Chapter 40 Management of Wound Dehiscence in Spinal Tumor Surgery
INTRODUCTION
The development of wound dehiscence and infection after spinal surgery for tumors is sometimes encountered. The incidence of superficial or deep wound infections is very high in patients who received preoperative radiation. The rate of infection in this patient group is about 10%. Patients requiring operation within a 6-week period after irradiation are at extremely high risk for developing wound complications.1 Acute radiation effects include inhibition of fibroblast outgrowth, resulting in a reduction of collagen formation and tensile wound strength. Many surgeons emphasize the importance of avoiding preoperative radiotherapy in patients who are candidates for surgery as a first-line therapy.2 The other risk factors for wound-related complications include the use of instrumentation, medical comorbidities, malnourishment, negative protein balance, multiple prior operations, and high-dose corticosteroid agents.1 In many patients, little can be done to ameliorate these risk factors; however, timely intervention may decrease the risk of other morbidities such as septicemia and deep venous thrombosis. In the past, wound correction has required serial debridements and raised significant concerns regarding instrumentation salvage.3 Recently, several authors have shown that instrumentation salvage is possible when using techniques such as serial debridements with antibiotic-impregnated beads, irrigation-suction systems, or muscle flaps.4 The trapezius or latissimus dorsi muscle turnover flaps and paraspinous advancement flaps are commonly used to treat wound dehiscence in patients undergoing surgery for spinal tumors. Flap selection for spinal wound repair is based on the anatomical location of the defect. The flap of choice for cervical spinal wound is the trapezius as a muscle only or as a musculocutaneous flap, based on the transverse cervical artery. For reconstruction of lower thoracic or upper lumbar spinal wounds, the latissimus dorsi muscle flap is the first choice. For the lower lumbar level wound, the paraspinal muscle flap is chosen. These muscle flaps provide vascularized muscle coverage that allows for instrumentation salvage with a limited number of wound debridements.
SURGICAL TECHNIQUES
TRAPEZIUS TRANSPOSITIONAL FLAP
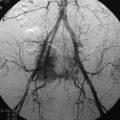
The trapezius muscle originates from the occipital bone and the seventh cervical and all thoracic vertebrae and inserts into the spine of the scapula, acromion, and clavicle. The muscle suspends the shoulder girdle and assists in raising and rotating the shoulder. The accessory nerve provides the innervation. The main blood supply to the trapezius muscle and overlying skin is from superficial and deep descending branches of the transverse cervical artery. The transverse cervical artery enters the trapezius muscle at the base of the neck and descends vertically along the deep surface of the trapezius muscle. The superficial descending branch often arises directly from the thyrocervical trunk and is named the transverse cervical artery. In this event, the deep descending branch arises directly from the subclavian artery and is termed the dorsal scapular artery. Generally, this area is not dissected during routine vertical trapezius flap harvesting. The dorsal scapular artery runs under the levator muscle, and its major branch penetrates between the rhomboid muscles and descends along the deep surface of the trapezius to supply the lower third of the trapezius.5 As a result, the muscle and overlying skin below the tip of the scapula generally receive their dominant blood supply from the dorsal scapular artery. The muscle and overlying skin above the tip of the scapula generally receive their dominant blood supply from the transverse cervical artery.6
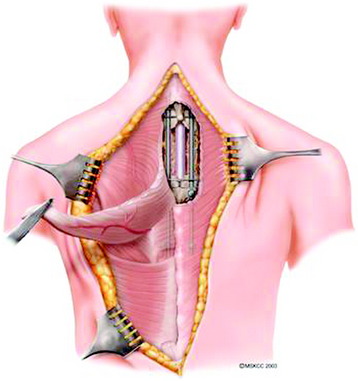
Trapezius muscle transpositional flaps are harvested through a midline incision in the region of the muscle, typically extending from the existing cervicothoracic incision inferiorly to the tip of the muscle. Skin and subcutaneous tissues are elevated, exposing the inferior half of the muscle, and the tissues are detached and elevated to the level of the scapula (Figs. 40-1 and 40-2). Care is taken to preserve the descending branch of the transverse cervical artery. The flap is turned on itself and transposed to obliterate the dead space and to cover the hardware of the cervical or upper thoracic defect (Fig. 40-3). As with all flaps, closed suction drains are placed under the skin flaps, and a layered skin closure is performed.
LATISSIMUS DORSI TRANSPOSITIONAL FLAP
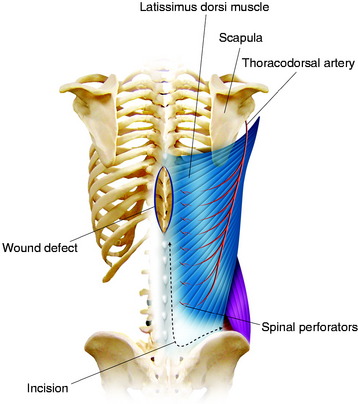
The transposition technique is first described (Figs. 40-4 and 40-5).7 After an appropriate size is outlined, the distal portion of the flap is incised. The incision is carried down through the thoracolumbar fascia. The dissection moves in this subfascial plane and in a cephalic direction until sufficient tissue is mobilized to meet the requirements of the defect. The lower attachment of the latissimus dorsi is incised; however, the spinal attachment to the muscle is preserved as much as possible. The humeral attachment is never released. Care should be taken to preserve as many posterior perforators of the lumbar and lower intercostal arteries as possible while still transferring a quantity of tissue that conforms well to the wound bed. To achieve wound closure in more caudal wounds, an island gluteus maximus flap based on its superior gluteal vascular pedicle can be transposed. A latissimus dorsi fasciocutaneous flap can cover fairly large defects if the wound is located mostly in the lower thoracic and upper to mid-lumbar area. In the lower thoracic and lumbar areas of the back, the local options for muscle flap reconstruction are anatomically limited. Essentially, three muscles lie nearby: the erector spinae muscle group, the latissimus dorsi muscle, and the gluteus maximus muscle.
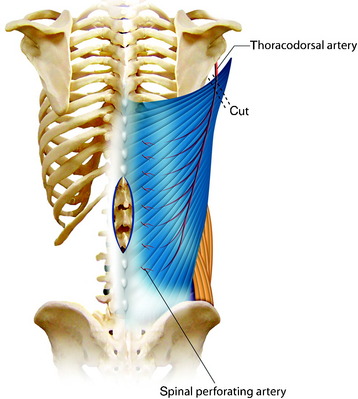
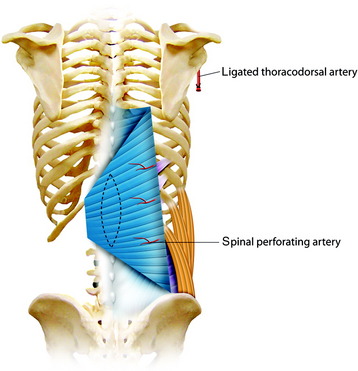
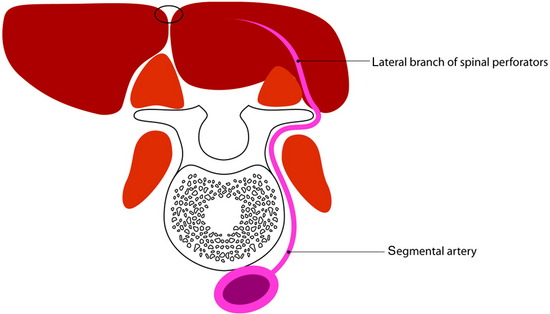
For the turnover (reverse) flap, the thoracolumbar pedicle is detached from the humerus and the flap is raised off the serratus muscle based on the two to three large intercostal perforating vessels that enter the muscle lateral to the paraspinous muscle mass (Fig. 40-6).8 The thoracodorsal artery, vein, and nerve are separated. The proximal portion of the flap is transposed to cover the defect. Anatomical studies have shown that the latissimus dorsi muscle has a dual blood supply. The dominant vascular branch contains the thoracodorsal artery as a branch of the subscapular artery. The latissimus dorsi muscle also receives a significant supply from the spinal perforator arteries that insert in the muscle close to the midline. If the latissimus dorsi muscle is detached at its insertion to the humerus, its vascular supply is then based on the secondary segmental perforators.
The skin incision must be made with the consideration of wide exposure of the wound site and the insertion site of the humerus. Once the incision has been made, it is advisable first to expose the lateral edge of the muscle and to mobilize the muscle proximally toward the humerus. The vascular nerve bundle of the thoracodorsal artery, vein, and nerve is exposed, tied off, and then detached. The detached proximal part of the latissimus dorsi muscle is dissected away from the belly of the teres major muscle and mobilized in the medial and distal directions (Fig. 40-7). The spinal perforator vessels are found about 5 cm from the midline, and three large intercostal perforator vessels arise from the 9th, 10th, and 11th intercostal spaces.9 After the proximal muscle belly has been turned back and tucked into the defect in the back, the muscle belly is sutured to the edges of the defect. Wound closure can be achieved directly by filling up the dead space with the muscle mass and adapting the edges of the wound, or by suturing the musculocutaneous material filling the wound to the edges of the wound, or using split-thickness skin grafts on one or both sides. Two drains are inserted into the donor site and left in place until the total volume of secretions is less than 50 mL per day.
PARASPINOUS MUSCLE ADVANCEMENT FLAP10
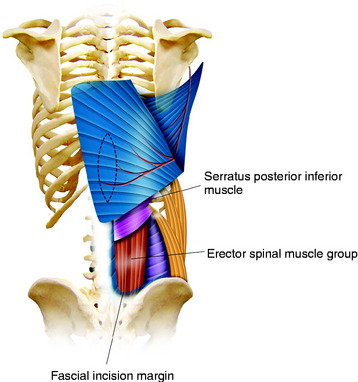
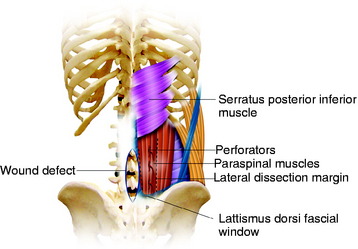
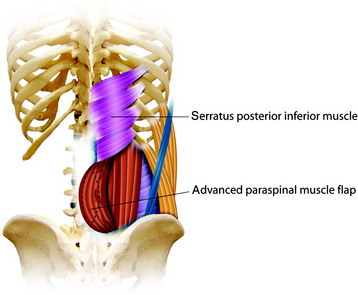
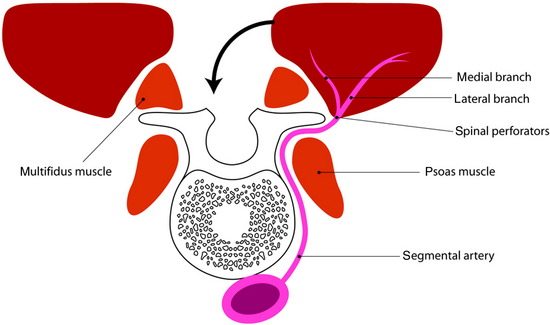
The paraspinous muscles are also known as the erector spinae muscles, which include the longissimus, iliocostalis, and spinalis portions. The paraspinous muscles are located deep to the latissimus dorsi muscles and thoracolumbar fascia. The paraspinous muscles originate inferiorly from the spinous processes and iliac crest and insert superiorly into the posteromedial aspect of the ribs. Creation of the paraspinous advancement flap is an ideal method for closing lower lumbar wound defects. The iliocostalis, spinalis, and longissimus muscles are mobilized. The paraspinous muscles are found by incising the thoracolumbar fascia medially and then elevating the underlying serratus posterior inferior muscles (Fig. 40-8). The portion of the paraspinous muscle required for spinal bone or hardware coverage is released from its origin on the spine. Then this portion of the paraspinous muscle is bluntly elevated from the multifidus muscle, transverse processes, intertransversarii muscles, and transversalis fascia from the medial to the lateral direction. This blunt dissection allows for mobilization and advancement of the paraspinous muscles in the medial direction, while preserving its lateral perforator vessels (Figs 40-9 and 40-10). Sometimes perforator vessels, which enter the muscle along its deep surface, limit muscle advancement to the midline, necessitating ligation of the medial branch of perforator vessels (Fig. 40-11). The muscle is sutured together at the midline in a manner that directs the bulk of the muscle medially and deep, thereby obliterating as much dead space as possible. The advantages of the paraspinal muscle flap are its technical simplicity, short operative time, and no requirement for additional incision for muscle dissection. Most spinal wounds are long and narrow, and the paraspinal muscles have a similar alignment to the wound. The paraspinal muscles can act as a biomechanical active column even if they are advanced medially.
To prevent wound-related complications, several measures are used in primary operation.2 At the end of the surgical procedure, extensive wound irrigation is performed before obtaining a hemostatic closure. Better coverage of soft tissue is provided by mobilizing the paraspinal muscles with simple relaxing incisions. Meticulous closure of the fascia should be made with a nonabsorbable monofilament suture material. Finally, drains are placed in all wounds to help prevent hematoma or seroma formation.
Case I
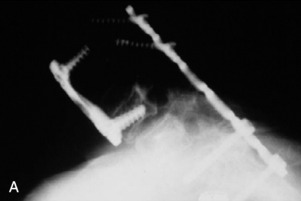
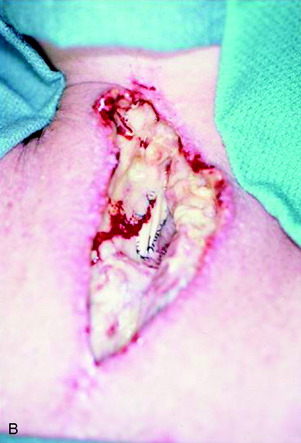
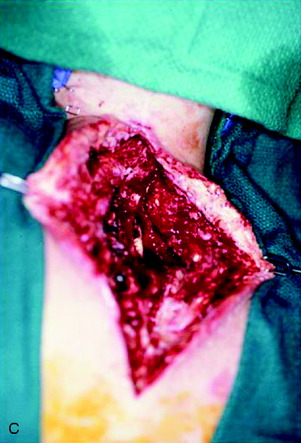
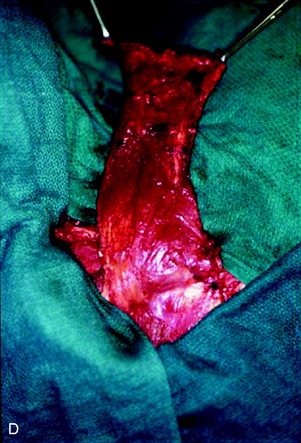
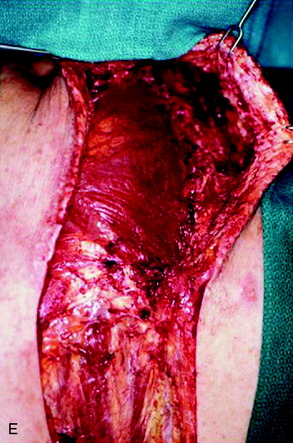
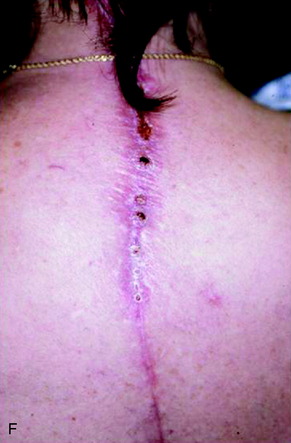
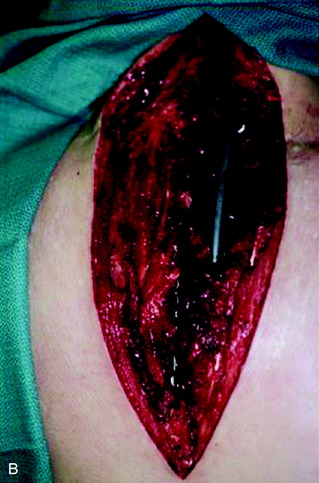
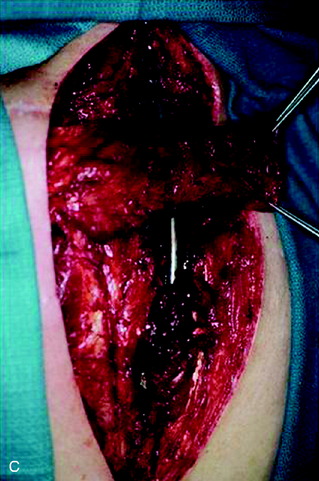
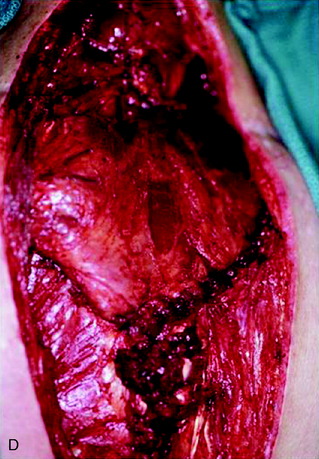
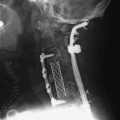
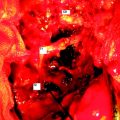
A 37-year-old woman was diagnosed with a malignant peripheral nerve sheath tumor. The mass was located at the C6–T1 levels and brachial plexus. The patient underwent surgery at the brachial plexus first, and radiation therapy was performed. The cervical spine operation was performed 1 year later. After the cervical spine mass was removed, posterior fixation was performed from C5 to T3. One year later, the posterior spine was revisioned and anterior fixation was added (Fig. 40-12, A). One more year passed, then intradural recurrence was detected. The intradural resection was performed with duraplasty. Ten days later, wound dehiscence was detected (Fig. 40-12, B). Several wound debridements were performed (Fig. 40-12, C). The trapezius muscle flap was elevated from the insertion of the spine (Fig. 40-12, D). The trapezius muscle turnover flap was inserted into the defect (Fig. 40-12, E). The wound healed completely (Fig. 40-12, F).
Case II
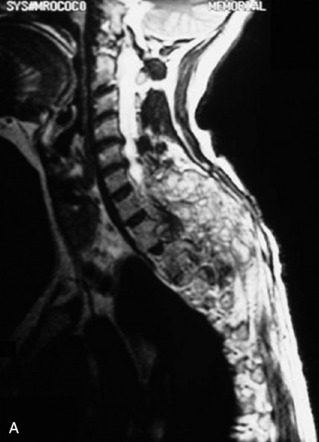
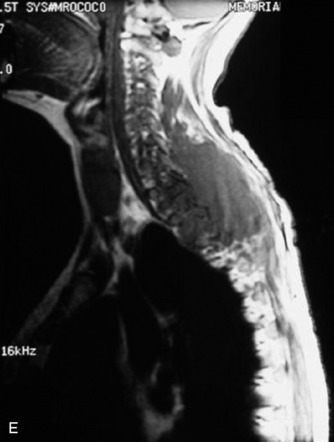
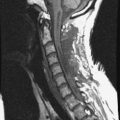
A 39-year-old woman who was diagnosed with chondrosarcoma of the right scapula underwent a right scapulectomy initially. One year later, a recurrent mass developed in the trapezius muscle and was resected. Seven months later, a left lung wedge resection was performed because of lung metastasis. Six months after the lung operation, posterior spine surgery was performed from C4 to T5 (Fig. 40-13, A). Postoperatively, the wound became infected and did not heal. The wound was open and the instruments were exposed (Fig. 40-13, B). The trapezius muscle flap was mobilized from the lower portion to cover the defect (Fig. 40-13, C). The mobilized muscle flap covered the defect completely (Fig. 40-13, D). Eventually, the wound healed and soft tissue coverage was completed on follow-up magnetic resonance imaging (MRI) (Fig. 40-13, E).