Chapter 30 Management of Pineal Region Tumors
Pineal region tumors encompass a diverse group of tumors that can arise from pineal parenchymal cells, supporting cells of the pineal gland, or glial cells from the midbrain and medial walls of the thalamus. These tumors occupy a central position that is equidistant from various cranial points traditionally used as routes of exposure. The deep central location places these tumors in intimate contact with important components of the deep venous system that lie dorsally, including the vein of Galen, the precentral cerebellar vein, and the internal cerebral veins.1 In some instances, there may be a dense attachment to these structures and the tela choroidea. The tumor is often fed by small-caliber branches of the posterior choroidal arteries and branches of the quadrigeminal arteries that generally do not supply any clinically significant areas of the brain.
Although pineal region tumors affect a relatively small number of patients, their variable histology and difficult surgical challenge has generated a comparatively large volume of literature. This difficulty is underscored by Cushing’s statement: “Personally, I have never succeeded in exposing a pineal tumor sufficiently well to justify an attempt to remove it.”2 Over the years, various supratentorial and infratentorial approaches have been developed by several prominent neurosurgeons including Dandy’s interhemispheric approach,3 Van Wagenen’s transventricular approach,4 and Poppen’s occipital transtentorial approach.5 The supracerebellar infratentorial approach was first described in 1926, when Krause reported three cases, each a different variety of tumor in the pineal or quadrigeminal region, which he approached through the posterior fossa, over the cerebellar hemispheres, and under the tentorium.6
In the 1970s, the increasing use of the operating microscope rekindled interest in direct surgical approaches to the pineal region.7–9 This led to considerable debate over the best surgical route to the pineal region. More important than the route, however, was that debates stimulated interest in operating on these tumors to identify their nature and remove them whenever possible.10,11 The more aggressive approach to pineal region tumors resulted in greater awareness of the histologic diversity of these tumors existing along an extensive continuum from benign to highly malignant.12 Some of these tumors are mixed in nature, simultaneously containing benign as well as malignant elements or even glial and pineal cell constituents.13 Despite advances in radiographic imaging and increased experience with tumor markers, preoperative diagnostic tests are insufficient and accurate determination of histologic typing requires operative intervention. With experience, the mortality and morbidity rates from the various surgical approaches dropped steadily and led to the current management philosophy for pineal tumors, which relies on an aggressive surgical approach for the removal of benign tumors and decompression and accurate histologic diagnosis of malignant tumors.14
Clinical Features
Pathology
The various cell types that comprise the mature pineal gland account for the diversity of histologic tumor subtypes that can occur in the pineal region. Lobules of pinealocytes surrounded by astrocytes form the pineal parenchyma with ependymal cells of the third ventricle lining the anterior border of the gland. Pineal tumors are grouped into four main categories:1 germ cell tumors,2 pineal cell tumors,3 glial cell tumors, and4 miscellaneous tumors (covering a wide range of histology). Each category contains tumors existing along a continuum from benign to malignant and can include mixed tumors of more than one cell type.15 The term pinealoma was originally used by Krabbe and is a misnomer because it originally pertained to germ cell tumors.16 Eventually this term was applied more generically to refer to any tumors of the pineal region. The term is now obsolete, in favor of pineal region tumors when a general reference is desired or an individual tumor’s histology in that region when specificity is preferred (e.g., astrocytoma of the pineal region).
Presentation
Tumors in the pineal region, regardless of their histology, generally become symptomatic by three mechanisms:15
1. Increased intracranial pressure from hydrocephalus.
Direct brain stem compression may lead to disturbances of extraocular movements, classically known as Parinaud’s syndrome.17 Involvement of the superior cerebellar peduncles can lead to ataxia and dysmetria. Hearing disturbances can occasionally occur, probably from compression of the inferior colliculi.18
Endocrine dysfunction is rare and may be caused by direct tumor involvement in the hypothalamus or from secondary effects of hydrocephalus.19 Diabetes insipidus and other neuroendocrine disturbances are often indicative of hypothalamic infiltration by tumor, even when not radiographically visualized.
Laboratory Diagnosis
α-Fetoprotein and β-hCG are markers of germ cell malignancy and should be measured in serum and cerebrospinal fluid, if possible, as part of the preoperative workup in all pineal region tumor patients.14,20,21 Elevation of germ cell markers is pathognomonic for the presence of a malignant germ cell tumor, and under these circumstances, histologic verification is unnecessary as surgery does not improve the outcome with radiation and chemotherapy.22 In patients with marker-positive germ cell tumors, measurement of marker levels can also be useful to monitor therapeutic response and as a sensitive early sign of tumor recurrence. The absence of germ cell markers should be interpreted cautiously because malignant germ cell tumors such as germinomas and embryonal cell carcinomas cannot be ruled out.
α-Fetoprotein, normally associated with fetal yolk sac elements, is markedly elevated with endodermal sinus tumors, whereas smaller elevations occur with embryonal cell carcinomas and immature teratomas. β-hCG, normally secreted by placental trophoblastic tissue, is markedly elevated with choriocarcinomas, with smaller elevations associated with embryonal cell carcinomas and those occasional germinomas containing syncytiotrophoblastic giant cells.23 Most germinomas are nonsecretory and carry a better prognosis than β-hCG positive germinomas.24
Imaging
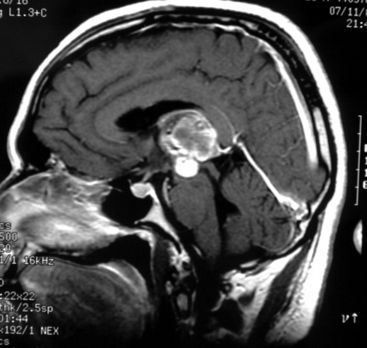
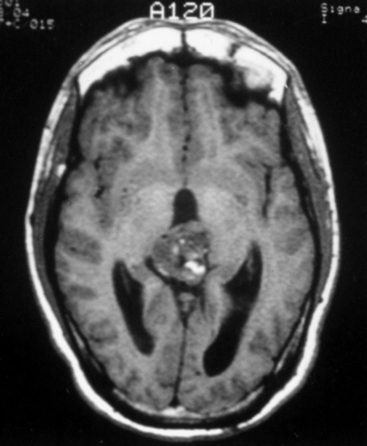
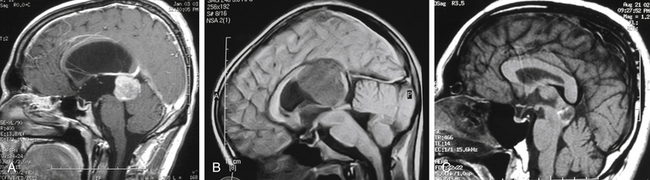
The standard diagnostic workup includes magnetic resonance imaging (MRI), with and without contrast, which has proven to be the most accurate diagnostic examination. MRI provides information on type, size, and extent of the tumor, as well as anatomic features such as degree of invasion, vascularity and the anatomic relationships of the tumor with its surroundings (Fig. 30-1). Some tumors can be suspected from the appearance of the scans, particularly teratomas, which contain multiple germ layers (Fig. 30-2). Angiography is only performed if the MRI suggests a vascular lesion such as a vein of Galen aneurysm or arteriovenous malformation. Despite this broad diagnostic armamentarium, the exact histologic nature of the tumor cannot be reliably determined without surgery.15
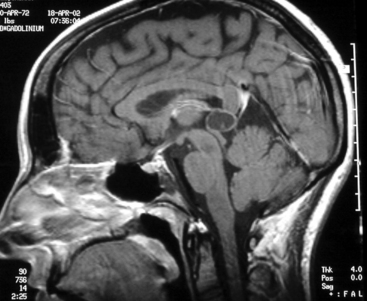
The increasing use of MRI has revealed a large number of patients with lesions of the pineal gland that are mostly cystic but contain a small amount of solid tissue (Fig. 30-3).25,26 In most cases, the aqueduct has not been compromised and the patients are not symptomatic from their lesion. Initially, they were considered to be low-grade cystic astrocytomas but, after surgical removal, were found to be composed of normal astrocytes and normal pineal cells. Histologically, these are pineal cysts and are normal anatomic variations of the pineal gland. As experience with pineal cysts has increased, it is clear that they should be managed conservatively with serial MRI scans and without surgery. Surgery is reserved for lesions that are symptomatic, progressing in size, or causing aqueductal obstruction.
Surgical Considerations
Surgical Anatomy
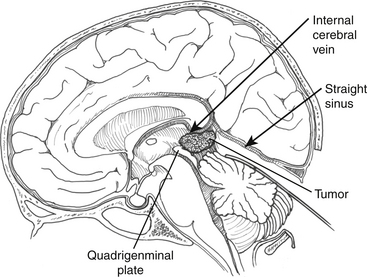
Most tumors arise from and are attached to the undersurface of the velum interpositum, which includes the choroid plexus, deep venous system, and choroidal arteries. Depending on the degree of invasion of these important midline structures, the attachment may be minimal or comprehensive. Tumors rarely extend above the velum interpositum for any significant distance. Therefore, the blood supply comes from within the velum interpositum, mainly through the posterior medial and lateral choroidal arteries with anastomoses to the pericallosal arteries and quadrigeminal arteries.1,27
Some tumors extend to the foramen of Monro, but most are centered at the pineal gland, extending to the midportion of the third ventricle and posteriorly to compress the anterior portion of the cerebellum. In rare instances, the internal cerebral veins are ventral to the tumor. Mostly, however, vein of Galen, internal cerebral veins, Rosenthal’s vein, and precentral cerebellar vein surround or cap the periphery of these tumors. The quadrigeminal plate may give rise to an exophytic astrocytoma or be infiltrated by the more malignant tumors of the pineal region, encompassing the aqueduct in the course of tumor growth. The most important aspects of the anatomy, which can be gleaned by radiographic imaging, are the relationship of the tumor to the third ventricle and quadrigeminal cistern, and the lateral and superior extent of the tumor. These features determine the route of the operation and the degree of difficulty likely to be encountered during surgery.1
Management of Hydrocephalus
Most patients present with obstructive hydrocephalus, a problem that may be managed in several ways.14 When a complete tumor removal is anticipated and a permanent shunt may not be necessary, the hydrocephalus can be managed with a ventricular drain placed at the time of tumor surgery. The ventricular drain can be removed or converted to a permanent shunt on postoperative day 2 or 3, depending on which circumstances prevail. Occasionally, no drain is necessary and the hydrocephalus resolves after complete tumor removal. The drain can be removed or converted to a shunt in the postoperative period as the circumstances dictate. Patients with more advanced symptomatology should be managed with an image-guided stereotactic endoscopic third ventriculostomy to allow a gradual reduction in intracranial pressure and resolution of symptoms.28 This method is preferable to ventriculoperitoneal shunting as it eliminates potential complications such as infection, overshunting, and peritoneal seeding of malignant cells.
Operative Approaches: Biopsy versus Open Resection
The wide diversity of pathology that can occur in the pineal region makes histologic diagnosis a necessity to optimize patient management decisions.14 Tissue histology has important implications for decisions concerning adjuvant therapy, metastatic workup, prognosis, and long-term follow-up. Cerebrospinal fluid cytology and radiographic examination are not sufficiently consistent to supplant the need for tissue diagnosis. The exception to mandatory histologic diagnosis is patients with elevated germ cell markers who can be treated with chemotherapy and radiation without a biopsy.23,29 Some of these patients require a delayed surgical resection to remove residual radiographic abnormalities that may represent residual tumor.30
Experienced neurosurgeons using current microsurgical techniques can expect favorable results with open surgery.14 Open procedures have the advantage of obtaining larger amounts of tissue to provide more extensive tissue sampling. This is particularly important for pineal region tumors where heterogeneity and mixed cell populations are common. This diversity is problematic for even experienced neuropathologists who can best resolve the subtleties of histologic diagnosis when free from the constraints of limited tissue sampling.31–33 Additionally, open procedures provide a clinical advantage by facilitating tumor removal.14,34,35 This is particularly important for the one third of patients with benign pineal tumors, in whom resection is usually complete but can also be useful for patients with malignant tumors in whom debulking may provide a more favorable response to adjuvant therapy and a better long-term prognosis. Additional advantages of aggressive tumor debulking include the potential to relieve hydrocephalus without additional procedures and the ability to control the risks of postoperative hemorrhage into an incompletely resected tumor bed. A disadvantage of open procedures is the relatively higher surgical morbidity compared with that of stereotactic biopsy, at least in the short term. This short-term disadvantage, however, may be a reasonable concession for the long-term advantage of better tumor control.
Any discussion about the relative risks and benefits of open procedures must recognize that the highly favorable outcome for patient with pineal tumor assumes an advanced level of experience, judgment, and expertise. These sophisticated surgical procedures are not recommended for novice surgeons as they can expect significantly less favorable outcomes. Stereotactic procedures, in contrast, can be appealing because of their ease of performance; however, the pineal region is among the most hazardous areas in the brain to safely biopsy, and careful forethought must be given to planning the target and trajectory to minimize hemorrhagic risks.36 The potential for hemorrhage is increased because of several mechanisms including bleeding from any of several pial surfaces that must be traversed, bleeding in highly vascular tumors, damage to the deep venous system, and bleeding into the ventricle where the tissue turgor is insufficient to tamponade minor bleeding.37,38 Despite this increased risk, several series have validated the effectiveness of stereotactic biopsy for these tumors.36,39
Stereotactic Biopsy
The most common surgical trajectory to the pineal region is via an anterolaterosuperior approach anterior to the coronal suture and lateral to the midpupillary line.40 This trajectory passes through the frontal lobe and the internal capsule. The ependyma of the lateral ventricle and the internal cerebral vein should be avoided. An alternative approach is a posterolaterosuperior approach through the parieto-occipital junction, which is best suited for large tumors that have a lateral extension.
Endoscopy
Advances in endoscopic technique have led to investigations of biopsies via this method.41,42 Endoscopy is sometimes performed in conjunction with an endoscopic third ventriculostomy to relieve hydrocephalus. Performing a ventriculostomy and biopsy simultaneously requires the use of a flexible endoscope because of the limited trajectory to the tumor through Monro’s foramen. A rigid endoscope can be used but would necessitate a second burr hole and trajectory with a more inferior entry point on the forehead. The risks of an endoscopic biopsy are considerable due to the limited tissue sampling and difficulty achieving hemostasis within the ventricle. Even minor bleeding can obscure the operative field, making it difficult to identify the target. In general, given these limitations, a stereotactic biopsy is preferable to endoscopic biopsy in situations in which diagnostic tissue is desired.
Surgical Approaches
Operative Considerations
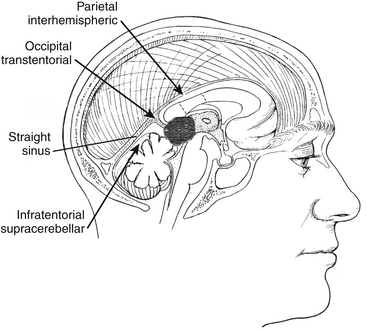
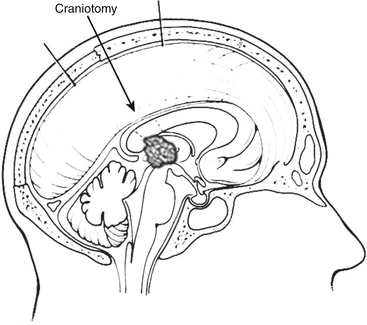
Common approaches to the pineal region include infratentorial supracerebellar, occipital transtentorial, and transcallosal interhemispheric (Fig. 30-4).15 The best approach depends on the anatomic location as well as the degree of familiarity and confidence that the surgeon has with a given approach (Fig. 30-5).
Generally, the infratentorial supracerebellar approach is preferred for several reasons:
1. The approach is to the center of the tumor, which begins at the midline and grows eccentrically.
2. The approach is ventral to the velum interpositum and the deep venous system to which the tumor is often adherent. This minimizes the risk of damage to the vascular drainage of this critical region.
3. The exposure in the sitting position is comparable with that of other routes.
1. Tumors that extend superiorly, involving or destroying the posterior aspect of the corpus callosum and deflecting the deep venous system in a dorsolateral direction
2. Tumors that extend laterally to the region of the trigone
3. Tumors that extend inferiorly into the quadrigeminal plate
4. In rare cases in which the tumor displaces the deep venous system in a ventral direction (often seen with meningiomas)
The transcallosal interhemispheric approach can provide extensive exposure, although the subtentorial portion of the tumor on the contralateral side of the approach is not easily visualized. This approach requires retraction of the parietal lobe and the disruption of bridging veins between parietal lobe and the sagittal sinus, creating the potential for venous infarct and retraction injury. Additionally, the veins of the deep venous system usually overlie the tumor, forcing the surgeon to work around them to avoid injury. Like the transcallosal approach, the occipital transtentorial approach has the disadvantage of encountering the deep venous system overlying the tumor. Once the tentorium is divided, however, this approach permits a wide view of the pineal region with particularly good visualization of the quadrigeminal plate. A major drawback is the high frequency of visual field deficits associated with this approach.43
Patient Positioning
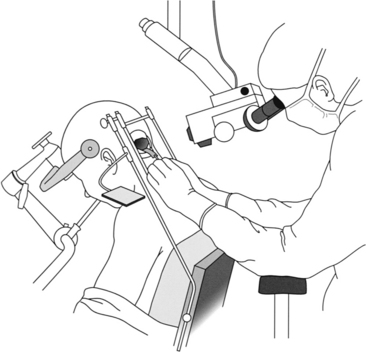
The sitting position is used most often for the infratentorial supracerebellar approach. This position enables gravity to work in the surgeon’s favor by helping tumor dissection from the roof of the third ventricle and minimizing blood pooling in the operative field. It does carry the risk of air embolus or ventricular and cortical collapse with subsequent subdural hematoma or air. However, with proper precautions, these complications are infrequent. The occipital transtentorial approach often uses the three-quarter prone and lateral decubitus position, which, although avoiding many of the complications of the sitting position, does not allow gravity to work in the surgeon’s favor. The Concorde position was developed to combine aspects of both the prone and semisitting positions but still has the disadvantage of blood pooling in the operative field.44
Lateral Position
The three quarter prone position is a useful variant of this approach. It is an extension of the lateral position with the head at an oblique 45-degree angle with a nondominant hemisphere dependent. It is particularly used for the occipital transtentorial approach as the nondominant hemisphere is retracted by gravity. Compared to the sitting position, the surgeon’s hands are on a horizontal plane and less prone to fatigue. This position can be cumbersome to set up and it is important to avoid unnecessary pressure on the dependent axilla by placing in an axillary roll and supporting the right arm in a sling-like fashion. A supporting roll is placed under the left thorax and the head pin holder is used to support the head slightly extended and rotated to the left at a 45-degree oblique angle. The patient must be securely strapped at the table, which will facilitate rotation of the table as needed.
Approaches
Supracerebellar-Infratentorial Approach
Once the patient is optimal in the sitting position, a self-retaining retractor, such as Greenberg’s Universal Retractor, is fixed via a bar to the operating table on the left side (Figs. 30-6 and 30-7). The bars are then arranged in a rectangular configuration framing the operative field, facilitating placement of a retractor in the inferior position to depress the cerebellum. A cottonoid tray is fixed to the self-retaining retractor system and held by one of the retractor’s arms.
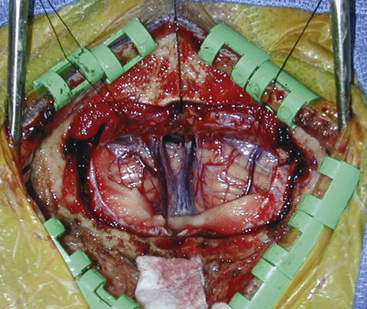
A long midline incision is used, extending approximately from the spinous process of the third vertebral body up into the occipital region, so that the pericranium and muscle attachments can be elevated on each side without disrupting their continuity. Freeing up the muscle layer facilitates closure later. A wide craniotomy is performed to include the lateral sinuses and torcula but without extending to the foramen magnum. Generally, using a high-speed air drill to perform a suboccipital craniotomy is preferred so that the bone flap may be replaced at the end of the procedure. A craniotome is used to turn the flap after first exposing each of the dural sinuses to avoid tears. Brain relaxation and cerebellar retraction can be facilitated by mannitol, ventricular drainage, or removal of cerebrospinal from the cisterna magna. It is preferable to open the dura on either side of the midline so that the cerebellar falx and accompanying cerebellar sinus can be ligated and divided. The dural opening should extend bilaterally up to the lateral sinus (Fig. 30-8).
Once the dura is opened, all bridging veins over the dorsal surface of the cerebellum, including the hemispheres and vermis, can be sacrificed to free the cerebellum from the tentorium. The weight of cottonoids and a copper retractor, along with gravitational forces that are exerted in the sitting position, allow sufficient depression of the cerebellum to establish an unobstructed corridor to the pineal region under the tentorium. At this point, the operating microscope should be brought to the operating table. A microscope capable of varying the objective length from 275 to 400 mm is desirable because the operating distance for the surgeon changes throughout the operation, going from the dorsal surface of the cerebellum to the center of the third ventricle. The use of a freestanding armrest is also helpful in minimizing fatigue.
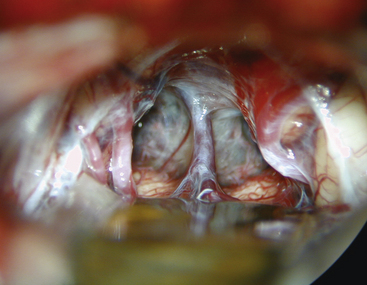
The arachnoid in the quadrigeminal region and around the incisura is usually thickened and opaque in the presence of tumors and must be opened by microdissection techniques to expose the surface of the tumor. The precentral cerebellar vein, which can be seen extending from the edge of the vermis to Galen’s vein, should be cauterized and divided (Fig. 30-9). Galen’s great vein and the internal cerebral veins are well above the tumor and are not encountered in these initial maneuvers. Laterally, the medial aspect of the temporal lobe and Rosenthal’s veins can be seen as they course upward toward the confluence of veins in these regions. In general terms, the trajectory of the operation is toward the velum interpositum. This must be considered when attempting to remove segments of the tumor in the inferior portion of the third ventricle or directly over the quadrigeminal plate in relation to the anterior lobe of the cerebellum. The thickened arachnoid should be opened widely to appreciate the underlying anatomy. Using sharp dissection, the arachnoidal opening must be kept close to the anterior surface of the vermis and cerebellar hemisphere to avoid injuring the deep venous system, keeping in mind that the initial trajectory is toward Galen’s vein. The anterior portion of the cerebellum is retracted by the inferior self-retaining retractor to expose the posterior surface of the tumor.
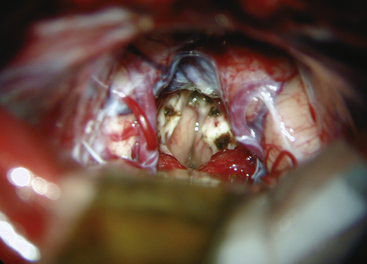
Because many of these tumors extend well into the third ventricle, even to the region of Monro’s foramen, long instruments are required to reach the anterior margins of the tumor. With tumors of firm consistency, a Cavitron with a long, curved tip can be helpful for debulking. A long focal length is desirable to accommodate the large instrument and maneuver into the operative field. The most difficult dissection involves the inferior portion of the tumor, where it is adherent to the collicular region of the dorsal midbrain. Small dental mirrors and angled instruments may be required to remove this portion of the tumor. Similarly, these techniques may be helpful for supratentorial tumor extensions. Further exposure can be gained by incising the tentorium if necessary. Tumors that are benign or encapsulated can usually be removed completely (Fig. 30-10). With tumors that are incompletely resected, particularly vascular ones such as pineal cell tumors, meticulous hemostasis is crucial. Surgicel is the preferred hemostatic agent, and it should be placed carefully so that it does not float and obstruct the aqueduct when the third ventricle fills with cerebrospinal fluid.
Occipital-Transtentorial Approach
A U-shaped right occipital scalp flap or a linear incision centered in the midline can be used. The craniotomy should extend across the sagittal sinus to the contralateral occipital lobe and should begin just above the torcula. Although some surgeons tried to avoid crossing the sagittal sinus, the bony overhang can limit the operative view. A burr hole is placed and slotted over the sagittal sinus just above the torcula and then a second burr hole slotted over the sagittal sinus 6-10 cm above this. The craniotomy is extended 1 to 2 cm left of midline providing an adequate exposure of the right occipital lobe. If the patient is positioned correctly, the nondominant occipital lobe will retract easily with gravity and brain relaxation. There are rarely any bridging veins near the occipital pole and minimal retraction is desirable to avoid hemianopsia. If the brain is tight, mannitol in ventricular drainage can be useful adjuncts.
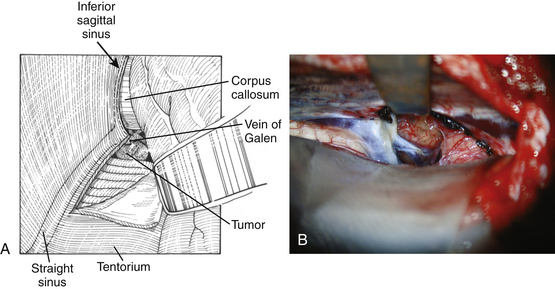
The dura is opened in a U-shaped fashion and reflected towards the sagittal sinus. The interhemispheric fissure is accessed and the occipital lobe is gently retracted. Under the microscope, the straight sinus is identified and the tentorium can be divided adjacent and parallel to the straight sinus (Fig. 30-11). The tentorium can often be highly vascular and it is a CO2 laser or unipolar cautery can facilitate the tentorial opening being careful to avoid damage to the underlying cerebellum and brain stem. If necessary, the falx can be retracted or even divided although this is rarely necessary. Once the tentorium is divided, the arachnoid overlying the tumor and the quadrigeminal cisterns can be seen and widely opened. Similar to what is described earlier in the supracerebellar approach, the tumor is entered dorsally, internally debulked, then gradually peeled away from the surrounding brain stem, deep venous system, and thalamus. Whenever possible, the plane between the tumor capsule and the surrounding structures should be developed.
Transcallosal, Interhemispheric Approach
The transcallosal interhemispheric approach (Fig. 30-12) provides a wide exposure between the falx and brain along the parietal occipital junction. One of the major drawbacks to this approach is the presence of bridging veins which can cause serious venous infarct if damaged or divided. Normally, one vein can be sacrificed if necessary. A generous craniotomy is desirable to provide some flexibility in the trajectory to the tumor if bridging veins are in the way. Although the sitting approach is often used for this approach, the lateral and prone positions can also be used.
Once through the corpus callosum, the deep venous system is identified and the tumor is internally debulked. As with the other approaches, once the tumor is internally debulked, it is helpful to work along the tumor capsule to avoid damage to the surrounding structures. There is considerable debate whether or not one internal cerebral vein can be safely dissected. Although there may be times when it is possible to get away with this, it is not desirable and should be avoided at all expenses.
Complications of Surgery
Serious complications of pineal tumor surgery, regardless of the route used, are related to the nature of the tumor and its potential for intra- or postoperative hemorrhage.15 Hemorrhage has played a major role in most of the surgery-related deaths and can occur with a delay of as long as several postoperative days. This phenomenon is most prevalent with malignant pineal cell tumors (pineoblastomas), which tend to be soft and highly vascular. Hemorrhage can occur before surgery as a so-called pineal apoplexy or can be associated with stereotactic biopsy.
Surgical Results
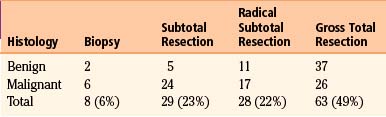
Among large series of pineal region tumors in the microscopic era, operative mortality ranges from 0% to 8% and permanent morbidity from 0% to 25% (Table 30-1).14 Pineal region tumors are among the most difficult surgical challenges, and outcome will vary significantly with the expertise and experience of the individual surgeon. For benign tumors, surgical resection is the treatment of choice with complete surgical removal likely to result in excellent long-term survival and likely cure (Table 30-2).15 With malignant tumors, the impact of surgery is less delineated, although the degree of resection can correlate with improved prognosis.14
TABLE 30-1 Surgical Outcome for 128 Pineal Region Tumors at New York Neurological Institute, 1990–2009
| Operative-related deaths, 2% |
| Permanent major morbidity, 1% |
| Transient major morbidity, 5% |
Adjuvant Therapy
Radiation Therapy
Patients with malignant germ cell or pineal cell tumors require radiation therapy. The recommended radiation dose is 5500 cGy given in 180-cGy daily fractions with 4000 cGy to the ventricular system and an additional 1500 cGy to the tumor bed.14,23,45–47 Recent studies suggest that using a more limited field of radiation that avoids the adverse side effects of ventricular exposure may be sufficiently efficacious; however, these studies lack long-term follow-up.48
Radiation therapy may be withheld for the rare, histologically benign pineocytoma or ependymoma that has been completely resected.49 This distinction is based on the intraoperative observation of a well-circumscribed tumor that is well differentiated histologically. Surgical resection alone provides excellent long-term control in this group; however, careful follow-up is necessary so that radiation or radiosurgery can be offered at the first sign of recurrence. Pediatric patients are particularly vulnerable to adverse radiation effects.50 In an effort to reduce the dose of radiation and minimize these effects, treatment strategies combining reduced radiation with chemotherapy are being investigated.51
Prophylactic spinal radiation is controversial and the current trend is to avoid spinal radiation unless there is documented evidence of spinal seeding or when pineoblastoma is present.15 In patients with radiographic documentation of spinal seeding, a dose of 3500 cGy is recommended to the spine.
Chemotherapy
Patients with nongerminomatous malignant germ cell tumors have benefited most from advances in chemotherapy. Current chemotherapy regimens have resulted in reasonable expectations for long-term survival.52 Additionally, patients with germinomas containing syncytiotrophoblastic giant cells have a less favorable prognosis and may benefit from more aggressive treatment with chemotherapy in addition to radiotherapy.24
Most of the germ cell chemotherapy regimens have been extrapolated from experience treating germ cell tumors of extracranial origin in which success has been remarkable.23 Recent studies, using VP-16 (etoposide) in place of vinblastine and bleomycin to avoid the pulmonary toxicity have shown improved response rate with less morbidity. Currently a regimen of cisplatin or carboplatin with etoposide is among the most widely used.
Chemotherapy for pineal cell tumors has mostly been relegated to recurrent or disseminated pineal cell tumors.45 Combined radiation and chemotherapy may be beneficial for pediatric patients with malignant pineal cell tumors.53
Radiosurgery
Radiosurgery has become more commonplace in the management of pineal tumors.54,55 Several studies have clearly documented the relative safety of this method, although long-term follow-up results are currently lacking. Radiosurgery is generally limited to tumors less than 3 cm in diameter.
Distinct differences in radiobiologic effects between radiosurgery and conventional fractionated radiation must be considered when choosing optimal therapeutic strategies. Germinomas, for example, have excellent long-term response to fractionated radiation, and it is unlikely that radiosurgery will improve on these results. Additionally, because radiosurgery provides no therapeutic coverage to the ventricular system, tumors such as pineal cell and germ cell tumors are particularly vulnerable to ventricular recurrence. Radiosurgery may have its greatest benefit in providing a local boost to the tumor bed so that the radiation exposure to the ventricles and surrounding brain can be reduced.56 It may also be useful for tumors that recur locally.
Bruce J.N., Ogden A.T. Surgical strategies for treating patients with pineal region tumors. J Neurooncol. 2004;69:221-236.
Bruce J.N., Stein B.M. Surgical management pineal region tumors. Acta Neurochir (Wien). 1995;134:130-135.
Chernov M.F., Kamikawa S., Yamane F., et al. Neurofiberscopic biopsy of tumors of the pineal region and posterior third ventricle: indications, technique, complications, and results. Neurosurgery. 2006;59:267-277. discussion 267-277
Edwards M.S., Hudgins R.J., Wilson C.B., et al. Pineal region tumors in children. J Neurosurg. 1988;68:689-697.
Jakacki R.I., Zeltzer P.M., Boyett J.M., et al. Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: a report of the Childrens Cancer Group. J Clin Oncol. 1995;13:1377-1383.
Kobayashi T., Kida Y., Mori Y. Stereotactic gamma radiosurgery for pineal and related tumors. J Neurooncol. 2001;54:301-309.
Konovalov A.N., Pitskhelauri D.I. Principles of treatment of the pineal region tumors. Surg Neurol. 2003;59:250-268.
Kreth F., Schatz C., Pagenstecher A., et al. Stereotactic management of lesions of the pineal region. Neurosurgery. 1996;39:280-291.
Kyritsis A.P. Management of primary intracranial germ cell tumors. J Neurooncol. 2010;96:143-149.
Lutterbach J., Fauchon F., Schild S.E., et al. Malignant pineal parenchymal tumors in adult patients: patterns of care and prognostic factors. Neurosurgery. 2002;51:44-55. discussion 55-56
Sands S.A., Kellie S.J., Davidow A.L., et al. Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: from the First International CNS Germ-Cell Tumor Study. Neuro Oncol. 2001;3:174-183.
Sawamura Y., de Tribolet N., Ishii N., Abe H. Management of primary intracranial germinomas: diagnostic surgery or radical resection? J Neurosurg. 1997;87:262-266.
Schild S.E., Scheithauer B.W., Haddock M.G., et al. Histologically confirmed pineal tumors and other germ cell tumors of the brain. Cancer. 1996;78:2564-2571.
Stein B.M. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg. 1971;35:197-202.
Stein B.M., Bruce J.N. Surgical management of pineal region tumors. Clin Neurosurg. 1992;39:509-532.
Weiner H.L., Lichtenbaum R.A., Wisoff J.H., et al. Delayed surgical resection of central nervous system germ cell tumors. Neurosurgery. 2002;50:727-733. discussion 733-734
Yamini B., Refai D., Rubin C.M., Frim D.M. Initial endoscopic management of pineal region tumors and associated hydrocephalus: clinical series and literature review. J Neurosurg. 2004;100:437-441.
1. Yamamoto I. Pineal region tumor: surgical anatomy and approach. J Neurooncol. 2001;54:263-275.
2. Cushing H. Intracranial tumors: notes upon a series of two thousand verfied cases with surgical mortality pertaining thereto. Springfield, IL: Charles C. Thomas; 1932.
3. Dandy W. Operative experience in cases of pineal tumor. Arch Surg. 1936;33:19-46.
4. Van Wagenen WP. A surgical approach for the removal of certain pineal tumors. Surg Gynecol Obstet. 1931;53:216-220.
5. Poppen JL. The right occipital approach to a pinealoma. J Neurosurg. 1966;25:706-710.
6. Krause F. Operative Frielegung der Vierhugel, nebst Beobachtungen uber Hirndruck und Dekompression. Zentrabl Chir. 1926;53:2812-2819.
7. Jameson K. Excision of pineal tumors. J Neurosurg. 1971;35:550-553.
8. Reid WS, Clark WK. Comparison of the infratentorial and transtentorial approaches to the pineal region. Neurosurgery. 1978;3:1-8.
9. Stein BM. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg. 1971;35:197-202.
10. Sano K. Pineal region tumors: problems in pathology and treatment. Clin Neurosurg. 1984;30:59-89.
11. Stein BM, Fetell MR. Therapeutic modalities for pineal region tumors. Clin Neurosurg. 1985;32:445-455.
12. Bruce JN. Management of pineal region tumors. Neurosurg Q. 1993;3:103-119.
13. Bruce JN, Connolly ES, Stein BM. Pineal and germ cell tumors. In: Kaye AH, Laws ER. Brain Tumors. London: Churchill Livingstone; 1995:725-755.
14. Bruce JN, Ogden AT. Surgical strategies for treating patients with pineal region tumors. J Neurooncol. 2004;69:221-236.
15. Stein BM, Bruce JN. Surgical management of pineal region tumors. Clin Neurosurg. 1992;39:509-532.
16. Krabbe KH. The pineal gland, especially in relation to the problem on its supposed significance in sexual development. Endocrinology. 1923;7:379-414.
17. Parinaud H. Paralysis of the movement of convergence of the eyes. Brain. 1886;9:330-341.
18. Missori P, Delfini R, Cantore G. Tinnitus and hearing loss in pineal region tumours. Acta Neurochir. 1995;135:154-158.
19. Fetell MR, Stein BM. Neuroendocrine aspects of pineal tumors. In: Zimmeman EA, Abrams GM. Neurologic Clinics: Neuroendocrinology and Brain Peptides. Philadelphia: WB Saunders; 1986:877-905.
20. Allen JC, Nisselbaum J, Epstein F, et al. Alphafetoprotein and human chorionic gonadotropin determination in cerebrospinal fluid. An aid to the diagnosis and management of intracranial germ-cell tumors. J Neurosurg. 1979;51:368-374.
21. Weiner HL, Finlay JL. Surgery in the management of primary intracranial germ cell tumors. Childs Nerv Syst. 1999;15:770-773.
22. Sawamura Y, de Tribolet N, Ishii N, Abe H. Management of primary intracranial germinomas: diagnostic surgery or radical resection? J Neurosurg. 1997;87:262-266.
23. Kyritsis AP. Management of primary intracranial germ cell tumors. J Neurooncol. 2010;96:143-149.
24. Uematsu Y, Tsuura Y, Miyamoto K, et al. The recurrence of primary intracranial germinomas. Special reference to germinoma with STGC (syncytiotrophoblastic giant cell). J Neurooncol. 1992;13:247-256.
25. Fetell MR, Bruce JN, Burke AM, et al. Non-neoplastic pineal cysts. Neurology. 1991;41:1034-1040.
26. Di Costanzo A, Tedeschi G, Di Salle F, et al. Pineal cysts: an incidental MRI finding? J Neurol Neurosurg Psychiatry. 1993;56:207-208.
27. Quest DO, Kleriga E. Microsurgical anatomy of the pineal region. Neurosurgery. 1980;6:385-390.
28. Goodman R. Magnetic resonance imaging-directed stereotactic endoscopic third ventriculostomy. Neurosurgery. 1993;32:1043-1047.
29. Choi JU, Kim DS, Chung SS, Kim TS. Treatment of germ cell tumors in the pineal region. Childs Nerv Syst. 1998;14:41-48.
30. Weiner HL, Lichtenbaum RA, Wisoff JH, et al. Delayed surgical resection of central nervous system germ cell tumors. Neurosurgery. 2002;50:727-733. discussion 733-724
31. Chandrasoma PT, Smith MM, Apuzzo MLJ. Stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimen. Neurosurgery. 1989;24:160-165.
32. Edwards MS, Hudgins RJ, Wilson CB, et al. Pineal region tumors in children. J Neurosurg. 1988;68:689-697.
33. Kraichoke S, Cosgrove M, Chadrasoma PT. Granulomatous inflammation in pineal germinoma. Am J Surg Pathol. 1988;12:655-660.
34. Bruce JN, Stein BM. Surgical management pineal region tumors. Acta Neurochir (Wien). 1995;134:130-135.
35. Konovalov AN, Pitskhelauri DI. Principles of treatment of the pineal region tumors. Surg Neurol. 2003;59:250-268.
36. Dempsey PK, Kondziolka D, Lunsford LD. Stereotactic diagnosis and treatment of pineal region tumours and vascular malformations. Acta Neurochir (Wien). 1992;116:14-22.
37. Favre J, Taha JM, Burchiel KJ. An analysis of the respective risks of hematoma formation in 361 consecutive morphological and functional stereotactic procedures. Neurosurgery. 2002;50:48-56. discussion 56-57
38. Field M, Witham TF, Flickinger JC, et al. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J Neurosurg. 2001;94:545-551.
39. Kreth F, Schatz C, Pagenstecher A, et al. Stereotactic management of lesions of the pineal region. Neurosurgery. 1996;39:280-291.
40. Maciunas R. Stereotactic biopsy of pineal region lesions. In: Kaye A, Black P. Operative Neurosurgery. London: Churchill Livingstone; 2000:841-848.
41. Chernov MF, Kamikawa S, Yamane F. Neurofiberscopic biopsy of tumors of the pineal region and posterior third ventricle: indications, technique, complications, and results. Neurosurgery. 2006;59:267-277. discussion 267-277
42. Yamini B, Refai D, Rubin CM, Frim DM. Initial endoscopic management of pineal region tumors and associated hydrocephalus: clinical series and literature review. J Neurosurg. 2004;100:437-441.
43. Nazzaro JM, Shults WT, Neuwelt EA. Neuro-ophthalmological function of patients with pineal region tumors approached transtentorially in the semisitting position. J Neurosurg. 1992;76:746-751.
44. Kobayashi S, Sugita K, Tanaka Y, Kyoshima K. Infratentorial approach to the pineal region in the prone position: Concorde position. J Neurosurg. 1983;58:141-143.
45. Lutterbach J, Fauchon F, Schild SE, et al. Malignant pineal parenchymal tumors in adult patients: patterns of care and prognostic factors. Neurosurgery. 2002;51:44-55. discussion 55-56
46. Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol. 1997;32:71-80.
47. Schild SE, Scheithauer BW, Haddock MG, et al. Histologically confirmed pineal tumors and other germ cell tumors of the brain. Cancer. 1996;78:2564-2571.
48. Yen SH, Chen YW, Huang PI, et al. Optimal treatment for intracranial germinoma: can we lower radiation dose without chemotherapy? Int J Radiat Oncol Biol Phys. 2009;77:980-987.
49. Vaquero J, Ramiro J, Martinez R, et al. Clinicopathological experience with pineocytomas: report of five surgically treated cases. Neurosurgery. 1990;27:612-619.
50. Sands SA, Kellie SJ, Davidow AL, et al. Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: from the First International CNS Germ-Cell Tumor Study. Neuro Oncol. 2001;3:174-183.
51. Calaminus G, Bamberg M, Baranzelli M, et al. Intracranial germ cell tumors: a comprehensive update of the European data. Neuropediatrics. 1994;25:26-32.
52. Calaminus G, Bamberg M, Jurgens H, et al. Impact of surgery, chemotherapy and irradiation on long term outcome of intracranial malignant non-germinomatous germ cell tumors: results of the German Cooperative Trial MAKEI 89. Klin Padiatr. 2004;216:141-149.
53. Jakacki RI, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: a report of the Childrens Cancer Group. J Clin Oncol. 1995;13:1377-1383.
54. Hasegawa T, Kondziolka D, Hadjipanayis CG, et al. The role of radiosurgery for the treatment of pineal parenchymal tumors. Neurosurgery. 2002;51:880-889.
55. Kobayashi T, Kida Y, Mori Y. Stereotactic gamma radiosurgery for pineal and related tumors. J Neurooncol. 2001;54:301-309.
56. Casentini L, Colombo F, Pozza F, Benedetti A. Combined radiosurgery and external radiotherapy of intracranial germinomas. Surg Neurol. 1990;34:79-86.