Management of Type 2 Diabetes Mellitus
Pharmacotherapy of Type 2 Diabetes
Insulin Sensitizers With Predominant Action in the Liver: Biguanides
Insulin Sensitizers With Predominant Action in Peripheral Insulin-Sensitive Tissues: Thiazolidinediones
Carbohydrate Absorption Inhibitors: α-Glucosidase Inhibitors
Practical Aspects of Initiating and Progressively Managing Type 2 Diabetes
Place of Glucose Management in the Treatment of Type 2 Diabetes
A comprehensive review of all the subtleties of diabetes management is beyond the scope of any chapter. The epidemiology, pathophysiology, and diagnosis of diabetes and its complications will not be reviewed, though arguably an understanding of these issues is integral in all treatment decisions. Treatment guidelines, lifestyle interventions, pharmacotherapy, the principles of cardiovascular risk-factor management, and upcoming trials that will inform treatment decisions in the future will be examined. An excellent source of information on these issues, which is updated annually, is the American Diabetes Association’s (ADA) Clinical Practice Recommendations.1 It is published as the first supplement to the journal Diabetes Care each January and is available online at www.diabetes.org by clicking “For Health Care Professionals.”
Over the last decade, a fundamental transformation of the principles of management of type 2 diabetes has occurred. Driven by a large number of multicenter randomized clinical trials documenting improved outcomes associated with glucose, blood pressure, and lipid management, guidelines have been established for diabetes treatment; increasingly, adherence to these guidelines is monitored and in some cases enforced by insurers and health care systems. In parallel, there has been a change in the level of concern about diabetes as a public health issue, and as a result, there have been changes in attitudes among patients and providers about its treatment. This has been driven by the recognition that we are in the midst of an epidemic of diabetes, well established in the United States2 and just emerging in much of the developing world.3 The estimated lifetime risk for developing diabetes for individuals born in the year 2000 in the United States is 32.8% for males and 38.5% for females, with Hispanic Americans having estimated lifetime risks for diabetes approximating 50%.4 The morbidity, mortality, and expense associated with diabetes are staggering. In Western society, people with diabetes are three times more likely to be hospitalized than nondiabetic individuals. In the United States, diabetes is the leading cause of blindness and accounts for over 40% of the new cases of end-stage renal disease. The risk of heart disease and stroke is two to four times higher, and the risk of lower-extremity amputation is approximately 20 times higher for people with diabetes than for those without diabetes.5 Although diabetes is the seventh leading cause of death in the United States, this is clearly an underestimate. Despite the fact that over 70% of people with diabetes die of heart disease and stroke, only approximately 10% have diabetes listed as a contributing cause on death certificates.6 Tragically, this enormous burden of death and disability has not been reduced by huge health care expenditures. In fact, the epidemic of diabetes is one of the drivers of increasing health care costs, with annual disbursements for people with diabetes approximately two to three times higher than expected in the absence of diabetes and accounting for at least 10% of health care expenditures in the United States.7 There is evidence that increased effort to control diabetes and its comorbidities can even reduce costs associated with diabetes and that a public health approach to diabetes can reduce the burden of complications of diabetes.8
Glucose Treatment Guidelines
Guidelines are optimally driven by the results of randomized multicenter clinical trials. Prospective randomized clinical trials have documented improved rates of microvascular complications in patients with diabetes treated to lower glycemic targets. In the U.K. Prospective Diabetes Study (UKPDS),9 patients with new-onset diabetes were treated with diet and exercise for 3 months, with an average reduction in glycosylated hemoglobin (Hb) or HbA1c (A1C) from approximately 9% to 7% (upper limit of normal 6%). Those with fasting plasma glucose (FPG) greater than 108 mg/dL (6 mM) after the dietary intervention were randomly assigned to one of two treatment policies. In the standard intervention, subjects continued the lifestyle intervention, and pharmacologic therapy was initiated only if the FPG reached 270 mg/dL (15 mM) or the patient became symptomatic. In the more intensive treatment program, all patients were randomly assigned to treatment with either sulfonylurea, metformin, or insulin as initial therapy, and doses increased in an effort to achieve an FPG less than 108 mg/dL. Additional agents were employed only if the patients became symptomatic or FPG became greater than 15 mM (270 mg/dL). As a consequence of the design, although the A1C fell initially to about 6% during the first year, over the average 10 years of follow-up, it rose to approximately 8% in the intensive treatment group. The average A1C in the standard treatment group was approximately 1% higher throughout the study. The risk of severe hypoglycemia was small—on the order of 1% to 5% per year in the insulin-treated group—and weight gain was modest; both were higher in patients randomly assigned to insulin and lower in those receiving metformin.10 Associated with this improvement in glycemic control, there was a reduction in the risk of microvascular complications (retinopathy, nephropathy, and neuropathy) in the intensive group. Although there was a trend toward reduced rates of macrovascular events in the more intensively treated group, it did not reach statistical significance.
Similar reductions in microvascular events were observed in another trial of entirely different design and much smaller size. In the Kumamoto study, Japanese patients of normal weight with type 2 diabetes treated with insulin were randomly assigned to standard treatment or an intensive program of insulin therapy designed to achieve normal glycemia. The control group maintained A1C values at approximately 9%, whereas A1C in the intensive group was reduced to approximately 7%, and that separation was maintained for 6 years. Again, there was a modest increased risk of hypoglycemia and weight gain, a reduction in microvascular complications, and a statistically non-significant trend toward reduced rates of vascular endpoints in the more intensively treated patients.11
In 2008, three studies examining the effects of two levels of glycemic control on cardiovascular endpoints in type 2 diabetes were reported. Action to Control Cardiovascular Risk in Diabetes (ACCORD),12 Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE),13 and the Veterans Affairs Diabetes Trial (VADT)14 each randomized middle-aged and older individuals at high risk for cardiovascular events. ACCORD and VADT aimed for an A1C target of less than 6%, using complex combinations of oral agents and insulin. ADVANCE aimed for an A1C target of less than or equal to 6.5%, using a somewhat less intensive approach based on the addition of the sulfonylurea gliclazide. None of the trials demonstrated a statistically significant benefit on combined vascular endpoints. ACCORD demonstrated a 22% increase in total mortality, while VADT had numerically more deaths in the intensive group (hazard ratio 1.07). Both VADTs and ADVANCE showed modest reductions in microvascular outcomes. In these studies, there were suggestions that people without clinical cardiovascular disease (CVD), shorter-duration disease and lower baseline A1C had greater benefits from the more intensive glucose-lowering strategies. Furthermore, a 10-year follow-up of the UKPDS cohort showed that the relative benefit of more intensive management of glucose at the end of the randomized portion of the trial was maintained, resulting in the emergence of statistically significant benefits on CVD endpoints and total mortality.15 Meta-analysis of cardiovascular outcomes in randomized trials suggests that with an average A1C reduction of 0.9%, there is a 17% reduction in nonfatal myocardial infarction and a 15% reduction in coronary heart disease (CHD), without significant effects on stroke or all-cause mortality. However, as discussed earlier, there is significant heterogeneity in the result with respect to mortality across trials, the etiology of which is completely uncertain.16
In Table 22-1, guidelines from the ADA1 and the American College of Endocrinology17 (ACE) are presented. The ADA, along with the American Heart Association and the American College of Cardiology Foundation, examined the results of the recently published cardiovascular outcomes studies.18 They reaffirmed the general A1C goal of less than 7% to reduce the incidence and progression of microvascular disease. Though no trial has demonstrated CVD benefit to improved glycemic control, they suggested that the general goal of less than 7% appeared reasonable from the perspective of macrovascular disease risk. However, they highlighted the need for individualization, suggesting that lower A1C goals than the general goal of less than 7% could be suggested for patients with short-duration disease, long life expectancy, and no significant CVD, if they could be achieved without significant hypoglycemia or other adverse effects of treatment. Furthermore, they suggested that less stringent goals may be appropriate for those with a history of severe hypoglycemia, limited life expectancy, advanced complications, extensive comorbid conditions, or those who have difficulties achieving the general A1C target of less than 7% despite concerted effort, including patient education, monitoring, and effective doses of multiple glucose-lowering agents including insulin.
Table 22-1
Guidelines for Targets in Glycemia Management from the American Diabetes Association (ADA) and American College of Endocrinology (ACE)

*The ADA further recommends: (1) goals should be individualized; (2) certain populations (children, pregnant women, and elderly) require special considerations; (3) less intensive goals may be indicated in patients with severe or frequent hypoglycemia; (4) based on epidemiologic analysis, more stringent glycemic goals (i.e., a normal A1C, <6%) may further reduce complications at the cost of increased risk of hypoglycemia; and (5) postprandial glucose may be targeted if A1C goals are not met despite reaching preprandial glucose goals.
†Postprandial glucose measurements should be made 1 to 2 hours after the beginning of the meal, generally peak levels in patients with diabetes.
Adapted from American Diabetes Association: Standards of medical care in diabetes, Diabetes Care 32:S13–S61, 2009; and American College of Endocrinologists: American College of Endocrinology Medical Guidelines for Clinical Practice for the Management of Diabetes Mellitus, Endocr Pract 13(Suppl 1):16–34, 2007.
With respect to fasting, premeal, or postprandial targets, there is little support for any particular level of glycemic control in the management of type 2 diabetes insofar as no large-scale outcome study has targeted particular levels of glucose with home glucose monitoring. The ADA target of fasting and premeal plasma glucose levels of 70 to 130 mg/dL (3.9 to 7.2 mM) is based on an estimate of the range of average glucose values that would be associated with a low risk of hypoglycemia and an A1C less than 7%. The American College of Endocrinology target of less than 110 mg/dL (6 mM) is an effort to achieve normal levels of glycemia. However, it should be recognized that consistent fasting and premeal glucose levels less than 110 mg/dL would be expected to be associated with an HbA1c of approximately 5.5% or lower.19
There are limited studies in which even safety, much less a clinical outcome, is documented for targeting a particular level of postprandial glucose. There are effective A1C-lowering agents that primarily target postprandial glucose levels. Monitoring postprandial glucose levels may allow more effective dose adjustment of these agents, though even this has not been demonstrated in clinical trials. Certainly there are patients with diabetes who have average fasting glucose levels within targets but whose A1C remains elevated. Monitoring and specifically treating postprandial elevations in these patients may provide improvements in A1C, perhaps with a lower risk of hypoglycemia and weight gain than further lowering fasting and premeal glucose levels. The ACE guidelines recommend targeting a 2-hour postprandial glucose level less than 140 mg/dL (7.8 mM) in an effort to achieve near-normal glycemia. Consistent postprandial glucose values less than 140 mg/dL would be associated with average A1C levels of approximately 5% or lower; 2-hour postprandial glucose levels of less than 180 mg/dL (10 mM) would generally be associated with A1C levels of 6% to 7% and is the recommended postprandial target of the ADA.1,19
Lifestyle Intervention
Education of Patients
Arguably, over the last 10 years, nothing has changed more fundamentally in diabetes care than the emphasis on lifestyle intervention. For decades, physicians and patients paid lip service to the notion that lifestyle intervention is important. Now we have significant clinical trial evidence that each component of lifestyle intervention, when appropriately administered, can contribute to improved outcomes.20,21 Furthermore, since the Balanced Budget Act of 1997 and the passage of complementary legislation by most state governments, lifestyle intervention has become a covered benefit for most patients with diabetes in the United States.
As defined by the ADA,22 diabetes self-management education is the process of providing the person with diabetes the knowledge and skills needed to perform self-care, manage crises, and make lifestyle changes. As a result of this process, the patient must become a knowledgeable and active participant in the management of his or her disease. To achieve this rather daunting goal, patients and providers work together in a long-term, ongoing process. Comprehensive diabetes education should be individualized, with emphasis on the issues highlighted in Table 22-2. There are many more specialized topics relevant to almost all patients, such as how to adjust therapy when eating out or during travel, as well as how to access available local health care resources and negotiate the complexity of health care financing in the United States. Although only limited studies are published to date, as a body of work, they do provide support for the concept that diabetes education can be cost effective and can improve outcomes.21–23
Table 22-2
Curricular Areas Which Should Be Addressed in Diabetes Self-Management Education
Pathophysiology of the patient’s diabetes and its relationship to treatment options
Incorporating appropriate nutritional management in the treatment program
Incorporating physical activity into the treatment program
Using medications (if applicable) for therapeutic effectiveness
Monitoring blood glucose, urine ketones (when appropriate), and using the results to improve control
Preventing, detecting, and treating acute complications, including “sick-day rules” and hypoglycemia management
Preventing, detecting, and treating chronic complications
Goal setting to promote health and problem solving for daily living
Integrating psychosocial adjustment to daily life
Promoting preconception care, management during pregnancy, and gestational diabetes management (if applicable)
Adapted from American Diabetes Association: Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications, Diabetes Care 25:202–212, 2002.
A team of providers is generally required to optimally implement the process of diabetes self-management education, because the amount of information that needs to be exchanged is large and the range of expertise required is broad. It is generally impossible to cover the recommended content fully in the context of several or even many brief encounters with a physician in an office setting. Potential providers in a team care approach could include nurses, dietitians, exercise specialists, behavioral therapists, pharmacists, and other medical specialists including diabetologists or endocrinologists, podiatrists, medical subspecialists, obstetrician-gynecologists, psychiatrists, and surgeons. In the diabetes self-care process, the potential role of the community where the patient lives and works is enormous. At a minimum, this community includes family, friends, employers, health care systems, and health care insurers. Each member of the team has a role to play in the process, and it is useful to review these roles frequently (Table 22-3). The primary role of the providers in this process is to provide guidance in goal setting to manage the risk of complications, suggest strategies to achieve goals and techniques to overcome barriers, provide training in skills, and screen for complications. For this process to be a success, the patient must commit to the principles of self-care, participate fully in the development of a treatment plan, make ongoing decisions regarding self-care from day to day, and communicate honestly and with sufficient frequency with the team.
Table 22-3
Team Care: Roles of the Players
Role of Providers
To be a source of accurate information and to refer to and coordinate with other sources of information as necessary
To provide guidance in developing goals of treatment
To screen for complications and evaluate progress in meeting treatment goals
To help develop strategies to achieve treatment goals and avoid complications
Fortunately, barriers to providing team care are becoming less daunting, in large measure because of the rapidly expanding number of diabetes education programs and improved insurance coverage for services. The American Association of Diabetes Educators (800-TEAM-UP4; www.diabeteseducator.org) and the ADA (800-DIABETES; www.diabetes.org) can provide information regarding diabetes educators and education programs nationwide.
For diabetes care to be effective, communication and mutual respect among the patient-centered team is critical. Unfortunately, in many communities, the full benefit of the consultation and ongoing care with diabetes educators, nurses, dietitians, pharmacists, medical consultants, and primary care providers is not achieved because of overly hierarchical approaches to care. Non–health care professionals, such as lay or peer supporters, can provide benefit to patients in developing effective diabetes self-care behaviors. Key functions of such efforts have been identified and include assistance in managing and living with diabetes in daily life, social and emotional support, and linkage to clinical care.23
Perhaps some of the most overlooked contributors to ineffective care in the setting of type 2 diabetes are the relatively common barriers created by psychiatric, neurocognitive function, and adjustment disorders, which are largely responsive to psychosocial therapies.24
Nutrition
The ADA has published technical reviews that exhaustively document the literature regarding the effect of medical nutrition therapy and specific advice on diabetes-related outcomes such as A1C and weight, as well as a position statement.25–27 These are summarized in Table 22-4. An individually negotiated nutrition program in which each patient’s circumstances, preferences, cultural background, and the overall treatment program are considered is most likely to result in optimal outcomes. Ideally, a registered dietitian with specific skill and experience in implementing nutrition therapy in diabetes management should work collaboratively with the patient and other health care team members in providing medical nutrition therapy. For optimum outcomes, this counseling should be performed over a series of visits initially, with intermittent follow-up thereafter. Analogously, physicians and other members of the health care team need to support the nutritional plan developed collaboratively.
Table 22-4
ADA Nutritional Principles and Recommendations
Carbohydrates
• Foods containing carbohydrate from whole grains, fruits, vegetables, and low-fat milk should be included in a healthy diet.
• With regard to the glycemic effects of carbohydrates, the total amount of carbohydrate in meals or snacks is more important than the source or type. However, attention to glycemic index can provide additional benefit over that observed when total carbohydrate is considered alone.
• Since sucrose does not increase glycemia to a greater extent than isocaloric amounts of starch, sucrose, and sucrose-containing foods in the context of a mixed meal, they do not need to be restricted by people with diabetes; however, they should be substituted for other carbohydrate sources in the context of an appropriate meal plan or, if added, covered with insulin or other glucose-lowering medication.
• Nonnutritive sweeteners are safe when consumed within the acceptable daily intake levels established by the U.S. Food and Drug Administration.
• Individuals receiving fixed daily insulin doses should try to be consistent in day-to-day carbohydrate intake.
• Individuals receiving intensive insulin therapy should adjust their premeal insulin doses based on the carbohydrate content of meals.
• As with the general public, consumption of dietary fiber is to be encouraged; however, there is no reason to recommend that people with diabetes consume a greater amount of fiber than others.
• Low-carbohydrate diets are not recommended in the management of diabetes, though they can be useful in reducing triglycerides. Although dietary carbohydrate is the major contributor to postprandial glucose concentration, it is an important source of energy, water-soluble vitamins and minerals, and fiber. Restricting total carbohydrate to less than 130 g/d is not recommended.
Proteins
• In people with controlled type 2 diabetes, ingested protein does not increase plasma glucose concentrations, although protein is just as potent a stimulant of insulin secretion as carbohydrate.
• For persons with diabetes, especially those not in optimal glucose control, the protein requirement may be greater than the recommended dietary allowance but not greater than usual intake.
Fats
• Less than 10% of energy intake should be derived from saturated fats. Some individuals (i.e., persons with LDL cholesterol ≥100 mg/dL) may benefit from lowering saturated fat intake to <7% of energy intake.
• Dietary cholesterol intake should be <300 mg/d. Some individuals (i.e., persons with LDL cholesterol ≥100 mg/dL) may benefit from lowering dietary cholesterol to <200 mg/d.
• To lower LDL cholesterol, energy derived from saturated fat can be reduced if weight loss is desirable or replaced with either carbohydrate or monounsaturated fat when weight loss is not a goal.
• Intake of trans unsaturated fatty acids should be minimized.
• Reduced-fat diets, when maintained long term, contribute to modest loss of weight and improvement in dyslipidemia.
• Two to three servings of fish per week provide dietary n-3 polyunsaturated fat and can be recommended.
• Polyunsaturated fat intake should be ≈10% of energy intake.
Energy Balance
• In insulin-resistant individuals, reduced energy intake and modest weight loss improve insulin resistance and glycemia in the short term.
• Weight loss is recommended for all overweight (BMI 25-29.9 kg/m2) or obese (BMI ≥ 30.0 kg/m2) adults who have, or who are at risk for developing, type 2 diabetes. The primary approach for achieving weight loss is therapeutic lifestyle change, which includes a reduction in energy intake and an increase in physical activity.
• A moderate decrease in caloric intake (500-1000 kcal/d) will result in a slow but progressive weight loss (1-2 lb/wk). For most patients, weight-loss diets should supply at least 1000-1200 kcal/d for women and 1200-1600 kcal/d for men.
• Structured programs that emphasize lifestyle changes, including education, reduced fat (<30% of daily energy) and energy intake, regular physical activity, and regular participant contact, can produce long-term weight loss on the order of 5%-7% of starting weight.
• Exercise and behavior modification are most useful as adjuncts to other weight-loss strategies. Exercise is helpful in maintenance of weight loss.
• Standard weight reduction diets, when used alone, are unlikely to produce long-term weight loss. Structured intensive lifestyle programs are necessary.
Micronutrients and Alcohol
• There is no clear evidence of benefit from vitamin or mineral supplementation in people with diabetes who do not have underlying deficiencies. Exceptions include folate for prevention of birth defects and calcium for prevention of bone disease.
• Routine supplementation of the diet with antioxidants is not advised because of uncertainties related to long-term efficacy and safety.
• If individuals choose to drink alcohol, daily intake should be limited to one drink for women and two drinks for men. One drink is defined as 12 oz of beer, 5 oz of wine, or 1.5 oz of distilled spirits. To reduce risk of hypoglycemia, alcohol should be consumed with food.
Adapted from Klein S, Sheard NF, Pi-Sunyer X, et al: Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition, Diabetes Care 27:2067–2073, 2004; Gillespie SJ, Kulkarni KD, Daly AE: Using carbohydrate counting in diabetes clinical practice, J Am Diet Assoc 98:897–905, 1998; and Egede LE, Ye K, Zhang D, et al: The prevalence and pattern of complementary and alternative medicine use in individuals with diabetes, Diabetes Care 25:324–329, 2002.
In general, the critical nutrient for glycemic control is carbohydrate. Essentially all carbohydrates consumed are converted to glucose in the gut and require the action of insulin to be cleared from the circulation. A dietary technique called carbohydrate counting can be used in patients with type 2 diabetes to facilitate consistent carbohydrate intake or to allow insulin dose adjustment in response to changes in carbohydrates consumed.28 Although this technique requires less insulin than fixed meal dosing and may help curb weight gain, it has not been shown to improve glycemic control or reduce the rate of hypoglycemia in patients with type 2 diabetes.29 Whereas the β cell in type 2 diabetes has generally lost its responsiveness to glucose, the second phase of insulin secretion is largely spared in type 2 diabetes and is in part driven by amino acids and fatty acids. Therefore, including some protein and fat in each meal and snack may be useful.
Dietary protein similarly has a minimal impact on glucose levels, although as mentioned, amino acids do promote insulin secretion. Metabolism of protein results in the formation of acids and nitrogenous waste that may result in bone demineralization and glomerular hyperfiltration. At least 0.8 g of high-quality protein per kilogram is generally recommended. Protein restriction in the setting of kidney disease has been recommended and is more fully discussed in Chapter 28. There is no evidence that protein intake materially effects the risk of developing kidney disease in patients with diabetes.
The role of vitamins, trace minerals, and nutritional supplements in the treatment of diabetes is poorly understood. There are some patients and providers who are absolutely convinced of the utility of soluble fiber, magnesium, chromium, zinc, folic acid, pyridoxine, cyanocobalamin, vitamin A, vitamin C, vitamin E, vanadium, selenium, garlic, and others. Clinical trial data to support their safety and efficacy are inconclusive. Many patients are convinced that nutritional supplementation is healthful, and it is often counterproductive to engage in scholarly discussion of the nature of the evidence base for their decision. At a minimum, discussion should include the documented efficacy of lifestyle and pharmacologic interventions and the idea that these efforts should not be left by the wayside when budgetary constraints affect potentially more effective interventions.30,31 A multivitamin/mineral preparation may be reasonable for most patients with diabetes. A recent randomized control trial in patients with diabetes demonstrated fewer self-reported infections and related absenteeism.32 Studies demonstrating the benefits of B-vitamin supplementation on restenosis after angioplasty33 have recently been called into question; the possibility has been raised that such therapy could increase rates of restenosis after stent placement.34
Although there are proponents of a wide range of dietary composition, there are few data to support these recommendations from long-term outcome studies of prescribed diets. Mixed meals containing 10% to 20% of calories from protein, no more than 10% of calories from saturated fat, no more than 10% from polyunsaturated fats, and the remainder largely from monounsaturated fats (seeds, nuts, avocados, olives, olive oil, canola oil) and carbohydrates, particularly whole grains, fruit, vegetables, and low-fat milk, are probably most reasonable. High-carbohydrate, low-fat diets, although historically recommended by many health organizations, have been shown to increase postprandial blood glucose and triglyceride levels, elevate fasting triglyceride levels, and decrease high-density lipoprotein (HDL) cholesterol levels in insulin-resistant people, including those with type 2 diabetes. Several studies have demonstrated improved lipid levels and blood glucose control in both short- and intermediate-term studies in which total fat intake approaches 45% of calories and carbohydrate intake is as low as 40% of calories. Reducing fat or carbohydrate intake in obese individuals will not necessarily lead to reduced calories. Since weight loss will occur only in the setting of caloric restriction, arguably the most appropriate approach is to limit intake of both fat and highly processed, easily digestible carbohydrates. The treatment of obesity is discussed in Chapter 2; the principles discussed are appropriate when type 2 diabetes is complicated by obesity. To date, short-term studies of medical nutrition therapy, physical activity, and comprehensive lifestyle approaches have been shown to improve the control of classic CVD risk factors, as well as intermediate markers of CVD risk such as C-reactive protein; no long-term, large-scale study of intentional weight loss has been powered to examine CVD endpoints. Look AHEAD (Action for Health in Diabetes) will examine CVD events for up to 11.5 years in a study in which patients with type 2 diabetes 45 to 74 years of age with a body mass index = 25 kg/m2 will be recruited. Patients will be randomized to a 4-year intensive weight loss program (calorie restriction and physical activity) or to diabetes support and education. With planned recruitment of 5000 patients at 16 centers over 2.5 years, the study is designed to provide a 0.90 probability of detecting an 18% difference in major CVD event rates between arms.35
Exercise
There is a substantial body of literature supporting exercise as a modality of treatment in type 2 diabetes, including a recent technical review by the ADA.36,37 The recommendations of this technical review are summarized in Table 22-5. Exercise is perhaps the single most important lifestyle intervention in diabetes, because it is associated with improved glycemic control, insulin sensitivity, cardiovascular fitness, and cardiac remodeling. Aerobic exercise and resistance (strength) training both have a positive impact on glucose control. Improvements in glycemic control are generally apparent immediately, become maximal after a few weeks of consistent exercise, but only persist for 3 to 6 days after the cessation of training. To maintain effects on glycemia, a minimum of three exercise sessions a week is suggested, with no more than 2 days rest between sessions.
Table 22-5
ADA Recommendations Regarding Physical Activity in People with Type 2 Diabetes
Indications for Graded Exercise Test With ECG Monitoring
In the absence of contraindications, a graded exercise test with ECG monitoring should be seriously considered before undertaking aerobic physical activity with an intensity exceeding the demands of everyday living (more intense than brisk walking) in previously sedentary diabetic individuals whose 10-year risk of a coronary event is ≥10%. This risk could be estimated directly using the UKPDS Risk Engine (www.dtu.ox.ac.uk/riskengine/download.htm) and would correspond approximately to meeting any of the following criteria:
• Age >40 years, with or without CVD risk factors other than diabetes
These criteria should not be construed as a recommendation against stress testing for individuals without the above risk factors or for those who are planning less intense exercise. [Note: The most recent ADA recommendations 1 do not advocate routine stress testing in this setting.]
Aerobic Exercise
The amount and intensity recommended for aerobic exercise vary according to goals.
• To improve glycemic control, assist with weight maintenance, and reduce risk of CVD, recommend at least 150 min/wk of moderate-intensity aerobic physical activity (40% to 60% of VO2max or 50% to 70% of maximum heart rate) and/or at least 90 min/wk of vigorous aerobic exercise (>60% of VO2max or >70% of maximum heart rate). The physical activity should be distributed over at least 3 days/wk and with no more than 2 consecutive days without physical activity.
• Performing ≥4 hr/wk of moderate to vigorous aerobic and/or resistance exercise is associated with greater CVD risk reduction compared with lower volumes of activity.
• For long-term maintenance of major weight loss (≥13.6 kg [30 lb]), larger volumes of exercise (7 hr/wk of moderate or vigorous aerobic physical activity) may be helpful.
Resistance Exercise
• In the absence of contraindications, people with type 2 diabetes should be encouraged to perform resistance exercise three times a week, including all major muscle groups, progressing to three sets of 8-10 repetitions at a weight that cannot be lifted greater than 8-10 times.
• To ensure resistance exercises are performed correctly, maximize health benefits, and minimize the risk of injury, initial supervision and periodic reassessments by a qualified exercise specialist are recommended.
CAD, Coronary artery disease; CVD, cardiovascular disease; ECG, electrocardiograph.
Adapted from Sigal RJ, Kenny GP, Wasserman DH, et al: Physical activity/exercise and type 2 diabetes, Diabetes Care 27:2518–2539, 2004.
Recommendations regarding stress testing before initiating an exercise program in previously sedentary people are provided in some detail in Table 22-5. The utility of such treadmill tests in potentially low-risk populations is limited by their poor sensitivity and specificity.38,39 It should also be noted that in an older asymptomatic population with normal electrocardiograms, adenosine technetium-99m sestamibi single-photon emission-computed tomography myocardial perfusion imaging revealed that over 20% of patients studied had silent ischemia. However, in 5-year follow-up, there was a spontaneous remission of abnormalities in many, generally very low event rates, and no difference in outcomes based on whether stress testing was performed or not.40 If the planned exercise program does not involve more strenuous (both intensity and duration) activity than the patient has engaged in recently but merely involves more frequent activity, screening cardiovascular stress testing is unlikely to be particularly useful.
Self-Monitoring of Blood Glucose
Self-monitoring of blood glucose (SMBG) has not been demonstrated in clinical trials to substantially change outcomes in non-insulin-treated type 2 diabetes when evaluated in isolation and may be associated with decreased quality of life.41,42 However, many diabetes self-management programs have been associated with improved glycemic control. In all of these, SMBG was integral to the overall process, suggesting that SMBG is at least a component of effective therapy. The frequency and type of glucose monitoring should be determined in collaboration with the patient, taking into account the overall treatment plan and goals, the patient’s abilities, and the stage of diabetes. SMBG can theoretically improve the safety of treatment with insulin or sulfonylureas, since it allows the identification of minimal or asymptomatic episodes of hypoglycemia. Coupled with appropriate education, dose adjustments, or modest changes in the lifestyle plan, SMBG can minimize risk of severe hypoglycemia or weight gain. Although severe hypoglycemia is relatively rare in type 2 diabetes, it is more common in the elderly and can have devastating consequences, such as physical injury as a result of trauma or a change in the perceived ability of a patient to continue to live independently as a result of confusion or loss of consciousness. In general, it is optimal to have patients use glucose monitoring to assess the nature of any hypoglycemic symptoms they experience. Many patients are fearful of hypoglycemia and routinely consume extra calories in response to a variety of life’s circumstances—such as when they are hungry, sweaty, nervous, or upset—without documenting hypoglycemia. Most “hypoglycemic” symptoms in patients with type 2 diabetes are not related to hypoglycemia and do not need to be treated with calorie consumption. Counseling patients to carry commercially available glucose tablets and glucose monitoring equipment at all times and to take a 15-g dose of carbohydrate for documented mild to moderate hypoglycemia helps patients avoid excessive calorie intake and recreational consumption of sweets for treatment of hypoglycemia.
Finally, one of the most difficult areas for health care providers to remain current is in the area of available equipment and supplies, particularly for glucose monitoring. Diabetes educators often have demonstration models and a robust understanding of patient characteristics that match well or poorly with particular devices; they can be exceptionally helpful to patients in selecting appropriate equipment. A useful resource in this regard is the annual Resource Guide, which comes out as the January issue of Diabetes Forecast, a magazine for lay people with diabetes and their families. It is available online at http://forecast.diabetes.org/magazine/archive to find the most recent January issue.
Pharmacotherapy of Type 2 Diabetes
The revolution in the treatment of type 2 diabetes since 1995 in the United States has been driven by the release of multiple new classes of drugs that independently address different pathophysiologic mechanisms that contribute to the development of diabetes. The relative benefits of lifestyle intervention and the eight classes of drugs available for the management of type 2 diabetes are found in Table 22-6. This area has been the subject of extensive reviews and recent guidelines from professional groups.43–44 Because of limitations of space, basic principles are summarized with recent reviews, along with limited additional references derived from intensive management in the setting of randomized clinical trials and clinical practice.
Table 22-6
Comparisons of Therapies for Type 2 Diabetes
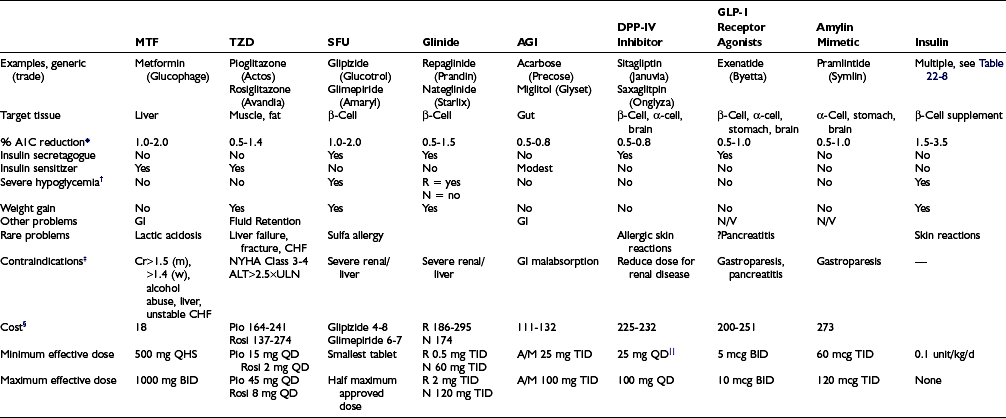
*Reduction as monotherapy; the reduction in A1C is proportionate to baseline A1C.43
†In the absence of cotreatment with agents that otherwise cause hypoglycemia.
§Generic price where available, per month at min-max dose, Consumer Reports, http://www.consumerreports.org/health/best-buy-drugs/type2diabetes.htm.
Insulin Sensitizers with Predominant Action in the Liver: Biguanides
Metformin is the only biguanide available in the United States and the subject of a recent review.45 Phenformin was removed from the United States market in the 1970s because of deaths associated with lactic acidosis. Metformin activates adenosine monophosphate–activated protein kinase (AMPK), a critical molecule in insulin signaling with effects on metabolism and energy balance. Metformin reduces hepatic insulin resistance, gluconeogenesis, and glucose release.46
The issue of greatest concern regarding metformin therapy is lactic acidosis, which is quite rare and occurs almost exclusively in patients who are otherwise at high risk of developing lactic acidosis.47 When used prudently, the risk of lactic acidosis is virtually zero. Patients at increased risk of developing lactic acidosis due to baseline medical conditions should avoid taking metformin.48 The package insert suggests that metformin is absolutely contraindicated in patients with renal insufficiency, because the drug is cleared renally.49 The drug should not be used in males with a serum creatinine greater than or equal to 1.5 mg/dL or in females at 1.4 mg/dL. There have been recent suggestions that metformin dosing should be based on estimated glomerular filtration rate (eGFR), with contraindication to use in stage 4 to 5 chronic kidney disease (eGFR < 30 mL/min/1.73 m2) and caution with eGFR less than 50 mL/min/1.73 m2.50
Metformin was originally contraindicated in patients with congestive heart failure requiring treatment. However, recent reports suggest improved morbidity and mortality in patients with stable compensated heart failure.51 This prompted the U.S. Food and Drug Administration (FDA) to recommend a change in labeling in 2005 to allow its use in patients with stable compensated heart failure without other contraindications to its use.52
Metformin has the strongest record of achievement of antidiabetic agents in outcome studies in patients with type 2 diabetes. In the UKPDS, among overweight subjects, those randomly assigned to metformin not only had improvements in microvascular complications similar to those of subjects randomly assigned to insulin and sulfonylurea but also exhibited a reduction in diabetes-related deaths and myocardial infarction.10 The validity of this observation has been challenged because of unusual responses in a subsequent subrandomization. Beneficial effects of metformin on macrovascular complications through glucose-independent mechanisms are plausible and suggested by consistent observations, such as metformin-associated reductions in low-density lipoprotein (LDL) and procoagulant factors.53
Insulin Sensitizers with Predominant Action in Peripheral Insulin-Sensitive Tissues: Thiazolidinediones
The thiazolidinedione class of drugs, often termed TZDs or glitazones, has engendered great enthusiasm and controversy since the first agent, troglitazone, was approved in 1997.54–55 Troglitazone was withdrawn from the U.S. market in the year 2000 as a result of rare cases of fulminant hepatic necrosis, and because pioglitazone and rosiglitazone appear to be safer. This class of oral antidiabetic agents is believed to work by binding to and modulating the activity of a family of nuclear transcription factors termed peroxisome proliferator–activated receptors (PPARs), particularly PPAR-γ. They are associated with slow improvement in glycemic control over weeks to months in parallel with an improvement in insulin sensitivity and reduction of free fatty acid (FFA) levels. Pioglitazone and rosiglitazone are generally well tolerated, with weight gain and fluid retention the most common adverse effects. There is no substantial evidence that these newer agents are associated with hepatotoxicity, but this record of safety has been established in the setting of careful liver function test monitoring. Therefore, it is important to continue to recommend that glitazones not be used in patients with active hepatocellular disease or with unexplained serum alanine aminotransferase levels greater than 2.5 times the upper limit of normal; routine monitoring of liver function tests with these glitazones is no longer recommended.56,57
A promising attribute of the glitazones that has generated great interest is an effect to improve insulin secretory dynamics in subjects with diabetes and impaired glucose tolerance. Initial reports suggest that these agents provide for more durable glycemic control than other antidiabetic agents.58 These latter observations provide hope that pioglitazone and rosiglitazone may be useful not only in diabetes prevention but also in halting the progression of established diabetes, thus reducing the need for additional drug therapy over time.59
Early studies from small, short-term, randomized controlled trials suggested that the glitazone agents have important cardiovascular benefits, including reduced progression of carotid intimal medial thickness, decreased rates of in-stent restenosis in the setting of percutaneous coronary intervention, normalization of vascular endothelial function, improvements in dyslipidemia, reduction of blood pressure, improvements in fibrinolytic and coagulation parameters, and reduction in inflammatory markers.54,55 Data from large multicenter trials examining hard clinical endpoints have revealed potential differences in cardiovascular effects between the two agents. Pioglitazone did not significantly reduce the primary composite endpoint of all-cause mortality, nonfatal myocardial infarction, stroke, acute coronary syndrome, endovascular or surgical intervention in the coronary or leg arteries, and amputation above the ankle over a period of nearly 3 years.60 Although the clinical relevance is debatable, the secondary endpoint, all-cause mortality, nonfatal myocardial infarction, and stroke was significantly decreased. Meta-analyses and interim analyses of ongoing studies indicate either harmful or neutral cardiovascular outcomes from rosiglitazone and either neutral or beneficial outcomes from pioglitazone.54–55 As a result, the FDA requested that the prescribing information for rosiglitazone be updated with a black box warning indicating the possible induction of myocardial ischemia with this drug and advised against co-administration with insulin or in patients with heart disease who are taking nitrates, both instances associated with particular risk.61 Very recently, a randomized trial of rosiglitazone versus metformin or sulfonylurea demonstrated no difference in cardiovascular deaths or hospitalizations.62 The major difference between these two glitazones is related to lipid effects; pioglitazone leads to greater increases in HDL cholesterol and smaller increases in LDL cholesterol than rosiglitazone and lowers triglycerides, while rosiglitazone tends to raise triglycerides.63 A recent large observational study suggests that among people older than age 65, pioglitazone is associated with a significantly lower risk of heart failure and death than rosiglitazone.64
The adverse effect that has engendered the most concern regarding this class of drugs is fluid retention and its manifestations: peripheral edema, a decrease in hemoglobin concentration, and heart failure.65 In the registration trials for rosiglitazone and pioglitazone, patient withdrawals for edema or heart failure were less than 1%. The patients most likely to experience edema are those treated with insulin and those with preexisting edema. Women, obese patients, and those with known ischemic heart disease, heart failure, diastolic dysfunction, or renal insufficiency also seem to be at increased risk. It is prudent to start high-risk patients with the lowest marketed dose (pioglitazone 15 mg daily or rosiglitazone 2 mg daily). Increasing edema can be treated with restricted sodium intake or diuretics, and limited data suggest particular benefit from amiloride or spironolactone.66 It should be noted that fluid retention to the point of congestive heart failure requiring hospitalization has been confirmed in large randomized controlled trials.62,67 Findings such as these have prompted the FDA to recommend strengthening the product labeling of pioglitazone and rosiglitazone to caution against use in patients with any degree of heart failure. However, no study has demonstrated worsening myocardial structure or function with these drugs.
Finally, increased fracture rates were reported in clinical trials of both rosiglitazone and pioglitazone, mainly in women.58,68 Distal sites were primarily affected in these studies, but small randomized controlled trials identified the loss of bone density (using DEXA scanning) at the lumbar spine as well.68 Preclinical studies suggest that activation of PPAR-γ inhibits bone formation by diverting stem cells from the osteogenic to the adipocytic lineage and may stimulate the development of osteoclasts. Currently, no data are available with respect to the prevention or management of thiazolidinedione-related bone loss, but prudent measures would include at a minimum an assessment of risk factors and appropriate bone density screening.
Sulfonylureas
The sulfonylureas have been available since the 1950s. They have a relatively quick onset of action and variable duration of action. There are numerous choices available (Table 22-7) which can be divided into first- and second-generation agents. In general, the second-generation agents (glipizide, glyburide, and glimepiride) are more potent and as a result have fewer adverse effects and drug-drug interactions. Extended-release glipizide and glimepiride are preferred agents because they can be dosed once daily in the vast majority of patients and involve a relatively low risk of hypoglycemia and weight gain. Glyburide has a higher risk of hypoglycemia and has been associated with an abrogation of ischemic preconditioning, a protective autoregulatory mechanism in the heart, mediated by interaction with the vascular and cardiac SUR2 receptors.69
Table 22-7
Characteristics of Sulfonylureas
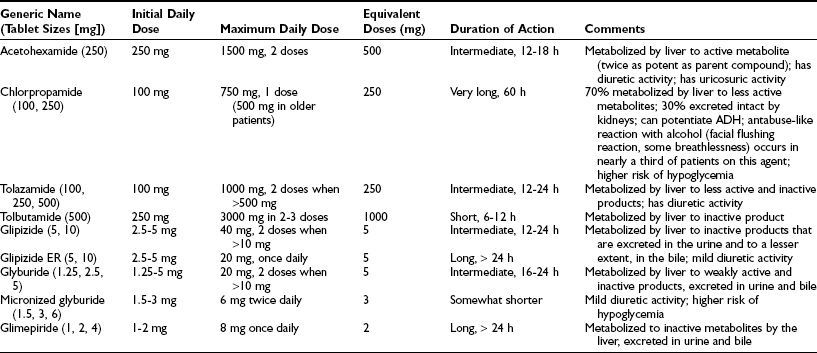
Adapted from Facts and Comparisons, Drug Information Monthly Update Service, St Louis, JB Lippincott.
There has also been concern regarding the potential for these agents to accelerate the progression of β cell failure, a key feature in the pathogenesis of type 2 diabetes.62,70–71 Human data are difficult to acquire because only indirect approximations of β-cell mass are available. Metformin and thiazolidinediones do appear to preserve glycemic control better than glyburide, but it is unknown whether this is a class effect.58 Nevertheless, the long-term safety and low cost continue to justify a solid role for sulfonylureas in the medical management of type 2 diabetes.
Nateglinide
The rationale for stimulating insulin secretion in a way that minimizes fasting hyperinsulinemia and maximizes postprandial control is compelling.72 These agents theoretically would be advantageous in comparison to sulfonylureas for patients with erratic eating patterns, renal dysfunction, or who otherwise are at increased risk of hypoglycemia. Clinical studies demonstrate that repaglinide leads to a reduction in endothelial dysfunction73 and carotid intima media thickness74 relative to sulfonylureas, observations that are attributed to their specificity for postprandial glucose. In addition to these potential cardiovascular advantages, these newer “glinide” agents demonstrate little binding to the vascular smooth muscle and cardiac SUR2 receptors. However, the use in the United States of these newer agents has been modest, in part because of the need for multiple daily doses, greater expense than with sulfonylureas, and lack of head-to-head comparative studies that demonstrate superiority over newer sulfonylureas, which have similarly been shown to have low potential for producing hypoglycemia and weight gain.
Carbohydrate Absorption Inhibitors: α-Glucosidase Inhibitors
α-Glucosidase inhibitors (AGIs) inhibit the terminal step of carbohydrate digestion at the brush border of the intestinal epithelium. When administered with the first bite of a carbohydrate-containing meal, carbohydrate absorption is shifted more distally in the intestine and is therefore delayed, allowing the sluggish insulin secretory dynamics characteristic of type 2 diabetes to catch up with carbohydrate absorption. There are two currently marketed agents in the United States: acarbose and miglitol. Acarbose is largely not absorbed from the intestine, whereas miglitol is. Their use has been limited by a number of factors, including frequent gastrointestinal complaints, the need to administer the medication at the beginning of each meal, and modest reductions in fasting glucose and A1C. These concerns should be balanced against the ability of the AGIs to lower glucose in virtually everyone, without hypoglycemia or weight gain. The major adverse effects are flatulence, abdominal distress or distension, and diarrhea. These result from excessive blockade of carbohydrate absorption in the small bowel, leading to fermentation and gas production in the colon. To maximize the likelihood that these agents will be tolerated, start with a low dose, such as one-fourth of the maximum dose once daily, and increase over a period of weeks to months to one-fourth to half the maximal doses with each meal. A recent study in prediabetic patients demonstrated a statistically significant reduction in cardiovascular endpoints and new hypertension associated with treatment with acarbose, suggesting that modulating postprandial excursions or perhaps nonglycemic effects of the drug may provide for benefits vis-à-vis cardiovascular disease.75 This notion is further supported by the observation in a single-center subgroup analysis from the same study that progression of intima media thickness is also slowed by acarbose.76 The AGIs are contraindicated in patients with chronic intestinal conditions, particularly inflammatory bowel disease.
Incretin-Based Therapies
Incretins are peptide hormones released by the gut that have a multitude of beneficial effects on glucose metabolism. Several recent reviews of these therapies are available.77–79 Incretins promote insulin secretion in response to an oral carbohydrate load in excess to that observed from intravenous carbohydrate exposure, a phenomenon called the incretin effect. Glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide 1 (GLP-1) account for the majority of the incretin effect, the latter being most critical. GLP-1 exhibits glucose-lowering effects through stimulation of insulin secretion, inhibition of glucagon production, slowing gastric emptying, and improving satiety. The insulin secretory effect is glucose-dependent, meaning that the β-cell stimulatory effect is abolished as the glucose level approaches hypoglycemia. Despite the inhibitory effect on glucagon, the counterregulatory hormone response to hypoglycemia is preserved with GLP-1 activity. GLP-1 has important β-cell protective effects in preclinical studies, suggesting that therapeutic approaches may prevent diabetes or slow the progression of β-cell failure, but this requires confirmation in humans. Frankly, many authorities believe that clinically significant benefits on preservation or augmentation of β-cell mass is unlikely. GLP-1 also has binding sites in the appetite centers of the hypothalamus and confers an important regulatory effect on food intake and weight. The enzyme, dipeptidyl peptidase IV (DPP-IV) rapidly inactivates GLP-1 and GIP. Therefore, therapeutic approaches involve agents that are resistant to degradation by DPP-IV (such as exenatide) or that potentiate GLP-1 levels through inhibition of DPP-IV (DPP-IV inhibitors).
Exenatide
Exenatide is a synthetic form of a naturally occurring GLP-1 receptor agonist that is found in the saliva of the Gila monster and exhibits all of the effects of naturally occurring GLP-1. Exenatide is approved as monotherapy or for add-on therapy to sulfonylureas, metformin, or thiazolidinediones.80 It is not currently approved or in combination with insulin, because limited data are available. Exenatide effectively reduces postprandial and fasting glucose and exhibits sustained reductions in HbA1c of approximately 1.0% and weight loss of 2.6 to 3.6 kg over 6 months and about 5 kg over 2 to 3 years, at least in those who continue therapy. It is available in a prefilled pen and dosed twice daily subcutaneously up to 60 minutes before the main meals. The most common side effects of exenatide are nausea, vomiting, and diarrhea and are minimized through slow titration. Gastrointestinal side effects generally diminish with continued use. Patients should initiate therapy with the 5-mcg dose in the 60 minutes before breakfast and supper. Pre-lunch dosing may be substituted for the pre-breakfast dose, so long as lunch and supper are separated by at least 6 hours. The dose should be increased to 10 mcg after 1 month, provided that nausea is minimal. Because of increased risk of side effects, contraindications include gastroparesis and severe renal disease (creatinine clearance <30 mL/min). Postmarketing cases of acute pancreatitis have been reported in association with exenatide, but a causal link has not been proven, nor has a mechanism been established. Nevertheless, it is recommended that exenatide not be restarted following any case of pancreatitis. Hypoglycemia is uncommon except in combination with sulfonylureas. In summary, exenatide has important therapeutic advantages, including weight loss, lack of hypoglycemia, and relatively few contraindications. However, the injectable route, side-effect profile, and expense ultimately limit its use as a first-line agent. Long-acting investigational GLP-1 receptor agonists (liraglutide and exenatide in a once-weekly preparation) are under review by the FDA; they lower fasting glucose to a greater extent and are associated with greater reduction in A1C and better tolerability.
Dipeptidyl Peptidase IV Inhibitors
Currently, only two DPP-IV inhibitors, sitagliptin and saxagliptin, are approved for use, but numerous DPP-IV inhibitors are in late stages of development, including vildagliptin and alogliptin. The currently available agents are approved for use as monotherapy and combination therapy, though not with insulin.81,81a The usual dose is the maximum dose, which is administered without titration. Owing to pharmacokinetic considerations, lesser-sized tablets are available and recommended in patients with estimated GFR less than 50 to 60 mL/min/1.73 m2. This class of drugs is very well tolerated and has very few contraindications. Side effects are uncommon but may include nasopharyngitis and headache. Serious hypersensitivity reactions have been reported in aftermarket surveillance, including anaphylaxis, angioedema, and exfoliative skin conditions with sitagliptin, but causality is difficult to substantiate because of the rarity of events. The weight neutrality, lack of hypoglycemia, broad applicability, tolerability, and ease of use create a unique niche for DPP-IV inhibitors. However, as with exenatide, the precise role of these agents in diabetes management has not been established. Cost, modest efficacy, and uncertain long-term safety remain barriers to use.
Pramlintide
Pramlintide is currently only approved for use in conjunction with basal-bolus insulin regimens in patients with type 1 or type 2 diabetes who are not meeting glycemic targets.82 Pramlintide was originally only available by vial and syringe but recently, a multi-use pen with several dose increments that facilitate titration became an option. For type 2 diabetes, the starting dose is 60 mcg (10 units) subcutaneously before major meals (those exceeding 30 g of carbohydrates), increasing to 120 mcg (20 units) if a patient has been nausea-free for 3 to 7 days. The major side effect is nausea, but it appears to be less intense than that observed with exenatide and also appears to be minimized with slow titration. Patients who have difficulty with 60 mcg may benefit from the lower-dose pen intended for the treatment of type 1 diabetes, which starts at a dose of 15 mcg and increases to 30 mcg. It is advisable that the prandial insulin dose be decreased by 50% at the initiation of pramlintide, although ultimately, most patients need a smaller decrement. Postprandial glucose levels generally peak later and at a smaller amplitude following pramlintide administration than with prandial insulin alone. This may lead to early hypoglycemia (in the first 30 minutes) followed later by hyperglycemia when administered in conjunction with typical rapid-acting insulins. Consequently, the timing of the prandial insulin dose may also require adjustment. The drug is contraindicated in patients with gastroparesis, history of severe hypoglycemia, or hypoglycemia unawareness. Because of the complexity of the regimen—resulting from multiple injections and the need for active concomitant titration of insulin—pramlintide is only recommended for highly motivated patients. Furthermore, its use is only advisable for patients in need of modest refinement of glycemic control. Additional study is needed to determine whether there is utility for monotherapy or concomitant use with basal insulin alone or oral agents.
Insulins
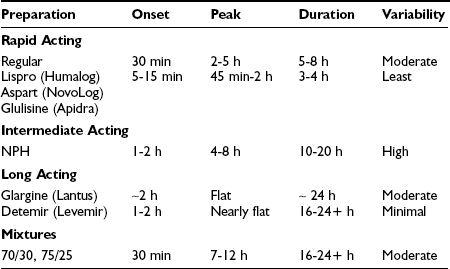
Insulin has been commercially available since the early 1920s and is arguably still the mainstay of therapy for the majority of people with type 2 diabetes worldwide. Subcutaneous injection of insulin in type 2 diabetes can be used to supplement endogenous production of insulin, both in the fasting state to modulate hepatic glucose production and in the postprandial state to facilitate glucose clearance into muscle and fat for storage. Currently, the vast majority of insulin used worldwide is of recombinant human origin; animal-source insulin is difficult to obtain and more expensive. The available formulations largely differ in their pharmacokinetics, as reviewed in Table 22-8.
There are now three rapid-acting insulin analogs—lispro, aspart, and glulisine—each with onset of action in 5 to 15 minutes, near-peak activity from approximately 45 minutes to 2 hours, and a duration of activity of approximately 4 hours, with slightly more prolonged activity at higher doses. These three molecules have a different structural modification that disrupts the “tail structure” of the insulin molecule; as a result, they do not exhibit as great a tendency to form dimers and hexamers at high concentration. Marketing suggests that there are differences between agents, but on the background of endogenous insulin secretion in the setting of type 2 diabetes, clinically relevant differences in glycemic control or adverse effects have not been demonstrated. These rapid-acting analogs are associated with better postprandial glucose control than regular human insulin, as well as a reduction in the risk of hypoglycemia in some studies. They should be administered up to 15 minutes before meals or even immediately after meals. Dosing after meals in combination with techniques such as carbohydrate counting may be helpful in overweight patients with type 2 diabetes who often have no idea what they will eat, effectively until they are done eating. Carbohydrate counting may be cumbersome for some patients, and a fixed-dose prandial insulin regimen that is adjusted weekly may result in similar improvements in glycemic control.83 However, insulin requirements are lower with carbohydrate counting, and weight gain tends to be less.
Detemir is the most recently developed insulin analog. It is relatively rapidly absorbed but has a prolonged duration of action, attributable in large part to its acylation at position B29 with a 14-carbon fatty acid (myristic acid). As a result of this structure, detemir is highly protein bound in interstitial fluid and plasma. Like glargine, detemir exhibits a nearly peakless profile, but the duration of action is several hours shorter, at least in patients with type 1 diabetes.84 In patients with type 2 diabetes, insulin-clamp studies demonstrate that the duration of both basal insulin analogs is dose dependent, lasting more than 24 hours in most patients.85 This indicates that detemir can be dosed once daily in most patients if they require over 0.4 units/kg. In the only head-to-head clinical trial in patients with type 2 diabetes, detemir exhibited similar improvements in glycemic control as glargine when either was added to existing oral agents.86 However, about half of patients on detemir received twice-daily dosing. Although larger doses were required to attain glycemic control, detemir was associated with slightly less weight gain than glargine. This has been attributed to the lipophilic properties of detemir, which may facilitate entry into the hypothalamic appetite centers in the brain. Detemir has also been shown in carefully controlled conditions to display less day-to-day variability in insulin action than NPH or glargine.84,87
Basal Insulin Monotherapy
Patient self-titration algorithms employing once-daily basal insulin are highly effective, empower patients, and may reduce the amount of time required by the provider in managing therapy. The Treat to Target Study recently documented the safety and effectiveness of bedtime NPH or glargine insulin as a single bedtime injection added to oral agents in the setting of poor glycemic control (A1C > 7.5%) on one or two oral agents.88 At randomization, patients added 10 units of NPH or glargine insulin at bedtime to their baseline oral antidiabetic agents and were instructed to increase the dose by 2 to 8 units per week based upon the average of the last two fasting glucose readings. Small decreases in insulin dose (2 to 4 units) were allowed if there was a severe hypoglycemic event or documented glucose less than 56 mg/dL. The mean A1C achieved was identical and approximately 7% in both groups. There was a statistically significant lower incidence of nocturnal hypoglycemia in the group treated with bedtime glargine when compared to those treated with NPH. A simpler algorithm is to have patients monitor fasting glucose at home on a daily basis and increase the nightly dose of NPH, glargine, or detemir insulin by 1 unit every day if the morning glucose is above 100 mg/dL.89 In patients who achieve control of their fasting glucose, many will maintain reasonable control through the rest of the day. Once-nightly insulin has become the most commonly prescribed form of insulin therapy in type 2 diabetes in the United States. It results in a similar reduction in HbA1c and lower incidence of hypoglycemia and weight gain than a strategy of initiating prandial insulin alone.90,91
Premixed Insulin
Premixed insulin formulations provide greater convenience and accuracy than those mixed by patients. Premixed formulations available in the United States include 70/30 mixtures of NPH and regular insulin, 75/25 mixtures of NPH and lispro insulin, and a 70/30 mixture of insulin aspart with NPH. Premixed insulin provides a profile of activity as expected from the addition of the activities of its components. Mixtures of 75/25 and 70/30 are traditionally dosed twice daily, usually two-thirds of the total daily dose before breakfast and a third of the total daily dose before supper. However, adding a comparatively small dose of 70/30 aspart at lunch may offer incremental glycemic benefit in patients failing twice-daily therapy.92 The role of premixed insulins in the management of type 2 diabetes has been widely debated. They have advantages with respect to convenience in administering the injection and disadvantages with respect to the inconvenience of having to be more rigid in the timing and composition of meals to achieve excellent control. In head-to-head studies, premixed insulins tend to reduce A1C to a greater degree than basal insulin alone, but at the expense of more hypoglycemia and possibly weight gain.91,93
Basal-Bolus Insulin
As A1C drops below 8%, postprandial glucose becomes an increasing component of overall control.94 On basal insulin alone, glucose levels may increase throughout the day before returning to more normal levels overnight. In this setting, a large proportion of patients will need to add rapid-acting insulin with one or more meals to achieve optimal glycemic control. When mealtime insulin is added to previously optimized basal insulin, simultaneous reductions in the basal insulin are often required. In such patients, the addition of prandial insulin analog produces slightly greater reduction in A1C than switching to a premixed insulin regimen.95 The details of implementing basal-bolus insulin are discussed in Chapter 19. The major qualitative difference in using multiple daily injection techniques in the setting of type 2 diabetes is that patients do not necessarily need to take an injection with each meal and snack, as is generally the case in type 1 diabetes. Many do very well taking rapid-acting insulin with larger or carbohydrate-rich meals, particularly at breakfast and/or supper, and relying on oral agents to tide them over through the rest of the day. Similarly, some patients do reasonably well overnight while fasting and merely require meal-associated insulin to maintain glucose levels during the day. It should be noted that in typical insulin-resistant overweight patients with type 2 diabetes, it has not been clearly demonstrated that there are clinically significant benefits to multiple daily injection regimens versus once- or twice-daily insulin.
Practical Aspects of Initiating and Progressively Managing Type 2 Diabetes
A significant challenge in clinical decision making in diabetes is that the increased availability of therapeutic options for antidiabetic therapy is ahead of adequate prospective outcome studies to determine optimal treatment strategies. Each class of drugs and even agents within each class has advantages and limitations, and individual issues may significantly affect the appropriate choice of therapy in particular patients. Table 22-6 highlights some of the relative advantages and disadvantages of various agents and classes. The choice of therapy and its adjustment ultimately require individualization. Of greater importance overall is appropriate initiation and adjustment of insulin therapy than the particular regimen patients receive.
Strategies
Insulin Avoidance/β-Cell Preservation Strategy
It is important to try to dispel notions that insulin therapy is difficult, ominous, or fraught with peril by highlighting its efficacy and the great strides that have been made in insulin formulations and delivery devices. Type 2 diabetes is a progressive disease, and most patients will eventually require insulin therapy due to progressive β-cell failure.96 Several agents have purported β-cell protective effects. This has been best demonstrated for thiazolidinediones and to a lesser extent metformin and is hypothesized to be a benefit of incretin-based therapies. Clearly glyburide and probably sulfonylurea agents as a class, when compared with insulin sensitizers, are associated with increased rates of β-cell failure.97 The use of these agents may be a suitable strategy, particularly for patients with a long life expectancy or those with recently diagnosed type 2 diabetes. A large trial examined the durability of various oral agents when used as monotherapy.58 The cumulative incidence of monotherapy failure at 5 years of therapy was 15% with rosiglitazone, 21% with metformin, and 34% with glyburide (P < 0.001 for both comparisons with rosiglitazone).
Minimal Insulin Resistance Strategy
Insulin resistance is a key component of the pathophysiology of type 2 diabetes and may contribute directly to atherosclerotic complications. There are no clinical data to suggest that exogenous insulin is associated with adverse side effects or long-term complications beyond its hypoglycemic effects and the associated weight gain. Nevertheless, insulin requirements can be minimized with the addition of insulin sensitizers in particular. The thiazolidinediones have the greatest efficacy in reducing insulin resistance, with lesser effects demonstrated for metformin. Although all oral agents increase peripheral insulin levels less than injected insulin, it should be noted that a recent study demonstrated no benefit with respect to death or CVD events between an insulin-providing and an insulin-sensitizing strategy.98
Guidelines
It is a disappointing reality that despite multiple effective agents for treating hyperglycemia, only 57% of patients with diabetes reach minimum treatment goals for A1C specified by professional organizations.99 The reasons for this are multiple, but the ever-increasing range and number of agents, as well as the increasing complexity of care can contribute to therapeutic inertia among providers and patients. The ADA and the European Association for the Study of Diabetes (EASD) published a consensus statement for step-wise, goal-directed management in order to assist patients and providers with managing hyperglycemia.43 The choice of individual agents is based upon efficacy in lowering A1C, long-term safety and durability, side effects and contraindications, ease of use, and cost.
Guidelines from the American Association of Clinical Endocrinologists (AACE) also recommend early goal-directed therapy, but stress that the choice of agents should be individualized, particularly with respect to the risk of hypoglycemia.44 In this guideline, initial monotherapy is preferred only for those patients with an A1C between 6% and 7%. If the initial A1C is between 7% and 8%, the guidelines recommend starting combination therapy. Insulin therapy should be added when the A1C exceeds 10% or in patients whose A1C exceeds 6.5% on two non-insulin drugs.
Future Directions
Novel pharmaceutical agents, including glucagon receptor antagonists, inhibitors of gluconeogenic and glycogenolytic pathways, glucokinase activators, sodium-glucose cotransporter inhibitors, acetyl-CoA carboxylase inhibitors, selective PPAR-γ modulators, modifiers of lipid metabolism, and antiobesity agents are areas of early pharmaceutical development.78
Novel methods of insulin delivery similarly have generated a great deal of enthusiasm among patients, particularly techniques to deliver insulin orally or by inhalation.100 However, the first inhaled insulin product to reach the market was shortly discontinued because of low utilization.
Additional DPP-IV inhibitors, as well as DPP-IV-resistant analogs and naturally occurring incretin mimetics are being investigated in clinical trials.78
Recently there has been a call for more rigorous testing of diabetes therapeutics prior to FDA approval.101 This was partly a reaction to perceived safety concerns of drugs that are approved solely on the basis of surrogate outcomes (A1C) instead of demonstrating a reduction in clinical endpoints. It is also in recognition of a growing number of already available therapeutic choices, some of which have a proven longstanding safety record when used appropriately and low cost. This position is debated, however, given that many patients still are unable to achieve glycemic targets easily.
Place of Glucose Management in the Treatment of Type 2 Diabetes
The UKPDS clearly demonstrated that good glycemic control reduces the incidence of microvascular complications in patients with type 2 diabetes.9,10 However, recent trials have demonstrated that even intensive glycemic control targeting an A1C (6% to 6.5%) lower than current guidelines does not reduce the risk of cardiovascular complications.12–14 Possible exceptions include younger patients with shorter duration of diabetes, no known vascular disease, and A1C < 8% at baseline. In newly diagnosed patients with type 2 diabetes, early effective glycemic control appears to have a legacy effect on later development of microvascular and macrovascular complications, as well as mortality, even if glycemic control is not maintained.15 As a result, a statement published by the ADA, the American College of Cardiology Foundation, and the American Heart Association recommended that a target A1C less than 7% is appropriate for most patients with diabetes to reduce the risk of microvascular complications.18 However, the consensus suggests that a higher target might be reasonable for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, or those with longstanding diabetes in whom the general goal is difficult to attain despite intensive efforts. Conversely, a target closer to normal (6%) might be indicated if it can be achieved without significant hypoglycemia or other adverse effects of treatment in patients with short duration of diabetes, long life expectancy, and no significant cardiovascular disease.
Nevertheless, the importance of managing dyslipidemia, hypertension, and the procoagulant state in the setting of diabetes to reduce cardiovascular events has been much more clearly demonstrated than the case for treating hyperglycemia. Since cardiovascular disease is the ultimate cause of death in the overwhelming majority of patients with diabetes, blood pressure, lipid, and antiplatelet therapies are therefore critical components of care.1 Recommended targets for lipid and blood pressure management are presented in Table 22-9. Lifestyle measures targeting weight reduction for overweight patients may be the most cost-effective and safest mode of therapy and should be reinforced at every visit. Cigarette smoking is the strongest risk factor for macrovascular disease in the general population, as well as for patients with diabetes. All patients with diabetes should be counseled not to start smoking or to quit if they are smoking.
Table 22-9
Targets for Cardiovascular Risk Reduction in Diabetes
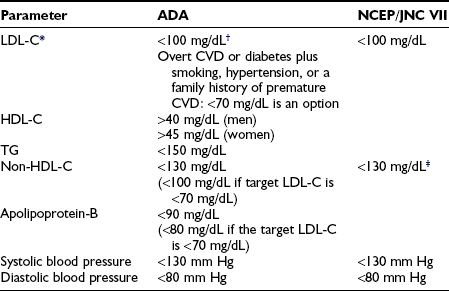
*LDL-C is the primary target of therapy except in the case of severe hypertriglyceridemia.
†Non-HDL cholesterol becomes a secondary target of therapy when triglyceride levels exceed 200 mg/dL.
‡In those with clinical CVD or older than 40 years of age with other CVD risk factors, pharmacologic treatment using a statin should be added to lifestyle therapy regardless of baseline lipid levels.
Data from American Diabetes Association: Standards of medical care in diabetes, Diabetes Care 27:S15–S35, 2004; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, JAMA 289:2560–2572, 2003; and Grundy SM, Cleeman JI, Merz CN, et al: Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines, J Am Coll Cardiol 44:720–732, 2004.
Comprehensive cardiovascular risk assessment and intervention deserves equal or greater attention to efforts at controlling glycemia in patients with type 2 diabetes.102 Today, achieving glycemic, lipid, and blood pressure goals is certainly within the grasp of each patient and his or her health care team. Unfortunately, most patients do not achieve comprehensive control. When patients understand the goals of therapy and their rationale, they can be a driving force to focus the health care team to provide strategies that are acceptable to achieve those goals. The epidemic of diabetes and obesity, coupled with the predicted early death and disability that follow, threatens to overwhelm our health care system. Practical, systematic approaches to stem this tide are desperately needed.103
References
1. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2009;32:S13–S61.
2. Engelgau, MM, Geiss, LS, Saaddine, JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950.
3. Wild, S, Roglic, G, Green, A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053.
4. Narayan, KM, Boyle, JP, Thompson, TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890.
5. Bjork, S. The cost of diabetes and diabetes care. Diabetes Res Clin Pract. 2001;54(Suppl 1):S13–S18.
6. Gu, K, Cowie, CC, Harris, MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998;21:1138–1145.
7. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31:596–615.
8. CDC Cost-Effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287:2542–2551.
9. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
10. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
11. Ohkubo, Y, Kishikawa, H, Araki, E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117.
12. Action to Control Cardiovascular Risk in Diabetes Study GroupGerstein, HC, Miller, ME, Byington, RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559.
13. ADVANCE Collaborative GroupPatel, A, MacMahon, S, Chalmers, J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
14. Duckworth, W, Abraira, C, Moritz, T, et al. Intensive glucose control and complications in American veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139.
15. Holman, RR, Paul, SK, Bethel, MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589.
16. Ray, KK, Seshasai, SR, Wijesuriya, S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772.
17. American College of Endocrinologists. American College of Endocrinology consensus statement on guidelines for glycemic control. Endocr Pract. 2002;8(Suppl 1):5–11.
18. Skyler, JS, Bergenstal, R, Bonow, RO, et alAmerican Diabetes AssociationAmerican College of Cardiology FoundationAmerican Heart Association. intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–192.
19. Rohlfing, CL, Wiedmeyer, HM, Little, RR, et al. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–278.
20. Klonoff, DC, Schwartz, DM. An economic analysis of interventions for diabetes. Diabetes Care. 2000;23:390–404.
21. Norris, SL, Engelgau, MM, Narayan, KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561–587.
22. Funnell, MM, Brown, TL, Childs, BP, et al. National standards for diabetes self-management education. Diabetes Care. 2009 Jan;32(Suppl 1):S87–S94.
23. Fisher, EB, Earp, JA, Maman, S, et al. Cross-cultural and international adaptation of peer support for diabetes management. Fam Pract. 2009 Mar 10. [[Epub ahead of print]].
24. Delamater, AM, Jacobson, AM, Anderson, B, et al. Psychosocial therapies in diabetes: report of the Psychosocial Therapies Working Group. Diabetes Care. 2001;24:1286–1292.
25. American Diabetes AssociationBantle, JP, Wylie-Rosett, J, Albright, AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008 Jan;31(Suppl 1):S61–S78.
26. Sheard, NF, Clark, NG, Brand-Miller, JC, et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the American Diabetes Association. Diabetes Care. 2004;27:2266–2271.
27. Klein, S, Sheard, NF, Pi-Sunyer, X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067–2073.
28. Gillespie, SJ, Kulkarni, KD, Daly, AE. Using carbohydrate counting in diabetes clinical practice. J Am Diet Assoc. 1998;98:897–905.
29. Bergenstal, RM, Johnson, M, Powers, MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care. 2008 Jul;31(7):1305–1310.
30. Egede, LE, Ye, K, Zhang, D, et al. The prevalence and pattern of complementary and alternative medicine use in individuals with diabetes. Diabetes Care. 2002;25:324–329.
31. Ernst, E. Complementary medicine: its hidden risks. Diabetes Care. 2001;24:1486–1488.
32. Barringer, TA, Kirk, JK, Santaniello, AC, et al. Effect of a multivitamin and mineral supplement on infection and quality of life. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:365–371.
33. Schnyder, G, Roffi, M, Pin, R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345:1593–1600.
34. Lange, H, Suryapranata, H, De Luca, G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350:2673–2681.
35. Ryan, DH, Espeland, MA, Foster, GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628.
36. Boule, NG, Haddad, E, Kenny, GP, et al. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–1227.
37. Sigal, RJ, Kenny, GP, Wasserman, DH, et al. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27:2518–2539.
38. Inzucchi, SE. Noninvasive assessment of the diabetic patient for coronary artery disease. Diabetes Care. 2001;24:1519–1521.
39. American Diabetes Association. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10–11 February 1998, Miami, Florida. Diabetes Care. 1998;21:1551–1559.
40. Young, LH, Wackers, FJ, Chyun, DA, et alDIAD Investigators. cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009 Apr 15;301(15):1547–1555.
41. Towfigh, A, Romanova, M, Weinreb, JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: a meta-analysis. Am J Manag Care. 2008 Jul;14(7):468–475.
42. Farmer, AJ, Wade, AN, French, DP, et alDiGEM Trial Group. blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial. Health Technol Assess. 2009 Feb;13(15):iii–iv. [ix–xi, 1–50].
43. Nathan, DM, Buse, JB, Davidson, MB, et alAmerican Diabetes AssociationEuropean Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
44. Rodbard, HW, Blonde, L, Braithwaite, SS, et alAACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13:1–68.
45. Setter, SM, Iltz, JL, Thams, J, et al. Metformin hydrochloride in the treatment of type 2 diabetes mellitus: a clinical review with a focus on dual therapy. Clin Ther. 2003;25:2991–3026.
46. Leverve, XM, Guigas, B, Detaille, D, et al. Mitochondrial metabolism and type-2 diabetes: a specific target of metformin. Diabetes Metab. 29, 2003. [6S88–6S94].
47. Misbin, RI. The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes Care. 2004;27:1791–1793.
48. Stades, AME, Heikens, JT, Erkelens, DW, et al. Metformin and lactic acidosis: cause or coincidence? A review of case reports. J Int Med. 2004;255:179–187.
49. Glucophage/Glucophage XR [package insert], Princeton, NJ, Bristol-Myers Squibb Company, January 2009. http://packageinserts.bms.com/pi/pi_glucophage.pdf. [accessed August 25, 2009].
50. Shaw, JS, Wilmot, RL, Kilpatrick, ES. Establishing pragmatic estimated GFR thresholds to guide metformin prescribing. Diabet Med. 2007 Oct;24(10):1160–1163.
51. Tahrani, AA, Varughese, GI, Scarpello, JH, et al. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ. 2007 Sep 8;335(7618):508–512.
52. Inzucchi, SE, Masoudi, FA, McGuire, DK. Metformin in heart failure. Diabetes Care. 2007;30:e129.
53. Wulffele, MG, Kooy, A, de Zeeuw, D, et al. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med. 2004;256:1–14.
54. McGuire, DK, Inzucchi, SE. New drugs for the treatment of diabetes mellitus: part I: thiazolidinediones and their evolving cardiovascular implications. Circulation. 2008;117:440–449.
55. Ceriello, A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab Res Rev. 2008;24:14–26.
56. ACTOS (package insert). Takeda Pharmaceuticals America. Lincolnshire, IL, December 2003. Available at http://www.actos.com/pi.pdf. [Accessed September 12, 2004].
57. Avandia (package insert). GlaxoSmithKline. Research Triangle Park, NC, August 2004. Available at http://us.gsk.com/products/assets/us_avandia.pdf. [Accessed September 12, 2004].
58. Kahn, SE, Haffner, SM, Heise, MA, et al. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443.
59. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105.
60. Dormandy, JA, Charbonnel, B, Eckland, DJ, et al. PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289.
61. http://www.fda.gov/Cder/drug/InfoSheets/HCP/rosiglitazone200707HCP.htm [Site accessed 12/2/08].
62. Home, PD, Pocock, SJ, Beck-Nielsen, H, et alRECORD Study Team. rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009 Jun 20;373(9681):2125–2135.
63. Goldberg, RB, Kendall, DM, Deeg, MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554.
64. Juurlink, DN, Gomes, T, Lipscombe, LL, et al. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ. 2009 Aug 18;339:b2942.
65. Nesto, RW, Bell, D, Bonow, RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure. A consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108:2941–2948.
66. Karalliedde, J, Buckingham, R, Starkie, M, et al. Effect of various diuretic treatments on rosiglitazone-induced fluid retention. J Am Soc Nephrol. 2006;17:3482–3490.
67. Ryden, L, Thrainsdottir, I, Swedberg, K. Adjudication of serious heart failure in patients from PROactive. Lancet. 2007;369:189–190.
68. Grey, A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19:129–137.
69. Riddle, MC. Editorial: Sulfonylureas differ in effects on ischemic preconditioning—is it time to retire glyburide? J Clin Endocrinol Metab. 2003;88:528–530.
70. Del Prato, S, Pulizzi, N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism. 2006;55:S20–S27.
71. Sawada, F, Inoguchi, T, Tsubouchi, H, et al. Differential effect of sulfonylureas on production of reactive oxygen species and apoptosis in cultured pancreatic beta-cell line, MIN6. Metabolism. 2008;57:1038–1045.
72. Blicklé, JF. Meglitinide analogues: a review of clinical data focused on recent trials. Diabetes Metab. 2006;32:113–120.
73. Manzella, D, Grella, R, Abbatecola, AM, et al. Repaglinide administration improves brachial reactivity in type 2 diabetic patients. Diabetes Care. 2005;28:366–371.
74. Esposito, K, Giugliano, D, Nappo, F, et al. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–219.
75. Chiasson, JL, Josse, RG, Gomis, R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA. 2003;290:486–494.
76. Hanefeld, M, Chiasson, JL, Koehler, C, et al. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004;35:1073–1078.
77. Riddle, MC, Drucker, DJ. Emerging therapies mimicking the effects of amylin and glucagon-like peptide 1. Diabetes Care. 2006;29:435–449.
78. Inzucchi, SE, McGuire, DK. New drugs for the treatment of diabetes: part II: incretin-based therapy and beyond. Circulation. 2008;117:574–584.
79. Drucker, DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343.
80. Exenatide prescribing information. http://pi.lilly.com/us/byetta-pi.pdf. [Accessed 12/3/08].
81. Sitagliptin prescribing information. http://www.merck.com/product/usa/pi_circulars/j/januvia/januvia_pi.pdf. [Accessed 12/3/08].
81a. Saxagliptin prescribing information. http://packageinserts.bms.com/pi/pi_onglyza.pdf. [Accessed 8/25/2009].
82. Pramlintide prescribing information. https://www.symlin.com/pdf/SYMLIN-pi-combined.pdf. [Accessed 12/3/08].
83. Bergenstal, RM, Johnson, M, Powers, MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm to carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care. 2008;31:1305–1310.
84. Owens, DR, Bolli, GB. Beyond the era of NPH insulin—long-acting insulin analogs: chemistry, comparative pharmacology, and clinical application. Diabetes Tech Ther. 2008;10:333–349.
85. Klein, O, Lynge, J, Endahl, L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290–299.
86. Rosenstock, J, Davies, M, Home, PD, et al. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51:408–416.
87. Heise, T, Nosek, L, Ronn, BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–1620.
88. Riddle, MC, Rosenstock, J, Gerich, J. Insulin Glargine 4002 Study Investigators: The Treat-To-Target Trial: Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086.
89. Gerstein, HC, Yale, JF, Harris, SB, et al. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med. 2006 Jul;23(7):736–742.
90. Bretzel, RG, Nuber, U, Landgraf, W, et al. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet. 2008;371:1073–1084.
91. Holman, RR, Thorne, KI, Farmer, AJ, et al4-T Study Group. addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–1730.
92. Garber, AJ, Wahlen, J, Wahl, T, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab. 2006;8:58–66.
93. Robbins, DC, Beisswenger, PJ, Ceriello, A, et al. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther. 2007;29:2349–2364.
94. Monnier, L, Lapinski, H, Colette, C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881–885.
95. Rosenstock, J, Ahmann, AJ, Colon, G, et al. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care. 2008;31:20–25.
96. UKPDS Study Group. (UKPDS 16), Overview of six years’ therapy of type 2 diabetes—a progressive disease. Diabetes. 1995;44:1249–1258.
97. Del Prato, S, Wishner, WJ, Gromada, J, et al. Beta-cell mass plasticity in type 2 diabetes. Diabetes Obes Metab. 2004;6:319–531.
98. BARI 2D Study GroupFrye, RL, August, P, Brooks, MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009 Jun 11;360(24):2503–2515.
99. Ong, KL, Cheung, BM, Wong, LY, et al. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol. 2008;18:222–229.
100. Brunton, S. Insulin delivery systems: reducing barriers to insulin therapy and advancing diabetes mellitus treatment. Am J Med. 2008;121:S35–S41.
101. Nathan, DM. Finding new treatments for diabetes: how many, how fast … how good? N Engl J Med. 2007;356:437–440.
102. Gaede, P, Lund-Andersen, H, Parving, H-H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591.
103. Narayan, KM, Gregg, EW, Engelgau, MM, et al. Translation research for chronic disease: the case of diabetes. Diabetes Care. 2000;23:1794–1798.