Chapter 205 Management of Thoracic Outlet Syndrome
Thoracic outlet syndrome (TOS) refers to a group of complex symptoms in the upper extremity caused by compression of the brachial plexus and subclavian artery and vein between the interscalene triangle and the inferior border of the axilla. A specific, treatable disorder is not diagnosed frequently in patients complaining of diffuse numbness, chronic pain of the head and neck, and pain and weakness of the upper extremities. These patients live with discomfort, uncertainty, and disability, carrying the reputation of “malingerers.”1 Persistent symptoms may lead to devastating functional, emotional, and financial impairment. Loss of job and lifelong disability are potential outcomes of this syndrome if not treated successfully.2 Sixty-three percent of these patients are usually observed by general practitioners, and 37% are observed by consultants in other specialties.3
The first description of symptoms related to a supplementary cervical rib was reported by Willshire in I860.4 In 1906, Murphy suggested that the anterior scalene muscle could produce these symptoms5 and, in 1956, Peet was the first to describe the term “thoracic outlet syndrome.”6
Predisposing factors for TOS include (1) exaggerated and repetitive motion, (2) preexisting bone, muscle tendinous anomaly, and (3) direct impact. The compression may be due to abnormal structures, such as cervical rib or clavicular malunion or by otherwise normal structures, such as the first rib, the seventh cervical transverse processes, the anterior or middle scalene muscle, or the pectoralis minor tendon. A traumatic event is described as a precipitating factor in 21% of TOS patients.7 This condition is observed in baseball players such as pitchers, catchers, and first-basemen, and other athletes such as kayakers, weight lifters, and golfers, as well as volleyball, karate, and tennis players,8 who require exaggerated shoulder motion in their athletic activities. Other patients have a more insidious onset of symptoms caused by particular job-related routine tasks. A teacher may indicate that symptoms are brought on by writing on a blackboard. A cellist may ascribe symptoms to holding the left arm in an abducted position. A painter may ascribe symptoms to working on a ceiling. Repeated arm exertion is required by auto mechanics, construction workers, and many others. Many experienced clinicians have noted that individuals (women in particular) with long necks and sloping shoulders are prone to the development of TOS symptoms.
The median age of patients suffering from TOS is 29 years.3 Most patients are women.3,9 This syndrome has been described also in children, although this is rare.10 The syndrome is occasionally precipitated by an injury or lifting a heavy weight in people aged over 50 years.
Exaggerated and repetitive motions can cause muscle hypertrophy and, as a result, subject the adjacent artery, vein, and brachial plexus to compression. The complexity of multiple symptoms in the upper extremity is caused by (1) painful brachial plexus compression, (2) vascular compression, (3) denervation of hand intrinsic muscles, or (4) venous obstruction.11 Symptoms are more frequent in the dominant hand of patients with bilateral cervical ribs,12 although most of the cervical ribs are bilateral and slightly predominant on the left.13,14
TOS is divided into two categories: the neurogenic type and the vascular type.
Neurogenic TOS presents a complex of brachial plexus syndromes. Most patients exhibit symptoms of brachial plexus compression. The dominant symptoms are pain and paresthesias (88% and 83%, respectively) of the hand and forearm (especially ulnar nerve distribution). Most of these patients have bone anomalies at the base of the neck, such as a prominent C7 transverse process or rudimentary cervical rib and associated fibrous bands extending to the first thoracic rib that provoke compression of the lower brachial plexus (nerve roots C8–T1, lower trunk). Neurogenic TOS, with and without bone anomalies, can evoke pain in the distribution of the shoulder, neck, and radial aspect of the arm in the uncommon upper brachial plexus distribution (nerve roots C5 and C6), as well as chronic pain that does not follow a confined dermatomal pattern. Other symptoms include fatigability (23%) and brachial plexus tenderness (32%).7
Disputed TOS15 is a category that embraces a multiplicity of vague organic and functional disorders of the upper limb, for which no other diagnosis has been reached.16 Symptomatology can be quite variable but often involves chronic pain that may or may not follow a confined dermatomal pattern. The diagnosis at present must rely on positive clinical signs and symptoms with or without radiologic or electrophysiologic signs. Exclusion of alternative diagnoses and proper patient selection is the key to diagnosis of disputed TOS.
Vascular TOS includes syndromes due to subclavian artery and vein complications, accounting for 1% to 10% of all patients with TOS,17,18 but may result in significant long-term disability. The majority of arterial cases of TOS are associated with the presence of a cervical rib, which may be incomplete or rudimentary, or an abnormal first rib.13,17 The arterial type of TOS may present symptoms of thromboembolism,19 lead to symptoms such as arm claudication or threaten arm viability. Occlusion of the subclavian artery also may be asymptomatic due to the rich collateral network. Subclavian artery pathology due to compression at the thoracic outlet is sometimes found to be the cause of an incidentally discovered, asymptomatic subclavicular bruit.
Subclavian vein compression occurs in the costoclavicular space and may be caused also by an exostosis or hypertrophic callus that develops after fracture of the first rib or clavicle.19 Patients with subclavian vein compression usually present with edema and pain of the hand and arm resulting from subclavian vein thrombosis.
Treatment
Concerning the issue of treatment, questions arise as to whether surgery is indicated and, if indicated, what surgical technique and approach should be used. Most surgeons routinely attempt conservative treatment for patients with TOS before considering surgical decompression. Patients who have bone or soft tissue anomalies that compress arteries but are free of arterial damage may be managed conservatively by avoiding strenuous or repetitive arm movement. However, surgical decompression should be considered before arterial injury occurs.19 Generally, at the time of surgical intervention, median duration of symptoms is 24 months (range 1–180).3 Surgical decompression is more successful when TOS is traumatic or subacute,2,20 suggesting that previous physical hard work has an influence on surgical results. Laborers with TOS are less likely to show successful results from surgical intervention, and they are unlikely to return to their original occupation and therefore may be required to pursue retraining for a non–labor-intensive occupation.2 A study that compared the outcomes of the nonoperative and operative workers, the majority of whom were diagnosed with disputed neurogenic TOS, showed that the surgically treated patients were three to four times more likely to be work disabled at follow-up.21 The treatment of spontaneous thrombosis of the subclavian vein has been conservative, but 50% of patients treated conservatively have persistent disabling symptoms.22 If vascular complications of TOS occur, aggressive, immediate surgical or endovascular intervention is needed.23
Surgical Anatomy
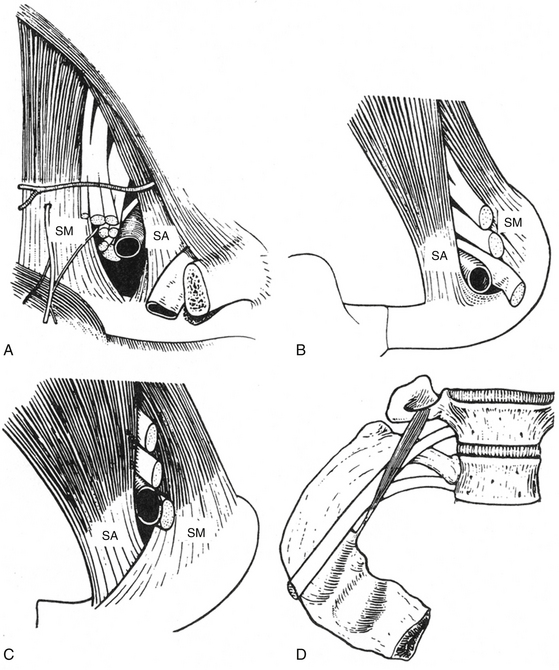
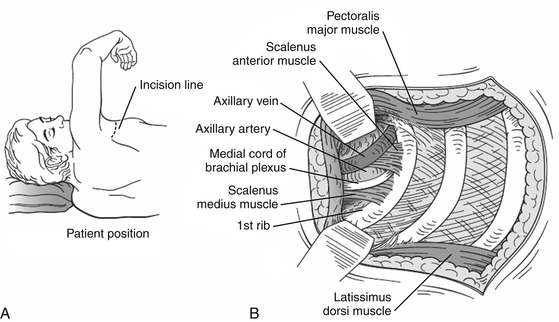
The anatomy of the thoracic outlet extends from the intervertebral foramina and superior mediastinum to the axilla.24 The apex of the axilla is bounded by the clavicle and subclavius muscle anterolaterally, and the upper border of the scapula and subscapularis muscle dorsally. The anterolateral border of the first rib is medial. The brachial plexus and subclavian vessels leave this area and pass beneath the coracoid process. The nerve roots of the brachial plexus and the subclavian artery pass through the scalene hiatus (interscalene triangle) formed by the scalenus medius posteriorly, the scalenus anterior anteriorly, and the first rib (or cervical rib) inferiorly (Fig. 205-1A). It is important to note that the subclavian vein runs anterior to the anterior scalene muscle. Under normal circumstances, there is ample room for their passage, but congenital variations affecting the arrangement of ribs and scalene muscles in this area are responsible for almost all the compression lesions of the lower trunk and subclavian artery that fall into the category of TOS. Immediately distal to the interscalene triangle, the neurovascular bundle enters the costoclavicular triangle, which is bordered anteriorly by the middle third of the clavicle, posteromedially by the first rib, and posterolaterally by the upper border of the scapula. The neurovascular bundle then enters the subcoracoid space, also referred to as the retropectoralis minor space, beneath the coracoid process deep to the pectoralis minor tendon.
Pathogenesis
Compression and/or irritation of the brachial plexus within the thoracic outlet have been well described in three spaces: the interscalene triangle, the costoclavicular space, and the subpectoral tunnel.25 Magnetic resonance imaging (MRI)26 and computed tomography (CT) angiography studies also indicate that compression and/or irritation of the brachial plexus occurs within each of these spaces.27 Scalenus medius is normally inserted into the superior aspect of the first rib posterior to the vascular and neural groove and scalenus anterior into the scalene tubercle, which lies to the front of the groove separating the subclavian artery from the vein. In cases of TOS the scalene insertions may overlap or fuse, presenting sharp tendinous ridges over which the nerves and artery must pass28 (Fig. 205-1B and C). The presence of a cervical rib further alters the insertion of these muscles. If the rib is complete, the muscles are inserted into the cervical rib as they would be into the normal first rib; but when the cervical rib is incomplete, scalenus medius may be partly inserted into it and may continue as a tight fibrous band to be inserted into the first rib. This fibrous extension, known as the scalenus medius band, corresponds to the continuation of the cervical rib whose bony development has been arrested and is one of the more common findings in TOS.29,30 Scalenus minimus, normally a small, inconstant muscle extending from the seventh cervical transverse process to the inner margin of the first rib, has occasionally been implicated as a source of nerve pressure as the first thoracic nerve root passes between this muscle and the scalenus medius31 (Fig. 205-1D). The arterial syndrome, on the other hand, is more likely to be associated with the complete cervical rib over which the artery must pass. The incomplete rib cannot extend far enough to exert pressure on the artery.
Brachial plexus compression (most often involving the lower trunk) within the thoracic outlet may occur in the following anatomic sites32:
1. Where it rubs against a sharp tendinous posterior border of the scalenus anterior
2. Where it is compressed between the clavicle and a normal or abnormal rib, especially in cases when the costoclavicular space is reduced by an incomplete abnormal rib or callus about a healed fracture of the clavicle
3. Where it is crossed by the firm, free posterior border of Sibson’s fascia (the suprapleural membrane, a band of fibrous connective tissue that runs between the C7 transverse process and the apex of the parietal pleura)
4. Where it crosses the crescentic tendinous fibers of the scalenus medius
5. Where it is compressed in the intermuscular cleft between the scalenus medius and scalenus anterior
6. Where it is wedged in the narrow tendinous angle between the scalenus medius and scalenus minimus, and/or the scalenus medius and scalenus anterior
7. Where it crosses an abnormal rib or the ligamentous extensions associated with such anomaly
8. Where it rubs across a first rib or at the site where an incomplete cervical rib articulates with the upper surface of the first rib
Diagnosis
Clinical Presentation and Physical Examination
Many patients with suspected TOS fail to exhibit a clear-cut neurophysiologic and radiologic picture. Pathologies may present with signs and symptoms that mimic TOS, including cervical disc disease, cervical spondylosis, peripheral nerve entrapment disorders such as cubital tunnel syndrome, carpal tunnel syndrome, radial tunnel syndrome and pronator syndrome, nerve sheath tumors, Pancoast’s syndrome from bronchogenic carcinoma and brachial plexus metastases from breast carcinoma or other primary tumors.33 The diagnosis of TOS is best reached by careful clinical, radiologic, and electrophysiologic assessment.
The pain of TOS has no characteristic pattern and may be absent in cases with a severe neurologic deficit. The pain here is more common during the day. Typically, it can be aggravated by a variety of repetitive physical activities (particularly overhead), cold, and carrying heavy weights, but this is also true for such a wide range of disorders affecting the neck and arm that the character of the pain alone rarely is specific to the diagnosis. Also, coincidence of arm pain and a cervical rib is not sufficient for a diagnosis of TOS, although in cases with long-term follow-up it can suggest TOS.
Typically, patients complain about changes in manual dexterity or handwriting. Commonly, they experience similar symptoms with overhead use of the arm such as reaching to a high shelf or holding a hair dryer. Sometimes, driving or lifting weights provokes symptoms. In these cases, hypothenar, interosseous, or adductor pollicis weakness of a subtle degree is often detected. The muscle power of little and ring fingers may be weak, and less commonly, the median motor nerve distribution of the lower trunk may be affected. However, it is usually not observed in the absence of weak ulnar innervated musculature. Muscles supplied by the median, ulnar, and radial nerves may all be involved. It has been recognized that selective thenar muscle wasting, particularly of the abductor pollicis brevis and opponens pollicis, is visible in TOS patients.34 In classic neurogenic TOS, sensory disturbances are found in ulnar nerve distribution and motor disturbances in median and at times in ulnar distributions. Some patients exhibit significant weakness and atrophy of intrinsic hand muscles, the so-called Gilliatt-Sumner hand,35 in which a dramatic degree of atrophy occurs in the abductor pollicis brevis and lesser atrophy in the interossei and the hypothenar muscles. However, about half of TOS patients have no motor deficit at all. The sensory deficit, if present, usually affects the medial forearm, hypothenar eminence, and little and ring fingers. It essentially includes the ulnar nerve sensory distribution and that of the medial cutaneous nerve of the forearm. As with the motor findings, those of the sensory deficit may be subtle or even absent in symptomatic patients. The finding of neurologic deficits within the territory of more than one peripheral nerve points to the possibility of brachial plexus involvement.
Other clinical signs of importance are tenderness to pressure over the lower trunk at the site of compression. A cervical rib may produce a visible and palpable swelling. Vasomotor disturbances such as skin color and temperature changes may be seen in advanced cases, presumably related to compression of sympathetic fibers in the lower trunk, C8, and/or T1. Unilateral Raynaud’s phenomenon due to a sympathetic dystrophy sometimes occurs with neurologic TOS without involvement of the subclavian artery.36 Some patients report that they feel a cold arm, intermittent swelling, and fatigue of the limb when exercising, pallor, and/or cyanosis of the affected hand with diminished or absent distal pulses, which may reflect arterial compression. A supraclavicular mass or bruit may also be present. Thrombosis of the subclavian vein, also known as Paget-von Schrotter syndrome, manifests as upper extremity edema and cyanosis, with distended superficial veins of the shoulder and chest, often without complaints of pain.18 Some patients exhibit acute thrombosis of the subclavian vein, or so-called “effort thrombosis,” which should be treated aggressively with intravenous catheterization using thrombolytic agents and then anticoagulation.37
Clinical Diagnostic Tests
Positive Tinel’s sign (pain or tingling on plexus percussion) over the brachial plexus beneath the scalene muscles and positional provocative maneuvers, and bruit or diminished or loss of radial artery pulse are helpful in increasing clinical suspicion, but many asymptomatic persons have these findings.38 Positional provocative maneuvers have sensitivity and specificity of 72% and 53%, respectively,39 although false-positive results were described in 45% for the Adson test, 77% in the Roos test, and 61% in the SCP (supraclavicular pressure) test.40
Classic Positional Provocative Maneuvers
Classic positional provocative maneuvers are also useful (Fig. 205-2). The Adson test is performed with the patient seated and the examiner palpating the radial pulse with the patient’s arm dependent. The neck is turned with full extension toward the side of the lesion, while a deep breath is taken. In a positive test the radial pulse is reduced or lost.
Electrophysiologic Evaluation
Electrophysiologic evaluation can be of potential value in the diagnosis of TOS; however, it should always be interpreted in the context of clinical findings. For true neurogenic TOS, electrodiagnosis has traditionally rested on the findings of nerve conduction studies showing prominent loss of the median compound muscle action potential and ulnar sensory nerve action potential amplitudes, with less involvement of the ulnar compound muscle action potential (CMAP) in the setting of a normal median sensory nerve action potential (SNAP).41 Levin et al.42 and Cruz-Martinez and Arpa43 showed that medial antebrachial cutaneous SNAP is important in the electrodiagnosis of TOS. Abnormal nerve conduction velocities of the medial antebrachial cutaneous nerve, in the absence of other electrophysiologic findings, suggest neurogenic TOS.44,45 Reduction of the ulnar SNAP amplitude is a nearly universal finding in this disorder, although the ulnar SNAP is less affected than the medial antebrachial cutaneous response. Many patients with TOS show normal electrophysiologic studies46; this is the typical finding in the disputed TOS cases. Lai et al.7 showed that the finding of abnormal preoperative somatosensory-evoked potentials (SSEPs) correlates with a favorable outcome after surgery for TOS. Patients (93%) with abnormal preoperative SSEPs showed good postoperative outcomes compared to patients (60%) with normal preoperative SSEPs.
Imaging Studies of TOS
Plain Radiographs and CT Scan with Three-Dimensional Reconstruction
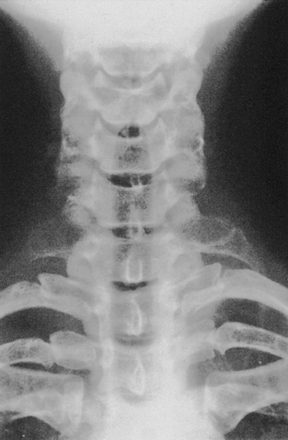
Bone abnormalities (cervical rib, malunited clavicular fracture, abnormal first thoracic rib) are clearly demonstrated by x-ray and CT scan. Classic TOS imaging of the cervical spine in most cases shows the presence of a cervical rib (Fig. 205-3) usually incomplete, an elongated transverse process of the seventh cervical vertebra, or rarely, a deformity of the first rib. Although the incidence of cervical ribs in the general population is about 1%,47 less than 20% of operative cases have significant osseous abnormalities.48
MRI can be of potential value in the diagnosis of TOS as follows: (1) demonstrating deviation or distortion of nerves or blood vessels, (2) suggesting the presence of radiographically invisible bands, and (3) disclosing other causes of TOS apart from ribs or bands.49 MRI is acquired with the symptomatic arm in neutral and in the provocative maneuvers.50 More recently, Filler and others have reported that MR neurography can directly image the nerve elements of the brachial plexus and have correlated preoperative imaging findings with operative findings and clinical outcomes.51
Evaluation of Vascular Insufficiency
Evaluation of vascular insufficiency includes Doppler pressures at the brachial and wrist levels or duplex ultrasound (US) that can evaluate arterial patency in a wide range of provocative positions. CT/MR angiogram and venogram are selectively used, because of their better sensitivity for intraluminal lesions. Venography is mandatory for patients who are suspected of having acute thrombosis of the subclavian vein.52 These patients have the acute onset of severe pain in the arm, accompanied by swelling and cyanosis. Immediate treatment is essential because initial treatment with intravenous catheterization, thrombolytic agents, and anticoagulation can reverse the process.
Sonographic Evaluation of Brachial Plexus
The successful attempts to use US for imaging of the brachial plexus anatomy53,54 reinforce the relevance of US study of brachial pathology. The advantages of sonography of the brachial plexus are related to its ability to directly visualize the nerve, providing fine details such as nerve compression, detecting the presence of scar tissue, and its relation to the nerve, demarcation of changes in nerve diameter (thickening), and texture (infiltration). Visualization of vascularity and adjacent circulation on US does not require the injection of contrast medium. Sonography is easily tolerated by the patient and is cost effective. In TOS, ultrasound is capable of demonstrating nerve or vascular compression during the hyperabduction maneuver which disappears again with lowering of the elevated arm.55
Initial Management, Indications, and Timing of Surgery
Conservative Treatment
Exercises that include overhead use of the arms or shoulder, deep massage in the supraclavicular fossa, and sleeping in positions with the arms overhead can aggravate the patient’s condition. Some patients habitually have poor posture. In those patients, the trapezius muscle is often atrophic and weak, and this allows the scapula to droop on the involved side. What is needed is a program of gently progressive strengthening exercises for the shoulder girdle and scalene musculature that the patient can learn to perform at home. All exercises are performed with the arms below shoulder height while avoiding shoulder back movements. For patients who are obese, weight reduction helps improve posture and lessen the load on shoulder girdles. Weight reduction and regular physical exercise coupled with physiotherapy in the form of postural and shoulder-bracing exercises are the mainstay.56 Water exercises and swimming are recommended. There must be close communication between the patient and the therapist so that exercise corrections can be made when necessary.
In some cases, the authors have used scalene muscle blocks for pain relief and also as additional diagnostic tests. It has been reported that patients with suspected diagnoses of fibromyalgia, complex regional pain syndrome, and/or depression do not show positive responses to scalene muscle blocks, while patients with positive responses are more likely to respond to surgery.57 Conservative management of TOS may be successful in 50% to 90% of cases.58,59 The positive results of conservative treatment are based on the patient’s home and/or work behavior.60 The authors’ experience suggests that the conservative course of treatment, which might include psychological counseling, should be pursued for at least 3 to 6 months unless symptoms worsen, or until it is readily apparent that there has been no improvement. In cases with significant compression, as indicated by severe muscle atrophy, conservative therapy will not suffice and surgery is indicated.
Indications for Surgery in TOS
1. Failure of a supervised exercise and postural program to improve activities of daily living
2. Continued intractable pain that is exacerbated by use of the arm
3. Progressive neurologic deficit
4. Impending vascular catastrophe
5. Following successful initial treatment of subclavian vein thrombosis
6. Operation is required urgently in cases of critical ischemia
Surgical Approaches
Surgical approaches for treatment of the above diffuse complex symptoms have been in use for more than 30 years. In 1966, Roos61 introduced and popularized the transaxillary route for TOS and this method became popular for resection of the first and/or cervical rib.62–64 Similar complications and long-term results were found between transaxillary61 and other surgical approaches: scalenotomy and cervical rib and first rib resection with removal of fibrous neuromuscular bands65 by supraclavicular,66,67 infraclavicular,68 and transthoracic approaches.69 In addition, in some cases, an incorrect diagnosis or inappropriate surgical approach could be responsible for failure of the surgery.
The selection of surgical approach is controversial and subject to the training and experience of the individual surgeon. In patients with upper root symptoms, the preferred operation is excision of the anterior and middle scalene muscles and any associated fibrous bands performed via a supraclavicular approach. Many surgeons believe that transaxillary first rib resection is indicated in patients with lower root and vascular symptoms. When both upper and lower root syndromes are present, the procedure may include both scalenotomy and first rib resection with section of fibrous bands and resection of a cervical rib, when present.62,70–72 Among the various surgical approaches, only the anterior supraclavicular, transaxillary, and posterior subscapular approaches have stood the test of time.
Anterior Supraclavicular Approach
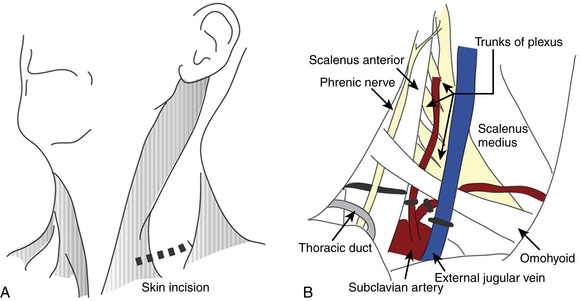
This approach, which allows a wide exposure of the supraclavicular plexus and the middle two thirds of the first rib, is favored by most neurosurgeons. The operation is performed under general anesthesia. The patient is positioned supine with a roll under the ipsilateral shoulder and the head turned 30 degrees toward the opposite side. An oblique 6- to 8-cm transverse incision is made 2 cm above the clavicle, at the base of the posterior triangle of the neck. The medial extent of the incision is the midpoint of the sternocleidomastoid (Fig. 205-4A). The platysma is divided in line with the skin incision. The supraclavicular nerves are identified and protected. The external jugular vein is retracted or ligated. The sternocleidomastoid is retracted medially. The omohyoid muscle is identified and retracted laterally. The supraclavicular fat pad is then reflected carefully from medial to lateral. Often, large lymphatic vessels are encountered within the fat pad, and they must be either preserved or, more often, coagulated. Few transverse cervical and suprascapular vessels often need to be ligated. The phrenic nerve is identified on the anterior surface of the anterior scalene and meticulously protected. The upper trunk of the brachial plexus is apparent. Then, the anterior scalene muscle is resected inferiorly. The middle trunk is then apparent. It is dissected distally to the juncture between the clavicle and the first rib and proximally to the transverse processes. The dissection continues medially and deeper until the lower trunk is identified and dissected. The subclavian artery is found inferiorly running in the plane of the brachial plexus. The trunks of the brachial plexus are dissected, neurolyzed, and gently retracted to expose the neck of the first rib and the transverse process of C7 (Fig. 205-4B). The firm fibrous bands between the first or cervical rib and the brachial plexus elements and subclavian artery are removed. This includes division of an anomalous muscle, the scalenus minimus, if present, and Sibson’s fascia (the suprapleural membrane), which may impinge on the lower trunk quite proximally. If necessary, the first rib can be identified and resected as well.
Intraoperative EMG recordings are used to help identify nerves by the muscles that contract when that particular nerve is stimulated. We generally73 record CMAPs from APB and first dorsal interosseous (FDIO) muscles at the beginning of the operation, during the operation, and after removal of fibrous bands, scar tissue, and neurolysis. Nerve action potentials (NAPs) may also be used to test damaged nerve segments.
It is believed by some authors4 that the pathomechanism of TOS may include more than one site of compression, each increasing the influence of the others, causing a “multiple crush phenomenon.” This theory, while unproven, explains the need for extensive neurolysis rather than routine approaches, which are based on the decompression of a specific anatomic structure (i.e., the rib or the scalenus muscle). Historically, simple sectioning of the anterior scalene muscle alone had a very high failure rate, and thus most surgeons currently will explore the entire region in search of additional areas of nerve compression. The supraclavicular approach is most appropriate for treating the multiple potential sites of brachial plexus, subclavian artery, and vein compression. From our previous experience,73 this approach allows the best exposure for neurolysis and decompression of the neurovascular complex as well as an excellent view and possibility to remove adhesions, scar tissue, or fibrous bands without further traumatizing the brachial plexus and vessels.
Posterior Subscapular Approach
In the posterior subscapular approach,74 the trapezius muscle is divided longitudinally away from its nerve supply. The levator scapulae, the rhomboideus minor, and major muscles are exposed and divided away from the edge of the scapula. Thus, the posterior chest wall is exposed (Fig. 205-5). The first rib is removed from the costotransverse articulation. The posterior and middle scalene muscles are released from their origin at the transverse spinous processes. After removal of these muscles superiorly, the roots of spinal nerves and the trunks of the brachial plexus are exposed. This approach, which requires extensive postoperative physical therapy, is particularly useful in patients who have undergone prior anterior approaches or radiation therapy to the area.
Transaxillary Approach
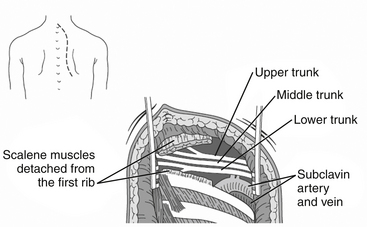
In 1966, Roos61 introduced the transaxillary route for TOS in the presence of a cervical rib. This approach is used by most thoracic and vascular surgeons and allows complete resection of the first rib. The patient is placed in the posterolateral position with the arm elevated above the head. An incision is made over the first palpable rib (usually the third rib) in the axillary fossa. The axillary fat, lymph nodes, and vessels are dissected away, and the anterior and middle scalene muscles are divided (Fig. 205-6). The cervical or first rib is identified and resected. The disadvantage of this approach is the limited exposure of the neurovascular elements, behind which congenital bands may be located. Endoscope-assisted transaxillary techniques have been developed and have been reported to be safe and effective.75,76
Case Illustration: Operative Treatment of TOS
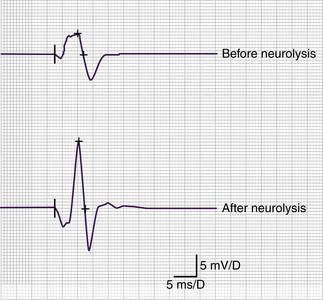
The operation was performed under general anesthesia without muscle relaxant agents so that nerve stimulation and recording could be performed. Using a supraclavicular approach, release and neurolysis of the C8 and T1 spinal nerves and lower trunk were performed. The fibrous bands between the rudimentary cervical rib and the brachial plexus elements, as well as the subclavian artery were removed. The cervical rib itself did not impinge on the plexus in this case and thus was not resected. After the release of the plexus elements, an increasing amplitude of CMAP was discovered (Fig. 205-7). Surgical results were studied with a follow-up period of 5 years.
Clinical Outcomes Following Surgery
Unfortunately, the surgical literature for TOS is littered with retrospective series in which surgeons report their own loosely defined results in patients with a wide variety of chronic upper extremity pain syndromes. Some series have reported good or excellent results in more than 80% of patients,3,64,71,77 but the most significant postoperative problem remains the recurrence or persistence of symptoms. More recent publications show resolution of pain and/or paresthesias in 50% to 60% of patients and partial response in another 20% to 30%.78–80
The experience of David Kline and colleagues at the Louisiana State University Health Sciences Center (LSUHSC), using posterior subscapular or anterior supraclavicular approaches in the treatment of 99 patients with disputed neurogenic TOS, showed that 65% had relief of pain and/or paresthesias and 33% had partial improvement in pain. Of the 35 patients with preoperative motor deficits, 24 (69%) improved and 11 (31%) were unchanged. Cervical ribs or elongated C7 transverse processes were found in 38% of cases. Of the 32 surgically treated patients with true neurogenic TOS, 19% had experienced resolution of pain, 47% partial improvement of pain, and 79% had improvement in motor deficit.79
A retrospective analysis of outcomes of 668 primary operations on 491 patients at up to 15 years since surgery showed similar results between supraclavicular and transaxillary approaches with a lower complication rate in patients that underwent the supraclavicular approach (with or without rib resection).81 In a small, randomized trial comparing supraclavicular neuroplasty to transaxillary first rib resection in patients with disputed neurogenic TOS, the results for pain relief were better using the transaxillary approach.82
Using the posterior subscapular approach for true neurogenic TOS, Kline reported improvement of pain in 54% and motor function in 67% of patients at 1 year.83 Surgical treatment of true neurogenic TOS with radiographic abnormalities using the supraclavicular approach showed complete resolution of pain in 60% and marked improvement of pain in 20% of patients. Sensory function improved in 75%, hand function in 72%, and muscle strength in 65% of patients.80 Leffert, using the transaxillary approach, reported that 35% of patients were totally asymptomatic and 35% had substantial pain relief and required no analgesics. About 15% derived no benefit from surgery.48
Recurrent TOS
Recurrence of symptoms is especially relevant when looking at outcome, as it has been suggested that the mean time for recurrence is within 6 months.84 Postoperative fixation of brachial plexus nerves to surrounding structures by stiff scar tissue and a first rib remnant are the usual mechanisms. Recurrence of symptoms after simple scalenotomy or partial scalenotomy performed via a supraclavicular approach, were reported in as high as 20% of the operated patients, in most cases due to the reattachment of the muscle to the first rib through scar tissue formation, involving both nerve roots and vessels.85,86 In several cases, the recurrence of symptoms after first rib resection appeared to be a result of a retained first rib segment. Partial rib regeneration or bony spike protrusion from the end of the rib remnant itself are important factors for incomplete decompression and allow the development of scar tissue and/or scalene musculature reattachment.28,87,88 Modification of the cervical rib resection surgery (a subperiosteal resection of the middle third of the cervical rib)3 decreased the complication rate; however, there is a possibility that reossification in the periosteal bed will lead to recurrent symptoms.89 Recurrence of symptoms following either transaxillary rib resection or scalenectomy is reported in 15% to 20% of cases.71,85,88 The success rate for operative treatment of recurrent TOS was 84% at 3 months but decreased to 50% at 3 to 5 years.88 One report showed that a combined primary transaxillary rib resection, and supraclavicular scalenectomy decreased the rate of recurrence to 5% to 10%.90
Complications
Postoperative complications occur in 14% to 34.5% of patients,64,91 including pneumothorax, hemothorax, chylothorax, pleural effusions, wound infections, wound hematomas, nerve injuries, and hypertrophic scars. Posterior subscapular approach complications include injury of long thoracic, dorsal scapular, and spinal accessory nerves during extensive muscle dissection, with a 5% incidence of scapular winging.74,92,93
Supraclavicular approach complications include injury of supraclavicular nerves with numbness over the supraclavicular region, lasting approximately 6 weeks, as well as painful neuromas and/or neuropathic pain,93 phrenic nerve injury (3% to 6%), pneumothorax (1% to 2%), chylothorax (1% to 2%),94,95 and vascular injury (1% to 2%) when the first rib is removed.94
Transaxillary approach complications include paresthesias and/or hypersensitivity in the distribution of the intercostobrachial nerve, brachial plexus injury (1% to 3%), venous injury (2%), pneumothorax (9%), Horner’s syndrome, and damage to the thoracic duct.18,93,94,96
Conclusion
Thoracic outlet syndrome refers to a group of complex symptoms in the upper extremity caused by compression of the brachial plexus and the subclavian artery and vein. Persistent symptoms may lead to loss of job and lifelong disability, causing devastating functional, emotional, and financial impairment if not treated successfully. Most patients are managed conservatively at first with a variety of modalities. Avoidance of provocative activities, directed physical therapy, comprehensive pain management, and anesthetic blocks, some with ultrasound guidance, should be pursued in patients with pain as the primary presentation. The careful selection of patients for surgery by a coordinated team comprised of a neurosurgeon, vascular surgeon, radiologist, neurophysiologist, and rehabilitation and pain management specialist can yield satisfactory results.
Abdellaoui A., Atwan M., Reid F., et al. Endoscopic assisted transaxillary first rib resection. Interact Cardiovasc Thorac Surg. 2007;6:644-646.
Atasoy E. Recurrent thoracic outlet syndrome. Hand Clin. 2004;20:99-105.
Chang D.C., Lidor A.O., Matsen S.L., et al. Reported in-hospital complications following rib resections for neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2007;21:564-570.
Cruz-Martinez A., Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41:253-256.
Demondion X., Bacqueville E., Paul C., et al. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227:461-468.
Demondion X., Herbinet P., Van Sint Jan S., et al. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26:1735-1750.
Filler A.G. MR neurography and brachial plexus neurolysis in the management of thoracic outlet syndromes. In: Yao J.S.T., Pearce W.H. Advances in Vascular Surgery. Chicago: Precept Press, 2002. pp. 499-523
Franklin G.M., Fulton-Kehoe D., Bradley C., et al. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54:1252-1257.
Fulford D.E., Baguneid M.S., Ibrahim M.R., et al. Outcome of transaxillary rib resection for thoracic outlet syndrome—a 10 year experience. Cardiovasc Surg. 2001;9:620-624.
Gillard J., Perez-Cousin M., Hachulla E., et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68:416-424.
Graif M., Martinoli C., Rochkind S., et al. Sonographic evaluation of brachial plexus pathology. Eur Radiol. 2004;14:193-200.
Janjua R.M., Tender G.C., Tiel R.L., et al. Thoracic outlet syndrome. In: Slutsky D.J., Hentz V.R. Peripheral Nerve Surgery: Practical Applications in the Upper Extremity. Philadelphia: Churchill Livingstone, 2008. pp. 285-298
Jordan S.E., Ahn S.S., Gelabert H.A. Differentiation of thoracic outlet syndrome from treatment-resistant cervical brachial pain syndromes: development and utilization of a questionnaire, clinical examination and ultrasound evaluation. Pain Physician. 2007;10:441-452.
Machanic B.I., Sanders R.J. Medial antebrachial cutaneous nerve measurements to diagnose neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2008;22:248-254.
Martinez B.D., Wiegand C.S., Evans P., et al. Computer-assisted instrumentation during endoscopic transaxillary first rib resection for thoracic outlet syndrome: a safe alternate approach. Vascular. 2005;13:327-335.
Maxey T.S., Reece T.B., Ellman P.I., et al. Safety and efficacy of the supraclavicular approach to thoracic outlet decompression. Ann Thorac Surg. 2003;76:396-399. discussion 399-400
Murovic J.A., Kim D.H., Kim S.H., et al. Thoracic outlet syndrome: part two. Neurosurg Q. 2007;17:13-18.
Nannapaneni R., Marks S.M. Neurogenic thoracic outlet syndrome. Br J Neurosurg. 2003;17:144-148.
Nord K.M., Kapoor P., Fisher J., et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48:67-74.
Rochkind S., Shemesh M., Patish H., et al. Thoracic outlet syndrome: a multidisciplinary problem with a perspective for microsurgical management without rib resection. Acta Neurochir Suppl. 2007;100:145-147.
Sanders R.J., Hammond S.L., Rao N.M. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46:601-604.
Seror P. Medial antebrachial cutaneous nerve conduction study, a new tool to demonstrate mild lower brachial plexus lesions. A report of 16 cases. Clin Neurophysiol. 2004;115:2316-2322.
Tender G.C., Kline D.G. Posterior subscapular approach to the brachial plexus. Neurosurgery. 2005;57:377-381. discussion 377-381
Yang J., Letts M. Thoracic outlet syndrome in children. J Pediatr Orthoped. 1996;16:514-517.
1. Dellon AL. The results of supraclavicular brachial plexus neurolysis (without first rib resection) in management of post-traumatic “thoracic outlet syndrome.”. J Reconstr Microsurg. 1993;9:11-17.
2. Goff CD, Parent FN, Sato DT, et al. A comparison of surgery for neurogenic thoracic outlet syndrome between laborers and nonlaborers. Am J Surg. 1998;176:215-218.
3. Sharan D., Moulton A., Greatrex GH. Two-surgeon approach to thoracic outlet syndrome: long-term outcome. J R Soc Med. 1999;92:239-243.
4. Willshire WH. Supernumerary first rib: clinical records. Lancet. 1860;2:633.
5. Murphy JB. The clinical significance of cervical ribs. Surg Gynecol Obstet. 1906;3:515.
6. Peet RM, Hendricksen JD, Guderson TP. Thoracic outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31:281-287.
7. Lai DT, Walsh J., Harris JP. Predicting outcomes in thoracic outlet syndrome. Med J Austr. 1995;162:345-347.
8. McCarty WJ, Yao JS, Schafer MF, et al. Upper extremity arterial injury in athletes. J Vasc Surg. 1989;9:317-327.
9. Wilbourn AJ. Brachial plexus disorders. In: Dyck PJ, Thomas PK, Griffin JW, et al. Peripherial Neurophathy. Philadelphia: WB Saunders, 1993., pp. 911-950
10. Yang J., Letts M. Thoracic outlet syndrome in children. J Pediatr Orthoped. 1996;16:514-517.
11. Wilbourn AJ, Porter JM. Thoracic outlet syndrome. In: Weiner MA, editor. Spine: State of the Art Reviews. Philadelphia: Hanley and Belfus, 1988., pp. 597-626
12. Short DW. The subclavian artery in 16 patients with complete cervical ribs. J Cardiovasc Surg. 1975;16:135-141.
13. Durham JR, Yao J.S.T., Pearce WH, et al. Arterial injuries in the thoracic outlet syndrome. J Vasc Surg. 1995;21:57-70.
14. Etter LE. Osseous abnormalities of the thoracic cage seen in forty thousand consecutive chest photoroentgenograms. Am J Roentgenol Radium Ther. 1944;51:359-363.
15. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11:66-74.
16. Cherington M. A conservative point of view of the thoracic outlet syndrome. Am J Surg. 1989;158:394-395.
17. Sanders RJ, Haug C. Review of arterial thoracic outlet syndrome with a report of five new instances. Surg Gynecol Obstet. 1991;173:415-425.
18. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46:601-604.
19. Hood DB, Kuehne J., Yellin AE, et al. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63:913-917.
20. Toso C., Robert J., Berney T., et al. Thoracic outlet syndrome: influence of personal history and surgical technique on long-term results. Eur J Cardiothorac Surg. 1999;16:44-47.
21. Franklin GM, Fulton-Kehoe D., Bradley C., et al. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54:1252-1257.
22. Becker DM, Philbrick JT, Walker FB. Axillary and subclavian venous thrombosis. Prognosis and treatment. Arch Intern Med. 1991;151:1934-1943.
23. Azakie A., McElhinney DB, Thompson RW, et al. Surgical management of subclavian-vein thrombosis as a result of thoracic outlet compression. J Vasc Surg. 1998;28:777-781.
24. Nichols HM. Anatomical structures of the thoracic outlet. Clin Orthop. 1967;51:17-25.
25. Ranney D. Thoracic outlet: an anatomical redefinition that makes clinical sense. Clin Anat. 1996;9:50-52.
26. Demondion X., Bacqueville E., Paul C., et al. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227:461-468.
27. Remy-Jardin M., Remy J., Masson P., et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174:1667-1674.
28. Telford ED, Mottershead S. Pressure at the cervico-brachial junction—an operative and anatomical study. J Bone Joint Surg Am. 1948;30B:249-265.
29. Bonney G. The scalenus medius band: A contribution to the study of thoracic outlet syndrome. J Bone Joint Surg Br. 1965;47:268-272.
30. Sargent P. Some points in the surgery of cervical ribs. Proc R Soc Med. 1913;6:117-126.
31. Lascelles RG, Mohr PD, Neary D., et al. The thoracic outlet syndrome. Brain. 1977;100:601-612.
32. Sunderland S. Nerve Injuries and Their Repair. Edinburgh, London, Melbourne and New York: Churchill Livingstone; 1991.
33. Campbell JN, Naff NJ, Dellon AL. Thoracic outlet syndrome. Neurosurgical perspective. Neurosurg Clin North Am. 1991;2:227-233.
34. Wilson SA. Some points in the symptomatology of cervical rib, with especial reference to muscular wasting. Proc R Soc Med. 1913;6:133-138.
35. Gilliatt RW, Le Quesne PM, Logue V., et al. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33:615-624.
36. Connell JL, Doyle JC, Gurry JF. The vascular complications of cervical rib. Aust N Z J Surg. 1980;50:125-130.
37. Shuttleworth RD, van der Merwe DM, Mitchell WL. Subclavian vein stenosis and axillary vein ‘effort thrombosis.’ Age and the first rib bypass collateral, thrombolytic therapy and first rib resection. S Afr Med J. 1987;71:564-566.
38. Lascelles RG, Schady W. The thoracic outlet syndrome. In: Vinken PJ, Bruyn GW. Handbook of Clinical Neurology. Amsterdam: Elsevier, 1987., pp. 119-131
39. Gillard J., Perez-Cousin M., Hachulla E., et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68:416-424.
40. Nord KM, Kapoor P., Fisher J., et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48:67-74.
41. McLafferty RB, Edwards JM, Taylor LM, et al. Diagnosis and long-term clinical outcome in patients diagnosed with hand ischemia. J Vasc Surg. 1995;22:361-369.
42. Levin KH, Wilbourn AJ, Maggiano HJ. Cervical rib and median sternotomy—related brachial plexopathies: a reassessment. Neurol. 1998;50:1407-1413.
43. Cruz-Martinez A., Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41:253-256.
44. Machanic BI, Sanders RJ. Medial antebrachial cutaneous nerve measurements to diagnose neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2008;22:248-254.
45. Seror P. Medial antebrachial cutaneous nerve conduction study, a new tool to demonstrate mild lower brachial plexus lesions. A report of 16 cases. Clin Neurophysiol. 2004;115:2316-2322.
46. Aminoff MJ. Electromyography in Clinical Practice, 3rd ed. New York: Churchill Livingstone; 1998.
47. Pollack EW. Surgical anatomy of the thoracic outlet syndrome. Surg Gynecol Obstet. 1980;150:97-103.
48. Leffert RD. Thoracic outlet syndrome. In: Omer GE, Spinner M., Van Beek AL. Management of Peripheral Nerve Problem. Philadelphia: Saunders, 1998., pp. 494-500
49. Panegyres PK, Moore N., Gibson R., et al. Thoracic outlet syndromes and magnetic resonance imaging. Brain. 1993;116:823-841.
50. Demondion X., Herbinet P., Van Sint Jan S., et al. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26:1735-1750.
51. Filler AG. MR neurography and brachial plexus neurolysis in the management of thoracic outlet syndromes. In: Yao J.S.T., Pearce WH. Advances in Vascular Surgery. Chicago: Precept Press, 2002. pp. 499-523
52. Sanders RJ, Haug C. Subclavian vein obstruction and thoracic outlet syndrome: a review of etiology and management. Ann Vasc Surg. 1990;4:397-410.
53. Sheppard DJ, Lyer RB, Fenstermacher MJ. Brachial plexus: demonstration at US. Radiology. 1998;208:402-406.
54. Graif M., Martinoli C., Rochkind S., et al. Sonographic evaluation of brachial plexus pathology. Eur Radiol. 2004;14:193-200.
55. Rochkind S, Alon M, Graif M, et al. Microsurgical Management of Thoracic Outlet Syndrome without Rib Resection. Paper presented at XVl International Symposium on Brachial Plexus Surgery Club A. Narakas, Luxembourg, May 21–23, 2009 (abstract).
56. Peet RM, Henriksen JD, Anderson TP, et al. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31:281-287.
57. Jordan SE, Ahn SS, Gelabert HA. Differentiation of thoracic outlet syndrome from treatment-resistant cervical brachial pain syndromes: development and utilization of a questionnaire, clinical examination and ultrasound evaluation. Pain Physician. 2007;10:441-452.
58. Aligne C., Barral X. Rehabilitation of patients with thoracic outlet syndrome. Ann Vasc Surg. 1992;6:381-389.
59. Kenny RA, Traynor GB, Withington D., et al. Thoracic outlet syndrome: a useful exercise treatment option. Am J Surg. 1993;165:282-284.
60. Vanti C., Natalini L., Romeo A., et al. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43:55-70.
61. Roos DB. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann Surg. 1966;163:354-358.
62. Roos DB. The place for scalenotomy and first-rib resection in thoracic outlet syndrome. Surgery. 1982;92:1077-1084.
63. Roos DB. Thoracic outlet syndrome: update. Am J Surg. 1987;154:568-573.
64. Davies AH, Walton J., Stuart E., et al. Surgical management of the thoracic outlet compression syndrome. Br J Surg. 1991;78:1193-1195.
65. Roos DB. Pathophysiology of congenital anomalies in thoracic outlet syndrome. Acta Chir Belg. 1980;79:353-361.
66. Falconer MA, Li WP. Resection of the first rib in costoclavicular compression of the brachial plexus. Lancet. 1962;1:59-63.
67. Hempel GK, Rusher AH, Wheeleer CG, et al. Supraclavicular resection of the first rib for thoracic outlet syndrome. Am J Surg. 1981;141:213-215.
68. Gol A., Patrick DW, McNeel DP. Relief of costoclavicular syndrome by infraclavicular removal of first rib. J Neurosurg. 1968;28:81-84.
69. Prêtre R., Spiliopoulos A., Mégevand R. Transthoracic approach in the thoracic outlet syndrome: an alternative operative route for removal of the first rib. Surgery. 1989;106:856-860.
70. Qvarfordt PG, Ehrenfeld WK, Stoney RJ. Supraclavicular radical scalenectomy and transaxillary first rib resection for the thoracic outlet syndrome. A combined approach. Am J Surg. 1984;148:111-116.
71. Sanders RJ, Monsour JW, Gerber WF, et al. Scalenectomy versus first rib resection for treatment of the thoracic outlet syndrome. Surgery. 1979;85:109-121.
72. Fulford DE, Baguneid MS, Ibrahim MR, et al. Outcome of transaxillary rib resection for thoracic outlet syndrome—a 10 year experience. Cardiovasc Surg. 2001;9:620-624.
73. Rochkind S., Shemesh M., Patish H., et al. Thoracic outlet syndrome: a multidisciplinary problem with a perspective for microsurgical management without rib resection. Acta Neurochir Suppl. 2007;100:145-147.
74. Dubuisson AS, Kline DG, Weinshel SS. Posterior subscapular approach to the brachial plexus. Report of 102 patients. J Neurosurg. 1993;79:319-330.
75. Abdellaoui A., Atwan M., Reid F., et al. Endoscopic assisted transaxillary first rib resection. Interact Cardiovasc Thorac Surg. 2007;6:644-646.
76. Martinez BD, Wiegand CS, Evans P., et al. Computer-assisted instrumentation during endoscopic transaxillary first rib resection for thoracic outlet syndrome: a safe alternate approach. Vascular. 2005;13:327-335.
77. Urschel HCJr., Razzuk MA. Management of the thoracic outlet syndrome. N Engl J Med. 1972;286:1140-1143.
78. Maxey TS, Reece TB, Ellman PI, et al. Safety and efficacy of the supraclavicular approach to thoracic outlet decompression. Ann Thorac Surg. 2003;76:396-399. discussion 399-400
79. Murovic JA, Kim DH, Kim SH, et al. Thoracic outlet syndrome: part two. Neurosurg Q. 2007;17:13-18.
80. Nannapaneni R., Marks SM. Neurogenic thoracic outlet syndrome. Br J Neurosurg. 2003;17:144-148.
81. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10:626-634.
82. Sheth RN, Campbell JN. Surgical treatment of thoracic outlet syndrome: a randomized trial comparing two operations. J Neurosurg Spine. 2005;3:355-363.
83. Kline DG. Thoracic outlet syndrome, Kim DH, Midha R., Murovic JA, Spinner RJ. Nerve Injuries, 2nd ed., Philadelphia: Saunders, Elsevier, 2008., pp. 373-390
84. Sessions RT. Reoperation for thoracic outlet syndrome. J Cardiovasc Surg. 1989;30:434-444.
85. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10:626-634.
86. Green RM, McNamare J., Ouriel K. Long-term follow-up after thoracic outlet decompression: an analysis of factors determining outcome. J Vasc Surg. 1991;14:739-746.
87. Urshel HCJr., Razzuk MA. The failed operation for outlet syndrome: the difficulty of diagnosis and management. Thorac Surg. 1986;42:523-528.
88. Sanders RJ, Haung CE, Pearce WH. Recurrent thoracic outlet syndrome. J Vasc Surg. 1990;12:390-400.
89. Kashyap VS, Ahn SS, Machleder HI. Thoracic outlet neurovascular compression: approuches to anatomic decompression and their limitations. Semin Vasc Surg. 1999;11:116-122.
90. Atasoy E. Recurrent thoracic outlet syndrome. Hand Clinics. 2004;20:99-105.
91. Mingoli A., Feldhaus RJ, Farina C., et al. Long-term outcome after transaxillary approach for thoracic outlet syndrome. Surgery. 1995;118:840-844.
92. Tender GC, Kline DG. Posterior subscapular approach to the brachial plexus. Neurosurgery. 2005;57:377-381. discussion 377-381
93. Mackinnon SE, Novak CB. Thoracic outlet syndrome. Curr Probl Surg. 2002;39:1070-1145.
94. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10:626-634.
95. Janjua RM, Tender GC, Tiel RL, et al. Thoracic outlet syndrome. In: Slutsky DJ, Hentz VR. Peripheral Nerve Surgery: Practical Applications in the Upper Extremity. Philadelphia: Churchill Livingstone, Elsevier, 2008., pp. 285-298
96. Chang DC, Lidor AO, Matsen SL, et al. Reported in-hospital complications following rib resections for neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2007;21:564-570.