Chapter 10 Management of Recurrent Gliomas
Confirmation of Recurrence
Differential Diagnosis
• A distinctly new tumor may arise at the site of an eradicated tumor. This is more likely to occur if there is a genetic predisposition to tumor development shared by cells in the area; for example, multiple gliomas may occur in a patient with tuberous sclerosis.
• A tumor of related histology may supplant the original tumor; for example, the astrocytic component may replace the oligodendrocytic component as the predominant subtype of a mixed glioma, or a gliosarcoma may arise from a previously treated glioblastoma.
• The initial therapy may induce a secondary tumor of a different type; for example, a glioblastoma in the radiation field of a low-grade glioma.
• Non-neoplastic lesions may mimic tumor growth; for example, an abscess or granuloma at the site of resection of a tumor induced by treatment of the original tumor, or radiation necrosis following focal high-dose irradiation.1,2
Malignant Progression
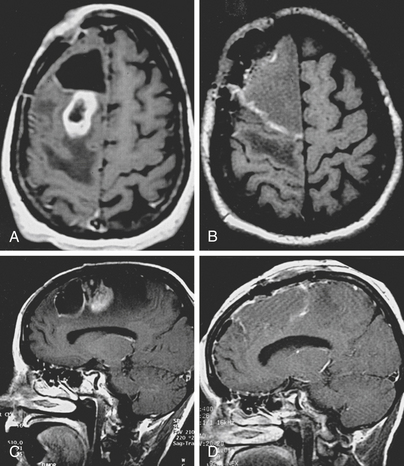
The first scenario is the renewed growth of a low grade tumor. When low grade gliomas regrow after therapy, approximately half remain nonanaplastic, but the other 50% have progressed to a more malignant form.3 Molecular analyses have delineated genetic correlates of this progression.4 Enlarging low grade tumors will usually resemble the original tumor on imaging studies. When progression in grade has occurred, the new tumor may also resemble the old one, especially if the original tumor enhanced with contrast. Enhancement is highly predictive of likelihood of recurrence; low grade enhancing tumors are 6–8 times more likely to recur than nonenhancing ones.3 Most commonly, new malignant growth in a previously nonenhancing glioma enhances and thus is readily identified. In one study, only 30% (16/42) of low-grade tumors enhanced initially, but 92% (22/24) enhanced at recurrence.3 Occasionally, an enlarging malignant focus may not enhance. It might, however, be apparent as a region of hypermetabolism on a 2-deoxyglucose or 11-C methionine PET study, or have an increased rate of enhancement on a dynamic MRI scan, increased activity on a dual-isotope, single-photon emission computerized tomogram (SPECT), or increased choline signal on magnetic resonance spectroscopy (MRS).5–7 The differential specificity of each of these new modalities is approximately 80% to 90%. Usually, however, histologic analysis after biopsy or resection is warranted to verify malignant transformation.8
Radiation Effects
The second scenario that causes diagnostic difficulty is renewed enlargement of a tumor mass following radiation. Often, CT and MR imaging inadequately distinguish recurrent tumor from radiation-induced enlargement. Usually only large, very malignant tumors grow sufficiently fast to show significant enlargement during, or within 3 months of completing, a course of radiation. When this does occur, the prognosis is particularly poor.9
Radiation can cause tumor enlargement in three ways:1 through an early reaction, occurring during or shortly after irradiation, which is likely to be edema;2 through an early delayed reaction arising a few weeks to a few months after radiation which involves edema and demyelination; and3 through a late delayed reaction that occurs 6 to 24 months after radiation and reflects radiation-induced necrosis.10 Regional teletherapy to a dose of 60 Gy is the current standard radiation treatment for most gliomas.11 Although these doses have a low risk of inducing radiation necrosis, regional early and early delayed effects are relatively common. In most cases, tissue swelling represents edema and is transient. Acute symptoms from early and early delayed effects of radiation usually respond quickly to a short course of corticosteroids. The low density, T1 hypointense, T2 hyperintense regions of edema correspond to the area irradiated. Chronically, these volumes of brain will demonstrate parenchymal atrophy, enlargement of subarachnoid spaces, and ex vacuo ventricular dilatation. Dementia with apathy, inanition, and memory loss and decline in fine motor control are the clinical correlates. In the absence of new tumor growth, enhancement on CT and MRI beyond the initial resection margin is infrequent; when it does occur, it is patchy, irregularly marginated, and it can be distinguished from the more focal appearance of recurrent tumor.
In contrast, the late delayed effect of radiation-induced necrosis appears at about the time malignant tumors might be expected to recur.12 It is thus more likely to be mistaken for recurrent tumor growth. The risk of radiation necrosis increases with the volume of tissue treated, the dose delivered, and the fraction size.13 Radiation necrosis following fractionated treatment to doses less than 70 Gy is rare, but it is much more common following brachytherapy or radiosurgery, which deliver high doses of radiation to relatively small volumes over a short time period.12,14,15 A common protocol for brachytherapy is a 50- to 60-Gy boost (to 60 Gy of regional external beam radiotherapy) to a 0- to 5-cm tumor delivered over approximately 1 week. The radiosurgery equivalent is a 10- to 20-Gy boost to a 0- to 3-cm diameter tumor delivered in less than 1 hour.16 Necrosis is radiographically and pathologically evident in almost all cases and symptomatic in about half.
Whether it arises from higher doses of fractionated radiotherapy, brachytherapy, or radiosurgery, radiation necrosis is often difficult to distinguish from recurrent tumor. It forms a ring contrast-enhancing mass that resembles a malignant tumor. It has a CT hypodense, T1 hypointense, T2 hyperintense center; an enhancing annular region; and a hypodense, T1 hypointense, T2 hyperintense surround. The surround corresponds to edema that strikingly radiates along white matter tracts. The similarity of this appearance to that of recurrent tumors and the time course of its occurrence frequently necessitates additional measures to differentiate radiation-induced necrosis from recurrent tumor. A variety of functional neurodiagnostic imaging techniques attempt to distinguish between these two possibilities. These include PET scans, SPECT studies, cerebral blood volume mapping, and MRS. Regions of high activity are thought to distinguish recurrent tumor from relatively metabolically inactive and hypovascular radiation necrosis.6 Although specificity in differentiating tumor from radiation necrosis of up to 100% has been claimed, in many cases these studies are inconclusive and the diagnosis is revealed either by the clinical course or by analysis of a pathology specimen.
When an enlarging mass, which is either recurrent tumor, radiation necrosis, or both, becomes symptomatic, corticosteroid therapy is required.17 Up to half the patients receiving brachytherapy and radiosurgery for a malignant glioma develop symptoms that either prove refractory to corticosteroids or require debilitating long-term steroid use.12,18,19 Surgery for resection of an enlarging, symptomatic mass is needed in 20% to 40% of cases following brachytherapy or radiosurgery of a malignant glioma. At reoperation for presumed radiation necrosis following focal radiation treatment of a malignant glioma, necrosis without tumor was found in 5% of cases, tumor alone in 29%, and a mixture of radiation necrosis and tumor in 66%.12 In almost all cases, the tumor that is seen is of reduced viability.20,21
Causes of Recurrence
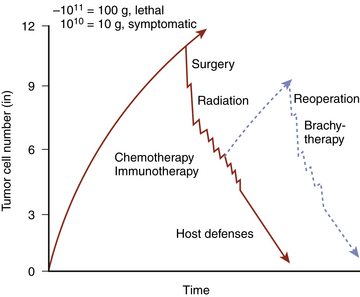
Renewed growth of a brain tumor following surgery and possibly radiation and chemotherapy indicates failure of these therapies to reduce the tumor mass to a size and cell number that would permit its eradication by the patient’s immune system (Fig. 10-1).22 Failure arises from a number of factors that limit the efficacy of each modality.
Recurrence after Surgery
Surgery may fail because of anatomic considerations, pathologic features, or errors in judgment or technique. The involvement of critical structures may limit the initial resection: involvement of the optic pathways, diencephalon, internal capsule, brain stem, or eloquent cortex by a glioma often precludes complete removal. Tumor recurrence, despite removal of all macroscopically evident tumor, can occur if there is microscopic infiltration of adjacent structures. Even low grade cerebral gliomas are usually infiltrative. Anaplastic astrocytomas characteristically are widely invasive. Finally, errors in judgment, such as preoperatively underestimating the amount of tumor that can be safely removed or intraoperatively failing to remove tumor that was targeted, result in potentially resectable tumor being left as a nidus of regrowth.
Recurrence after Radiation
Radiation therapy may fail because of inadequate targeting, underutilization of tolerable dose, or radiation resistance of the tumor cells. Proximity to critical anatomical structures can also limit maximal allowable radiation dosage. Furthermore, the correlations between imaging abnormality and tumor extent are incomplete. Pathologic studies have shown that individual tumor cells can be found throughout and even beyond CT hypodense and MRI T2 hyperintense areas of malignant glioma.23,24 For each individual case, the extent of brain invasion from the contrast-enhancing margin of a malignant glioma is incompletely known. The choice of field size for irradiation of such lesions is difficult and relies as much on the trade-off between target volume and tolerable dose as on the accurate delineation of tumor boundaries. Failure to include an adequate annulus of tissue about the tumor to accommodate imaging uncertainty and technical error may leave tumor cells incompletely irradiated.
Even if the maximal dose tolerated by infiltrative surrounding brain is delivered, tumor cells may remain viable. Hypoxic, nonproliferating cells and tumor stem cells are particularly radioresistant; with time or change in the physiologic conditions following therapy, re-entry of cells into the cell cycle permits the proliferation that results in clinically apparent tumor recurrence.25,26 Analysis of patterns of failure demonstrates that, even with maximal tolerable doses of photon radiation of 70 to 80 Gy, almost all malignant astrocytomas fail centrally.27 A study of high dose fractionated proton irradiation following radical resection of glioblastomas showed the following:1 a dose between 80 and 90 Gy is sufficient to prevent tumor regrowth;2 outside of this high dose volume, tumor regrows, usually in areas receiving between 60 and 70 Gy;3 enlargement of the high dose volume to include more peripheral areas is likely to induce unacceptably high levels of symptomatic radiation-related necrosis.28
Recurrence after Chemotherapy
Chemotherapy fails as a result of inadequate drug delivery, toxicity, or cell resistance. The blood–brain barrier is deficient in the contrast-enhancing region of the tumor, but surrounding brain usually has an intact blood–brain barrier; lipid-insoluble drugs thus have limited access to tumor cells infiltrating peripheral regions. The margin between drug efficacy and neurotoxicity, bone marrow suppression, pulmonary injury, and gastrointestinal side-effects is often narrow. Non-cycling cells and tumor stem cells are resistant to cell-cycle specific drugs, and potentially vulnerable cells often rapidly develop biochemical means of resistance to chemotherapeutic agents.25,26,29
Even if these therapies significantly reduce the tumor burden, the patient’s immune response may be rendered ineffective by chemotherapy and by the tumor’s secretion of factors antagonistic to immune function, such as IL-10, prostaglandin E2, and transforming growth factor (TGF)-beta 2, and its expression of apoptosis-inducing molecules, such as Fas ligand (FasL) and galectin-1.30 Each of these limitations of each component of multimodality therapy may contributes to failure to prevent tumor regrowth. At the time of tumor recurrence, consideration of these reasons for failure is essential to assessment of prognosis and to the choice of subsequent therapy.
Prognostic Implications of Residual and Recurrent Tumor
Residual Tumor
Cytoreductive surgery is a fundamental part of the treatment of most systemic malignancies.31 In most cases, there is a strong relationship between the extent of resection and outcome. For gliomas, correlation between extent of resection or, more significantly, size of residual tumor, and outcome measures, such as interval to tumor progression and survival, has been strongly suggested by retrospective and prospective series, but not by randomized clinical trials.32 More recently, one study, reported a significant survival advantage when 98% or greater resection is achieved.33
Correlation of survival with extent of resection for low grade gliomas has been suggested by retrospective uncontrolled reviews and comparisons with historical controls.3,34–36 One study of 461 adult patients with low grade cerebral gliomas found that gross total surgical removal correlated with length of survival.37 Another reported median survival duration of 7.4 years following maximal surgical resection. The median survival of a subgroup patients with hemispheric tumors compared favorably (10 years vs. 8 years) with that of a comparable series treated with biopsy and radiation alone.3,35 Additional studies have demonstrated that extensive resection of low grade gliomas delays tumor recurrence.36,38
For high grade gliomas, the correlations between the extent of resection at the initial operation and1 the time to tumor recurrence and2 the duration of patient survival have been disputed.39 Historical reports and reviews of large series have noted the association of survival and extent of resection for both astrocytomas and oligodendrogliomas.11,40–43 Extensive reviews of the literature, however, have failed to locate randomized, controlled clinical trials comparing survival after biopsy with that after radical resection of malignant gliomas.44,45 Nevertheless, the benefit of surgical cytoreduction has been strongly suggested by the following findings:
1. Reviews of multicentered trials have shown that the more complete the resection, the longer the patient lived.46–48
2. In a study of 243 patients, multivariate regression analysis identified extent of resection as an important prognostic factor (p < 0.0001) for survival.49
3. In a retrospective review of 1215 patients with WHO grade III or IV, increasing extent of surgical resection was associated with improved survival independent of age, degree of disability WHO grade, or subsequent treatment modalities used.50
4. Single center studies have confirmed this relationship: in one study containing 21 glioblastomas and 10 anaplastic astrocytomas, median duration of survival after gross total resection was 90 weeks versus only 43 weeks following subtotal resection, and the 2-year survival rates were 19% and 0%, respectively, even though the two groups were well matched for other prognostically significant variables;34,51 in another study, patients with gross total resection of malignant glioma lived longer (76 vs. 19 weeks) than those who underwent only a biopsy, even after correction for tumor accessibility and all other prognostically significant variables52 and one large recent series showed that GTR (>98%) significantly increases the duration of survival.33
5. In two larger series, patients with resected cortical and subcortical grade IV gliomas lived longer (50.6 vs. 33.0 weeks53 and 39.5 vs. 32.0 weeks54 after surgery and radiation than those who underwent biopsy and radiation.
6. Small postoperative tumor volume has been shown to correlate with longer time to tumor progression after surgery55 and longer patient survival.56,57
Recurrent Tumor
Regrowth of tumors after an initial response (diminution or stability) to surgery and radiation therapy is ominous. This is particularly true if the growth is more rapid or more infiltrative than that of the original tumor. Such growth often exhibits changes in the basic biology of the tumor that make it less responsive to subsequent therapy. A short interval between initial treatment and recurrence of symptoms often indicates rapid regrowth and a poor prognosis. Factors to be considered in estimating prognosis include the biology of the tumor (its pathology, growth rate, and invasiveness), its resectability, its prior response to radiation and chemotherapy, and the age and performance status of the patient.58 Estimates of the recurrent tumor’s size, growth rate, invasiveness, and location must be made in assessing its potential for causing both neurologic deficit and death. Reappearance of a slowly growing, well-demarcated frontal convexity oligodendroglioma with deletions of chromosomes 1p and 19q in a middle-aged patient of good neurologic condition after a 10-year interval of postsurgical quiescence clearly carries a prognosis very different from that of diffuse diencephalic spread of a glioblastoma multiforme in an elderly patient with a poor performance status 3 months after treatment with surgery, radiation, and chemotherapy.59
Therapy of Recurrent Glial Tumors
Therapy of Recurrent Gliomas
The choice of therapy of a recurrent glioma is based on a comparison of the natural history of the regrowing tumor with the risk–benefit ratio of potential therapies. Recurrent gliomas warrant aggressive multimodality therapy if the patient is in good neurologic and general medical condition and therapeutic options offer a realistic chance for significant improvement in neurologic status or extension of survival.60
Patterns of Recurrence of Gliomas
When gliomas recur, most do so locally. Historically, more than 80% of recurrent glioblastoma multiforme arose within 2 cm of the original margin of contrast-enhancing tumor.61,62 In one series, over 90% of glioma cases showed recurrence at the original tumor location, while 5% developed multiple lesions after treatment.63 In another study of 36 patients with malignant gliomas receiving 70 to 80 Gy of fractionated radiation, 32 (89%) had central (at least 95% of the recurrent tumor within the volume receiving at least 95% of the maximum dose) or in-field (at least 80% of tumor within this highest dose volume) recurrence, and 3 (8%) had marginal recurrence. Only one (3%) fell predominantly outside the high dose range. Seven patients had multiple sites of recurrence, but only one had a large recurrence outside the high dose volume.27 This tendency to recur locally is a function of tumor cell distribution. There is a gradient of tumor cell density in which tumor cell number decreases rapidly at increasing distances from the contrast-enhancing rim of solid tumor. Thus, although individual tumor cells spread through the brain at great distances from the primary site, there are so many more cells locally that the odds favor local reaccumulation of tumor mass.23,24
Factors contributing to the likelihood of local recurrence include the following:
1. Relative predominance of tumor cell mass in the region
2. Statistical likelihood that a local cell will be the cell that first develops a competitive proliferative advantage
3. Possibility that the physiologic milieu (hypervascularity and increased permeability, disrupted tissue architecture, and paracrine growth factor stimuli) at the site is particularly conducive to regrowth.
As tumor cell proliferation resumes at the initial tumor site, cells again spread rapidly and diffusely. Tumor cell proliferation resumes at distant sites as a result of the influx of these new, mitotically active cells or the renewed growth of cells that spread before the initial treatment.63 Biologic therapeutic agents may also affect the pattern of recurrence; recent experience with bevacizumab (Avastin) suggests that tumors treated with this inhibitor of VEGF are more likely to recur as diffusely infiltrative lesions distant from the original site of tumor.64 Consequently, treatments targeting local recurrence alone will, at best, be briefly palliative. Treatment of tumor recurrence thus usually involves a combination of modalities aimed at both local and distant disease.
Epidemiology of Recurrent Glioblastoma
The heterogeneity in defining recurrence and the variability of treatment algorithms employed at different institutions result in a vague profile of recurrent glioblastoma multiforme.65 In a multi-center trial of reoperation for resection and placement of cavitary biodegradable BCNU-wafer in 222 patients with recurrent glioblastoma and a Karnofsky Performance Score of at least 60, the median interval from initial diagnosis to tumor recurrence was 12 months.66 Among a cohort of 301 patients with GBM, 223 patients had tumor recurrence at a median interval from initial diagnosis of 4.9 months;67 64% of these had a Karnovsky Performance Score greater than 70 at the time of recurrence.
Glioblastoma recurrence is demonstrated on imaging obtained in routine surveillance or in response to new or recurring symptoms. In a questionnaire-based study of patients with recurrent glioblastoma or anaplastic astrocytoma and a KPS >70, self-reported symptoms included fatigue, uncertainty about the future, motor difficulties, drowsiness, communication difficulties, and headache.68 While most symptoms likely reflected tumor recurrence, confounding factors such as radiation necrosis and steroid treatment may have contributed to generalized fatigue, and pain and uncertainty about the future may have resulted from the diagnosis alone, independent of current tumor status. Incoordination, weakness, visual loss, and pain were reported more frequently by patients with recurrent glioblastoma than by those with anaplastic astrocytoma, providing evidence that more aggressive disease will cause greater neurological deficit.
Therapy of Recurrent Malignant Glioma
Choice of therapy for a recurrent glioma must consider the tumor’s current and previous histology, previous treatment, and location and the patient’s age, medical and neurologic conditions, and preferences. An enlarging lesion that was originally a low grade glioma should undergo biopsy (stereotactically or, if resection is anatomically feasible, by open craniotomy) to confirm histology (Fig. 10-2). If the tumor remains low grade and a large part of the lesion can be resected without inflicting significant neurologic deficit, it should be removed; if previously irradiated to significantly less than maximal tolerable dose, the tumor bed and surrounding area should receive fractionated radiotherapy. The longer the interval since the initial radiotherapy, the higher the dose that can be given safely at recurrence. If the tumor is inaccessible to surgery, radiation alone should be prescribed. If a low grade tumor previously irradiated to a maximal tolerable dose recurs as a low grade glioma, it should be resected, if possible. If it is inaccessible, stereotactically delivered focal radiation is an attractive option.69,70
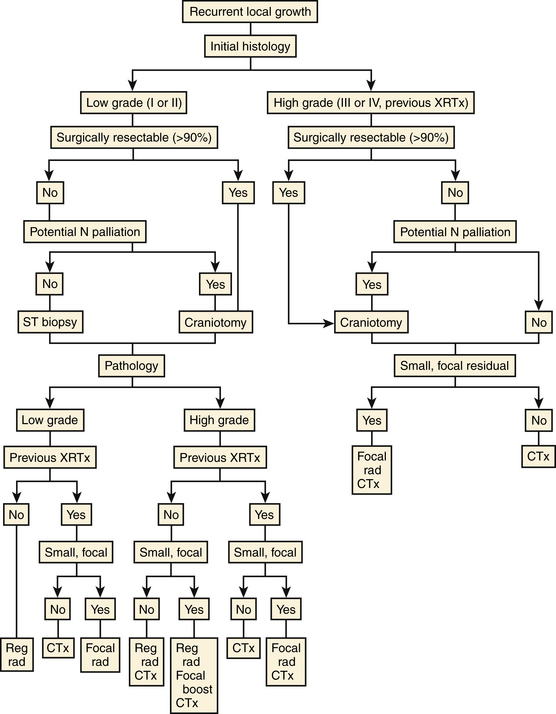
FIGURE 10-2 Recurrent glioma. Decisions in the management of a recurrent tumor should consider grade, resectability, and prior therapy. N, neurologic; reg rad, regional fractionated radiation therapy; focal rad/boost, stereotactic radiosurgery, brachytherapy, or radiotherapy; small, focal, less than 10 cm3, radiographically demarcated; CTx, chemotherapy; ST, stereostatic; XRTx, radiotherapy.
Surgery
If the low grade tumor recurs as a high grade tumor or if a high grade tumor recurs, reoperation should be attempted if the patient has a Karnofsky score of at least 70 and removal of all or almost all of the contrast-enhancing tumor is potentially attainable, or if the tumor mass is causing neurologic symptoms that might be palliated by its reduction. Removal of tumor may improve the patient’s quality of life by alleviating neurologic deficit or permitting reduction of steroid dose. It may also prolong survival by reducing tumor burden and improving response to radiation, chemotherapy, immunotherapy, or biologic therapy.71,72
Radiation Therapy
If the tumor was not irradiated previously, the tumor bed and its annular margin should receive regional radiotherapy. Even when radiotherapy has been used initially, it is an option at recurrence, but doses permitted under standard guidelines for conventional fractionation and volumes are unlikely to be high enough to be effective. Other possibilities include highly conformal conventionally fractionated radiotherapy (e.g., IMRT, intensity modulated radiation therapy), hypofractionated stereotactic radiotherapy, interstitial brachytherapy, and stereotactic radiosurgery.72–76 In one study, the use of highly conformal teletherapy for re-irradiation of recurrent gliomas (mean re-irradiation dose of 38 Gy, range 30.6–59.4 Gy) at a median time of 38 months (range 9–234 months) produced radiographic stability or regression and neurologic improvement in two thirds of the patients.75 In another study, 10 patients with recurrent malignant gliomas treated with intensity-modulated radiation therapy (daily fractions of 5 Gy to a total median dose of 30 Gy) demonstrated median overall survival duration of 10.1 months from the time of treatment, with 1- and 2-year survival rates of 50% and 33%, respectively.76
Hypofractionated stereotactic radiotherapy (SRT) combines high dose per fraction with stereotactic targeting. Hypofractionated SRT (e.g., 20–30 Gy in 2–5-Gy fractions) of recurrent malignant gliomas resulted in a median duration of subsequent survival of 9.3 months (15.4 months for grade III tumors and 7.9 months for grade IV tumors) in one study.77 Another protocol that delivered 20 to 50 Gy in 5-Gy fractions to 29 patients with recurrent high grade astrocytomas resulted in a median duration of survival after retreatment of 11 months.78 Steroid dependent toxicity occurred in 36% of patients, reoperation was required in 6%, and a total dose in excess of 40 Gy predicted radiation damage (p < 0.005). Another study used 24 Gy in 3 Gy fractions, 30 Gy in 3 Gy fractions, and 35 Gy in 3.5 Gy fractions to boost previously irradiated residual or recurrent malignant gliomas at a mean of 3.1 months (range 1–46 months) after standard treatment to 60 Gy. Sixty percent of patients required less steroid and 45% improved neurologically. Eighty percent of those receiving 30 or 35 Gy responded. The median duration of survival was 10.5 months. No grade 3 toxicity occurred. Reoperation was not performed.79 A fourth protocol combined fractionated stereotactic radiosurgery and taxol as a radiosensitizer for recurrent malignant gliomas. It resulted in median survival duration of 14.2 months in 14 selected patients.80 These four studies suggest that hypofractionated SRT has moderate efficacy and acceptable safety in selected patients.
Although several studies suggested promise for brachytherapy in treating glioblastomas, both initially and at the time of recurrence, tumors in these studies were highly selected for small size and focality, two features that would make them appropriate for stereotactic radiosurgery or radiotherapy, less invasive techniques with equivalent results.12,81,82 In a retrospective comparison of interstitial brachytherapy and radiosurgery for recurrent glioblastomas, the median durations of survival in the two groups were similar (11.5 months and 10.2 months, respectively). Patterns of failure were similar. The actuarial risks of reoperation for necrosis at 12 and 24 months were 33% and 48% respectively, after radiosurgery, and 54% and 65%, respectively, after brachytherapy, with the caveat that the brachytherapy group had larger tumors and longer follow-up.83,84
Other forms of focal radiation therapy of recurrent gliomas such as photodynamic therapy (PDT), boron neutron capture therapy (BNCT), intraoperative radiation (IORT), and radiolabeled monoclonal antibodies to tumor cells’ surface receptors have been studied.85–90 A dozen clinical trials have measured the safety and efficacy of irradiation of a tumor’s resection cavity by 131I-labeled mAb 81C6 (anti-tenascin C). Promising results for this technique during initial resection suggest its potential utility during reoperation as well.90
Chemotherapy
Chemotherapy of infiltrative gliomas at diagnosis and at recurrence is often valuable. Temozolamide (Temodar, TMZ), an oral DNA-methylating agent with a benefit-toxicity profile superior to that of antecedent alternative intravenous alkylating agents such as BCNU and CCNU, has become the drug of choice. TMZ is appropriate for those low grade tumors not treated by chemotherapy at the time of initial presentation; one study reported a response rate of 47%.91 If the tumor recurs despite TMZ, a protracted schedule of TMZ administration or strategies using CCNU alone, PCV, bevacizumib (Avastin) alone or bevacizumib combined with other drugs (e.g., Irinotecan) can be given.64,92–95
For Grade III tumors, TMZ is also recommended.96 Perhaps reflective of the favorable biology of the tumor, TMZ of anaplastic oligodendrogliomas with deletions of chromosomes 1p and 19q is particularly effective.59,97 In the initial treatment of patients with GBM, the addition of TMZ to radiotherapy increases the percentage of patients surviving at 2 years to 26.5% from 10.4% for radiotherapy alone and the median duration of survival to 14.6 months from 12.1 months.98 At the time of renewed growth of a high grade tumor, if the tumor has not previously been exposed to TMZ, TMZ should be given. If TMZ had been used but it was discontinued prior to either tumor progression or treatment-limiting toxicity occurring, rechallenge with TMZ or a single nitrosurea agent would be appropriate.99 Patients treated for recurrent or progressive GBM with TMZ showed an overall response rate of 19% and mean time to progression of 11.7 weeks.99 Similarly, treatment of recurrent GBM with a standard TMZ regimen (150 to 200 mg/m2 × 5 days in 28-day cycles) produced a progression-free survival rate of 6 months (PFS-6) of 21% vs. 8% following procarbazine.100 A more intensive regimen (150 mg/m2 daily on a week on/week off cycle) yielded a PFS-6 of 48% with an overall PFS-12 of 81%101 and combinations of TMZ with the matrix metalloproteinase inhibitor, marimastat, or 13-cis-retinoic acid, produced rates of PFS-6 of 39% and 32%, respectively.102 If TMZ was used to treat the primary tumor and toxicity occurred, then an agent with a different toxicity risk profile should be used.95
Limitations of the efficacy of TMZ and related alkylating agents (BCNU, CCNU) reflect cellular drug resistance mechanisms, including the suppression of DNA repair mechanisms. The MGMT cytoprotective repair protein removes TMZ-induced methyl adducts at the O6-guanine in DNA.103,104 Administration of O6-benzlguanine to suppress this DNA repair has increased the cytotoxicity of TMZ in preclinical models.105
Intracavitary implantation of wafers of drug polymers attempts to enhance local drug delivery while avoiding systemic side effects. The initial randomized, double blinded clinical trial with intracavitary BCNU wafers showed longer median survival (31 vs. 23 weeks) and improved survival rates 6 months after treatment of recurrent GBM in the BCNU arm relative to the placebo arm, but the survival curves converged at longer follow-up, and higher rates of symptomatic edema, infection (3.6% vs. 0.9%) and seizures (37.3% vs. 28.6%) were noted.66
Chemotherapy offers little benefit to patients whose tumors recur a second time.106 Nor is multiagent chemotherapy more beneficial than single-agent chemotherapy.107 And hematologic toxicity is worse with more complex combinatorial agents.108–110
For cancer patients, quality of life is an important consideration. Patients suffering from recurrent GBM reported greater satisfaction and a higher health-related quality of life (HRQOL) when treated with TMZ than with PCV.68 Choice of chemotherapy should consider such findings as well as efficacy, toxicity, and cost.
Biologic and Immune Therapies
Delineation of the molecular pathways of glial tumorigenesis has fostered development and testing of small molecule inhibitors and monoclonal antibodies targeting their components. Erlotinib and gefitinib, inhibitors of the epidermal growth factor receptor (EGFR)—a tyrosine kinase amplified or mutated in a high percentage of glioblastomas—are two examples of such molecular-based therapeutic agents. However, recent phase II trials have failed to demonstrate convincing effect. Gefitinib provided rates of progression-free survival at 8 months (PFS-6) of 14% in a prospective study of 28 patients with recurrent or progressive high-grade glioma and of 13% in 53 patients with recurrent glioblastoma.111,112 A randomized, phase II trial conducted by the European Organization for Research and Treatment of Cancer found a PFS-6 of 12%, for patients with recurrent glioblastomas treated with gefitinib compared to a PFS-6 of 24% for the control group treated with either BCNU or TMZ.113 Disappointment over the failure of EGFR inhibitors as single agents has not dimmed optimism that they may be more valuable when used in conjunction with other therapeutic agents.
EGFRvIII, a constitutively active form of the receptor resulting from its most common deletion (found in each 30% of GBM), has been targeted by small inhibitory molecules and monoclonal antibodies specific for the mutated receptor. Tyrphostin, an inhibitor of EGFR more active against the mutated than against the wild-type receptor, has significantly delayed tumor recurrence in animal models and is entering the clinical phase of trials.114 And mAbs raised against the variant receptor have shown antitumor effects in cell culture.115
Since EGFR is an initial component of the PI3Kinase pathway, which is crucial to cell survival, proliferation and motility, tumor cells, when chronically activated by the EGFRvIII mutation, become dependent on PI3k, and thus potentially sensitized to its disruption.116,117 Interestingly, the PTEN tumor–suppressor protein, an inhibitor of the P13K pathway, is often lost in glioblastoma.118 Based on these observations, it has been hypothesized that while possession of the EGFRvIII mutation would sensitize tumors to EGFR kinase inhibitors, loss of PTEN would mitigate this effect by disassociating EGFR inhibition from inhibition of the downstream P13K pathway. Coexpression of EGFRvIII and PTEN at both mRNA and protein levels was significantly associated with a clinical response in glioblastomas treated with EGFR kinase inhibitors.117 Thus, although inhibition of EGFR activity and the PI3K pathway may not be effective against all GBM, it may hold promise against tumors, at diagnosis or at recurrence, selected for having predisposing molecular lesions.
Targeting the tumor’s vasculature is another promising strategy.119 Bevacizumab (Avastin), an inhibitor of vascular endothelial growth factor (VEGF) with antiangiogenic and antiedema effects, rapidly reduces contrast enhancement at the site of tumor. This response is associated with increased time to tumor progression: bevacizumib and irinotecan produced a PFS-6 rate of 46% in patients with recurrent glioblastoma.120 Despite other similarly impressive bevacizumib-induced increases in time to local recurrence of contrast-enhancing tumor, lengthened overall survival has not been observed. This suggests that the rapid, often substantial, reduction of contrast enhancement induced by bevacizumab may reflect decreases in vascular permeability rather than true regression of tumor. Furthermore, there is increasing concern both that the suppression of angiogenesis during treatment is transient and that treatment may favor more diffuse, initially hypoangiogenic growth.64 Despite these concerns, an advisory committee to the Food and Drug Administration recently unanimously recommended approval of bevacizumib for treatment of glioblastoma.
Immunotherapy also holds great promise for patients with malignant gliomas.121 Efforts to enhance the immune response to tumors include both passive and active strategies.122 Passive approaches include implantation of modified immune cells into a resection cavity. One early study employing lymphokine-activated killer (LAK) cells and IL-2 reported a median duration of survival of 53 weeks after reoperation and implantation compared with 26 weeks following reoperation and chemotherapy.123 Another implanting the LAK cells without the IL-2 in 40 patients with recurrent glioblastomas reported a median duration of survival of 9 months and a 1-year survival rate of 34%.124 Adoptive immunotherapy, a variety of passive immunotherapy, is receiving great interest. Tissue harvested at surgery is disaggregated and various constituents (simple lysate, DNA, proteins, etc.) are used to prime dendritic cells harvested from the patient’s blood or tumor.125,126 Active immune strategies employ vaccination. Clinical testing of a vaccine to EGFR vIII has progressed to a phase III trial based on safety and efficacy against newly diagnosed GBM in phase I and II trials.127 Confirmation of the actual clinical benefit of these and other efforts to enhance the immune response awaits completion of phase III trials.
Monoclonal antibodies to other tumor cell surface receptors have also been used to block other components of growth factor signaling pathways (e.g., PDGF), to deliver toxins specifically to tumor cells (e.g., toxin linked to EGF or IL-13), and, as mentioned above, to selectively irradiate tumor sites (e.g., I131-mAb).90,128 For example, Pseudomonas exotoxin has been targeted to the IL-13 receptors highly expressed on malignant glioma cells by injecting the toxin conjugated to IL-13 into the resection cavity of recurrent, malignant glioma. Pseudomonas exotoxin conjugated to TGFalpha also has been investigated in a phase I trial, with relative safety but mixed results in terms of efficacy. Overall, toxin delivery by cytokines has demonstrated relative safety and variable efficacy.
Cells carrying therapeutic genes have also been used. Preliminary gene therapy studies using ganciclovir activated by a thymidine kinase gene delivered by a retrovirus produced by a modified mouse fibroblast packaging cell line have proved feasibility and safety but have not yet achieved proof of mechanism or demonstrated efficacy.129,130 In a small study that examined the effects of an intratumoral injection of retroviral vector-producing cells combined with intravenous ganciclovir, the authors obtained a 1-year survival rate of 25% with tumor response in 50% of the cases.131
Numerous such strategies utilize reoperation to harvest tissue for molecular analysis or to guide tumor specific therapies and as a source of agents for vaccination. Reoperation also presents an opportunity to implant drug polymers, immune stimulants, toxins, viruses, radioisotopes, therapeutic cells, and infusion catheters for subsequent delivery of therapeutic agents (Fig. 10-3).66,123, 128, 129, 131
Reoperation for Malignant Glioma
Rationale for Reoperation
Early reoperation, within months of the initial procedure, might be indicated for complications such as intracerebral, subdural, or epidural hematoma, wound dehiscence and infection, or hydrocephalus and CSF leakage. Occasionally, failure to identify and remove readily accessible tumor mass might warrant reoperation. In the Royal Melbourne Hospital experience, 5 of 200 patients underwent early reoperation.132 More frequently, true tumor recurrence after an interval of response to the initial therapy is the reason for considering reoperation. Reoperation is justified if it produces sustained improvement of neurologic condition and quality of life or significant increase of response rates to adjuvant therapy. Palliation of neurologic symptoms by surgery results from reduction of the local mass effect produced by the tumor and tumor-induced edema. Steroid dose may be able to be reduced and steroid side effects diminished.
Multiple studies have shown that initial surgical cytoreduction of a malignant glioma can both improve neurologic deficits and promote maintenance of high-performance status. One review of 82 patients examined five categories of neurologic function in each patient. Preoperatively, 191 neurologic deficits were noted. Postoperatively, 151 deficits were improved or stable and 40 were worse.133 Another study showed that patients undergoing gross total resection of their malignant gliomas were likely to have improved neurologic function (97% of 36 patients had either improved or stable neurologic examinations), higher functional status (mean KPS score improvement of 6.8%), and extended maintenance of good functional status (185 weeks).34,51 A third study confirmed that more extensive resection was correlated with better immediate postoperative performance, lower 1-month mortality rate, and longer survival: 43% of patients with malignant gliomas improved, 50% remained unchanged, and 7% suffered deterioration in their neurologic condition following resection of at least 75% of their tumor. A more limited resection proved inferior (28% improved, 51% were unchanged, and 21% were worse).49
Similar results can be achieved by reoperation. In one series, 45% of patients had an improved Karnofsky score following reoperation.108 In another series focusing on reoperation, when gross tumor resection was achieved, 82%32,39 of patients had improvement or stability in their Karnofsky score.134 In a third, patients with Karnofsky scores of 50 or less also underwent reoperation. Two-thirds improved from a dependent to an independent state and the median duration of survival was similar to that for all patients undergoing reoperation.135
The doubling rate of malignant gliomas is so high, however, that the benefit from reoperation will be very brief unless adjuvant therapies are able to induce remission of tumor growth. Surgical resection is especially beneficial when reduction of tumor burden improves the response rate to such adjuvant therapies. One early study of multidrug chemotherapy following reoperation for recurrent malignant gliomas used multivariate analysis to identify prognostic factors. During chemotherapy, disease stabilization or partial response occurred in 29 of 51 (57%) patients. Median time to tumor progression was 19 weeks for all pathologies; it was 32 weeks for patients with anaplastic astrocytomas and 13 weeks for those with glioblastoma multiforme. Median survival time was 40 weeks for all pathologies; it was 79 weeks for patients with anaplastic astrocytoma and 33 weeks for those with glioblastoma multiforme. Thirty-five percent of patients had serious chemotoxicity, but none had permanent morbidity or mortality. Factors associated with a longer median time to tumor progression included a higher Karnofsky score, lower grade of initial histology, lack of prior chemotherapy, less myelotoxicity, smaller postoperative tumor volume, greater extent of resection, and a local rather than diffuse pattern of recurrence. Those associated with a longer median survival time were a higher Karnofsky score, anaplastic astrocytoma rather than glioblastoma at recurrence, greater and myelotoxicity, and lobar rather than central location of the tumor.72
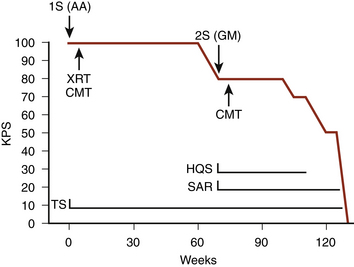
More recent studies from the University of California-San Francisco (UCSF), Memorial Sloan-Kettering, the University of Washington, and Johns Hopkins University have shown that reoperation followed by chemotherapy leads to stabilization of performance score for significant intervals.66,108,136,137 At UCSF, 44% of patients with glioblastomas maintained a performance level of at least 70 (a level consistent with self care and judged to be survival of high quality (58%)) for at least 6 months after reoperation; 18% maintained this level for at least a year; and 3 patients did so for longer than 3 years. Most (52% of 31) patients with anaplastic astrocytomas maintained this performance level for at least 12 months after reoperation; 13% had more than 4 years of high quality survival. Approximately 90% of the survival after reoperation for anaplastic astrocytoma was of high quality136 (Fig. 10-4). In the Memorial Sloan-Kettering group, the median duration of maintenance of independent status (Karnofsky score of at least 80) was 34 weeks. In the Seattle series, patients with a Karnofsky score of at least 70 maintained this high level of function for an average of 37 weeks after reoperation for glioblastoma and for 70 weeks after reoperation for anaplastic astrocytoma.137
A more complete resection of recurrent tumor increases the duration as well as the quality of patient survival.60,134 Support for reoperation is found in comparisons of the outcomes in cases in which different degrees of tumor removal were accomplished and in comparisons of the survival of patients following reoperation with that of historical controls not receiving reoperation. Patients in whom gross total resection of a glioblastoma is achieved survive longer (45.6 vs. 25.6 weeks) than do those receiving near-total or subtotal resections; for anaplastic astrocytomas, the effect of extent of resection is similar (87.5 vs. 55.7 weeks).137 Comparable results were obtained by another group in which the median duration of survival for GBM patients after gross total resection was 76 weeks and for anaplastic astrocytoma was 33 months.138 In the Sloan-Kettering series that grouped glioblastomas and anaplastic astrocytomas together, a similar difference was found (51.2 vs. 23.3 weeks).108 In the UCSF series, survival of patients undergoing reoperation and chemotherapy for either anaplastic astrocytoma or glioblastoma was longer than that of patients receiving chemotherapy alone at the time of tumor recurrence.136
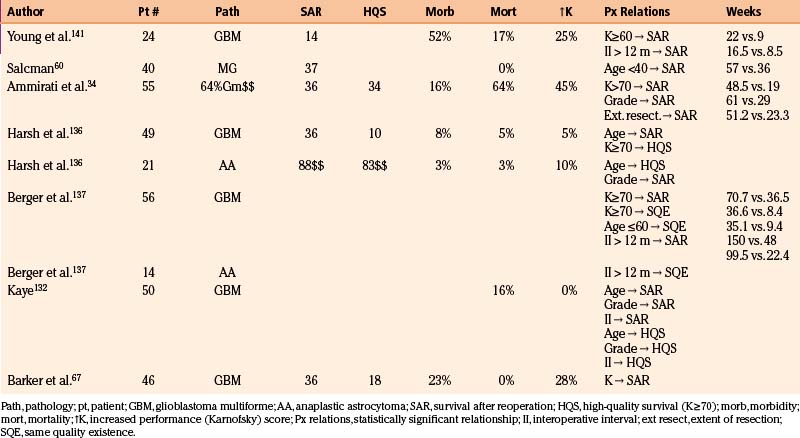
A later study from UCSF67 evaluated 46 patients (15.3%) who underwent reoperation among a group of 301 patients with glioblastoma multiforme. The actuarial rate of reoperation was 15% at 1 year and 31% at 2 years after the initial diagnosis. Patients who were younger (p = 0.01) and who had received an extensive resection initially were more likely to undergo reoperation. KPS scores after reoperation were improved in 28% of patients, unchanged in 49%, and worse in 23%. There was no perioperative mortality. Eight (17.4%) underwent a third operation. A high preoperative KPS score was the only factor predictive of longer survival after reoperation. Similar to the earlier study, the median duration of survival after reoperation was 36 weeks: 61% were alive at 6 months and 24% at 21 months after reoperation. The median period of high-quality survival was 18 weeks (Table 10-1). A subset of 32 patients who underwent reoperation within 45 days of first tumor recurrence was compared with a control group of 141 patients who did not. The median duration of survival after recurrence was 18.7 weeks longer (42.4 vs. 23.7 weeks) in the group undergoing reoperation (p < 0.05, hazard ratio = 0.67, 95% confidence interval = 0.44–1.00) even when controlled for age and KPS score at the time of recurrence. The 13-week difference in survival was potentially due to selection bias. Adjusting for resectability by eliminating from the control group those who underwent biopsy only as the first operation and those whose recurrent tumor was separate from the original tumor reduced the statistical significance of this difference (p = 0.12; hazard ratio = 0.71, 95% confidence interval = 0.46–1.09). The Cox multivariate proportional hazards model adjusted for resectability predicted that a typical 55-year-old man with a KPS score of 80 likely would survive 8 weeks longer (35 vs. 27 weeks) with reoperation than without it. Selection bias was also reduced by stratifying reoperation and control groups by propensity to undergo reoperation. This analysis identified reoperation (p = 0.03, hazard ratio = 0.64, 95% confidence interval = 0.42–0.96) as well as KPS score as statistically significant predictors of longer survival.67
| Definition | Percent | Criteria |
|---|---|---|
| Able to carry on normal activity and to work. No special care is needed | 100 | Normal; no complaints; no evidence of disease. |
| 90 | Able to carry on normal activity; minor signs or symptoms of disease. | |
| 80 | Normal activity with effort; some signs or symptoms of disease. | |
| Unable to work. Able to live at home, care for most personal needs. A varying amount of assistance is needed. | 70 | Cares for self. Unable to carry on normal activity or to do active work. |
| 60 | Requires occasional assistance, but is able to care for most of his or her needs. | |
| 50 | Requires considerable assistance and frequent medical care. | |
| Unable to care for self. Requires equivalent of institutional or hospital care. Disease may be progressing rapidly. | 40 | Disabled; requires special care and assistance. |
| 30 | Severely disabled; hospitalization is indicated although death is not imminent. | |
| 20 | Very sick; hospitalization necessary; active supportive treatment necessary. | |
| 10 | Moribund; fatal processes progressing rapidly. | |
| 0 | Dead. |
From Karnofsky D, Burchenal JH, Armistead GC Jr, et al. Triethylene melamine in the treatment of neoplastic disease. Arch Intern Med. 1951;87:477-516.
The benefit of reoperative surgery is also suggested by experience with brachytherapy. Patients undergoing reoperation for tumor recurrence and/or radiation necrosis following brachytherapy for glioblastomas, either initially or at first recurrence, survived longer than those not receiving reoperation (median total survival of 120 vs. 62 weeks for patients with primarily treated tumors and 90 vs. 37 weeks for patients treated with brachytherapy at the time of first recurrence).12
Reoperation as a part of the multimodality treatment of recurrent gliomas is further supported by study of long-term survivors of glioblastoma multiforme. A review of the UCSF experience identified 22 of 449 (5%) patients with glioblastomas who survived at least 5 years after diagnosis. Sixteen of 22 had tumor recurrence that was treated; and 9 underwent between one and three reoperations. For 8 of the 16 patients with treated recurrence, survival after treatment of recurrence (median of 4.5 years) was longer than the remission produced by the initial treatment.138
The benefit to survival from reoperation is not above dispute. As noted above, multivariate analyses of chemotherapy studies have found that extent of resection and smaller postoperative volume are associated with prolongation of time to tumor progression but not of survival.32 A similar study of survival after progression of malignant gliomas identified high KPS score and age less than 50 years as independent prognostic factors.139 Those who underwent reoperation (58/143 patients) lived longer (median of 35 weeks vs. 16 weeks, p < 0.005 in univariate analysis) after tumor recurrence than those treated without reoperation, but multivariate analysis identified only a trend toward reduction of risk of death (hazard ratio = 0.74; 95% confidence interval = 0.50–1.11; p = 0.014) following reoperation. Randomized controlled trials using prognostic indices for analysis of outcomes are needed to evaluate definitively the benefits of reoperation.140
Selection of Patients for Reoperation
Case selection is critical to outcome. The patient’s profile of prognostic factors, the predicted tolerance of the procedure, and the feasibility of extensive tumor resection without undue risk of new neurologic morbidity must all be considered. Multiple characteristics have been identified as predictive of a good response to reoperation (Table 10-2). Foremost among these are tumor histology, patient age, performance status, interoperative interval, and extent of resection.
The prognostic significance of tumor grade is evident in most series. Median duration of survival after reoperation was 88 weeks for patients with anaplastic astrocytomas but only 36 weeks for those with glioblastomas at UCSF, and 61 weeks and 29 weeks, respectively, at Sloan-Kettering.108,136
The effect of age may overwhelm that of tumor grade. In one series, survival after reoperation was 57 weeks for those younger than 40 years but only 36 weeks for older patients.60 Other authors found an association between youth and total survival duration after diagnosis and between youth and quality of survival after reoperation, but not between youth and duration of survival after reoperation.108,136
Preoperative performance score significantly affects outcome. At Kentucky, median duration of survival after reoperation was 22 weeks for patients with performance scores of at least 70 but only 9 weeks for more disabled patients.141 In the Seattle series, for glioblastomas, survival after reoperation was almost twice as long (71 vs. 36 weeks) for patients with Karnofsky scores of at least 70 than for those with lower scores.137
The prognostic importance of the interval between initial treatment and recurrence is disputed.142 The Kentucky series found survival to be twice as long if the interval between operations exceeded 6 months. In Seattle, a threefold difference (150 vs. 48 weeks for glioblastoma, and 164 vs. 52 weeks for anaplastic astrocytomas) was noted when the time to progression exceeded 3 years.137 Others, however, have found either no relation, or an inverse relation, between the interoperative interval and duration of survival after reoperation.60,108,136
In choosing patients for reoperation and subsequent therapy, consideration of the individual patient’s profile of these prognostically significant factors permits a reasonable estimate of the likelihood of benefit from the procedure. Quality of life is a very important determinant of benefit as perceived by the patient, family, and society. Reoperation and subsequent multimodal therapy should be chosen only if maintenance of a reasonable level of function is anticipated (Figure 10-4).
Reoperative Exposure
In planning the needed exposure, the tumor can be located by its relationship to the margins of the craniotomy and to the cortical pattern of gyri and sulci on the preoperative MRI scan, by intraoperative frameless stereotaxy, or by intraoperative MRI.143,144 The procedure should be planned in advance to ensure adequate skin opening, craniotomy, and durotomy to expose the recurrent mass. All may need enlarging because of the increased extent of the tumor or a desire to perform corticography for mapping of motor or speech function.
Localization of the subcortical extent of the tumor is then undertaken. Again, the preoperative imaging studies and stereotactic techniques are of value.145 Brain shift occurring during dural opening and tumor resection must be considered when using these techniques. Intraoperative MRI or ultrasonography may help correct for this.144,146,147 Tumor may also be found by locating a cystic resection cavity or encephalomalacic brain left after the previous operation. In that almost all tumors recur within 2 cm of the original tumor’s margin, exposure of the initial tumor’s surgical bed will usually reveal at least part of the recurrent mass.
Electrocorticographic mapping of motor, sensory, and speech areas may reduce the chance of inflicting neurologic deficit and may encourage a more extensive resection by revealing the relationship of the site of cortical traverse and of the subsequent subcortical dissection to eloquent brain (Fig. 10-5). This technique is often more difficult at the time of reoperation because of cortical disruption by the tumor and prior surgery.148 However, long term reshapings of language, sensory, and motor maps in some patients have allowed gross total resection of recurrent gliomas without neurologic sequelae.149,150
Once the tumor mass has been removed, the margins of resection should be inspected to verify completeness of the excision. The margins should be free of tumor that is more firm, glassy, opaque, and hypervascular. Biopsies of the surrounding edematous brain should be sent for frozen-section analysis to verify absence of tumor. If solid tumor or tumor infiltrating into noneloquent areas remains, it should be removed. In some cases, extension of tumor into eloquent areas or diencephalic structures will preclude resection of the entire mass. In such cases, the tumor should be divided. This often entails coagulation of numerous strands of small, thin-walled blood vessels. This is particularly true if the extension is in the direction of the vascular supply, such as medial extension of a temporal lobe tumor toward the posterior aspect of the sylvian fissure. Particular care should be taken to coagulate and sharply divide these vessels. Tearing them without prior coagulation will leave a loose end that will retract and continue to bleed. Such loose ends should be directly coagulated rather than tamponaded with hemostatic packing, which may encourage deeper dissection of a hematoma.
Ammirati M., Galicich J.H., Arbit E., Liao Y. Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery. 1987;21:607-614.
Ammirati M., Vick N., Liao Y.L., et al. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987;21:201-206.
Barker F.G.II, Chang S.M., Gutin P.H., et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42:709-720. discussion 709-723
Barker F.G.II, Prados M.D., Chang S.M., et al. Radiation response and survival time in patients with glioblastoma multiforme. J Neurosurg. 1996;84:442-448.
Berger M.S., Tucker A., Spence A., Winn H.R. Reoperation for glioma. Clin Neurosurg. 1992;39:172-186.
Brandes A.A., Tosoni A., Franceschi E., et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27:1275-1279.
Brem H., Piantadosi S., Burger P.C., et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345:1008-1012.
Cairncross J.G., Ueki K., Zlatescu M.C., et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473-1479.
Chandler K.L., Prados M.D., Malec M., Wilson C.B. Long-term survival in patients with glioblastoma multiforme. Neurosurgery. 1993;32:716-720. discussion 720
Coffey R.J., Lunsford L.D., Taylor F.H. Survival after stereotactic biopsy of malignant gliomas. Neurosurgery. 1988;22:465-473.
Fitzek M.M., Thornton A.F., Rabinov J.D., et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91:251-260.
Harsh G.R.IV, Levin V.A., Gutin P.H., et al. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615-621.
Hochberg F.H., Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907-911.
Kelly P.J., Daumas-Duport C., Scheithauer B.W., et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62:450-459.
Kim H.K., Thornton A.F., Greenberg H.S., et al. Results of re-irradiation of primary intracranial neoplasms with three-dimensional conformal therapy. Am J Clin Oncol. 1997;20:358-363.
Liau L.M., Prins R.M., Kiertscher S.M., et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515-5525.
McGirt M.J., Chaichana K.L., Gathinji M., et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156-162.
Nazzaro J.M., Neuwelt E.A. The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg. 1990;73:331-344.
Norden A.D., Young G.S., Setayesh K., et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779-787.
Rostomily R.C., Spence A.M., Duong D., et al. Multimodality management of recurrent adult malignant gliomas: results of a phase II multiagent chemotherapy study and analysis of cytoreductive surgery. Neurosurgery. 1994;35:378-388. discussion 388
Salcman M., Kaplan R.S., Ducker T.B., et al. Effect of age and reoperation on survival in the combined modality treatment of malignant astrocytoma. Neurosurgery. 1982;10:454-463.
Shrieve D.C., Alexander E.III, Wen P.Y., et al. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36:275-282. discussion 282-284
Smith J.S., Tachibana I., Passe S.M., et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246-1256.
Stupp R., Mason W.P., van den Bent M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
Yu J.S., Liu G., Ying H., et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973-4979.
1. Buckley S.C., Broome J.C. A foreign body reaction to Surgicel(R) mimicking an abscess or tumour recurrence. Br J Neurosurg. 1995;9:561-563.
2. Vogelsang J.P., Wehe A., Markakis E. Postoperative intracranial abscess—clinical aspects in the differential diagnosis to early recurrence of malignant glioma. Clin Neurol Neurosurg. 1998;100:11-14.
3. McCormack B.M., Miller D.C., Budzilovich G.N., et al. Treatment and survival of low-grade astrocytoma in adults—1977-1988. Neurosurgery. 1992;31:636-642. discussion 642
4. Ohgaki H., Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445-1453.
5. Wong T.Z., van der Westhuizen G.J., Coleman R.E. Positron emission tomography imaging of brain tumors. Neuroimaging Clin North Am. 2002;12:615-626.
6. Alexiou G.A., Tsiouris S., Polyzoidis K.S., et al. Scintigraphic assessment of recurrent glioma with focus on proliferation. Nucl Med Commun. 2008;29:840-841.
7. Hu L.S., Baxter L.C., Smith K.A., et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR. 2009;30:552-558.
8. Hsu D.W., Louis D.N., Efird J.T., Hedley-Whyte E.T. Use of MIB-1 (Ki-67) immunoreactivity in differentiating grade II and grade III gliomas. J Neuropathol Exp Neurol. 1997;56:857-865.
9. Barker F.G.II, Prados M.D., Chang S.M., et al. Radiation response and survival time in patients with glioblastoma multiforme. J Neurosurg. 1996;84:442-448.
10. Leibel S.A., Sheline G.E. Radiation therapy for neoplasms of the brain. J Neurosurg. 1987;66:1-22.
11. Walker M.D., Alexander E.Jr., Hunt W.E., et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333-343.
12. Scharfen C.O., Sneed P.K., Wara W.M., et al. High activity iodine-125 interstitial implant for gliomas. Int J Radiat Oncol Biol Physics. 1992;24:583-591.
13. Marks J.E., Baglan R.J., Prassad S.C., Blank W.F. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Physics. 1981;7:243-252.
14. Loeffler J.S., Alexander E.III, Hochberg F.H., et al. Clinical patterns of failure following stereotactic interstitial irradiation for malignant gliomas. Int J Radiat Oncol Biol Physics. 1990;19:1455-1462.
15. Loeffler J.S., Alexander E.III, Wen P.Y., et al. Results of stereotactic brachytherapy used in the initial management of patients with glioblastoma. J Natl Cancer Inst. 1990;82:1918-1921.
16. Loeffler J.S., Alexander E.III, Shea W.M., et al. Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol. 1992;10:1379-1385.
17. Edwards M.S., Wilson C.B. Treatment of radiation necrosis. In: Gilbert H.A., Kagan A.R. Radiation Damage to the Nervous System a Delayed Therapeutic Hazard. New York: Raven Press; 1980:120-143.
18. Combs S.E., Widmer V., Thilmann C., et al. Stereotactic radiosurgery (SRS): treatment option for recurrent glioblastoma multiforme (GBM). Cancer. 2005;104:2168-2173.
19. Biswas T., Okunieff P., Schell M.C., et al. Stereotactic radiosurgery for glioblastoma: retrospective analysis. Radiat Oncol (London). 2009;4:11.
20. Daumas-Duport C., Blond S., Vedrenee C., et al. Radiolesion versus recurrence: bioptic data in 30 gliomas after interstitial implant or combined interstitial and external radiation treatment. Acta Neurochir. 1984;33:291-299.
21. Rosenblum ML, Chiu-Liu H, Davis RL, et al. Radiation necrosis versus tumor recurrence following interstitial brachytherapy: utility of tissue culture studies. Presented at American Association of Neurological Surgeons, Atlanta, GA, October 21-25, 1985.
22. Harsh G.R., Wilson C.B. Neuroepithelial tumors in adults. In: Youmans J.R., editor. Neurological Surgery. Philadelphia: W.B. Saunders; 1990:3040-3136.
23. Kelly P.J., Daumas-Duport C., Scheithauer B.W., et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62:450-459.
24. Burger P.C., Heinz E.R., Shibata T., Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988;68:698-704.
25. Hoshino T. A commentary on the biology and growth kinetics of low-grade and high-grade gliomas. J Neurosurg. 1984;61:895-900.
26. Dirks P.B. Cancer: stem cells and brain tumours. Nature. 2006;444:687-688.
27. Lee S.W., Fraass B.A., Marsh L.H., et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Physics. 1999;43:79-88.
28. Fitzek M.M., Thornton A.F., Rabinov J.D., et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91:251-260.
29. Kornblith P.L., Walker M. Chemotherapy for malignant gliomas. J Neurosurg. 1988;68:1-17.
30. Bower R., Lim M., Harsh G.R. Immunotherapy for gliomas: Part I. tumor-induced immunosuppression and cytokine therapy. Contemp Neurosurg. 2007;29:1-6.
31. DeVita V.T.Jr. The James Ewing lecture. The relationship between tumor mass and resistance to chemotherapy. Implications for surgical adjuvant treatment of cancer. Cancer. 1983;51:1209-1220.
32. Rostomily R.C., Spence A.M., Duong D., et al. Multimodality management of recurrent adult malignant gliomas: results of a phase II multiagent chemotherapy study and analysis of cytoreductive surgery. Neurosurgery. 1994;35:378-388. discussion 388
33. Hentschel S.J., Sawaya R. Optimizing outcomes with maximal surgical resection of malignant gliomas. Cancer Control. 2003;10:109-114.
34. Ammirati M., Vick N., Liao Y.L., Ciric I., Mikhael M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987;21:201-206.
35. Vertosick F.T.Jr., Selker R.G., Arena V.C. Survival of patients with well-differentiated astrocytomas diagnosed in the era of computed tomography. Neurosurgery. 1991;28:496-501.
36. Chang E.F., Clark A., Jensen R.L., et al. Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg. 2009;111:203-210.
37. Laws E.R.Jr., Taylor W.F., Clifton M.B., Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61:665-673.
38. Berger M.S., Rostomily R.C. Low grade gliomas: functional mapping resection strategies, extent of resection, and outcome. J Neuro-oncol. 1997;34:85-101.
39. Coffey R.J., Lunsford L.D., Taylor F.H. Survival after stereotactic biopsy of malignant gliomas. Neurosurgery. 1988;22:465-473.
40. Jelsma R., Bucy P.C. Glioblastoma multiforme: its treatment and some factors effecting survival. Arch Neurol. 1969;20:161-171.
41. Chang C.H., Horton J., Schoenfeld D., et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52:997-1007.
42. Nelson D.F., Nelson J.S., Davis D.R., et al. Survival and prognosis of patients with astrocytoma with atypical or anaplastic features. J Neuro-oncol. 1985;3:99-103.
43. Shaw E.G., Scheithauer B.W., O’Fallon J.R., et al. Oligodendrogliomas: the Mayo Clinic experience. J Neurosurg. 1992;76:428-434.
44. Nazzaro J.M., Neuwelt E.A. The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg. 1990;73:331-344.
45. Quigley M.R., Maroon J.C. The relationship between survival and the extent of the resection in patients with supratentorial malignant gliomas. Neurosurgery. 1991;29:385-388. discussion 388-389
46. Shapiro W.R. Treatment of neuroectodermal brain tumors. Ann Neurol. 1982;12:231-237.
47. Wood J.R., Green S.B., Shapiro W.R. The prognostic importance of tumor size in malignant gliomas: a computed tomographic scan study by the Brain Tumor Cooperative Group. J Clin Oncol. 1988;6:338-343.
48. Simpson J.R., Horton J., Scott C., et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Physics. 1993;26:239-244.
49. Vecht C.J., Avezaat C.J., van Putten W.L., et al. The influence of the extent of surgery on the neurological function and survival in malignant glioma. A retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry. 1990;53:466-471.
50. McGirt M.J., Chaichana K.L., Gathinji M., et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156-162.
51. Ciric I., Ammirati M., Vick N., Mikhael M. Supratentorial gliomas: surgical considerations and immediate postoperative results. Gross total resection versus partial resection. Neurosurgery. 1987;21:21-26.
52. Winger M.J., Macdonald D.R., Cairncross J.G. Supratentorial anaplastic gliomas in adults. The prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989;71:487-493.
53. Devaux B.C., O’Fallon J.R., Kelly P.J. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg. 1993;78:767-775.
54. Kreth F.W., Warnke P.C., Scheremet R., Ostertag C.B. Surgical resection and radiation therapy versus biopsy and radiation therapy in the treatment of glioblastoma multiforme. J Neurosurg. 1993;78:762-766.
55. Levin V.A., Hoffman W.F., Heilbron D.C., Norman D. Prognostic significance of the pretreatment CT scan on time to progression for patients with malignant gliomas. J Neurosurg. 1980;52:642-647.
56. Andreou J., George A.E., Wise A., et al. CT prognostic criteria of survival after malignant glioma surgery. AJNR. 1983;4:488-490.
57. Rostomily R.C., Keles G.E., Berger M.S. Radical surgery in the management of low-grade and high-grade gliomas. Baillieres Clin Neurol. 1996;5:345-369.
58. Karnofsky D.A., Burchenal J.H., Armistead G.C.Jr., et al. Triethylene melamine in the treatment of neoplastic disease: a compound with nitrogen-mustardlike activity suitable for oral and intravenous use. JAMA. 1951;87:477-516.
59. Smith J.S., Perry A., Borell T.J., et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636-645.
60. Salcman M., Kaplan R.S., Ducker T.B., et al. Effect of age and reoperation on survival in the combined modality treatment of malignant astrocytoma. Neurosurgery. 1982;10:454-463.
61. Hochberg F.H., Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907-911.
62. Wallner K.E., Galicich J.H., Krol G., et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Physics. 1989;16:1405-1409.
63. Choucair A.K., Levin V.A., Gutin P.H., et al. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg. 1986;65:654-658.
64. Narayana A., Kelly P., Golfinos J., et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110:173-180.
65. Hou L.C., Veeravagu A., Hsu A.R., Tse V.C. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20:E5.
66. Brem H., Piantadosi S., Burger P.C., et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345:1008-1012.
67. Barker F.G.II, Chang S.M., Gutin P.H., et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42:709-720. discussion 720-723
68. Osoba D., Brada M., Prados M.D., Yung W.K. Effect of disease burden on health-related quality of life in patients with malignant gliomas. Neuro-oncology. 2000;2:221-228.
69. Ostertag C.B. Biopsy and interstitial radiation therapy of cerebral gliomas. Ital J Neurol Sci. 1983;2:121-128.
70. Mayer R., Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Physics. 2008;70:1350-1360.
71. Vick N.A., Ciric I.S., Eller T.W., Cozzens J.W., Walsh A. Reoperation for malignant astrocytoma. Neurology. 1989;39:430-432.
72. Brem H., Mahaley M.S.Jr., Vick N.A., et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74:441-446.
73. Arcicasa M., Roncadin M., Bidoli E., et al. Reirradiation and lomustine in patients with relapsed high-grade gliomas. Int J Radiat Oncol Biol Physics. 1999;43:789-793.
74. Hayat K., Jones B., Bisbrown G., Baria K., Pigott T. Retreatment of patients with intracranial gliomas by external beam radiotherapy and cytotoxic chemotherapy. Clin Oncol (R Coll Radiol). 1997;9:158-163.
75. Kim H.K., Thornton A.F., Greenberg H.S., et al. Results of re-irradiation of primary intracranial neoplasms with three-dimensional conformal therapy. Am J Clin OncolAm J Clin Oncol. 1997;20:358-363.
76. Voynov G., Kaufman S., Hong T., et al. Treatment of recurrent malignant gliomas with stereotactic intensity modulated radiation therapy. Am J Clin OncolAm J Clin Oncol. 2002;25:606-611.
77. Vordermark D., Kolbl O., Ruprecht K., et al. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer. 2005;5:55.
78. Shepherd S.F., Laing R.W., Cosgrove V.P., et al. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Physics. 1997;37:393-398.
79. Hudes R.S., Corn B.W., Werner-Wasik M., et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Physics. 1999;43:293-298.
80. Lederman G., Arbit E., Odaimi M., et al. Fractionated stereotactic radiosurgery and concurrent taxol in recurrent glioblastoma multiforme: a preliminary report. Int J Radiat Oncol Biol Physics. 1998;40:661-666.
81. McDermott M.W., Sneed P.K., Gutin P.H. Interstitial brachytherapy for malignant brain tumors. Semin Surg Oncol. 1998;14:79-87.
82. Gaspar L.E., Zamorano L.J., Shamsa F., et al. Permanent 125iodine implants for recurrent malignant gliomas. Int J Radiat Oncol Biol Physics. 1999;43:977-982.
83. Shrieve D.C., Alexander E.III, Wen P.Y., et al. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36:275-282. discussion 282-284
84. Alexander E.III, Loeffler J.S. Radiosurgery for primary malignant brain tumors. Semin Surg Oncol. 1998;14:43-52.
85. Hara A., Nishimura Y., Sakai N., et al. Effectiveness of intraoperative radiation therapy for recurrent supratentorial low grade glioma. J Neuro-oncol. 1995;25:239-243.
86. Muller P.J., Wilson B.C. Photodynamic therapy for recurrent supratentorial gliomas. Semin Surg Oncol. 1995;11:346-354.
87. Popovic E.A., Kaye A.H., Hill J.S. Photodynamic therapy of brain tumors. J Clin Laser Med Surg. 1996;14:251-261.
88. Bigner D.D., Brown M.T., Friedman A.H.II, et al. Iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment of patients with recurrent malignant gliomas: phase I trial results. J Clin Oncol. 1998;16:2202-2212.
89. Chanana A.D., Capala J., Chadha M., et al. Boron neutron capture therapy for glioblastoma multiforme: interim results from the phase I/II dose-escalation studies. Neurosurgery. 1999;44:1182-1192. discussion 1192-1193
90. Reardon D.A., Zalutsky M.R., Bigner D.D. Antitenascin-C monoclonal antibody radioimmunotherapy for malignant glioma patients. Exp Rev Anticancer Ther. 2007;7:675-687.
91. Pace A., Vidiri A., Galie E., et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. 2003;14:1722-1726.
92. Triebels V.H., Taphoorn M.J., Brandes A.A., et al. Salvage PCV chemotherapy for temozolomide-resistant oligodendrogliomas. Neurology. 2004;63:904-906.
93. Kesari S., Schiff D., Drappatz J., et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res. 2009;15:330-337.
94. Norden A.D., Young G.S., Setayesh K., et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779-787.
95. Taillibert S., Vincent L.A., Granger B., et al. Bevacizumab and irinotecan for recurrent oligodendroglial tumors. Neurology. 2009;72:1601-1606.
96. Chinot O.L., Honore S., Dufour H., et al. Safety and efficacy of temozolomide in patients with recurrent anaplastic oligodendrogliomas after standard radiotherapy and chemotherapy. J Clin Oncol. 2001;19:2449-2455.
97. Cairncross J.G., Ueki K., Zlatescu M.C., et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473-1479.
98. Stupp R., Mason W.P., van den Bent M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
99. Brandes A.A., Ermani M., Basso U., et al. Temozolomide in patients with glioblastoma at second relapse after first line nitrosourea-procarbazine failure: a phase II study. Oncology. 2002;63:38-41.
100. Yung W.K., Albright R.E., Olson J., et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588-593.
101. Wick W., Steinbach J.P., Kuker W.M., et al. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62:2113-2115.
102. Jaeckle K.A., Hess K.R., Yung W.K., et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2003;21:2305-2311.
103. Zhang M., Chakravarti A. Novel radiation-enhancing agents in malignant gliomas. Semin Radiat Oncol. 2006;16:29-37.
104. Brandes A.A., Tosoni A., Franceschi E., et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27:1275-1279.
105. Pepponi R., Marra G., Fuggetta M.P., et al. The effect of O6-alkylguanine-DNA alkyltransferase and mismatch repair activities on the sensitivity of human melanoma cells to temozolomide, 1,3-bis(2-chloroethyl)1-nitrosourea, and cisplatin. J Pharmacol Exp Therapeutics. 2003;304:661-668.
106. Hau P., Baumgart U., Pfeifer K., et al. Salvage therapy in patients with glioblastoma: is there any benefit? Cancer. 2003;98:2678-2686.
107. Nieder C., Grosu A.L., Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26:397-409.
108. Ammirati M., Galicich J.H., Arbit E., Liao Y. Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery. 1987;21:607-614.
109. Poisson M., Pereon Y., Chiras J., Delattre J.Y. Treatment of recurrent malignant supratentorial gliomas with carboplatin (CBDCA). J Neuro-oncol. 1991;10:139-144.
110. Sanson M., Ameri A., Monjour A., et al. Treatment of recurrent malignant supratentorial gliomas with ifosfamide, carboplatin and etoposide: a phase II study. Eur J Cancer. 1996;32A:2229-2235.
111. Franceschi E., Cavallo G., Lonardi S., et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer. 2007;96:1047-1051.
112. Rich J.N., Reardon D.A., Peery T., et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133-142.
113. van den Bent M.J., Kros J.M. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66:1074-1081.
114. Han Y., Caday C.G., Nanda A., et al. Tyrphostin AG. 1478 preferentially inhibits human glioma cells expressing truncated rather than wild-type epidermal growth factor receptors. Cancer Res. 1996;56:3859-3861.
115. Aldape K.D., Ballman K., Furth A., et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700-707.
116. Weinstein I.B. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science (New York). 2002;297:63-64.
117. Mellinghoff I.K., Wang M.Y., Vivanco I., et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012-2024.
118. Smith J.S., Tachibana I., Passe S.M., et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246-1256.
119. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186.
120. Vredenburgh J.J., Desjardins A., Herndon J.E.II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722-4729.
121. Hall W.A. Extending survival in gliomas: surgical resection or immunotherapy? Surg Neurol. 2004;61:145-148.
122. Mitra S., Li G., Harsh G.R.4th. Passive antibody-mediated immunotherapy for the treatment of malignant gliomas. Neurosurg Clin North Am. 2010;21:67-76.
123. Hayes R.L., Koslow M., Hiesiger E.M., et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76:840-852.
124. Dillman R.O., Duma C.M., Schiltz P.M., et al. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother. 2004;27:398-404.
125. Yu J.S., Liu G., Ying H., et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973-4979.
126. Liau L.M., Prins R.M., Kiertscher S.M., et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515-5525.
127. Sampson J.H., Archer G.E., Mitchell D.A., et al. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20:267-275.
128. Sampson J.H., Akabani G., Archer G.E., et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro-oncology. 2008;10:320-329.
129. Ram Z., Culver K., Oshiro E. Summary of results and conclusions of the gene therapy of malignant brain tumors: a clinical study. J Neurosurg. 1995;82:343A.
130. Harsh G.R., Deisboeck T.S., Louis D.N., et al. Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: a gene-marking and neuropathological study. J Neurosurg. 2000;92:804-811.
131. Colombo F., Barzon L., Franchin E., et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12:835-848.
132. Kaye A.H. Malignant brain tumors. In: Rothenberg R.E., editor. Reoperative Surgery. New York: McGraw-Hill; 1992:51-76.
133. Shapiro W.R., Green S.B., Burger P.C., et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989;71:1-9.
134. Wallner K.E., Galicich J.H., Malkin M.G., et al. Inability of computed tomography appearance of recurrent malignant astrocytoma to predict survival following reoperation. J Clin Oncol. 1989;7:1492-1496.
135. Sipos L., Afra D. Re-operations of supratentorial anaplastic astrocytomas. Acta Neurochir. 1997;139:99-104.
136. Harsh G.R.IV, Levin V.A., Gutin P.H., et al. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615-621.
137. Berger M.S., Tucker A., Spence A., Winn H.R. Reoperation for glioma. Clin NeurosurgClin Neurosurg. 1992;39:172-186.
138. Chandler K.L., Prados M.D., Malec M., Wilson C.B. Long-term survival in patients with glioblastoma multiforme. Neurosurgery. 1993;32:716-720. discussion 720
139. Stromblad L.G., Anderson H., Malmstrom P., Salford L.G. Reoperation for malignant astrocytomas: personal experience and a review of the literature. Br J Neurosurg. 1993;7:623-633.
140. Latif A.Z., Signorini D., Gregor A., Grant R., Ironside J.W., Whittle I.R. Application of the MRC brain tumour prognostic index to patients with malignant glioma not managed in randomised control trial. J Neurol Neurosurg Psychiatry. 1998;64:747-750.
141. Young B., Oldfield E.H., Markesbery W.R., et al. Reoperation for glioblastoma. J Neurosurg. 1981;55:917-921.
142. Wilson C.B. Reoperation for primary tumors. Semin Oncol. 1975;2:19-20.
143. Barnett G.H., Kormos D.W., Steiner C.P., Weisenberger J. Use of a frameless, armless stereotactic wand for brain tumor localization with two-dimensional and three-dimensional neuroimaging. Neurosurgery. 1993;33:674-678.
144. Black P.M., Alexander E.3rd, Martin C., et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999;45:423-431. discussion 431-433
145. Golfinos J.G., Fitzpatrick B.C., Smith L.R., Spetzler R.F. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg. 1995;83:197-205.
146. LeRoux P.D., Berger M.S., Ojemann G.A., et al. Correlation of intraoperative ultrasound tumor volumes and margins with preoperative computerized tomography scans. An intraoperative method to enhance tumor resection. J Neurosurg. 1989;71:691-698.
147. Hatiboglu M.A., Weinberg J.S., Suki D., et al. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery. 2009;64:1073-1081. discussion 1081
148. Berger M.S., Ojemann G.A., Lettich E. Neurophysiological monitoring during astrocytoma surgery. Neurosurg Clin North Am. 1990;1:65-80.
149. Duffau H., Denvil D., Capelle L. Long term reshaping of language, sensory, and motor maps after glioma resection: a new parameter to integrate in the surgical strategy. J Neurol Neurosurg Psychiatry. 2002;72:511-516.
150. Robles S.G., Gatignol P., Lehericy S., Duffau H. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization grade II gliomas in eloquent areas. J Neurosurg. 2008;109:615-624.