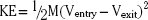Chapter 134 Management of Penetrating and Blast Injuries of the Nervous System
Historical Aspects
The basic principles used in the management of penetrating brain injuries (PBIs) were developed while treating casualties during the wars of the past century. Modern antiseptic techniques were first applied to head injured patients during the Anglo-Boer war.1 During World War I, Harvey Cushing implemented en-block bone resection, suction debridement, magnet removal of metallic fragments, and two-layered closure of penetrating head injuries to effect a decrease in mortality from 54% to 28%.2,3 During World War II, widespread use of early antibiotics dramatically reduced rates of infection.4–7 Korean War experiences emphasized the role of early evacuation of patients with head injuries.8
In the Vietnam conflict, a wealth of experience and data were obtained, often in a prospective fashion.9 Routine use of skull x-rays and angiography allowed better visualization of penetrating bone and metallic fragments, which were thought to be associated with increased rates of infection and seizures. Military medical doctrine advocated the pursuit of all retained bone fragments even when this entailed multiple operations.9 This dogma was later questioned when follow-up studies of Vietnam veterans with retained fragments failed to find increased rates of either infectious complications10 or epilepsy.11
The routine international use of diagnostic computed tomography (CT) scanning coincided with the Arab-Israeli conflict in Lebanon. Its use in medical decision making prompted a less aggressive initial surgical approach in the absence of traumatic mass effect.12 Long-term analysis of Israeli survivors found 48% had retrained in-driven intracranial bone and further questioned the need to aggressively remove all penetrating fragments.13 The Iran-Iraq war also led to a wealth of published data, including reports on the causes of infections and the vascular complications of penetrating head injuries.14–16 Closed-head-injury data from bomb blasts in the Lebanon conflict17 and more recent reports from the Iraq Conflict18 emphasize the complexity of blast injuries, which combine the elements of both penetrating and blast effects.
Ballistics
The kinetic energy (KE) released by a missile fragment is related to its mass (M) and velocity (V) and may be estimated by the formula19:
This equation highlights the importance of missile velocity relative to mass. Most modern high-velocity rifles surpass 2500 ft/sec (762 m/sec), whereas most handguns fire 800 to 1400 ft/sec (244 to 427 m/sec). Shrapnel typically has a velocity of 600 feet/second (183 m/sec).20 A missile’s energy is greatest at the moment it is launched, and decays with time and distance. Therefore, it is the impact velocity of the projectile that more accurately reflects the true wounding potential of the missile Fig. 134-1. The actual energy delivered to tissue is determined not just by the kinetic energy available to the projectile (as expressed by the above equation) but also by the deformation and fragmentation of the missile itself.21 Weapons and ammunition are designed to create friction-free flight in air but to enhance resistance to passage in tissue. Expansion, tumbling, yaw, and fragmentation all enhance energy delivery to the tissue.20 Bone fragments driven into neural elements may act as secondary missiles. Thus even a tangential missile strike can achieve a release of energy leading to significant injury to the central nervous system (CNS).
Pathophysiology
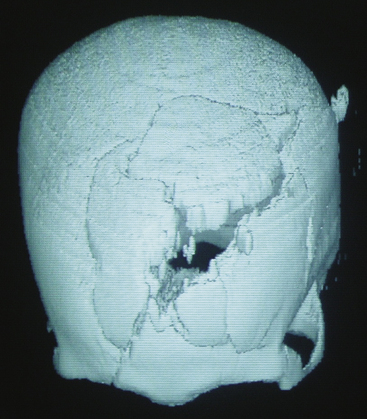
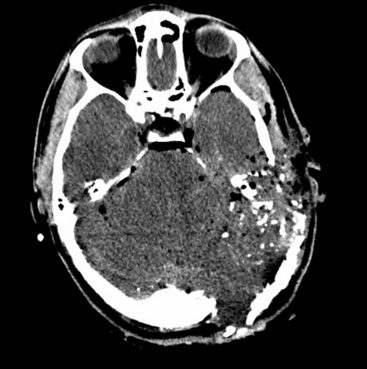
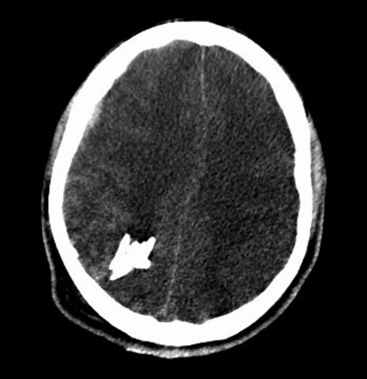
The biomechanical effects of a missile strike on tissue were originally described in 1945 by E. Newton Harvey22 and more recently simulated in cold gel blocks by Martin Fackler and colleagues.23 Carey utilized a cat model to study the wounding effects of a projectile through an intact skull.24 First, a direct crush injury results in a permanent cylindrical cavity. Pressure waves resulting from kinetic energy transfer, termed “ordinary pressure waves,” are propelled radially from the missile path. These create a temporary cavity lasting approximately 20 milliseconds as the walls of the permanent cavity first expand, then contract. The ordinary pressure waves sometimes result in stretch injury to adjacent tissues even at distances far removed from the projectile tract. Collapse of the temporary cavity is followed by extravasation of blood from around the missile track, which may expand to occupy a much larger space than the initial cavity Figs. 134-2 and 134-3. In perforating injuries, the exit wound is inevitably larger than the entrance wound.
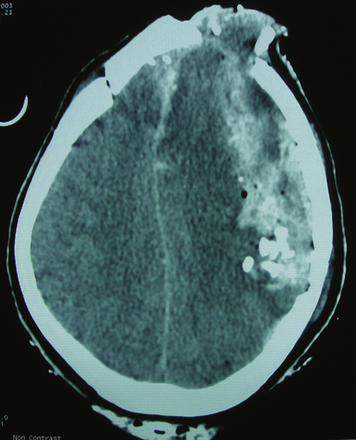
FIGURE 134-2 Axial computed tomography from patient in Fig. 134-1. Note hemorrhage into the temporary projectile cavity with surrounding contusions from stretch injury.
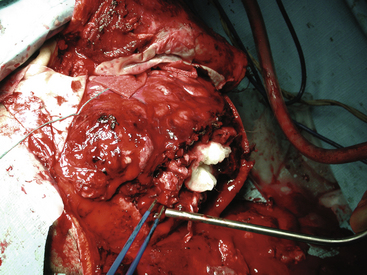
FIGURE 134-3 Intraoperative photo from patient in Fig. 134-1. Massive tissue injury and swelling are seen after high-velocity gunshot wound through the left hemisphere of the brain.
Animal models have been utilized to study the effects of a missile strike to the brain on the physiologic parameters of intracranial pressure (ICP), mean arterial blood pressure (MABP), and cerebral perfusion pressure (CPP). In a monkey model, Crockard and colleagues25 showed that a rapid rise in ICP immediately follows penetrating injury, peaking 2 to 5 minutes after injury and declining gradually thereafter. Carey and colleagues,26 utilizing his feline model, demonstrated that a marked rise in MABP in the first minute after injury actually results in a short-lived rise in CPP; however, the rise in ICP is more sustained over time and larger in magnitude relative to baseline values than the rise in MABP, and it leads to a reduction in CPP. Levett and colleagues, using Crockard’s model, showed that CPP values fell within 1 minute of injury to 41 mm Hg from a baseline of 90 mm Hg.27 In this model, cerebral blood flow was also reduced to a level more than 50% below control values following penetrating injury. These pathophysiologic changes following PBI likely contribute to further neuronal damage and lend the theoretical justification for monitoring of ICP and CPP after PBI.
Carey and colleagues noted that severe respiratory changes occur after projectile injury, even when the missile is of low energy and the tract is distant from the brain stem.26 Carey hypothesized that the location of the medullary respiratory center (directly below the fluid-filled fourth ventricle) may make those neurons more susceptible to the ordinary pressure waves generated by a missile. Above a certain level of energy, a missile often produces an apnea which is fatal unless the animal is supported by mechanical ventilation. The probable correlate in human injuries may be a significant factor responsible for mortality in PBI. Carey’s experiments suggest that only a narrow window exists between the level of energy required for a missile to penetrate the skull and the level that causes fatal apnea.
Initial Assessment and Resuscitation
Advanced Trauma Life Support (ATLS) principles guide initial assessment and resuscitation of the patient with penetrating neurologic injuries.28 Steps include the following:
1. Acute control of including airway, breathing, and circulation
4. A brief initial neurologic evaluation, including the Glasgow Coma Scale (GCS),29 assessment of the pupils, and an evaluation for any focal deficit
5. Assessment of the cranium and face for external injuries
6. Evaluation of the spine for deformities and/or open abnormalities
7. Concomitant head-to-toe evaluation for other life- or limb-threatening injuries
After primary survey for life-threatening injuries, vital signs are monitored frequently and isotonic intravenous fluids are administered as needed. Blood products should be immediately available to replace lost volume. Hypotension without tachycardia warrants consideration of spinal shock. Oxygenation should be monitored with continuous pulse oximetry. Blood is drawn for laboratory tests including arterial blood gas, blood count, electrolytes, coagulation profile, and blood type analysis. Chest x-ray should follow intubation of the trachea. Decompression of the stomach and bladder also allow monitoring of outputs. Most modern trauma centers have replaced routine cervical and pelvis x-rays with whole-body CT scans. Cervical and thoracolumbar spine precautions are maintained in multitrauma patients and those with unclear histories. Radiologic clearance of the cervical spine may not be necessary in patients with isolated penetrating brain injuries.30
Initial Medical Management
Anticonvulsants
Active seizures may lead to acute deterioration, and should be treated aggressively with intravenous diazepam or lorazepam. It is appropriate to administer a loading dose of anticonvulsant medication intravenously to all patients with PBI. These patients suffer higher rates of seizures than those with nonpenetrating traumatic brain injury (TBI).11,31–34 The rate of seizures after PBI has been reported as high as 30% to 50%, with 4% to 10% of seizures occurring within the first week after injury.11,31 Temkin and colleagues have demonstrated in a randomized trial the efficacy of phenytoin in reducing the incidence of seizures in the first week after head injury.34 Their study included patients with penetrating head injury. The use of anticonvulsant prophylaxis beyond 1 week is controversial. Salazar and associates reported a seizure rate of 53% in their study of Vietnam veterans followed over a 15-year period, with 18% of patients having their first seizure more than 5 years after injury.11 Although no studies have demonstrated the efficacy of prophylactic anticonvulsants in preventing late seizures, the high rate of late epilepsy in this population leads many authors to recommend continuing prophylaxis for 1 year or more after injury.35–38 Of interest, Temkin’s most recent meta-analysis of seizure prophylaxis in all traumatic brain injuries suggests that although Carbamazepine or Phenytoin suppress early seizures by as much as 25%, no tested medications have a positive effect on late seizures, even during treatment.39 The most recent opinion about long-term management of these seizures is to manage them the same way one would treat non-traumatic seizures of the same type.40
Antibiotics
Initiation of prophylactic broad-spectrum antibiotic therapy is recommended in penetrating neurologic injuries. The rate of infection is high because of contamination from foreign objects, skin, hair, clothing, and bone fragments, which all may lodge in the wound tract. In the preantibiotic era, rates of infection were nearly 60%, as reported by Whitaker during World War I.41 When penicillin was introduced in a successive fashion to the various theaters of war during World War II, reported rates of infection dropped to 6% to 13%.4 More recent reports from the military and civilian literature, based on routine use of modern broad-spectrum antibiotics, document rates of infection between 1% and 11%, with a trend toward higher rates in the military setting.10,14,42–51 Reports on causative organisms identify Staphylococcus species, Streptococcus, Acinetobacter, Escherichia coli, Klebsiella, and Enterobacter as the common organisms identified in cultures.5,42,44,52 Important risk factors for the development of infection include CSF leak,42,53 air sinus wounds,54 and wound dehiscence.10,44
The issue of which antibiotic regimen is best suited for prophylaxis is not settled. In a survey of American neurosurgeons, Kaufman and colleagues reported the use of a cephalosporin by 87% of respondents, chloramphenicol by 24%, penicillin by 16%, an aminoglycoside by 12%, and vancomycin by 6%.55 The issue of optimal duration of prophylaxis is also unsettled, with very little evidence in the literature to support a recommendation.56 Both the issues of choice of antibiotic and duration of treatment continue to merit a prospective, randomized controlled study.
Initial Imaging
Once the patient is stabilized and the initial neurologic evaluation completed, the patient is taken to the CT suite. Computed tomography is the examination of choice because the extent of brain injury (including intracranial hematomas and air), the extent of bone injury (including in-driven bone chips), and the presence of foreign bodies are all easily identified. The wound tract is readily visualized, and the presence of cerebral edema is noted. The size of the ventricles and cisterns is also noted. In spine injuries, bony detail and injury to surrounding structures is seen optimally. The utility of CT in both the military and civilian settings has been well-documented.12,13,57–59 Head CT findings associated with poor prognosis include bihemispheric injury,50,58,60–64 transventricular path of the projectile,12 intraventricular hemorrhage,61,64 diffuse cerebral edema, and effacement of cisterns Fig. 134-4.65
In rare cases, the patient is hemodynamically unstable because of abdominal or thoracic injuries, and must be taken urgently to the operating theater by the trauma team without CT scan. X-rays of the skull may help to define the projectile tract, the extent of bony injury, and the presence of intracranial air. In such circumstances the neurosurgeon’s clinical judgment and experience are important and dictate further therapy. In some cases a conservative approach is warranted, which may include placement of an ICP monitor until the patient is hemodynamically stable enough to proceed to CT. In the rare case when anisocoria is present, burr holes or an exploratory craniotomy may be undertaken with the aim of evacuating a life-threatening hematoma. Intraoperative ultrasound is useful to localize an intracerebral clot in such cases. In cranial stab wounds, angiography should be considered immediately to look for commonly seen vascular injuries.66 In other types of penetrating trauma, angiography can usually be postponed until after the patient has been stabilized and initial neurosurgical intervention is completed.
Decision Making in the Patient with Poor Prognosis
After stabilization and imaging, a decision concerning further therapy will need to be made. The most difficult clinical scenario exists when the postresuscitation GCS is between 3 and 5. In the civilian setting, between 38% and 81% of patients present in this condition.50,60,61,65,67 Published reports document poor rates of survival in patients with a GCS score of 3 to 5 and even lower rates of neurologically good outcomes.50,60,61,63,65 In the case of a patient who has a postresuscitation GCS score of 3 with dilated and nonreactive pupils without a mass lesion on CT, most neurosurgeons agree that no surgical intervention is warranted.58,68,69 Grahm and colleagues prospectively studied 100 patients with gunshot wounds to the head in a civilian setting.60 They aggressively treated all patients with evidence of neurologic function after resuscitation and found no satisfactory outcome in the group with a postresuscitation GCS score of 3 to 5. They concluded that no further therapy should be offered to a patient with a postresuscitation GCS score of 3 to 5 who did not have evidence of a large, surgically treatable hematoma on CT scan. In a study by Kaufman and colleagues involving two centers, the outcome of 412 patients presenting with a GCS score of 3 to 5 were evaluated. Of these, 4 had a neurologically good outcome.63 In the study by Nagib and colleagues, 1 of 28 patients presenting with a GCS score of 3 to 5 had a favorable outcome.50 Miner and colleagues, in their study of gunshot wounds in a pediatric population, found that decerebrate or decorticate posturing in children did not predict a poor outcome.70 In military series, the results in patients with a GCS score of 3 to 5 are better, with rates of good outcome reaching 15% to 34%.12,14 Thus it is important to keep in mind that, although few, some patients with a GCS score of 4 to 5, or even a GCS score of 3 with evidence of brain stem function, may have neurologically meaningful outcomes. An ethical dilemma arises when considering whether the tremendous societal costs incurred in the aggressive treatment of all patients in this group are justified given the poor outcomes and low rates of survival. No across-the-board recommendation can be made based on the current literature. Though each case will need to be considered individually, most agree that an aggressive approach to treatment is warranted as long as some evidence of motor or brain stem function is demonstrated.71
Surgical Management
The general guidelines of surgical treatment of penetrating injures of the nervous system include:
1. Adequate debridement of devitalized tissue
3. Removal of accessible in-driven bone fragments and foreign bodies
6. Complete closure of the scalp and coverage of the spinal dura
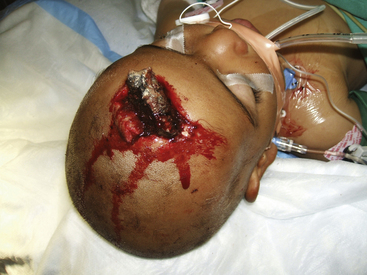
All operative procedures for penetrating head and spine injuries should begin with wide prepping and draping of the injured area to allow for adequate exposure Fig. 134-5. Thorough but gentle irrigation is used to flush out all tissues extruding from the wound site. Two strategies are acceptable in planning the initial scalp incision for head injuries. A traditional large trauma flap may be utilized, especially in cases where CT demonstrates a large hematoma. In cases where the entry site is punctuate, the skin flap may incorporate it. This allows the necrotic edges to be excised, the healthy tissue advanced, and the wound closed primarily. When there is a large laceration at the entry site, this may be extended in lieu of a traditional incision. This is preferable in cases where flap vascularity would otherwise be jeopardized. More extensive scalp injuries may require the use of rotation skin flaps for closure. Consultation with a plastic surgeon is helpful in cases of extensive scalp injuries and should be undertaken prior to the initial incision in order to avoid difficulty with wound closure at the end of operation.
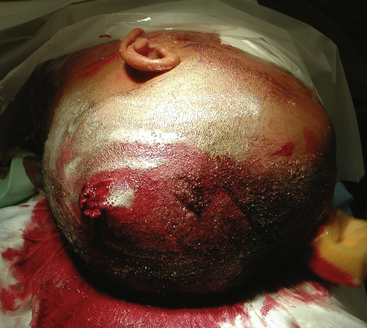
FIGURE 134-5 A large left hemisphere scalp flap is planned for this patient with a perforating frontoparietal gunshot wound.
Adequate exposure of the bony defect is essential. The underlying dural injury often extends beyond the margins of the bone injury. The bone opening should extend beyond the limits of the visible bone injury until intact dura can be visualized. When an underlying hematoma is present, the bone flap should encompass the limits of its extent to allow adequate hemostasis. At the site of penetration, adequate debridement of the bony edges should be performed. Once the dura is exposed, herniating necrotic brain tissue often extrudes from the dural defect. This can be gently irrigated free with saline. The tract of penetration through the brain is gently flushed through the dural defect, removing necrotic tissue as well as any loose foreign objects within the necrotic cavity. At this point the dural defect may need to be extended to allow for adequate debridement of the injured brain. Blood clots are aspirated. A thorough exploration of the resulting cavity is conducted. Accessible bone fragments and foreign bodies are removed. Although CT may demonstrate deeper retained fragments, exploration should not continue beyond the point of safe hemostasis. This is consistent with the approach advocating preservation of viable brain tissue over the removal of all retained fragments.12,13 Meticulous hemostasis is accomplished using bipolar coagulation. Hemostatic agents should be irrigated from the cavity to avoid adding to the mass effect Fig. 134-6.
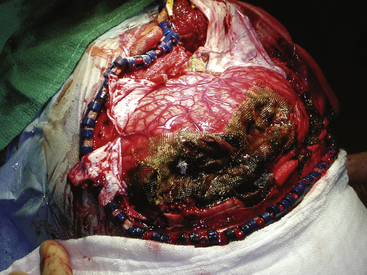
FIGURE 134-6 Intraoperative photo from patient in Fig. 134-5. High hemispheric penetrating injuries are often well tolerated but associated with significant swelling during the acute phase of the injury.
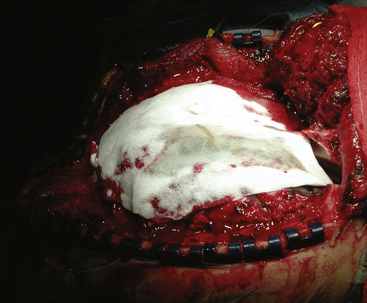
Dural repair is undertaken next. Wartime discussions argue that watertight closure may not be necessary,72–74 but Carey concludes that failure to achieve a watertight closure results in higher complication rates.75 Necrotic dural edges should be removed. Primary closure of the dura is often not possible. Autologous grafts may be utilized in order to obtain a watertight closure. Temporalis fascia or pericranium are local autologous options. If these are not available, a fascia lata graft may be harvested from the thigh or transversalis fascia may be harvested from the abdomen. Allograft dura or cadaveric pericardium may also be used when autologous options are not practical Fig. 134-7.
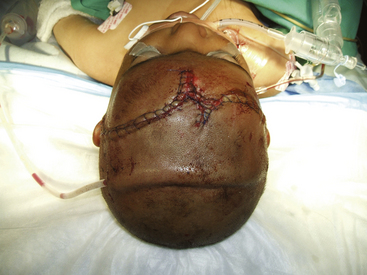
Once dural closure is obtained, the bone flap or fragments can be replaced or left out. In cases where severe brain swelling cannot be controlled despite all operative and medical measures, the bone flap should not be replaced. In cases where replacement is feasible, sizeable retrieved bone fragments may be thoroughly cleaned and replaced at the site of defect. They can be assembled and held in place using miniplates, titanium wires, or other fixation devices. Cranioplasty utilizing foreign materials is discouraged to avoid infectious risks. A generous subgaleal drain should be inserted. The galea is closed with absorbable suture. The skin may be closed with nylon suture or a skin stapler. Some cases will require the use of a rotation flap to cover the defect. A skin graft may be used to cover the resulting pericranial defect but should never cover the wound site (Figs. 134-8 and 134-9). The scalp closure serves as a crucial barrier in preventing infection.
Intracranial Pressure Monitoring
Studies that include data on ICP after PBI indicate that intracranial hypertension is common after these injuries. Crockard studied ICP very early after PBI and found that elevated ICP was associated with high mortality.76 Patients without elevated ICP did well. Nagib and colleagues studied patients with civilian head injuries and found that patients with elevated ICP refractory to treatment measures invariably died.50 The majority of patients in whom ICP responded to treatment survived, and those who did not have significant elevations of ICP had better outcomes. Miner and colleagues studied a pediatric population and found that 75% of children with ICP above 40 mm Hg that was refractory to treatment died, whereas the rest were left with severe or moderate deficits.70 Patients with ICP controllable below 20 mm Hg had good outcomes or only moderate disability 6 months after injury. In their experience with PBI from bomb blasts, Rosenthal and colleagues found an elevated mortality in patients with elevated ICP that was refractory to treatment (Rosenthal G, Segal R, Umansky F, unpublished data. 2004.) Patients in whom ICP responded to treatment had better outcomes. Higher ICP values were seen in patients with intraventricular blood, brain edema, and large hematomas. After thorough review of the existing literature, the Brain Trauma Foundation recommended ICP monitoring as a standard for patients with severe head injuries (GCS 3–8).78 Ventriculostomy remains the gold standard because it allows therapeutic drainage of CSF. This is especially important when intraventricular blood is present. No prospective studies have documented the effectiveness of routine catheter exchanges with prolonged ventricular drainage (>5–7 days). The exiting ventricular catheter should always be tunneled at least 5 cm from its puncture site. Zabramski and colleagues found that the use of a ventricular catheter impregnated with rifampin and minocycline reduces the rate of infection in patients undergoing ventriculostomy.79 These more expensive catheters are routinely available. In austere settings or cases where the ventricles cannot be cannulated, placement of an intraparenchymal ICP monitor is an acceptable alternative.
Bone Fragments
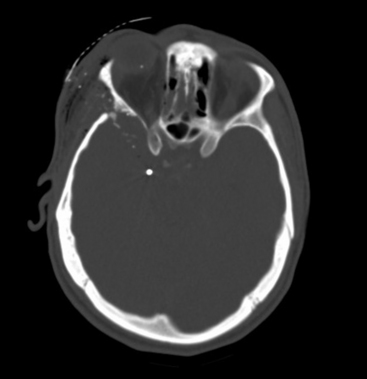
The need to remove all bone fragments has been a controversial issue in the surgical management of penetrating injuries of the brain. In the era when imaging was limited to plain radiographs of the skull, retained bone fragments were considered evidence of inadequate debridement and were thought to predispose to post-traumatic epilepsy and infection. During the Vietnam War, United States military doctrine dictated removal of all retained fragments. Patients often underwent repeat operation to remove retained fragments.9 CT scans entered routine clinical use during the Lebanon conflict. Most patients were evacuated to the Rambam Medical Center in Haifa within 2 hours. The Israeli experience there led to a more conservative approach. Initial surgery was limited to debridement, removal of large hematomas, dural repair, and ICP monitoring.12 Postoperative CT often demonstrated retained bone fragments. These were not pursued. No increase in the rate of infectious complications was seen. There was no correlation between retained bone fragments and epilepsy.12 Long-term follow-up from the Vietnam Head Injury Study (VHIS) helped clarify the issue.10 In an analysis of 1221 patients, only 37 cases of brain abscess were documented. Although 11 of these patients had retained bone fragments, in all these cases additional risk factors for infection (CSF fistula, wound closure complications, air sinus injuries, or multiple surgical procedures) were present. Data from the VHIS also failed to find a relationship between retained bone fragments and epilepsy.11 Based on these findings, only easily accessible bone fragments should be removed at surgery. No potentially viable brain tissue should be sacrificed to pursue distant fragments (Figs. 134-10 and 134-11).
Dural Sinus Injuries
Injury to a dural sinus is one of the most concerning penetrating neurologic injuries. A high level of suspicion should arise when an injury occurs in proximity to the midline of the cranial vault or the torcular Herophili. Digital exploration of the wound should be avoided. This may exacerbate bleeding from the sinus by dislodging any tamponading fragments. When a venous sinus is injured, profuse bleeding should be treated immediately with a compressive bandage and elevation of the head. CT often reveals either a foreign body or in-driven bone fragments adjacent to or perforating the sinus. The tract of a missile may also transverse the sinus. Subdural blood often accompanies this injury. Before proceeding to surgery, preparations must be made for the possibility of a massive hemorrhage. Packed red blood cells and fresh frozen plasma should be on hand in the operating room. The point of dural laceration should not be disturbed until good exposure has been achieved.80 When the injury is bleeding actively, hemostasis can often be achieved by placing thrombin-soaked Gelfoam and a Cottonoid patty over the injured site. Repair of the sinus may be necessary when the injury is more extensive. The sinus should be clipped with a temporary vascular clip proximally and distally. This allows for a tear to be identified and repaired. Primary repair is the preferred option when possible. When the injury to the sinus is more extensive, a dural, periosteal or muscle flap or graft may be sutured into place to repair the defect.81 Ligation may be considered if the injury involves the anterior third of the superior sagittal sinus (Fig. 134-12). Reconstruction of the sinus with a vein graft or a silastic tube has been described82 and may be necessary in cases of transection along the posterior two thirds of the superior sagittal sinus. One small series describes a patency rate of 91% and a mortality rate of 9% using these techniques.83
Postoperative Management
Patients with penetrating neurologic injuries require intensive care postoperatively in a neurosurgical or general ICU setting. Attention must be focused on adequate oxygenation and blood pressure stability. This is especially important in patients with multisystem injuries. During the initial postinjury period, sedation should be maintained with short-acting agents such as propofol to allow frequent neurologic assessments. Once a stable exam has been observed, longer acting agents such as midazolam or lorazepam are reasonable. Refractory intracranial pressure warrants radiologic reassessment and precludes cessation of sedation for physical examinations. Once surgical lesions are adequately addressed, medical management can be optimized using parenchymal and traditional monitoring alone. ICP monitoring remains the standard of care. Cerebral blood flow, cerebral oxygenation, and cerebral microdialysis monitoring are valid options to consider as well.84,85
Routine treatment of elevated ICP includes modest hyperventilation (pCO2 30–35 mm Hg), CSF drainage, and mannitol or hypertonic saline boluses. Barbiturate coma or further surgical decompression should be considered in refractory cases. Questions remain whether the underlying physiologic measures responsible for ICP elevation are the same in PBI and blunt TBI. It has been suggested that failure of cerebral autoregulation plays an important role in the etiology of elevated ICP early after PBI.76 Studies of effective treatment regimes for elevated ICP in PBI and their effect on outcome are yet to be done.
Coagulopathy is a concern following penetrating head injury. Risk factors for development of coagulopathy include the extent of brain injury,61 massive blood loss in the multi–system-injured patient, acidosis, and hypothermia. Patients at risk should have fibrinogen levels, fibrin split products, and D dimers checked, in addition to routine studies of prothrombin time, activated partial thromboplastin time, and platelet count. The presence of disseminated intravascular coagulation (DIC) has been shown to be associated with risk of delayed intracranial hemorrhage, and it may impair hemostasis intraoperatively.86 In the patient with DIC, plasma factors should be replaced until correction of the coagulation parameters is achieved. Recent reports have suggested that recombinant factor VIIa (rFactor VIIa) may be efficacious in stabilizing patients with exsanguinating hemorrhage.87–89 However, the published literature regarding the use of rFactor VIIa to correct coagulopathy in the neurosurgical population is limited.90 Concerns exist, including thrombosis (most ominously of the venous sinuses),91 and caution is indicated until further studies are completed.92
Post-Traumatic Complications
Cerebrospinal Fluid Leakage
The variable most highly correlated with delayed CNS infection is acute or delayed CSF leakage. This has been corroborated by several authors, including Arendall and Meirowsky’s evaluation of casualties from the Korean War.54 Brandvold and colleagues reported the same finding in analyzing data from the Israeli experience in Lebanon,12 as did Aarabi and colleagues in their experience with casualties from the Iran-Iraq War.42 Meirowsky and colleagues, analyzing data from the Vietnam conflict, documented a 49.5% incidence of infection in patients who leaked CSF, as opposed to a rate of 4.6% in those who did not.53 Only half of patients experienced leaks from the wound site; the remainder had rhinorrhea or otorrhea from skull base fractures or air sinus injuries. Mortality in patients who leaked CSF was 22.8%, compared with 4.6% in those who did not leak. A majority of leaks (72%) appeared within 2 weeks after injury. The prompt treatment of CSF fistula is imperative. CSF drainage by ventricular or lumbar catheter is the first option. If there is a persistent leak from the skull base, craniotomy may be required. Preoperative imaging with intrathecal contrast, thin-slice CT, and coronal reconstructions is often helpful in defining the site of bony defect.93,94 When not contraindicated by retained ferromagnetic fragments, MRI may also help to identify the site of CSF leakage.95 An extradural search for the site of dural injury and bony defect are then undertaken. Primary dural repair is often not feasible. Surgical options include placing a vascularized periosteal flap, plugging of the bony defect with fat or muscle together with fibrin glue or other sealants, oversewing the dura with a fascial graft, or ligating the thecal sack in patients with complete spinal paralysis distal to the laceration. Unless contraindicated, patients should have a ventricular or lumbar drain maintained for several days postoperatively.
Migration of Intracranial Fragments
Intracerebral migration of a metallic fragment is a rare complication of penetrating head injury.96,97 When migration through the brain parenchyma occurs, neurologic deterioration may result.98,99 Crossing of the midline has also been reported.93 If migration has been documented, removal of the offending fragment is indicated to prevent further injury. Rosenthal and colleagues recommend neuronavigation systems in planning the surgical approach to remove retained metallic fragments.77
The risk of migration is high when a fragment is lodged within the ventricular system.100 This may lead to obstructive hydrocephalus.101 Removal of such a fragment is warranted and may be assisted by judicious positioning of the patient preoperatively to lodge the fragment in the occipital horn of the lateral ventricle.100
Post-traumatic Cerebrovascular Lesions
In PBI the vasculature may be damaged either directly by the projectile, or by shearing forces generated by the pressure wave of the expanding and contracting temporary cavity. In most cases a traumatic pseudoaneurysm results from complete disruption of the arterial wall with periarterial hematoma formation. In these false aneurysms, the defect is bound only by hematoma, fibrin, and surrounding brain tissue. In rare cases, a true traumatic intracranial aneurysm results from partial damage to the arterial wall. The incidence of post-traumatic intracranial aneurysms (TICAs) in PBI is uncertain. Rates of incidence in penetrating missile wounds are reported between 3% and 8%.16,102–104 The rate of vascular injury in stab wounds is much higher, and approached 35% in large series from South Africa.66,105 Traumatic aneurysms typically occur along the vessel’s length and not at the bifurcation (as in berry aneurysms). Most typically they occur along branches of the middle or anterior cerebral arteries, or the supraclinoid carotid artery.16,106
Computed tomography angiography may be used to screen for traumatic aneurysms, but conventional angiography is the diagnostic study of choice. The varying reported rates of TICA may depend in part on the timing of the angiogram. There is no consensus regarding the optimal timing of angiography. Although some authors advocate angiography in the immediate postinjury period,103 others note that these lesions may only become angiographically and clinically evident much longer after the initial insult.106 A negative angiogram performed soon after injury does not rule out the development of a TICA. The study should be repeated if vasospasm is noted on the angiogram because this may hide a vascular lesion. The clinician must maintain a high index of suspicion for post-traumatic vascular injury in PBI. Any patient who develops a delayed intracerebral or subarachnoid hemorrhage or has otherwise unexplained neurologic deterioration must be suspected of harboring a TICA. Higher incidences have also been reported in cases where the projectile crosses two dural compartments or involves the facial, orbital, or pterional regions.102 Angiography should be considered in all such cases.
Post-traumatic aneurysms have a high rate of repeat hemorrhage.16,106 The rate of mortality in untreated post-traumatic aneurysms has been reported to be as high as 41%.107 When a TICA is diagnosed, prompt therapy is indicated. The goal is exclusion of the lesion from the circulation. Endovascular therapy is an excellent first-line therapeutic option. In the rare case of a true aneurysm, coiling of the lesion will be possible. In most cases, the TICA will need to be trapped either by endovascular coils or surgically. Most patients will tolerate the sacrifice of a distal branch of the middle or anterior cerebral artery, but in some cases a bypass procedure will be required.108,109 When surgery is performed, proximal control is crucial because these lesions have a tendency to rupture and dissection may be difficult in the injured brain. As in standard aneurysmal surgery, the use of a cerebral protectant is useful.
Cranial Stab Wounds
Although knives are by far the most common weapon in stab assaults, objects as innocuous as a pencil110,111 or a sewing needle may penetrate the cranium.112 The orbit is a common site for penetration into the cranial vault, especially in children.111–114 Because less kinetic energy is transmitted by a stab weapon, the injury is usually limited to the site of penetration. The site of penetration, its depth, and its trajectory are important factors in determining the extent of damage (Figs. 134-13 and 134-14). Intracerebral hematoma and vascular injury are a common result. In two large series from South Africa, rates of vascular injury were between 31% and 35%.66,115 Most often the patient will present with the weapon removed prior to arrival to a medical facility.66 In all cases, the radiologic evaluation should begin with a plain x-ray of the skull and CT. Both will demonstrate the characteristic “slot” fracture associated with a stab wound. CT shows the extent and location of intracerebral hematoma, subarachnoid hemorrhage, and in-driven bone fragments. When the weapon is still embedded in the skull, conventional or CT angiography is essential and should be obtained prior to any attempted removal.116,117 Approximately one third of patients will have a vascular injury.115 In all cases, care must be taken in the removal of the penetrating object, because vigorous manipulation will often increase the damage to brain structures. Cross-matched blood should be available in the operating room. Proximal and distal control of the vessel should be considered prior to craniotomy. Removal of impaled objects should only be performed in the operating theater66,115,117 after the scalp incision and bone flap are completed. The trajectory of removal must follow precisely that of the weapon’s entry. Once the foreign body is removed, surgical management is similar to that for missile injuries, with debridement of the tract, removal of any hematomas or accessible bone chips, and watertight closure of the dura. In a large series of 330 patients, du Trevou and van Dellen demonstrated the utility of early angiography in cranial stab wounds and concluded that there is no advantage to delaying angiography.66 The information gained from the angiogram can be utilized to achieve primary repair of the vascular lesion during the initial surgery or to expedite endovascular therapy. This is of paramount importance, given the high rates of mortality associated with a secondary bleed from a traumatic vascular lesion.66
Outcomes
The most epidemiologically accurate data on civilian gunshot injuries has been reported by Siccardi and colleagues because they included all gunshot victims in their series, including those who expired at the scene.118 Patients who died underwent autopsy so that data were obtained concerning the extent of their brain injuries. Seventy-three percent of patients died before reaching the hospital and 12% died within 3 hours of injury. All other civilian series reporting outcomes refer only to patients who survived to reach the emergency room, and their results must be viewed in the light of Siccardi’s important findings. In recent series of civilian penetrating brain injuries, mortality rates varied from 51% to 75%.50,58,60–62 Rates of mortality appear higher in self-inflicted injuries than in assaults in most series.50,60,61,118,119 Although rates of mortality are high in PBI, favorable outcome in survivors, defined as good recovery or moderate disability, is common (74%).120
Important factors influencing outcome include postresuscitation GCS score, age, and initial CT findings. The postresuscitation GCS score is perhaps the single most consistent factor among studies that is shown to influence outcome. Patients with a postresuscitation GCS score of 3 to 5 have extremely high rates of mortality in reported civilian series.50,60,61,67,118,121 Reported rates of recovery, including moderate disability, have ranged from 0% to 9% in this group. In fact, some authors have questioned the rationale for aggressive intervention in these patients given the dismal prognosis.60 In contrast, patients with postresuscitation GCS scores of 6 to 8 have outcomes from good recovery to moderate disability in 13% to 52% of cases, whereas those presenting with GCS score of 9 to 15 attain this result in 56% to 100% of cases.50,60,61,118,121
The influence of age on outcome in PBI is less clear. Two studies demonstrate worse outcomes in patients over 49 years of age.61,118 In other reports no statistically significant relationship was found between age and outcome.51,62
The findings on initial CT scan are also correlated with outcome. Factors which have been reported to be associated with poor outcome include bihemispheric injury,50,58,60–64 multilobar injury,12,50,51,57,118 transventricular trajectory,12 and intraventricular hemorrhage.61,64 Siccardi’s data, which included postmortem analysis of patients who died before CT could be performed, reported a mortality of 92% and 98% in patients with bihemispheric injuries and intraventricular blood, respectively.118 Subarachnoid hemorrhage is another factor reported as a correlate of unfavorable outcome.122 The presence of intracerebral hemorrhage has not been shown to be associated with worsened outcome.12,120
Aarabi B. Management of traumatic aneurysms caused by high-velocity missile head wounds. Neurosurg Clin North Am. 1995;6:775-797.
Aarabi B. Surgical outcome in 435 patients who sustained missile head wounds during the Iran-Iraq War. Neurosurgery. 1990;27:692-695.
Aldrich E.F., Eisenberg H.M., Saydjari C., et al. Predictors of mortality in severely head-injured patients with civilian gunshot wounds: a report from the NIH Traumatic Coma Data Bank. Surg Neurol. 1992;38(6):418-423.
American College of Surgeons Committee on Trauma. ATLS (Advanced Trauma for Life Support) for Doctors Student Manual, 8th ed. Chicago: American College of Surgeons; 2002.
Amirjamshidi A., Rahmat H., Abbassioun K. Traumatic aneurysms and arteriovenous fistulas of intracranial vessels associated with penetrating head injuries occurring during war: principles and pitfalls in diagnosis and management: a survey of 31 cases and review of the literature. J Neurosurg. 1996;84(5):769-780.
Antibiotic prophylaxis for penetrating brain injury. J Trauma. 2001;51(suppl 2):S34-S40.
Bell R., et al. Military traumatic brain and spinal column injury: a 5-year study of the impact blast and other military grade weaponry on the central nervous system. J Trauma. 2009;66:S104-S111.
Bhatia A., Gupta A.K. Neuromonitoring in the intensive care unit. I. Intracranial pressure and cerebral blood flow monitoring. Intensive Care Med. 2007;33:1263-1271.
Bhatia A., Gupta A.K. Neuromonitoring in the intensive care unit. II. Cerebral oxygenation monitoring and microdialysis. Intensive Care Med. 2007;33:1322-1328.
Brain Trauma Foundation. AANS, and Joint Section on Neurotrauma and Critical Care. Indications for intracranial pressure monitoring. J Neurotrauma. 2000;17(6-7):479-491.
Brierbrauer K., Tindall S. Gunshot wounds to the head and spine, Part I. Contemp Neurosurg. 1987;9(20):1-5.
Brierbrauer K., Tindall S. Gunshot wounds to the head and spine, Part II. Contemp Neurosurg. 1987;9(21):1-6.
Carey M.E., Sarna G.S., Farrell J.B., et al. Experimental missile wound to the brain. J Neurosurg. 1989;71(5 Pt 1):754-764.
Carey M.E. The treatment of wartime brain wounds: traditional versus minimal debridement. Surg Neurol. 2003;60(2):112-119.
Cushing H. Notes on penetrating wounds of the brain. BMJ. 1918;1:221-226.
Hammon W. Analysis of 2187 consecutive penetrating wounds of the brain in Vietnam. J Neurosurg. 1971;34:127-131.
Harvey E.N., Butler E., McMillen J., et al. Mechanisms of wounding. War Med. 1945;8:91-104.
Kapapa T., Konig K., Heissler H.E., Schatzmann C., et al. The use of recombinant activated factor VII in neurosurgery. Surgical Neurology. 2009;71:172-179.
Kapp J.P., Gielchinsky I., Deardourff S.L. Operative techniques for management of lesions involving the dural venous sinuses. Surg Neurol. 1977;7(6):339-342.
Kieck C.F., de Villiers J.C. Vascular lesions due to transcranial stab wounds. J Neurosurg. 1984;60(1):42-46.
Martin J., Campbell E. Early complications following penetrating wounds of the skull. J Neurosurg. 1946;2:58-73.
Prognosis in penetrating head injury. J Trauma. 2001;52:544-586.
Swan K.G., Swan R.C. Wound ballistics for the civilian surgeon. Surg Ann. 1985;17:163-187.
Temkin N.R. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(suppl 2):10-13.
Whitaker R. Gunshot wounds of the cranium: with special reference to those of the brain. Br J Surg. 1916;3:708-735.
1. de Villiers J. The management of missile injuries of the head during the Anglo-Boer War. Br J Neurosurg. 1987;1:53-61.
2. Cushing H. A study of a series of wounds involving the brain and its enveloping structures. Br J Surg. 1918;5:558-684.
3. Cushing H. Notes on penetrating wounds of the brain. BMJ. 1918;1:221-226.
4. Slemon H. Forward neurosurgery in Italy. J Neurosurg. 1945;2:332-339.
5. Haynes W. Penetrating brain wounds: analysis of 342. cases. J Neurosurg. 1945;2:365-378.
6. Martin J., Campbell E. Early complications following penetrating wounds of the skull. J Neurosurg. 1946;2:58-73.
7. Munslow R. Penetrating head wounds: experiences from the Italian campaign. Ann Surg. 1946;123:180-189.
8. Lewin W. Missile head wounds in the Korean campaign: a survey of British casualties. Br J Surg. 1956;43:628-632.
9. Hammon W. Analysis of 2187 consecutive penetrating wounds of the brain in Vietnam. J Neurosurg. 1971;34:127-131.
10. Rish B.L., Caveness W.F., Dillon J.D., et al. Analysis of brain abscess after penetrating craniocerebral injuries in Vietnam. Neurosurgery. 1981;9(5):535-541.
11. Salazar A.M., Jabbari B., Vance S.C., et al. Epilepsy after penetrating head injury. I: Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35(10):1406-1414.
12. Brandvold B., Levi L., Feinsod M., et al. Penetrating craniocerebral injuries in the Israeli involvement in the Lebanese conflict, 1982-1985: analysis of a less aggressive surgical approach. J Neurosurg. 1990;72(1):15-21.
13. Levi L., Borovich B., Guilburd J.N., et al. Wartime neurosurgical experience in Lebanon, 1982-1985. I: penetrating craniocerebral injuries. Isr J Med Sci. 1990;26:548-554.
14. Aarabi B. Surgical outcome in 435. patients who sustained missile head wounds during the Iran-Iraq War. Neurosurgery. 1990;27:692-695.
15. Aarabi B. Causes of infections in penetrating head wounds in the Iran-Iraq War. Neurosurgery. 1989;25:923-926.
16. Aarabi B. Traumatic aneurysms of brain due to high velocity missile head wounds. Neurosurgery. 1988;22:1056-1063.
17. Levi L., Borovich B., Guilburd J.N., et al. Wartime neurosurgical experience in Lebanon, 1982-1985. II: closed craniocerebral injuries. Isr J Med Sci. 1990;26:555-558.
18. Bell R., et al. Military traumatic brain and spinal column injury: a 5-year study of the impact blast and other military grade weaponry on the central nervous system. J Trauma. 2009;66:S104-S111.
19. Hopkinson D.A., Marshall T.K. Firearm injuries. Br J Surg. 1967;54(5):344-353.
20. Swan K.G., Swan R.C. Wound ballistics for the civilian surgeon. Surg Ann. 1985;17:163-187.
21. Allen I.V., Scott R., Tanner J.A. Experimental high-velocity missile head injury. Injury. 1982;14:183-193.
22. Harvey E.N., Butler E., McMillen J., et al. Mechanisms of wounding. War Med. 1945;8:91-104.
23. Fackler M.L., Bellamy R.F., Malinowski J.A. The wound profile: illustration of the missile-tissue interaction. J Trauma. 1988;28(suppl 1):S21-S29.
24. Carey M.E., Sarna G.S., Farrell J.B., et al. Experimental missile wound to the brain. J Neurosurg. 1989;71(5 Pt 1):754-764.
25. Crockard H., Brown F., Calica A., et al. Physiological consequences of experimental cerebral missile injury and use of data analysis to predict survival. J Neurosurg. 1977;46:784-794.
26. Carey M.E., Sarna G.S., Farrell J.B., et al. Experimental missile wound to the brain. J Neurosurg. 1989;71(5 Pt 1):754-764.
27. Levett J.M., Johns L.M., Replogle R.L., et al. Cardiovascular effects of experimental cerebral missile injury in primates. Surg Neurol. 1980;13(1):59-64.
28. American College of Surgeons Committee on Trauma. ATLS (Advanced Trauma for Life Support) for Doctors Student Manual, 8th ed., Chicago: American College of Surgeons, 2002.
29. Teasdale G., Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81-84.
30. Kennedy F.R., Gonzalez P., Beitler A., et al. Incidence of cervical spine injury in patients with gunshot wounds to the head. South Med J. 1994;87(6):621-623.
31. Caveness W.F., Meirowsky A.M., Rish B.L., et al. The nature of posttraumatic epilepsy. J Neurosurg. 1979;50(5):545-553.
32. Annegers J.F., Hauser W.A., Coan S.P., et al. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338(1):20-24.
33. Glotzner F.L., Haubitz I., Miltner F., et al. Seizure prevention using carbamazepine following severe brain injuries. Neurochirurgia (Stuttg). 1983;26(3):66-79.
34. Temkin N.R., Dikmen S.S., Wilensky A.J., et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497-502.
35. Cooper P.. Gunshot Wounds of the Brain, Cooper P., editor. Head Injury, 3rd ed., Baltimore: Williams & Wilkins, 1993.
36. George E., Dagi T.. Penetrating missile injuries of the head, Schmidek H., Sweet W. Operative Neurosurgical Techniques: Indications, Methods, and Results, 2nd ed., Orlando: Grune & Stratton, 1988.
37. Kaufman H.H. Treatment of civilian gunshot wounds to the head. Neurosurg Clin North Am. 1991;2(2):387-397.
38. Saba M. Surgical management of gunshot wounds of the head. In: Schmidek H., Sweet W. Operative Neurosurgical Techniques: Indications, Methods, and Results. 2nd ed. Orlando: Grune & Stratton; 1988:37-48.
39. Temkin N.R. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(suppl 2):10-13.
40. Langendorf F.G., Pedley T.A., Temkin N.R. Posttraumatic seizures. In: Engel Jr J., Pedley T.A. Epilepsy: a comprehensive textbook. Philadelphia: Lippincott Williams & Wilkins; 2008:2537-2542.
41. Whitaker R. Gunshot wounds of the cranium: with special reference to those of the brain. Br J Surg. 1916;3:708-735.
42. Aarabi B., Taghipour M., Alibaii E., et al. Central nervous system infections after military missile head wounds. Neurosurgery. 1998;42(3):500-507. discussion 507-509
43. Taha J.M., Haddad F.S., Brown J.A. Intracranial infection after missile injuries to the brain: report of 30 cases from the Lebanese conflict. Neurosurgery. 1991;29(6):864-868.
44. Aarabi B. Comparative study of bacteriological contamination between primary and secondary exploration of missile head wounds. Neurosurgery. 1987;20(4):610-616.
45. Benzel E.C., Day W.T., Kesterson L., et al. Civilian craniocerebral gunshot wounds. Neurosurgery. 1991;29(1):67-71. discussion 71-72
46. Byrnes D.P., Crockard H.A., Gordon D.S., et al. Penetrating craniocerebral missile injuries in the civil disturbances in Northern Ireland. Br J Surg. 1974;61(3):169-176.
47. Helling T.S., McNabney W.K., Whittaker C.K., et al. The role of early surgical intervention in civilian gunshot wounds to the head. J Trauma. 1992;32(3):398-400.
48. Hubschmann O., Shapiro K., Baden M., et al. Craniocerebral gunshot injuries in civilian practice: prognostic criteria and surgical management: experience with 82 cases. J Trauma. 1979;19(1):6-12.
49. Lillard P.L. Five years experience with penetrating craniocerebral gunshot wounds. Surg Neurol. 1978;9(2):79-83.
50. Nagib M.G., Rockswold G.L., Sherman R.S., et al. Civilian gunshot wounds to the brain: prognosis and management. Neurosurgery. 1986;18(5):533-537.
51. Suddaby L., Weir B., Forsyth C. The management of .22. caliber gunshot wounds of the brain: a review of 49. cases. Can J Neurol Sci. 1987;14(3):268-272.
52. Carey M., Young H., Mathis J., et al. A bacteriological study of craniocerebral missile wounds from Vietnam. J Neurosurg. 1971;34:145-154.
53. Meirowsky A.M., Caveness W.F., Dillon J.D., et al. Cerebrospinal fluid fistulas complicating missile wounds of the brain. J Neurosurg. 1981;54(1):44-48.
54. Arendall R.E., Meirowsky A.M. Air sinus wounds: an analysis of 163. consecutive cases incurred in the Korean War, 1950-1952. Neurosurgery. 1983;13(4):377-380.
55. Kaufman H.H., Schwab K., Salazar A.M. A national survey of neurosurgical care for penetrating head injury. Surg Neurol. 1991;36(5):370-377.
56. Antibiotic prophylaxis for penetrating brain injury. J Trauma. 2001;51(suppl 2):S34-S40.
57. Shoung H.M., Sichez J.P., Pertuiset B. The early prognosis of craniocerebral gunshot wounds in civilian practice as an aid to the choice of treatment: a series of 56. cases studied by computerized tomography. Acta Neurochir (Wien). 1985;74(1-2):27-30.
58. Clark W.C., Muhlbauer M.S., Watridge C.B., et al. Analysis of 76. civilian craniocerebral gunshot wounds. J Neurosurg. 1986;65(1):9-14.
59. Cooper P.R., Maravilla K., Cone J. Computerized tomographic scan and gunshot wounds of the head: indications and radiographic findings. Neurosurgery. 1979;4(5):373-380.
60. Grahm T.W., Williams F.C.Jr., Harrington T., et al. Civilian gunshot wounds to the head: a prospective study. Neurosurgery. 1990;27(5):696-700. discussion 700
61. Kaufman H.H., Makela M.E., Lee K.F., et al. Gunshot wounds to the head: a perspective. Neurosurgery. 1986;18(6):689-695.
62. Jacobs D.G., Brandt C.P., Piotrowski J.J., et al. Transcranial gunshot wounds: cost and consequences. Am Surg. 1995;61(8):647-653. discussion 653-654
63. Kaufman H., Levy M., Stone J., et al. Patients with Glasgow Coma Scale scores 3, 4, 5. after gunshot wounds to the brain. Neurosurg Clin North Am. 1995;6:701-714.
64. Shaffrey M.E., Polin R.S., Phillips C.D., et al. Classification of civilian craniocerebral gunshot wounds: a multivariate analysis predictive of mortality. J Neurotrauma. 1992;9(suppl 1):S279-S285.
65. Aldrich E.F., Eisenberg H.M., Saydjari C., et al. Predictors of mortality in severely head-injured patients with civilian gunshot wounds: a report from the NIH Traumatic Coma Data Bank. Surg Neurol. 1992;38(6):418-423.
66. du Trevou M.D., van Dellen J.R. Penetrating stab wounds to the brain: the timing of angiography in patients presenting with the weapon already removed. Neurosurgery. 1992;31(5):905-911. discussion 911-912
67. Mancuso P., Chiaramonte I., Passanisi M., et al. Craniocerebral gunshot wounds in civilians: report on 40. cases. J Neurosurg Sci. 1988;32(4):189-194.
68. Brierbrauer K., Tindall S. Gunshot wounds to the head and spine, Part I. Contemp Neurosurg. 1987;9(20):1-5.
69. Brierbrauer K., Tindall S. Gunshot wounds to the head and spine, Part II. Contemp Neurosurg. 1987;9(21):1-6.
70. Miner M.E., Ewing-Cobbs L., Kopaniky D.R., et al. The results of treatment of gunshot wounds to the brain in children. Neurosurgery. 1990;26(1):20-24. discussion 24-25
71. Kelly D., Nikas D., Becker D. Diagnosis and treatment of moderate and severe head injuries in adults. In: Youmans J., editor. Neurological Surgery. Philadelphia: WB Saunders, 1996.
72. Taha J.M., Saba M.I., Brown J.A. Missile injuries to the brain treated by simple wound closure: results of a protocol during the Lebanese conflict. Neurosurgery. 1991;29:380-384.
73. Brandvold B., Levi L., Feinsod M., George E. Penetrating craniocerebral injuries in the Israeli involvement in the Lebanese conflict, 1982-1985. J Neurosurg. 1990;72:15-21.
74. Amirjamshidi A. Minimal debridement or simple wound closure as the only surgical treatment in war victims with low-velocity penetrating head injuries. Indications and management protocol based upon more than 8. years follow up of 99. cases from Iran-Iraq conflict. Surg Neurol. 2003;60:105-111.
75. Carey M.E. The treatment of wartime brain wounds: traditional versus minimal debridement. Surg Neurol. 2003;60(2):112-119.
76. Crockard H.A. Early intracranial pressure studies in gunshot wounds of the brain. J Trauma. 1975;15(4):339-347.
78. Brain Trauma Foundation. AANS, and Joint Section on Neurotrauma and Critical Care. Indications for intracranial pressure monitoring. J Neurotrauma. 2000;17:479-491.
79. Zabramski J.M., Whiting D., Darouiche R.O., et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98(4):725-730.
80. Meirowsky A.M. Wounds of dural sinuses. J Neurosurg. 1953;10(5):496-514.
81. Cooper P. Depressed skull fracture. In: Apuzzo M., editor. Brain Surgery: Complication Avoidance and Management. New York: Churchill Livingstone; 1993:1273-1282.
82. Donaghy R.M., Wallman L.J., Flanagan M.J., et al. Sagittal sinus repair: technical note. J Neurosurg. 1973;38(2):244-248.
83. Kapp J.P., Gielchinsky I., Deardourff S.L. Operative techniques for management of lesions involving the dural venous sinuses. Surg Neurol. 1977;7(6):339-342.
84. Bhatia A., Gupta A.K. Neuromonitoring in the intensive care unit. I. Intracranial pressure and cerebral blood flow monitoring. Intensive Care Med. 2007;33:1263-1271.
85. Bhatia A., Gupta A.K. Neuromonitoring in the intensive care unit. II. Cerebral oxygenation monitoring and microdialysis. Intensive Care Med. 2007;33:1322-1328.
86. Kaufman H.H., Moake J.L., Olson J.D., et al. Delayed and recurrent intracranial hematomas related to disseminated intravascular clotting and fibrinolysis in head injury. Neurosurgery. 1980;7(5):445-449.
87. Dutton R.P., Hess J.R., Scalea T.M. Recombinant factor VIIa for control of hemorrhage: early experience in critically ill trauma patients. J Clin Anesth. 2003;15(3):184-188.
88. Eikelboom J.W., Bird R., Blythe D., et al. Recombinant activated factor VII for the treatment of life-threatening haemorrhage. Blood Coagul Fibrinolysis. 2003;14(8):713-717.
89. Bouwmeester F.W., Jonkhoff A.R., Verheijen R.H., et al. Successful treatment of life-threatening postpartum hemorrhage with recombinant activated factor VII. Obstet Gynecol. 2003;101(6):1174-1176.
90. Park P., Fewel M.E., Garton H.J., et al. Recombinant activated factor VII for the rapid correction of coagulopathy in nonhemophilic neurosurgical patients. Neurosurgery. 2003;53(1):34-38. discussion 38-39
91. Siegel L.J., Gerigk L., Tuettenberg J., et al. Cerebral sinus thrombosis in a trauma patient after recombinant activated factor VII infusion. Anesthesiology. 2004;100(2):441-443.
92. Kapapa T., Konig K., Heissler H.E., Schatzmann C., et al. The use of recombinant activated factor VII in neurosurgery. Surg Neurol. 2009;71:172-179.
93. Manelfe C., Cellerier P., Sobel D., et al. Cerebrospinal fluid rhinorrhea: evaluation with metrizamide cisternography. AJR Am J Roentgenol. 1982;138(3):471-476.
94. Ozgen T., Tekkok I.H., Cila A., et al. CT cisternography in evaluation of cerebrospinal fluid rhinorrhea. Neuroradiology. 1990;32(6):481-484.
95. el Gammal T., Brooks B.S. MR cisternography: initial experience in 41. cases. AJNR Am J Neuroradiol. 1994;15(9):1647-1656.
96. Bearcroft P.W., Freer C.E. CT of a peripatetic intracranial foreign body. Neuroradiology. 1995;37(7):542-544.
97. Alessi G., Aiyer S., Nathoo N. Home-made gun injury: spontaneous version and anterior migration of bullet. Br J Neurosurg. 2002;16(4):381-384.
98. Zafonte R.D., Watanabe T., Mann N.R. Moving bullet syndrome: a complication of penetrating head injury. Arch Phys Med Rehabil. 1998;79(11):1469-1472.
99. Duman H., Ziyal I.M., Canpolat A. Spontaneous subfalcial transcallosal migration of a missile to the contralateral hemisphere causing deterioration in neurological status: case report. Neurol Med Chir (Tokyo). 2002;42(8):332-333.
100. Milhorat T.H., Elowitz E.H., Johnson R.W., et al. Spontaneous movement of bullets in the brain. Neurosurgery. 1993;32(1):140-143.
101. Furlow L., Bender M., Teuber H. Movable foreign body within the cerebral ventricle. J Neurosurg. 1947;4:380-386.
102. Aarabi B. Management of traumatic aneurysms caused by high-velocity missile head wounds. Neurosurg Clin North Am. 1995;6(4):775-797.
103. Amirjamshidi A., Rahmat H., Abbassioun K. Traumatic aneurysms and arteriovenous fistulas of intracranial vessels associated with penetrating head injuries occurring during war: principles and pitfalls in diagnosis and management: a survey of 31 cases and review of the literature. J Neurosurg. 1996;84(5):769-780.
104. Rahimizadeh A., Abtahi H., Daylami M.S., et al. Traumatic cerebral aneurysms caused by shell fragments: report of four cases and review of the literature. Acta Neurochir (Wien). 1987;84(3-4):93-98.
105. Kieck C.F., de Villiers J.C. Vascular lesions due to transcranial stab wounds. J Neurosurg. 1984;60(1):42-46.
106. Haddad F.S., Haddad G.F., Taha J. Traumatic intracranial aneurysms caused by missiles: their presentation and management. Neurosurgery. 1991;28(1):1-7.
107. Fleischer A.S., Patton J.M., Tindall G.T. Cerebral aneurysms of traumatic origin. Surg Neurol. 1975;4(2):233-239.
108. Batjer H., Giller C., Kopitnik T., et al. Intracranial and cervical vascular injuries, Cooper P.R., editor. Head Injury, 3rd ed., Baltimore: Williams & Wilkins, 1993.
109. Spetzler R.F., Owen M.P. Extracranial-intracranial arterial bypass to a single branch of the middle cerebral artery in the management of a traumatic aneurysm. Neurosurgery. 1979;4(4):334-337.
110. Bursick D.M., Selker R.G. Intracranial pencil injuries. Surg Neurol. 1981;16(6):427-431.
111. Ildan F., Bagdatoglu H., Boyar B., et al. The nonsurgical management of a penetrating orbitocranial injury reaching the brain stem: case report. J Trauma. 1994;36(1):116-118.
112. Ashkenazi E., Mualem N., Umansky F. Successful removal of an intracranial needle by an ophthalmologic magnet: case report. J Trauma. 1990;30(1):114-115.
113. Di Roio C., Jourdan C., Mottolese C., et al. Craniocerebral injury resulting from transorbital stick penetration in children. Childs Nerv Syst. 2000;16(8):503-506. discussion 507
114. Matsumoto S., Hasuo K., Mizushima A., et al. Intracranial penetrating injuries via the optic canal. AJNR Am J Neuroradiol. 1998;19(6):1163-1165.
115. Kieck C.F., de Villiers J.C. Vascular lesions due to transcranial stab wounds. J Neurosurg. 1984;60(1):42-46.
116. Bullock R., van Dellen J.R. Acute carotid-cavernous fistula with retained knife blade after transorbital stab wound. Surg Neurol. 1985;24(5):555-558.
117. van Dellen J.R., Lipschitz R. Stab wounds of the skull. Surg Neurol. 1978;10(2):110-114.
118. Siccardi D., Cavaliere R., Pau A., et al. Penetrating craniocerebral missile injuries in civilians: a retrospective analysis of 314 cases. Surg Neurol. 1991;35(6):455-460.
119. Levi L., Linn S., Feinsod M. Penetrating craniocerebral injuries in civilians. Br J Neurosurg. 1991;5(3):241-247.
120. Prognosis in penetrating head injury. J Trauma. 2001;52:544-586.
121. Levy M.L., Masri L.S., Levy K.M., et al. Penetrating craniocerebral injury resultant from gunshot wounds: gang-related injury in children and adolescents. Neurosurgery. 1993;33(6):1018-1024. discussion 1024-1025
122. Levy M.L., Rezai A., Masri L.S., et al. The significance of subarachnoid hemorrhage after penetrating craniocerebral injury: correlations with angiography and outcome in a civilian population. Neurosurgery. 1993;32(4):532-540.