Chapter 38 Management of the Neck
Anatomy of the Lymphatic System of the Neck
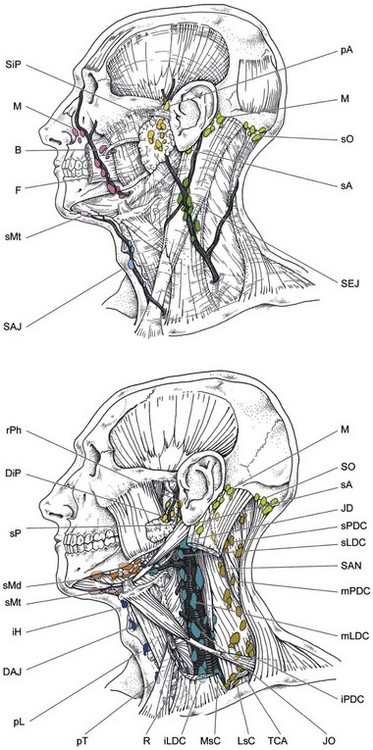
The head and neck region has a rich network of lymphatic vessels draining from the base of the skull through the jugular nodes, spinal accessory nodes, and transverse cervical nodes to the venous jugulosubclavian confluence or the thoracic duct on the left side and the lymphatic duct on the right side.1,2 A comprehensive anatomic description of this network was made by Rouvière more than 50 years ago.1 The whole lymphatic system of the neck is contained in the celluloadipose tissue delineated by the aponeurosis enveloping the muscles, vessels, and nerves (Fig. 38-1). The lymphatic drainage is mainly ipsilateral, but structures such as the soft palate, tonsils, base of the tongue, posterior pharyngeal wall, and especially the nasopharynx have bilateral drainage. On the other hand, sites such as the true vocal cord, paranasal sinuses, and middle ear have few or no lymphatic vessels at all.
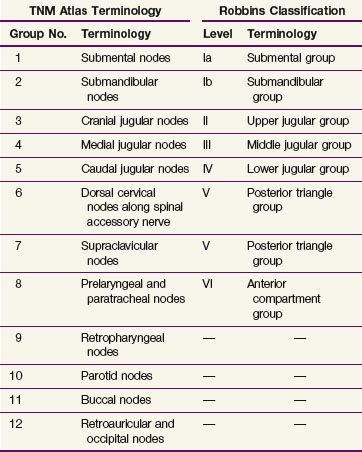
The nomenclature of head and neck lymph nodes has been complicated by various confusing synonyms that are still in use in major textbooks and articles. Several expert bodies have proposed the adoption of systematic classifications aimed at standardizing the terminology. Following the description by Rouvière, the TNM (primary tumor, regional nodes, metastases) atlas proposed a terminology that divides the head and neck lymph nodes into 12 groups.3 In parallel to this classification, the Committee for Head and Neck Surgery and Oncology of the American Academy for Otolaryngology–Head and Neck Surgery has been working on a classification (the so-called Robbins classification), dividing the neck into six levels, including eight node groups.4 This classification is based on the description of a level system that has been used for a long time by the Head and Neck Service at the Memorial Sloan-Kettering Cancer Center (MSKCC).5 Because one of the objectives in developing the Robbins classification was to create a standardized system of terminology for neck dissection procedures, only the lymph node groups routinely removed during neck dissection were considered. The terminology proposed by Robbins was recommended by the International Union Against Cancer (UICC).6 A comparison of the TNM system and the Robbins terminology is shown in Table 38-1. The major advantage of the Robbins classification over the TNM terminology is the definition of the boundaries of the node levels. The delineation of these boundaries is based on anatomic structures such as major blood vessels, muscles, nerves, bones, and cartilage that are easily identifiable by a surgeon during neck dissection procedures. The anatomic boundaries are oriented to a patient lying in a supine position with the neck in a surgical position (i.e., hyperextension) to better individualize the anatomic structures.
TABLE 38-1 Comparison of the TNM Atlas Terminology and the Robbins Classification of the Lymph Nodes of the Neck
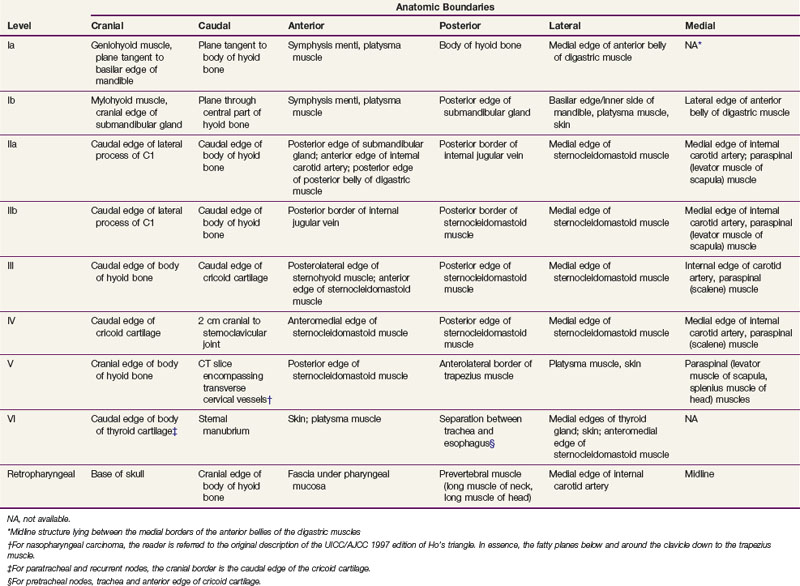
Level IV includes the lower jugular lymph nodes located around the inferior third of the internal jugular vein from the caudal limit of level III to the clavicle caudally. The anterior and posterior limits are the same as for level III (i.e., the lateral border of the sternohyoid muscle and the posterior edge of the sternocleidomastoid muscle, respectively). Laterally, level IV is limited by the sternocleidomastoid muscle and medially by the internal carotid artery and paraspinal muscles. Level IV contains a variable number of nodes and receives efferent lymphatics primarily from levels III and V; some efferent lymphatics from the retropharyngeal, pretracheal, and recurrent laryngeal nodes; and collecting lymphatics from the hypopharynx, larynx, and thyroid gland.1 Level IV nodes are at high risk for harboring metastases from cancers of the hypopharynx, larynx, and cervical esophagus.
Level V includes the lymph nodes of the posterior triangle group. This group includes the lymph nodes located along the lower part of the spinal accessory nerve and the transverse cervical vessels. Level V is limited cranially by the convergence of the sternocleidomastoid muscle and the trapezius muscles, caudally by the clavicle, anteriorly by the posterior border of the sternocleidomastoid muscle, and posteriorly by the anterior border of the trapezius muscle. Laterally, level V is limited by the platysma muscle and the skin and medially by the splenius capitis, levator scapulae, and scaleni (posterior, medial, and anterior) muscles. Level V is currently subdivided into levels Va and Vb. The distinction between the upper posterior triangle (level Va) and the lower posterior triangle (level Vb) allows lymph node involvement of the lower two-thirds of the spinal accessory nerve chain to be differentiated from that of the transverse cervical vessel chain.7,8 A horizontal plane defined by the caudal edge of the cricoid cartilage separates these two compartments. The demarcation between the posterior end of level IIb and the uppermost part of level Va has still not been clearly defined. The American Academy for Otolaryngology–Head and Neck Surgery defined the posterior boundary of level IIb as the posterior border of the sternocleidomastoid muscle and the apex of the convergence of the sternocleidomastoid muscle and the trapezius muscles as the cranial boundary of level Va. However, the uppermost part of level Va contains superficial occipital lymph nodes and, inconsistently, one subfascial lymph node close to the occipital attachment of the sternocleidomastoid muscle.1
These lymph nodes collect lymphatics from the occipital scalp and the postauricular and nuchal regions. They are not involved in the drainage of head and neck cancers except for skin tumors. Consequently, Hamoir and colleagues9 proposed to subdivide the level Va nodes into two sublevels: the apex of level V, or level Vas (superior), and level Vai (inferior). The border between level Vas and level Vai should be the lower two-thirds of the spinal accessory nerve. From a radiologic point of view, a horizontal plane defined by the upper edge of the body of the hyoid bone appears to be a reliable landmark to separate the two sections. Dissection of the apex (level Vas) does not seem to be required in mucosal head and neck squamous cell carcinoma. It should be considered only in cases of skin cancer of the posterior scalp and posterior neck. Level V receives efferent lymphatics from the occipital and postauricular nodes and from the occipital and parietal scalp, skin of the lateral and posterior neck and shoulder, nasopharynx, and oropharynx (tonsils and base of the tongue). Level V lymph nodes are at high risk for harboring metastases from cancers of the nasopharynx and oropharynx. Nodes in level Va are more often associated with primary cancers of the nasopharynx, oropharynx, or cutaneous structures of the posterior scalp, whereas those in level Vb are more commonly associated with tumors arising in the thyroid gland.
Imaging of the Neck
The armamentarium available for the imaging workup of the metastatic cervical lymph nodes includes computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography, and positron emission tomography (PET).10 Nodal imaging is mandatory in a pretreatment workup because clinical assessment of the nodal status in patients with a thick and/or small neck has a low sensitivity and because deeply located nodes remain inaccessible in all patients.11
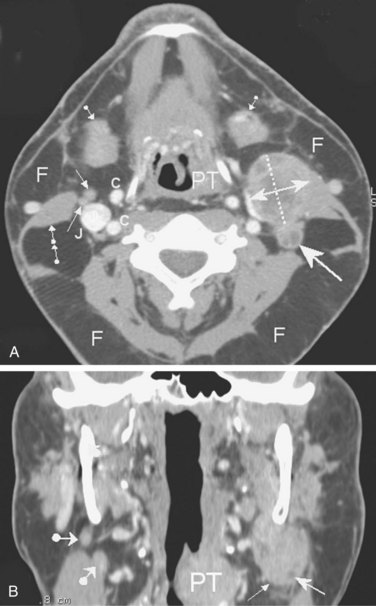
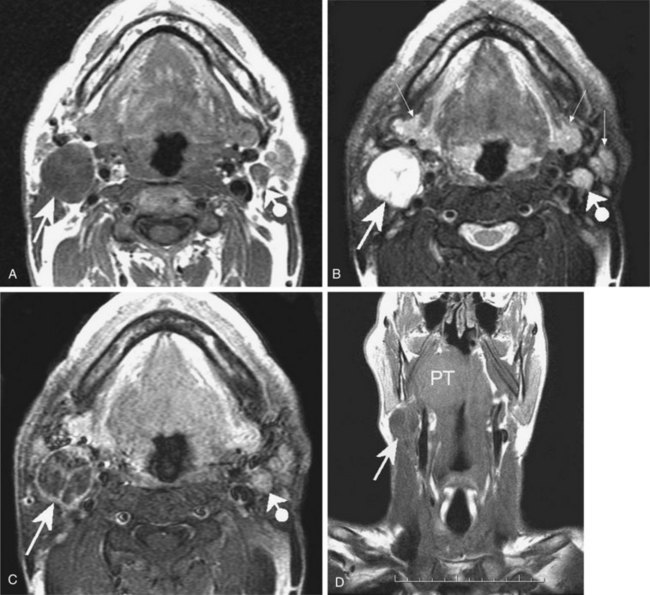
CT and MRI are standard cross-sectional imaging modalities through which anatomic “slices” of the entire neck depict the contours and internal structure of the nodes (Figs. 38-2 and 38-3). MRI may have an advantage over CT scanning because of the higher spontaneous contrast it reveals between fatty and nonfatty tissues. The multiplanar capability of MRI has been an advantage of this technique since its introduction into clinical use in the early 1980s. However, the multirow detector technology and the spiral acquisition modality of newer-generation CT systems have boosted the multiplanar reformatting capabilities of CT scans, which now equal those of MR images. The major criteria for nodal malignancy using CT and/or MRI include the size of the nodes (a short axis longer than 10 mm) and the presence of a central necrosis (hypodensity on CT images; hypointensity on T1-weighted MR images and hyperintensity on T2-weighted MR images). Central necrosis is well highlighted by intravenous contrast agent perfusion, which enhances nodal areas with arterial blood supply on both CT and MR images (see Figs. 38-2A and 38-3C). However, the two techniques share common weaknesses: the inability to detect micrometastatic deposits within normal-sized nodes (false-negative results) and the risk for inappropriate classification of malignancy in nodes that are enlarged by benign reactive changes (false-positive results). Thus far, neither MRI nor CT can provide perfect diagnostic accuracy for nodal metastatic workup. It has been shown in a large series of patients that the two techniques have an almost similar and unsatisfactory performance.12
Ultrasonography has long been regarded as a low-cost, widely available, and innocuous alternative to CT/MRI, with the additional advantages of color Doppler flow–encoded vascular architecture depiction and fine-needle aspiration guidance. However, time demands and operator skill requirements are limiting factors, as well as the unresolved technical difficulties of fusing two-dimensional ultrasound data and three-dimensional CT/MRI data for radiotherapy planning. Ultrasound is also less efficient than CT and MRI at detecting nodal necrosis,13 and deeply located nodes may be poorly accessible to the technique.
Research is underway to improve the diagnostic accuracy of MRI, including experimental lymphophilic contrast agents, magnetization transfer imaging (MTI), free water diffusion-weighted imaging (DWI), magnetic resonance spectroscopy (MRS), and bolus tracking perfusion-weighted imaging (PWI).14–16 Currently, only the DWI MR technique has made a definite breakthrough in clinical routine by yielding fast-to-acquire and easy-to-process accurate quantitative data that significantly add to pretherapeutic nodal staging attempts,17–19 early prediction of therapeutic response,20 and post-treatment early detection of residual/recurrent tumor.21 Many think that molecular imaging probes targeting either specific membrane antigens or metabolic pathways of tumor cells could be the definitive contributors to perfect diagnostic accuracy in nodal metastatic workup, in which case MRI and PET will be competitors.
PET using fluorodeoxyglucose (FDG) as a tracer has become the most available technique for “metabolic imaging” by enhancing foci of increased glucose uptake. However, PET alone provides only restricted diagnostic accuracy. The limitations of PET can be offset by coregistering the information on anatomic CT or MR images22 (Fig. 38-4). Integrated PET/CT combines the poor anatomic localization of PET with the accurate morphologic data provided by CT. The results of a recent large, prospective study showed that FDG-PET significantly improved the staging of HNSCC because of its detection of metastatic or additional disease.23 However, a recent meta-analysis totaling 1236 patients demonstrated that the accuracy of FDG-PET was only marginally superior to that of CT or MRI; this study calls into question the routine value of FDG-PET for nodal staging.24 In addition to research on dedicated tissue-specific MR contrast agents, research is under way on PET radiopharmaceutical tracers that would allow the assessment of hypoxia, angiogenesis, apoptosis, and receptor status; these studies make up the second main field of research in oncology imaging.25 Using anatomic imaging studies to detect tumor recurrences in previously treated primary sites is unsatisfactory because extensive unspecific post-therapeutic changes may mask small foci of neoplastic recurrence.26 On the other hand, very promising data have been reported with the use of FDG-PET in this setting.27 Similar concepts apply to nodal metastases, and it may be possible that molecular and metabolic imaging methods will also become standard in nodal relapse detection. The key imaging concept for the radiation oncologist’s day-to-day practice is the capability of superimposing PET/MRI metabolic/molecular mapping onto CT/MRI anatomic images to improve the delineation of the irradiation target.28 PET-CT and PET-MRI fusions are the founding paradigms that have now emerged into the clinical routine29 (see Figs. 38-3 and 38-4).
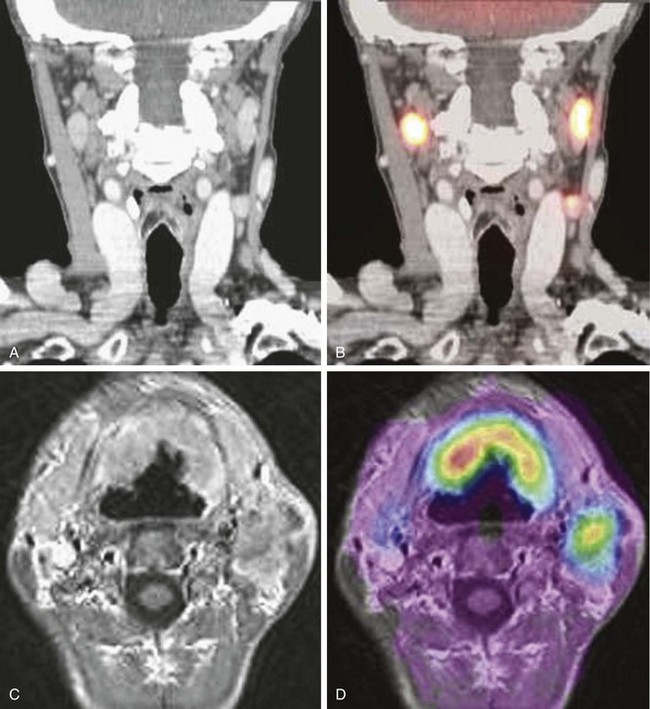
Figure 38-4 CT-MRI-PET image fusion. A, Postcontrast, reformatted CT image in the coronal plane shows bilateral, mildly enlarged metastatic nodes in the carotid-jugular chains. Tumoral involvement of the nodes remains speculative because of the borderline short-axis diameter and absence of obvious necrotic changes. B, Superimposition of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) data on a CT image demonstrates increased glucose uptake within the nodes. C, Postcontrast, T1-weighted, axial transverse MR image from three-dimensional gradient-echo acquisition using spoiled gradients (SPGR) shows bilateral anterior, horseshoe-sized oropharyngeal tumor and metastatic left-sided nodes in level II. D, Superimposition of FDG-PET data on an MR image demonstrates increased glucose uptake within the primary tumor and metastatic nodes.
Courtesy M. Lonneux, MD, PhD.
Staging of Neck Node Metastases
The seventh edition (2009) of the American Joint Committee on Cancer (AJCC) staging for neck node metastases30 is presented in Table 38-2. This classification does not apply to nasopharyngeal, thyroid, or skin cancers. The classification for nodal staging applies whether the modality used for neck assessment is clinical examination or imaging. The routine use of CT or MRI and—in expert hands—ultrasound is recommended especially for assessing nodes not clinically identifiable (e.g., retropharyngeal, intraparotid, or superior mediastinal nodes) or for patients for whom clinical palpation of the neck is less sensitive (e.g., those who have a thick or small neck).31 Last, it should be emphasized that the Nx classification applies only when the neck has not been assessed or could not be assessed.
TABLE 38-2 American Joint Committee on Cancer Staging for Neck Node Metastasis
| Category | Definition |
|---|---|
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral node, ≤3 cm in greatest dimension |
| N2a | Metastasis in a single ipsilateral node, >3 cm but ≤6 cm in greatest dimension |
| N2b | Metastasis in multiple ipsilateral nodes, ≤6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral nodes, ≤6 cm in greatest dimension |
| N3 | Metastasis in a lymph node >6 cm in greatest dimension |
From Sobin L, Gospodarowicz M, Wittekind C: UICC/TNM Classification of Malignant Tumors (7th ed.) New York, 2009, Wiley-Blackwell.
Incidence and Distribution of Neck Node Metastases
Clinical and Radiologic Assessment
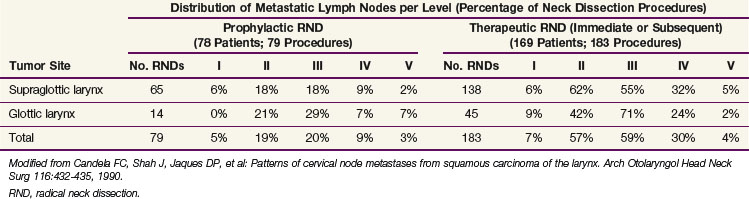
The metastatic spread of head and neck tumors into the cervical lymph nodes is rather consistent and follows predictable pathways, at least in the neck that has not been violated by previous surgery or radiotherapy. In Table 38-3, the frequency of metastatic lymph nodes is expressed as a percentage of node-positive patients.32,33
TABLE 38-3 Distribution of Clinical Metastatic Neck Nodes from Oral Cavity and Pharyngolaryngeal Squamous Cell Carcinomas
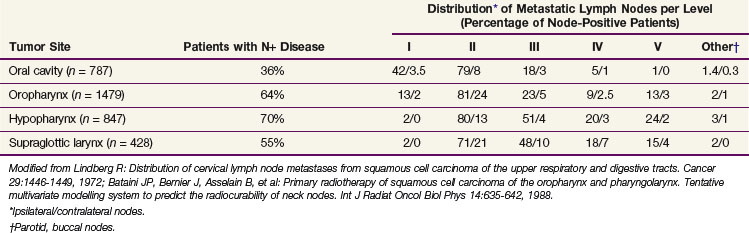
Metastatic lymph node involvement in the neck depends on the size of the primary tumor, increasing with the T category. In the series reported by Bataini and associates,32 44% of patients with a T1 tumor had clinical lymph node involvement; this increased to 70% for patients with T4 lesions. There are, however, no data suggesting that the relative distribution of involved neck levels varies with the T category.
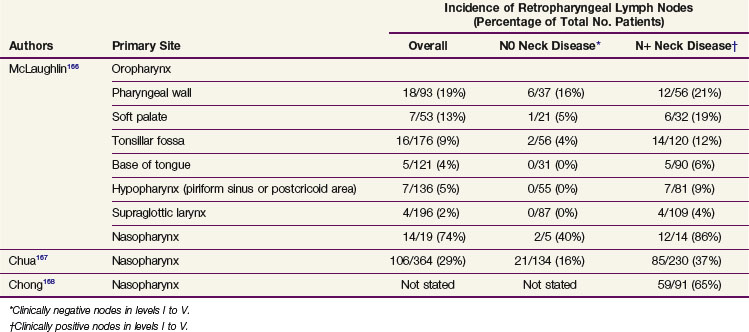
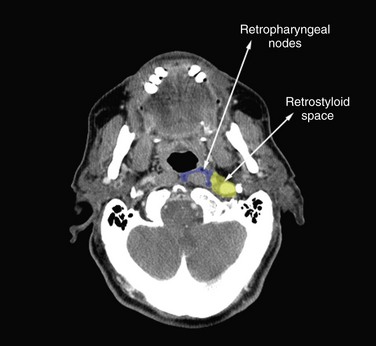
Retropharyngeal lymph nodes represent a special entity because they are usually not clinically detectable. The incidence of retropharyngeal lymph node involvement can be estimated only from series in which CT or MRI of the retropharynx has been systematically performed as part of the diagnostic procedure. Retropharyngeal node involvement occurs in primary tumors arising from (or invading) the mucosa of the occipital and cervical somites, such as the nasopharynx, pharyngeal wall, and soft palate (Table 38-4). The incidence of retropharyngeal lymph node involvement is higher in patients in whom involvement of other neck node levels has also been documented. However, in patients with clinical stage N0 nasopharyngeal tumors and, to a lesser extent, in patients with pharyngeal wall tumors, the incidence of retropharyngeal node involvement is still significant—between 16% and 40%. Also, as already described for the other lymph node levels, involvement depends on the T category and is typically lower for T1 tumors. Accurate figures are not available.
Pathologic Lymph Node Metastases
The distribution of pathologic lymph node metastasis in patients with primary tumors of the oral cavity, oropharynx, hypopharynx, and larynx can be derived from retrospective series in which a systematic radical neck node dissection was proposed as part of the initial treatment procedures.34–37 Retrospective series are essentially biased regarding patient and treatment selection, but these series from the Head and Neck Department at MSKCC are the largest and most consistent data ever published on that matter. The results of these retrospective studies are shown in Tables 38-5 to 38-8. The data are presented in terms of the number of neck dissections with positive lymph nodes divided by the total number of neck dissection procedures and expressed as a percentage. Most patients (>99% for patients with N0 tumors and 95% for patients with N+ tumors) had unilateral treatment only, and no distinction between the ipsilateral and contralateral neck was made.
TABLE 38-5 Incidence of Pathologic Lymph Node Metastasis in Squamous Cell Carcinomas of the Oral Cavity
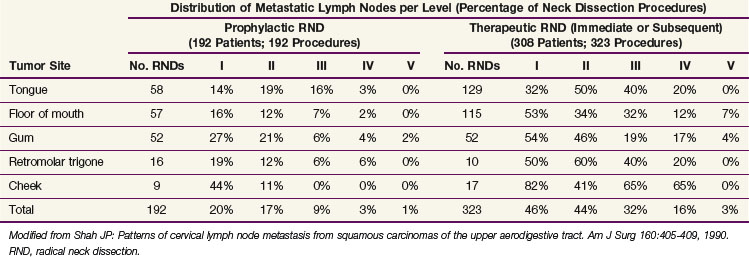
TABLE 38-6 Incidence of Pathologic Lymph Node Metastasis in Squamous Cell Carcinomas of the Oropharynx
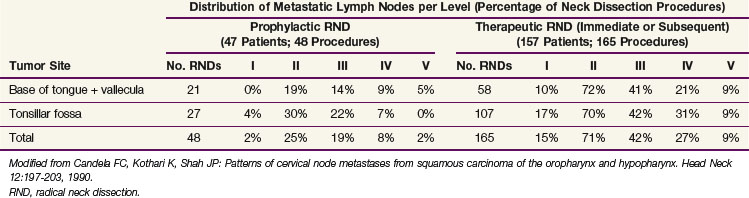
TABLE 38-7 Incidence of Pathologic Lymph Node Metastasis in Squamous Cell Carcinomas of the Hypopharynx
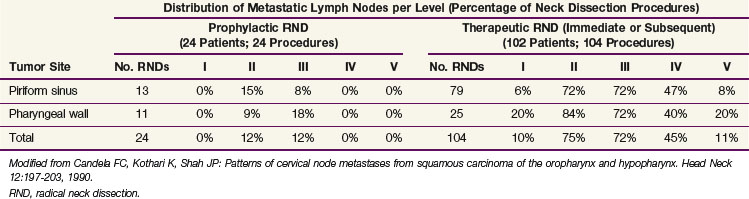
The pattern of metastatic node distribution in patients who underwent therapeutic neck dissection was similar to that observed in patients with N0 tumors, with the difference that significant pathologic infiltration of an additional nodal level was typically observed—level IV for oral cavity tumors and levels I and V for oropharyngeal, hypopharyngeal and, to a lesser extent, laryngeal tumors. Overall, this observation illustrates the gradual infiltration of node levels in the neck. This concept is well illustrated by the prevalence of metastases in level V. In the MSKCC series,38 the prevalence of pathologic infiltration in level V was quite low, averaging 3% in 1277 neck dissections in patients with oral cavity, oropharyngeal, hypopharyngeal, and laryngeal tumors. The prevalence peaked at 11% for hypopharyngeal tumors with pathologically positive nodes (see Table 38-7). A thorough analysis of level V infiltration showed that for all tumor sites pooled together, infiltration of level V without metastases in levels I to IV was observed in only one patient (0.2%). This patient had a hypopharyngeal tumor. Infiltration in level V remained below 1% when a single pathologically confirmed positive node was also observed in levels I to III, but reached 16% when a single pathologically confirmed positive node was also observed in level IV. When more than one level was infiltrated, the probability of level V involvement progressively increased, reaching 40% when levels I to IV were all involved. The pattern of involvement of level I is also a good illustration of the concept of gradual node infiltration. In the MSKCC series, pathologic involvement of level I was found in only 2% of patients with clinical stage N0 oropharyngeal tumors (see Table 38-6) and was not observed in patients with clinical stage N0 hypopharyngeal tumors (see Table 38-7). On the other hand, in patients with clinically positive nodes, metastases in level I were reported in 15% and 10% of patients with oropharyngeal and hypopharyngeal tumors, respectively.
Incidence of Skip Metastases in the Neck
Skip metastases are metastases that do not progress in an orderly manner from one level to the next (e.g., from level I to level II). Depending on their frequency, skip metastases in patients with clinical stage N0 tumors may have a profound implication for the therapeutic management of the neck. In the series from the MSKCC,37 8 of 343 patients with clinical stage N0 tumors (2.5%) developed skip metastases. Seven of these patients had oral cavity tumors that metastasized in level IV or V only. One patient had a laryngeal tumor. These low figures are in good agreement with a rate of neck failure outside the dissected levels of 3% (2 of 64 lesions) observed in patients with pathologic N0 lesions treated at the same institution by supraomohyoid neck dissection.39 Most of these patients had tumors of the oral cavity. None of them received postoperative radiation therapy because they were all free of metastases. Byers and colleagues40 carefully evaluated the frequency of skip metastases in 270 patients primarily treated by surgery at the M.D. Anderson Cancer Center from 1970 to 1990 for squamous cell carcinoma of the oral tongue. Of these patients, 12 had metastases in level III only, 9 had metastases in level IV only, and 2 had metastases in level IIb (i.e., nodes that are far enough posterior to the internal jugular vein). In addition, in 90 of the patients who had pathologic stage N0 tumors after selective neck dissection of levels I to III and who did not receive postoperative radiation therapy, 9 subsequently developed recurrence in level IV Altogether (in levels IIb, III, and IV), the frequency of skip metastases reached 12% (32 of 270 lesions). If one excludes the skip metastases in level IIb and III, the frequency reached only 7% (18 of 270 lesions).
Incidence and Pattern of Node Distribution in the Contralateral Neck
There are very few data available on the pattern of pathologic node distribution in the contralateral neck. Bilateral neck dissection was performed only when the surgeon thought there was a high risk of contralateral node involvement (e.g., with tumors of the oral cavity or oropharynx reaching the midline or extending beyond it, or with hypopharyngeal and supraglottic tumors). Obviously, in such cases bilateral radical neck dissection was never performed, so an accurate estimate of the pattern of node involvement in levels I to V of the contralateral neck is not possible. Furthermore, in almost every study, data on both sides of the neck were pooled for presentation. Kowalski41 presented data on 90 patients who underwent bilateral supraomohyoid neck dissection and in whom the pattern of node distribution in each side of the neck was reported separately. Most of these patients had squamous cell carcinoma of the lip or oral cavity. In the ipsilateral neck, pathologic infiltration in levels I, II, and III reached 20%, 15%, and 15%, respectively. In the contralateral neck, corresponding values reached 13%, 11%, and 0%, respectively. These figures are in good agreement with data on clinical node distribution showing that both sides of the neck exhibited a similar pattern of node distribution but with a lower incidence in the contralateral neck.
Foote and colleagues42 reported the rate of contralateral neck failure in a limited series of 46 patients with clinical stage N0 base of the tongue tumors treated by some form of glossectomy and ipsilateral neck dissection. None of these patients received postoperative radiation therapy. Ten patients (22%) had contralateral neck recurrence, and the most common sites were levels II, III, and IV. It appears that in two of these patients, recurrence was also observed at the primary site. The development of delayed contralateral neck metastases was not related to the clinical or pathologic extent of the base of the tongue tumor. O’Sullivan and associates43 reported a retrospective series of 228 patients with tonsillar carcinoma who were treated for the primary tumor and in the ipsilateral neck only with radiation therapy. The vast majority of these patients had T1 to T2 and N0 to N1 disease. Contralateral recurrence in the neck was observed in only eight patients (2%), including five patients with local recurrence as well. None of these neck failures occurred in the 133 patients with stage N0 tumors. Although not significant because of the small number of events, involvement of a midline structure (i.e., soft palate and base of the tongue) appeared to be a prognostic factor for contralateral neck recurrence. Similar results were reported in a series of 101 node-negative tonsil carcinoma (mainly, stage T1 to T3) cases treated unilaterally.44 Only two neck recurrences were observed in the contralateral neck.
Recommendations for Selection of Target Volumes in the Neck
Metastatic lymph node involvement of primary squamous cell carcinoma of the oral cavity, pharynx, and larynx typically follows a predictive pattern. Data on clinical and pathologic neck node distribution as well as on neck recurrence after selective dissection procedures support the concept that not all neck node levels should be treated as part of the initial management strategy of head and neck primaries of squamous cell origin.45,46 However, the data on which such a concept is based have come from retrospective series and so may include possible biases (e.g., in patient selection, use of a series from the preimaging area, and so on) that could limit their validity.
Tables 38-9 to 38-12 present recommendations for the selection of clinical target volumes in the neck for pharyngolaryngeal squamous cell carcinomas. These guidelines can be applied whether the treatment modality is surgery or radiotherapy. A complete discussion on choosing between these two modalities is beyond the scope of this chapter, but factors to consider include the neck stage, the treatment option for the primary tumor, the performance status of the patient, and the institutional policy agreed on by a multidisciplinary head and neck tumor board.
TABLE 38-9 Recommendations for Selection of Clinical Target Volume in the Neck for Oral Cavity Tumors
| Nodal Category (AJCC) | Levels to Be Included in CTV | |
|---|---|---|
| Ipsilateral Neck | Contralateral Neck | |
| N0-1 (in level I, II, or III) | I, II,* III, + IV† | I, II,* III, + IV† |
| N2a-b | I, II, III, IV, V‡ | I, II,* III, + IV for anterior tongue tumor |
| N2c | According to N stage on each side of the neck | According to N stage on each side of the neck |
| N3 | I, II, III, IV, V ± adjacent structures according to clinical and radiologic data | I, II,* III, + IV for anterior tongue tumor |
AJCC, American Joint Committee on Cancer; CTV, clinical target volume.
* Level IIb could be omitted for stage N0 tumors.
† For anterior tongue tumor and any tumor with extension to the oropharynx (e.g., anterior tonsillar pillar, tonsillar fossae, base of tongue).
‡ Level V could be omitted if only levels I to III are involved.
TABLE 38-10 Recommendations for Selection of Clinical Target Volume in the Neck for Oropharyngeal Tumors
| Nodal Category (AJCC) | Levels to Be Included in the CTV | |
|---|---|---|
| Ipsilateral Neck | Contralateral Neck | |
| N0-1 (in level II, III, or IV) | (Ib),* II, III, IV, + RP for posterior pharyngeal wall tumor | II, III, IV, + RP for posterior pharyngeal wall tumor |
| N2a-b | Ib, II, III, IV, V, + RP | II, III, IV, + RP for posterior pharyngeal wall tumor |
| N2c | According to N stage on each side of the neck | According to N stage on each side of the neck |
| N3 | I, II, III, IV, V, + RP ± adjacent structures according to clinical and radiologic data | II, III, IV, + RP for posterior pharyngeal wall tumor |
AJCC, American Joint Committee on Cancer; CTV, clinical target volume; RP, retropharyngeal nodes.
* Any tumor with extension to the oral cavity (e.g., retromolar trigone, mobile tongue, inferior gum, oral side of anterior tonsillar pillar).
TABLE 38-11 Recommendations for Selection of Clinical Target Volume in the Neck for Hypopharyngeal Tumors
| Nodal Category (AJCC) | Levels to Be Included in the CTV | |
|---|---|---|
| Ipsilateral Neck | Contralateral Neck | |
| N0 | II,* III, IV, + RP for posterior pharyngeal wall tumor + VI for apex of piriform sinus or esophageal extension | II,* III, IV, + RP for posterior pharyngeal wall tumor + VI for esophageal extension |
| N1N2a-b | Ib, II, III, IV, V, + RP + VI for piriform sinus or esophageal extension | II,* III, IV, + RP for posterior pharyngeal wall tumor + VI for esophageal extension |
| N2c | According to N stage on each side of the neck | According to N stage on each side of the neck |
| N3 | I, II, III, IV, V, + RP + VI for piriform sinus or esophageal extension ± adjacent structures according to clinical and radiologic data | II,* III, IV, + RP for posterior pharyngeal wall tumor + VI for esophageal extension |
AJCC, American Joint Committee on Cancer; CTV, clinical target volume; RP, retropharyngeal (nodes).
* Level IIb could be omitted for stage N0 tumors.
TABLE 38-12 Recommendations for Selection of Clinical Target Volume in the Neck for Laryngeal Tumors (Stage T1 N0 Glottic Carcinoma Excluded)
| Nodal Category (AJCC) | Levels to Be Included in the CTV | |
|---|---|---|
| Ipsilateral Neck | Contralateral Neck | |
| N0-1 (in level II, III, or IV) | II,* III, IV, + VI for transglottic or subglottic extension | II,* III, IV, + VI for transglottic or subglottic extension |
| N2a-b | II, III, IV, V, + VI for transglottic or subglottic extension | II,* III, IV, + VI for transglottic or subglottic extension |
| N2c | According to N stage on each side of the neck | According to N stage on each side of the neck |
| N3 | Ib, II, III, IV, V, + VI for transglottic or subglottic extension ± adjacent structures according to clinical and radiologic data | II,* III, IV, + VI for transglottic or subglottic extension |
AJCC, American Joint Committee on Cancer; CTV, clinical target volume.
* Level IIb could be omitted for stage N0 tumors.
Selective treatment of the neck is appropriate for patients with clinical N0 HNSCC of the oral cavity, oropharynx, hypopharynx, and larynx.39,46,47 Typically, level I to III nodes should be treated for oral cavity tumors, and level II to IV nodes should be treated for oropharyngeal, hypopharyngeal, and laryngeal tumors. Robbins7 suggested that elective treatment of level IIb nodes is probably not necessary for patients with clinical stage N0 primary tumors of the oral cavity, larynx, or hypopharynx. However, Byers and associates40 suggested that level IV nodes be included in the treatment of the mobile tongue because of the high incidence (10%) of skip metastases. Retropharyngeal nodes should be treated in tumors of the posterior pharyngeal wall. For subglottic tumors, tumors with subglottic or transglottic extension, or hypopharyngeal tumors with esophageal extension, level VI nodes should also be included in the treatment volume.
As proposed by Byers,47 similar guidelines could be recommended for patients who have N1 tumors without radiologic evidence of extracapsular infiltration. However, when an involved lymph node is located at a boundary of a level that has not been selected for the target volume, it is recommended to extend the selection to include the adjacent level.48 Typically, this applies only for oropharyngeal tumors with a single lymph node involved in level II at the boundary with level Ib or for an oral cavity tumor with an N1 node in level III at the boundary with level IV.
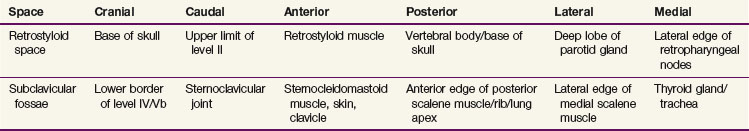
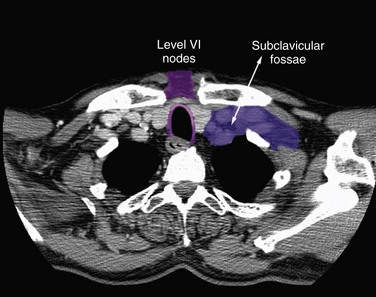
For patients with multiple nodes (N2b), the available data suggest that adequate treatment should include nodes in levels I to V. Level I nodes could, however, be omitted for laryngeal tumors, and level V nodes omitted for oral cavity tumors with neck involvement limited to levels I to III. Prophylactic treatment of the retropharyngeal nodes should be systematically performed for oropharyngeal and hypopharyngeal tumors. Patients with N0 disease should have level VI nodes treated for subglottic tumors, tumors with subglottic or transglottic extension, or hypopharyngeal tumors with esophageal extension. It has been proposed that patients with nodes in the upper neck (i.e., upper level II nodes) have the upper limit of the target volume extended to include the retrostyloid space.48 Similarly, the subclavicular fossae should be included in the target volume in cases of lower neck involvement (i.e., level IV or Vb nodes).48
Prophylactic treatment of the contralateral neck for N0 disease is still a gray zone and is likely to be based on clinical judgment rather than on strong scientific evidence. Typically, patients with midline tumors or tumors originating from or extending to a site that has bilateral lymphatic drainage (e.g., the base of the tongue, vallecula, posterior pharyngeal wall) are thought to benefit from bilateral neck treatment, whereas well-lateralized tumors (e.g., the lateral border of the tongue, retromolar trigone, tonsillar fossa) can be spared contralateral treatment. It has also been reported that the risk of contralateral neck metastases increased with involvement of the ipsilateral neck in patients with tumors of the pharynx and larynx.49 Putting all these data together, it would seem advisable to restrict treatment to the ipsilateral neck for tumors of the lower gum (not approaching the midline), lateral floor of the mouth, lateral border of the mobile tongue, upper gum, cheek, retromolar trigone, tonsillar fossa (without extension to the base of the tongue, soft palate, or posterior pillar), and lateral wall of the piriform sinus. In other situations in which prophylactic contralateral neck treatment is recommended, selection of the node levels to be treated should follow rules similar to those for the ipsilateral neck.
In principle, a similar approach should apply for the definition of the node levels to be irradiated postoperatively. However, if the selection criteria for postoperative radiotherapy are capsular rupture, a metastatic node over 3 cm in diameter, or more than one metastatic node, then irradiation of levels I to V will typically be performed. As for primary irradiation, the retrostyloid space and subclavicular fossae should be included in the target volume, depending on the location of the metastatic nodes.48 For laryngeal tumors, level I nodes could be omitted. For oral cavity tumors with metastatic nodes located in level I and/or II only, postoperative irradiation of level V could be omitted. Retropharyngeal and paratracheal nodes should be treated as mentioned earlier.
Neck Node Dissection Procedures and Sentinel Node Biopsy
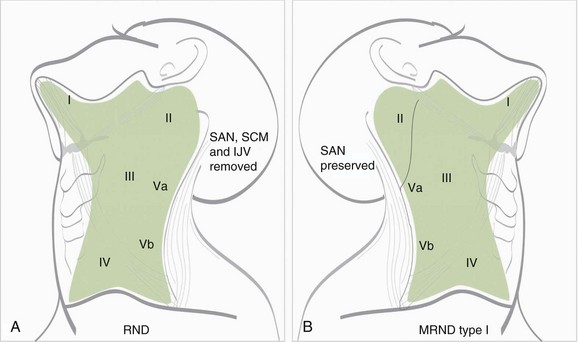
In 1991, based on the definition of the neck level, the Committee for Head and Neck Surgery and Oncology of the American Academy for Otolaryngology–Head and Neck Surgery made several recommendations for neck dissection terminology. The main objective was to develop standardized terminology limited to the use of a few defined procedures in which the lymphatic and nonlymphatic structures removed were unambiguously described. Such recommendations had to correlate with the biology of neck metastases and meet the standards of oncologic principles. The goal of each type of neck dissection is to remove the lymphatic structures (nodes and vessels) that are poorly individualized in the fatty tissue of the neck. When it is oncologically sound, some or all nonlymphatic structures of the neck—such as the internal jugular vein, spinal accessory nerve, sternocleidomastoid muscles, and submandibular glands—may be preserved. Since the 1991 classification, revisions have been proposed; the current neck dissection terminology and definitions7,8,50,51 are summarized in Table 38-13.
TABLE 38-13 Classification of Neck Dissection: Definitions and Terminology
| Type of Neck Dissection | Lymph Node Levels Resected | Nonlymphatic Structures Removed |
|---|---|---|
| Radical neck dissection | I, II, III, IV, V | SCM, IJV, SAN |
| Modified radical neck dissection | I, II, III, IV, V | Preservation of one or more of the following: SCM, IJV, SAN |
| Selective neck dissection | Preservation of one or more of the following: I, II, III, IV, V. Parentheses are used to denote levels or sublevels removed: SND (I-IV) | None |
| Extended neck dissection | Resection of one or more or additional lymph node group not routinely removed by radical neck dissection (e.g., parapharyngeal, paratracheal nodes) | Resection of one or more nonlymphatic structures not routinely removed by radical neck dissection (e.g., carotid artery, hypoglossal nerve, overlying skin) |
IJV, internal jugular vein; SAN, spinal accessory nerve; SCM, sternocleidomastoid muscle.
Adapted from Robbins KT: Classification of neck dissection. Current concepts and future considerations. Otolaryngol Clin North Am 31:639-656, 1998.
Modified RND refers to the removal of all lymph nodes routinely resected by RND but sparing one or more nonlymphatic structures (i.e., spinal accessory nerve, internal jugular vein, sternocleidomastoid muscles) usually removed during RND (Fig. 38-5). Medina52 subclassified modified RND into three types: type I preserves the spinal accessory nerve only; type II preserves the spinal accessory nerve and sternocleidomastoid muscles; and type III preserves the spinal accessory nerve, sternocleidomastoid muscles, and internal jugular vein. Type III is also called by European authors “functional neck dissection,” as first described by Suarez53 and popularized by Bocca and associates.54,55 However, in their classic description, the submandibular gland was not removed.
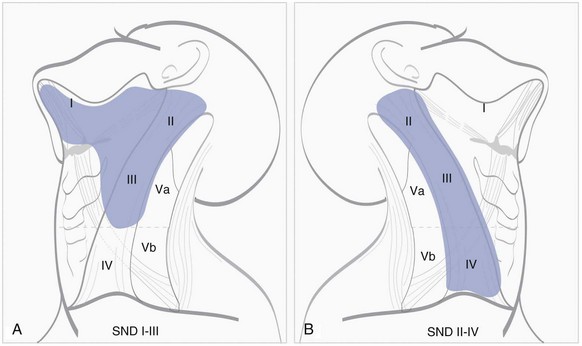
Selective neck dissection (SND) is dissection with preservation of one or more lymph node levels routinely resected in RND (Fig. 38-6). To avoid confusion in terminology for the different subtypes of SND, the 2002 revision of the node dissection classification excluded “named” node dissections (e.g., supraomohyoid node dissection, posterolateral node dissection) and proposed that the term selective neck dissection (SND) be followed in parentheses by the node levels or sublevels removed, for example, SND(I to III).50
A promising method for detecting occult micrometastases in the neck is lymphoscintigraphy associated with sentinel lymph node biopsy (SLNB). The concept is based on identification of the primary echelon of lymphatic drainage followed by the harvest of the sentinel lymph node within this basin only, assuming that if the sentinel lymph node is negative, there is no need for a comprehensive neck dissection. The technique was initially proposed for the detection of lymph node invasion in cutaneous melanoma, and thereafter in various sites.56–58 SLNB of cervical lymph nodes was introduced as a minimally invasive diagnostic procedure, able to predict more accurately the nodal status in node-negative patients with oral or oropharyngeal squamous cell carcinoma.59 The sentinel lymph node can be identified by peritumoral injection of a radioactive tracer.60 The tracer mimics lymph spread and accumulates in the first-echelon node. With the help of lymphoscintigraphy and a gamma probe, the sentinel lymph node is localized and selectively excised. Histologic evaluation of the sentinel lymph node allows for pathologic staging of the neck. Additionally, lymphoscintigraphy and SLNB provide information on the presence of atypical basins or lymphatic flow that is not predictable and would typically not be addressed by a selective node dissection.53
Sentinel lymph node biopsy has been shown to improve staging of clinical stage N0 tumors in patients with early squamous cell carcinoma of the oral cavity and oropharynx by identifying micrometastases.61,62 Several centers have now adopted SLNB alone as a staging tool for early clinical stage N0, oral and oropharyngeal squamous cell carcinomas.61 With experienced teams, the negative predictive value is 96%.62 Because sentinel lymph nodes are worked up thoroughly, a high number of inapparent metastases are revealed. Occult metastases are, by definition, clinical stage N0 neck metastases detected by histologic testing. They are further divided into macrometastases (>2 mm in largest dimension), micrometastases (<2 mm in greatest dimension), and isolated tumor cells.63–65
Isolated tumor cells are recognized as a separate entity, and are single tumor cells or small clusters of tumor cells not more than 0.2 mm in greatest dimension. Such cells are typically detected after immunohistochemical or molecular biologic testing only, and they do not show evidence of metastatic activity (e.g., proliferation or stromal reaction) or penetration of vascular or lymphatic sinus walls.64 The debate continues whether patients with micrometastases or isolated tumor cells in sentinel lymph nodes have a risk similar to that for patients with metastases in the neck who have clinically invaded lymph nodes. There is evidence supporting further treatment to the neck in patients with micrometastases in sentinel lymph nodes.65 Regarding isolated tumor cells only, a watchful waiting policy could be warranted, although there is a low risk for metastases in nonsentinel lymph nodes. Larger series are required to establish the usefulness of micrometastases and isolated tumor cells and to define an acceptable level of wrongly down-staged isolated tumor cell disease in patients who have other metastases in the neck.
Neck Node Delineation and Irradiation Techniques
Delineation of the Clinical Target Volume
Several authors have proposed recommendations for the delineation of the neck node levels.51,66,67–69 It is beyond the scope of this chapter to compare them all. In the radiation oncology community, however, the so-called Brussels and Rotterdam guidelines have emerged as the most widely used.66,67,68 Detailed comparison of these guidelines reveals a few important discrepancies, preventing uniform target volume delineation in the neck among radiation oncologists. A critical review of the two proposals was undertaken in collaboration with representatives of the major European and North American clinical cooperative groups to generate an international set of guidelines for the delineation of the neck node levels in a node-negative neck.70 The correspondence between these guidelines and neck dissection procedures was further validated.71 Last, a few amendments were proposed to take into account the specific situation of a node-positive and postoperative neck.48,72
The consensus guidelines for the delineation of levels I to VI nodes and the retropharyngeal lymph nodes are presented in Table 38-14 and Figure 38-7. The volumes delineated in Figure 38-7 correspond to the clinical target volume (CTV) and do not include margins for organ motion or setup inaccuracy. The boundaries are based on a patient lying supine with the head in a “neutral” position. The terms cranial and caudal refer to structures closer to the cephalic and pedal ends, respectively. The terms anterior and posterior were chosen to be less confusing than the terms ventral and dorsal, respectively.
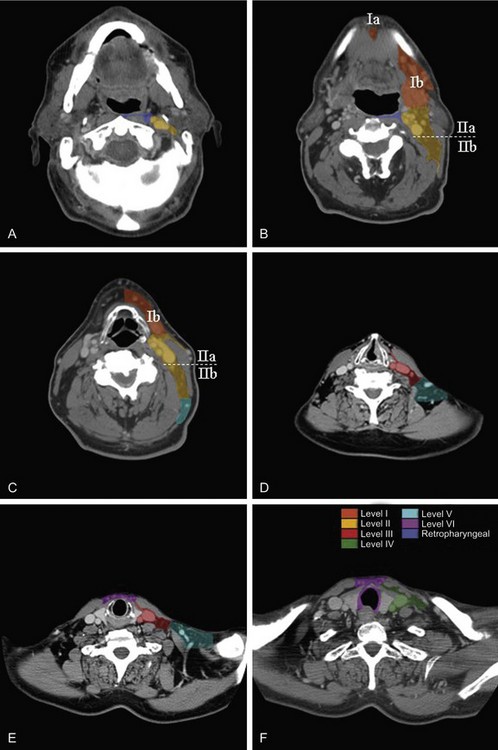
Figure 38-7 CT scan of a patient with a stage T1N0M0 glottic squamous cell carcinoma (see tumor in D). The examination was performed with a dual-detector spiral CT scanner (Elscint Twin, Haifa, Israel) using a slice thickness of 2.7 mm, an interval reconstruction of 2 mm, and a pitch of 0.7. Contrast medium was injected intravenously at a rate of 2 mL per second, with a total amount of 100 mL. Sections were taken at the level of the bottom edge of C1 (A), upper edge of C3 (B), middle of C4 (C), bottom edge of C6 (D), bottom edge of C7 (E), and middle of D1 (F). Neck node levels were drawn on each CT slice using the radiologic boundaries detailed in Table 38-14. Each node level corresponds to the clinical target volume and does not include any security margin for organ motion or setup inaccuracy.
It is beyond the scope of this chapter to discuss in depth the various boundaries of all the node levels. The reader is referred to the original publication.70 A few specific issues, however, merit attention. The upper limit of level II was set at the caudal edge of the lateral process of the first vertebra, an easier landmark to determine than the surgical landmark, the insertion of the posterior belly of the digastric muscle to the mastoid. The caudal limit of level IV was set arbitrarily 2 cm cranially to the cranial edge of the sternoclavicular joint because the dissection of level IV typically does not go all the way down to the clavicle and definitely never reaches the medial portion of the clavicle at the level of the sternoclavicular joint. The cranial limit of level V (i.e., the base of the skull) that was commonly accepted and depicted has been questioned. Hamoir and colleagues9 challenged the necessity to treat the uppermost part of level Va in mucosal HNSCC. They proposed to divide the level Va nodes into two sublevels: level Vas (superior) and level Vai (inferior) nodes, using the lower two thirds of the spinal accessory nerve as the cranial limit of level V. From a radiologic point of view, a horizontal plane crossing the cranial edge of the body of the hyoid bone appears to be a reliable landmark for separating level Vas and level Vai. For the caudal limit of level V, it appears from a critical examination of neck dissection procedures that surgeons never dissect the neck further down than the cervical transverse vessels. It was therefore agreed to set the caudal limit of level V at CT slices encompassing the cervical transverse vessels.
Last, because the dissection of level V does not extend all the way to the anterior edge of the trapezius muscle, it was proposed to use a virtual line joining the anterolateral border of both trapezius muscles as the posterior limit of level V. The retropharyngeal space is bounded anteriorly by the pharyngeal constrictor muscles and posteriorly by the prevertebral fascia. For the sake of simplicity and consistency, it was proposed to use the fascia below the pharyngeal mucosa as the anterior limit and the prevertebral muscles (longus colli and longus capitis) as the posterior limit. Typically, retropharyngeal nodes are divided into a medial and a lateral group. The medial group is an inconsistent group that consists of one or two lymph nodes intercalated in or near the midline, and it was proposed that it could be omitted from the delineation of the retropharyngeal clinical target volume.73
As already discussed, in some clinical situations, it was proposed to extend the delineation of the “standard” neck node levels to include the retrostyloid space and/or the subclavicular fossae. The anatomic boundaries of these spaces are presented in Table 38-15 and illustrated in Figures 38-8 and 38-9. If extracapsular extension is suspected, it was proposed to include the entire sternocleidomastoid muscle in the target volume, at least in the entire invaded level.48 Another proposal was to adopt a 1-cm margin around the gross tumor volume (GTV) to take into account the microscopic spread outside of the nodes.72 This proposal would typically apply for the delineation of the therapeutic nodal clinical target volume.
Irradiation Techniques
With the use of three-dimensional conformal radiotherapy (3D CRT) and intensity-modulated radiotherapy (IMRT), there is no longer a standard recipe for setting up field sizes and borders according to bony landmarks. Instead, the irradiation technique should be selected and adapted so that the entire planning target volume (PTV) receives the prescribed dose within the adopted dose-volume constraints and in full consideration of the International Commission on Radiation Units and Measurements (ICRU) recommendations.73a
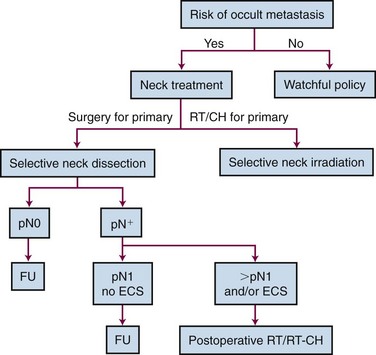
Control of the N0 Neck
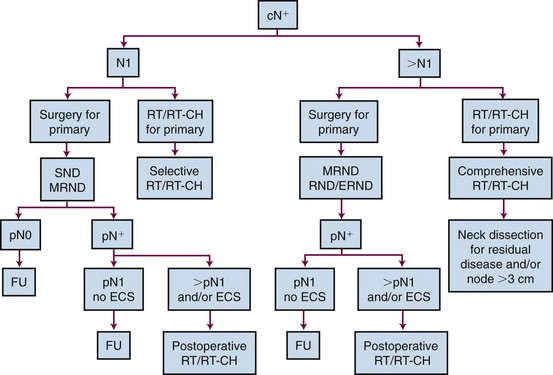
Guidelines generally recommend performing prophylactic treatment of the neck in patients with primary HNSCC clinically staged at N0 but having a probability of 20% or more of occult lymph node metastases.74 Elective neck dissection and elective neck irradiation are equally effective in controlling stage N0 lesions of the neck. The choice between these two procedures generally depends on the treatment modality chosen for the primary tumor, which in turn mainly depends on the institutional policy. However, the basic rule that should govern the choice between surgery and radiotherapy (RT) is to favor a single-modality treatment if possible, avoiding overtreatment. For example, supraglottic laryngectomy with selective neck node dissection is just as effective as primary radiation therapy of the larynx and neck for treating a T1 or T2N0 supraglottic laryngeal tumor. For such a disease stage, the need for postoperative radiotherapy is quite low. Conversely, for a T3N0 supraglottic laryngeal tumor, a conservative treatment approach with primary radiotherapy or concomitant chemoradiation should be favored because of the necessity of postlaryngectomy RT and the nonsuperiority of the surgical approach (Fig. 38-10).
Neck Control after Neck Node Dissection
Selective neck dissection (SND) has become more widely used despite some concerns that it may not be as effective as a more comprehensive form of neck dissection, such as a modified radical neck dissection (RND). Anticipating the conclusions that could be drawn from the MSKCC data with regard to the extent of the neck dissection, several groups have been performing SND since the 1950s.39,41,47,75–85 Such selective neck procedures were initially proposed for clinically node-negative patients and later extended to clinically node-positive patients. These studies are biased because the patients treated by a selective procedure were probably highly selected with regard to the tumor site, tumor stage, and nodal status. In addition, in most of these patients, postoperative RT was usually performed in the presence of high-risk features for primary tumor or neck recurrence, such as resection, multiple node involvement, large node infiltration, or extracapsular spread. It is likely that the irradiated field encompassed the node levels that were not dissected but that could be at risk for microscopic infiltration.
Originally, SND was typically considered a method for accurately staging neck disease but not one that could affect regional control and survival rates. A retrospective review of 359 patients with stage T1 to T2 N0 squamous cell carcinoma of the oral cavity and oropharynx treated at the University of Pittsburgh showed significant improvement in regional control, disease-free survival (DFS), and regional recurrence-free survival rates for the group of patients who underwent SND along with primary tumor excision as compared with another group of patients who underwent excision of the primary tumor only.78 The patients who underwent SND were three times more likely to receive postoperative RT, most likely because of identification of adverse prognostic factors in the pathologic specimen. In a prospective randomized trial comparing modified RND and SND(I to III) (supraomohyoid nodes) for clinically node-negative patients with T2 to T4 tumors of the oral cavity, the Brazilian Head and Neck Cancer Study Group was unable to show any difference either in 5-year actuarial overall survival (OS) or in the rate of neck failure.75 Postoperative irradiation was delivered in cases with a positive margin of the primary tumor and/or positive lymph nodes. study is biased by the fact that patients included in the SND group and found to have a positive lymph node (on frozen section) during the neck dissection were offered amodified RND.
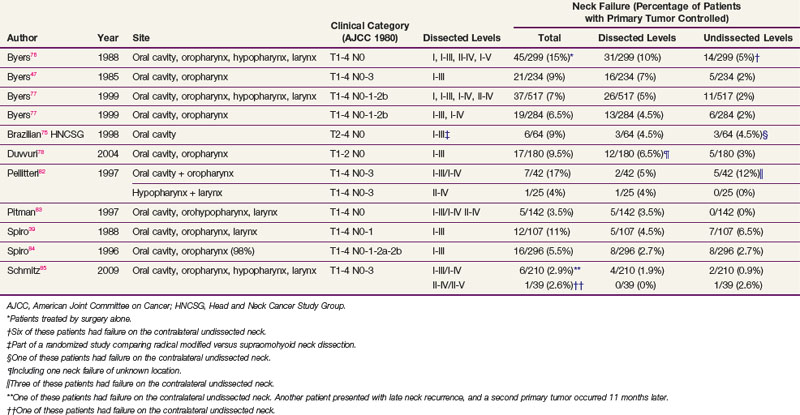
With these limitations in mind, in some of these studies the level of the neck recurrence was reported, allowing an estimate of the failure rate in the neck inside and outside the dissected levels (Table 38-16). In most of these studies, neck recurrence was reported only in patients in whom the primary tumor had been controlled, excluding neck recurrence as a result of reseeding from the recurrent primary tumor. After SND(I to III) or SND(II to IV), the rate of neck failure in nondissected levels was low, typically below 10%. Consequently, SND can be considered the optimal procedure for surgically managing N0 neck disease in patients with a high risk of occult lymph node metastasis.
Neck Control after Radiotherapy
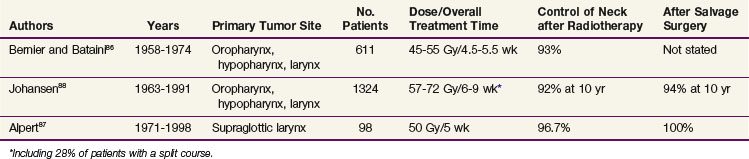
Table 38-17 presents the percentage of neck recurrences in large retrospective series of pharyngolaryngeal squamous cell carcinomas treated with conventional fractionated RT.86–88 Some of the patients reported in these series were treated in the late 1950s, so the data must be interpreted with caution because of the likelihood of large uncertainties about the absolute dose calculation and dose distribution. Altogether, the neck control rates reached more than 92% after radiotherapy. After salvage surgery, the ultimate neck control rate reached a range of 94% to 100%. As expected, because of the high probability of regional control obtained with standard fractionation regimens, altered fractionation regimens or combined chemoradiation regimens did not improve the neck control rates.89–90 All of these studies were performed using two-dimensional irradiation techniques, that is, with target volumes extending typically from the base of the skull to the clavicles.
With the introduction of 3D CRT, IMRT, and selective neck irradiation, one important issue is the potential risk of geographic miss outside of the irradiated volumes. Eisbruch and associates91 reported a series of 135 patients treated bilaterally from 1994 to 2002 with 3D CRT or IMRT for primary tumors located mainly in the oropharynx (n = 80) and without node metastasis on the contralateral neck. Of these, 73 patients received postoperative RT, but none had neck node dissection on the contralateral neck. On the contralateral neck, the CTV typically included the level II to IV and retropharyngeal nodes. For the contralateral level II nodes, the upper limit was set at the junction between the posterior belly of the digastric muscle and the jugular vein. The median prophylactic dose was 50.4 Gy in fractions of 1.8 or 2 Gy. With a median follow-up of 30 months (range, 6 to 105 months), 15 patients had a regional recurrence (of these, 6 also had a primary tumor recurrence), 11 on the ipsilateral side and 4 on the contralateral side. Only 1 of the 15 patients presented a retropharyngeal node recurrence marginal to the CTV. Using a similar treatment philosophy, Bussels and colleagues92 did not report any recurrence on the ipsilateral side of the neck with N0 disease treated with parotid-sparing 3D CRT in a series of 72 patients with oral cavity and pharyngolaryngeal squamous cell carcinoma. Chao and associates93 also looked at the pattern of recurrence in a series of 126 patients treated postoperatively (n = 74) or primarily (n = 52) for HNSCC by IMRT from 1997 to 2000. In this series, the lower neck (below the thyroid notch) was treated with a “traditional” anterior field. With a median follow-up of 26 months, 17 recurrences (13%) were observed. Six of these patients had recurrence outside of the target volumes, of which only one was in the lower neck of a patient with N0 disease.
Control of the N1 to N3 Neck
Neck Control after Surgery Alone
The surgical management of N1 neck tumors is controversial (Fig. 38-11). Traditionally, a comprehensive neck dissection (RND and modified RND) has been the surgical standard for patients presenting with neck disease. Andersen and associates94 reported that the rates of regional recurrences in the dissected neck following RND or modified RND type I for N1 or N2 disease were similar. Selective procedures have, however, gained popularity. In the retrospective study by Byers and colleagues,77 including 517 SND procedures mainly for patients with N0 or N1 neck disease, 50 patients had pathologic N1 disease. Of these patients, 36 received postoperative radiotherapy for the presence of risk factors associated either with the tumor or the nodal site and only 1 (3%) presented with a regional recurrence. In patients who did not receive irradiation despite the presence of risk factors, 5 of 14 (36%) had neck failure. In a large retrospective review of 296 selective node dissections in levels I to III, Spiro and colleagues84 reported a rate of regional failure of 6.5% in patients staged with a pathologically positive neck. Most patients with pathologically invaded lymph nodes had postoperative radiotherapy. Recently, Schmitz and colleagues85 reported a regional failure rate of 8% in neck disease of pathologic N1 without better regional control in the necks treated with postoperative irradiation, suggesting that postoperative irradiation is not justified in pathologic N1 neck disease without extracapsular spread. With the inherent limitation of retrospective studies, it appears that SND for patients with limited neck disease is a safe procedure, providing that postoperative irradiation is given in the presence of risk factors for regional relapse.
Despite the use of aggressive single and combined treatment protocols, patients with advanced metastatic neck disease still have a poor prognosis because of the high risk of regional failure and distant metastases.95,96 However, the concept of using a less than radical procedure has gained acceptance even for advanced regional disease. Khafif and associates97 reported the results of 118 patients with N2 to N3 disease treated with RND or modified RND and was not able to find any difference in OS between the two groups. The recurrence rate in the modified RND group increased significantly in comparison with the group of patients treated with standard RND (52% vs. 33%), but some modified RND procedures were not really comprehensive. In a study comparing RND and modified RND (type I) in 212 patients with N2 and N3 lesions, the MSKCC group reported an overall 5-year neck control rate of 86% and a 5-year actuarial survival rate of 61%.94 No difference was found between the two groups. Adjuvant postoperative RT enhances regional control but does not seem to significantly improve survival rates.98 Investigators of the Royal Prince Alfred Hospital in Sydney, Australia, reported the outcome of 181 patients who had 233 neck dissections for N2 to N3 disease (163 extended RND, RND, or modified RND and 70 SND).99 Postoperative RT to the neck was given in 82% of the patients. At 5 years, control of disease in the treated neck was achieved in 86%. Adjuvant RT significantly improved neck control rates (p = .004) but did not alter survival rates.
The utility of extended RND depends on whether acceptable control rates are attainable without prohibitive morbidity. Shaha100 described the results in 40 patients with N2 to N3 tumors treated by extended RND combined with postoperative RT. The regional control rate reached 70% at 2 years, with one perioperative death. The morbidity of extended RND depends on the additional structure or structures resected. If a common carotid artery is removed, the perioperative mortality rate ranges from 6% to 58%.101–102 However, when the structures sacrificed are of no major neurovascular significance (e.g., parotid gland lymph nodes, paraspinal muscles), the additional morbidity is minimal.
Neck Control after Primary Radiotherapy
The lower probability of regional control of the positive neck with RT has been documented by several retrospective series.86,88,103 In a series from the Institut Curie in Paris,86 1646 patients with squamous cell carcinoma of the oropharynx and pharyngolarynx had a 3-year regional control probability of 98%, 90%, 88%, and 71% for N0, N1, N2, and N3 lesions (AJCC 1976 classification), respectively. The nodal size was an even more discriminating factor, with nodal failure rates of 6%, 14%, and 39% for nodes below 3 cm, between 4 and 7 cm, and more than 7 cm, respectively.103 In this series, 75% of the neck nodes were treated by a form of concomitant boost approach with total doses in the range of 70 to 85 Gy in 5 to 6 weeks. In the series of 458 node-positive patients with squamous cell carcinoma of the larynx and pharynx treated at Aarhus University Hospital from 1963 to 1991, the 5-year neck node control rate reached 68%, 68%, and 56% for N1, N2, and N3 lesions (UICC 1982 stage), respectively.88
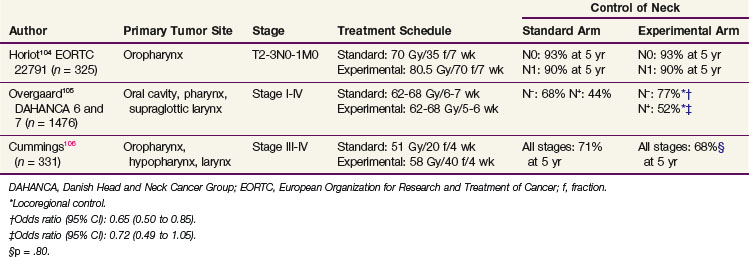
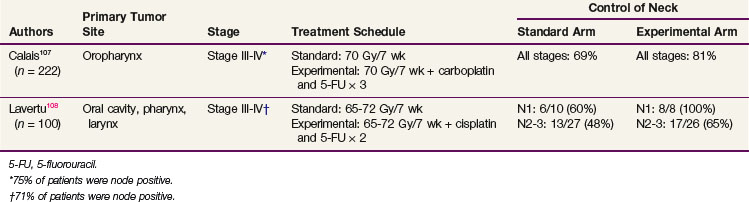
A key issue in the management of positive neck disease by RT is whether these rather poor results are improved by hyperfractionated or accelerated regimens or concomitant chemoradiation. In randomized studies comparing a standard fractionated regimen with an altered fractionated regimen, no improvement was observed between the two arms104,105,106 (Table 38-18). In the European Organization for Research and Treatment of Cancer (EORTC) 22791 study, only patients with N0 or N1 neck disease were included, and because of the very good control of neck disease in the standard arm, it was not surprising that the hyperfractionated regimen did not bring any benefit.104 In the Danish Head and Neck Cancer Group (DAHANCA) 6 and 7 trials, no significant regional improvement was observed with the accelerated regimen in the node-positive patients.105 Similarly, despite a benefit in OS, the Toronto trial did not observe any increase in the control of the neck with an accelerated hyperfractionated regimen.106 Contrary to results achieved with altered fractionation regimens, concomitant chemoradiation regimens appear to have an impact on the control of the neck107,108 (Table 38-19). In the study by Calais and associates,107 all neck stages were analyzed together. However, because 75% of the patients were node positive, it is very unlikely that the 12% improvement rate resulted only from a beneficial effect in the patients with N0 lesions. In the study by Lavertu and associates,108 which included fewer patients, the improvement in node control was observed for all positive neck categories.
Indications for Postoperative Irradiation and Postoperative Chemoradiation
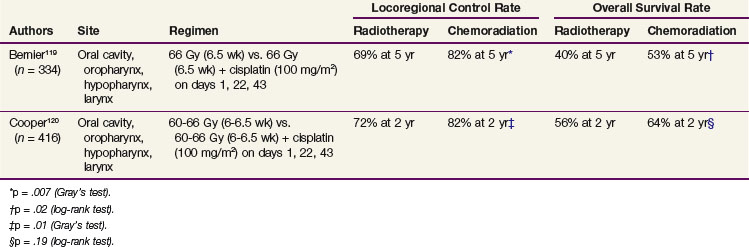
The benefit of postoperative RT in HNSCC progressively emerged in the 1970s and 1980s as a standard of care for patients at high risk of locoregional relapse after surgery.109–112 Prognostic indicators for locoregional relapse after surgery include the primary disease site, surgical margins at the primary site, presence of perineural invasion, number of metastatic lymph nodes, and presence of extracapsular rupture.113,114 Based on the clustering of these pathologic factors, the M.D. Anderson Cancer Center proposed to stratify patients into three risk categories, conditioning the need for postoperative RT115 (Table 38-20). In the absence of any risk factor, the need for postoperative RT could not be shown. Patients with extracapsular rupture or a combination of two or more risk factors were identified as being at high risk of locoregional relapse, and for these patients a randomized study showed the benefit of a radiation dose of 63 Gy (in 35 fractions) as compared with 57.6 Gy (in 32 fractions). For patients with only one risk factor other than extracapsular rupture, a dose of 57.6 Gy was shown to be optimal. A subsequent study from the same group further validated the use of these categories of risk factors and also individualized the time between surgery and the start of postoperative RT and the total treatment time (from surgery to the end of RT) as additional risk factors.116 In this study, it was also shown that patients with a high risk of relapse benefited from accelerated treatment (63 Gy in 5 weeks vs. 63 Gy in 7 weeks) in terms of both locoregional control and survival.
TABLE 38-20 Prognostic Factors for Locoregional Relapse after Surgery
| Moderate Risk | High Risk |
|---|---|
From Peters LJ, Goepfert H, Ang KK, et al: Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys 26:3-11, 1993.
With the need to further improve locoregional control rates after surgery and postoperative RT, few trials combining postoperative concomitant chemotherapy and RT were reported in the 1990s.117,118 Although the results favored the combined approach, these studies did not really influence the pattern of care of patients primarily treated with surgery. The European Organization for Research and Treatment of Cancer (EORTC) and the Radiation Therapy Oncology Group (RTOG) conducted similarly designed studies aimed at assessing the benefit of postoperative RT (60 to 66 Gy) combined with cisplatin (100 mg/m2) given on days 1, 22, and 43 for patients with a variety of risk factors that were slightly different between the two trials.119,120 In the EORTC study, a highly statistically significant benefit in favor of the combined treatment was observed for both locoregional control and OS (Table 38-21). In the RTOG study, the benefit in locoregional control probability did not translate into a statistically significant difference in survival rates. Combined-modality treatment did not decrease the incidence of distant metastasis in either of these studies. In both studies, the concomitant use of chemotherapy significantly enhanced the acute local toxicity of radiotherapy, and only half the patients could actually receive the full treatment as planned. A meta-analysis of these two studies demonstrated a statistically significant benefit of combined chemoradiation but only in patients who had positive surgical margins and/or extracapsular spread, that is, patients with the highest risk of relapse after surgery.121 For the other patients, radiotherapy alone can still be considered as a standard of care.
Indications for Postradiotherapy Neck Node Dissection
Advances in chemoradiation for advanced head and neck carcinoma have shown that organ preservation is feasible without compromising DFS and OS.122,123 This strategy has led to controversial issues concerning the role of node dissection after RT or chemoradiation for patients with N2 to N3 disease at initial diagnosis. A residual neck mass may be present in as many as 30% to 50% of patients after completion of chemoradiation. For these patients, irrespective of the neck stage, there seems to be a consensus in the literature favoring an immediate neck node dissection because of the very low probability of achieving neck control with salvage surgery when recurrence develops.124
Whether a neck dissection should be proposed for all patients with N2 to N3 disease at diagnosis or only for those without a complete response is still a matter of debate.125,126,127,128–133 Proponents of systematic neck dissection argue that it improves regional control and disease-free survival rates, that a clinical response cannot be used to predict a pathologic response, and that in the event of neck disease recurrence, salvage surgery is associated with very poor results. On the contrary, there are many arguments in favor of performing neck dissection as a function of the response to chemoradiation, such as a very low probability of isolated neck disease recurrence following a complete response,134 the absence of survival benefit in adding neck dissection in complete responders,130 a high rate of futile neck dissections (up to 80% of negative pathologic specimens) with the inherent morbidity, and the absence of a randomized trial showing the superiority of one approach.
This controversy seems to be fueled by the difficulty of assessing residual neck disease after an organ-preservation protocol. In this respect, the use of FDG-PET scanning has gained some interest. In a study including 41 patients treated with definite RT (with or without concomitant chemotherapy), a negative PET scan after RT was highly correlated with a negative pathologic finding after neck node dissection, fine-needle aspiration, or with the absence of neck relapse (negative predictive value of 100% for maximum standardized uptake values <3).135 In another study including 24 patients staged with FDG-PET after induction chemotherapy followed by chemoradiation, the negative predictive value of a PET scan reached only 73%.136 These contrasting results could partly be explained by a lack of standardization with regard to the quality of PET imaging, the timing of PET after RT, and the timing of the neck dissection. Despite reluctance of surgical teams to delay postradiotherapy neck dissection for more than 6 to 8 weeks because of fears that more fibrosis may develop, thus making surgery more difficult and increasing the risk of postoperative complications, it is recognized that the most appropriate time to perform PET is 12 to 15 weeks following RT.137–139 The utility of PET after chemoradiation in patients with residual lymph node disease was clearly demonstrated by two retrospective studies.138,139 In both studies, PET scans were obtained at a median time of 12 to 13 weeks after completion of RT. In these studies, the negative predictive value reached 97% and 100%, and the positive predictive value reached 71% and 62.5%, respectively. Based on these data, it may be concluded that a negative PET scan closely correlates with a negative pathologic neck node status. Last, a cost-effectiveness analysis on the use of CT and PET/CT for assessing the need for adjuvant neck dissection was reported comparing three stategies: dissection in all patients, dissection in patients with residual disease on CT scanning, and dissection in patients with residual disease on PET/CT scanning.140 Probabilistic sensitivity analyses confirmed that the PET/CT strategy was more cost-effective than the other two options. A definite answer on the issue of postradiotherapy neck dissection will only come from prospective randomized studies. Such a study is ongoing in England, comparing SND after chemoradiation with neck dissection in patients with a positive FDG-PET scan.
Irrespective of whether or not neck dissection should be performed after chemoradiation, little attention has been paid to the use of procedures less extensive than RND or modified RND in this setting. Although the concept of SND is widely accepted for patients with limited regional disease when surgery is proposed as primary treatment, its use in advanced nodal disease remains oncologically unsound. After chemoradiation, one could hypothesize that preoperative treatment was effective prophylaxis for all levels at risk for microscopic infiltration and that only the levels in which residual disease is still anticipated require neck dissection. In patients with advanced regional disease, Robbins and colleagues141 reported 33 SNDs performed after chemoradiation. In all cases, it was possible to completely excise all residual positive lymph nodes with negative margins after pathologic examination of the whole specimen. There was only one neck recurrence. Stenson and colleagues142 reported 56 SNDs performed in patients after concomitant chemoradiation. Thirty-five percent of patients had positive lymph nodes in specimens, whereas only one patient experienced recurrence in the neck. Other studies support the use of SND after chemoradiation, even in patients with initially advanced regional disease.143 In these studies, the rate of major postoperative complications was less than 10% and comparable to the rate of complications after primary surgery.129,142,144,145 Despite the absence of a prospective study comparing SND with more comprehensive node dissection after organ preservation protocols, one would intuitively expect less fibrosis, shoulder dysfunction, and neck deformity in patients who underwent limited neck surgery.
Late Complications after Neck Treatment
Complications of Neck Node Dissection
In addition to the medical complications inherent in any surgical procedure, neck dissection may potentially be associated with some specific perioperative complications and late complications or sequelae. The description of perioperative complications is beyond the scope of this chapter. Readers interested in such topics will find detailed information in a textbook dedicated to head and neck surgery.146
Recognized complications after neck dissection include numbness and/or burning of the neck and ear, neck pain, shoulder discomfort, neck tightness, lower lip weakness, and cosmetic disfigurement. In 2001, Shah and associates147 proposed a neck dissection–specific quality-of-life questionnaire used in a series of patients who had undergone neck dissection as part of their treatment.147 Some of these patients had been previously treated by chemotherapy (25%) or RT (50%). Neck tightness and shoulder discomfort had the greatest effect on quality of life. Advanced tumor stage, often requiring more radical surgery and use of chemotherapy or RT, was associated with a worse quality of life after neck dissection. Overall, the quality of life after neck dissection tends to improve with time.147
The most noticeable sequela induced by spinal accessory nerve resection is the decreased ability to abduct the shoulder above 90 degrees. However, any type of neck dissection may result in impairment of shoulder function.148 Studies reported that preservation of the spinal accessory nerve in modified RND and SND was associated with a better quality of life and fewer shoulder disorders in both the short and long term.147 Using the University of Washington quality-of-life questionnaire, the Liverpool group reported that little morbidity associated with shoulder dysfunction was observed in patients following a unilateral level I to III or I to IV neck dissection in comparison with patients undergoing primary surgery without neck dissection.149 However, unilateral neck dissection extending to level V and bilateral SND(I to III or I to IV) were associated with statistically significantly worse shoulder dysfunction. Adjuvant RT seemed to have a detrimental effect on shoulder dysfunction, whether or not the patient had a unilateral SND.
In another series, assessment of patients who underwent neck dissection with or without RT at least 1 year previously revealed that neck pain was present in one third of patients, shoulder pain in 37%, and loss of sensation in 65% (related to the number of dissected levels and to RT).150 A prospective study on objective assessment after treatment concluded that adjuvant RT had no effect on shoulder joint function and that shoulder disability was inevitable in all types of neck dissections, whether or not the spinal accessory nerve was spared. However, shoulder function was better after SND and modified RND than after RND, although electromyographic findings were similar after either RND or modified RND and SND.151 The benefit of postoperative physical therapy has been stressed, and it should be started in the early postoperative period after all types of neck dissections.152
Complications of Neck Irradiation
The probability of grade 3 to 4 (RTOG late morbidity scale) subcutaneous fibrosis in the neck is rather low after standard RT. From randomized studies performed in the 1990s, it can be estimated at around 3%.107,153,154,155 After accelerated or hyperfractionated treatments, no increase in grade 3 to 4 subcutaneous toxicity was observed, providing that enough time was left between fractions.154 In an EORTC trial with only a 4-hour interfraction time, a 50% risk of fibrosis was documented at 5 years after treatment.156 After concomitant chemoradiation, randomized trials reported a substantial increase in late skin morbidity, reaching figures of around 10%.107
Clinically indolent hypothyroidism in patients irradiated on the lower neck has been reported at rates of up to 24%.157,158 Most patients usually develop hypothyroidism within 1 year after treatment. Postoperative RT—especially after laryngeal surgery—including partial thyroidectomy, has been shown to be a risk factor. Yearly thyroid function testing (i.e., thyroid-stimulating–hormone level) is advised in the follow-up of patients with irradiation to the neck.
Carotid artery stenosis after neck irradiation has been reported by several authors, but very few studies have investigated the incidence, disease patterns, and risk factors. Matched control Doppler ultrasound examinations have reported significant carotid stenosis in 30% to 50% of patients with previous irradiation to the neck.159 Compared with the general population, a relative risk of stroke of 5.6 has been reported in patients with previous irradiation to the neck.160 This relative risk was further increased for patients older than 60 years and with follow-up longer than 10 years. Increased attention to the clinical signs of carotid stenosis together with proper management of the other risk factors (e.g., diabetes, hypertension, hypercholesterolemia, smoking, obesity) should contribute to a decreased incidence of stroke and neurologic sequelae in this patient population.
All of the published data on late complications are from the era before IMRT was available. With the use of modern RT technique, a major reduction in late complications is anticipated, mainly through a reduction in the volume of normal tissue irradiated at a high dose and a reduction of the “uncontrolled” hot spot within or outside of the PTV. The introduction of IMRT has, however, raised the controversial concern of an increased risk of radiation-induced secondary neoplasms because a larger volume of normal tissue might be irradiated at a lower dose in comparison with standard two-dimensional techniques. Also, the delivery of a specified dose to the isocenter from modulated fields requires a longer beam time compared with the same dose delivered to a nonmodulated field. The IMRT treatment plan then results in an increase in the number of monitor units by a factor of 2 to 3, thus increasing the dose outside the boundary of the primary collimator caused by leakage and scattered radiation.161 As a consequence, the total body dose received is substantially increased. It is estimated that an additional 0.5% of surviving patients will develop a secondary malignancy as a result of an increased volume of normal tissue receiving a small radiation dose. This number needs to be added to the 0.25% of surviving patients who subsequently develop a radiation-induced malignancy. In all, it is estimated that about 0.75% of surviving patients are expected to develop a secondary malignancy as a result of the switch to IMRT, which is approximately twofold greater than the incidence observed after more conventional RT.162 Whatever the contribution of IMRT in the induction of secondary cancers may be, we should bear in mind that even if IMRT increases the probability of locoregional control and the potential for increased cause-specific survival, this group of patients experiences comorbidities and increased risk for a second primary tumor associated with their lifestyle. This may decrease the relative importance of radiation-induced secondary malignancies.
Management of Recurrent Neck Disease
Whether treated by RT, surgery, or the combination of both, the prognosis of patients with recurrence of disease in the neck remains abysmal. Recurrent neck disease is quite invariably associated with unfavorable prognostic factors. Extracapsular spread is almost always reported and multiple lymph node levels are frequently involved.163 Neck disease recurrences are often unresectable because of involvement of the wall of the common carotid artery or the internal carotid artery, the paraspinal muscles, and the cranial nerves. Even when salvage surgery is attempted, the inability to achieve a complete resection with clear margins is generally the rule.
Very few studies have specifically addressed the problem of recurrent neck disease following curative treatment in HNSCC. Godden and colleagues163 retrospectively reviewed the charts of 35 patients with recurrent neck disease. More than 80% of patients had primary surgery and the rest were treated with RT. Of the patients treated with primary surgery, 50% had postoperative RT. A neck dissection was performed in 18 patients at initial presentation. Recurrence was managed by neck dissection in 25 patients and, among them, 18 had postoperative RT. Ten patients (29%) were considered inoperable. Of the 18 patients who had an initial neck dissection, 9 tumors (50%) recurred in level II, which had been previously cleared. Neck recurrence seemed to be more related to residual disease after the first neck dissection. This high rate of level II recurrence stresses the necessity of adequate training for surgeons performing neck dissection procedures. In this series, ultimate control of neck disease was obtained in only 5 of the 35 patients and 4-year OS did not exceed 20%.
The likelihood of successful salvage treatment in patients with neck recurrence after primary RT is very low. Bernier and Bataini86 reviewed 116 patients with isolated nodal failure after RT alone for oropharyngeal, hypopharyngeal, and laryngeal carcinoma. Fourteen patients had salvage neck dissection and 18 were reirradiated on the neck. Only one patient (1%) was successfully salvaged. The University of Florida reviewed the medical records of 51 patients who experienced recurrent disease in the neck only.164 Only 18 patients (35%) underwent salvage treatment; chemotherapy alone was used in 4 patients, chemotherapy and neck dissection in 1 patient, neck dissection alone in 11 patients, and neck dissection with postoperative RT in 2 patients. After salvage treatment, all patients had relapse of disease (locally, regionally, or distantly). The control rate for neck disease at 5 years was 9% for the group who underwent salvage treatment, which was similar to the rate of neck disease control for the whole population. For the whole group of patients, absolute and cause-specific survival rates reached 10% at 5 years for both end points. However, at 3 years, patients who received salvage treatment had absolute and cause-specific survival rates of 44%. In comparison, none of the 33 remaining patients was alive at 3 years.
Some institutions have evaluated salvage treatment with aggressive combined modality approaches that include low-dose reirradiation (preferably preoperative concurrent chemoradiation) combined with an attempt at gross resection plus intraoperative radiotherapy (IORT) with either electrons (IOERT) or high-dose-rate brachytherapy (HDR-IORT).165 Although the series involve small numbers of patients, early results suggest potential improvements in both locoregional control and survival rates compared with those of standard salvage approaches, and further evaluation is warranted.
1 Rouvière H. Anatomie humaine descriptive et topographique, ed 6. Paris: Masson et Cie; 1948. pp 226-230
4 Robbins KT, Medina JE, Wolfe GT, et al. Standardizing neck dissection terminology. Official report of the academy’s committee for head and neck surgery and oncology. Arch Otolaryngol Head Neck Surg. 1991;117:601-605.
7 Robbins KT. Classification of neck dissection: current concepts and future considerations. Otolaryngol Clin North Am. 1998;31:639-656.
10 Coche EE, Duprez T, Lonneux M. Imaging the lymph nodes: CT, MRI, and PET. In: Grégoire V, Scaillet P, Ang K, editors. Clinical Target Volumes in Conformal and Intensity Modulated Radiation Therapy: A Clinical Guide to Cancer Treatment. New York: Springer-Verlag; 2003:37-67.
24 Kyzas PA, Evangelou E, Denaxa-Kyza D, et al. 18 F-Fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. 2008;100:712-720.
28 Daisne JF, Duprez T, Weynand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004;233:93-100.
32 Bataini JP, Bernier J, Brugere J, et al. Natural history of neck disease in patients with squamous cell carcinoma of the oropharynx and pharyngolarynx. Radiother Oncol. 1985;3:245-255.
33 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446-1449.
34 Candela FC, Kothari K, Shah JP. Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck. 1990;12:197-203.
35 Candela FC, Shah J, Jaques DP, et al. Patterns of cervical node metastases from squamous carcinoma of the larynx. Arch Otolaryngol Head Neck Surg. 1990;116:432-435.
36 Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer. 1990;66:109-113.
37 Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405-409.
45 Byers RM. Neck dissection: concepts, controversies, and technique. Semin Surg Oncol. 1991;7:9-13.
46 Clayman GL, Frank DK. Selective neck dissection of anatomically appropriate levels is as efficacious as modified radical neck dissection for elective treatment of the clinically negative neck in patients with squamous cell carcinoma of the upper respiratory and digestive tracts. Arch Otolaryngol Head Neck Surg. 1998;124:348-352.
48 Grégoire V, Hamoir M, Levendag P, et al. Proposal for the delineation of the nodal CTV in the node-positive and the postoperative neck. Radiother Oncol. 2006;79:15-20.
66 Gregoire V, Coche E, Cosnard G, et al. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy: proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56:135-150.
70 Grégoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227-236.
86 Bernier J, Bataini JP. Regional outcome in oropharyngeal and pharyngolaryngeal cancer treated with high dose per fraction radiotherapy: analysis of neck disease response in 1646 cases. Radiother Oncol. 1986;6:87-103.
87 Alpert TE, Morbidini-Gaffney S, Chung CT, et al. Radiotherapy for the clinically negative neck in supraglottic laryngeal cancer. Cancer J. 2004;10:335-338.
88 Johansen LV, Grau C, Overgaard J. Nodal control and surgical salvage after primary radiotherapy in 1782 patients with laryngeal and pharyngeal carcinoma. Acta Oncol. 2004;43:486-494.
98 Shah JP. Cervical lymph node metastases—diagnostic, therapeutic, and prognostic implications. Oncology (Huntingt). 1990;4:61-69.
104 Horiot JC, Le Fur R, N’Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25:231-241.
105 Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933-940.
107 Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081-2086.
116 Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571-578.
119 Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-1952.
120 Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937-1944.
127 Clayman GL, Johnson CJ2nd, Morrison W, et al. The role of neck dissection after chemoradiotherapy for oropharyngeal cancer with advanced nodal disease. Arch Otolaryngol Head Neck Surg. 2001;127:135-139.
147 Shah S, Har-El G, Rosenfeld RM. Short-term and long-term quality of life after neck dissection. Head Neck. 2001;23:954-961.
154 Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyper-fractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7-16.
162 Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83-88.
1 Rouvière H. Anatomie humaine descriptive et topographique, ed 6. Paris: Masson et Cie; 1948.
2 Vidic B, Suarez-Quian C. Anatomy of the head and neck. In: Harrison LB, Sessions RB, Ki Hong W, editors. Head and Neck Cancer: A Multidisciplinary Approach. Philadelphia: Lippincott-Raven; 1998:79-114.
3 Spiessl B, Beahrs OH, Hermanek P, et al. TNM Atlas: Illustrated Guide to the TNM/PTNM Classification of Malignant Tumours. 2nd rev, ed 3. Springer, Berlin/Heidelberg/New York, 1992..
4 Robbins KT, Medina JE, Wolfe GT, et al. Standardizing neck dissection terminology. Official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg. 1991;117:601-605.
5 Shah JP, Strong E, Spiro RH, et al. Surgical grand rounds. Neck dissection. Current status and future possibilities. Clin Bull. 1981;11:25-33.
6 Hermanek P, Henson DE, Hutter RVP, Sobin LH, editors. TNM Supplement: A Commentary on Uniform Use. Berlin/Heidelberg/New York: Springer, 1993.
7 Robbins KT. Classification of neck dissection. Current concepts and future considerations. Otolaryngol Clin North Am. 1998;31:639-656.
8 Robbins KT. Integrating radiological criteria into the classification of cervical lymph node disease. Arch Otolaryngol Head Neck Surg. 1999;125:385-387.
9 Hamoir M, Desuter G, Gregoire V, et al. A proposal for redefining the boundaries of level V. Is dissection of the apex of level V necessary in mucosal cell carcinoma of the head and neck? Arch Otolaryngol Head Neck Surg. 2002;128:1381-1383.
10 Coche EE, Duprez T, Lonneux M. Imaging the lymph nodes: CT, MRI, and PET. In: Grégoire V, Scaillet P, Ang K, editors. Clinical Target Volumes in Conformal and Intensity Modulated Radiation Therapy. New York: Springer-Verlag; 2002:37-67.
11 Ishikawa M, Anzai Y. MR imaging of lymph nodes in the head and neck. Neuroimaging Clin North Am. 2004;14:679-694.
12 Curtin HD, Ishwaran H, Mancuso AA, et al. Comparison of CT and MR imaging in staging of neck metastases. Radiology. 1998;207:123-130.
13 King AD, Tse GMK, Ahuja AT, et al. Necrosis in metastatic neck nodes. Diagnostic accuracy of CT, MR imaging, and US. Radiology. 2004;230:720-726.
14 Star-Lack JM, Adalsteinsson E, Adam MF, et al. In vivo 1H MR spectroscopy of human head and neck lymph node metastasis and comparison with oxygen tension measurements. AJNR Am J Neuroradiol. 2000;21:183-193.
15 Fischbein NJ, Noworolski SM, Henry RG, et al. Assessment of metastatic cervical adenopathy using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2003;24:301-311.
16 Sumi M, Sakihama N, Sumi T, et al. Discrimination of metastatic cervical lymph node with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol. 2003;24:1627-1634.
17 Dirix P, Vandecaveye V, De Keyzer F, et al. Diffusion-weighted MRI for nodal staging of head and neck squamous cell carcinoma. Impact on radiotherapy planning. Int J Radiat Oncol Biol Phys. 2010;76:761-766.
18 Vandecaveye V, De Keyzer F, Vander Poorten V, et al. Head and neck squamous cell carcinoma. Value of diffusion-weighted MR imaging for nodal staging. Radiology. 2009;251:134-146.
19 King AD, Ahuja AT, Yeung DKW, et al. Malignant cervical lymphadenopathy. Diagnostic accuracy of diffusion-weighted MR imaging. Radiology. 2007;245:806-813.
20 Kato H, Kanematsu M, Tanaka O, et al. Head and neck squamous cell carcinoma. Usefulness of diffusion-weighted MR imaging in the prediction of a neoadjuvant therapeutic effect. Eur Radiol. 2009;19:103-109.
21 Abdel-Razek AAK, Kandeel AY, Soliman N, et al. Role of diffusion-weighted echo-planar MR imaging in differentiation of residual or recurrent head and neck tumors and posttreatment changes. AJNR Am J Neuroradiol. 2007;28:1146-1152.
22 Schroder H, Yeung HW, Gonen M, et al. Head and neck cancer. Clinical usefulness and accuracy of PET-CT image fusion. Radiology. 2004;231:65-72.
23 Lonneux M, Hamoir M, Reychler H, et al. PET-FDG improves staging and patient management in head and neck squamous-cell carcinoma patients. A multi-center prospective study. J Clin Oncol. 2010;28:1190-1195.
24 Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JPA. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma. A meta-analysis. J Natl Cancer Inst. 2008;100:712-720.
25 Torabi M, Aquino SL, Harishinghani MG. Current concepts in lymph node imaging. J Nucl Med. 2004;45:1509-1518.
26 Van de Wiele C, Versijpt J, Dierckx RA, et al. Tc-99m-labelled HL91 versus CT and biopsy for the visualization of tumor recurrence of squamous head and neck carcinoma. Nucl Med Commun. 2001;22:269-275.
27 Lonneux M, Lawson G, Ide C, et al. Positron emission tomography with fluorodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope. 2000;110:1493-1497.
28 Daisne JF, Duprez T, Weynand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma. Comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004;233:93-100.
29 Schwartz DL, Ford E, Rajendran J, et al. FDG-PET/CT imaging for preradiotherapy staging of head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:129-136.
30 Sobin L, Gospodarowicz M, Wittekind C, editors. UICC/TNM Classification of Malignant Tumors, ed 7, New York: Wiley-Blackwell, 2009.
31 O’Sullivan B, Shah J. New TNM staging criteria for head and neck tumors. Semin Surg Oncol. 2003;21:30-42.
32 Bataini JP, Bernier J, Brugere J, et al. Natural history of neck disease in patients with squamous cell carcinoma of the oropharynx and pharyngolarynx. Radiother Oncol. 1985;3:245-255.
33 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446-1449.
34 Candela FC, Kothari K, Shah JP. Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck. 1990;12:197-203.
35 Candela FC, Shah J, Jaques DP, et al. Patterns of cervical node metastases from squamous carcinoma of the larynx. Arch Otolaryngol Head Neck Surg. 1990;116:432-435.
36 Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer. 1990;66:109-113.
37 Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405-409.
38 Davidson BJ, Kulkarny V, Delacure MD, et al. Posterior triangle metastases of squamous cell carcinoma of the upper aerodigestive tract. Am J Surg. 1993;166:395-398.
39 Spiro JD, Spiro RH, Shah JP, et al. Critical assessment of supra-omohyoid neck dissection. Am J Surg. 1988;156:286-289.
40 Byers RM, Weber RS, Andrews T, et al. Frequency and therapeutic implications of “skip metastases” in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19:14-19.
41 Kowalski LP, Magrin J, Waksman G, et al. Supraomohyoid neck dissection in the treatment of head and neck tumors. Survival results in 212 cases. Arch Otolaryngol Head Neck Surg. 1993;119:958-963.
42 Foote RL, Olsen KD, Davis DL, et al. Base of tongue carcinoma. Patterns of failure and predictors of recurrence after surgery alone. Head Neck. 1993;15:300-307.
43 O’Sullivan B, Warde P, Grice B, et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys. 2001;51:332-343.
44 Jackson SM, Hay JH, Flores AD, et al. Cancer of the tonsil. The results of ipsilateral radiation treatment. Radiother Oncol. 1999;51:123-128.
45 Byers RM. Neck dissection: concepts, controversies, and technique. Semin Surg Oncol. 1991;7:9-13.
46 Clayman GL, Frank DK. Selective neck dissection of anatomically appropriate levels is as efficacious as modified radical neck dissection for elective treatment of the clinically negative neck in patients with squamous cell carcinoma of the upper respiratory and digestive tracts. Arch Otolaryngol Head Neck Surg. 1998;124:348-352.
47 Byers RM. Modified neck dissection. A study of 967 cases from 1970 to 1980. Am J Surg. 1985;150:414-421.
48 Grégoire V, Hamoir M, Levendag P, et al. Proposal for the delineation of the nodal CTV in the node-positive and the postoperative neck. Radiother Oncol. 2006;79:15-20.
49 Marks JE, Devineni VR, Harvey J, et al. The risk of contralateral lymphatic metastases for cancers of the larynx and pharynx. Am J Otolaryngol. 1992;13:34-39.
50 Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update. Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128:751-758.
51 Som PM, Curtin HD, Mancuso AA. An imaging-based classification for the cervical nodes designed as an adjunct to recent clinically based nodal classifications. Arch Otolaryngol Head Neck Surg. 1999;125:388-396.
52 Medina JE. A rational classification of neck dissections. Otolaryngol Head Neck Surg. 1989;100:169-176.
53 Suarez O. El problema de las metastasis linfaticas y alejadas del cancer de laringe e hipofaringe. Rev Otorinolaringol. 1963;23:83-89.
54 Bocca E, Pignataro O. A conservation technique in radical neck dissection. Ann Otol Rhinol Laryngol. 1967;76:975-987.
55 Bocca E, Pignataro O, Sasaki CT. Functional neck dissection. A description of operative technique. Arch Otolaryngol Head Neck Surg. 1980;106:524-527.
56 Morton DL, Wen DR, Wong JH, et al. Technical details of intra-operative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392-399.
57 Pan D, Narayan D, Ariyan S. Merkel cell carcinoma. Five case reports using sentinel lymph node biopsy and a review of 110 new cases. Plast Reconstr Surg. 2002;110:1259-1265.
58 Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546-553.
59 Shoaib T, Soutar DS, MacDonald DG, et al. The accuracy of head and neck carcinoma sentinel lymph node biopsy in the clinically N0 neck. Cancer. 2001;91:2077-2083.
60 Pitman KT, Johnson JT, Brown ML, et al. Sentinel lymph node biopsy in head and neck squamous cell carcinoma. Laryngoscope. 2002;112:2101-2113.
61 Ross GL, Soutar DS, MacDonald DG, et al. Improved staging of cervical metastasis in clinically negative patients with head and neck squamous cell carcinoma. Ann Surg Oncol. 2004;11:213-218.
62 Stoekli SJ, Pfalz M, Steinert H, et al. Histopathological features of occult metastasis detected by sentinel lymph node biopsy in oral and oropharyngeal squamous cell carcinoma. Laryngoscope. 2002;112:111-115.
63 Hermanek P, Hutter RVP, Sobin LH, Wittekind C. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668-2673.
64 Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. International Union Against Cancer (UICC), ed 6. New York: Wiley-Liss; 2002. pp 10-13
65 Atula T, Hunter KD, Cooper LA, et al. Micrometastases and isolated tumour cells in sentinel lymph nodes in oral and oropharyngeal squamous cell carcinoma. Eur J Surg Oncol. 2009;35:532-538.
66 Gregoire V, Coche E, Cosnard G, et al. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56:135-150.
67 Nowak PJ, Wijers OB, Lagerwaard FJ, et al. A three-dimensional CT-based target definition for elective irradiation of the neck. Int J Radiat Oncol Biol Phys. 1999;45:33-39.
68 Wijers OB, Levendag PC, Tan T, et al. A simplified CT-based definition of the lymph node levels in the node negative neck. Radiother Oncol. 1999;52:35-42.
69 Martinez-Monge R, Fernandes PS, Gupta N, et al. Cross-sectional nodal atlas. A tool for the definition of clinical target volumes in three-dimensional radiation therapy planning. Radiology. 1999;211:815-828.
70 Grégoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck. DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227-236.
71 Levendag P, Grégoire V, Hamoir M, et al. Intraoperative validation of CT-based lymph nodal levels, sublevels IIA and IIB. Is it of clinical relevance in selective radiation therapy? Int J Radiat Oncol Biol Phys. 2005;62:690-699.
72 Apisarnthanarax S, Elliott D, El Naggar AK, et al. Determining optimal clinical target volume margins in head and neck cancer based on microscopic extracapsular extension of metastatic neck nodes. Int J Radiat Oncol Biol Phys. 2006;64:678-683.
73 Bussels B, Hermans R, Rijnders A, Van den Bogaert W. Retropharyngeal nodes in squamous cell carcinoma of the oropharynx. Implications for target volume delineation. Radiother Oncol. 2004;73(Suppl 1):S179.
73a Grégoire V, Mackie R. ICRU report 83: Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). editors]. J ICRU. 2010;10:1.
74 Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994;120:699-702.
75 Brazilian Head and Neck Cancer Study Group. Results of a prospective trial on elective modified radical classical versus supraomohyoid neck dissection in the management of oral squamous carcinoma. Am J Surg. 1998;176:422-427.
76 Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10:160-167.
77 Byers RM, Clayman GL, McGill D, et al. Selective neck dissections for squamous carcinoma of the upper aerodigestive tract. Patterns of regional failure. Head Neck. 1999;21:499-505.
78 Duvvuri U, Simental AAJr, DAngelo G, et al. Elective neck dissection and survival in patients with squamous cell carcinoma of the oral cavity and oropharynx. Laryngoscope. 2004;114:2228-2234.
79 Jesse RH, Ballantyne AJ, Larson D. Radical or modified neck dissection. A therapeutic dilemma. Am J Surg. 1978;136:516-519.
80 Lingeman RE, Helmus C, Stephens R, et al. Neck dissection. Radical or conservative. Ann Otol. 1977;86:737-744.
81 Medina JE, Byers RM. Supraomohyoid neck dissection. Rationale, indications, and surgical technique. Head Neck. 1989;11:111-122.
82 Pellitteri PK, Robbins KT, Neuman T. Expanded application of selective neck dissection with regard to nodal status. Head Neck. 1997;19:260-265.
83 Pitman KT, Johnson JT, Myers EN. Effectiveness of selective neck dissection for management of the clinically negative neck. Arch Otolaryngol Head Neck Surg. 1997;123:917-922.
84 Spiro RH, Morgan GJ, Strong EW, et al. Supraomohyoid neck dissection. Am J Surg. 1996;172:650-653.
85 Schmitz S, Machiels JP, Weynand B, et al. Results of selective neck dissection in the primary management of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2009;266:437-443.
86 Bernier J, Bataini JP. Regional outcome in oropharyngeal and pharyngolaryngeal cancer treated with high dose per fraction radiotherapy. Analysis of neck disease response in 1646 cases. Radiother Oncol. 1986;6:87-103.
87 Alpert TE, Morbidini-Gaffney S, Chung CT, et al. Radiotherapy for the clinically negative neck in supraglottic laryngeal cancer. Cancer J. 2004;10:335-338.
88 Johansen LV, Grau C, Overgaard J. Nodal control and surgical salvage after primary radiotherapy in 1782 patients with laryngeal and pharyngeal carcinoma. Acta Oncol. 2004;43:486-494.
89 Nakfoor BM, Spiro IJ, Wang CC, et al. Results of accelerated radiotherapy for supraglottic carcinoma. A Massachusetts General Hospital and Massachusetts Eye and Ear Infirmary experience. Head Neck. 1998;20:379-384.
90 Dische S, Saunders M, Barrett A, et al. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol. 1997;44:123-136.
91 Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer. Implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28-42.
92 Bussels B, Maes A, Hermans R, et al. Recurrences after conformal parotid-sparing radiotherapy for head and neck cancer. Radiother Oncol. 2004;72:119-127.
93 Chao KS, Ozyigit G, Tran BN, et al. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55:312-321.
94 Andersen PE, Shah JP, Cambronero E, et al. The role of comprehensive neck dissection with preservation of the spinal accessory nerve in the clinically positive neck. Am J Surg. 1994;168:499-502.
95 Merino OR, Lindberg RD, Fletcher GH. An analysis of distant metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1977;40:145-151.
96 Carew JF, Singh B, Shah JP. Cervical lymph nodes. In: Shah JP, Johnson NW, Batsakis JG, Dunitz M, editors. Oral cancer. New York: Thieme; 2003:215-249.
97 Khafif RA, Gelbfish GA, Asase DK, et al. Modified radical neck dissection in cancer of the mouth, pharynx, and larynx. Head Neck. 1990;12:476-482.
98 Shah JP. Cervical lymph node metastases—diagnostic, therapeutic, and prognostic implications. Oncology (Huntingt). 1990;4:61-69.
99 Clark J, Li W, Smith G, et al. Outcome of treatment for advanced cervical metastatic squamous cell carcinoma. Head Neck. 2005;27:87-94.
100 Shaha AR. Extended neck dissection. J Surg Oncol. 1990;45:229-233.
101 Brennan JA, Jafek BW. Elective carotid artery resection for advanced squamous cell carcinoma of the neck. Laryngoscope. 1994;104:259-263.
102 Maves MD, Bruns MD, Keenan MJ. Carotid artery resection for head and neck cancer. Ann Otol Rhinol Laryngol. 1992;101:778-781.
103 Bataini JP, Bernier J, Asselain B, et al. Primary radiotherapy of squamous cell carcinoma of the oropharynx and pharyngolarynx. Tentative multivariate modelling system to predict the radiocurability of neck nodes. Int J Radiat Oncol Biol Phys. 1988;14:635-642.
104 Horiot JC, Le Fur R, N’Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma. Final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25:231-241.
105 Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck. DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933-940.
106 Cummings B, OSullivan B, Keane T, et al. 5-Year results of 4 week/twice daily radiation schedule. The Toronto Trial. [Abstract.]. Radiother Oncol. 2000;56:S8.
107 Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081-2086.
108 Lavertu P, Bonafede JP, Adelstein DJ, et al. Comparison of surgical complications after organ-preservation therapy in patients with stage III or IV squamous cell head and neck cancer. Arch Otolaryngol Head Neck Surg. 1998;124:401-406.
109 Nisi KW, Foote RL, Bonner JA, et al. Adjuvant radiotherapy for squamous cell carcinoma of the tongue base. Improved local-regional disease control compared with surgery alone. Int J Radiat Oncol Biol Phys. 1998;41:371-377.
110 Lundahl RE, Foote RL, Bonner JA, et al. Combined neck dissection and postoperative radiation therapy in the management of the high-risk neck. A matched-pair analysis. Int J Radiat Oncol Biol Phys. 1998;40:529-534.
111 Vikram B, Strong EW, Shah JP, et al. Failure in the neck following multimodality treatment for advanced head and neck cancer. Head Neck Surg. 1984;6:724-729.
112 Dixit S, Vyas RK, Toparani RB, et al. Surgery versus surgery and postoperative radiotherapy in squamous cell carcinoma of the buccal mucosa. A comparative study. Ann Surg Oncol. 1998;5:502-510.
113 Amdur RJ, Parsons JT, Mendenhall WM, et al. Postoperative irradiation for squamous cell carcinoma of the head and neck: an analysis of treatment results and complications. Int J Radiat Oncol Biol Phys. 1989;16:25-36.
114 Parsons JT, Mendenhall WM, Stringer SP, et al. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997;39:137-148.
115 Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer. First report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26:3-11.
116 Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571-578.
117 Haffty BG, Son YH, Sasaki CT, et al. Mitomycin C as an adjunct to postoperative radiation therapy in squamous cell carcinoma of the head and neck. Results from two randomized clinical trials. Int J Radiat Oncol Biol Phys. 1993;27:241-250.
118 Bachaud JM, Cohen-Jonathan E, Alzieu C, et al. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma. Final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36:999-1004.
119 Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-1952.
120 Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937-1944.
121 Bernier J, Cooper J, Pajak T, et al. Defining risk levels in locally advanced head and neck cancers. A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck. 2005;27:843-850.
122 Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
123 Lefebvre JL, Lartigau E. Preservation of form and function during management of cancer of the larynx and hypopharynx. World J Surg. 2003;27:811-816.
124 Mendenhall WM, Villaret DB, Amdur RJ, et al. Planned neck dissection after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck. 2002;24:1012-1018.
125 Narayan K, Crane CH, Kleid S, et al. Planned neck dissection as an adjunct to the management of patients with advanced neck disease treated with definitive radiotherapy. For some or for all? Head Neck. 1999;21:606-613.
126 Stenson KM, Haraf DJ, Pelzer H, et al. The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy. The feasibility of selective neck dissection. Arch Otolaryngol Head Neck Surg. 2000;126:950-956.
127 Clayman GL, Johnson CJ2nd, Morrison W, et al. The role of neck dissection after chemoradiotherapy for oropharyngeal cancer with advanced nodal disease. Arch Otolaryngol Head Neck Surg. 2001;127:135-139.
128 McHam SA, Adelstein DJ, Rybicki LA, et al. Who merits a neck dissection after definitive chemoradiotherapy for N2-N3 squamous cell head and neck cancer? Head Neck. 2003;25:791-798.
129 Brizel DM, Prosnitz RG, Hunter S, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58:1418-1423.
130 Argiris A, Stenson KM, Brockstein BE, et al. Neck dissection in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck. 2004;26:447-455.
131 Goguen LA, Posner MR, Tishler RB, et al. Examining the need for neck dissection in the era of chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:526-531.
132 Forest VI, Nguyen-Tan PF, Tabet JC, et al. Role of neck dissection following concurrent chemoradiation for advanced head and neck carcinoma. Head Neck. 2006;28:1099-1105.
133 Vedrine PO, Thariat J, Hitier M, et al. Need for neck dissection after chemoradiotherapy? A study of the French GETTEC Group. Laryngoscope. 2008;118:1775-1780.
134 Lambrecht M, Dirix P, Van den Bogaert W, Nuyts S. Incidence of isolated regional recurrence after definitive (chemo-) radiotherapy for head and neck squamous cell carcinoma. Radioth Oncol. 2009;93:498-502.
135 Yao M, Graham MM, Hoffman HT, et al. The role of postradiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1001-1010.
136 McCollum AD, Burrell SC, Haddad RI, et al. Positron emission tomography with 18F-fluorodeoxyglucose to predict pathologic response after induction chemotherapy and definitive chemoradiotherapy in head and neck cancer. Head Neck. 2004;26:890-896.
137 Yao M, Buatti JM, Dornfeld KJ, et al. Can post-RT FDG PET accurately predict the pathologic status in neck dissection after radiation for locally advanced head and neck cancer? In regard to Rogers et al (Int J Radiat Oncol Biol Phys 58:694-697, 2004). Int J Radiat Oncol Biol Phys. 2005;61:306-307. author reply 61:307, 2005
138 Yao M, Hoffman HT, Chang K, et al. Is planned neck dissection necessary for head and neck cancer after intensity-modulated radiotherapy? Int J Radiat Oncol Biol Phys. 2007;68:707-713.
139 Porceddu SV, Jarmolowski E, Hicks RJ, et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo)radiotherapy in head and neck cancer. Head Neck. 2005;27:175-181.
140 Sher DJ, Tishler RB, Annino D, Punglia RS. Cost-effectiveness of CT and PET-CT for determining the need of adjuvant neck dissection in locally advanced head and neck cancer. Ann Oncol. 2010;21:1072-1077.
141 Robbins KT, Doweck I, Samant S, Viera F. Effectiveness of superselective and selective neck dissection for advanced nodal metastases after chemoradiation. Arch Otolaryngol Head Neck Surg. 2005;131:965-969.
142 Stenson KM, Huo D, Blair E, et al. Planned post-chemoradiation neck dissection. Significance of radiation dose. Laryngoscope. 2006;116(1):33-36.
143 Doweck I, Robbins KT, Mendenhall WM, et al. Neck-level-specific nodal metastases in oropharyngeal cancer. Is there any role for selective neck dissection after definitive radiotherapy? Head Neck. 2003;25:960-967.
144 Stenson KM, Haraf DJ, Pelzer H, et al. The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy. The feasibility of selective neck dissection. Arch Otolaryngol Head Neck Surg. 2000;126(8):950-956. Review
145 Proctor E, Robbins KT, Viera F, et al. Postoperative complications after chemoradiation for advanced head and neck cancer. Head Neck. 2004;26:272-277.
146 Medina JE, Houck JR, O’Malley BB. Management of cervical lymph nodes in squamous cell carcinoma. In: Harrison LB, Sessions RB, Ki Hong W, editors. Head and Neck Cancer: A Multi-disciplinary Approach. Philadelphia: Lippincott-Raven; 1998:353-378.
147 Shah S, Har-El G, Rosenfeld RM. Short-term and long-term quality of life after neck dissection. Head Neck. 2001;23:954-961.
148 Leipzig B, Suen JY, English JL, et al. Functional evaluation of the spinal accessory nerve after neck dissection. Am J Surg. 1983;146:526-530.
149 Laverick S, Lowe D, Brown JS, et al. The impact of neck dissection on health-related quality of life. Arch Otolaryngol Head Neck Surg. 2004;130:149-154.
150 van Wilgen CP, Dijkstra PU, van der Laan BF, et al. Morbidity of the neck after head and neck cancer therapy. Head Neck. 2004;26:785-791.
151 Erisen L, Basel B, Irdesel J, et al. Shoulder function after accessory nerve-sparing neck dissections. Head Neck. 2004;26:967-971.
152 Blessing R, Mann W, Beck C. How important is preservation of the accessory nerve in neck dissection? Laryngol Rhinol Otol (Stuttg). 1986;65:403-405.
153 Lee DJ, Cosmatos D, Marcial VA, et al. Results of an RTOG phase III trial (RTOG 85-27) comparing radiotherapy plus etanidazole with radiotherapy alone for locally advanced head and neck carcinomas. Int J Radiat Oncol Biol Phys. 1995;32:567-576.
154 Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyper-fractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas. First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7-16.
155 Trotti A. Toxicity in head and neck cancer. A review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47:1-12.
156 Horiot JC, Bontemps P, van den Bogaert W, et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers. Results of the EORTC 22851 randomized trial. Radiother Oncol. 1997;44:111-121.
157 Kumpulainen EJ, Hirvikoski PP, Virtaniemi JA, et al. Hypothyroidism after radiotherapy for laryngeal cancer. Radiother Oncol. 2000;57:97-101.
158 Sinard RJ, Tobin EJ, Mazzaferri EL, et al. Hypothyroidism after treatment for nonthyroid head and neck cancer. Arch Otolaryngol Head Neck Surg. 2000;126:652-657.
159 Abayomi OJ. Neck irradiation, carotid injury and its consequences. Oral Oncol. 2004;40:872-878.
160 Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282-288.
161 Williams BC, Hounsella R. X-ray linkage considerations for IMRT. Br J Radiol. 2001;74:98-102.
162 Hall EJ, Wuu CS. Radiation-induced second cancers. The impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83-88.
163 Godden DR, Ribeiro NF, Hassanein K, et al. Recurrent neck disease in oral cancer. J Oral Maxillofac Surg. 2002;60:748-753.
164 Mabanta SR, Mendenhall WM, Stringer SP, et al. Salvage treatment for neck recurrence after irradiation alone for head and neck squamous cell carcinoma with clinically positive neck nodes. Head Neck. 1999;21:591-594.
165 Foote RL, Garrett P, Rate W, et al. IORT for head and neck cancer. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intra-operative Irradiation: Techniques and Results. Totowa. NJ: Humana Press; 1999:471-497.
166 McLaughlin MP, Mendenhall WM, Mancuso AA, et al. Retropharyngeal adenopathy as a predictor of outcome in squamous cell carcinoma of the head and neck. Head Neck. 1995;17(3):190-198.
167 Chua DT, Sham JS, Kwong DL, et al. Retropharyngeal lymphadenopathy in patients with nasopharyngeal carcinoma: a computed tomography-based study. Cancer. 1997;79(5):869-877.
168 Chong VF, Fan YF, Khoo JB. Retropharyngeal lymphadenopathy in nasopharyngeal carcinoma. Eur J Radiol. 1995;21(2):100-105.