Mechanical Ventilation and Noninvasive Ventilatory Support
Perspective
The decision to intubate is discussed in Chapter 1 and in various other places throughout this textbook in the context of individual conditions. This chapter describes the modalities and techniques of noninvasive and invasive mechanical ventilation.
Principles of Mechanical Ventilation
Physiology of Positive Pressure Breathing
Transition from negative pressure breathing to positive pressure breathing affects cardiovascular and pulmonary physiology and can have significant clinical consequences. Pressure changes in the thoracic cavity directly affect pressures in the chambers of the heart. During spontaneous inspiration, venous return and preload are augmented, cardiac output is increased, and there is an increased pressure gradient between the left ventricle and the aorta. With the initiation of positive-pressure ventilation (PPV), venous return is diminished, cardiac output falls, and there is a decreased pressure gradient between the left ventricle and the aorta.1 Relative hypotension can occur after ventilatory support has been initiated, and this may be exaggerated in patients with clinical hypovolemia or vasodilatory states.2
Invasive Mechanical Ventilation
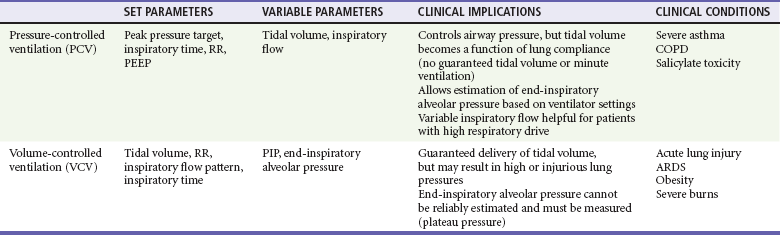
In VCV a breath is defined by delivery of a set tidal volume to the lungs. Inspiratory flow is fixed, and inhalation ends when a preset tidal volume has been delivered; peak inspiratory pressures (PIPs) and end-inspiratory alveolar pressures vary based on lung compliance and set tidal volume. The main benefit to VCV is the ability to control tidal volume and minute ventilation, but it may be problematic by causing high peak pressures when the compliance of the respiratory system is poor. Clinically, poor respiratory system compliance occurs in conditions that increase lung or chest wall stiffness. Such conditions include pulmonary edema, acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), pneumothorax, and obesity.3
When a physician is choosing between pressure-cycled ventilation versus volume-cycled ventilation, it is important to consider the underlying reason for mechanical ventilation. Volume-cycled ventilation should be used when strict control of tidal volume is mandated. Specifically, this includes patients with known ALI or ARDS, in whom low–tidal volume strategies have been proven to reduce mortality.4 In addition, patients with decreased chest wall compliance should be placed on VCV to ensure that adequate tidal volume is delivered. This includes patients with morbid obesity or severe chest wall burns. Conversely, in conditions in which strict control of airway pressure is desired, pressure-cycled ventilation should be used. As detailed earlier, this includes patients with asthma or COPD. In addition, because inspiratory flow is not limited in pressure-cycled ventilation, this strategy may be preferred to volume-cycled ventilation in patients with a high respiratory drive, such as patients with salicylate overdose. For patients who do not require strict control of either pressure or volume, similar ventilation mechanics can generally be achieved with either pressure-cycled or volume-cycled ventilation (Table 2-1).
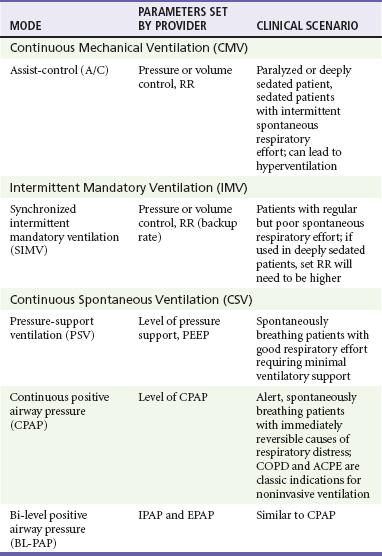
Ventilator mode refers specifically to the amount of respiratory support provided by the ventilator but more commonly represents a combination of the type of breath to be given and the way the breath is to be initiated. The most common ventilator modes can be categorized on the basis of how often the ventilator will initiate a breath for the patient and can be divided broadly into continuous mechanical ventilation (CMV), intermittent mechanical ventilation (IMV), and continuous spontaneous ventilation (CSV).5 CMV and IMV can be delivered via pressure-control or volume-control methods. In CSV, no mandatory breaths are delivered to a patient; the size of the breath is determined by the effort of the patient and can be augmented with applied pressure to the airway. These methods are compared in Table 2-2. Other, more complex modes of ventilation include proportional-assist ventilation (PAV) and airway pressure release ventilation (APRV), though these generally are not used in the emergency department (ED).
CMV is intended to provide full ventilatory support for patients with little or no spontaneous respiratory activity. CMV, also referred to as assist-control (A/C) ventilation, provides a preset number of breaths per minute. In addition to preset breaths, A/C will also deliver a breath in response to patient effort. In this mode, patients can trigger a breath at any rate but will always receive at least the preset number of breaths. Notably, when a patient initiates a breath, he or she receives the full breath as set on the ventilator. For the promotion of ventilator synchrony, a spontaneous patient-initiated breath will take priority over a preset breath, meaning that if the ventilator is set to deliver 12 breaths/min, a breath is provided every 5 seconds in the absence of spontaneous inspiratory effort. When the patient makes a spontaneous effort, the ventilator provides an additional breath and the timer resets for another 5 seconds. A/C ventilation is the most useful initial mode of mechanical ventilation in ED patients, as many patients are initially paralyzed and sedated and do not interact with the ventilator. One of the biggest challenges with A/C ventilation, however, is that patient-initiated breaths are not proportional to patient effort; when inspiratory effort is detected, a full-sized breath is delivered. Clinically, this requires adequate sedation of patients when ventilated in A/C mode to prevent spontaneous respiratory efforts that will result in hyperventilation, air trapping, hypotension, and poor ventilator synchrony.6
Positive End-Expiratory Pressure
Regardless of the ventilatory mode chosen, PEEP is often used during invasive mechanical ventilation. PEEP refers to the maintenance of positive airway pressure after the completion of passive exhalation. During acute respiratory failure, lung volumes are typically decreased; application of PEEP increases functional residual capacity (FRC), improves oxygenation, and decreases intrapulmonary shunt. Use of PEEP also reduces portions of nonaerated lung that may contribute to the development of ventilator-induced lung injury7 Notably, PEEP increases both intrapulmonary and intrathoracic pressure and may affect pulmonary and cardiovascular physiology. Potential adverse effects of PEEP include decreased cardiac output, lung overdistention, and pneumothorax.
Management
General Approach: Noninvasive versus Invasive Ventilation
The decision to intubate carries significant implications for patients, including potentially life-threatening complications related to airway management and subsequent complications related to intensive care unit (ICU) care.8 NPPV is an appealing option for patients requiring ventilatory assistance with potentially reversible conditions when tracheal intubation is not immediately necessary, or as a therapeutic adjunct for patients with “do-not-intubate” directives.9,10 In appropriately selected patients, NPPV obviates intubation in greater than 50% of cases and improves survival.8,11 Need for emergency intubation is a contraindication to NPPV, except as a means to improve preoxygenation in preparation for intubation. Other relative contraindications include decreased level of consciousness, lack of respiratory drive, increased secretions, hemodynamic instability, and conditions, such as facial trauma, that would prevent an adequate mask seal.12,13 If NPPV is initiated, patients should be reassessed frequently for progress of therapy, tolerance of the mode of support, and any signs of clinical deterioration that indicate a need for intubation.
Patients most likely to respond to NPPV in the ED are those with more readily reversible causes of their distress, such as COPD exacerbation or cardiogenic pulmonary edema in which fatigue is a significant factor. Robust evidence suggests benefit of NPPV for both conditions. In patients with acute COPD exacerbations, NPPV decreases the need for subsequent intubation, hospital length of stay, and mortality when compared with standard therapy. Notably, though helpful in most patients with COPD exacerbation, NPPV has been shown to have the largest benefit for patients with hypercapnic acidosis and pH below 7.3.14–17 Treatment failure, defined as subsequent need for intubation, is predicted by a Glasgow Coma Scale score of less than 11, a sustained arterial pH less than 7.25, and tachypnea greater than 30 breaths/min.18
In patients with acute cardiogenic pulmonary edema (ACPE), NPPV reduces the work of breathing while simultaneously improving cardiac output; application of NPPV causes elevations in intrathoracic pressure that decrease both left ventricular (LV) ejection pressure and LV transmural pressure. This results in afterload reduction. In addition, decreases in RV preload may improve LV compliance via ventricular interdependence.1–3,19,20 Compared with standard therapy, multiple studies and several meta-analyses have confirmed decreased need for intubation as well as decreased mortality for patients with ACPE treated with NPPV. Benefits were independent of whether patients received CPAP or BL-PAP, and despite suggestions from early clinical data, no increased rate of acute myocardial infarction occurred in patients receiving any form of NPPV.21–24 Though either modality can be used, a recent ED-based study suggested faster clinical improvement with BL-PAP.25 Specific predictors of failure of NPPV in congestive heart failure (CHF) have not been systematically examined.
Use of NPPV in other patients with respiratory compromise, including asthma and pneumonia, is not well studied, though limited preliminary data suggest that NPPV may be beneficial for patients with acute asthma exacerbations.26–28 Studies have failed to establish a role for NPPV in pneumonia. Although no data suggest harm from NPPV, the presence of pneumonia has been shown to be an independent risk factor for failure of noninvasive ventilation.29–32
Approach to Initial Ventilator Settings
Initial settings for noninvasive ventilation should be determined by the amount of ventilatory assistance required by the patient, as well as patient comfort and cooperation with the therapy. The first consideration in the use of NPPV is whether to provide support in the form of CPAP or BL-PAP. This was discussed earlier, and there is no clear benefit of one over the other. Support will be given by a full-face (oronasal) mask or nasal mask; this choice is determined by patient comfort, feelings of claustrophobia, and the need for the patient to effectively cough or speak. Inspiratory support (IPAP) can be initiated at 10 cm H2O, and expiratory support (EPAP) can be initiated at 5 cm H2O. Subsequent titration of these parameters is based on the patient’s clinical response, particularly pressure tolerance, respiratory rate, and oxyhemoglobin saturation. Though blood gas analysis is confirmatory, improvement in clinical condition can be observed by decrease in work of breathing, good patient-ventilator synchrony, and patient report. If required, EPAP and IPAP can be adjusted by 1 to 2 cm H2O at a time based on clinical response.12 If work of breathing is unchanged, increases in IPAP and EPAP reduce hypercarbia by increasing tidal volume and minute ventilation while increasing oxygenation by combating atelectasis and promoting alveolar recruitment. IPAP greater than 20 cm H2O can be uncomfortable and can cause gastric insufflation and should be avoided.12,33
For the intubated patient, initial ventilator settings should facilitate ventilation that improves gas exchange, promotes ventilatory synchrony, and minimizes the potential for complications. For an apneic or paralyzed patient, full ventilatory support is required; for this reason, A/C is the recommended mode of initial ventilation. Specific required settings depend on whether the patient is receiving PCV or VCV, but the principles underlying selection of settings are similar. Reasonable initial ventilator settings should deliver a tidal volume of 6 to 8 mL/kg of estimated ideal body weight (IBW) at rate of 12 to 14 breaths/min. If VCV is used, tidal volume can be set directly, and if PCV is used, tidal volume is determined by adjusting the targeted pressure to be delivered; initial pressure targets should not exceed 30 cm H2O. Initial FIO2 should be set at 1.0 but generally can be adjusted down quickly to maintain an oxygen saturation of 90% or greater. PEEP is routinely given and is set initially at 5 cm H2O.6 Settings for specific clinical conditions, such as status asthmaticus, are discussed later.
Ongoing Management
Mechanical ventilation requires monitoring and regular adjustment to ensure appropriate gas exchange, safe delivery of desired tidal volume, and prevention of metabolic derangement. Changes to ventilator settings are guided dynamically by multiple factors, including pulse oximetry, end-tidal carbon dioxide (ETCO2) monitoring, ventilation pressures, and blood gases. For the adequacy of ventilation to be monitored, capnography may be used; arterial blood gases should be measured 15 to 20 minutes after initiation of ventilatory support to correlate ETCO2 with PCO2. Notably, venous blood gases generally correlate well with pH and PCO2 of arterial samples,34,35 though this correlation may be unreliable in critically ill patients.36,37 Although there is variation in agreement between capnography and blood gas values, capnography generally correlates well with PCO2 of arterial samples and may be useful for ventilator adjustment after initial correlation has been established.38–41 In the event of uncertainty, arterial blood gases remain the definitive test for evaluating PaO2 and PCO2. Minute ventilation can subsequently be altered by adjusting tidal volume or respiratory rate. To avoid oxygen toxicity, FIO2 should be reduced to the lowest level that provides acceptable (>90%) oxygen saturation. In many instances, increases in PEEP will allow better oxygenation for a given FIO2 but may worsen hypotension or increase intrathoracic pressure.
Other Management Considerations
Aside from specific ventilator management, considerations in the care of the intubated patient include analgesia and sedation, potential neuromuscular paralysis, and secretion management. After intubation, the primary goals of care in the ED are sustained effective ventilation and patient comfort. Intubation causes pain and anxiety for patients, and both analgesia and sedation are required to promote patient comfort and patient-ventilator synchrony.42 In initiating sedation (see later), sedation should be titrated to comfort and therapeutic goals while avoiding both oversedation and undersedation. Desired level of sedation will differ based on patient tolerance and the clinical scenario: assuming comfort is maintained, lighter sedation may be useful in patients requiring serial neurologic examinations, whereas deep sedation maybe more beneficial for patients with severe hypoxemia or ventilator dyssynchrony. Several clinical scales have been established for this purpose; the reliability of the Richmond Agitation-Sedation Scale (RASS) has been validated in multiple studies.43,44 Sedation should be maintained at the highest RASS score at which the patient is comfortable (between 0 and −5) and should be serially readdressed. Any paralyzed patient should remain deeply sedated (Table 2-3).
Table 2-3
The Richmond Agitation-Sedation Scale (RASS)
| SCORE | TERM | DESCRIPTION |
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement, fights ventilator |
| +1 | Restless | Anxious, but movements not aggressive or vigorous |
| 0 | Calm | Alert and calm |
| −1 | Drowsy | Not fully alert, but has sustained awakening (>10 sec) |
| −2 | Light sedation | Briefly awakens with eye contact to voice (<10 sec) |
| −3 | Moderate sedation | Movement or eye opening to voice but no eye contact |
| −4 | Deep sedation | No response to voice but movement or eye opening with physical stimulation |
| −5 | Unarousable | No response to voice or physical stimulation |
Adapted from Ely EW, et al: Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003; 289:2983-2991.
After rapid sequence intubation, additional neuromuscular blocking agents (NBMAs) should generally be used only when poor ventilator synchrony interferes with ventilation sedation and analgesia. This may be particularly true in patients with ARDS, in whom the use of NMBAs has been associated with shorter duration of ventilation and improved mortality.45 With proper sedation and analgesia, however, neuromuscular blockade usually is not required. If needed, longer-acting agents such as rocuronium and vecuronium should be used; note that impaired hepatic or renal function may increase duration of paralysis.
Analgesia is achieved by generous doses of opioid medications; fentanyl and morphine remain the most commonly used agents for analgesia in critically ill patients.46 Opioids are associated with dose-dependent respiratory depression, a side effect that may be particularly beneficial for patients experiencing ventilator dyssynchrony. Both morphine and fentanyl can be used for analgesia, though dosage requirements will vary based on tolerance and drug metabolism. Sedation and analgesia should therefore be titrated with a standard sedation scale, as discussed earlier. Notably, the active metabolite of morphine (morphine-6-glucuronide) is renally cleared and has potent sedative effects. For this reason, fentanyl may be preferred in patients with renal insufficiency. Remifentanil is an ultra-short-acting opiate that is metabolized by nonspecific plasma esterases. The predictable metabolism and short half-life of remifentanil make it an emerging choice for sedation, and ICU data demonstrate decreased duration of ventilation with remifentanil compared with morphine when used for analgesia in the ICU.47
Sedation after intubation can be accomplished via multiple pharmacologic modalities, and though there are limited data about ED sedation practices, lorazepam and midazolam are the most commonly used agents in the ICU.46 Benzodiazepines exert dose-dependent clinical effects by binding γ-aminobutyric acid (GABA) receptors, first producing anxiolysis, then sedation and hypnosis.48 Benzodiazepines also cause respiratory depression, which is potentiated by concomitant opioid administration. For this reason, a sedation regimen of both opioids and benzodiazepines may improve ventilator dyssynchrony while providing an anxiolytic and amnestic effect. Benzodiazepines can be administered either as repeated boluses or by continuous infusion, though in critically ill patients, benzodiazepines have altered pharmacokinetics that result in tissue accumulation and prolonged sedation. This is particularly true in obese patients and in patients with renal or hepatic insufficiency.49–53 For this reason, sedation with benzodiazepines should be attempted with intermittent bolus administration before a continuous infusion is used.
Similar to benzodiazepines, propofol binds to the GABA receptor to induce sedation. Propofol is lipophilic, and the ability of the drug to rapidly penetrate the blood-brain barrier and distribute into peripheral tissues is responsible for both the rapidity and the short duration of clinical effect.54 Unlike benzodiazepines, the clearance of propofol is minimally affected in critically ill patients. In addition, propofol can precipitate hypotension by increasing venous capacitance, a side effect that is exaggerated in hypovolemic patients. For these reasons, propofol should be given as an infusion rather than as a bolus, initiated at low doses (0.1 mg/kg/min) and titrated to desired level of sedation. In comparison to benzodiazepines, continuous infusions of propofol have been demonstrated to decrease duration of mechanical ventilation,55,56 suggesting that propofol may confer benefit when compared with benzodiazepines in the sedation regimen for mechanically ventilated patients.
Other medications for the sedation of ventilated patients in the ED include dexmedetomidine and haloperidol. Dexmedetomidine is a centrally acting alpha2-agonist with sedative and analgesic properties, largely distinguished from other sedative agents by a negligible impact on respiratory drive, even with simultaneous opioid administration. It is administered as a loading dose followed by a continuous infusion and can precipitate bradycardia and relative hypertension.57 Studies have demonstrated dexmedetomidine to be beneficial in facilitating use of noninvasive ventilation, as well as awake fiberoptic intubations.58–60 When dexmedetomidine was compared with continuous infusion of midazolam, a large, multicenter evaluation demonstrated that dexmedetomidine was associated with shorter duration of mechanical ventilation, as well as decreased sedation-associated delirium.61 Although not systematically studied in the ventilated ED patients, dexmedetomidine is an emerging alternative for sedation strategy for critically ill patients and may be considered as an alternative to traditional modalities in clinical settings in which agitation or anxiety limit therapeutic goals.
Haloperidol, commonly used as a sedative for agitated patients, can also be used as an adjunct to traditional sedation regimens in mechanically ventilated patients. The use of haloperidol may be particularly useful in patients who remain acutely agitated after large doses of other sedative medications, especially because it does not affect hemodynamics. Recent limited data have suggested a mortality benefit for mechanically ventilated patients who receive haloperidol, though this requires further study.62 Notably, however, haloperidol does not have any analgesic or amnestic properties and cannot be used as a single therapy for sedation in critically ill patients.
Other ED considerations in the care of the ventilated patient include secretion management and steps to reduce potential ventilator-associated pneumonia (VAP). Management of secretions is achieved via regular endotracheal suctioning, recognizing a balance between secretion clearance and the disruption of ventilation. In addition, a nasogastric or orogastric tube should be placed for gastrointestinal decompression. Finally, evidence demonstrates benefit in the prevention of VAP from placing the patient in the semirecumbent position by elevating the head of the bed.63 Limited data suggest that use of VAP care “bundles,” including elevation of the head of the bed, have decreased incidence of VAP in the ICU; this may warrant further study to determine benefit in the ED.64 A recent meta-analysis also demonstrated a decrease in the incidence of VAP with continuous aspiration of subglottic secretions. This is done via a specialized endotracheal tube for this purpose, and, though not routinely used in the ED, this technique may be a direction for future investigation.65
Complications of Positive-Pressure Ventilation
PPV also has a direct impact on the lungs. Whether delivered as a set volume or set pressure, invasive PPV forcibly distends the lung and can be injurious. Injuries from elevated lung volume or lung pressure are known as volutrauma and barotrauma, respectively, and contribute to the development of VILI. VILI is mitigated by limiting pathologic stretch on the alveoli; current data support that maximum “safe” end-inspiratory alveolar pressures are 30 to 32 cm H2O,4,66–69 though this continues to be actively researched. Barotrauma can also manifest overtly with pneumothorax or pneumomediastinum, but this is relatively uncommon.69
Troubleshooting the Ventilator
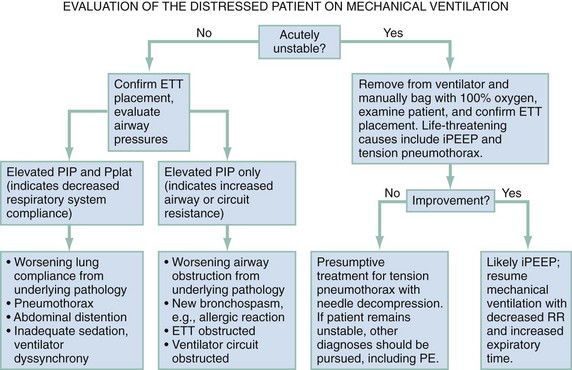
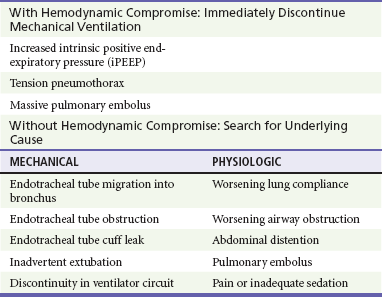
When a patient’s condition suddenly deteriorates during mechanical ventilation, a systematic approach should be applied to immediately assess for life-threatening conditions (Fig. 2-1). The first step in evaluating the ventilated patient who has a change in clinical status is to assess vital signs. Patients with acute hemodynamic compromise or acute hypoxia should be removed from the ventilator and bagged manually on 100% oxygen. Tension pneumothorax, increased iPEEP, and accidental extubation are the most life-threatening concerns in this situation and must be immediately addressed. While the patient is bagged, the chest should be examined to ensure bilateral breath sounds. Changes in breath sounds may indicate a pneumothorax or a migration of the endotracheal tube. Clinical examination, oxygen saturation, and ETCO2 monitoring can be used as surrogates for tube placement, but suspicion of inadvertent extubation should prompt direct visualization. Acute hypotension can be precipitated by extreme elevations in intrathoracic pressure; compromise from iPEEP will improve once the patient has been disconnected from the ventilator, whereas hypotension from a tension pneumothorax will not. If the patient’s condition remains unstable after he or she has been disconnected from the ventilator circuit, a tension pneumothorax should be treated presumptively with needle decompression and eventual chest tube placement. Although unlikely, it is possible that a patient could sustain bilateral pneumothoraces, and this should be considered. If the patient remains unstable after chest decompression, sources for decompensation unrelated to the ventilator must be pursued.
Acute distress without hemodynamic changes can be precipitated by multiple factors, both mechanical and physiologic (Table 2-4). Again, the initial evaluation should begin by confirming position and patency of the endotracheal tube before other diagnoses are investigated, including evaluation of the tracheal balloon. Once tube placement has been confirmed, the next step in evaluation of ventilator-related causes of distress should focus on airway pressures. Acute decreases in PIP indicate discontinuity in the ventilator circuit, which could include inadvertent extubation. Patients with increased PIP can be considered in two categories: those with concomitant increases in Pplat, and those with unchanged Pplat. If both PIP and Pplat acutely increase, this suggests decreased compliance of the respiratory system. Elevated PIP with unchanged Pplat indicates problems with increased airway resistance, either in the lungs or the ventilator circuit. Specific conditions that cause decreased respiratory system compliance or increased airway resistance are detailed in Figure 2-1.
Special Clinical Circumstances
Acute Exacerbation of Chronic Obstructive Pulmonary Disease
Strategies for managing intubated patients with COPD focus on improving gas exchange while minimizing iPEEP. Reduction of iPEEP is achieved by decreasing airway resistance with bronchodilators and corticosteroids and ensuring adequate expiratory time during mechanical ventilation. Adequate expiratory time is achieved by decreasing respiratory rate, tidal volume, and inspiratory time. Adequate oxygenation (saturation of 90%) is achieved while minimizing barotrauma by deliberately reducing minute ventilation, so-called “permissive hypercapnia.” No data suggest the advantage of PCV versus VCV, and either method can be used. The ideal ratio of inspiratory-to-expiratory time (I/E ratio) is variable, but the ratio should initially be set at 1 : 4. Data in asthmatics suggest that expiratory times longer than 4 seconds have minimal impact on airflow.70 iPEEP can also result in poor patient-ventilator synchrony, causing inadequate gas exchange. Initially, deep sedation and analgesia (or sometimes paralysis) are required to prevent ventilator asynchrony and permit effective ventilation. Corticosteroids often are indicated (see Chapter 74). NBMAs are avoided if possible, as patients receiving both NMBA and corticosteroids are at higher risk for “polymyopathy of critical illness” and subsequent increased mortality.71
Status Asthmaticus
Concerns in ventilating the acute asthmatic generally parallel those for patients with COPD, with small notable differences. In acute asthma, respiratory failure is both a result of airway obstruction and airway inflammation. Furthermore, unlike COPD, airway obstruction is much less dynamic and occurs predominantly in large airways. In addition, acute inflammatory changes throughout the lung contribute to decreased lung compliance, which has a direct impact on lung pressures during ventilation.72 Strategies should focus on low respiratory rates with emphasis on maximizing expiratory time. The use of PEEP is debated and is largely thought to contribute to increased lung pressure. Though no data definitively exist supporting VCV over PCV, decreased lung compliance and potential iPEEP may make the delivery of adequate tidal volumes with PCV difficult. This is especially problematic for patients with severe, acute respiratory acidosis for whom adequate ventilation is essential. Recommendations for ventilator settings include VCV with tidal volumes of 6 to 8 mL/kg IBW, respiratory rate of 10 to 15 breaths per minute, and no PEEP.72 Decreased inspiratory time allows greater expiratory time and is achieved by increasing the inspiratory flow rate.
Acute Lung Injury and Acute Respiratory Distress Syndrome
ALI and ARDS are on a spectrum of inflammatory lung disease characterized by heterogeneous noncardiogenic pulmonary edema, hypoxia, and diffuse lung consolidation. Strictly, the difference between these conditions is defined by the ratio of arterial oxygen concentration to the fraction of inspired oxygen (PaO2/FIO2), with ALI being less severe than ARDS. ALI and ARDS can be caused by pulmonary or extrapulmonary inflammation, including VILI. Though epidemiologic data suggest that ALI is not common on initial presentation to the ED, incidence of ALI in ventilated patients is not uncommon.73 The impact of ventilation in the ED on development of lung injury or ARDS is unclear.74,75 Nonetheless, development of VILI has been associated with lung overdistention and alveolar injury,69,76–78 and attention to ventilation strategy in the ED is warranted. Studies confirm that decreased tidal volumes are of clear benefit in the management of patients with ALI and ARDS.4,66,67,79 The majority of studies examining low–tidal volume ventilation strategies, including the landmark ARDSnet trial in 2000, used 6 to 7 mL/kg tidal volumes based on IBW,4,66,69,80 though studies with 7 mL/kg did not demonstrate a difference in mortality. A meta-analysis of these data concluded that tidal volumes below 7 mL/kg and Pplat above 31 cm H2O conferred mortality benefit in patients with ALI or ARDS, though more recent work suggests that low–tidal volume ventilation may improve outcome for patients without lung injury as well.74,81 Level of PEEP in patients with ALI and ARDS continues to be actively researched. Therefore in patients with ALI or ARDS (PaO2/FIO2 < 300), low–tidal ventilation strategy should be used. Initial ventilator settings should be volume cycled, with tidal volumes based on 6 mL/kg of IBW. IBW can also be estimated by height.
Outcomes
Lastly, although the treatment of mechanically ventilated patients usually extends beyond the ED, delays in ICU admission can have significant implications on ED management of the critically ill ventilated patient as the role of emergency physicians extends beyond acute stabilization toward ongoing clinical management.82,83 In addition, when boarding times are long, patients intubated solely for airway protection may be candidates for extubation in the ED if the initial insult has been reversed.
References
1. Pinsky, MR. Cardiovascular issues in respiratory care. Chest. 2005;128(5 Suppl 2):592S–597S.
2. Steingrub, JS, Tidswell, M, Higgins, TL. Hemodynamic consequences of heart-lung interactions. J Intensive Care Med. 2003;18:92–99.
3. Grinnan, DC, Truwit, JD. Clinical review: Respiratory mechanics in spontaneous and assisted ventilation. Crit Care. 2005;9:472–484.
4. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308.
5. Chatburn, RL. Classification of ventilator modes: Update and proposal for implementation. Respir Care. 2007;52:301–323.
6. Bigatello, LM, Massachusetts General Hospital. Critical Care Handbook of the Massachusetts General Hospital, 5th ed. Philadelphia: Lippincott, Williams & Wilkins/Wolters Kluwer Health; 2009.
7. Brower, RG, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336.
8. Demoule, A, Girou, E, Richard, JC, Taille, S, Brochard, L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32:1756–1765.
9. Levy, M, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med. 2004;32:2002–2007.
10. Fernandez, R, Baigorri, F, Artigas, A. Noninvasive ventilation in patients with “do-not-intubate” orders: Medium-term efficacy depends critically on patient selection. Intensive Care Med. 2007;33:350–354.
11. Hess, DR. The evidence for noninvasive positive-pressure ventilation in the care of patients in acute respiratory failure: A systematic review of the literature. Respir Care. 2004;49:810–829.
12. Hostetler, MA. Use of noninvasive positive-pressure ventilation in the emergency department. Emerg Med Clin North Am. 2008;26:929–939.
13. American Thoracic Society, European Respiratory Society, European Society of Intensive Care Medicine, Société de Réanimation de Langue Française. International Consensus Conferences in Intensive Care Medicine: Noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 2001;163:283–291.
14. Keenan, SP, Powers, CE, McCormack, DG. Noninvasive positive-pressure ventilation in patients with milder chronic obstructive pulmonary disease exacerbations: A randomized controlled trial. Respir Care. 2005;50:610–616.
15. Keenan, SP, Sinuff, T, Cook, DJ, Hill, NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med. 2003;138:861–870.
16. Brochard, L, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822.
17. Ram, FS, Picot, J, Lightowler, J, Wedzicha, JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 1, 2004.
18. Confalonieri, M, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25:348–355.
19. Brinker, JA, et al. Leftward septal displacement during right ventricular loading in man. Circulation. 1980;61:626–633.
20. Lenique, F, et al. Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am J Respir Crit Care Med. 1997;155:500–505.
21. Ho, KM, Wong, K. A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: A meta-analysis. Crit Care. 2006;10:R49.
22. Seupaul, RA. Evidence-based emergency medicine/systematic review abstract. Should I consider treating patients with acute cardiogenic pulmonary edema with noninvasive positive-pressure ventilation? Ann Emerg Med. 2010;55:299–300.
23. Winck, JC, Azevedo, LF, Costa-Pereira, A, Antonelli, M, Wyatt, JC. Efficacy and safety of non-invasive ventilation in the treatment of acute cardiogenic pulmonary edema—a systematic review and meta-analysis. Crit Care. 2006;10:R69.
24. Weng, CL, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152:590–600.
25. Nouira, S, et al. Non-invasive pressure support ventilation and CPAP in cardiogenic pulmonary edema: A multicenter randomized study in the emergency department. Intensive Care Med. 2011;37:249–256.
26. Soroksky, A, Stav, D, Shpirer, I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest. 2003;123:1018–1025.
27. Fernández, MM, Villagrá, A, Blanch, L, Fernández, R. Non-invasive mechanical ventilation in status asthmaticus. Intensive Care Med. 2001;27:486–492.
28. Vital, FM, et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 3, 2008.
29. Keenan, SP, Sinuff, T, Cook, DJ, Hill, NS. Does noninvasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? A systematic review. Crit Care Med. 2004;32:2516–2523.
30. Carron, M, Freo, U, Zorzi, M, Ori, C. Predictors of failure of noninvasive ventilation in patients with severe community-acquired pneumonia. J Crit Care. 2010;25:540.
31. Antonelli, M, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: A multi-center study. Intensive Care Med. 2001;27:1718–1728.
32. Confalonieri, M, et al. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1585–1591.
33. Liesching, T, Kwok, H, Hill, NS. Acute applications of noninvasive positive pressure ventilation. Chest. 2003;124:699–713.
34. Chu, YC, et al. Prediction of arterial blood gas values from venous blood gas values in patients with acute respiratory failure receiving mechanical ventilation. J Formos Med Assoc. 2003;102:539–543.
35. Malatesha, G, Singh, NK, Bharija, A, Rehani, B, Goel, A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24:569–571.
36. Zhang, H, Vincent, JL. Arteriovenous differences in PCO2 and pH are good indicators of critical hypoperfusion. Am Rev Respir Dis. 1993;148(4 Pt 1):867–871.
37. Bakker, J, et al. Veno-arterial carbon dioxide gradient in human septic shock. Chest. 1992;101:509–515.
38. Corbo, J, Bijur, P, Lahn, M, Gallagher, EJ. Concordance between capnography and arterial blood gas measurements of carbon dioxide in acute asthma. Ann Emerg Med. 2005;46:323–327.
39. Plewa, MC, et al. Evaluation of capnography in nonintubated emergency department patients with respiratory distress. Acad Emerg Med. 1995;2:901–908.
40. Prause, G, et al. A comparison of the end-tidal-CO2 documented by capnometry and the arterial pCO2 in emergency patients. Resuscitation. 1997;35:145–148.
41. Hess, D, Agarwal, NN. Variability of blood gases, pulse oximeter saturation, and end-tidal carbon dioxide pressure in stable, mechanically ventilated trauma patients. J Clin Monit. 1992;8:111–1115.
42. Kress, JP, Hall, JB. Sedation in the mechanically ventilated patient. Crit Care Med. 2006;34:2541–2546.
43. Sessler, CN, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
44. Ely, EW, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
45. Papazian, L, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116.
46. Soliman, HM, Melot, C, Vincent, JL. Sedative and analgesic practice in the intensive care unit: The results of a European survey. Br J Anaesth. 2001;87:186–292.
47. Dahaba, AA, Grabner, T, Rehak, PH, List, WF, Metzler, H. Remifentanil versus morphine analgesia and sedation for mechanically ventilated critically ill patients: A randomized double blind study. Anesthesiology. 2004;101:640–646.
48. Amrein, R, Hetzel, W, Hartmann, D, Lorscheid, T. Clinical pharmacology of flumazenil. Eur J Anaesthesiol Suppl. 1988;2:65–80.
49. Byatt, CM, Lewis, LD, Dawling, S, Cochrane, GM. Accumulation of midazolam after repeated dosage in patients receiving mechanical ventilation in an intensive care unit. Br Med J (Clin Res Ed). 1984;289:799–800.
50. Oldenhof, H, de Jong, M, Steenhoek, A, Janknegt, R. Clinical pharmacokinetics of midazolam in intensive care patients, a wide interpatient variability? Clin Pharmacol Ther. 1988;43:263–269.
51. Bauer, TM, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet. 1995;346:145–147.
52. Spina, SP, Ensom, MH. Clinical pharmacokinetic monitoring of midazolam in critically ill patients. Pharmacotherapy. 2007;27:389–398.
53. Kollef, MH, et al. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548.
54. Shafer, SL. Advances in propofol pharmacokinetics and pharmacodynamics. J Clin Anesth. 1993;5(6 Suppl 1):14S–21S.
55. Carson, SS, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34:1326–1332.
56. Ostermann, ME, Keenan, SP, Seiferling, RA, Sibbald, WJ. Sedation in the intensive care unit: A systematic review. JAMA. 2000;283:1451–1459.
57. Panzer, O, Moitra, V, Sladen, RN. Pharmacology of sedative-analgesic agents: Dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral mu antagonists. Crit Care Clin. 2009;25:451–469.
58. Bergese, SD, et al. A Phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther. 2010;17:586–595.
59. Bergese, SD, et al. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22:35–40.
60. Takasaki, Y, Kido, T, Semba, K. Dexmedetomidine facilitates induction of noninvasive positive pressure ventilation for acute respiratory failure in patients with severe asthma. J Anesth. 2009;23:147–150.
61. Riker, RR, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009;301:489–499.
62. Milbrandt, EB, et al. Haloperidol use is associated with lower hospital mortality in mechanically ventilated patients. Crit Care Med. 2005;33:226–229.
63. Collard, HR, Saint, S, Matthay, MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med. 2003;138:494–501.
64. Morris, AC, et al. Reducing ventilator-associated pneumonia in intensive care: Impact of implementing a care bundle. Crit Care Med. 2011;39:2218–2224.
65. Muscedere, J, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: A systematic review and meta-analysis. Crit Care Med. 2011;39:1985–1991.
66. Amato, MB, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354.
67. Petrucci, N, Iacovelli, W. Ventilation with smaller tidal volumes: A quantitative systematic review of randomized controlled trials. Anesth Analg. 2004;99:193–200.
68. Hager, DL. Carpal tunnel syndrome in patients more than 65 years old. J Hand Surg Am. 2005;30:866–867.
69. Ramnath, VR, Hess, DR, Thompson, BT. Conventional mechanical ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med. 2006;27:601–613.
70. Leatherman, JW, McArthur, C, Shapiro, RS. Effect of prolongation of expiratory time on dynamic hyperinflation in mechanically ventilated patients with severe asthma. Crit Care Med. 2004;32:1542–1545.
71. Hermans, G, De Jonghe, B, Bruyninckx, F, Van den Berghe, G. Clinical review: Critical illness polyneuropathy and myopathy. Crit Care. 2008;12:238.
72. Medoff, BD. Invasive and noninvasive ventilation in patients with asthma. Respir Care. 2008;53:740–748.
73. Roupie, E, et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. SRLF Collaborative Group on Mechanical Ventilation. Société de Réanimation de Langue Française. Intensive Care Med. 1999;25:920–929.
74. Gajic, O, et al. Early identification of patients at risk of acute lung injury: Evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470.
75. Gajic, O, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824.
76. Hoegl, S, et al. Short-term exposure to high-pressure ventilation leads to pulmonary biotrauma and systemic inflammation in the rat. Int J Mol Med. 2008;21:513–519.
77. Dreyfuss, D, Saumon, G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323.
78. Slutsky, AS. Ventilator-induced lung injury: From barotrauma to biotrauma. Respir Care. 2005;50:646–659.
79. Brochard, L, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158:1831–1838.
80. Villar, J, Kacmarek, RM, Pérez-Méndez, L, Aguirre-Jaime, A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: A randomized, controlled trial. Crit Care Med. 2006;34:1311–1318.
81. Sundar, S, et al. Influence of low tidal volume ventilation on time to extubation in cardiac surgical patients. Anesthesiology. 2011;114:1102–1110.
82. Wood, S, Winters, ME. Care of the intubated emergency department patient. J Emerg Med. 2010;40:419–427.
83. McCarthy, ML, et al. Crowding delays treatment and lengthens emergency department length of stay, even among high-acuity patients. Ann Emerg Med. 2009;54:492–503.