch Ophthalmol. 1990;108:1524–1525.
108 Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106:1190–1195.
109 Fishman GA, Gilbert COT, Fiscella RG, et al. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol. 1989;107:1445–1452.
110 Apushkin MA, Fishman GA. Use of dorzolamide for patients with X-linked retinochisis. Retina. 2006;26:741–745.
111 Genead MA, Fishman GA, McAnany JJ. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc Ophthalmol. 2010;121:231–240.
112 Wolfensberger TJ, Chiang R, Takeuchi A, et al. Inhibition of membrane-bound carbonic anhydrase enhances subretinal fluid absorption and retinal adhesiveness. Graefes Arch Clin Exp Ophthalmol. 2000;238:76–80.
113 Fishman GA, Apushkin MA. Continued use of dorzolamide for the treatment of cystoid macular oedema in patients with retinitis pigmentosa. Br J Ophthalmol. 2007;91:743–745.
114 Genead MA, Fishman GA. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with retinitis pigmentosa and usher syndrome. Arch Ophthalmol. 2010;128:1146–1150.
115 Apushkin MA, Fishman GA, Grover S, et al. Rebound of cystoid macular edema with continued use of acetazolamide in patients with retinitis pigmentosa. Retina. 2007;27:1112–1118.
116 Thobani A, Fishman GA. The use of carbonic anhydrase inhibitors in the retreatment of cystic macular lesions in retinitis pigmentosa and X-linked retinoschisis. Retina. 2011;31:312–315.
117 Fang XY, Hayashi A, Cekic O, et al. Effect of Ca2+-free and Mg2+-free BSS plus solution on the retinal pigment epithelium and retina in rabbits. Am J Ophthalmol. 2001;131:481–488.
118 Chignell AH, Markham RHC. Retinal detachment surgery without cryotherapy. Br J Ophthalmol. 1981;65:371–373.
119 Machemer R. The importance of fluid absorption, traction, intraocular currents, and chorioretinal scars in the therapy of rhegmatogenous retinal detachments. Am J Ophthalmol. 1984;98:681–693.
120 Zauberman H, Rosell FG. Treatment of retinal detachment without inducing chorioretinal lesions. Trans Am Acad Ophthalmol Otolaryngol. 1975;79:835–844.
121 Kita M, Negi A, Kawano SI, et al. Photothermal, cryogenic, and diathermic effects on retinal adhesive force in vivo. Retina. 1991;11:441–444.
122 Kwon OW, Kim SY. Changes in adhesive force between the retina and the retinal pigment epithelium by laser photocoagulation in rabbits. Yonsei Med J. 1995;36:243–250.
123 Zhu ZR, Goodnight R, Sorgente N, et al. Cellular proliferation induced by subretinal injection of vitreous in the rabbit. Arch Ophthalmol. 1988;106:406–411.
124 Takeuchi A, Kricorian G, Marmor MF. When vitreous enters the subretinal space: implications for subretinal fluid protein. Retina. 1996;16:426–430.
125 Negi A, Marmor MF. The resorption of subretinal fluid after diffuse damage to the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1983;24:1475–1479.
126 Negi A, Marmor MF. Experimental serous retinal detachment and focal pigment epithelial damage. Arch Ophthalmol. 1984;102:445–449.
127 Tsukahara Y, Marmor MF. Experimental studies on the accumulation of subretinal fluid. Ophthalmologica. 1991;202:202–207.
128 Marmor MF. On the cause of serous detachments and acute central serous chorioretinopathy. Br J Ophthalmol. 1997;81:812–813.
129 Marmor MF, Yao XY. Conditions necessary for the formation of serous detachment: Experimental evidence from the cat. Arch Ophthalmol. 1994;112:830–838.
130 Kita M, Negi A, Kawano S, et al. Lamellar scleral resection enhances subretinal fluid absorption in eyes with choroidal congestion. Jpn J Ophthalmol. 1991;35:394–401.
131 Yao XY, Marmor MF. Induction of serous retinal detachment in rabbit eyes by pigment epithelial and choriocapillary injury. Arch Ophthalmol. 1992;110:541–546.
132 Chon CH, Yao XY, Dalal R, et al. An experimental model of retinal pigment epithelial and serous detachment. Retina. 1996;16:139–144.
133 De Venecia G, Jampol LM. The eye in accelerated hypertension. II. Localized serous detachments of the retina in patients. Arch Ophthalmol. 1984;102:68–73.
134 Gelber GS, Schatz H. Loss of vision due to central serous chorioretinopathy following psychological stress. Am J Psychiatry. 1987;144:46–50.
135 Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Retina. 1987;7:111–130.
136 Fastenberg DM, Ober RR. Central serous choroidopathy in pregnancy. Arch Ophthalmol. 1983;101:1055–1058.
137 Gass JDM, Little H. Bilateral bullous exudative retinal detachment complicating idiopathic central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology. 1995;102:737–747.
138 Wakakura M, Ishikawa S. Central serous chorioretinopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1984;68:329–331.
139 Yoshioka H, Katsume Y, Akune H. Experimental central serous chorioretinopathy in monkey eyes: fluorescein angiographic findings. Ophthalmologica. 1982;185:168–178.