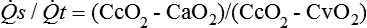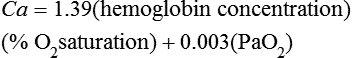Measurement and implications of the  s/
s/ t
t
Hypoxemia can be caused by a variety of factors (Box 32-1). This chapter will focus on the primary cause of hypoxemia—shunting, which may, at times, be due to ventilation/perfusion (![]() /
/![]() ) mismatch.
) mismatch.
Ventilation/perfusion mismatch
Ideally, pulmonary perfusion (![]() ) evenly matches alveolar ventilation at all levels of the lung; however, perfect matching does not occur because the distribution of ventilation and perfusion and the
) evenly matches alveolar ventilation at all levels of the lung; however, perfect matching does not occur because the distribution of ventilation and perfusion and the ![]() /
/![]() ratio vary throughout the lung. A normal lung has a
ratio vary throughout the lung. A normal lung has a ![]() /
/![]() ratio of approximately 0.8. A
ratio of approximately 0.8. A ![]() /
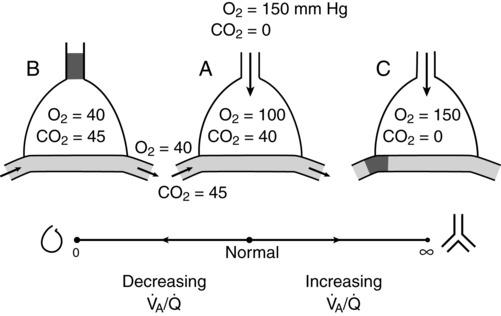
/![]() ratio of 0 (i.e., a shunt) exists when perfused alveoli have no ventilation and the values for PO2 and PCO2 of the trapped air are the same as those of mixed venous blood (PO2 = 40 mm Hg and PCO2 = 47 mm Hg). Conversely, a
ratio of 0 (i.e., a shunt) exists when perfused alveoli have no ventilation and the values for PO2 and PCO2 of the trapped air are the same as those of mixed venous blood (PO2 = 40 mm Hg and PCO2 = 47 mm Hg). Conversely, a ![]() /
/![]() ratio of ∞ exists when ventilated alveoli have no perfusion and, at sea level, the PO2 and PCO2 equal approximately 150 and 0 mm Hg, respectively. Nonperfused alveoli (i.e., alveolar dead space) is approximately 25 to 50 mL in a healthy 70-kg person. Figure 32-1 depicts the progression of a
ratio of ∞ exists when ventilated alveoli have no perfusion and, at sea level, the PO2 and PCO2 equal approximately 150 and 0 mm Hg, respectively. Nonperfused alveoli (i.e., alveolar dead space) is approximately 25 to 50 mL in a healthy 70-kg person. Figure 32-1 depicts the progression of a ![]() /
/![]() ratio from 0 to ∞; the normal, idealized, alveolar-capillary unit is shown as example A.
ratio from 0 to ∞; the normal, idealized, alveolar-capillary unit is shown as example A.
Shunting due to other causes
Hypoxemia that is the result of a physiologic or anatomic shunt cannot be corrected by having the patient breathe supplemental O2. The hemoglobin in blood that perfuses alveoli with a ![]() /
/![]() ratio of 1 will readily achieve 100% saturation; increasing the partial pressure of O2 in these alveoli will minimally increase the O2 content. Blood that perfuses alveoli with a
ratio of 1 will readily achieve 100% saturation; increasing the partial pressure of O2 in these alveoli will minimally increase the O2 content. Blood that perfuses alveoli with a ![]() /
/![]() ratio of 0 will not be exposed to any O2, no matter the fraction of inspired O2 (FIO2); therefore, no significant improvement in arterial oxygenation occurs (Figure 32-2).
ratio of 0 will not be exposed to any O2, no matter the fraction of inspired O2 (FIO2); therefore, no significant improvement in arterial oxygenation occurs (Figure 32-2).
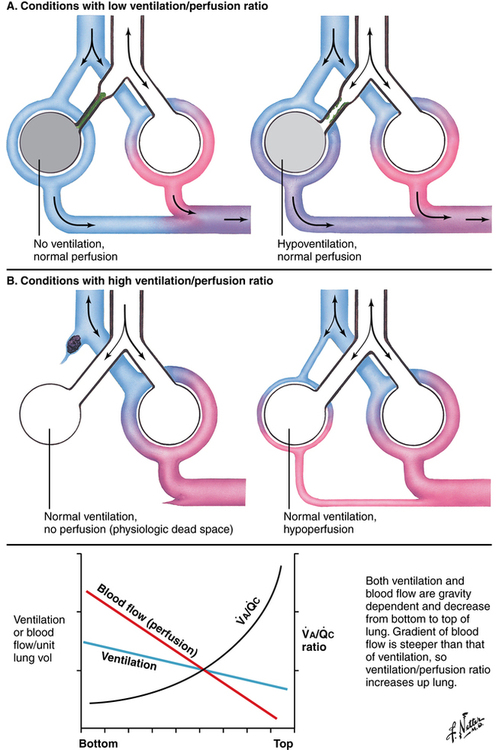
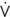 A/
A/ c), is lowest at the bottom of the lung and greatest at the top of the lung (B).
c), is lowest at the bottom of the lung and greatest at the top of the lung (B).  A/
A/ c is also affected by various other conditions affecting ventilation and perfusion (A and B). (Netter illustration from www.netterimages.com. © Elsevier Inc. All rights reserved.)
c is also affected by various other conditions affecting ventilation and perfusion (A and B). (Netter illustration from www.netterimages.com. © Elsevier Inc. All rights reserved.)