Management of Respiratory Failure
Goals of Supportive Therapy for Gas Exchange
Maintenance of Carbon Dioxide Elimination
If the degree of CO2 retention is sufficiently great to cause a marked decrease in the patient’s pH (<7.25–7.30), ventilatory assistance with a mechanical ventilator is often necessary.* Similarly, if marked CO2 retention has impaired the patient’s mental status, ventilatory assistance is indicated. For the patient who has a good chance of rapid reversal of CO2 retention with therapy (assuming the level of CO2 retention is not life threatening), this therapy is often attempted first, with the hope of avoiding mechanical ventilation.
Maintenance of Oxygenation
However, patients with chronic hypercapnia may be subject to further increases in PCO2 when they receive supplemental O2 (see Chapter 18). If PCO2 rises to an unacceptably high range, the patient may require intubation and assisted ventilation with a mechanical ventilator to maintain an acceptable PCO2. Fortunately, this complication is infrequent with judicious use of supplemental O2.
Mechanical Ventilation
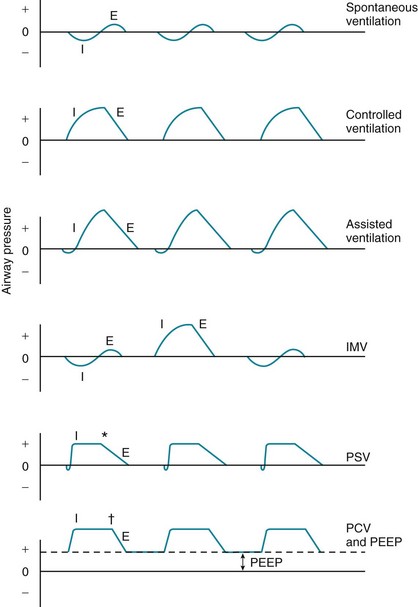
Several ventilatory patterns or modes are available with most mechanical ventilators when used in a volume-cycled fashion (Fig. 29-1). In controlled ventilation, ventilation is supplied entirely by the ventilator at a respiratory rate, tidal volume, and inspired O2 concentration chosen by the physician. If the patient attempts to take a spontaneous breath between the machine-delivered breaths, he or she does not receive any inspired gas. This type of ventilation is uncomfortable for the conscious patient capable of initiating inspiration and therefore can only be used for patients who are comatose, anesthetized, or unable to make any inspiratory effort.
Complications of Mechanical Ventilation
Intubation and mechanical ventilation of patients in respiratory failure are not without risks or complications (Table 29-1). The procedure of intubation can be complicated acutely by problems such as arrhythmias, laryngospasm, and malposition of the endotracheal tube (either in the esophagus or in a mainstem bronchus). When a tube remains in the trachea for days to weeks, complications affecting the larynx and trachea can occur. Vocal cord ulcerations and laryngeal stenosis and granulomas may develop. The trachea is subject to ulcerations, stenosis, and tracheomalacia (degeneration of supporting tissues in the tracheal wall) resulting from pressure applied by the inflated balloon at the end of the tube. As a precaution to decrease tracheal complications, tubes are made with cuffs that minimize the pressure exerted on the tracheal wall and the resulting pressure necrosis. For prolonged ventilatory support (weeks to months), a tracheostomy tube placed directly into the trachea through an incision in the neck has some advantages over prolonged orotracheal or nasotracheal intubation, including patient comfort and prevention of further vocal cord injury.
Table 29-1
COMPLICATIONS OF INTUBATION AND MECHANICAL VENTILATION
ASSOCIATED WITH INTUBATION
ASSOCIATED WITH ENDOTRACHEAL OR TRACHEOSTOMY TUBES
ASSOCIATED WITH MECHANICAL VENTILATION
Administration of positive pressure by a mechanical ventilator has its own attendant problems. Patients receiving positive-pressure ventilation are subject to barotrauma—traumatic changes such as pneumothorax or pneumomediastinum occurring as a result of high alveolar pressures. Because alveolar overdistention with rupture is currently thought to be the cause of these complications, the term volutrauma is now often used instead of barotrauma. Development of a pneumothorax in patients receiving mechanical ventilation can have catastrophic consequences if not detected and treated quickly. The ventilator continues to deliver gas under positive pressure, and the gas enters the pleural space through the rupture. The pressure in the pleural space and thorax increases, and a tension pneumothorax results (see Chapter 15), which severely diminishes venous return and cardiac output and causes rapid cardiovascular collapse. In such situations, a tube, catheter, or needle must be immediately inserted to decompress the pleural space, allow resumption of venous return, and enable reexpansion of the lung.
Concern has been raised that the excessive volumes and high levels of pressure provided to the alveoli may be injurious to alveolar structures. Because lung injury in ARDS is often heterogeneously distributed (see Chapter 28), inspired gas is preferentially distributed to the more normal, more compliant alveoli than to the abnormal, less compliant alveoli. This puts the more normal alveoli at particular risk for overdistention. At the same time, more diseased alveoli are subject to collapse (atelectasis) during the expiratory phase of the respiratory cycle because of intraalveolar fluid and/or a disrupted or insufficient surfactant layer. Alveoli that are open during inspiration but collapse during expiration are subject to abnormal shear stresses during the repetitive process of opening and closing, a complication termed atelectrauma.
Noninvasive Ventilatory Support for Acute Respiratory Failure
When patients with acute respiratory failure require mechanical ventilation, support traditionally has been provided by positive pressure administered through a tube placed into the trachea (i.e., endotracheal tube). However, use of the tube is associated with risks and complications, such as patient discomfort from the tube itself, injury to the airway mucosa, and development of lower respiratory tract infection (see Table 29-1). An alternative form of support for acute respiratory failure does not require use of an endotracheal tube; rather, positive pressure is provided through a tightly fitting mask placed over the face. This approach has been used for support of patients with a variety of types of acute respiratory failure, including patients with cardiogenic pulmonary edema, those with hypercapnic acute exacerbation of chronic obstructive pulmonary disease, and patients who are not considered suitable candidates for intubation. However, noninvasive ventilatory support is not appropriate if patients are unable to protect their airway; it is most useful when respiratory failure most likely is readily reversible and therefore of relatively short duration.
Ambrosino, N, Vagheggini, G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J. 2008;31:874–886.
Boles, J-M, Bion, J, Connors, A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056.
Briel, M, Meade, M, Mercat, A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873.
Caples, SM, Gay, PC. Noninvasive positive pressure ventilation in the intensive care unit: a concise review. Crit Care Med. 2005;33:2651–2658.
Chandra, D, Stamm, JA, Taylor, B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159.
Esan, A, Hess, DR, Raoof, S, et al. Severe hypoxemic respiratory failure: Part 1–Ventilatory strategies. Chest. 2010;137:1203–1216.
Fan, E, Needham, DM, Stewart, TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA. 2005;294:2889–2896.
Garpestad, E, Brennan, J, Hill, NS. Noninvasive ventilation for critical care. Chest. 2007;132:711–720.
Girard, TD, Bernard, GR. Mechanical ventilation in ARDS. A state-of-the-art review. Chest. 2007;131:921–929.
Khirani, S, Georgopoulos, D, Rossi, A, et al. Ventilator support in chronic obstructive pulmonary disease: invasive and noninvasive. Eur Respir Mon. 2006;11(monograph 38):401–429.
Liesching, T, Kwok, H, Hill, NS. Acute applications of noninvasive positive pressure ventilation. Chest. 2003;124:699–713.
Lorente, L, Blot, S, Rello, J. New issues and controversies in the prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2010;182:870–876.
MacIntyre, NR, Cook, DJ, Ely, EW, Jr., et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(Suppl):375S–395S.
Malhotra, A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357:1113–1120.
McConville, JF, Kress, JP. Weaning patients from the ventilator. N Engl J Med. 2012;367:2233–2239.
Pinhu, L, Whitehead, T, Evans, T, et al. Ventilator-associated lung injury. Lancet. 2003;361:332–340.
Putensen, C, Theuerkauf, N, Zinserling, J, et al. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566–576.
Tobin, MJ. Advances in mechanical ventilation. N Engl J Med. 2001;344:1986–1996.
Tobin, MJ, Laghi, F, Jubran, A. Narrative review: ventilator-induced respiratory muscle weakness. Ann Intern Med. 2010;153:240–245.
Vlahakis, NE, Hubmayr, RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med. 2005;171:1328–1342.
Consensus Conference Report. clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation. Chest. 1999;116:521–534.
Hill, NS. Clinical applications of body ventilators. Chest. 1986;90:897.
Howard, RS, Davidson, C. Long-term ventilation in neurogenic respiratory failure. J Neurol Neurosurg Psychiatry. 2003;74(Suppl 3):iii24–30.
Kennedy, JD, Martin, AJ. Chronic respiratory failure and neuromuscular disease. Pediatr Clin North Am. 2009;56:261–273.
MacIntyre, NR, Epstein, SK, Carson, S, et al. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128:3937–3954.
Make, BJ, Hill, NS, Goldberg, AI, et al. Mechanical ventilation beyond the intensive care unit. Report of a consensus conference of the American College of Chest Physicians. Chest. 1998;113(Suppl):289S–344S.
Nelson, JE, Cox, CE, Hope, AA, et al. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454.
Ozsancak, A, D’Ambrosio, C, Hill, NS. Nocturnal noninvasive ventilation. Chest. 2008;133:1275–1286.
Simonds, AK. Home ventilation. Eur Respir J Suppl. 2003;47:38s–46s.
Wallis, C, Paton, JY, Beaton, S, et al. Children on long-term ventilatory support: 10 years of progress. Arch Dis Child. 2011;96:998–1002.
Ahmad, S, Shlobin, OA, Nathan, SD. Pulmonary complications of lung transplantation. Chest. 2011;139:402–411.
Kotloff, RM, Thabut, G. Lung transplantation. Am J Respir Crit Care Med. 2011;184:159–171.
Nicod, LP. Mechanisms of airway obliteration after lung transplantation. Proc Am Thorac Soc. 2006;3:444–449.
Orens, JB, Estenne, M, Arcasoy, S, et al. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755.
Pierson, RN, III. Lung transplantation: current status and challenges. Transplantation. 2006;81:1609–1615.
Solomon, M, Grasemann, H, Keshavjee, S. Pediatric lung transplantation. Pediatr Clin North Am. 2010;57:375–391.
Todd, JL, Palmer, SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140:502–508.
*To initiate ventilatory support, the patient generally is intubated (i.e., a flexible plastic endotracheal tube is placed through the nose or mouth, through the vocal cords, and into the trachea), and a mechanical ventilator is connected to this tube. An alternative method of assisting ventilation, called noninvasive positive-pressure ventilation (NIPPV), is described later in this chapter.