Chapter 188 Management of Spinal Cord Tumors and Arteriovenous Malformations
Intramedullary Spinal Cord Tumors
History of Surgery
The first reported attempt to resect an intramedullary spinal cord tumor was described in 1890.1 The patient died in the early postoperative period. As a result, these tumors were considered essentially nonsurgical lesions until the first described successful resection of an intramedullary ependymoma in 1907 by Anton von Eiselsberg. After undergoing a gross total resection, the patient regained and maintained ambulation with long-term follow-up.2
As the new wave of intramedullary spinal cord tumor surgery progressed, Elsberg and Beer advocated attempts at gross total resection by identifying safe tissue planes with blunt dissection.3 They reported an aborted first attempt at resection of an intramedullary tumor in which hemodynamic instability was encountered after myelotomy was performed. Upon returning to the operating room 1 wk later, Elsberg found that the tumor had extruded through the myelotomy defect with minimal spinal cord manipulation, and the patient regained function. Subsequently, Elsberg advocated such a two-stage approach for all intramedullary tumors in his seminal text, Diagnosis and Treatment of Surgical Diseases of the Spinal Cord and Its Membranes, in 1916.4 While there are still many surgeons who practice this “two-stage” approach, technological advances have allowed many more intramedullary lesions to be resected with good outcomes in one stage. Further series demonstrated reliably that ependymomas are more likely to be safely completely resected, while astrocytomas frequently lack a necessary clear plane between neoplastic and normal tissue (Table 188-1).
Significant Technical Advances
Technical advances account for a large part of the reason that intramedullary tumors are now seen as surgically treatable diseases. The development of the binocular operating microscope in the 1920s, and its first neurosurgical use by Theodore Kurze, revolutionized all aspects of neurosurgery.5 It has become absolutely mandatory in the resection of intramedullary lesions.
Bipolar cautery was first developed in the 1930s and expanded in use with Leonard Malis.6 It allowed for the precise coagulation of tumor vessels while sparing vessels supplying the cord. It also directed electrical current between two forceps tips, protecting surrounding neural tissue from thermal injury. It is indispensable in identifying the natural tumor/cord cleavage plane, if one exists.
Advanced Imaging
Myelography was the gold standard in diagnosis of spinal cord tumors until the advent of MRI. MRI was developed in the 1970s by teams of scientists, and was being applied to the spinal cord by the 1980s. Previously, indirect evidence of spinal cord tumor included cord expansion or myelographic block. With MRI, the spinal cord and tumor tissue could be directly visualized, and described by their signal characteristics.7 As a result, pre-operative diagnosis and planning can often be accomplished, and help direct the care of the patient. MRI is also useful in distinguishing non-neoplastic and nonsurgical lesions from surgical lesions, sparing unnecessary risk for many patients.8 Preoperative diffusion tensor imaging (DTI) tractography may be able to help determine which tumors will have a clear plane of demarcation with non-neoplastic tissue, and which may then safely be resected.9,10 Intraoperative imaging with ultrasound can help localize the optimal site for myelotomy,11 and intraoperative MRI may eventually be helpful to assess the extent of resection.
Intraoperative Neurophysiological Monitoring
Intraoperative neurophysiological monitoring plays a crucial role in the safe resection of intramedullary lesions. Somatosensory evoked potentials (SSEPs), used intraoperatively since the 1970s, record the electrical conductivity along the dorsal columns. Thus they can be used to help identify the midline myelotomy site if the gross anatomy and visual landmarks are found to be distorted. Once the myelotomy is made, it is not unusual to lose SSEP recording, which typically correlates with a loss of vibration and proprioception in the appropriate limbs. With time and rehabilitation, patients learn to compensate for this lost function and can resume normal activities. Use of intraoperative SSEP prior to making the myelotomy may allow for precise identification of the midline between the two dorsal columns and may decrease the morbidity from injury to this tract.12
Transcranial MEP can also be recorded by an epidural electrode located distal to the resection level. This measures the D wave in a quantitative manner, allowing for graded rather than all-or-none interpretation of the electrical signals. It can be recorded with no detectable muscle movement and thus can be monitored continuously during resection. A drop in amplitude greater than 50% is considered significant and warrants a pause in the surgery, at least until signals recover. A surgeon may aggressively seek out a dissection plane as long as the D wave remains stable. Typically, a loss of peripheral muscle MEP with preserved epidural D wave indicates a transient loss of function that may be severe but is likely to recover.13,14
Surgical Technique
In 1962, Turnbull stated: “A surgeon exploring a spinal cord for a suspected intramedullary tumor must be prepared to face a formidable problem and also have the courage of conviction to make every attempt to remove the tumor. Anything less than this, with a cursory inspection of the spinal cord or aspiration thereof, can only create problems of a more complex nature for the subsequent surgical effort to remove such tumors.”15 These words remain true and are the guiding principle in intramedullary spinal cord tumor surgery today.
Surgery remains the primary treatment for intramedullary tumors. Radiotherapy has little to offer as a primary treatment, even for malignant tumors. Radiation treatment has its most significant role as adjuvant treatment of World Health Organization (WHO) grades III and IV tumors.16,17 Regarding the timing of surgery, there is little to be gained by allowing further tumor growth prior to offering surgery. The surgical results are generally predicated on the preoperative condition of the patient, no matter how large the tumor. If the patient preoperatively has minimal neurological findings, then the postoperative course should be gratifying, especially if the tumor can be resected grossly. Those patients who arrive for surgery with significant preoperative deficits may regain little of their lost function even after a technically successful operation.
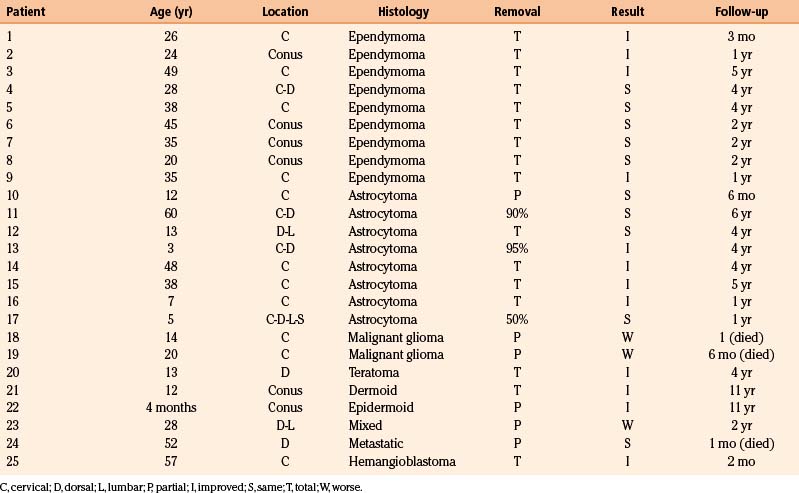
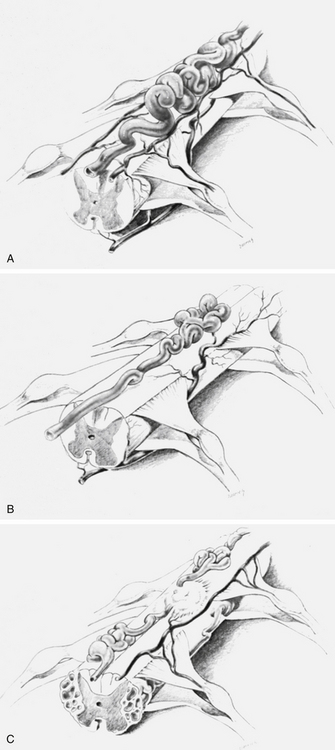
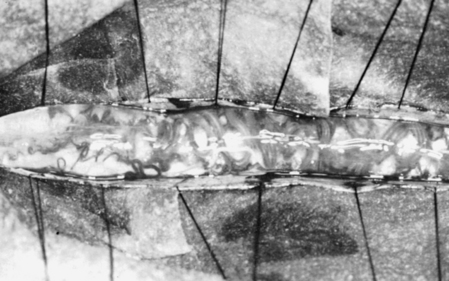
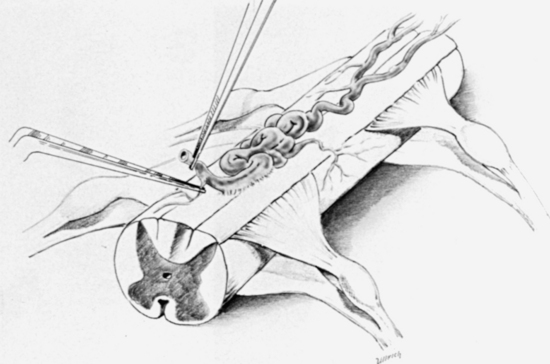
The dorsal surface of the cord at the area of greatest enlargement is then inspected for the site of the myelotomy. Generally, myelotomies are performed in the midline with a preference for the thinnest and most avascular areas. When the spinal cord is rotated and the midline is difficult to identify, the bilateral dorsal root entry zones can be identified and thereby help to define the midline to perform the myelotomy. Intraoperative dorsal column monitoring can be useful in determining the location for the midline myelotomy.12 At times, however, it is more expeditious to use a paramedian approach (Fig. 188-1).
Following the myelotomy, 6-0 or 7-0 traction sutures are placed through the pial margins on either side and sutured to the edges of the opened dura to expose the interior of the spinal cord (see Fig. 188-1). Generally, the tumor is visible a few millimeters under the dorsal surface of the spinal cord. There is often a soft gliotic interface between the tumor and the spinal cord proper. With the use of an operating microscope, microsurgical techniques, bipolar cautery, small suction tubes, and various dissectors, a plane is developed around the margin of the tumor, taking care to retract primarily on the tumor and not on the spinal cord. Gentle use of plated bayoneted forceps can help tease out natural planes between normal and neoplastic tissue. The surgeon works to one or the other end of the tumor. At the pole where the tumor is the narrowest, it may be possible to grasp the end and gently extract it from the interior of the cord. All the fine vascular adhesions to the spinal cord, especially on the ventral aspect of the tumor, should be cauterized with bipolar cautery and sharply divided. No blunt dissection should be carried out in areas where vascular channels connect the tumor to the spinal cord. Most tumors are relatively avascular and present little of hemorrhage or the loss of control of large blood vessels. It is important to keep the operative field meticulously dry so that the plane between the tumor and the spinal cord may be readily identified (see Fig. 188-1).
If the tumor is too large to remove en bloc or necessitates too much retraction on the spinal cord, its interior may be decompressed. The ultrasonic aspirator may be used to debulk the tumor, facilitating dissection around its capsule and its removal. This has a minor disadvantage of spilling the contents of the tumor into the dissection plane or obscuring the dissection plane by bleeding. In most instances, however, because the tumors are relatively avascular, bleeding is not a major problem, even with the use of the ultrasonic aspirator. Gradually, the entire tumor may be removed. For those tumors (usually astrocytomas) that are infiltrating, a debulking procedure may be valuable, especially in children. Carbon dioxide and argon lasers have also been used for this purpose.18 More recently, the development of sapphire-tipped Nd:YAG (neodymium-doped yttrium-aluminum-garnet) and argon lasers has allowed very focused transmission of energy located at the tip itself. This can be useful in creating the initial myelotomy as well as in dissecting along the tumor-normal interface, with minimal manipulation of the spinal cord.19–21
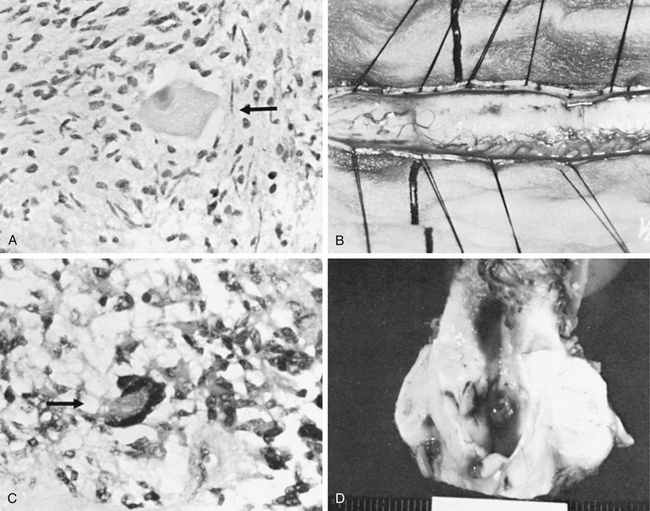
Cystic collections at the margins of the tumor, as in the case of ependymomas, facilitate the tumor resection. No attempt should be made to remove the wall of the cyst, which is thin and non-neoplastic. If there is any doubt about the totality of removal, small biopsies may be obtained from the margin of the resection and evaluated by frozen sectioning during surgery. In most instances, the margins are well-defined and there is no question about the removal of the tumor (see Figs. 93-4 and 93-6). Malignant tumors such as the glioblastoma respond poorly to surgery and do not appear to respond to radiation therapy. In such cases, heroic lifesaving efforts at cordotomy have been attempted, with dismal results.22,23
A number of aspects concerning the surgical removal and management of intramedullary spinal cord tumors must be emphasized. The surgeon should assume that the majority of all intramedullary cord tumors are benign and resectable. Intraoperative judgment should be based on the gross appearance rather than the histological tumor characteristics, because some low-grade astrocytomas are well-circumscribed and resectable. An inadequate myelotomy may fail to reveal a clear plane, and frozen section specimens made from a small piece of tumor taken through a limited myelotomy may lead to an erroneous tissue diagnosis and thus be misleading. This problem often occurs with tanycytic ependymomas, which may be mistaken for astrocytomas on frozen sections. Therefore, if a clear plane is identified between tumor and normal tissue, the surgeon should continue with the resection as long as electrophysiological monitoring is stable. On the other hand, if the frozen histologic diagnosis is ependymoma, then every attempt must be made to identify a plane, even if one is not initially obvious, and to remove the tumor in total. This is particularly true for large tumors that initially seem infiltrative or that severely compress the surrounding spinal cord such that it is almost unrecognizable. It is sometimes amazing that this thin ribbon of cord tissue can function reasonably well and recover some of its lost function. Therefore, even very large and extensive ependymomas should not deter the surgeon from complete removal. Numerous studies have demonstrated that identification of dissection planes is a major factor in determining the completeness of resection and the eventual long-term prognosis for patients with such tumors.24–26
Clinical Presentation
Although intramedullary tumors occur most commonly in adults,21 a significant incidence has been reported in children.27,28 The symptomatology in both age groups is similar. Slowly growing tumors may displace much of the spinal cord substance before becoming symptomatic, but ultimately an end point is reached when compensatory ability fails and marked neurological deterioration develops rapidly. Persistent pain involving the dorsal root dermatomes in the area of tumor involvement is often the signature of an intramedullary neoplasm. Dysfunction of the posterior column may occur in a progressive fashion, with sensory dysesthesias in the arms, torso, and legs, depending on the site of the intraspinal neoplasm. Sacral sparing may be present and is not an invariable finding with intramedullary neoplasms. Lower motor neuron symptoms and signs usually occur at the level of the tumor. Well-defined central cord syndromes, such as those seen in syringomyelia, with disassociated sensory loss and the classic signs of anterior horn cell involvement may be lacking. Children may present with scoliosis. The symptomatology generally is progressive, with few remissions or exacerbations. Symptoms are usually bilateral, but in rare instances neurological abnormalities are confined to one extremity. The duration of symptoms generally is measured in years, although some neoplasms may have histories of 6 months or less.
Diagnostic Evaluation
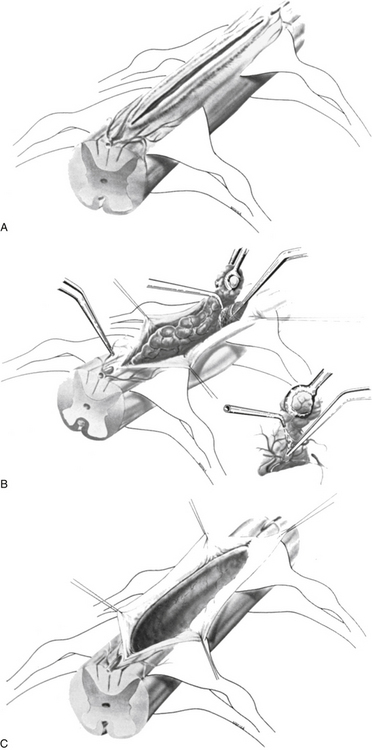
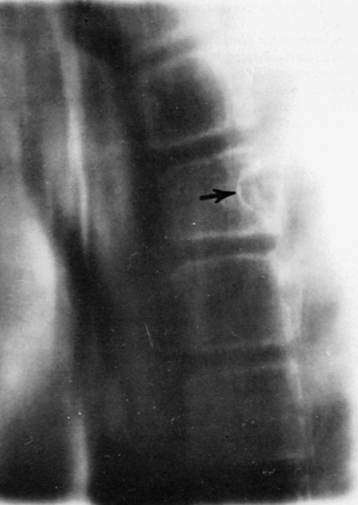
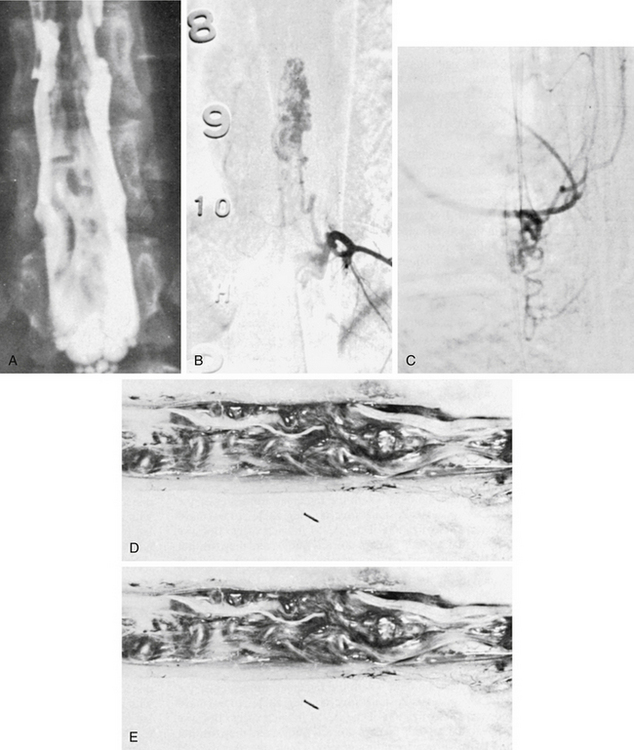
MRI, without or with gadolinium, is the standard of care in the diagnostic evaluation of intramedullary tumors of the spinal cord. A fusiform dilatation of the cord in the region of the tumor, with possible associated rostral and caudal cysts, is a typical finding on T1-weighted images. T2 hyperintensity can be seen in the substance of the tumor as well as in associated syrinx or spinal cord edema. Tumors often enhance with the administration of the gadolinium. Ependymomas are typically more circumscribed and enhanced more uniformly with the contrast, whereas fibrillary astrocytomas are somewhat more diffuse and enhance more irregularly. An ependymoma is likely to be more centrally placed within the spinal cord compared to an astrocytoma and is more often associated with rostral and caudal cysts. Hemangioblastomas present on or close to the surface of the cord enhance brilliantly with contrast material and are nearly always associated with a cyst. They may also be associated with syrinx, often at a location distant from the tumor itself. Ependymomas are more commonly found in the cervical cord or conus, whereas astrocytomas are seen in the thoracic spinal cord (Fig. 188-2). DTI is an advanced form of MRI tractography that can provide preoperative assessment of tumor respectability and, to some extent, pathological diagnosis.8,9
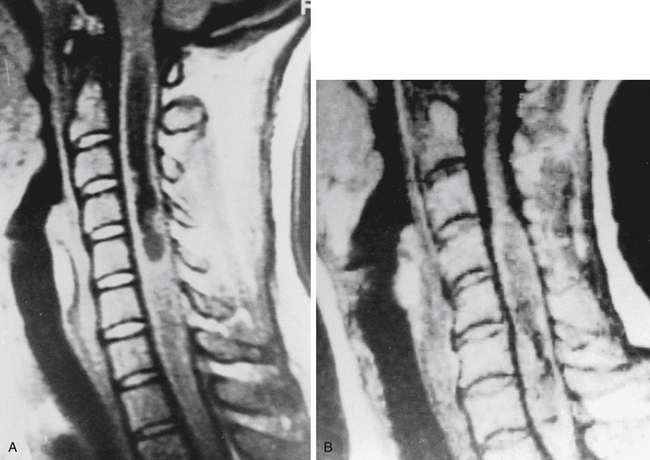
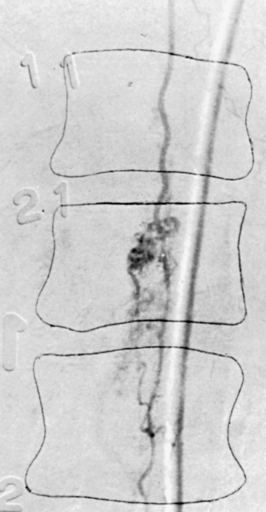
In patients for whom MRI may be contraindicated, myelography and computed tomographic myelography can be useful in the preoperative assessment. A fusiform dilatation of the cord in the region of the tumor can be seen, rarely with a complete myelographic block (Fig. 188-3). If a complete block is present, contrast may be instilled from the opposite end of the spinal canal to define the complete extent of the tumor. Dilated venous channels at the caudal aspect of small intramedullary tumors suggest the differential diagnosis of a vascular malformation. This differential is rarely a problem with high-definition MRI, and spinal angiography has not been of additional diagnostic value except for the identification of an intramedullary hemangioblastoma or an intramedullary AVM, which can mimic a vascular intramedullary tumor. Angiography has been carried out in the presence of dilated vessels and has been helpful in the diagnosis of hemangioblastomas and AVMs.29
Surgical Pathology
Intramedullary spinal cord tumors account for one third of primary intraspinal neoplasms.21,30,31 Astrocytomas and ependymomas constitute the largest group of intramedullary tumors and occur with approximately equal frequency, although astrocytomas are more common in childhood and ependymomas are more prevalent in the adult population. The tumors are generally low-grade and extend over many segments of the spinal cord. Astrocytomas most commonly appear in the cervical and thoracic regions of the spinal cord, whereas the ependymomas have a higher incidence in the caudal regions because of their prevalence in the conus medullaris and filum terminale.31
Intramedullary ependymomas often have a distinct plane between the neoplasm and spinal cord tissue. These tumors generally are soft and solid and have a pseudocapsule. They are not highly vascular and may have necrotic areas. Astrocytomas usually are infiltrative, with an ill-defined margin between the neoplasm and the normal spinal cord tissue. Where a well-defined margin between the neoplasm and the normal spinal cord exists, a pseudocapsule is found. This variety is similar to the cerebellar astrocytoma, which has a uniformly soft consistency and minimal vascularity sometimes associated with cysts containing yellow fluid high in protein. At times, astrocytomas may appear to have a plane between the tumor and the normal cord tissue, yet pathologically they demonstrate infiltration (Fig. 188-4).
Ependymomas and astrocytomas are the most common intramedullary spinal cord tumors, comprising 80% to 90% of all such tumors.32 In most series, ependymomas are nearly twice as common as astrocytomas. The majority are low-grade, with only uncommon glioblastomas. Hemangiomas are the next most common, with 20% of these being associated with von Hippel-Lindau syndrome. Intramedullary teratomas are rare and generally occur in the lumbosacral region. Intradural metastases and lymphomas are also very rare.
Outcomes
The primary concerns with surgical removal of intramedullary spinal cord tumors are neurological outcome, progression-free survival (PFS), and long-term sequelae such as postoperative kyphotic deformity. Tumor pathology has been shown to correlate with PFS.25 Hemangioblastomas carry the longest PFS rate, with 5-year PFS of 82%, followed by ependymoma with 5-year PFS of 60% to 70%. Astrocytomas have shorter expected PFS, with the likelihood of survival decreasing with increasing WHO astrocytoma grade. For ependymomas and hemangioblastomas, gross total resection correlates with longer PFS. Identification of a surgical plane between neoplastic and normal tissue has frequently been cited as a prognostic factor favoring longer PFS for all tumor histologies.25,33
With respect to acute intraoperative neurological decline, increased patient age and intraoperative change in MEPs have been found to be the best predictors of worsening postoperative function.25 Approximately one third of patients may be expected to experience such a decline, but most return to their preoperative baseline within 1 month. Factors thought to influence the likelihood of progressive postoperative kyphotic deformity include patient age, with incidence much higher in pediatric patients than in the adult population (50% to 80% vs.10%).33,34 An increasing number of laminectomized levels, the extent of facetectomy, cervical spine location, preoperative deformity, and postoperative radiation have all been correlated with an increased likelihood of postoperative deformity.34 Use of laminoplasty rather than laminectomy has not been shown to decrease the likelihood of progressive deformity.33
Spinal Cord Arteriovenous Malformations
AVMs of the spinal cord are relatively rare, being only one tenth as common as cerebral AVMs and one tenth as common as primary spinal neoplasms.35 Multiple different types exist, and they can be categorized according to numerous classification systems. Each can be described by its own demographics and pathophysiology and may require different treatment plans. Many spinal cord vascular malformations are fisutulas rather than truly nidal AVMs.
Anatomy and Terminology
The spinal branch of each intercostal artery divides into dural and radicular arteries after penetrating the outer dural layers covering the nerve roots in the intervertebral foramina. The dural arteries supply blood to the spinal and nerve root dura, and the radicular arteries supply the anterior and posterior nerve roots. At some levels, the segmental artery also gives rise to a medullary branch that penetrates the nerve sheath dura, travels along the nerve roots, and supplies the anterior spinal artery or one of the paired posterolateral spinal arteries.36 The venous drainage of the spinal cord is carried by a rich network of radially oriented veins with anastomotic connections to the coronal plexus and longitudinal veins. These are drained segmentally by radicular veins of varying caliber, which penetrate the dura near the axilla, and drain blood back toward the vena cava.37 In childhood, the spinal cord surface veins generally correspond to the arterial pattern, with predominant anterior and paired dorsolateral vessels, and an additional median longitudinal venous channel exists dorsally.38 In adulthood, progressive ectasia and tortuosity occur, adding complexity to interpretation of the venous anatomy.
In the most commonly used nomenclature system, that developed by Oldfield and Doppman,39,40 spinal AVMs are classified according to vascular and nidal location. Each has its own clinical presentation, epidemiology, and preferred treatment modality. Type I lesions are the most common and represent dural AVFs. These are typically low-flow lesions with an abnormal intradural connection between a dorsal radicular artery and a medullary or radicular vein. Arterialization of the coronal venous plexus outflow leads to intramedullary venous hypertension with concomitant myelopathy. The fistula is often located near the level of the intervertebral foramen and often along the dorsal nerve root dura.
Type II lesions, also known as glomus malformations, are true intraparenchymal AVMs of the spinal cord. They are characterized by a compact nidus located in the spinal cord parenchyma and sometimes extending into an extramedullary component as well. The malformations are angiographically and surgically well-visualized and well-defined41,42 (Figs. 93-5 and 93-6).
Type III lesions, known as juvenile-type or metameric AVMs, are more diffuse, high-flow AVMs and may completely encircle the spinal cord (Fig. 188-5). These lesions often permeate the spinal cord and adjacent vertebral bodies at multiple levels, along with involvement of paraspinal structures, making their obliteration nearly impossible (Fig. 188-5). They have multiple levels of arterial contributions that may be visualized by arteriography. Flow is rapid, and the blood supply of the AVM and the cord may be closely related, making ligation or embolization of supplying arteries too risky to undertake without significant risk to the spinal cord.
Type IV lesions, also known as perimedullary AVFs, were originally described by Djindjian and colleagues38 and later restudied by Heros et al43 and Mourier and colleagues.44 Type IV lesions are now recognized with increasing frequency as angiographic techniques become more precise. Type IV lesions are further subclassified into three subtypes, described by Mourier and colleagues as follows: (1) a single AVF of the conus medullaris or filum terminale fed by the anterior spinal artery, (2) a small group of AVFs of the conus medullaris fed by both anterior and posterior spinal arteries, and (3) a single giant AVF of the cervical or thoracic cord fed by both anterior and posterior spinal arteries. Combining the classification system of Di Chiro and Doppman with that of Mourier and colleagues, we refer to the three categories of type IV lesions as A, B, and C, respectively.
Other vascular lesions of the spinal cord include cavernous angiomas, which are not true AVMs. AVMs of the posterior fossa can have prominent and symptomatic spinal venous drainage45 but are not truly primary spinal arteriovenous lesions. Vertebral artery epidural AVFs are typically posttraumatic lesions and are not discussed further in this chapter.
The classification scheme described above is most commonly used to describe spinal cord AVMs. However, because this system fails to fully describe some lesions that fall between the classifications, other systems have been proposed. Spetzler et al46 proposed a modified classification system for spinal cord AVMs that is centered on anatomical localization. Lesions are first differentiated into fistulas and true nidal AVMs.
Clinical Presentation and Natural History
The clinical presentation of spinal AVMs can be varied and nonspecific. Thus, after disc disease, tumor, and demyelinating disease are excluded, the diagnosis of an AVM may not be considered until considerable time has passed and symptoms have progressed significantly. Initially,47 spinal AVMs were thought to occur most commonly in males older than 40 years of age, primarily in the lower thoracic and thoracolumbar regions. Localized pain was the earliest symptom in one half of patients; lower-extremity weakness in one third; and bowel, bladder, and sexual dysfunction being uncommon early symptoms. By the time of diagnosis, however, 95% of patients had developed leg weakness and 93% had bowel, bladder, or sexual dysfunction. When patients with spinal AVMs were followed to observe the natural history of the AVMs,48 19% of patients were severely disabled by 6 months after presenting with lower-extremity symptoms and 50% were disabled by 3 years. Sixty-eight percent of patients developed bladder-voiding difficulties. With recognition of differences between spinal cord AVFs and different types of AVMs, it became clear that nidal AVMs present at a younger age, more commonly in the cervical and thoracic regions, and with temporal progression different from that of AVFs.49
Spinal AVMs and AVFs produce many symptoms due to compression, venous hypertension, vascular steal, and hemorrhage.49 The specific clinical syndrome depends on the type of lesion. Types II and III lesions are true high-flow AVMs and, like their intracranial counterparts, produce symptoms by causing hemorrhage or arterial steal. Feeding artery aneurysms may be seen (Fig. 188-6).50,51 Accordingly, deficits can be catastrophic and sudden in onset in the case of hemorrhage or insidious and progressive in the case of vascular steal. They present in children and young adults equally between the sexes. Types I and IV lesions typically have an insidious onset in middle age or older, with progressive and relentless deterioration, mainly in men. They are most commonly found in the thoracolumbar region. Pain, lower-extremity weakness, and urinary complaints are the most frequent symptoms. Low-flow type I fistulas present as a result of venous hypertension and concomitant decreased cord perfusion or because of infarction due to venous congestion.52,53
Although spinal AVFs typically present with insidious, progressive symptoms of myelopathy, a small percentage of patients may present with spontaneous intracranial subarachnoid hemorrhage. Typically, this is seen with cervical type I AVFs.54,55 In patients with angiographically negative intracranial subarachnoid hemorrhage, the diagnosis of a spinal dural AVF should be considered. Catheterization of bilateral vertebral arteries should ensure that bilateral proximal vessels are free of fistulas, and MRI of the cervical region should be performed to exclude these uncommon lesions.
Diagnosis
Keeping the possibility of the diagnosis of spinal cord AVM in mind is the key to diagnosis. Patients presenting with myelopathy or pain in whom all other workup has been negative may warrant a search for such lesions. The specific location of pain or symptoms, as well as the age and sex of the patient, may give a clue regarding the specific type of malformation present. Equally important as making the diagnosis is identifying the spinal levels at which the abnormal arteriovenous connections are located, in order to devise a treatment plan. Even localized pain, however, is often not a reliable indication of the levels of involvement.56 On physical examination, two signs are helpful if present. A bruit over the spine is virtually diagnostic57 of a type II or III AVM, and the presence of a cutaneous angioma may help to localize the level of the lesions.58 Definitive diagnostic imaging must be obtained to confirm the diagnosis, however.
The gold standard for diagnostic imaging of spinal AVMs is catheter-based, two-dimensional, subtraction spinal arteriography.59,60 Angiography can confirm the diagnosis, classify the lesion, localize the levels, and assist in treatment planning. It produces images with outstanding spatial and temporal resolution.61–63 Spinal angiography must be comprehensive to be of use in evaluating a spinal AVMs, and bilateral injections should be performed along the extent of the AVM. The critical features that should be angiographically defined are (1) precise definition of the level and location of all the arterial feeders, (2) determination of the predominant venous drainage pattern, (3) detection of venous and arterial aneurysms, and (4) delineation of the anterior and posterior spinal arteries in the region of the AVM. However, spinal angiography carries some risk. Long radiation exposure times, nephrotoxic contrast agents, the amount of time and labor required, and the risk of spinal cord infarction are all known. If there is no myelographic or MRI clue to the location of the AVM, the procedure can be painstaking.
In the past, spinal myelography with iophendylate (Pantopaque) was often diagnostic.47,64 False-positive diagnoses were possible, however, because of webbing or trabeculation of the dorsal arachnoid or occasionally tortuous nerve roots, spinal metastases, and dilated vessels associated with intramedullary tumors (Fig. 188-7). Myelography is now performed with water-soluble contrast agents and, together with computed tomography, sometimes facilitates diagnosis.65 CT myelography can help localize levels to help guide selective spinal angiography.
Despite the great strides in spinal angiography, in some instances, arteriography may fail to define the precise arterial contribution to an extensive juvenile malformation of dilated arterialized veins extending over multiple segments of the spinal cord. In other instances, it may be difficult to tell on the basis of angiography whether the lesion is intra- or extramedullary in location.66 Even superselective angiography may occasionally fail to distinguish dural from intrathecal type IV spinal arteriovenous lesions.67 For these reasons, as well as for its noninvasive nature, MRI and MR angiography (MRA) have more recently been added to the preoperative diagnostic armamentarium. MRA has been found to be able to localize many fistulous connections and to differentiate between intra- and extramedullary components, but it has the limitation of poor temporal resolution.68–70 Diffusion tensor tractography is of limited clinical use for spinal AVMs at this time but has demonstrated that intramedullary components of AVMs may displace, separate, or interrupt the long fiber tracts and thus may be of some use in preoperative planning.71
Treatment
Treatment of spinal AVMs is either surgical or endovascular. Some AVMs may be followed conservatively if the risks of treatment outweigh the benefits. Although the ideal goal is always complete obliteration of a spinal AVM, in many cases partial obliteration over multiple treatments may satisfy the need to relieve symptoms and minimize the risk of injury to the spinal cord. As newer techniques have evolved, the number of cases treated by using endovascular techniques rather than traditional surgery has increased from 50% in 1995 to 66% in 2006.72
Embolization
Endovascular therapeutic technologies have been developed to attempt to improve access to spinal vascular lesions, which can be difficult to access surgically, especially when they are ventral and intramedullary and encased in the bony spinal canal. Endovascular technology has been changing at a rapid pace since its introduction as a therapeutic option for spinal AVMs in the 1960s. The major advancements have been in the development of microcatheters and the introduction of novel embolization substances. Early attempts to treat spinal AVMs were limited by the inability to navigate tortuous vessels with traditional catheters and to select only the small feeding arteries of the malformation itself, preserving the normal arterial supply to the spinal cord. Newer, smaller microcatheters, which can be directed into the microvasculature by using microguidewires or by flow-directed means, continue to evolve and allow for more selective catheterization.73
The earliest studies that evaluated embolization of spinal AVMs produced unfavorable results, in part because of the use of particulate embolic agents such as silk and polyvinyl alcohol (PVA). The development of liquid adhesives such as isobutyl 2-cyanoacrylate and n-butyl cyanoacrylate acrylic glues led to better initial success and lower recanalization rates due to better vessel penetration.74,75 The liquid adhesives carry their own risks, however, including the risk of catheter adhesion, premature venous occlusion, and spinal cord infarction.76 The introduction of newer, nonadhesive liquid embolic substrates, such as Onyx, has shown promising results.76,77 Onyx allows for a slower injection, prolonged injection time, lower risk of catheter adhesion and breakage, and lower risk of premature venous occlusion76 compared to the commonly used liquid adhesives. Embolization techniques are of limited utility when vessels are especially tortuous, small, and numerous and when main feeder arteries share their origin with major contributors to the anterior spinal artery.
Surgical Excision
The cornerstone of treatment for spinal AVMs has traditionally been microsurgical obliteration.61,68,78–83 The lesions lend themselves to surgery when they lie on the dorsal surface of the spinal cord and are thereby accessible (Figs. 188-9 and 188-10; see also Fig. 188-8). The low-flow and low-pressure systems may have limited arterial contribution with a significant venous component that is readily visualized on the surface of the spinal cord. In other cases, the AVM invades portions of the spinal cord. Even in such instances, the AVM occasionally may be removed microsurgically with satisfactory results.41,84–86
The patient is given dexamethasone intravenously just before surgery. Choice of position is left to the surgeon and is usually prone. The surgeon and assistant work together across the operating table. The extent of the lesion as identified by preoperative spinal angiography determines the exposure. The laminectomy should be broad and the dura opened widely with preservation of the arachnoid until the extent of the lesion is determined by visualization through the arachnoid. The operating microscope is always used from the point of dural opening. The arachnoid is then opened widely with small scissors or an arachnoid knife. It may be necessary to gently rotate the cord, cutting the dentate ligament and grasping it to see the lateral supply to the malformation. In many cases of long dorsal AVMs, the malformation may extend over different regions of the spinal cord, and a few cases have been described in which the lesion extended the entire length of the spinal cord. As is frequently described,83,87 it may not be necessary or possible to remove the entire malformation. Interrupting the fistula between the major arterial supply and the arterialized venous system may suffice.
Microbipolar cautery is used with constant irrigation during the occlusion of the AVM vessels. Only the largest vessels are clipped, and, from a practical point of view, this means none, one, or two arteries during the removal of the usual spinal AVM. It is extremely difficult to use the standard metallic clips in the removal of an intramedullary lesion, which must be accomplished by bipolar cautery. Lesions are approached from the arterial side first. The venous flow, even though arterialized, is often slow and can be managed easily should rupture occur on the venous side. We see no advantage in attacking the lesion primarily from the venous side if definitive arterial contributions are easily visualized. The lesion is generally peeled away from the spinal cord while additional arterial contributions are coagulated and divided (see Fig. 188-9). In general, malformations located within the spinal cord are associated with large venous aneurysms, often partially thrombosed. These are typically rather fragile and friable, and it is undesirable to threaten the venous drainage or the aneurysm more than necessary to collapse it, so that it may be removed safely from within the spinal cord.
Lesser surgical procedures such as decompressive laminectomy with or without opening the dura appear to have little or no merit in the treatment of these malformations. In fact, such operative explorations may be detrimental in that they make future definitive surgery more difficult. If the lesion is found to be inoperable and all that can be offered is decompression with opening of the dura, then the dura should be closed with a patch graft of fascia to prevent adhesions to the surface of the spinal cord. If there is a possibility that surgery will be performed in the future, it is mandatory to close the dura, because the adhesions that will otherwise form will preclude subsequent, definitive surgical ventures.
Traditional surgery for AVMs has been evolving with improved technology. Intraoperative angiography has been advocated by some, both as means of improved intraoperative localization and for confirmation of surgical obliteration of the lesion.88,89 However, intraoperative, catheter-based spinal angiography requires additional time, personnel, and resources and poses significant technical challenges. A new technique of intraoperative angiography is the use of indocyanine green (ICG) fluorescent dye.90 In this technique, ICG is injected as a bolus into a peripheral vein and delineates the anatomy of the AVM within seconds.91 All that is needed is the appropriate optical filter attached to the operating microscope.
The use of intraoperative frameless neuronavigation in the surgical treatment of spinal AVMs is expected to advance in an effort to minimize exposure and localize the lesion precisely. Combining preoperative static images with advanced intraoperative images is expected to improve utilization of these techniques for spinal AVMs. Efforts to optimize surgical techniques and minimize injury to the surrounding spinal cord tissue by using microrobotic manipulators are under investigation as well.92
Special Considerations
Type I Malformations: Dural Arteriovenous Fistulas
Type I low-flow dural AVFs can be treated either by surgery or by embolization. Traditional surgery, with identification and obliteration of the superficial dorsal fistula between the radicular artery and vein, has been advocated as preferable because of the relative ease and safety of the procedure, the low recanalization rate, and the initially higher recanalization rate with particle embolization. PVA particle embolization led to low initial obliteration success rates and to recanalization rates as high as 83%.74,84 Embolization with newer liquid adhesives has led to higher initial success rates varying between 30% and 90%, however, with lower recanalization rates below 20%.85 The likelihood of recanalization decreases when penetration of embolic material into the proximal vein is seen. Use of newer agents such as Onyx is expected to improve the outcomes after endovascular treatment, but long-term studies are still lacking. In comparison, one meta-analysis showed that 98% of fistulas treated surgically were obliterated with no recanalization.86 Both surgery and endovascular treatment were associated with few complications.
The large, coiled, arterialized veins associated with these lesions are fed by one or two primary arteries, which are part of the dorsal radiculomedullary system. Previous practice was to gradually sweep the entire malformation off the dorsal aspect of the cord, interrupting all arterial feeders to it until the venous system turned blue. This practice has been all but abandoned in favor of a more limited resection of the AVF in or near the dura,79,87 based on the concept that the arteriovenous connection is in or near the dura. Clinically, this more limited procedure has led to dramatic improvement in many cases, and postoperative arteriographic studies have shown no visualization of the AVM in such cases.
At present, observations at the time of surgery should be considered in determining the extent of the surgery. In general, the major coils of veins do not need to be obliterated. There is some evidence that this stripping procedure may cause compromise of the normal cord blood supply and ischemic changes beyond the area of the malformation. After the stripping of large, coiled veins from the dorsal surface of the cord, the cord appears significantly devascularized (see Fig. 188-10). When the major arterial fistula is interrupted, the circulation has a more appealing appearance, and postoperative angiography demonstrates complete obliteration.67
Type II Malformations: Glomus Malformations
Type II malformations are typically high-flow intramedullary lesions with multiple feeding vessels off the anterior and posterior spinal arteries draining into medullary veins. Although their intramedullary nature makes surgery less attractive and riskier than in type I malformations, they are also less responsive to traditional embolization. Most of the studies evaluating embolization involved the older particle substrates such as PVA and demonstrated moderate success rates with high recanalization rates and symptomatic recurrence after a few months, necessitating multiple repeated embolizations.74,93 Most patients improved or stabilized with these treatments, however, and embolization is sometimes advocated with plans for repeated treatments as needed or as part of preoperative staging to assist with open resection. Long-term results with the use of newer substrates such as Onyx are still pending.76 When technically feasible, resection seems to be the mainstay of treatment for glomus malformations, with some authors reporting 80% to 92% complete resection rates and low morbidity.46,85 Although recurrences were seen, they tended to be asymptomatic, and symptom progression was halted with surgery.
Malformations that are primarily intramedullary are usually glomus in configuration. A rare one will be associated with a long dorsal type of malformation in conjunction with the primary intramedullary lesion. These lesions are managed in the same fashion as removing a glomus from the dorsal or dorsolateral surface of the cord with the additional considerations akin to removal of an intramedullary tumor. The locus of the lesion is visualized as the cord is exposed. A myelotomy is made in a longitudinal direction from the polar aspects of the lesion, allowing visualization of the rostral and caudal margins of the lesion. Dissection is carried carefully around the appropriate margin, depending on the anatomy of the venous drainage and arterial supply as determined by angiography and intraoperative observations. These lesions are preferably approached from the arterial side using bipolar cautery for interruption of the arterial feeders while the malformation is rolled toward the venous pedicle. Occasionally, the venous pedicle is in direct relationship to the major supply from the anterior spinal artery. It should be possible to approach the lesion from the arterial side, and once this is interrupted, the venous pedicle is easy to manage. Clips are rarely used. Sharp dissection is always preferred in the removal, as opposed to a blunt or tearing type of dissection. These lesions are frequently associated with a large aneurysmal dilatation, often partially thrombosed, but still active and thin-walled, predisposing the patient to intraoperative hemorrhage secondary to excessive manipulation. Careful use of a broad bipolar tip under irrigation may be used to shrink venous aneurysms, but care must be taken that the wall is not violated. In many cases, the venous aneurysm may be gently teased out of the cord intact. The venous aneurysm is often on the edge of the shunt component of the malformation. The cavity left by removal of these lesions is similar in size and configuration to that left by the total removal of a large intramedullary tumor. It is remarkable how thin the surrounding cord structure can be and still result in a functional neurological state.
Type III Malformations: Juvenile Malformations
Type III lesions are the most difficult to treat, with high-flow, large malformations typically involving the paraspinal structures. Neurological deficits are secondary to a combination of venous congestion, hemorrhage, spinal cord compression, and vascular steal. Treatment for such lesions is typically considered to be palliative in nature, with the goal of stabilizing the lesion and the symptoms.86,93 Staged endovascular partial treatment of these malformations may serve to reduce the size or compression of the lesion, alleviate venous congestion, decrease flow, or eliminate associated aneurysms.94 Half of patients with partially treated type III AVMs neurologically stabilize or improve, although they may need repeated treatment in the future.76
Type IV Malformations: Perimedullary Malformations
Perimedullary malformations resemble the type I dural AVFs, except that they comprise smaller, perimedullary, intradural fistulas and are divided into types A, B, and C as described above. Because the type A lesions are small, low-flow, and comprise a single small fistula fed by the anterior spinal artery, they can prohibit successful selective catheterization.93 Thus, though endovascular treatment has been described previously,95,96 treatment is typically surgical.97 For types B and C lesions, surgical treatment remains the mainstay, but larger series of endovascular cases are still lacking, and smaller series have shown some success.95,96
Akopov S.E., Schievink W.I. History of spinal cord vascular malformations and their treatment. Semin Cerebrovasc Dis Stroke. 2002;2:178-185.
Ali S., Cashen T.A., Carroll T.J., et al. Time-resolved spinal MR angiography: initial clinical experience in the evaluation of spinal arteriovenous shunts. AJNR Am J Neuroradiol. 2007;28:1806-1810.
Bederson J.B., Spetzler R.F. Pathophysiology of type I spinal dural arteriovenous malformations. BNI Q. 1996;12:23-32.
Biondi A., Merland J.J., Hodes J.E., et al. Aneurysms of spinal arteries associated with intramedullary arteriovenous malformations. II. Results of AVM endovascular treatment and hemodynamic considerations. AJNR Am J Neuroradiol. 1992;13:923-931.
Chaloupka J.C. Future directions in the evaluation and management of spinal cord vascular malformations. Semin Cerebrovasc Dis Stroke. 2002;2:245-256.
Cho K.T., Lee D.Y., Chung C.K., et al. Treatment of spinal cord perimedullary arteriovenous fistula: embolization versus surgery. Neurosurgery. 2005;56:232-241.
Colby G.P., Coon A.L., Sciubba D.M., et al. Intraoperative indocyanine green angiography for obliteration of a spinal dural arteriovenous fistula. J Neurosurg Spine. 2009;11:705-709.
Constantini S., Miller D.C., Allen J.C., et al. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93:183-193.
Corkill R.A., Mitsos A.P., Molyneux A.J. Embolization of spinal intramedullary arteriovenous malformations using the liquid embolic agent, Onyx: a single-center experience in a series of 17 patients. J Neurosurg Spine. 2007;7:478-485.
da Costa L., Dehdashti A.R., terBrugge K.G. Spinal cord vascular shunts: spinal cord vascular malformations and dural arteriovenous fistulas. Neurosurg Focus. 2009;26:E6.
Garcés-Ambrossi G.L., McGirt M.J., Mehta V.A., et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine. 2009;11:591-599.
Karikari I.O., Nimjee S.M., Hodges T.R., et al. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery. 2011;68:188-197.
Koenig E., Thron A., Schrader V., Dichgans J. Spinal arteriovenous malformations and fistulae: clinical, neuroradiological and neurophysiological findings. J Neurol. 1989;236:260-266.
Krings T., Lasjaunias P.L., Hans F.J., et al. Imaging in spinal vascular disease. Neuroimaging Clin N Am.. 2007;17:57-72.
Kucia E.J., Bambakidis N.C., Chang S.W., Spetzler R.F. Surgical technique and outcomes in the treatment of spinal cord ependymomas, part 1: intramedullary ependymomas. Neurosurgery. 2011;68(Suppl 1 Operative):57-63.
Lad S.P., Santarelli J.G., Patil C.G., Steinberg G.K., Boakye M. National trends in spinal arteriovenous malformations. Neurosurg Focus. 2009;26:1-5.
Medel R., Crowley R.W., Dumont A.S. Endovascular management of spinal vascular malformations: history and literature review. Neurosurg Focus. 2009;26:E7.
Narvid J., Hetts S.W., Larsen D., et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008;62:159-166.
Nogueira R.G., Dabus G., Rabinov J.D., et al. Onyx embolization for the treatment of spinal dural arteriovenous fistulae: initial experience with long-term follow-up: technical case report. Neurosurgery. 2009;64:E197-E198.
Sciubba D.M., Liang D., Kothbauer K.F., et al. The evolution of intramedullary spinal cord tumor surgery. Neurosurgery. 2009;65(suppl 6):84-91.
Spetzler R.F., Detwiler P.W., Riina H.A., Porter R.W. Modified classification of spinal cord vascular lesions. J Neurosurg Spine. 2002;96:145-156.
Steinmetz M.P., Chow M.M., Krishnaney A.A., et al. Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery. 2004;55:77-87.
Veznedaroglu E., Nelson P.K., Jabbour P.M., Rosenwasser R.H. Endovascular treatment of spinal cord arteriovenous malformations. Neurosurgery. 2006;59(5 suppl 3):S202-S209.
Yanni D.S., Ulkatan S., Deletis V., et al. Utility of neurophysiological monitoring using dorsal column mapping in intramedullary spinal cord surgery. J Neurosurg Spine. 2010;12:623-628.
1. Church A., Eisendrath D.W. A contribution to spinal cord surgery. Am J Med Sci. 1892;103:403-405.
2. von Eiselsberg A. Vienna Weekly Clinics of Surgery [in German]. 1910;10:375.
3. Elsberg C.A., Beer E. The operability of intramedullary tumors of the spinal cord: a report of two operations with remarks upon the extrusion of the spinal cord. Am J Med Sci. 1911;142:636-647.
4. Elsberg C.A. Diagnosis and Treatment of Surgical Diseases of the Spinal Cord and Its Membranes. Philadelphia, PA: W.B. Saunders; 1916.
5. Kriss T.C., Kriss V.M. History of the operating microscope: from magnifying glass to microneurosurgery. Neurosurgery. 1998;42:899-908.
6. Malis L.I. Electrosurgery and bipolar technology. Neurosurgery. 2006;58:ONS1-ONS12.
7. Norman D., Mills C.M., Brant-Zawadzki M., et al. Magnetic resonance imaging of the spinal cord and canal: potentials and limitations. AJR Am J Roentgenol. 1983;141:1147-1152.
8. Solmaz İ, Önal M.B., Civelek E., et al. Intramedullary lumbar lesion mimicking spinal cord tumor: a case of non-neoplastic intramedullary spinal cord lesion. Eur Spine J. 2010;19(suppl 2):S169-S173.
9. Setzer M., Murtagh R.D., Murtagh F.R., et al. Diffusion tensor imaging tractography in patients with intramedullary tumors: comparison with intraoperative findings and value for prediction of tumor resectability. J Neurosurg Spine. 2010;13:371-380.
10. Vargas M.I., Delavelle J., Jlassi H., et al. Clinical applications of diffusion tensor tractography of the spinal cord. Neuroradiology. 2008;50:25-29.
11. Chandler W.F., Knake J.E. Intraoperative use of ultrasound in neurosurgery. Clin Neurosurg. 1983;31:550-563.
12. Yanni D.S., Ulkatan S., Deletis V., et al. Utility of neurophysiological monitoring using dorsal column mapping in intramedullary spinal cord surgery. J Neurosurg Spine. 2010;12:623-628.
13. Morota N., Deletis V., Constantini S., et al. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery. 1997;41:1327-1336.
14. Kothbauer K.F. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin. 2007;37:407-414.
15. Turnbull F. Intramedullary tumors of the spinal cord. Clin Neurosurg. 1962;8:237-247.
16. Harrop J.S., Ganju A., Groff M., Bilsky M. Primary intramedullary tumors of the spinal cord. Spine. 2009;34(Suppl 22):S69-S77.
17. Kucia E.J., Bambakidis N.C., Chang S.W., Spetzler R.F. Surgical technique and outcomes in the treatment of spinal cord ependymomas, part 1: intramedullary ependymomas. Neurosurgery. 2011;68(Suppl 1 Operative):57-63.
18. Powers S.K., Edwards S.B., Boggan J.E., et al. Use of the argon surgical laser in neurosurgery. J Neurosurg. 1984;60:523-530.
19. Jallo G.I., Kothbauer K.F., Epstein F.J. Contact laser microsurgery. Childs Nerv Syst. 2002;18:333-336.
20. Kothbauer K.F. Neurosurgical management of intramedullary spinal cord tumors in children. Pediatr Neurosurg. 2007;43:222-235.
21. Sciubba D.M., Liang D., Kothbauer K.F., et al. The evolution of intramedullary spinal cord tumor surgery. Neurosurgery. 2009;65(suppl 6):84-91.
22. Nakamura M., Tsuji O., Fujiyoshi K., et al. Cordotomy for patients with thoracic malignant astrocytoma. J Neurosurg Spine. 2010;13:418-423.
23. Ewelt C., Stummer W., Klink B., et al. Cordectomy as final treatment option for diffuse intramedullary malignant glioma using 5-ALA fluorescence-guided resection. Clin Neurol Neurosurg. 2010;112:357-361.
24. Boström A., von Lehe M., Hartmann W., et al. Surgery for spinal cord ependymomas: outcome and prognostic factors. Neurosurgery. 2011;68:302-309.
25. Karikari I.O., Nimjee S.M., Hodges T.R., et al. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery. 2011;68:188-197.
26. Garcés-Ambrossi G.L., McGirt M.J., Mehta V.A., et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine. 2009;11:591-599.
27. Yao K.C., McGirt M.J., Chaichana K.L., et al. Risk factors for progressive spinal deformity following resection of intramedullary spinal cord tumors in children: an analysis of 161 consecutive cases. J Neurosurg. 2007;107(suppl 6):463-468.
28. Epstein F., Epstein N. Surgical treatment of spinal cord astrocytomas of childhood: a series of 19 patients. J Neurosurg. 1982;57:685-689.
29. Yaşargil M.G., Antić J., Laciga R., et al. The microsurgical removal of intramedullary spinal hemangioblastomas: report of twelve cases and a review of the literature. Surg Neurol. 1976;3:141-148.
30. Kan S., Fox A.J., Viñuela F., et al. Delayed CT metrizamide enhancement of syringomyelia secondary to tumor. AJNR Am J Neuroradiol. 1983;4:73-78.
31. Aghayev K., Vrionis F., Chamberlain M.C. Adult intradural primary spinal cord tumors. J Natl Compr Canc Netw. 2011;9:434-437.
32. Chamberlain M.C., Tredway T.L. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep. 2011;11:320-328.
33. Constantini S., Miller D.C., Allen J.C., et al. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93(suppl 2):183-193.
34. Fassett D.R., Clark R., Brockmeyer D.L., Schmidt M.H. Cervical spine deformity associated with resection of spinal cord tumors. Neurosurg Focus. 2006;20:E2.
35. Rodesch G., Lasjaunias P. Spinal cord arteriovenous shunts: from imaging to management. Eur J Radiol. 2003;46:221-232.
36. Thron A.K., Rossberg C., Mironov A. Vascular Anatomy of the Spinal Cord: Neuroradiological Investigations and Clinical Syndromes. New York, NY: Springer-Verlag; 1988.
37. Crock H.V., Yoshizawa H. The Blood Supply of the Vertebral Column and Spinal Cord in Man. New York, NY: Springer; 1977.
38. Djindjian M., Djindjian R., Rey A., et al. Intradural extramedullary spinal arterio-venous malformations fed by the anterior spinal artery. Surg Neurol. 1977;8:85-93.
39. Doppman J.L., Di Chiro G., Ommaya A.K. Selective Arteriography of the Spinal Cord. St. Louis, MO: W.H. Green; 1969.
40. Oldfield E.H., Doppman J.L. Spinal arteriovenous malformations. Clin Neurosurg. 1988;34:161-183.
41. Doppman J.L. The nidus concept for spinal cord arteriovenous malformations: a surgical recommendation based on angiographic observations. Br J Radiol. 1971;44:758-763.
42. Cogen P., Stein B.M. Spinal cord arteriovenous malformations with significant intramedullary components. J Neurosurg. 1983:59471-59478.
43. Heros R.C., Debrun G.M., Ojemann R.G., et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg. 1986;64:134-139.
44. Mourier K.L., Gobin Y.P., George B., et al. Intradural perimedullary arteriovenous fistulae: results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 1993;32:885-891.
45. Mahagne M.H., Rogopoulos A., Paquis P., et al. Intracranial dural arteriovenous fistula with spinal venous drainage [in French]. Rev Neurol (Paris). 1992;148:789-792.
46. Spetzler R.F., Detwiler P.W., Riina H.A., Porter R.W. Modified classification of spinal cord vascular lesions. J Neurosurg Spine. 2002;96:145-156.
47. Aminoff M.J., Logue V. Clinical features of spinal vascular malformations. Brain. 1974;97:197-210.
48. Aminoff M.J., Logue V. The prognosis of patients with spinal vascular malformations. Brain. 1974;97:211-218.
49. Rosenblum B., Oldfield E.H., Doppman J.L., Di Chiro G. Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVM’s in 81 patients. J Neurosurg. 1987;67:795-802.
50. Biondi A., Merland J.J., Hodes J.E., et al. Aneurysms of spinal arteries associated with intramedullary arteriovenous malformations. I. Angiographic and clinical aspects. AJNR Am J Neuroradiol. 1992;13:913-922.
51. Biondi A., Merland J.J., Hodes J.E., et al. Aneurysms of spinal arteries associated with intramedullary arteriovenous malformations. II. Results of AVM endovascular treatment and hemodynamic considerations. AJNR Am J Neuroradiol. 1992;13:923-931.
52. Jellema K., Tijssen C.C., van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129:3150-3164.
53. Koenig E., Thron A., Schrader V., Dichgans J. Spinal arteriovenous malformations and fistulae: clinical, neuroradiological and neurophysiological findings. J Neurol. 1989;236:260-266.
54. Aviv R.I., Shad A., Tomlinson G., et al. Cervical dural arteriovenous fistulae manifesting as subarachnoid hemorrhage: report of two cases and literature review. AJNR Am J Neuroradiol. 2004;25:854-858.
55. Fassett D.R., Rammos S.K., Patel P., et al. Intracranial subarachnoid hemorrhage resulting from cervical spine dural arteriovenous fistulas: literature review and case presentation. Neurosurg Focus. 2009;26:E4.
56. Tobin W.D., Layton D.D. The diagnosis and natural history of spinal cord arteriovenous malformations. Mayo Clin Proc. 1976;51:637-646.
57. Matthews W.B. The spinal bruit. Lancet. 1959;2:1117-1118.
58. Doppman J.L., Wirth F.P.Jr., Di Chiro G., Ommaya A.K. Value of cutaneous angiomas in the arteriographic localization of spinal-cord arteriovenous malformations. N Engl J Med. 1969;281:1440-1444.
59. Doppman J.L., Krudy A.G., Miller D.L., et al. Intraarterial digital subtraction angiography of spinal arteriovenous malformations. AJR Am J Roentgenol. 1983;4:1081-1085.
60. Akopov S.E., Schievink W.I. History of spinal cord vascular malformations and their treatment. Semin Cerebrovasc Dis Stroke. 2002;2:178-185.
61. Cullen S., Krings T., Ozanne A., et al. Diagnosis and endovascular treatment of pediatric spinal arteriovenous shunts. Neuroimaging Clin N Am. 2007;17:207-221.
62. Krings T., Lasjaunias P.L., Hans F.J., et al. Imaging in spinal vascular disease. Neuroimaging Clin N Am. 2007;17:57-72.
63. Narvid J., Hetts S.W., Larsen D., et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008;62:159-166.
64. Wyburn-Mason R. The Vascular Abnormalities and Tumors of the Spinal Cord and Its Membranes. London, UK: Kimpton; 1943.
65. Di Chiro G., Doppman J.L., Wener L. Computed tomography of spinal cord arteriovenous malformations. Radiology. 1977;123:351-354.
66. Kasdon D.L., Wolpert S.M., Stein B.M. Surgical and angiographic localization of spinal arteriovenous malformations. Surg Neurol. 1976;5:279-283.
67. Bederson J.B., Spetzler R.F. Pathophysiology of type I spinal dural arteriovenous malformations. BNI Q. 1996;12:23-32.
68. Ali S., Cashen T.A., Carroll T.J., et al. Time-resolved spinal MR angiography: initial clinical experience in the evaluation of spinal arteriovenous shunts. AJNR Am J Neuroradiol. 2007;28:1806-1810.
69. Mull M., Nijenhuis R.J., Backes W.H., et al. Value and limitations of contrast-enhanced MR angiography in spinal arteriovenous malformations and dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2007;28:1249-1258.
70. Sharma A.K., Westesson P.L. Preoperative evaluation of spinal vascular malformation by MR angiography: how reliable is the technique. Case report and review of literature. Clin Neurol Neurosurg. 2008;110:521-524.
71. Ozanne A., Krings T., Facon D., et al. MR diffusion tensor imaging and fiber tracking in spinal cord arteriovenous malformations: a preliminary study. AJNR Am J Neuroradiol. 2007;28:1271-1279.
72. Lad S.P., Santarelli J.G., Patil C.G., et al. National trends in spinal arteriovenous malformations. Neurosurg Focus. 2009;26:1-5.
73. Chaloupka J.C. Future directions in the evaluation and management of spinal cord vascular malformations. Semin Cerebrovasc Dis Stroke. 2002;2:245-256.
74. Hall W.A., Oldfield E.H., Doppman J.L. Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg. 1989;70:714-720.
75. Niimi Y., Berenstein A. Endovascular treatment of spinal vascular malformations. Neurosurg Clin N Am. 1999;10:47-71.
76. Corkill R.A., Mitsos A.P., Molyneux A.J. Embolization of spinal intramedullary arteriovenous malformations using the liquid embolic agent, Onyx: a single-center experience in a series of 17 patients. J Neurosurg Spine. 2007;7:478-485.
77. Krayenbühl H., Yaşargil M.G., McClintock H.G. Treatment of spinal cord vascular malformations by surgical excision. J Neurosurg. 1969;30:427-435.
78. Luessenhop A.J., Dela Cruz T. The surgical excision of spinal intradural vascular malformations. J Neurosurg. 1969;30:552-559.
79. Ommaya A.K., Di Chiro G., Doppman J.L. Ligation of arterial supply in the treatment of spinal cord arteriovenous malformations. J Neurosurg. 1969;30:679-692.
80. Houdart R., Djindjian R., Hurth M. Vascular malformations of the spinal cord: the anatomic and therapeutic significance of arteriography. J Neurosurg. 1966;24:583-594.
81. Kunc Z., Bret J. Diagnosis and treatment of vascular malformations of the spinal cord. J Neurosurg. 1969;30:436-445.
82. Yaşargil R.W., DeLong W.B., Guarnaschelli J.J. Complete microsurgical excision of cervical extramedullary and intramedullary vascular malformations. Surg Neurol. 1975;4:211-224.
83. Latchaw T.W., Harris R.D., Chou S.N., Gold L.H. Combined embolization and operation in the treatment of cervical arteriovenous malformations. Neurosurgery. 1980;6:131-137.
84. Morgan M.K., Marsh W.R. Management of spinal dural arteriovenous malformations. J Neurosurg. 1989;70:832-836.
85. Narvid J., Hetts S.W., Larsen D., et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008;62:159-166.
86. Steinmetz M.P., Chow M.M., Krishnaney A.A., et al. Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery. 2004;55:77-87.
87. Oldfield E.H., Di Chiro G., Quindlen E.A., et al. Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dura fistula. J Neurosurg. 1983;59:1019-1030.
88. Ayad M., Ulm A.J., Yao T., et al. Real-time image guidance for open vascular neurosurgery using digital angiographic roadmapping. Neurosurgery. 2007;61(suppl 3):55-61.
89. Xia Y., Ishii K., Nakamura M., et al. The validity of intraoperative angiography for the treatment of spinal arteriovenous fistula. J Spinal Disord Tech. 2007;20:442-448.
90. Raabe A., Beck J., Gerlach R., et al. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132-139.
91. Colby G.P., Coon A.L., Sciubba D.M., et al. Intraoperative indocyanine green angiography for obliteration of a spinal dural arteriovenous fistula. J Neurosurg Spine. 2009;11:705-709.
92. Nakamura M., Tamaki N., Tamura S., et al. Image-guided microsurgery with the Mehrkoordinaten Manipulator system for cerebral arteriovenous malformations. J Clin Neurosci. 2000;7(suppl 1):10-13.
93. Hodes J.E., Merland J.J., Casasco A., et al. Spinal vascular malformations: endovascular therapy. Neurosurg Clin N Am. 1999;10:139-152.
94. da Costa L., Dehdashti A.R., terBrugge K.G. Spinal cord vascular shunts: spinal cord vascular malformations and dural arteriovenous fistulas. Neurosurg Focus. 2009;26:E6.
95. Cho K.T., Lee D.Y., Chung C.K., et al. Treatment of spinal cord perimedullary arteriovenous fistula: embolization versus surgery. Neurosurgery. 2005;56:232-241.
96. Oran I., Parildar M., Derbent A. Treatment of slow-flow (type I) perimedullary spinal arteriovenous fistulas with special reference to embolization. AJNR Am J Neuroradiol. 2005;26:2582-2586.
97. Medel R., Crowley R.W., Dumont A.S. Endovascular management of spinal vascular malformations: history and literature review. Neurosurg Focus. 2009;26:E7.