Chapter 26 Massive Hemoptysis
Hemoptysis is the coughing up of blood or bloodstained sputum. Massive hemoptysis is variably defined as 200 to 1000 ml of blood expectorated in 24 hours; 600 ml is a widely accepted figure.1,2 Blood loss is rarely measured and is difficult to quantify. A more practical definition of massive hemoptysis is that which is acutely life-threatening. Although massive hemoptysis is uncommon, it is potentially lethal and has a mortality rate of between 9% and 59%.1 The management of patients with massive hemoptysis requires a team approach, with involvement from specialists in intensive care, pulmonology, interventional radiology, and thoracic surgery.
ETIOLOGY
The causes of massive hemoptysis are listed in Table 26-1. Tuberculosis is the most common cause worldwide, but in developed countries chronic inflammatory lung diseases (e.g., bronchiectasis) and lung cancer are the most common causes.
| Neoplastic | Bronchial carcinoma |
| Metastatic lung cancer | |
| Leukemia | |
| Infectious | Bronchiectasis |
| Bronchitis | |
| Tuberculosis | |
| Fungal infections | |
| Paragonimiasis (parasitic lung fluke) | |
| Hydatid cyst | |
| Vascular | Pulmonary infarction or embolism |
| Mitral stenosis | |
| Bronchoarterial fistula | |
| Rupture of a thoracic aortic aneurysm | |
| Arteriovenous malformation | |
| Vasculitic | Wegener granulomatosis |
| Behçet disease | |
| Goodpasture syndrome | |
| Systemic lupus erythematosus | |
| Miscellaneous | Anticoagulant therapy |
| Coagulopathy | |
| Trauma | |
| Lymphangioleiomyomatosis | |
| Iatrogenic | Pulmonary artery rupture secondary to a pulmonary artery catheter |
| Malposition of a chest drain | |
| Tracheoarterial fistula |
Adapted from Jean-Baptiste E: Clinical assessment and management of massive hemoptysis. Crit Care Med 28:1642-1647, 2000.
DIAGNOSIS AND TREATMENT
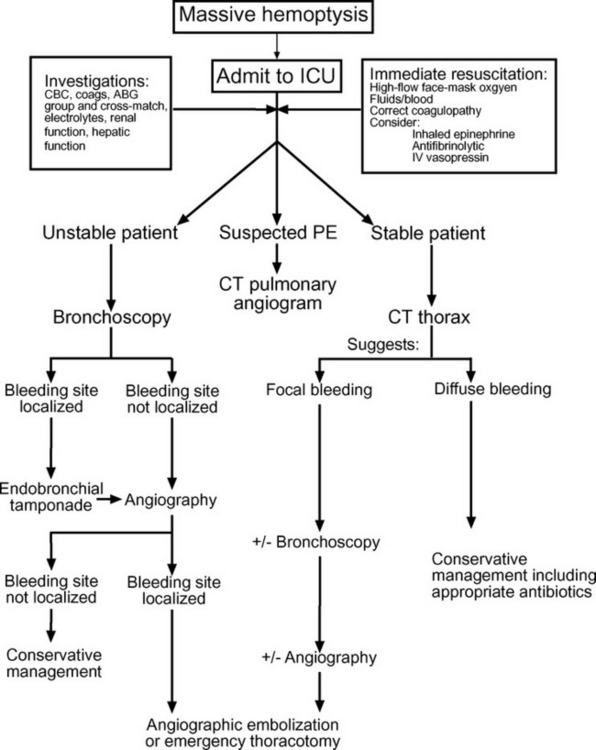
As in all situations in which a patient develops an acute critical illness, diagnosis and treatment must proceed in parallel. An algorithm for the diagnosis and management of massive hemoptysis is provided in Figure 26-1.
Immediate Resuscitation and Lung Isolation
As little as a few hundred milliliters of blood within the alveolar space can cause impaired gas exchange. Thus, massive hemoptysis will cause death by asphyxia well before hypovolemic shock develops. The primary goal during resuscitation is to achieve adequate oxygenation.
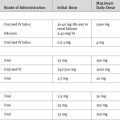
If it is known which lung is bleeding, the patient should be positioned on his or her side, with the bleeding lung dependent so as to minimize soiling of the nonbleeding lung. Other maneuvers that can be tried include antifibrinolytic therapy (see Chapter 30), nebulized epinephrine (5 mg in 5 ml doses repeated every 15 minutes), and intravenous vasopressin. The recommended dose of vasopressin in this situation (20 units over 15 min followed by an infusion of 0.2 units/min.3) is very high and must be used with caution in patients with cardiac disease or hypovolemia.
Following intubation, fiberoptic bronchoscopy should be performed to identify the site of bleeding and, if necessary, to achieve endobronchial control. Endobronchial control involves isolation of the bleeding lung and selective ventilation of the nonbleeding lung. It is required if bleeding remains active despite intubation. Two methods to achieve endobronchial control are (1) selective endobronchial intubation and (2) the use of a bronchial blocker. Endobronchial intubation is achieved by advancing the endotracheal tube into the left or right main bronchus under bronchoscopic guidance. This technique works best when selective intubation of the left main bronchus is required (for bleeding from the right lung) because with intubation of the right main bronchus (for bleeding from the left lung), the cuff of the endotracheal tube is likely to occlude the origin of the right upper lobe bronchus. A number of purpose-designed endobronchial blockers are available for lung isolation. One device, the Arndt endobronchial blocker (Cook Critical Care, Bloomington, IN), features a balloon-tipped catheter with an endoscopic snare (Fig. 26-2). With the bronchoscope placed through the snare, the tip of the catheter can be directed over the bronchoscope into the left or right main bronchus. The balloon is then inflated, and lung isolation is achieved.
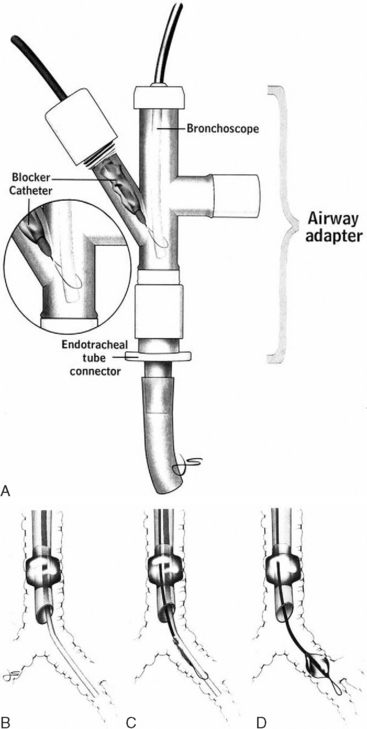
Figure 26.2 Arndt Endobronchial Blocker (Cook Critical Care, Bloomington, IN.) See text for details.
(From Grocott HP, Scales G, Schinderle D, et al: A new technique for lung isolation in acute thoracic trauma. J Trauma 49:940-942, 2000.)
As an alternative to bronchoscopically guided endobronchial control, insertion of a double-lumen tracheobronchial tube may be considered (see Chapter 40, Fig. 40-6). However, in patients with massive hemoptysis, accurate placement of these tubes can be very difficult, and physicians who are unfamiliar with these devices should not attempt this procedure. These techniques of lung isolation are only temporizing measures to allow adequate gas exchange to be achieved while more definitive diagnosis and treatment are organized.
Diagnosis, Localization, and Control of the Bleeding Site
Chest Radiography
On a chest radiograph, bleeding within the pulmonary parenchyma produces a diffuse alveolar infiltrate that resembles pneumonia. This may allow identification of the pulmonary segments that are involved. The chest radiograph may also demonstrate other relevant pathologies such as a tumor or cavity lesions. However, in 20% to 40% of patients with hemoptysis, the chest radiograph is normal or nonlocalizing.4,5
Bronchoscopy
Fiberoptic bronchoscopy can be performed at the bedside in patients who are intubated and ventilated, and it may allow accurate localization of the site of bleeding. Diagnostic accuracy may be improved by instilling dilute epinephrine (1:20,000) or ice-cold water into the working port of the bronchoscope to temporarily reduce bleeding. Once the bleeding site has been identified (and endobronchial control achieved; see earlier material), the patient may proceed to rigid bronchoscopy or, more commonly, to bronchial artery angiography.
Rigid bronchoscopy has some advantages over flexible fiberoptic bronchoscopy in patients with hemoptysis. It permits effective airway toilet, facilitates endobronchial control and, with techniques such as cold saline lavage, instillation of epinephrine, and laser photocoagulation, may allow hemostasis to be achieved.2 However, the procedure may be poorly tolerated in a critically unwell patient and may not be successful in controlling hemorrhage, particularly that due to peripheral lesions.
Computed Tomography Scanning
High-resolution contrast-enhanced CT scans may provide important diagnostic information that is not apparent on a chest radiograph, particularly the presence of bronchiectasis or small lung cancers.4,6 The CT scan may also suggest a diffuse microvascular cause of the bleeding (e.g., vasculitis or pulmonary venous hypertension secondary to mitral stenosis).
Bronchial Artery Angiography and Embolization
Selective bronchial artery angiography using coils, foam, or other materials allows identification of the bleeding vessel and its embolization. Indicators of a likely site of bleeding include extravasation of dye or the finding of aneurysmal or tortuous vessels. Acute control of hemorrhage is possible in more than 90% of cases.7,8 However, on occasion bleeding begins again within the first few days, so repeated embolization is required. Failure of bronchial artery embolization is usually due to the presence of collateral vessels from the phrenic artery, intercostal, mammary, and subclavian arteries. Embolization of the spinal artery causing spinal cord ischemia may occur when the spinal artery arises from a bronchial artery, although this risk is very low (<1%).9
Role of Surgery
If hemoptysis is massive, originates from a focal lesion, and is uncontrolled by bronchial artery embolization, emergency thoracotomy should be considered. Surgery is also the treatment of choice for bronchial adenoma, leaking aortic aneurysm (see Chapter 11), arteriovenous malformations, severe iatrogenic pulmonary arterial rupture, tracheoarterial fistula, hydatid cyst, cavitating tuberculosis, and aspergilloma resistant to other therapies.2 Where hemoptysis is due to trauma, emergency thoracotomy is indicated, with early clamping of the bronchovascular trunk of the involved lung to avoid air embolism.
Emergency thoracotomy performed during a bleeding crisis may necessitate pneumonectomy and has a high mortality rate. Thus, if surgery is indicated, but acute bleeding can be controlled by bronchial artery embolization, surgery should be delayed until the patient has recovered from the bleeding crisis. Surgery is contraindicated in patients with advanced lung cancer (see Chapter 12) and in those with poor cardiorespiratory reserve.
SPECIFIC CONDITIONS
Pulmonary Arterial Rupture
Mild hemoptysis resulting from the rupture of a pulmonary artery branch that has been caused by the use of a pulmonary artery catheter may be controlled by positive pressure ventilation, positive end-expiratory pressure, and treatment of any coagulation abnormalities. However, when significant hemoptysis or hemothorax occurs, and pulmonary artery rupture is confirmed, the mortality rate is high (33% to 80%).10 Balloon tamponade of the vessel has been described,11 but this technique is not recommended. Lung isolation and angiography with pulmonary artery embolization is frequently effective.12 If hemothorax is present or embolization fails, emergency thoracotomy is indicated.13
Bleeding Tracheotomy
Massive hemoptysis in a patient with a tracheotomy may indicate tracheoarterial fistulae, usually involving the brachiocephalic artery. Initial treatment should include overinflation of the tracheotomy tube cuff and anterior and downward pressure on the tracheotomy.1 Emergency surgery may be required.
Aspergilloma
Aspergilloma is a granuloma within the lung resulting from infection by a species of the fungus Aspergillus. Hemoptysis due to a bleeding aspergilloma may be controlled acutely by bronchial arterial embolization or, failing this, by surgical resection. As an alternative to surgery in patients with limited cardiorespiratory reserve, antifungal drugs such as amphotericin B may be directly instilled into the lesion, either percutaneously or transbronchially.
Small Vessel Vasculitis
Occasionally, hemoptysis develops due to microvascular bleeding as a consequence of immune-mediated inflammatory processes such as Wegener granulomatosis, Goodpasture syndrome, and systemic lupus erythematosus. Treatment is largely independent of cause and involves immunosuppression (corticosteroids, cyclophosphamide) with or without plasmapheresis, and gamma globulin.14
1 Lordan JL, Gascoigne A, Corris PA. The pulmonary physician in critical care * Illustrative case 7: Assessment and management of massive haemoptysis. Thorax. 2003;58:814-819.
2 Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28:1642-1647.
3 Jougon J, Ballester M, Delcambre F, et al. Massive hemoptysis: what place for medical and surgical treatment. Eur J Cardiothorac Surg. 2002;22:345-351.
4 Naidich DP, Funt S, Ettenger NA, et al. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology. 1990;177:357-362.
5 Marshall TJ, Flower CD, Jackson JE. The role of radiology in the investigation and management of patients with haemoptysis. Clin Radiol. 1996;51:391-400.
6 Millar AB, Boothroyd AE, Edwards D, et al. The role of computed tomography (CT) in the investigation of unexplained haemoptysis. Respir Med. 1992;86:39-44.
7 Cremaschi P, Nascimbene C, Vitulo P, et al. Therapeutic embolization of bronchial artery: a successful treatment in 209 cases of relapse hemoptysis. Angiology. 1993;44:295-299.
8 Tanaka N, Yamakado K, Murashima S, et al. Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system. J Vasc Interv Radiol. 1997;8:65-70.
9 Cowling MG, Belli AM. A potential pitfall in bronchial artery embolization. Clin Radiol. 1995;50:105-107.
10 Abreu AR, Campos MA, Krieger BP. Pulmonary artery rupture induced by a pulmonary artery catheter: a case report and review of the literature. J Intens Care Med. 2004;19:291-296.
11 Thomas R, Siproudhis L, Laurent JF, et al. Massive hemoptysis from iatrogenic balloon catheter rupture of pulmonary artery: successful early management by balloon tamponade. Crit Care Med. 1987;15:272-273.
12 Ray CEJr, Kaufman JA, Geller SC, et al. Embolization of pulmonary catheter-induced pulmonary artery pseudoaneurysms. Chest. 1996;110:1370-1373.
13 Kearney TJ, Shabot MM. Pulmonary artery rupture associated with the Swan-Ganz catheter. Chest. 1995;108:1349-1352.
14 Schwarz MI, Brown KK. Small vessel vasculitis of the lung. Thorax. 2000;55:502-510.
15 Grocott HP, Scales G, Schinderle D, et al. A new technique for lung isolation in acute thoracic trauma. J Trauma. 2000;49:940-942.