Chapter 97 Management of Shunt Infections
Hydrocephalus is one of the most common conditions in neurosurgical practice. Hydrocephalus prevalence in childhood ranges from 0.5 to 1 per 1,000 children.1,2 In the adult population, initial diagnosis is rather uncommon, and incidence is approximately 3.4 per 100,000.3
Since the introduction of cerebrospinal fluid (CSF) shunts in the 1940s,4 morbidity and mortality rates associated with shunt implantation have decreased significantly, from 50% to between 5% and 10%,5 and many shunted patients can lead a normal life. Shunt malfunction and infection, however, are the most common complications in hydrocephalus management, often having serious sequelae. Furthermore, shunt complications represent a significant cost to healthcare systems and a serious problem. According to recent reports, approximately 27,800 shunt implantation or revision procedures were performed in the United States in 2000 alone.6 Considering that each procedure costs the American healthcare system $35,000 on average and that infected patients represent an estimated 5% to 15% of shunted patients,1,7–9 the number of cases potentially requiring reoperation could range from 1,393.5 to 4,181 and cost between $48.76 million and $146.34 million annually. These patients require longer hospital stays, multiple diagnostic and treatment procedures, and antibiotic therapy, all of which have an impact on the healthcare system and could be averted with the right preventative measures. Moreover, the aforementioned complications are associated with high morbidity and mortality rates and the subsequent sequelae affecting patients for life.10
Several studies have tried to establish why these patients develop infections, and in some cases, the use of strict surgical techniques has reduced the incidence of infections nearly to zero.11
Shunt malfunction is reportedly around 25% to 35% during the first postimplantation year.12,13 Rates of infection are variable, ranging from 5% to 15% of all implanted shunts.1,7–9 Shunt infection can be ascribed to various factors; reviewing them individually assists in understanding how to minimize the risks of serious complications and implement an appropriate treatment.
Etiology of Hydrocephalus
The etiology of hydrocephalus as a risk factor for the development of infections has not been clearly shown. An association between the causal agent and a higher risk for shunt infection has rarely been found,11,14 although a recent review described an association between obstructive hydrocephalus and rate of complications.15
Hydrocephalus following perinatal bleeding has been associated with a higher incidence of infection,16 but we could not establish such an association in our series of 964 operated patients.
Patient Age and Nutritional Status
Many reports stress the importance of age as a risk factor for infectious complications in patients who have a CSF shunt.9,17 The risk of complications is mainly present in infants and elders. A multicenter study analyzing shunt complications in general found that younger children were at a higher risk of complications and malfunction; however, the study fails to specify whether these were infectious or obstructive.18
Nutritional status is yet another significant factor, as undernourished subjects seem to have a higher rate of infectious complications.19 Infant nutrition may also play an important role, and a lower incidence of infection has been described in breastfed infants.20
Surgical Technique
Shunt implantation is a procedure often performed by neurosurgical trainees who have inadequate experience in the technique; this factor may be critical for the development of postoperative infections.21
Many papers have shown that postoperative infection rates may be reduced by using a meticulous surgical technique.7,11,22 Where possible, the procedure should be carried out in a dedicated neurosurgical operating room and be the first procedure of the day. The paramedic personnel involved in the procedure should be trained in prosthesis manipulation and instructed to maximally restrict circulation into and out of the operating room. Entrance to and exit from the operating room would only be allowed in emergency situations; where possible, the number of personnel within the operating room should be restricted to four professionals—an anesthesiologist, a nurse, a surgeon experienced in the management of hydrocephalus, and an assistant. Contaminated elements should be carefully placed away from the sterile sector of the operating room.
We recommend giving prophylactic antibiotic therapy at the time of anesthetic induction, as well as during the first 24 postoperative hours. A meta-analysis of recent reports showed the effectiveness of prophylactic antibiotic therapy in the reduction of infection rates.23 The prophylactic antibiotic drugs scheme to be used should be designed after the bacterial flora prevailing in the healthcare site.
Many studies have investigated the association between antibiotic-impregnated shunt catheters and risk of infection. In vitro results24 reported antibiotic-impregnated catheters afford a lower colonization risk, but reports of studies conducted in humans showed controversial results. Some authors stress the contribution of these devices to lower postoperative infection rates and thus recommend them as an effective tool.25,26 Other reports have found no significant infection rate differences in patients who underwent the implantation of antibiotic-impregnated catheters.23,27 This kind of devices could be used in patients with a history of previous infection or in high-risk cases, but regardless of the circumstance, the previously described perioperative care steps should be followed carefully.
We recommend using forceps for implantation to avoid manipulation of the shunt system. Intraoperative preparation with povidone–iodine is useful, and some authors suggest immersing the shunt in a gentamicin solution bath.11 The implementation of this kind of protocol has already been described by other authors7,11,22 and proved to have high efficacy in preventing infection.
Presentation and Clinical Features of Shunt Infection
Presentation of shunt infections is highly variable, depending on the causal agent, the site of infection, and patient age. Many patients remain asymptomatic,28 whereas others are oligosymptomatic or show signs and symptoms of increased intracranial pressure due to shunt malfunction. In neonates, clinical features of shunt malfunction include bulging fontanelle, irritability, vomiting, fever, and feeding difficulty. In older children and adults, the signs and symptoms tend to be nonspecific, though fever, headache, vomiting, meningism, and abdominal pain may suggest shunt infection. Up to 50% of shunt malfunctions may be explained by an underlying infection.29
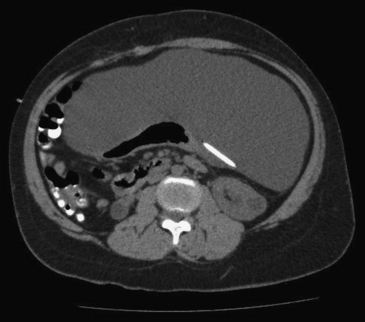
Occasionally, shunt infection signs are seen at the distal end, with abdominal symptoms that may vary from nontender focal peritoneal fluid collections to acute abdomen. The finding of abdominal fluid collections or pseudocysts is considered by many authors to be a sign suggestive of infection in patients with ventriculoperitoneal shunts,30,31 although cystic fluid cultures often test negative.32 Early diagnosis of this complication by means of an abdomen computed tomography (CT) scan (Fig. 97-1) affords an effective management by externalizing the catheter, removing the shunt or re-placing the peritoneal catheter laparoscopically.33,34
Infections associated with ventriculoatrial shunts are usually more severe and may cause bacteremia with a subsequent hemodynamic involvement on account of the close link between CSF and circulating blood flow, which could result in the formation of thrombi at the catheter tip and thus lead to endocarditis or thromboembolic events. Shunt nephritis is a glomerular disease produced by antibody deposits in the renal glomeruli and the production of complement, which is characterized by hematuria, anemia, liver and spleen enlargement, and nephrotic syndrome. Although shunt nephritis has a low incidence, it should be considered a potential diagnosis in patients who have a ventriculoatrial shunt.35
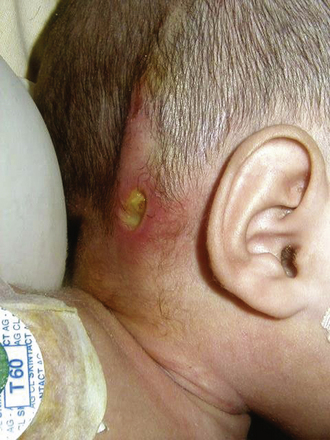
Infections caused by skin organisms may present with redness and edema along the length of the shunt, wound dehiscence and shunt exposure, or purulent discharge through the wound (Fig. 97-2). This kind of infection is commonly external and occurs in neonates when CSF accumulates, particularly when CSF fistulas are present.36 Thus, given the external nature of the infection in these cases, puncturing the valve would be inadvisable because it could lead to germ contamination of a sterile fluid.
Mechanisms for Entry of Bacteria Into Shunts
Because skin organisms are the most commonly identified causal agents in shunt infection, colonization of prosthetic materials by direct inoculation at the time of shunt implantation usually constitutes the infection mechanism. This accounts for the early presentation of infections postoperatively. Skin wounds may also cause shunt colonization. Blood spread may occur in the setting of bacteremia and become particularly important when there is a ventriculoatrial shunt; it may also be seen in patients with neurogenic bladder. Finally, infections by gram-negative organisms in particular may show a retrograde spread as a result of bowel perforation.37
Microbiology and Pathogenesis
CSF shunt infections are most commonly produced by certain low-virulence organisms present in skin flora. Common causal agents are coagulase-negative staphylococci, which are identified in 50% to 80% of shunt infections, together with coagulase-positive staphylococci.38–40 Staphylococci can synthesize and secrete a mucoid substance called slime. Slime has been shown to be associated with vascular catheter infections as it adheres to plastic or polymeric materials and induces organism growth. It may also act as a mechanical barrier to systemic antibiotic action.39,40 Although they are less common, gram-negative organisms are clinically important because they lead to higher morbidity and mortality rates.37,41 Such anaerobic diphtheroid agents as the Propionibacterium acnes are a less common source of infection but should be equally regarded as a potential causal agent, because they are usually found on the skin and can contaminate shunts with few signs and symptoms.41
Diagnosis
CSF sampling for culture is mandatory to establish the diagnosis of infection.
Diagnostic criteria for shunt infection include the following:42–44
1. Positive CSF culture with fluid obtained from the shunt in patients presenting clinically with acute bacterial meningitis or signs or symptoms of malfunction
2. At least one of the following parameters of bacterial inflammation of the CSF:
Wherever possible, and depending on the type of shunt that has been implanted, the sample should be obtained from the valve reservoir. CSF culture is a key element for diagnosis; because of the common presence of low-virulence organisms, cultures should be kept for a long time to allow identification of slow-growing organisms.45 CSF physical and chemical properties may raise clinical suspicion; CSF pleocytosis (particularly when neutrophils predominate), along with increased CSF protein levels and decreased CSF glucose levels, are suggestive of an ongoing bacterial infection.46 Leukocytosis, erythrocyte sedimentation rate (ESR) elevation, and increased C-reactive protein levels may also prove useful for diagnosis (we found that ESR elevation is also useful in adults).
Treatment
Controversy exists over what constitutes the best treatment for ventriculoperitoneal and ventriculoatrial shunt infections. Discussion is raised by whether the system should or should not be removed, since the ventricular catheter may adhere to the choroid plexus and removing the catheter may lead to intraventricular bleeding. Other factors to be taken into account when removing the shunt are patient dependence on the system and potential increase of intracranial pressure. CSF drainage should be ensured at the time of removal. Placement of an external ventricular drainage is associated with a risk for overinfection. Consequently, some authors suggest the implementation of less invasive treatment options, namely, intravenous and/or intrashunt antibiotic therapy.47 Intravenous antibiotic therapy with shunt removal and CSF external drainage or ventricular punctures followed by reimplantation of the shunt when the CSF is sterilized has proved more effective in patients with shunt infection.48 This is the most widely accepted procedure and the only one whose effectiveness has been demonstrated in a prospective randomized study comparing treatment options and a follow-up study.49,50 Other studies have also shown the success of this treatment option,51–54 which is reportedly close to 95%, with the lowest associated morbidity and mortality rates.48
Bacteria such as staphylococci produce vitronectin, a substance that mediates bacterial adhesion to the catheter, preventing antibiotic action and making shunt removal unavoidable.55 Some reports have shown a high cure rate when direct replacement of the shunt is performed and no external drainage is used before the replacement procedure.48
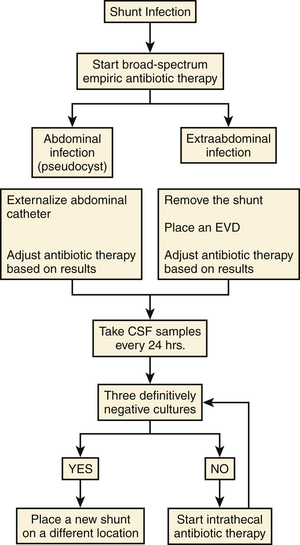
When intravenous antibiotic therapy does not provide successful control of the infection (persistence of positive cultures), the intrathecal route may become an administration alternative (Fig. 97-3).
Brown E., Edwards R., Pople I. Conservative management of patients with cerebrospinal fluid shunt infections. Neurosurgery. 2006;58:657-665.
Brown E.M., de Luvois J., Boyston R., et al. Distinguishing between chemical and bacterial meningitis in patients who have undergone neurosurgery. Clin Infec Dis. 2002;34:556-558.
Choksey M.S., Malik A. Zero tolerance to shunt infections: can it be achieved? J Neurol Neurosurg Psychiatry. 2004;75:87-91.
Choux M., Genitori L., Lang D., et al. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg. 1992;77:875-880.
Christiansen G.D., Simpson W.A., Bisno A.L., et al. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immunol. 1982;37:318-325.
Cochrane D.O., Kestle J.R. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg. 2003;38:295-301.
Desai A., Lollis S., Missios S., et al. How long should cerebrospinal fluid cultures be held to detect shunt infections? J Neurosurg Pediatrics. 2009;4:184-189.
Garner J.S., Jarvis W.R., Emori T.G., et al. CDC definition for nosocomial infection. Am J Infect Control. 1988;16:128-140.
James H.E., Walsh J.W., Wilson H.D., et al. The management of CSF shunt infection: a clinical experience. Acta Neurochir (Wien). 1981;59:157-166.
James H.E., Walsh J.W., Wilson H.D., et al. Prospective randomized study of therapy in cerebrospinal fluid shunt infection. Neurosurgery. 1980;7:459-463.
Jeelani N., Kulkarni A., DeSilva P., et al. Postoperative cerebrospinal fluid wound leakage as a predictor of shunt infection: a prospective analysis of 205 cases. J Neurosurg Pediatrics. 2009;4:166-169.
Lan C.C., Wong T.T., Chen S.J., et al. Early diagnosis of ventriculoperitoneal shunt infections and malfunctions in children with hydrocephalus. J Microbiol lmmunol lnfect. 2003;36:47-50.
Li D.Q., Lundberg F., Ljungh A. Characterization of vitronectin-binding proteins of Staphylococcus epidermidis. Curr Microbiol. 2001;42:361-367.
Nejat F., Tajik P., Ghodsi S.M., et al. Breastfeeding: a potential protective factor against ventriculoperitoneal shunt infection in young infants. J Neurosurg Pediatr. 2008;1:138-141.
Patwardhan R., Nanda A. Implanted ventricular shunts in the United States: the billion–dollar-a-year cost of hydrocephalus treatment. Neurosurgery. 2005;56:139-145.
Persson E.K., Anderson S., Wiklund L.M., et al. Hydrocephalus in children born in 1999-2002: epidemiology, outcome and ophthalmological findings. Childs Nerv Syst. 2007;23:1111-1118.
Persson E.K., Hagberg G., Uvebrant P. Hydrocephalus prevalence and outcome in a population-based cohort of children born in 1989-1998. Acta Paediatr. 2005;94:726-732.
Pirotte B., Lubansu A., Bruneau M., et al. Sterile surgical technique for shunt placement reduces the shunt infection rate in children: preliminary analysis of a prospective protocol in 115 consecutive procedures. Childs Nerv Syst. 2007;23:1251-1261.
Ratilal B., Costa J., Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatrics. 2008;1:48-56.
Richards H.K., Seeley H.M., Pickard J.D. Efficacy of antibiotic impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. J Neurosurg Pediatrics. 2009;4:389-393.
Schreffler R.T., Schreffler A.J., Wittler R.R. Treatment of cerebrospinal fluid shunt infections: a decision analysis. Pediatr Infect Dis J. 2002;21:632-636.
Shah S., Hall M., Slonim A., et al. A multicentric study of factors influencing cerebrospinal fluid shunt survival in infants and children. Neurosurgery. 2008;62:1095-1103.
Tisell M., Hoglund C., Wikkelso C. National and regional incidence of surgery for adult in Sweden. Acta Neurol Scan. 2005;112:72-75.
Vinchon M., Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22:692-697.
Wu Y., Green N., Wrensh M., et al. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery. 2007;61:557-563.
1. Gardner P., Leipzig T., Phillips P. Infections of central nervous system shunts. Med Clin North Am. 1985;69:297-314.
2. Persson E.K., Anderson S., Wiklund L.M., et al. Hydrocephalus in children born in 1999-2002: epidemiology, outcome and ophthalmological findings. Childs Nerv Syst. 2007;23:1111-1118.
3. Tisell M., Hoglund C., Wikkelso C. National and regional incidence of surgery for adult in Sweden. Acta Neurol Scan. 2005;112:72-75.
4. Hadenius A.M., Hagberg B., Hyttnäs-Bensch K., et al. Congenital hydrocephalus. II. Long-term prognosis of untreated hydrocephalus in infants. Nord Med. 1962;68:1515-1519.
5. Persson E.K., Hagberg G., Uvebrant P. Hydrocephalus prevalence and outcome in a population-based cohort of children born in 1989-1998. Acta Paediatr. 2005;94:726-732.
6. Patwardhan R., Nanda A. Implanted ventricular shunts in the United States: the billion–dollar-a-year cost of hydrocephalus treatment. Neurosurgery. 2005;56:139-145.
7. Choux M., Genitori L., Lang D., et al. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg. 1992;77:875-880.
8. Shurtleff D.B., Stuntz J.T., Hayden P.W. Experience with 1201 cerebrospinal fluid shunt procedures. Pediatr Neurosci. 1985/86;12:49-57.
9. George R., Leibrock L., Ebstein M. Long term analysis of cerebrospinal fluid shunt infections. A 25 years experience. J Neurosurg. 1979;51:804-811.
10. Vinchon M., Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22:692-697.
11. Choksey M.S., Malik A. Zero tolerance to shunt infections: can it be achieved? J Neurol Neurosurg Psychiatry. 2004;75:87-91.
12. Vinchon M., Fichten A., Delestret I., et al. Shunt revision for asymptomatic failure: surgical and clinical results. Neurosurgery. 2003;52:347-353.
13. Caldarelli M., Di Rocco C., La Marca F. Shunt complications in the first postoperative year in children with meningomyelocele. Childs Nerv Syst. 1996;12:748-754.
14. Kontny U., Hofling B., Gutjahr P., et al. CSF shunt infections in children. Infection. 1993;21:89-92.
15. Wu Y., Green N., Wrensh M., et al. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery. 2007;61:557-563.
16. Serlo W., Fernell E., Heikkinen E., et al. Functions and complications of shunts in different etiologies of childhood hydrocephalus. Childs Nerv Syst. 1990;6:92-94.
17. McGirt M.J., Zaas A., Fuchs H.E., et al. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36:858-862.
18. Shah S., Hall M., Slonim A., et al. A multicentric study of factors influencing cerebrospinal fluid shunt survival in infants and children. Neurosurgery. 2008;62:1095-1103.
19. Jain G., Mukerji G., Dixit A., et al. The impact of nutritional status on the outcome of Indian patients undergoing neurosurgical shunt surgery. Br J Nut. 2007;98:944-949.
20. Nejat F., Tajik P., Ghodsi S.M., et al. Breastfeeding: a potential protective factor against ventriculoperitoneal shunt infection in young infants. J Neurosurg Pediatr. 2008;1:138-141.
21. Cochrane D.O., Kestle J.R. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg. 2003;38:295-301.
22. Pirotte B., Lubansu A., Bruneau M., et al. Sterile surgical technique for shunt placement reduces the shunt infection rate in children: preliminary analysis of a prospective protocol in 115 consecutive procedures. Childs Nerv Syst. 2007;23:1251-1261.
23. Ratilal B., Costa J., Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatrics. 2008;1:48-56.
24. Bayston R., Lambert E. Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter-related infection. J Neurosurg. 1997;87:247-251.
25. Sciuba D., Lin L., Woodworth G., et al. Factors contributing to the medical costs of cerebrospinal fluid shunt infection treatment in pediatric patients with standard shunt components compared with those in patients with antibiotic impregnated components. Neurosurg Focus. 2007;22:E9.
26. Richards H.K., Seeley H.M., Pickard J.D. Efficacy of antibiotic impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. J Neurosurg Pediatrics. 2009;4:389-393.
27. Ritz R., Roser F., Morgalla M., et al. Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis. 2007;7:38.
28. Conen A., Walti L.N., Merlo A., et al. Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: a retrospective analysis over an 11-year period. Clin Infect Dis. 2008;47:73-82.
29. Walters B.C., Hoffman H.J., Hendrick E.B., et al. Cerebrospinal fluid shunt infection. J Neurosurg. 1984;60:1014-1021.
30. Gaskill S.J., Marlin A.E. Pseudocysts of the abdomen associated with ventriculoperitoneal shunts: a report of twelve cases and a review of the literature. Pediatr Neurosci. 1989;15:23-27.
31. Hahn Y.S., Engelhard H., McLone D. Abdominal CSF pseudocyst: clinical features and surgical management. Pediatr Neurosci. 1985;12:75-79.
32. Roitberg B.Z., Tomita T., McLone O.G. Abdominal cerebrospinal fluid pseudocyst: a complication of ventriculoperitoneal shunt in children. Pediatr Neurosurg. 1998;29:267-273.
33. Kusano T., Miyazato H., Shimoji H., et al. Revision of ventriculoperitoneal shunt under laparoscopic guidance in patients with hydrocephalus. Surg Laparoscop Endosc. 1998;8:474-476.
34. Nfonsam V., Chand B., Rosenblat S., et al. Laparoscopic management of distal ventriculoperitoneal shunt complications. Surg Endosc. 2008;22:1866-1870.
35. Stickler G.B., Shin M.H., Burke E.C., et al. Diffuse glomerulonephritis associated with infected ventriculoatrial shunt. N Engl J Med. 1968;279:1077-1082.
36. Jeelani N., Kulkarni A., DeSilva P., et al. Postoperative cerebrospinal fluid wound leakage as a predictor of shunt infection: a prospective analysis of 205 cases. J Neurosurg Pediatrics. 2009;4:166-169.
37. Stamos J.K., Kaufman B.A., Yogev R. Ventriculoperitoneal shunt infections with gram-negative bacteria. Neurosurgery. 1993;33:858-862.
38. Forward K.R., Fewer H.D., Stiver H.G. Cerebrospinal fluid shunt infections. J Neurosurg. 1983;59:389-393.
39. Christiansen G.D., Simpson W.A., Bisno A.L., et al. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immunol. 1982;37:318-325.
40. Sells C.J., Shurtleff D.B., Loeser J.D. Gram-negative cerebrospinal fluid shunt-associated infections. Pediatrics. 1977;59:614-618.
41. Everett E.D., Eickoff T.C., Simon R.H. Cerebrospinal fluid shunt infections with anaerobic diphtheroids (Propionibacterium species). J Neurosurg. 1976;44:580-585.
42. Brown E.M., de Luvois J., Boyston R., et al. Distinguishing between chemical and bacterial meningitis in patients who have undergone neurosurgery. Clin Infec Dis. 2002;34:556-558.
43. Odio C., McCracken G., Nelson J. CSF shunt infections in pediatrics: a seven year experience. Am J Dis Child. 1984;138:106-109.
44. Garner J.S., Jarvis W.R., Emori T.G., et al. CDC definition for nosocomial infection. Am J Infect Control. 1988;16:128-140.
45. Desai A., Lollis S., Missios S., et al. How long should cerebrospinal fluid cultures be held to detect shunt infections? J Neurosurg Pediatrics. 2009;4:184-189.
46. Lan C.C., Wong T.T., Chen S.J., et al. Early diagnosis of ventriculoperitoneal shunt infections and malfunctions in children with hydrocephalus. J Microbiol lmmunol lnfect. 2003;36:47-50.
47. Brown E., Edwards R., Pople I. Conservative management of Patients with cerebrospinal fluid shunt infections. Neurosurgery. 2006;58:657-665.
48. Schreffler R.T., Schreffler A.J., Wittler R.R. Treatment of cerebrospinal fluid shunt infections: a decision analysis. Pediatr Infect Dis J. 2002;21:632-636.
49. James H.E., Walsh J.W., Wilson H.D., et al. Prospective randomized study of therapy in cerebrospinal fluid shunt infection. Neurosurgery. 1980;7:459-463.
50. James H.E., Walsh J.W., Wilson H.D., et al. The management of CSF shunt infection: a clinical experience. Acta Neurochir (Wien). 1981;59:157-166.
51. Ronan A., Hogg G.G., Klug G.L. Cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 1995;14:782-786.
52. Nelson J.D. Cerebrospinal fluid shunt infections. Pediatr Infect Dis. 1984;3:S30-S32.
53. Swayne R., Rampling A., Newsom S.W. Intraventricular vancomycin for treatment of shunt-associated ventriculitis. J Antimicrob Chemother. 1987;19:249-253.
54. Yogev R. Cerebrospinal fluid shunt infections: a personal view. Pediatr lnfect Ois. 1985;4:113-118.
55. Li D.Q., Lundberg F., Ljungh A. Characterization of vitronectin-binding proteins of Staphylococcus epidermidis. Curr Microbiol. 2001;42:361-367.