Mechanical Ventilation
Introduction
Mechanical ventilation (MV) is an important tool for resuscitation of critically ill patients in the emergency department (ED). It is vital that ED practitioners have a thorough understanding of the basics of MV and to know when to apply these principles and how to support patients in respiratory or cardiac failure. Hospital overcrowding has led to a delay in transfer of mechanically ventilated patients out of the ED, and ventilator management often falls on the emergency medicine physician. Also, during nights and weekends in some facilities, the ED physician may be called on to troubleshoot or stabilize mechanically ventilated patients in the intensive care unit (ICU). The traditional view of MV as a prescription that fits virtually all patients equally should be discarded as a gross misunderstanding of pulmonary pathophysiology. Increasing evidence has shown that the mechanism of lung ventilation by MV may be as deleterious as it is helpful.1 Because patients remain in the ED while mechanically ventilated, ED clinicians should embrace the established paradigm of pulmonary-protective MV strategies as a cornerstone of care.
Basic Physiology
Minute Volume and Alveolar Ventilation
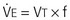
Normal 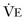 is 7 to 10 L/min. Vt can be further broken down into alveolar volume (Va) and dead space volume (Vds):
is 7 to 10 L/min. Vt can be further broken down into alveolar volume (Va) and dead space volume (Vds):
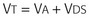
In healthy young persons, the anatomic dead space is accounted for by the trachea and the larger airways and is approximately 2.2 mL/kg lean body weight. In disease states, in addition to the anatomic dead space, there is a variable amount of “pathologic” dead space, which corresponds to ventilated alveoli and respiratory bronchioles that are not adequately perfused. The sum of anatomic and pathologic dead space is often referred to as physiologic dead space.
Alveolar minute ventilation (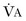 ) is the product of rate times Vt minus dead space:
) is the product of rate times Vt minus dead space:
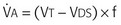
 and the rate of CO2 production by the body determine the partial pressure of CO2 in the alveoli (Paco2), which is approximately equal to systemic arterial CO2 tension.
and the rate of CO2 production by the body determine the partial pressure of CO2 in the alveoli (Paco2), which is approximately equal to systemic arterial CO2 tension.
Volume-Pressure Relationship
Conversely, this relationship also holds for volume. For example, a pressure of 20 cm H2O will create a certain volume based on the compliance of the system. Increasing pressure will result in higher volume. Decreasing pressure will result in lower volume. Decreasing compliance will result in lower volume. Increasing compliance will result in higher volume.
Airway Pressures
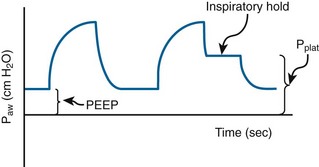
Plateau pressure is measured at the end of inspiration with a short breath-hold (Fig. 8-1). At this point no airflow should be occurring. This is considered a static pressure. By understanding the aforementioned volume-pressure relationship, one can easily deduce how plateau pressure is inversely related to respiratory system compliance and directly related to volume. Anything that decreases compliance will increase plateau pressure. Increasing compliance will decrease plateau pressure. Decreasing volume will decrease plateau pressure (a major tenet in lung-protective ventilation).
Peak Airway Pressure
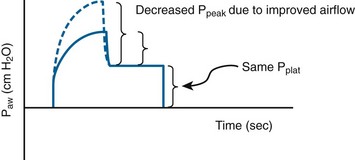
A physiologically appropriate means of detecting and monitoring bronchospasm is the peak-plateau gradient. A normal gradient is less than 4 cm H2O pressure, and elevated values indicate increased airway resistance. The efficacy of treatment with β2-agonists, steroids, intravenous magnesium, or diuresis may be assessed by monitoring the changes in this gradient (Fig. 8-2).
Positive End-Expiratory Pressure
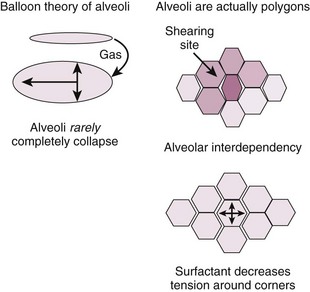
Extrinsic PEEP: When discussing MV and PEEP, most often authors are referring to extrinsic PEEP (PEEPe). This is also referred to as applied PEEP. It is the PEEP that is extrinsically applied by the ventilator. When PEEP is used without a subscript in this chapter, it refers to PEEPe. The useful PEEP range is from 3 to 20 cm H2O.2 PEEP is used to increase FRC and move the zero pressure point of each alveolar unit more proximally in the airway and thereby prevent early alveolar collapse.3 By so doing, PEEP increases the available number of alveolar units that can participate in gas exchange. The primary effect of PEEP on gas exchange is improvement in oxygenation, not removal of CO2. CO2 clearance is rather efficient and will be well preserved, even in hypoxic situations. By opening one alveolar unit, the tendency of adjacent units is to open as well (i.e., alveolar codependency) (Fig. 8-3).4 Excessive PEEP will compromise hemodynamics. There are two primary questions to ask when using PEEP to augment oxygenation: (1) What is the “optimal PEEP” and (2) Is the current amount of PEEP compromising the patient’s hemodynamics?
PEEP is not without untoward side effects, and increased levels of PEEP can lead to lung injury and hemodynamic compromise.5 Increased intrathoracic pressure can result in cardiac compression and collapse, principally of the right atrium. It is imperative that the patient be adequately volume-resuscitated because preload depletion compounds this problem. Desired levels of PEEP simply may not be possible because of deleterious effects on cardiac output.
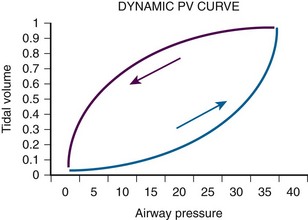
Another excellent method of determining the optimal PEEP is guided by assessing changes in plateau pressure with changes in PEEP. As PEEP is increased from a minimal level, the patient’s peak airway pressure and plateau pressure will increase by the amount of PEEPe. When the optimal PEEP for the lung units is achieved, plateau pressure will no longer increase. As the lung is optimally recruited, peak and plateau pressure may decrease because more volume of lung is available to receive a set Vt. Once this level is exceeded, there will be further increases in plateau pressure beyond the incremental increase in PEEP as the units overdistend. Therefore, the clinician must readily identify the plateau in this plateau pressure trend. The same relationship may be displayed graphically in the dynamic pressure-volume loop (Fig. 8-4). The lower limb of the loop represents the pressure required to open the alveolar units.6,7 In the absence of PEEP (or inadequate PEEP), this limb is prolonged and flattened and has an inflection point far to the right of the origin of the loop (Fig. 8-5). As PEEP is progressively increased, the inflection point travels to the left. When the optimal PEEP is achieved, there will be a rapid upstroke of the loop because the vast majority of the functional lung units are already open and ready to be ventilated (see Fig. 8-4). This strategy is known as the open lung model of MV.6
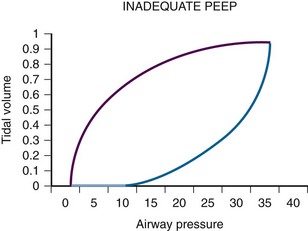
Figure 8-5 Inadequate positive end-expiratory pressure (PEEP) and the pressure-volume loop. Compare this curve with that in Figure 8-4. Note that the loop is initially flat (lower segment) along the x-axis. Once airway pressure is high enough to open the alveolar units, each increase in airway pressure is matched by a corresponding increase in tidal volume.
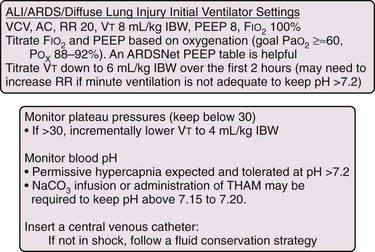
Irrespective of what technique is used, it is currently widely agreed that plateau pressure should not exceed 30 cm H2O. If respiratory system compliance is so low that plateau pressure exceeds 30 cm H2O, either PEEP or Vt has to be decreased. If this is not possible because of either recalcitrant hypoxia or acidosis, rescue therapies may need to be used (see the section “Acute Lung Injury and Acute Respiratory Distress Syndrome”).
Intrinsic PEEP: Intrinsic PEEP (PEEPi) is additional pressure that is generated within the airways from trapped gas that should have been exhaled but for various reasons (commonly obstruction to exhalation such as in chronic obstructive pulmonary disease [COPD]) was not. PEEPi is also referred to as auto-PEEP, dynamic hyperinflation, and breath stacking. For the remainder of this chapter it will be referred to as PEEPi.
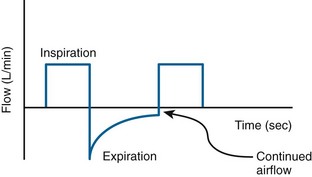
PEEPi can cause hemodynamic instability secondary to decreased venous return, just like high levels of PEEP.7 PEEPi may be detected in two ways: (1) evaluation of the flow-time trace or (2) disconnection of the patient from the ventilator and listening for additional exhaled gas after an exhalation should have occurred.6 The flow-time trace will demonstrate that the exhalation is not yet completed before the next breath has been initiated (Fig. 8-6).8
Equipment—standard Options
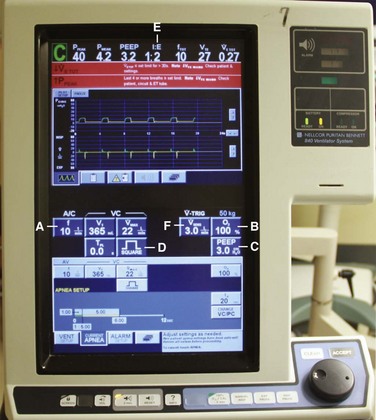
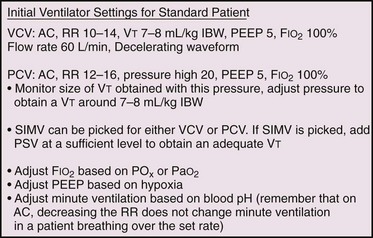
Perhaps one of the most confusing aspects of MV is the plethora of terms and acronyms that are used. Understanding the basic terminology helps clarify this subject. The following discussion explores machine features and settings. Regardless of which ventilator is used, a limited number of standard features are common to each (Fig. 8-7).
Fraction of Inspired Oxygen
All ventilators can deliver an adjustable fraction of inspired oxygen (Fio2). Recommendations are to set it initially at 1.0 because the act of transitioning from negative pressure ventilation (normal physiologic breathing) to positive pressure ventilation (PPV) may unpredictably alter ventilation-perfusion (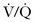 ) matching. Although initially an Fio2 of 100% is optimal, it is beneficial to quickly titrate Fio2 down because of the theoretical risk for oxygen toxicity. Make adjustments based on ABG analysis or pulse oximetry, with a goal of keeping arterial Po2 higher than 60 mm Hg or arterial oxygen saturation at 88% to 92% to avoid potential oxygen toxicity (see Table 3-3 in Chapter 3). Such adjustments may best be accomplished in the ICU rather than the ED, after the entire clinical scenario can be analyzed and all interventions are appropriately adjusted.
) matching. Although initially an Fio2 of 100% is optimal, it is beneficial to quickly titrate Fio2 down because of the theoretical risk for oxygen toxicity. Make adjustments based on ABG analysis or pulse oximetry, with a goal of keeping arterial Po2 higher than 60 mm Hg or arterial oxygen saturation at 88% to 92% to avoid potential oxygen toxicity (see Table 3-3 in Chapter 3). Such adjustments may best be accomplished in the ICU rather than the ED, after the entire clinical scenario can be analyzed and all interventions are appropriately adjusted.
Positive End-Expiratory Pressure
PEEPe is typically set at 5 to 8 cm H2O. Most patients should be started at a PEEP of 5 cm H2O, which is considered a physiologic level. It is used to offset the gradual loss of functional residual volume (FRC) in supine, mechanically ventilated patients. PEEP can be increased by 2 cm H2O every 10 to 15 minutes as needed or tolerated by patients who remain hypoxic. The initial goal is to reduce Fio2 to nontoxic levels. This goal is coming under increasing scrutiny as new information challenges the time frame and concept of O2-induced lung injury at Fio2 levels greater than 0.6.9 Exercise care when using PEEP levels higher than 8 cm H2O in the setting of elevated intracranial pressure (ICP),10 unilateral lung processes, hypotension, hypovolemia, or pulmonary embolism. High PEEP can potentially lead to hypotension as it increases intrathoracic pressure and decreases venous return and subsequently cardiac output.
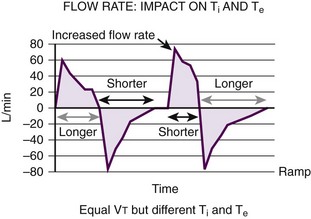
I/E Time Ratio
The normal I/E ratio in a spontaneously breathing, nonintubated patient is 1 : 4.11 Intubated patients commonly achieve I/E ratios of 1 : 2. Shorter ratios may lead to decreased exhalation by compromising Te. In its extreme form, inverse ratio ventilation (IRV), the normal pattern of breathing is reversed. A longer time is spent in inhalation to allow more time for oxygenation and recruitment. The decrease in Te can lead to air trapping, elevated mean airway pressure, and rising Pco2. These problems lead to hypercapnia, respiratory acidosis, and PEEPi12 (Fig. 8-8).
Sensitivity
This refers to the sensitivity of the trigger. If the trigger is set too high (not sensitive enough), the work of breathing incurred by the patient can be substantial. Some providers have been known to set the sensitivity at a high level if the patient is markedly overbreathing the set rate. This is not recommended because it causes an undue increase in the work of breathing. Many ventilators are set to a pressure trigger with a sensitivity of 1 to 3 cm H2O.13 If the sensitivity is set too low (too sensitive), the ventilator can “auto-trigger” (inappropriate initiation of machine-generated breaths) because of oscillating water in the ventilator tubing, hyperdynamic heartbeats, or patient movement.
Modes of Ventilation
Once some of the standard features are understood, the next step is determining the ventilator’s target. Most ventilators can be set to achieve spontaneous breathing, volume-targeted ventilation, pressure-targeted ventilation, or some combination. In volume-targeted ventilation, the ventilator is set to reach a determined volume regardless of the pressure required to do so. Pressure-targeted modes are set to reach a determined pressure regardless of the volume generated. Dual modes combine the benefits of both strategies (Fig. 8-9).
Pressure-Cycled Ventilation
An advantage of PCV is that airway pressure is tightly managed to limit or eliminate alveolar overdistention and to reduce ventilator-induced lung injury.14 It should be noted that the clinician does not control waveform or peak inspiratory flow. Patients can generate their desired flow rate and thus reduce air hunger. Pressure-targeted modes, which are growing in popularity, might have better pressure distribution, improved dissemination of airway pressure, and greater distribution of ventilation.14
Modes of Ventilation Commonly Used in the ED
Assist/Control Ventilation
Caution should be exercised to avoid auto-PEEP (also known as breath stacking) when using volume-targeted AC modes. Because each mechanically delivered breath is given at full Vt, patients with a high actual respiratory rate on AC may not have sufficient time to completely exhale between breaths. This results in progressive air trapping, which leads to an increase in auto-PEEP (PEEPi) (see Fig. 8-6). This is of clinical concern in patients with asthma, in whom auto-PEEP can significantly reduce cardiac output and even promote cardiovascular collapse.
Synchronized Intermittent Mechanical Ventilation
SIMV provides breaths at a preset rate (machine breath), similar to the AC mode. The patient can initiate an additional spontaneous breath between the mandated or preset number of ventilator-supported breaths. Such spontaneous breaths above the preset ventilatory rate are not supported by the ventilator, and the patient receives only a spontaneous Vt that reflects the depth and time spent in the patient-controlled inspiration. For each of these nonmandatory (i.e., spontaneous) breaths, the patient has a high work of breathing. SIMV is typically partnered with PSV to aid in spontaneous breathing support and to overcome the intrinsic resistance associated with MV. This mode was initially recommended by those who thought that as a patient’s need for mechanical ventilatory support decreased, the set respiratory rate could be decreased and the patient “weaned” to PSV alone and ensuing extubation. Subsequent data have shown that this method of liberation actually increases the number of ventilator days.15 The synchronized version of intermittent MV allows the ventilator to attempt to coordinate spontaneous and machine breaths to prevent it from delivering a scheduled breath on top of a spontaneous breath or during exhalation after a spontaneous breath. This could lead to elevated mean airway pressure, alveolar overdistention, and biotrauma.16
Advanced Modes of Mechanical Ventilation
Advanced modes of mechanical ventilation use a closed-loop ventilator logic that combines the features of volume- and pressure-targeted ventilation (Box 8-1). These modes automatically alter control variables, either breath to breath or within a breath, to ensure a minimum Vt or minute ventilation.17 Detailed explanation of these modes is beyond the scope of this chapter. If these modes are encountered, one should discuss options with a respiratory therapist and critical care medicine specialist.
Other Modes
High-frequency ventilation (HFV) attempts to achieve adequate gas exchange by using asymmetric velocity profiles when combining very high respiratory rates with Vt levels that are smaller than the volume of anatomic dead space. It is used more commonly in neonates and infants with neonatal respiratory failure. There has been renewed interest in using HFV in adult patients with ALI or ARDS under the rationale that the small Vt may cause less ventilator-associated lung injury. More trials are necessary to determine whether HFV can improve mortality outcomes in these patients.18
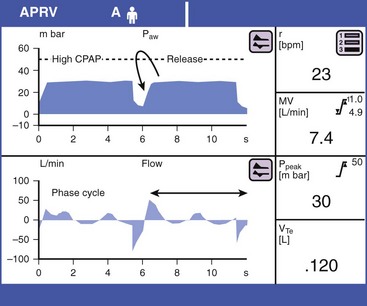
Airway Pressure Release Ventilation and Bi-Level Ventilation
Both these modes are proprietary names yet function in essentially the same manner. The clinician sets a pressure high, a pressure low, and a time at each level (time high and time low). Although at first glance this appears to be similar to PCV, it differs markedly in that the majority of time is spent at pressure high with brief periods at pressure low. The patient typically spends 4 to 6 seconds in time high. Pressure high may be as high as 40 cm H2O or greater. Ventilation occurs during the release from pressure high to pressure low. Time low is typically 0.2 to 0.8 second in restrictive lung disease and 0.8 to 1.5 seconds in obstructive lung disease. It is probably prudent to start at 0.8 and titrate to meet individual patient requirements. Time low is also referred to as the release phase.19 The long time that high-level pressure is maintained achieves oxygenation, and the short release period achieves clearance of CO2 (Fig. 8-10). The long time at high-level pressure results in substantial recruitment of alveoli from markedly different regional time constants at rather low gas flow rates. The establishment of PEEPi by the short release time enhances oxygenation. CO2 clearance is aided by recruitment of the patient’s lung at close to total lung capacity. Elastic recoil creates large-volume gas flow during the release period.
In a paralyzed patient, airway pressure release ventilation and bilevel ventilation (APRV/Bi-Level) are identical to pressure-targeted IRV. For these reasons, some have described this mode as inverse ratio ventilation. A major difference between APRV/Bi-Level and IRV is that IRV typically requires chemical paralysis or heavy sedation. APRV/Bi-Level is a fundamentally different mode from cyclic ventilation. This mode allows the patient to spontaneously breathe during all phases of the cycle, thus making it relatively more comfortable and reducing the level of sedation or paralysis needed. This mode is enabled to succeed by having a floating valve that is responsive to the patient’s needs, regardless of the location within the respiratory cycle. The patient is allowed to breathe in or out during the pressure high phase and during the release phase. Accordingly, the sequence is called a phase cycle. There is no set Ti or Te and no readily identifiable respiratory rate in the traditional sense. During the pressure high phase, patients may exhale 50 to 200 mL or more of gas as the lung volume becomes full of gas. This is not a full exhalation, and the release of excess gas should not be counted as a breath. APRV has been used successfully for neonatal, pediatric, and adult forms of respiratory failure. It is considered an alternative open–lung model approach to MV.19
Given the spontaneous nature of the mode, there should be virtually no need for continuous infusion of neuromuscular blocking drugs in patients placed on this mode of ventilation. This may result in a shorter length of ICU stay and a reduced incidence of prolonged neuromuscular blockade syndrome. The need for sedatives is reduced because patients are more comfortable on this spontaneous mode than on cyclic ventilation.20 APRV/Bi-Level has gained popularity in patients with hypoxemic respiratory failure because it improves oxygenation by optimizing alveolar recruitment and 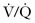 matching.21
matching.21
A common mistake with this mode is setting time low too long. This essentially mimics a pressure-targeted SIMV strategy. Transport of patients on APRV with pressure high greater than 20 cm H2O should occur with the patient attached to the ventilator instead of being hand-ventilated.22 Hand ventilation is unable to match the manner of gas delivery and pressure dynamics that the patient requires. Attempts at hand ventilation, even with an appropriately set PEEP valve, are frequently complicated by unexpected hypoxemia and hemodynamic instability.
Noninvasive Positive Pressure Ventilation
Nonetheless, negative pressure or “iron lungs” were the mainstay of ventilatory support for patients with chronic respiratory failure until as late as the mid-1980s. In the early 1980s, nasal continuous positive airway pressure (CPAP) was introduced to treat obstructive sleep apnea. These tightly fitting masks proved to be an effective means of assisting ventilation, and noninvasive positive pressure ventilation (NPPV) quickly displaced traditional negative pressure ventilation as the treatment of choice for chronic respiratory failure in patients with neuromuscular and chest wall deformities. Current NPPV devices are able to provide a set respiratory rate, set Vt, and set Fio2. The use of NPPV has also been integrated into the acute inpatient setting, where it is now used to treat acute respiratory failure.23
Definitions
As its name suggests, CPAP supplies continuous positive pressure via a tightly fitting face mask (Fig. 8-11). NPPV or bilevel provides an inspiratory positive airway pressure (IPAP) in addition to end-expiratory positive airway pressure (EPAP), and breaths are usually triggered by the patient (Fig. 8-12). On many such devices, backup rates may be set that deliver bilevel pressure, even if patients fail to initiate a breath.
Rationale for Using NPPV
The most important advantage of NPPV is avoiding the complications associated with invasive MV. It has been well documented that invasive MV increases the incidence of airway and lung injury and augments the risk for nosocomial pneumonia. NPPV avoids these complications by keeping the upper airway defense mechanisms intact and allows the patient to retain the ability to eat, clear secretions, and communicate normally when NPPV is used intermittently (NIPPV).23 NPPV has the potential to reduce the mortality of a selected group of patients with acute respiratory failure and may shorten hospital stays, thereby reducing cost. Specific to the ED, appropriate initiation of NPPV may avoid unnecessary intubation of certain patients, hence avoiding ICU admission, reducing cost, decreasing complications, and improving mortality.
Pathophysiologic Effects of NPPV
CPAP increases alveolar recruitment and size, thereby enhancing the area available for gas exchange, and improves the 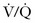 relationship. The term CPAP is synonymous with PEEPe and bilevel EPAP. It can also negate the effects of PEEPi. In patients with dynamic hyperinflation (such as asthma or COPD), an escalating PEEPi increases the magnitude of the drop in airway pressure that the patient must generate to trigger a breath. This causes increased work of breathing for the patient. Careful application of PEEPe can reduce this gradient and decrease the patient’s work of breathing. PPV also creates an increase in intrathoracic pressure. This causes preload to decrease as a result of diminished venous return and also decreases transmural pressure, which reduces afterload.24
relationship. The term CPAP is synonymous with PEEPe and bilevel EPAP. It can also negate the effects of PEEPi. In patients with dynamic hyperinflation (such as asthma or COPD), an escalating PEEPi increases the magnitude of the drop in airway pressure that the patient must generate to trigger a breath. This causes increased work of breathing for the patient. Careful application of PEEPe can reduce this gradient and decrease the patient’s work of breathing. PPV also creates an increase in intrathoracic pressure. This causes preload to decrease as a result of diminished venous return and also decreases transmural pressure, which reduces afterload.24
In NPPV, IPAP is similar to pressure support and, when combined with EPAP, further augments alveolar ventilation, thereby allowing some rest of the respiratory muscles during the inspiratory phase (Box 8-2).
Acute Exacerbation of Chronic Obstructive Pulmonary Disease: Numerous studies have shown that NPPV can reduce the need for intubation, length of hospital stay, and in-hospital mortality in patients with acute exacerbations of COPD.25,26 NPPV should be initiated early and along with standard medical therapy. If it is started late, after the failure of medical treatment, the benefits conferred by NPPV (hospital mortality, length of ICU stay, number of days on the ventilator, overall complications) are eliminated.27 Early implementation of NPPV in patients seen in the ED with an acute exacerbation of COPD and without contraindications should be considered the standard of care.
Acute Cardiogenic Pulmonary Edema: CPAP has been shown to produce a reduction in the rate of intubation and a trend toward decreasing mortality.28 CPAP and NPPV both reduce the risk of intubation in the ED.29 An early article described an increased rate of acute myocardial infarction in patients with acute cardiogenic pulmonary edema treated with NPPV,30 but several subsequent trials have refuted this increased risk for myocardial infarction.
Hypoxemic Respiratory Failure: Although some of the literature suggests that NPPV may be beneficial in the setting of acute hypoxemic respiratory failure, doubt still exists. A large multicenter trial of NPPV in patients with acute hypoxemic respiratory failure that excludes patients with cardiogenic pulmonary edema and COPD may help clarify the use of NPPV in this setting.
Immunosuppressed Patients: When faced with a severely immunosuppressed patient with acute hypoxemic respiratory failure in the ED, early initiation of NPPV may be beneficial in avoiding the serious complications of ET intubation. NPPV can keep upper airway defenses intact and minimize the risk for ventilator-associated pneumonia, which is universally fatal in these patients.31 NPPV has been shown to be associated with a lower rate of ET intubation, shorter ICU stay, and lower ICU mortality. In-house mortality did not differ significantly.32
“Do-Not-Intubate/Do-Not-Resuscitate” Patients: In do-not-intubate/do-not-resuscitate (DNR/DNI) patients willing to undergo NPPV, success would be measured by improved ventilation, oxygenation, and comfort. NPPV can provide support for the patient while the underlying cause of the respiratory failure is being treated. NPPV should be discontinued if it is not producing the desired response or if the patient is unable to tolerate this therapy. In these circumstances it should be a joint decision between the health care team, the patient, and the family to limit NPPV and transition toward comfort measures.
In patients who have chosen to forego any life-sustaining therapy and are receiving comfort care measures, NPPV might be used as a form of palliative care in an attempt to reduce the associated dyspnea. In this circumstance, the use of NPPV is considered successful if it alleviates the patient’s symptoms. If it causes any discomfort to the patient, it should be discontinued. This use of NPPV is controversial, and no studies have assessed the benefits of NPPV in these patients.33 Another use of NPPV in patients who have chosen comfort care measures is a time-limited trial to achieve the goal of survival until the arrival of family and friends. In this situation, NPPV would be used to provide life support until friends and family can achieve closure.
Initiation of NPPV
There is no standard approach to the initiation of NPPV. Different methods have been used in clinical trials, yet these methods have never been compared. There are two main strategies: a high-low approach and a low-high approach (Fig. 8-13).
Cautions with the Use of NPPV
Most studies involving NPPV have excluded patients who were hemodynamically unstable, had an altered level of consciousness, or were unable to protect their airway. This was based on the concern that a depressed sensorium would predispose the patient to aspiration. The International Consensus Conference in Intensive Care Medicine on Noninvasive Positive Pressure Ventilation in Acute Respiratory Failure held in April 2000 considered the presence of severe encephalopathy, as manifested by a Glasgow Coma Scale score lower than 10, to be a contraindication to NPPV.34
Other accepted contraindications to NPPV are listed in Box 8-3.
Recently, studies have looked specifically at the use of NPPV in patients with hypercapnic coma secondary to acute respiratory failure. This was based on the observation that some do-not-intubate patients who declined intubation had successful outcomes with NPPV therapy despite their initial comatose state. Diaz and colleagues conducted an observational study and found that success rates were comparable between the comatose and noncomatose group.35 Scala and coworkers performed two studies, both showing that NPPV could be used successfully in treating patients with exacerbations of COPD and hypercapnic encephalopathy. Their 2007 study showed that performance of NPPV by an experienced team led to similar short- and long-term survival, fewer nosocomial infections, and shorter durations of hospitalization than in patients who underwent MV.36,37 Of note, these studies were conducted in the ICU35 or specialized respiratory care units37 with a nursing ratio of at least 1 : 3. The patients were very closely monitored by staff while they received NPPV. This high nursing-to-patient ratio may not be feasible in a busy ED, however.
High-Flow Nasal Cannula
The high-flow nasal cannula (HFNC) is a relatively new oxygen delivery system. A conventional nasal cannula uses a low-flow system and at higher flow (>6 L/min) can cause nasal dryness, epistaxis, and patient discomfort. The HFNC system (Fig. 8-14) is a novel device that combines oxygen, pressurized air, and warm humidification to deliver tolerable flow of up to 40 L/min through a nasal cannula. Fio2 and flow rates can be adjusted. With higher flow, less room-air entrainment occurs, and the higher flow rates match the dyspneic patient’s increased minute ventilation.
Its use has been studied more extensively for neonatal respiratory care, where ongoing studies suggest that HFNC may be as effective as nasal CPAP in preterm neonates.38 One study looked at the effect of HFNC on exercise performance in adults with COPD.39 Currently, there are no published studies that have investigated the use of HFNC in patients with acute respiratory failure. Anecdotally, this system has been used with some success in adults in the ICU, with immunosuppressed patients being targeted in the hope of avoiding intubation. It seems to be better tolerated than NPPV, and at higher flow it is believed to provide a certain amount of continuous positive pressure. More studies will have to be performed before this technology becomes a mainstay of treatment in patients with acute respiratory failure.
Neuromuscular Blockade/Paralyzing Agent for Mechanically Ventilated Patients
The long-term use of chemical paralysis in the ICU has greatly diminished because of prolonged recovery secondary to drug and metabolite accumulation. Cases of prolonged paralysis, also known as critical illness myopathy, postparalysis syndrome, polyneuropathy of critical illness, and acute quadriplegic myopathy syndrome, lead to protracted ICU stays.40 In the ICU, daily discontinuation of NMBAs for a few hours or avoiding their use entirely has been recommended to potentially decrease the incidence of these conditions. When clinically feasible, it is advisable to stop administration of the NMBA in the ED and reassess the need for continued paralysis.
A number of drugs are available, but the nondepolarizing agents vecuronium and pancuronium are the most commonly prescribed agents for short-term paralysis in the ED.41 Both are given by intermittent bolus administration as needed. Continuous infusion, though potentially useful, is rarely used or indicated in the ED. Nondepolarizing NMBAs can be reversed by neostigmine at a dose of 0.035 to 0.07 mg/kg.
Sedation
See Box 8-4 for common sedatives and analgesics. It is recommended that only the minimal amount needed to achieve comfort be used. Sedation should be targeted to a specific sedation scale (such as the Richmond Agitation Sedation Scale). Agents with a quick offset are preferable to improve spontaneous awakening and for breathing trials once the patient is a candidate for extubation. Benzodiazepines should be avoided if possible. If paralyzing drugs are used for neuromuscular blockade, it is imperative to provide aggressive sedation because pain and anxiety in paralyzed patients cannot be evaluated. It is prudent to conclude that a paralyzed ventilated patient is awake and can hear and feel unless sedated.
Specific Disease Processes
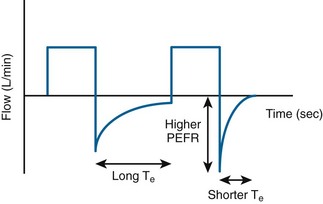
As asthma and COPD treatment (β-agonists, steroids) takes effect, peak pressure will begin to lower, the peak expiratory flow rate should increase, and the expiration flow time should shorten (Fig. 8-16). It is necessary to adequately sedate these patients because their hypercapnic state is a powerful stimulus to breath rapidly. Opiates such as fentanyl and sedatives such as propofol and ketamine have gained increased roles in these patients. Occasionally, one will be required to chemically weaken these patients with the use of paralytics to keep their respiratory rate and expiratory time controlled. This should be a final option and be done with the understanding that the side effects of steroids and paralytics can be quite devastating,42,43 but paralysis cannot be avoided in certain cases. In the ICU, paralysis is commonly titrated to an effect monitored by a peripheral nerve monitor applied over the ulnar or other peripheral nerve distribution.44 No blockade results in four twitches of the adductor pollicis muscle causing four supramaximal triggering stimuli; complete blockade yields no response. A common goal of blockade is to use enough of the agent to result in two twitches out of a “train of four.” A peripheral nerve monitor is not commonly used in the ED and is not a standard intervention during resuscitation and interim ED care.
Valuable information can be gained from flow-time curves and pressure-time curves. Improvement (Fig. 8-16) or worsening of airway obstruction and air trapping (see Fig. 8-6) can often be evident in these curves before becoming clinically apparent. In ideal situations, PEEPi can be measured with an end-expiratory hold. This measurement is often inconsistent and difficult to obtain. Some authors have recommended that PEEPe be applied at 80% of the measured PEEPi.45 Because of the difficulty in accurately measuring PEEPi and the potential hazard of adding too much PEEPe,46 others have suggested that it should be set at 50% of the measured PEEPi. If PEEPi becomes severe enough, it will begin to affect plateau pressure. Current recommendations for obstructive airway disease are to keep plateau pressure below 35 cm H2O,47,48 but many clinicians follow the ALI/ARDS recommendations and maintain plateau pressure at less than 30 cm H2O.
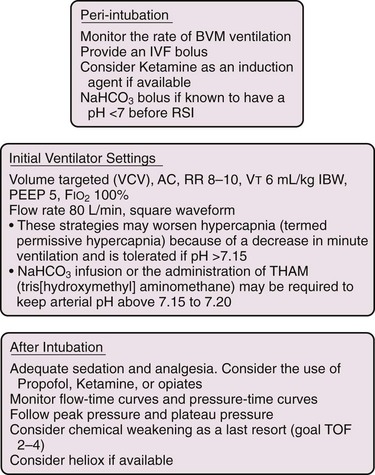
Though seemingly counterintuitive, decreasing the respiratory rate and Vt in a critically ill asthmatic may be beneficial and result in an acceptable elevation in Pco2, termed permissive hypercapnia.49 Hence, it may not be advisable to meet arbitrary Pco2 levels in a ventilated asthmatic but rather concentrate on maintaining acceptable oxygenation (Po2 >60 mm Hg, oxygen saturation of 88% to 92%) while minimizing PEEPi and optimizing plateau pressure.
If cardiovascular collapse occurs in a ventilated asthmatic with either pulseless electrical activity or sudden hypotension, a first step in troubleshooting is to remove the patient from the ventilator. This is both a diagnostic and a therapeutic maneuver for air trapping. Some clinicians also advocate fluid loading and rapid and deep chest compressions while the patient is disconnected from the ventilator to expel the excess volumes of air trapped by prior aggressive ventilation (Fig. 8-17).50 Tension pneumothorax must also be considered (see below).
Complications of MV
Pneumothorax
Pneumothorax that is not associated with trauma in a mechanically ventilated patient typically stems from alveolar overdistention (continuous or episodic) and leads to alveolar rupture and escape of gas into the pleural space.51 In patients receiving PPV, it is wise to drain the pleural space to prevent a simple pneumothorax from progressing to tension pneumothorax with hemodynamic compromise. Loculated pneumothoraces may be successfully drained percutaneously under ultrasound or computed tomography (CT) guidance. Successful drainage of air space disease leads to enhanced liberation from MV.52 Pneumothorax or tension pneumothorax may also result from aggressive bag-valve-mask ventilation. Patients with intrinsic lung disease such as COPD or asthma are more prone to the development of pneumothorax than the average patient is.53
A simple pneumothorax can be drained by surgical tube thoracostomy with a small-bore tube (24 Fr), a commercially available pneumothorax kit (Arrow), or a pigtail catheter placed into the pleural space via the Seldinger technique (see Chapter 9). Each of these catheters should be placed into a chest drainage collection unit that incorporates a water seal chamber and variable suction control. Treat persistent air leaks initially with continuous suction (usually suction at 20 cm H2O) to evacuate the pleural space and promote coaptation of the visceral and parietal pleurae. Reduce suction and place the chest tube on water seal only after resolution of any air leak. Remove the chest tube directly from the water seal if no pneumothorax is apparent on a chest film or after a test period of tube clamping and subsequent radiographic evaluation. The author favors a 4-hour period of clamping because recurrent pneumothorax is easier to treat by unclamping a tube than by placing a new one. Not all patients with a pneumothorax require invasive techniques to evacuate air from the pleural space. It is important to recognize that small pneumothoraces occurring in spontaneously breathing patients (i.e., negative pressure ventilation) may be reevaluated in 4 to 6 hours with a repeated chest radiograph and drained only if they are expanding. This option is not advised for patients who are on any form of PPV because a simple pneumothorax can rapidly become a tension pneumothorax with subsequent hypotension and death. Tension pneumothoraces may be recognized by tachycardia, hypotension, elevated peak airway pressure (if mechanically ventilated, tachypnea if not), jugular venous distention, thoracic resonance by percussion on the affected side, diminished or absent breath sounds on the affected side, and tracheal deviation away from the affected side. Because not all signs or symptoms are present in all patients, treatment should be dictated by the patient’s clinical condition.
Loculated pneumothoraces or fluid collections develop in certain patients. If the collections are either single or immediately adjacent to one another and readily identified, they may be drained under ultrasound guidance at the bedside.54 Loculations are frequently in inaccessible areas or are difficult to image with ultrasound. CT scanning of the thorax can provide precise anatomic definition of the presence and number of loculated collections and be used as a guide for the interventional radiologist. Successful treatment involving CT-guided drainage of loculated pleural collections (air and fluid) to assist in weaning of patients from mechanical ventilator support has been reported.52
Ventilator-Induced Lung Injury
There are several causes of ventilator-induced lung injury, including biotrauma, volutrauma, barotrauma, and atelectasis-related trauma. Biotrauma refers to the self-sustaining process of lung injury from MV that follows alveolar overdistention or rupture, alveolar hypoperfusion, and repetitive shear stress across alveolar walls. Originally, this problem was thought to be caused by too much pressure (barotrauma).55 Current principles hold that elevated airway pressure is a straightforward reflection of excess volume delivered to a lung that cannot accept excess gas (i.e., in volutrauma, excess volume is delivered).56 Lung injury is an inhomogeneous process with areas of normal lung immediately adjacent to diseased and injured segments.57 The healthy and compliant segments with shorter regional time constants will readily accept gas, but their neighbors with reduced compliance and longer regional time constants will not. The end result is overdistention of the compliant segments, alveolar injury, liberation of inflammatory cytokines and chemokines, activation of endothelin and arachidonic acid pathways, and the expression of adhesion molecules along the vascular endothelium.58 This leads to infiltration of inflammatory cells, release of destructive lysosomal enzymes, and induction of toxic oxygen metabolites. Avoiding this inflammatory cascade is an intelligent means of protecting a patient’s lungs from volume-induced lung injury. Such a notion has given rise to lung-protective ventilator strategies based on low-Vt ventilation (6 mL/kg IBW) and low plateau pressure (<30 cm H2O).59 Several studies have reported the development within hours of ventilator-induced lung injury in patients with normal lungs that were ventilated with larger Vt (12 mL/kg).60–64 Current recommendations are for all MV to be conducted with lower Vt than the once-standard 12 to 15 mL/kg. Patients with abnormal lungs (interstitial lung disease, lung resection, severe pneumonia, edema) or the presence of an ALI risk factor (sepsis, aspiration, transfusion) should be ventilated at a Vt of 6 mL/kg IBW. These patients may initially be markedly acidotic and may require starting Vt at 8 mL/kg IBW and titrating down to 6 mL/kg IBW within 2 hours. Those with normal lungs and no ALI risk factors should be started at a Vt of less than 10 mL/kg IBW.64
Troubleshooting
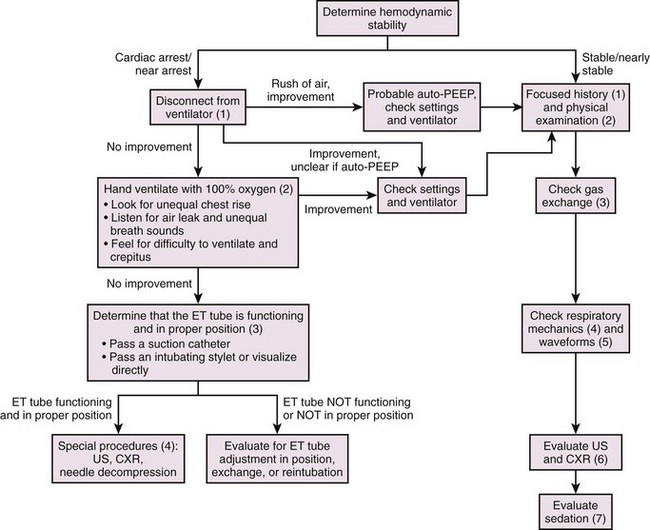
Determine Hemodynamic Stability
The initial step in managing a crashing ventilated patient is to determine the patient’s hemodynamic stability. A ventilated patient is, by definition, critically ill. The patient can fall anywhere on the spectrum from being ventilated for airway protection, with normal vital signs, blood pressure, and oxygen saturation, to being ventilated and in cardiac arrest. Determining where the patient is on this spectrum, including assessment for hypotension and hypoxia, will dictate how much time that the practitioner has to implement rescue strategies. In addition, it is important to anticipate the patient’s clinical course. The approach to a patient who is intubated for hypoxia stemming from pneumonia and whose blood pressure and oxygenation gradually trend down over a period of hours or days is different from the approach to a patient who is declining over a span of minutes (Fig. 8-19).
Cardiac Arrest and Near Arrest Patients
During stabilization of these patients it is important to keep in mind the original pathology that necessitated intubation. Sudden decompensation in an asthmatic is a common example of physician intervention worsening the scenario unless the pathophysiology of the arrest is appreciated (see Fig. 8-17). A crashing ventilated patient may simply be worsening from the primary pathology. A multitrauma patient may have an intrathoracic or intraabdominal catastrophe, and a septic patient may be deteriorating clinically from lack of source control.
It is also important to determine and address special circumstances that the ventilator can precipitate, the most significant of which are tension pneumothorax and severe auto-PEEP. Tension pneumothorax can lead to marked hypotension as a result of decreased cardiac output and marked hypoxia from 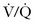 mismatch.63 Auto-PEEP (also referred to as PEEPi, breath stacking, or dynamic hyperinflation) is caused by trapped volume in the pulmonary system. If severe enough, it will eventually lead to increased intrathoracic pressure. This can cause hypotension and decreased cardiac output from decreased venous return and marked hypoxia from
mismatch.63 Auto-PEEP (also referred to as PEEPi, breath stacking, or dynamic hyperinflation) is caused by trapped volume in the pulmonary system. If severe enough, it will eventually lead to increased intrathoracic pressure. This can cause hypotension and decreased cardiac output from decreased venous return and marked hypoxia from 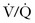 mismatch.64
mismatch.64
Step 2: Breathing—Hand-Ventilate with 100% Oxygen
Ensure that 100% oxygen is being delivered and limit the respiratory rate to 8 to 10 breaths/min. Particular attention should be paid to the delivery of hand ventilation. Inadvertent rates as high as 40 breaths/min are often used in codes.65,66 Excessive rates will increase intrathoracic pressure and lead to a decrease in venous return and cardiac output.67 Look at both sides of the chest to determine whether there is equal chest rise. Unequal chest rise can signify main stem intubation, pneumothorax, or a mucus plug. Listen for air escaping from the mouth or nose (a sign of an air leak). Listen over the epigastric area and in both axillae. Decreased breath sounds may provide clues regarding main stem intubation, pneumothorax, or an atelectatic lung. Feel for subcutaneous crepitus (a sign of pneumothorax) and assess for difficulty in hand ventilating (a sign of low dynamic or static respiratory system compliance).
Step 3: Airway—Determine That the ET Tube Is Functioning and in the Proper Position
The ET tube functions by providing a conduit to the lower part of the trachea. Its cuff attempts to create a seal between it and the inner wall of the trachea. To determine whether the ET tube is functioning properly, pass the suction catheter and listen for an air leak (Fig. 8-20). Easy passage of the suction catheter does not guarantee that the ET tube is in the trachea because the catheter may be passing down the esophagus. If it is difficult or not possible to pass the suction catheter, the ET tube is either dislodged or obstructed. Attempt to correct this by repositioning the head in the case of a twisted or bent ET tube or inserting a bite block if the patient is biting on the tube. Dislodged or obstructed ET tubes require reintubation. Patients with dislodged ET tubes should be treated as difficult intubations because unplanned extubations are notorious for causing trauma to the glottis and for leading to vocal cord edema.68
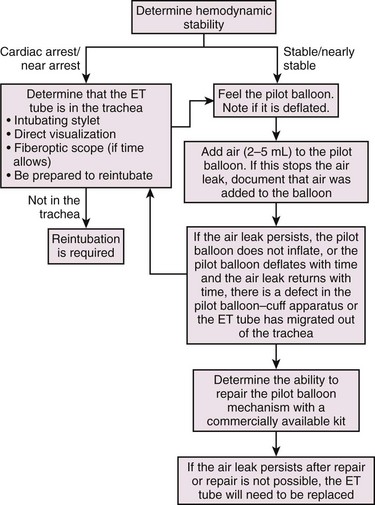
Figure 8-20 Troubleshooting an air leak. ET, Endotracheal.
Other simple techniques may be used to confirm that the ET tube is in the trachea. Direct visualization of the carina with a fiberoptic scope is an option, but this device is not typically readily available to the ED practitioner. Another quick and readily available technique is to pass an intubating stylet (gum elastic bougie or Eschmann introducer).69 The stylet is passed gently through the ET tube. If resistance is met at 30 cm, the ET tube is in the trachea. If the stylet passes beyond 35 cm without resistance, the ET tube is probably in the esophagus. If resistance is met too soon, the intubating stylet may be catching on the ET tube.
Step 4: Special Procedures
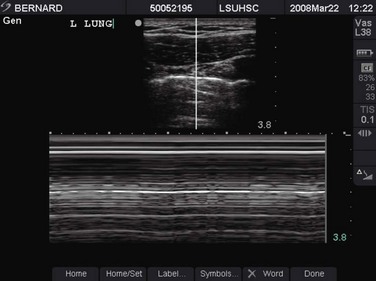
If the patient is still in cardiac arrest or near arrest after being disconnected from the ventilator, ensuring proper placement of the ET tube, and hand-ventilating with 100% oxygen, a clinical decision will be required regarding needle decompression of the chest. If time permits, a focused history from the bedside nurse, respiratory therapist, or paramedic and a focused physical examination will indicate which side of the chest to decompress. In addition, depending on the urgency of the situation, bedside ultrasound and chest radiography may be used. Bedside ultrasound has been shown to exclude pneumothorax in the presence of “lung slide.” This is depicted in M-mode as the “seashore sign” (Fig. 8-21) and, in its absence, as the “stratosphere sign/bar code sign” (Fig. 8-22).70–72
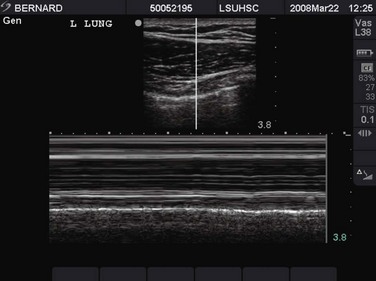
Figure 8-21 Ultrasound image of a lung slide (“seashore sign”) in M-mode. This patient does not have a pneumothorax.
At times, the clinical situation does not allow imaging studies, and the focused history and physical examination may not be helpful. In these cases, needle decompression of both sides of the chest should be considered if other more likely causes of acute decompensation are not found. It is important to remember that chest tube placement is required in patients after needle decompression.73–75
Stable and Nearly Stable Patients
Step 2: Perform a Focused Physical Examination
Airway: Look at the ET tube and determine whether it has migrated from its previous position. It is possible that the ET tube has migrated out of the trachea or into a main bronchus. Adjust if necessary. Listen for escaping air (an air leak) from the mouth or nose (see Fig. 8-20). This typically signifies that the tube has lost its seal with the trachea and occurs with extubation or cuff failure. Feel the pilot balloon. If it is deflated, the cuff is deflated. Add air to the pilot balloon. If this stops the air leak, make a note that air was added to the balloon. If the pilot balloon does not inflate or deflates with time, there is a defect in the pilot balloon-cuff apparatus, and the ET tube will probably need to be exchanged. Occasionally, it may be possible to repair the pilot balloon mechanism with commercially available kits. This is a good option in patients who are difficult to intubate.
Breathing: Look at both sides of the chest to determine whether there is equal chest rise. Unequal chest rise can signify main stem intubation, pneumothorax, or a mucus plug. Look at the ventilator tubing and determine whether there is an oscillating water collection. Listen for air escaping from the mouth or nose (a sign of an air leak). Listen over the epigastric area and in both axillae. Decreased breath sounds may provide clues regarding main stem intubation, pneumothorax, or an atelectatic lung. Feel for subcutaneous crepitus (a sign of pneumothorax).
Step 3: Assess Gas Exchange
Hypoxia can be diagnosed with pulse oximetry if the waveform is reliable. The waveform should not be highly variable and the frequency of the waveform should match the heart rate on the cardiac monitor. In a few instances, such as carbon monoxide poisoning, pulse oximetry is not reliable.76 In these cases or if the pulse oximeter is not picking it up, an ABG sample should be obtained. Patients with a Pao2/Fio2 ratio of less than 200 should be evaluated for ARDS. Those with a ratio between 200 and 300 should be evaluated for ALI.77 A lung-protective strategy should be implemented in those determined to have ALI or ARDS (see Fig. 8-18).78 Hypoventilation cannot be identified with pulse oximetry. ABG analysis is beneficial in this event.
Step 4: Check Respiratory Mechanics
Determine whether peak pressure and plateau pressure have changed from their previous values. These values should be obtained on volume-targeted modes. Airway pressure is a function of volume and respiratory system compliance. The respiratory system incorporates the ventilator circuit, ET tube, trachea, bronchi, pulmonary parenchyma, and chest wall. A set volume with a set system compliance results in a specific pressure. Peak pressure is a function of volume, resistance to airflow, and respiratory system compliance. Plateau pressure is obtained during an inspiratory pause, thus eliminating airflow, and therefore reflects only respiratory system compliance. An isolated increase in peak pressure is indicative of increased resistance to airflow. An isolated increase in plateau pressure is indicative of a decrease in respiratory system compliance. Note that plateau pressure can never be higher than peak pressure and that if plateau pressure rises, so will peak pressure. It is important to keep in mind the Δ relationship (peak pressure − plateau pressure). These measurements assume a comfortable patient, and peak pressure and plateau pressure values are not reliable in a “bucking” patient.79,80
Step 5: Observe Ventilator Waveforms
The two most helpful ventilator waveforms are the flow-time curve and the pressure-time curve. The flow-time curve can be used to detect air trapping. The pressure-time curve can be used to determine plateau pressure with an inspiratory hold (see Fig. 8-1).
Step 6: Imaging Studies—Chest Radiograph and Bedside Ultrasound
Evaluate the chest radiograph for ET tube position, main stem intubation, lung atelectasis, pneumothorax, and a worsening parenchymal process. Bedside ultrasound, if available, is typically quicker in evaluating for pneumothorax, but it will not provide information on the location of the ET tube, lung atelectasis, or parenchymal processes (see Figs. 8-21 and 8-22).
Step 7: Evaluate Sedation
Patients who display air hunger and have a high respiratory rate can be given a trial of opiates to relieve their symptoms. Proper sedation and analgesia are paramount in patients being treated with a strategy that allows or permits hypercapnia, such those with as status asthmaticus, and in patients being treated with lung-protective strategies, such as those with ARDS. Hypercapnia is a powerful stimulus to the respiratory drive, and opiates are often required to control respiratory rates. Patients who tend to be difficult to control (besides those with status asthmaticus and ARDS) include patients with hepatic encephalopathy or intracranial processes such as a mass effect and hemorrhage. Chemical weakening with intermittently dosed paralytics may be required if patients have undergone a good trial of sedation, analgesia, and ventilator changes and are still markedly tachypneic. Careful consideration should be given before this step because prolonged paralysis has been implicated in critical illness polyneuropathy.81,82 In addition, expert consultation should be obtained before prolonged paralysis of a neurosurgical patient. The goal in chemical paralysis in these patients is to weaken them enough to control their interaction with the ventilator. Usually, this does not require a full dose of the paralytic.
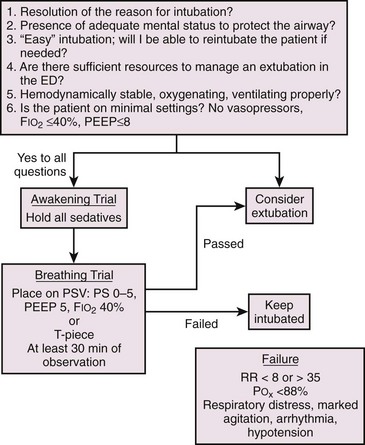
Liberation from the Ventilator
Occasionally, patients can be considered for extubation while still in the ED. Before extubating a patient, several questions should be answered in the affirmative (Fig. 8-23). Common scenarios include resolution of exacerbations of COPD or asthma, exacerbation of congestive heart failure, and metabolism of intoxicants. Patients who were difficult intubations or required multiple attempts should have a planned extubation. It may be prudent to allow the edema from a traumatic intubation to subside.
For patients intubated for exacerbation of COPD, NPPV should be considered. For patients with COPD who failed a spontaneous breathing trial, extubating to NPPV decreases mortality, hospital length of stay, the incidence of ventilator-associated pneumonia, and the total duration of mechanical ventilation.83
Conclusion
Mechanically ventilated patients are typically the most critically ill patients that the ED practitioner will manage. The underlying disease process that required intubation is typically life-threatening. When patients become unstable, the physician should take a stepwise approach toward determining whether the patient is deteriorating because of the underlying disease process or because of interaction with the ventilator. It is hoped that the approach presented here will assist practitioners with a framework to evaluate and stabilize crashing ventilated patients. For further information, Wood and Winters present an up-to-date review of the care of ventilated patients in the ED and evaluation of potential problems.84
References
1. Bailey, H, Kaplan, LJ. Barotrauma: eMedicine:Internal Medicine/Surgery/Ob-Gyn/Psychiatry [serial online]. http://www.emedicine.com, 1998. [Available at].
2. Broussarsar, M, Thierry, G, Jaber, S, et al. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28:406.
3. Ranieri, VM, Giuliani, R, Cinnella, G, et al. Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled MV. Am Rev Respir Dis. 1993;147:5.
4. Kreit, JW. Mechanics of the respiratory system. In: Grenvik A, Ayres SM, Holbrook PR, et al, eds. Textbook of Critical Care. 4th ed. Philadelphia: Saunders; 2000:1184.
5. Kirby, RR, Perry, JC, Calderwood, HW, et al. Cardiorespiratory effects of high positive end-expiratory pressure. Anesthesiology. 1975;43:533.
6. Kacmarek, RM. Strategies to optimize alveolar recruitment. Curr Opin Crit Care. 2001;7:15.
7. Pepe, P, Marini, JJ. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto-PEEP effect. Am Rev Respir Dis. 1982;126:166.
8. Venkataraman, ST, Orr, RA. Intra-hospital transport of critically ill patients. Crit Care Clin. 1992;8:525.
9. Capallier, G, Beuret, P, Clement, G, et al. Oxygen tolerance in patients with acute respiratory failure. Intensive Care Med. 1998;24:422.
10. Cooper, KR, Boswell, PA. Reduced functional residual capacity and abnormal oxygenation in patients with severe head injury. Chest. 1983;84:29–35.
11. Shapiro, M, Wison, K, Cesar, G, et al. Work of breathing through different sized endotracheal tubes. Crit Care Med. 1986;14:1028.
12. Mercat, A, Diehl, JL, Michard, F, et al. Extending inspiratory time in acute respiratory distress syndrome. Crit Care Med. 2001;29:40.
13. Sassoon, CS, Giron, AE, Ely, EA, et al. Inspiratory work of breathing on flow-by and demand-flow continuous positive airway pressure. Crit Care Med. 1989;17:1108–1114.
14. Wang, SH, Wei, TS. The outcome of early pressure-controlled inverse ratio ventilation on patients with severe acute respiratory distress syndrome in surgical intensive care unit. Am J Surg. 2002;183:151.
15. Esteban, A, Frutos, F, Tobin, MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345–350.
16. dos Santos, CC, Slutsky, AS. Mechanotransduction, ventilator-induced lung injury and multiple organ dysfunction syndrome. Intensive Care Med. 2000;26:638.
17. Branson, RD, Davis, K, Jr. Dual control modes: combining volume and pressure breaths. Respir Care Clin N Am. 2001;7:397–408. [viii].
18. Krishnan, JA, Brower, RG. High-frequency ventilation for acute lung injury and ARDS. Chest. 2000;118:795–807.
19. Habashi, NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med. 2005;33(3 suppl):S228–S240.
20. Kaplan, LJ, Bailey, H, Formosa, V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care. 2001;5:221.
21. Putensen, C, Rasanen, J, Lopez, FA. Ventilation-perfusion distributions during mechanical ventilation with superimposed spontaneous breathing in canine lung injury. Am J Respir Crit Care Med. 1994;150:101–108.
22. Kaplan, LJ, Bailey, H. Lesson learned from airway pressure release ventilation. Crit Care. 2001;5:S9.
23. Rajan, T, Hill, NS. Noninvasive positive pressure ventilation. In: Fink MP, Abraham L, Vincent JL, et al, eds. Textbook of Critical Care. 5th ed. Philadelphia: Saunders; 2005:519–526.
24. Cross, AM. Review of the role of non-invasive ventilation in the emergency department. J Accid Emerg Med. 2000;17:79–85.
25. Bott, J, Carroll, MP, Conway, JH, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557.
26. Brochard, L, Mancebo, J, Wysocki, M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822.
27. Conti, G, Antonelli, M, Navalesi, P, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707.
28. Pang, D, Keenan, SP, Cook, DJ, et al. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998;114:1185–1192.
29. Collins, SP, Mielniczuk, LM, Whittingham, HA, et al. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med. 2006;48:260–269. [269 e261-264].
30. Mehta, S, Jay, GD, Woolard, RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25:620–628.
31. Hilbert, G, Gruson, D, Vargas, F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–487.
32. Antonelli, M, Conti, G, Bufi, M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283:235–241.
33. Curtis, JR, Cook, DJ, Sinuff, T, et al. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med. 2007;35:932–939.
34. International Consensus Conferences in Intensive Care Medicine. Noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 2001;163:283–291.
35. Diaz, GG, Alcaraz, AC, Talavera, JC, et al. Noninvasive positive-pressure ventilation to treat hypercapnic coma secondary to respiratory failure. Chest. 2005;127:952–960.
36. Scala, R, Naldi, M, Archinucci, I, et al. Noninvasive positive pressure ventilation in patients with acute exacerbations of COPD and varying levels of consciousness. Chest. 2005;128:1657–1666.
37. Scala, R, Nava, S, Conti, G, et al. Noninvasive versus conventional ventilation to treat hypercapnic encephalopathy in chronic obstructive pulmonary disease. Intensive Care Med. 2007;33:2101–2108.
38. Sreenan, C, Lemke, RP, Hudson-Mason, A, et al. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics. 2001;107:1081–1083.
39. Chatila, W, Nugent, T, Vance, G, et al. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest. 2004;126:1108–1115.
40. Murray, MJ, Cowen, J, DeBlock, H, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002;30:142.
41. Rhoney, DH, Murry, KR. National survey of the use of sedating drugs, neuromuscular blocking agents, and reversal agents in the intensive care unit. J Intensive Care Med. 2003;18:139.
42. Douglass, JA, Tuxen, DV, Horne, M, et al. Myopathy in severe asthma. Am Rev Respir Dis. 1992;146:517–519.
43. Kupfer, Y, Namba, T, Kaldawi, E, et al. Prolonged weakness after long-term infusion of vecuronium bromide. Ann Intern Med. 1992;117:484–486.
44. Mazurek, AJ, Rae, B, Hann, S, et al. Rocuronium verus succinylcholine: are they equally effective during rapid-sequence induction of anesthesia? Anesth Analg. 1998;87:1259.
45. Gladwin, MT, Pierson, DJ. Mechanical ventilation of the patient with severe chronic obstructive pulmonary disease. Intensive Care Med. 1998;24:898–910.
46. Ranieri, VM, Giuliani, R, Cinnella, G, et al. Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilation. Am Rev Respir Dis. 1993;147:5–13.
47. Slutsky, AS. Mechanical ventilation. American College of Chest Physicians’ Consensus Conference. Chest. 1993;104:1833–1859.
48. Papiris, S, Kotanidou, A, Malagari, K, et al. Clinical review: severe asthma. Crit Care. 2002;6:30–44.
49. Leatherman, JW, McArthur, C, Shapiro, RS. Effects of prolongation of expiratory time on dynamic mechanical ventilation of patients with severe asthma. Crit Care Med. 2004;32:1542.
50. Kollef, MH. Lung hyperinflation caused by inappropriate ventilation resulting in electromechanical dissociation: a case report. Heart Lung. 1992;21:74–77.
51. Gammon, RB, Shin, MS, Grozdanovik, Z, et al. Clinical risk factors for pulmonary barotrauma. Am J Respir Crit Care Med. 1995;152:1235.
52. Kaplan, LJ, Trooskin, SZ, Santora, TA. Thoracic compartment syndrome. J Trauma. 1996;40:291.
53. Sahn, SA. Pleural disease in the intensive care unit. In: Grenvik A, Ayres SM, Holbrook PR, et al, eds. Textbook of Critical Care. 4th ed. Philadelphia: Saunders; 2000:1548.
54. Beckh, S, Bolcskei, PL, Lessnau, KD. Real-time chest ultrasonography: a comprehensive review for the pulmonologist. Chest. 2002;122:1759.
55. Peterson, GW, Baier, H. Incidence of pulmonary barotrauma in a medical ICU. Crit Care Med. 1983;11:67.
56. Brochard, LA, Rauss, S, Benito, G, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150:896.
57. Martin, A, Soloway, H, Simmons, R. Pathologic anatomy of the lungs following shock and trauma. J Trauma. 1968;8:687.
58. dos Santos, CC, Slutsky, AS. Mechanotransduction, ventilator-induced lung injury and multiple organ dysfunction syndrome. Intensive Care Med. 2000;26:638.
59. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301.
60. Gajic, O, Dara, SI, Mendez, JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817.
61. Gajic, O, Fructos-Vivar, F, Esteban, A, et al. Ventilator settings as a risk factor for acute respiratory syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922.
62. Schultz, MJ, Haitsma, JJ, Slutsky, AS, et al. What tidal volumes should be used in patients without acute lung injury? Anesthesiology. 2007;106:1226.
63. Chen, KY, Jerng, JS, Liao, WY, et al. Pneumothorax in the ICU: patient outcomes and prognostic factors. Chest. 2002;122:678–683.
64. Pepe, PE, Marini, JJ. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto-PEEP effect. Am Rev Respir Dis. 1982;126:166–170.
65. O’Neill, JF, Deakin, CD. Do we hyperventilate cardiac arrest patients? Resuscitation. 2007;73:82–85.
66. Abella, BS, Alvarado, JP, Myklebust, H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305–310.
67. Aufderheide, TP, Sigurdsson, G, Pirrallo, RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960–1965.
68. Christie, JM, Dethlefsen, M, Cane, RD. Unplanned endotracheal extubation in the intensive care unit. J Clin Anesth. 1996;8:289–293.
69. Bair, AE, Laurin, EG, Schmitt, BJ. An assessment of a tracheal tube introducer as an endotracheal tube placement confirmation device. Am J Emerg Med. 2005;23:754–758.
70. Lichtenstein, DA, Meziere, G, Lascols, N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33:1231–1238.
71. Blaivas, M, Lyon, M, Duggal, S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12:844–849.
72. Wilkerson, RG, Stone, MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17:11–17.
73. Harcke, HT, Pearse, LA, Levy, AD, et al. Chest wall thickness in military personnel: implications for needle thoracentesis in tension pneumothorax. Mil Med. 2007;172:1260–1263.
74. Givens, ML, Ayotte, K, Manifold, C. Needle thoracostomy: implications of computed tomography chest wall thickness. Acad Emerg Med. 2004;11:211–213.
75. Zengerink, I, Brink, PR, Laupland, KB, et al. Needle thoracostomy in the treatment of a tension pneumothorax in trauma patients: what size needle? J Trauma. 2008;64:111–114.
76. Lee, WW, Mayberry, K, Crapo, R, et al. The accuracy of pulse oximetry in the emergency department. Am J Emerg Med. 2000;18:427–431.
77. Bernard, GR, Artigas, A, Brigham, KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824.
78. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308.
79. Tobin, MJ. Advances in mechanical ventilation. N Engl J Med. 2001;344:1986–1996.
80. Tobin, MJ. Respiratory monitoring. JAMA. 1990;264:244–251.
81. Douglass, JA, Tuxen, DV, Horne, M, et al. Myopathy in severe asthma. Am Rev Respir Dis. 1992;146:517–519.
82. Kupfer, Y, Namba, T, Kaldawi, E, et al. Prolonged weakness after long-term infusion of vecuronium bromide. Ann Intern Med. 1992;117:484–486.
83. Burns, KE, Adhikari, NK, Meade, MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 4, 2003. [CD004127].
84. Woods, S, Winters, ME. Care of the intubated emergency department patient. J Emerg Med. 2011;40:419.

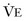 ).
). 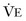 is the product of tidal volume (V
is the product of tidal volume (V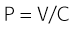
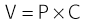


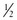 to
to 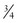 of PEEPi, which allows the patient to perform less work to trigger each inhalation. This process mandates frequent reassessment of PEEPi and manipulation of the ventilator during this dynamic period.
of PEEPi, which allows the patient to perform less work to trigger each inhalation. This process mandates frequent reassessment of PEEPi and manipulation of the ventilator during this dynamic period.